Abstract
AIM
To determine the patterns of metastasis and recurrence in thymic epithelial tumours based on longitudinal imaging studies, and to correlate the patterns with World Health Organization (WHO) histological classifications.
MATERIALS AND METHODS
Seventy-seven patients with histopathologically confirmed thymomas (n=62) and thymic carcinomas (n=15) who were followed with cross-sectional follow-up imaging after surgery were retrospectively studied. All cross-sectional imaging studies during the disease course were reviewed to identify metastasis or recurrence. The sites of involvement and the time of involvement measured from surgery were recorded.
RESULTS
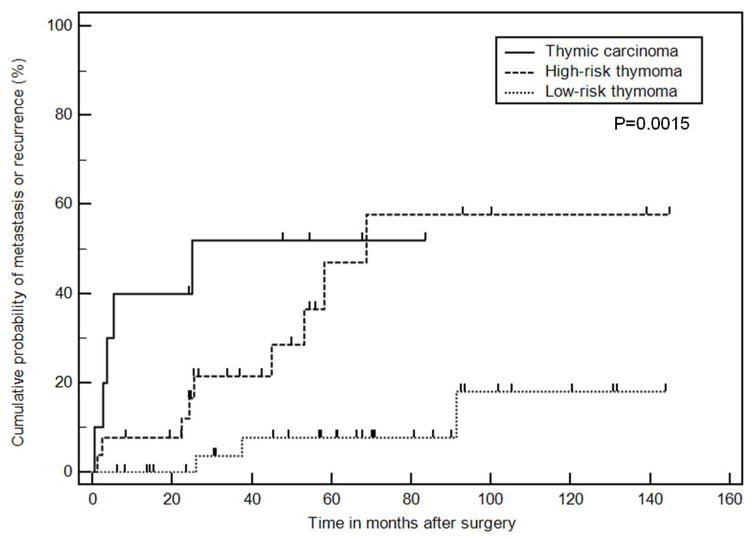
Metastasis or recurrence was noted in 24 (31%) of the 77 patients. Patients with metastasis or recurrence were significantly younger than those without (median age: 46 versus 60, respectively; p=0.0005), and more commonly had thymic carcinomas than thymomas (p=0.002). The most common site of involvement was the pleura (17/24), followed by the lung (9/24), and thoracic nodes (9/24). Abdominopelvic involvement was noted in 12 patients, most frequently in the liver (n=8). Lung metastasis was more common in thymic carcinomas than thymomas (p=0.0005). Time from surgery to the development of metastasis or recurrence was shortest in thymic carcinoma, followed by high-risk thymomas, and was longest in low-risk thymoma (median time in months: 25.1, 68.8, and not reached, respectively; p=0.0015).
CONCLUSIONS
The patterns of metastasis and recurrence of thymic epithelial tumours differ significantly across histological subgroups, with thymic carcinomas more commonly having metastasis with shorter length of time after surgery. The knowledge of different patterns of tumour spread may contribute to further understanding of the biological and clinical behaviours of these tumours.
INTRODUCTION
Thymic epithelial tumours, including thymomas and thymic carcinomas, are relatively uncommon tumours of the thorax, yet present a wide spectrum of clinical, histological, and imaging manifestations1–3. Classifications of thymic epithelial tumours have been an actively discussed topic for many years. The Masaoka–Koga staging system, described in 1981, is based on the presence of invasion or metastasis at surgery4, 5. The World Health Organization (WHO) classification system is based on histological findings, and subdivides thymomas into types A, AB, B1, B2, and B31, 3, 6, 7. Among them, type B2 and B3 tumours are considered to be “high-risk” thymomas, whereas type A, AB, and B1 tumours are considered “low-risk” thymomas”.8 Thymic carcinoma, which was formerly called type C, is now considered to be a separate entity. Although there are ongoing debates regarding the reproducibility and predictive value for outcomes of the system, the WHO classification is commonly used and often provides the histological basis for clinical and radiological studies of thymic epithelial tumours2, 3, 6, 8–14. Recently, there has been growing interest in studies of thymic tumours, as represented by the efforts of the International Thymic Malignancy Interest Group (ITMIG), to create a database of large numbers of patients to systematically address this challenging disease15.
