Abstract
Background
We reported earlier that the cytokine macrophage migration inhibitory factor (MIF) is a potential biomarker in burn injury. In the present study, we investigated the clinical significance in severely burned patients of expression levels the newly discovered MIF family member D-dopachrome tautomerase (DDT or MIF-2) and their common soluble receptor CD74 (sCD74).
Methods
DDT and sCD74 serum levels were measured 20 severely burned patients and 20 controls. Serum levels were correlated to the abbreviated burn severity index (ABSI) and TBSA followed by receiver operating characteristic (ROC) analysis. Data were supported by gene expression dataset analysis of 31 burn patients and 28 healthy controls.
Results
CD74 and DDT were increased in burn patients. Furthermore, CD74 and DDT also were elevated in septic non-survivors when compared to survivors. Serum levels of DDT showed a positive correlation with the ABSI and TBSA in the early stage after burn injury, and the predictive character of DDT was strongest at 24 hrs. Serum levels of CD74 only correlated with the ABSI five days post-injury.
Conclusions
DDT may assist in the monitoring of clinical outcome and prediction of sepsis during the early post-burn period. sCD74 and MIF, by contrast, have limited value as an early predictor of death due to their delayed response to burn injury.
Keywords: Burn, sepsis, DDT, CD74, MIF, biomarker
Introduction
Continued research and technological advances have led to a substantial improvement in the treatment of critically ill patients over the last three decades. A recently published comprehensive review, however, revealed that medical progress did not result in equivalent improvement in the survival rate of burn patients [1]. One of the leading causes of death in severely burned patients is sepsis and its sequelae and although a number of biomarkers have been proposed the early diagnosis still poses a difficulty in the management of septic patients [2]. Due to the complexity of burn injury, the search for a combination rather than a single biomarker appears to be sensible.
The cytokine macrophage migration inhibitory factor (MIF) has emerged as a promising biomarker as it is rapidly released from preformed intracellular pools in response to invasive stimuli such as microbial products and tissue damage (LPS) [3–5]. This unique characteristic distinguishes MIF from other transcriptionally activated and translated pro-inflammatory cytokines [6]. The type 2 transmembrane protein CD74 was the first receptor described to bind MIF [7, 8]. Genetic deletion of Mif, however, did not lead to a complete abrogation of CD74 activation in mouse models, suggesting the existence of another activating binding partner. This observation was recently explained by the discovery of the gene product of the D-dopachrome tautomerase (DDT, MIF-2) as a functional homolog of MIF and a new CD74 ligand [9]. Similar to MIF, DDT interacts with CD74 and activates pro-inflammatory cascades [10].
We earlier reported that, together with procalcitonin (PCT), MIF may be a biomarker for the prediction of sepsis and lethal outcome in burn patients [5]. A circulating soluble isoform of the MIF family receptor CD74 (sCD74) has recently been described [11], and since DDT and MIF share functional and molecular properties, we hypothesized that DDT and sCD74 may serve as informative biomarkers in burn patients. Therefore, we measured serum levels of sCD74 and DDT in severely burned patients and correlated the data with clinical parameters and outcome. Serum levels were further validated by analysis of DDT and CD74 gene expression.
Patients and Methods
Patients and Serum Samples
In a prospective study, we collected serum samples of patients treated in the Intensive Care Unit (ICU) of the RWTH-Aachen University Hospital Burn Center. All samples were collected after informed consent was provided by the patient or relatives. The study was approved by the local ethics committee (EK 104/08) and registered at ClinicalTrials.gov (Identifier: NCT02549079).
Patients with I – III degree burn injuries, a minimum total body surface area (TBSA) burn injury of greater than 10%, and older than 18 years were included in the study. Patients with pre-existing severe cardiovascular diseases, immunodeficiency, and malignancies were excluded. Patients were divided into survivors and non-survivors (Table 1). The serum samples were used for measurement of MIF levels that were previously reported [5].
Table 1.
Patient characteristics
| Patient | Age | Sex | Degree | TBSA | ABSI | Sepsis | Lethal |
|---|---|---|---|---|---|---|---|
| 1 | 49 | M | II | 20 | 6 | N | N |
| 2 | 67 | M | III | 80 | 16 | n/a | Y |
| 3 | 34 | F | II–III | 18 | 6 | N | N |
| 4 | 36 | M | II–III | 15 | 6 | N | N |
| 5 | 37 | F | II | 10 | 4 | N | N |
| 6 | 29 | M | III | 80 | 12 | Y | Y |
| 7 | 55 | M | II–III | 10 | 5 | N | N |
| 8 | 46 | M | II–III | 30 | 8 | N | N |
| 9 | 50 | M | II–III | 90 | 14 | n/a | Y |
| 10 | 38 | F | II–III | 85 | 14 | n/a | Y |
| 11 | 34 | M | I–III | 25 | 6 | N | N |
| 12 | 80 | F | II–III | 25 | 9 | N | N |
| 13 | 44 | M | II–III | 20 | 5 | N | N |
| 14 | 33 | M | II–III | 65 | 11 | N | N |
| 15 | 31 | F | II–III | 85 | 14 | N | N |
| 16 | 27 | M | III | 90 | 14 | Y | Y |
| 17 | 78 | F | III | 14 | 8 | N | N |
| 18 | 28 | M | II | 51 | 8 | N | N |
| 19 | 66 | F | III | 93 | 17 | Y | Y |
| 20 | 56 | M | II | 15 | 5 | N | N |
For each patient, blood samples were taken at five time points: 0h (time of admission to the ICU), 12h, 24h, 48h, and 120h. 20 healthy subjects were used for baseline measurements of DDT and CD74. The abbreviated burn severity index (ABSI) was assessed as a standardized scale for the severity of burn injury [12]. Sepsis was defined as the combination of systemic inflammatory response syndrom (SIRS) plus positive or suspected systemic infection [13].
DDT Enzyme-linked Immunosorbent Assay (ELISA)
The protein levels of DDT were measured by ELISA as previously reported [9]. Plates were read by an iMark™ Microplate Absorbance Reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
sCD74 ELISA
The circulating levels of sCD74 were measured from serum samples by ELISA as reported earlier [11]. Plates were read by an iMark™ Microplate Absorbance Reader.
Dataset Analysis
Gene expression analysis was performed by the software BRB-array tools (version 4.4.0 stable). mRNA expression data of DDT and CD74 were retrieved from the dataset GSE19743 which was downloaded from the gene expression omnibus (GEO) database. Total RNA was extracted from leukocytes isolated from peripheral blood samples and mRNA expression was analyzed on an Affymetrix U133 Plus 2.0 array platform.
Patients also were categorized according to their outcome into survivors and non-survivors. Log2-transformed expression data were exported by the BRB array tools software and differential expression of DDT, CD74 and calcitonin-related polypeptide alpha (CALCA), interleukin-1 receptor antagonist (IL-1ra), IL-6, IL-8, IL-10, tumor necrosis factor α (TNFα), was carried out by the analysis wizard tool comparing single classes.
Statistics
The software GraphPad Prism ® (GraphPad Software, Inc., La Jolla, CA, USA). All data are expressed as mean ± SEM. Statistical significance was calculated by student’s T-test and repeated measurement ANOVA with asterisks representing different levels of statistical significance: *p: <0.05, **: p <0.01 and ***p: < 0.001. Associations between CD74 and DDT levels and the TBSA and ABSI were calculated by Spearman’s rank correlation with assessment of linear regression and 95% confidence interval. Receiver operating characteristic (ROC) curves were computed to assess the accuracy of MIF, DDT, and CD74 for prediction of lethal outcome. MIF ELISA data was retrieved from a previous study and used for calculation of ROC curves [5].
Results
Patients
Out of 115 eligible patients, 93 patients were excluded based on their age, TBSA, medical preconditions, and other reasons (e.g. missing patient consent, death within first 24 hours). From 22 eligible patients, 2 patients were not studied further due to loss or insufficient volume of blood samples (Fig. 1).
Fig. 1. Flowchart.
Flowchart according to the Strobe recommendations
The mean age of the 20 included patients was 45.9 ± 3.7 years and mean TBSA was 46.05 ± 7.4 % (Table 1). Except for patient 11, all patients suffered at least second degree burns. Data were compared between the group of survivors and non-survivors. The group of non-survivors consisted of six patients who all showed a TBSA of at least 80%, ABSI scores 12, and septic conditions during medical treatment (for patients 2, 9, and 10 no clinical data regarding sepsis was available). Patients 6, 16, and 19 died within the first 120 hours, patients 2, 9, and 10 died within 10 days after burn injury.
DDT and sCD74 Serum Levels are Elevated in Burn Patients
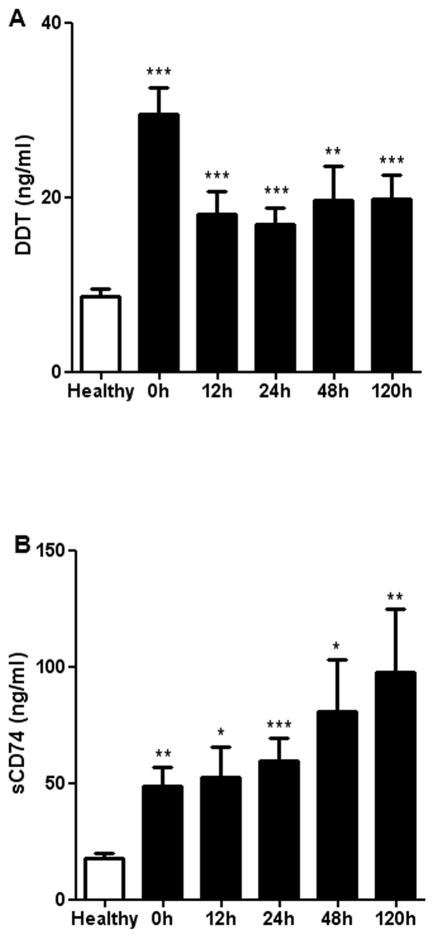
First, we measured circulating levels of DDT and sCD74 in the recruited cohort of burn patients and healthy controls. Patients with burn injuries showed significantly increased levels of DDT and sCD74 serum concentration when compared to healthy controls across all time points (Fig. 2). The dynamics of DDT and sCD74 release, however, are markedly different. While serum levels of sCD74 gradually increased over time, maximum DDT serum levels were observed at admission to ICU immediately after burn injury. After 12 hours, DDT serum levels decrease and remain at a plateau that was still significantly higher than DDT levels of the controls.
Fig. 2. Serum levels of DDT and sCD74 in burn patients.
Serum levels of DDT and sCD74 were measured in burn patients by ELISA over a time period of 120 hours. Healthy patients without burn injury served as controls. A Serum levels of DDT are highest at the time of admission and decrease within the first 12 hours. B Serum levels of sCD74 increase over time. * p <0.05, ** p <0.01, and *** p <0.001. Data represent mean ± SEM
DDT and sCD74 Serum Levels are Increased in Non-Survivors
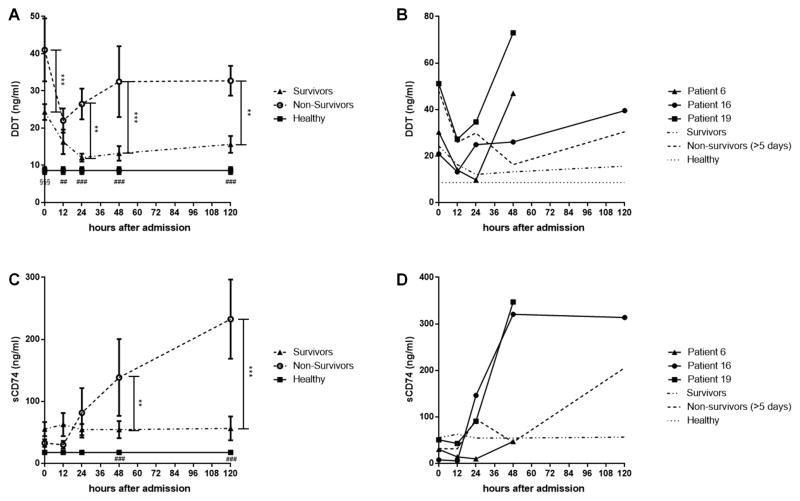
To evaluate the impact of DDT and sCD74 levels on the outcome, we compared DDT and sCD74 serum levels between survivors, non-survivors, and healthy controls (Fig. 3 a, c). Non-survivors showed a biphasic response of DDT to burn injury. After a decrease in the first 12 hours, DDT serum levels increased again until they reached a plateau after 48 hours. The difference between survivors and non-survivors was significant at 0h, 24h, 48h, and 120h. The difference between non-survivors and healthy controls was significant over the whole time period. In the group of survivors, by contrast, initially high DDT serum levels decreased in the first 24 hours and remained at a low plateau that was only a slightly higher level than the healthy control group. Except for the measurement on admission (where survivors showed significantly higher DDT serum levels than healthy controls), there was no significant difference between survivors and healthy controls. Similarly, sCD74 levels remained low over the whole time course in survivors which were again only minimally higher than the healthy control group (not significant). In non-survivors, however, sCD74 levels are low in the first 12 hours, but start to increase after 24 hours. At 48 and 120 hours, the difference in sCD74 levels between survivors and non-survivors/healthy controls was significant.
Fig. 3. Serum levels of DDT and sCD74 of survivors and non-survivors.