Imaging is an essential component of diagnosis and follow-up of patients with thymic tumours. Most prior imaging studies of thymic epithelial tumours have focused on the appearance of primary preoperative tumours and their correlation with the WHO classification or staging 8, 14, 16–20. In a study of 91 patients by Jeong et al.,8 computed tomography (CT) findings of primary tumours that were more commonly seen in thymoma type B2 and B3 (high-risk thymomas) and thymic carcinomas included a lobulate contour, heterogeneous internal enhancement, higher prevalence of calcification, mediastinal fat invasion, and great-vessel invasion8. The initial CT findings predictive of increased frequency of recurrence and metastasis included a lobulate or irregular contour, oval shape, mediastinal fat invasion or great-vessel invasion, and pleural seeding8. In a study using positron-emission tomography (PET) combined with CT, Benveniste et al.20 reported that a higher focal FDG uptake was able to distinguish type B3 thymoma and thymic carcinoma from other lower-grade thymomas.
In contrast to the well-studied imaging features of primary tumours at initial presentation in thymic epithelial tumours, there is a significant paucity of investigations of radiological patterns of metastasis and recurrence in these tumours. Among the few prior reports for prognostic factors of thymoma after surgical resection and relapse patterns focusing on clinical outcome, Huang et al.21 studied 120 patients with thymic epithelial tumours who underwent surgical intervention, and reported that thymic carcinoma patients had significantly shorter 5-year overall and progression-free survival than thymoma patients, and had earlier occurrence of relapse, which was more frequent at the distant sites. Given the important role of imaging in the follow-up evaluations of patients with thymic epithelial tumours, a systematic radiological investigation based on the longitudinal imaging data may help to provide additional important knowledge of these tumours and may contribute to improve patient management.
The purpose of the present study was to systematically review the longitudinal imaging studies of patients with histopathologically confirmed thymomas and thymic carcinomas, describe the radiological patterns of metastasis and recurrence, and correlate these patterns among subgroups of tumours according to WHO histological classifications.
MATERIALS AND METHODS
Patient population
The study population included 77 patients with a histopathological diagnosis of thymomas (n=62) and thymic carcinomas (n=15) between 2000 and 2012, who were followed with follow-up cross-sectional imaging study after surgery. At least one post-surgical follow-up scan and a minimum follow-up time of 6 months (measured from the date of surgery to the date of the last follow-up imaging) were required. Five patients whose primary tumours were found to be unresectable at surgery and had follow-up imaging were also included; all these patients had thoracoscopy or mediastinoscopy for histopathological diagnosis, and the dates of these procedures were used as an alternate for the date of surgery. Imaging and medical records of these patients were retrospectively reviewed with the approval of the institutional review board. A waiver of the requirement for informed consent was obtained.
Image analysis
All cross-sectional imaging studies, including CT, PET/CT, and magnetic resonance imaging (MRI), were reviewed for evidence of metastatic or recurrent disease by two radiologists in consensus with expertise in thoracic imaging. Radiologists were aware that the cohort had thymomas or thymic carcinomas, but did not have access to the histopathological types, WHO classifications, or clinical outcome at the time of review. All images were reviewed on the picture archiving communication system (PACS) workstation (Centricity, GE Healthcare, Barrington, IL, USA)22–24.
The anatomical sites of the radiologically detected metastatic and recurrent lesions were recorded, throughout the post-surgical follow-up time period in each patient. Each radiologically detected site was correlated with histopathological diagnosis for confirmation if available. The lesions without histopathological confirmation were considered to be metastatic if (1) they demonstrated interval change in size and were in keeping with the overall clinical scenario of the patient or (2) the lesion demonstrated 2-[18F]-fluoro-2-deoxy-D-glucose (FDG) uptake on PET/CT and was consistent with metastatic disease, as described previously25–27. Any indeterminate lesions were not included as metastasis or recurrence.
The primary thymic tumour was evaluated in patients whose initial imaging studies prior to surgery were available, according to the “standard report terms for chest CT reports of anterior mediastinal masses suspicious for thymomas” by Marom et al. 28. Variables included location, size, contour, attenuation, calcification, infiltration into surrounding mediastinal fat, abutment with mediastinal structure, vascular invasion, nodal, lung parenchymal involvement, presence or absence of pleural effusion/pleural nodule, and distant metastasis28.
Statistical analysis
Clinical characteristics, WHO histological classifications, and the imaging features of the primary tumour were compared between patients with and without metastasis or recurrence using a Wilcoxon rank sum test for continuous data, and a Fisher’s exact test for categorical data. The sites of metastatic involvements were also compared between thymomas and thymic carcinomas, and across three groups including thymic carcinomas, high-risk thymoma (B3 and B2), and low-risk thymoma (B1, AB, A) according to the simplified WHO classifications as described previously1, 8. Median time from surgery to metastasis or recurrence was calculated using the Kaplan–Meier method. A log-rank test was used to compare the differences of time to metastasis or recurrence among the different groups. All tests conducted were two-sided at the 0.05 significance level.