DDT and sCD74 serum levels were compared between surviving burn patients, non-surviving burn patients, and healthy controls. We further compared the levels between patients who died before 120 hours (patient 6, 16, 19) and patients who died between 120 hours and 10 days (“non-survivors (>5 days)”). A On admission, serum levels of DDT are significantly increased in non-survivors when compared to survivors and healthy controls. The DDT serum levels of survivors also are significantly higher when compared to controls. After a temporary decrease, DDT levels again increase in non-survivors. In survivors, DDT serum levels remain low with no statistical difference to healthy controls. B Patients 6, 16, and 19 who died within 5 days after admission show a rapid increase pre mortem when compared to patients who died after 5 days (“non-survivors (>5 days)”). C Serum levels of sCD74 are comparable in the early stage after burn injury but increase in non-survivors after 24 hours. Serum CD74 levels of survivors are slightly higher than healthy controls (statistically not significant). D Except for patient 6, patients who died within 5 days after admission (patients 16, 19) show higher levels of sCD74 when compared to patients who died after 5 days (“non-survivors (>5 days)”). Significant difference between non-survivors and survivors: ** p <0.01, *** p <0.001. Significant difference between non-survivors and healthy control: ## p <0.01, ### p <0.001. Significant difference between non-survivors and healthy control/survivor and healthy control: §§§ p <0.001. Data represent mean ± SEM
We also investigated DDT and sCD74 serum levels in patients who died within the first 120 hours (patients 6, 16, 19) and compared the values between survivors and non-survivors within the first ten days after burn injury (non –survivors: 2, 9, 10) (Figure 3 b, d). DDT and sCD74 serum levels of the patients were markedly elevated immediately before death in patients 6, 16, and 19. In patients 2, 9 and 10. By contrast, DDT and sCD74 serum levels were lower in the first 120 hours. The only exception was sCD74 serum levels of patient 16 which remained low.
DDT Serum Levels Correlate with ABSI and TBSA, and sCD74 Serum Levels Correlate with ABSI
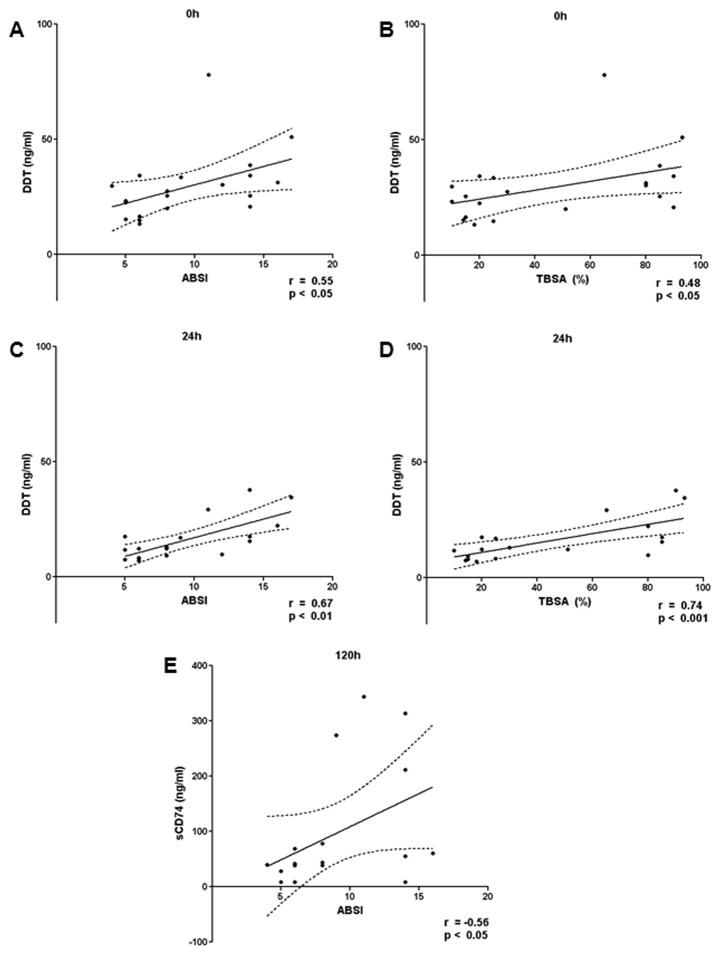
To evaluate the possible associations of DDT and sCD74 with TBSA and ABSI scores, we performed Spearman’s rank correlation analysis. DDT showed a significant positive correlation with ABSI and TBSA at 0h and 24h after ICU admission (Fig. 3). DDT positively correlated with ABSI and TBSA at all other time points but the correlations did not reach statistical significance (Supplementary Fig. 1 and 2). Serum levels of sCD74 positively correlated with the ABSI 120 hours after ICU admission (Fig. 4). No relevant correlations were observed at the other time points (Supplementary Fig. 3 and 4).
Fig. 4. Correlation between DDT/sCD74 serum levels and ABSI/TBSA.
DDT and sCD74 serum levels were correlated with ABSI and TBSA at each time point by Spearman’s rank correlation. While CD74 showed only one significant correlation, DDT was positively associated with ABSI and TBSA at 0h and 24h. Only significant correlations are shown with their respective p-value and Spearman r value. The complete correlation data is found in Supplementary Figure 1–4. A DDT and ABSI at 0h. B DDT and TBSA at 0h. C DDT and ABSI at 24h. D DDT and TBSA at 24h. E sCD74 and ABSI at 120h. ABSI – abbreviated burn severity index; TBSA – total burn surface area
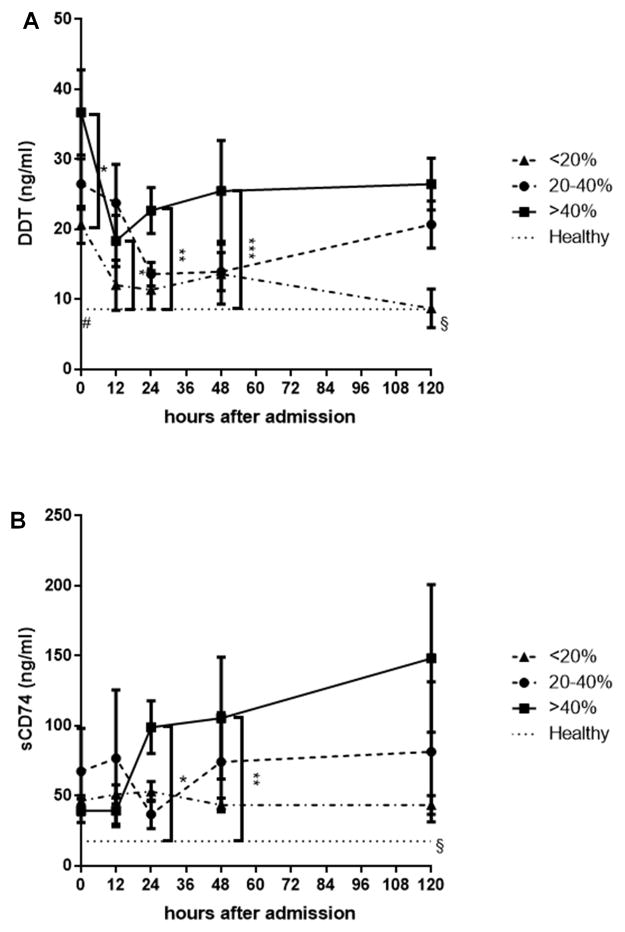
We further assessed the impact of the TBSA on serum DDT and sCD74 levels by dividing the patients into three groups according to their TBSA: TBSA <20%, TBSA 20–40%, and TBSA >40%. DDT serum levels were highest in the TBSA >40% group and significant differences were already observed at 0h. In the group of TBSA <20%, DDT levels normalized after five days whereas an increase was seen in the group of TBSA 20–40%. Serum CD74 serum levels also were highest in the group of TBSA >40%. The differences, however, became more evident after 24 hours while initial sCD74 levels were low regardless the TBSA
Potential of MIF, DDT and sCD74 as Predictors for Lethal Outcome
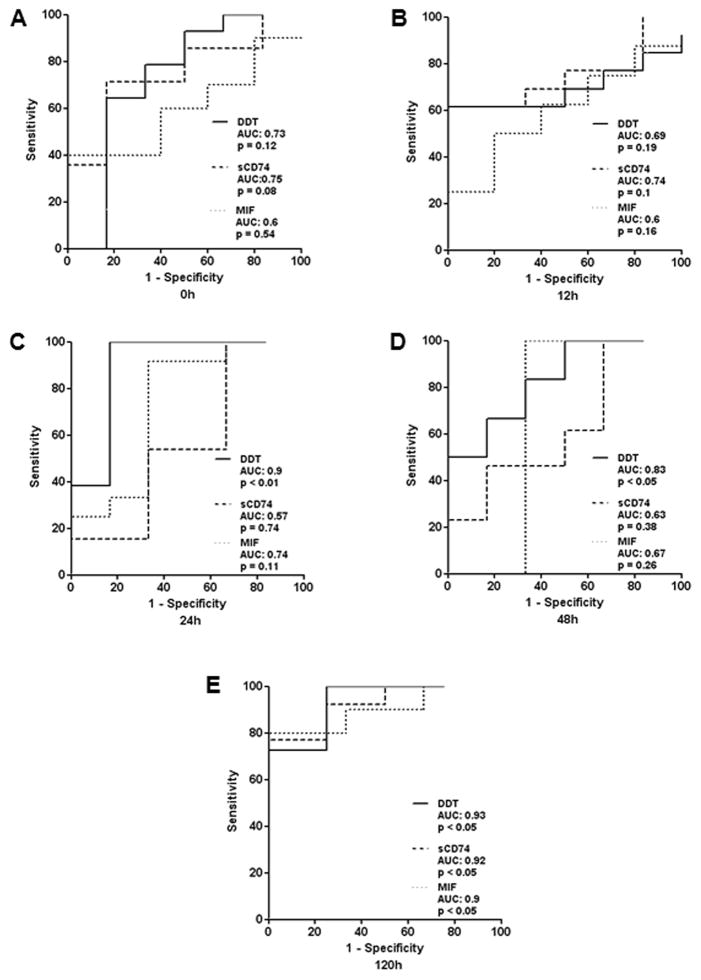
In order to assess the ability of serum DDT and sCD74 to predict lethal outcome in burn patients, ROC curves were calculated (Fig. 5). ROC curves for DDT were significant at 24h, 48h, and 120h. At 24h, a cutoff level of 19.97 ng/ml reached a sensitivity of 1 and a specificity of 0.833 with an area under the curve (AUC) of 0.9. At 48h and 120h, AUC curves showed a comparably low specificity (48h: 20.66 ng/ml - sensitivity of 0.833 / specificity of 0.667; 120h: 29.92 ng/ml – sensitivity of 1 / specificity of 0.75). In the case of sCD74, we detected the best predictive accuracy with highest sensitivity and specificity at a cutoff level of 43.7 ng/ml (sensitivity of 0.714 / specificity of 0.833) on admission to the ICU. AUC curves at 12h, 24h, and 48h showed a weak prediction of lethal outcome. The only significant ROC curve with a sensitivity of 0.769 and specificity of 1 and an AUC of 0.9 was found at 120h (cutoff level of 58.28 ng/ml).
Fig. 5. Impact of TBSA on DDT and sCD74 serum levels.
To evaluate the impact of burn size on DDT and sCD74 serum levels, we divided burn patients into three groups: TBSA <20%, TBSA 20–40%, and TBSA >40%. We compared the serum levels between three groups A DDT serum levels increased with burn size. Significant differences are already seen at 0h. In the group of TBSA <20%, DDT levels normalized after five days. B Serum CD74 serum levels also increased with the TBSA. The differences, however, became more evident in the later stages. * p <0.05, ** p <0.01, *** p <0.001. Significant difference between healthy and TBSA >40%/TBSA 20–40%: # p <0.05. Significant difference between TBSA >40% and TBSA <20%/healthy control: § p <0.05. Data represent mean ± SEM. TBSA – total burn surface area
We also compared ROC curves of serum DDT and sCD74 to ROC curves calculated from corresponding MIF serum levels to identify any potential advantages of a combination of DDT, sCD74, and MIF value in the prediction of lethal outcome [5]. Similar to CD74, MIF showed weak predictive power in the early stages and the only significant ROC curve was found at 120h at a cut-off value of 16.71 ng/ml with a sensitivity of 80%, specificity of 100%, and AUC of 0.9.
DDT and CD74 mRNA Expression Levels are Elevated in Burn Patients
Measurements of sCD74 and DDT serum levels by ELISA were complemented by mRNA expression analysis (Fig. 6). We analyzed a publically available dataset and compared the mRNA expression levels of CD74 and DDT with the software BRB-array tools as described previously [14].
Fig. 6. Predictive value of DDT and sCD74.