RESULTS
Frequency of metastasis and recurrence
A total of 835 scans (median number of scans: 8; range: 1–38 scans) for the cohort of 77 eligible patients was reviewed. The median time from surgery to the last follow-up scan was 57 months (range: 6.3–191.4 months). Among the 77 patients, 24 (31%) demonstrated metastasis or recurrence of thymomas or thymic carcinomas, which included five patients with metastasis away from the primary site on the preoperative scans.
Patient characteristics with and without metastasis are summarised in Table 1. Patients with metastasis or recurrence were significantly younger than those without (median age: 46 versus 60 years, respectively; p=0.0005). The distribution of WHO subtypes were significantly different between patients with and without metastasis or recurrence (p=0.002), with thymic carcinoma being significantly more common in patients with metastasis or recurrence (p=0.002). Among the 59 patients with thymomas with detailed WHO subtypes available, type B2 or B3 tumours were more common in the group with metastasis or recurrence (p=0.02). The length of follow-up, measured from the date of surgery to the date of the last follow-up imaging, did not differ significantly between the two groups (p=0.30; Table 1).
Table 1.
Disease characteristics of patients with and without metastasis/recurrence
| Patients with metastasis/recurrence (n=24) | Patients without metastasis/recurrence (n=53) | Total (n=77) | p-Value | ||
|---|---|---|---|---|---|
| Age | |||||
| Median | 46 | 60 | 55 | 0.0005a | |
| Range | 22–68 | 22–81 | 22–81 | ||
| Sex | |||||
| Male | 14 | 25 | 39 | 0.46 | |
| Female | 10 | 28 | 38 | ||
| WHO classification of thymic epithelial tumours | |||||
| Low-risk thymoma | A | 0 | 5 | 5 | 0.002a |
| AB | 1 | 17 | 18 | ||
| B1 | 2 | 8 | 10 | ||
| High-risk thymoma | B2 | 5 | 9 | 14 | |
| B3b | 4 | 8 | 12 | ||
| Thymic carcinoma | 10 | 5 | 15 | ||
| Not classified | 2 | 1 | 3 | ||
| Follow-up time (from the surgery date to the last follow-up imaging date) | |||||
| Median (months) | 62.3 | 56.0 | 57.0 | 0.30 | |
Significance was assumed at p<0.05.
Includes four patients classified as B2/B3, and one patient classified as AB/B2/B3.
WHO, World Health Organization.
Among 53 of the 77 patients who had preoperative scans available for review (median time between preoperative scan and surgery: 3.7 weeks), 16 patients had metastasis or recurrence, while 37 did not. Metastasis or recurrence was associated with baseline imaging features including the presence of a pleural nodule (p=0.002), infiltration of the surrounding fat (p=0.0007), and calcification in the primary tumour (p=0.05). Other findings, such as a lobulate contour (p=0.25), internal density (p=0.69), the longest diameter (p=0.08) of the primary tumour, or the presence of pleural effusion on the preoperative scan (p=0.27), were not associated with metastasis or recurrence.
Location of metastasis or recurrence
Table 2 lists the site of metastasis and recurrence of the 24 patients, dichotomised by the histopathological subtypes by thymomas and thymic carcinomas. The most common site of involvement was the pleura, noted in 17 patients (17/24, 71%; Fig. 1), followed by the lung (9/24, 38%), and thoracic lymph nodes (9/24, 38%). The most frequently involved thoracic nodes were the supraclavicular nodes (n=4; Fig. 2), followed by retrocrural nodes (n=3; with a coexistent axillary node in one, and a coexistent para-oesophageal node in another), and pericardial nodes (n=2). Notably, no mediastinal or hilar node involvement was noted except for supraclavicular and para-oesophageal nodes. Abdominopelvic involvement was noted in 12 patients. Among them, the liver was the most common site (n=8, 33%), followed by the abdominopelvic nodes (n=7, 29%), which included retroperitoneal (n=5), iliac (n=1), and both retroperitoneal and iliac nodes (n=1).
Table 2.