The predictive character of DDT and sCD74 serum levels was calculated by ROC curve analysis at the five different time points. For each curve, AUC and p-values are illustrated. Significant AUC curves for DDT are found at 24h, 48h, and 120h, whereas the only significant AUC curve for sCD74 was observed at 120h. A AUC curve at 0h. B AUC curve at 12h. C AUC curve at 24h. D AUC curve at 48h. E AUC curve at 120h. ROC – receiver operating characteristics; AUC – area under the curve
The GSE19743 dataset includes mRNA expression data of 57 burn patients and 63 healthy controls. Total RNA was isolated from leukocytes of peripheral blood samples of the patients. From the original dataset, only patients over the age of 18 years were included in the analysis, leaving 31 burn patients (mean age 40.42 ± 2.09 years, 25 male, 6 female) and 28 healthy controls (35.43 ± 2.06 years, 17 male, 11 female). 12 burn patients did not survive. The dataset provided gene expression at an early (49.93 ± 9.26 h) and late time point (430.5 ± 23.62h) for each patient.
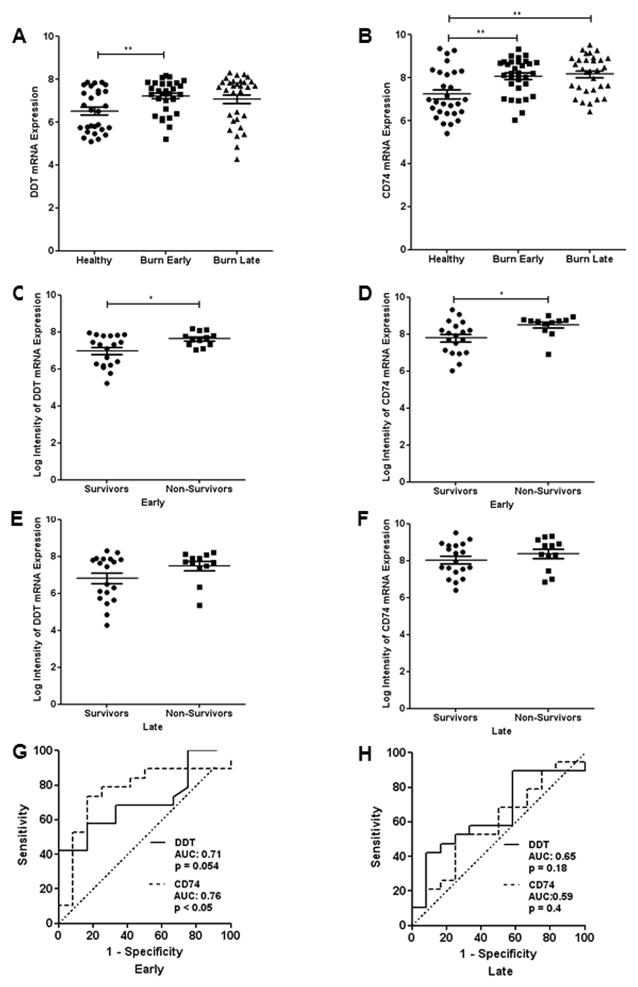
DDT and CD74 mRNA expression was significantly higher in burn patients when compared to healthy subjects at the early time point while only CD74 mRNA expression levels were significantly elevated at the late time point (Fig. 5). At the early time point, DDT and CD74 gene expression was significantly higher in survivors when compared to non-survivors. The GSE19743 dataset indicates that not only DDT and CD74 protein levels but also gene expression is up-regulated in the first days after burn injury and may predict lethality.
We found a significant ROC curve at the early time point for CD74. A cutoff level of 8.225 (log2 gene expression) showed a sensitivity of 0.737 and specificity of 0.833 with an AUC of 0.76. The ROC curve for DDT at the early time point narrowly missed significance (p = 0.054). The other ROC curves at the late time point did not reveal any meaningful predictive character of DDT and CD74.
Comparison of DDT and sCD74 mRNA expression and the Biomarkers CALCA, IL-1ra, IL-6, IL-8, IL-10, TNFα
Finally, the gene expression of CALCA, IL-1ra, IL-6, IL-8, IL-10, TNFα were analyzed in the GSE19743 dataset. CALCA is the gene encoding the inflammatory protein PCT. IL-1ra, IL-6, IL-8, IL-10, TNFα are cytokines that were proposed as biomarkers in burn injury.
Analog to the gene analysis of DDT and CD74, we first compared the gene expression between healthy donors and burn samples at the early (49.93 ± 9.26 h) and late 430.5 ± 23.62h) time point (Supplementary Figure 5). We found significant elevated IL1-ra, IL-10, and TNFα expression in early and late burn samples whereas IL-8 expression was significantly down-regulated at both time points. No differences were observed in IL-6 and CALCA gene expression. Next, we compared the differences between survivors and non-survivors at the early (Supplementary Figure 6) and late time point (Supplementary Figure 7), but could not show a significant increase of any of the cytokines. Considering the predictive accuracy for prediction of death, the only significant ROC curve was found for IL-1ra at the early time points whereas all other ROC curves showed weak predictive value of CALCA, IL-6, IL-8, IL-10, and TNFα (Supplementary Figure 8).
Discussion
DDT or MIF-2 is a newly discovered member of the MIF protein superfamily. As MIF’s only known homolog, DDT binds to the MIF receptor complex CD74/CD44 with high affinity [9]. MIF is a pro-inflammatory cytokine that serves as a biomarker for a variety of inflammatory, autoimmune, neoplastic, and metabolic diseases [4, 15, 16]. In this context, we recently investigated MIF’s role as a potential biomarker in burn patients and its predictive value for lethal outcome [5]. On admission, MIF serum levels were elevated in burn patients when compared to healthy controls and then varied in circulating level depending on clinical outcome. In survivors, MIF levels decreased over time to normal levels while in non-survivors MIF increased after an early decrease. PCT is one of the best studied biomarkers for sepsis and outcome in burn patients [17, 18]. Therefore, MIF levels also were evaluated in combination with PCT levels. A combination of PCT levels > 3 ng/ml and MIF levels > 20 ng/ml at 24h and 48h after admission correlated with sepsis and these patients died within five days. A combination of PCT levels > 3 ng/ml and MIF levels < 20 ng/ml was associated with sepsis and was predictive for lethal outcome between five to ten days.
We found an initial increase of serum DDT levels in burn patients immediately after admission to ICU. Compared to other inflammatory mediators, MIF and DDT are both located in preformed intracellular pools that are rapidly released in response to invasive or injurious stimuli [10]. The initially high circulating DDT levels decreased within 12 hours in all patients regardless to their outcome, which may be interpreted by depletion of preformed intracellular DDT pools without compensatory synthesis and release. Circulating DDT levels only increased again in non-survivors with highest levels immediately before death, whereas survivors demonstrated a normalization of serum levels that were comparable to healthy controls. The gene array analysis indicates that higher gene expression contributes to the sustained increase in DDT serum levels in non-survivors. In the early stages, serum DDT also positively correlates with TBSA and ABSI scores. Both, the TBSA and the ABSI score, which is used to rapidly estimate the severity and prognosis of burn injuries based on age, sex, TBSA, presence of inhalation injury, and full-thickness burns, correlate with the outcome in burn patients [16, 17]. We also showed that the burn size has significantly contributes to DDT serum levels. DDT’s strongest predictive power was detected 24 hours after ICU admission similarly to the strong predictive value of MIF and PCT at 24 hours and 48 hours [5]. While the sensitivity of DDT was high, low specificity restricted the overall predictive value. Although MIF and DDT are both stored in intracellular pools, DDT showed a higher predictive value than MIF whose only significant ROC curve was found at 120 hours. DDT may be considered as a biomarker for burn-related sepsis since in our study serum DDT only re-increased in septic burn patients and were particularly high immediately before death. These findings are in line with recent findings of Merk and colleagues who showed that DDT serum levels were positively associated with sepsis severity [9]. Furthermore, DDT serum levels in sepsis patients correlated with the APACHE II score which estimates the severity of disease and risk of death of ICU patients [19]. In this connection, increased intraoperative DDT levels also were associated with pneumonia, acute kidney injury and predictive for atrial fibrillation [16].
Although CD74 has been proposed as a potential biomarker in autoimmune hepatitis and complications that arise from cardiac surgery, its relevance in burn patients has not been investigated to date [11, 15, 20–22]. Soluble CD74 showed a different secretion pattern from DDT and MIF. We observed increased sCD74 serum levels and CD74 mRNA expression in peripheral leukocytes indicating that sCD74 may also be involved in systemic body response to burn injury. When serum levels of burn patients were divided into survivors and non-survivors, elevated sCD74 levels were mainly attributed to non-survivors and tended to be highest shortly before death. Survivors showed relatively low sCD74 levels with no statistically measurable difference to healthy controls. However, differences in sCD74 serum levels of survivors and non-survivors reached significance only at later stages of burn injury (48 and 120 hours after admission). Similarly, sCD74 levels did not show meaningful association with the ABSI score and TBSA except for a positive correlation with TBSA at 120h. As for MIF, the only significant ROC curve analysis was at 120 hours. Furthermore, the overall sensitivity, which is crucial to identify the probability of death, was low at time points. Therefore, sCD74 appears to have limited value as an early predictor for lethal outcome and may be regarded as a marker to monitor the clinical course and severity of burn patients at later time points together with MIF. sCD74 also may act as a biomarker for burn-related sepsis. In contrast to our findings, Cazalis et al. reported decreased mRNA levels of CD74 in septic shock patients and an association with increased risk of mortality [23]. This apparent contradiction may be explained by the dynamics of soluble factors which depend on the underlying insult. According to Orman et al. the behavior of cytokines after burn injuries differs significantly from other diseases or traumas (e.g. such as sepsis to cecal ligation and puncture treatment) [24].
It remains unknown whether DDT and sCD74 act as protective or detrimental factors in burn patients as we did not investigate any potential actions for these proteins in the enrolled patients. MIF and DDT are both released upon LPS stimulation, competitively bind to the CD74/CD44 MIF receptor complex, activate Src-family kinases, and initiate phosphorylation of extracellular-signal-regulated kinases (ERK) 1/2, which regulate further downstream actions [9, 25]. Importantly, MIF and DDT showed an additive activation of the ERK1/2 pathway, suggesting that the two proteins function synergistically. MIF is largely regarded as a pro-inflammatory cytokine and promotes expression of key cytokines such as tumor necrosis factor (TNF)-α [26]. Like MIF, neutralization of DDT protects mice from LPS-induced endotoxic shock [9]. Accordingly, DDT and MIF may act synergistically during burn injury. In the same vein, recent data received from cardiac surgery patients indicated that increased levels of DDT were associated with postoperative organ dysfunctions, which were suggested to be mediated through an enhanced recruitment of immune cells that may further amplify the peri-operative inflammatory response [16]. In contrast, MIF showed overall organo-protective characteristics, which may be due to the fact that DDT lacks two of three conserved cysteines (Cys 60 and Cys 81) that mediate intracellular MIF redox activity that may contribute to MIF’s beneficial properties in certain settings [27, 28].
On the other hand, evidence also supports a protective role of MIF and DDT through activation of adenosine monophosphate-activated protein kinase (AMPK) [29, 30]. Ischemic stress stimulates DDT release from murine hearts and protects the heart via CD74 and AMPK in an autocrine and parakrine manner [30]. However, in a burn model MIF exhibited a contrary, cardiodepressive effect [31]. Mice treated with anti-MIF showed ameliorated cardiac function after burn injury delineating the complexity of the role of MIF superfamily proteins in disease.
The function of sCD74 in burn injury also remains unknown. It was shown that sCD74 is released by stellate cells in the liver, binds to MIF and thereby neutralizes the bioactivity of MIF [11]. A potential neutralizing effect of CD74 in burn patients needs to be investigated in the future.
Common indicators and biomarkers that are routinely detected in clinical practice such as the body temperature, C-reactive protein (CRP), white blood cell count (WBC) are insufficient for the prediction and monitoring of sepsis and outcome in burned patients because of their nonspecific increase and delayed response to burn injury [17, 32]. Therefore, the identification of more specific biomarkers in the context of burn related sepsis is needed. PCT is the most thoroughly studied biomarker, which is routinely used in the clinical practice. A recent meta-analysis proposed that it is a beneficial marker for the diagnosis of sepsis in burn patients [33]. Lavrentieva et al. reported that maximum PCT levels were an independent predictor of outcome at day one and found a strong correlation with the onset of sepsis [34]. Other authors, however, discussed PCT’s limitation in the early diagnosis of septic burn patients [17]. A less well-established marker for the diagnosis of septic burn patients is IL-8, which was reported to correlate with sepsis, mortality, and infection [35]. We have earlier shown that IL-6 also may play a role in septic burn injuries while the exact mechanisms remain unknown [32]. Further biomarkers in septic burn patients include IL-1ra, IL-10, and TNFα [36]. In extension to these analyses, we aimed to evaluate the clinical value of DDT and CD74 compared to these afore-mentioned biomarkers. Therefore we studied the mRNA gene expression of CALCA, IL-1ra, IL-6, IL-8, IL-10, TNFα in the dataset. While IL-1ra, IL-8, IL-10, and TNFα showed significant changes in burned patients when compared to healthy controls, none of the genes were able to discriminate non-survivors from survivors. Furthermore, only IL-1ra showed a significant ROC curve for the prediction of death in the early burn samples. Taken together, these observations underline the value of DDT and CD74 in burn injury.
We acknowledge limitations of the present study. First, the number of serum samples included in this study is small and important parameters such as the severity of burn injury and age vary in our studied cohort. Second, our observation time only covered five days since the majority of patients were discharged or died after that time point so that no interpretation of long term results can be made. Measuring serum levels after 120 hours may have been helpful to distinguish the metabolic response of the early Ebb phase and the later hypermetabolic flow phase of burn injury [37]. A follow up of sCD74 and DDT serum levels after full recovery in survivors may have been beneficial to specify the significance of both factors in the aftercare. Third, the time of measurements refers to the time of admission to the ICU and not the time of injury which could not be exactly reconstructed with the available clinical information. Variation in the time of sample acquisition may especially affect the exact description of the dynamics of DDT at early time points due to its rapid release from preformed pools. Fourth, discrepancies between the time points of ELISA measurements and mRNA isolation for gene expression analysis exist. The late measurement was done almost 18 days after the injury on average, and lay far behind our latest ELISA measurement of 120 hours. Thus, the early time point must be considered as the more relevant for direct interpretation of our ELISA data. Thus, the present data should be considered as exploratory and encourage further validation in the future.
Conclusion
In conclusion, we have shown that both serum levels and gene expression of DDT and sCD74 are elevated in adults after burn injury. In non-survivors, DDT shows a biphasic curve with its highest serum levels at admission and a second increase after 24 hours, whereas sCD74 serum levels increase gradually over time. In contrast to sCD74 and MIF, which show limited value as a biomarker in the first days after burn injury, DDT may assist in monitoring clinical outcome and potentially predict sepsis during the early post-burn period.
Supplementary Material
Fig. 7. Gene array data of DDT and CD74 expression in burn patients.
Our ELISA data were supported by analysis of a gene array dataset. Gene expression of burn samples was measured at an early (49.93 ± 9.26h) and late time point (430.5 ± 23.62h). A DDT gene expression in burn patients compared to healthy controls. B CD74 gene expression in burn patients compared to healthy controls. C DDT gene expression in survivors and non-survivors at the early time point. D CD74 gene expression in survivors and non-survivors at the early time point. E DDT gene expression in survivors and non-survivors at the late time point. F CD74 gene expression in survivors and non-survivors at the late time point. G ROC curve analysis for CD74 and DDT expression at the early time point with their respective AUC and p-values. H ROC curve analysis for CD74 and DDT expression at the late time point with their respective AUC and p-values. ROC – receiver operating characteristics; AUC – area under the curve
Highlights.
DDT and sCD74 serum and mRNA levels are increased in burn patients
DDT is a potential biomarker for burn sepsis and a predictor for lethal outcome
sCD74 may rather be used to monitor burn patients due to its delayed up-regulation
Acknowledgments
Source of Funding:
Bong-Sung Kim is supported by “START”, a program for young scientists of the Medical Faculty at the RWTH Aachen University (Project number: 691346, START 2013-2) and the Research Fellowship Program of the German Research Foundation (Deutsche Forschungsgemeinschaft (DFG), GZ: KI 1973/1-1). Norbert Pallua is supported by DFG grant PA 1271/5-1. Jürgen Bernhagen is supported by DFG grants SFB1123/P03; SFB/TRR57-P07, and DFG BE1977/7-1. Richard Bucala is supported by National Institute of Health (NIH) R01 AI042310. The funding sources were not involved in the study design, collection, analysis, interpretation, writing, and subumission process of the manuscript.
Abbreviations
- ABSI
abbreviated burn severity index (ABSI)
- AMP
adenosine monophosphate-activated protein kinase
- AUC
area under the curve
- CALCA
calcitonin-related polypeptide alpha
- CRP
C-reactive protein
- DDT
D-dopachrome tautomerase
- ELISA
Enzyme-linked Immunosorbent Assay
- ERK
extracellular-signal-regulated kinases
- GEO
gene expression omnibus
- ICU
intensive care unit
- IL-1ra
interleukin-1 receptor antagonist
- IL-6
interleukin-6
- IL-8
interleukin 8
- IL-10
interleukin 10
- MIF
macrophage migration inhibitory factor
- PCT
procalcitonin
- ROC
receiver operating characteristic
- SIRS
systemic inflammatory response syndrome
- TBSA
total body surface area
- TNFα
tumor necrosis factor α
- WBC
white blood cell count
Footnotes
Conflict of Interest:
All authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tompkins RG. Survival from burns in the new millennium: 70 years’ experience from a single institution. Ann Surg. 2015;261:263–8. doi: 10.1097/SLA.0000000000000623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chipp E, Milner CS, Blackburn AV. Sepsis in burns: a review of current practice and future therapies. Ann Plast Surg. 2010;65:228–36. doi: 10.1097/SAP.0b013e3181c9c35c. [DOI] [PubMed] [Google Scholar]
- 3.Rex S, Kraemer S, Grieb G, Emontzpohl C, Soppert J, Goetzenich A, et al. The role of macrophage migration inhibitory factor in critical illness. Mini Rev Med Chem. 2014;14:1116–24. doi: 10.2174/1389557515666150203143736. [DOI] [PubMed] [Google Scholar]
- 4.Grieb G, Merk M, Bernhagen J, Bucala R. Macrophage migration inhibitory factor (MIF): a promising biomarker. Drug News Perspect. 2010;23:257–64. doi: 10.1358/dnp.2010.23.4.1453629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grieb G, Simons D, Piatkowski A, Bernhagen J, Steffens G, Pallua N. Macrophage migration inhibitory factor-A potential diagnostic tool in severe burn injuries? Burns : journal of the International Society for Burn Injuries. 2010;36:335–42. doi: 10.1016/j.burns.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 6.Calandra T, Roger T. Macrophage migration inhibitory factor: a regulator of innate immunity. Nat Rev Immunol. 2003;3:791–800. doi: 10.1038/nri1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, et al. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med. 2007;13:587–96. doi: 10.1038/nm1567. [DOI] [PubMed] [Google Scholar]
- 8.Leng L, Metz CN, Fang Y, Xu J, Donnelly S, Baugh J, et al. MIF signal transduction initiated by binding to CD74. J Exp Med. 2003;197:1467–76. doi: 10.1084/jem.20030286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Merk M, Zierow S, Leng L, Das R, Du X, Schulte W, et al. The D-dopachrome tautomerase (DDT) gene product is a cytokine and functional homolog of macrophage migration inhibitory factor (MIF) Proc Natl Acad Sci U S A. 2011;108:E577–85. doi: 10.1073/pnas.1102941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merk M, Mitchell RA, Endres S, Bucala R. D-dopachrome tautomerase (D-DT or MIF-2): doubling the MIF cytokine family. Cytokine. 2012;59:10–7. doi: 10.1016/j.cyto.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assis DN, Leng L, Du X, Zhang CK, Grieb G, Merk M, et al. The role of macrophage migration inhibitory factor in autoimmune liver disease. Hepatology. 2014;59:580–91. doi: 10.1002/hep.26664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobiasen J, Hiebert JM, Edlich RF. The abbreviated burn severity index. Ann Emerg Med. 1982;11:260–2. doi: 10.1016/s0196-0644(82)80096-6. [DOI] [PubMed] [Google Scholar]
- 13.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Li X, Jiang C, Xiao M, Zheng Y, Zhang J, et al. Data mining and bioinformatics of the expression data of esophageal squamous cell carcinoma. Cell Biochem Biophys. 2014;69:481–5. doi: 10.1007/s12013-014-9821-y. [DOI] [PubMed] [Google Scholar]
- 15.Grieb G, Kim BS, Simons D, Bernhagen J, Pallua N. MIF and CD74 - suitability as clinical biomarkers. Mini Rev Med Chem. 2014;14:1125–31. doi: 10.2174/1389557515666150203143317. [DOI] [PubMed] [Google Scholar]
- 16.Stoppe C, Rex S, Goetzenich A, Kraemer S, Emontzpohl C, Soppert J, et al. Interaction of MIF family proteins in myocardial ischemia/reperfusion damage and their influence on clinical outcome of cardiac surgery patients. Antioxid Redox Signal. 2015 doi: 10.1089/ars.2014.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren H, Li Y, Han C, Hu H. Serum procalcitonin as a diagnostic biomarker for sepsis in burned patients: A meta-analysis. Burns. 2015 doi: 10.1016/j.burns.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Kim HS, Yang HT, Hur J, Chun W, Ju YS, Shin SH, et al. Procalcitonin levels within 48 hours after burn injury as a prognostic factor. Ann Clin Lab Sci. 2012;42:57–64. [PubMed] [Google Scholar]
- 19.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 20.Beswick EJ, Das S, Pinchuk IV, Adegboyega P, Suarez G, Yamaoka Y, et al. Helicobacter pylori-induced IL-8 production by gastric epithelial cells up-regulates CD74 expression. J Immunol. 2005;175:171–6. doi: 10.4049/jimmunol.175.1.171. [DOI] [PubMed] [Google Scholar]
- 21.Degener T, Momburg F, Moller P. Differential expression of HLA-DR, HLA-DP, HLA-DQ and associated invariant chain (Ii) in normal colorectal mucosa, adenoma and carcinoma. Virchows Arch A Pathol Anat Histopathol. 1988;412:315–22. doi: 10.1007/BF00750257. [DOI] [PubMed] [Google Scholar]
- 22.Stoppe C, Rex S, Goetzenich A, Kraemer S, Emontzpohl C, Soppert J, et al. Interaction of MIF Family Proteins in Myocardial Ischemia/Reperfusion Damage and Their Influence on Clinical Outcome of Cardiac Surgery Patients. Antioxid Redox Signal. 2015 doi: 10.1089/ars.2014.6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cazalis MA, Friggeri A, Cave L, Demaret J, Barbalat V, Cerrato E, et al. Decreased HLA-DR antigen-associated invariant chain (CD74) mRNA expression predicts mortality after septic shock. Crit Care. 2013;17:R287. doi: 10.1186/cc13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orman MA, Nguyen TT, Ierapetritou MG, Berthiaume F, Androulakis IP. Comparison of the cytokine and chemokine dynamics of the early inflammatory response in models of burn injury and infection. Cytokine. 2011;55:362–71. doi: 10.1016/j.cyto.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lue H, Kapurniotu A, Fingerle-Rowson G, Roger T, Leng L, Thiele M, et al. Rapid and transient activation of the ERK MAPK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on JAB1/CSN5 and Src kinase activity. Cell Signal. 2006;18:688–703. doi: 10.1016/j.cellsig.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 26.Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–9. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- 27.Kleemann R, Kapurniotu A, Frank RW, Gessner A, Mischke R, Flieger O, et al. Disulfide analysis reveals a role for macrophage migration inhibitory factor (MIF) as thiol-protein oxidoreductase. J Mol Biol. 1998;280:85–102. doi: 10.1006/jmbi.1998.1864. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen MT, Beck J, Lue H, Funfzig H, Kleemann R, Koolwijk P, et al. A 16-residue peptide fragment of macrophage migration inhibitory factor, MIF-(50–65), exhibits redox activity and has MIF-like biological functions. J Biol Chem. 2003;278:33654–71. doi: 10.1074/jbc.M301735200. [DOI] [PubMed] [Google Scholar]
- 29.Miller EJ, Li J, Leng L, McDonald C, Atsumi T, Bucala R, et al. Macrophage migration inhibitory factor stimulates AMP-activated protein kinase in the ischaemic heart. Nature. 2008;451:578–82. doi: 10.1038/nature06504. [DOI] [PubMed] [Google Scholar]
- 30.Qi D, Atsina K, Qu L, Hu X, Wu X, Xu B, et al. The vestigial enzyme D-dopachrome tautomerase protects the heart against ischemic injury. J Clin Invest. 2014;124:3540–50. doi: 10.1172/JCI73061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willis MS, Carlson DL, Dimaio JM, White MD, White DJ, Adams GAt, et al. Macrophage migration inhibitory factor mediates late cardiac dysfunction after burn injury. Am J Physiol Heart Circ Physiol. 2005;288:H795–804. doi: 10.1152/ajpheart.00189.2004. [DOI] [PubMed] [Google Scholar]
- 32.Pallua N, von Heimburg D. Pathogenic role of interleukin-6 in the development of sepsis. Part I: Study in a standardized contact burn murine model. Critical care medicine. 2003;31:1490–4. doi: 10.1097/01.CCM.0000065724.51708.F5. [DOI] [PubMed] [Google Scholar]
- 33.Mann EA, Wood GL, Wade CE. Use of procalcitonin for the detection of sepsis in the critically ill burn patient: a systematic review of the literature. Burns : journal of the International Society for Burn Injuries. 2011;37:549–58. doi: 10.1016/j.burns.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Lavrentieva A, Papadopoulou S, Kioumis J, Kaimakamis E, Bitzani M. PCT as a diagnostic and prognostic tool in burn patients. Whether time course has a role in monitoring sepsis treatment. Burns : journal of the International Society for Burn Injuries. 2012;38:356–63. doi: 10.1016/j.burns.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 35.Kraft R, Herndon DN, Finnerty CC, Cox RA, Song J, Jeschke MG. Predictive Value of IL-8 for Sepsis and Severe Infections After Burn Injury: A Clinical Study. Shock. 2015;43:222–7. doi: 10.1097/SHK.0000000000000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruiz-Castilla M, Roca O, Masclans JR, Barret JP. Recent Advances in Biomarkers in Severe Burns. Shock. 2016;45:117–25. doi: 10.1097/SHK.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 37.Tredget EE, Yu YM. The metabolic effects of thermal injury. World journal of surgery. 1992;16:68–79. doi: 10.1007/BF02067117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.