Location of metastasis and recurrence
| Location | Thymoma (n=14) | Thymic (n=10) carcinoma | Total (n=24) |
|---|---|---|---|
| Thoracic | |||
| Pleura | 11 | 6 | 17 (71%) |
| Lung | 1 | 8 | 9 (38%) |
| Thoracic lymph node | 5 | 4 | 9 (38%) |
| Chest wall | 4 | 2 | 6 (25%) |
| Mediastinum (local recurrence at the primary tumour site) | 5 | 0 | 5 (21%) |
| Pericardium | 2 | 2 | 4 (17%) |
| Extrathoracic | |||
| Liver | 3 | 5 | 8 (33%) |
| Abdomino-pelvic lymph node | 3 | 4 | 7 (29%) |
| Peritoneum | 2 | 1 | 3 (12%) |
| Bone | 0 | 3 | 3 (12%) |
| Adrenal | 1 | 1 | 2 (8%) |
| Spleen | 0 | 1 | 1 (4%) |
| Brain | 0 | 1 | 1 (4%) |
Fig. 1.
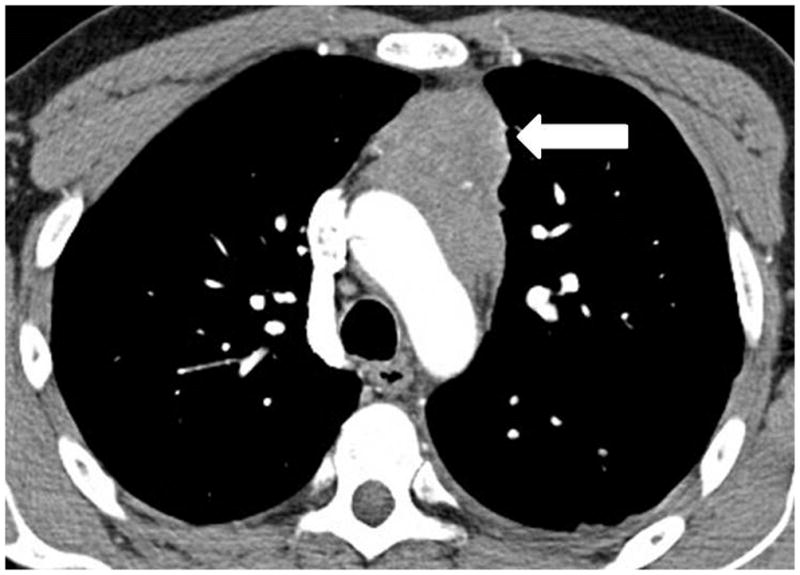
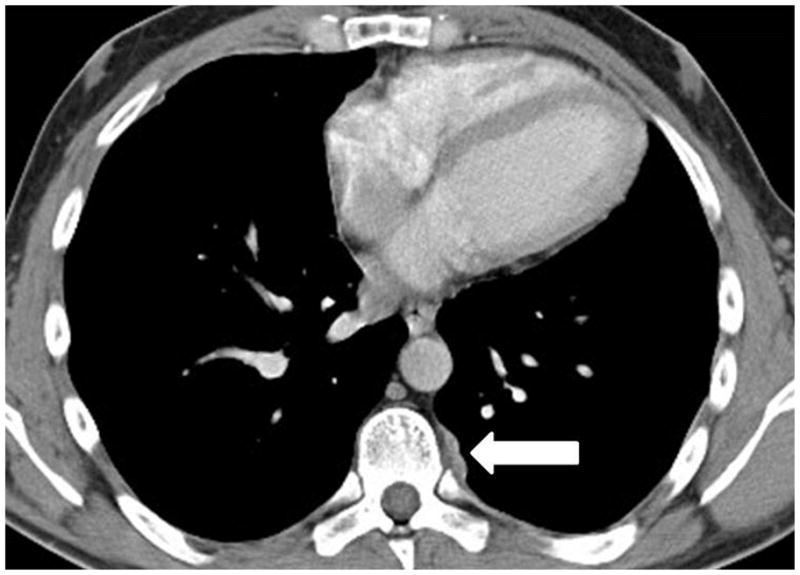
A 35-year-old man with thymoma type B3. (a) Preoperative chest CT demonstrated a large anterior mediastinal mass with a lobulate contour, abutting the aortic arch and infiltrating the surrounding fat. The patient was treated with neoadjuvant chemotherapy and radical thymectomy. (b) Follow-up CT 22 months after surgery demonstrated a new pleural lesion on the left (arrow), which was surgically resected and histopathologically confirmed to be metastatic thymoma.
Fig. 2.
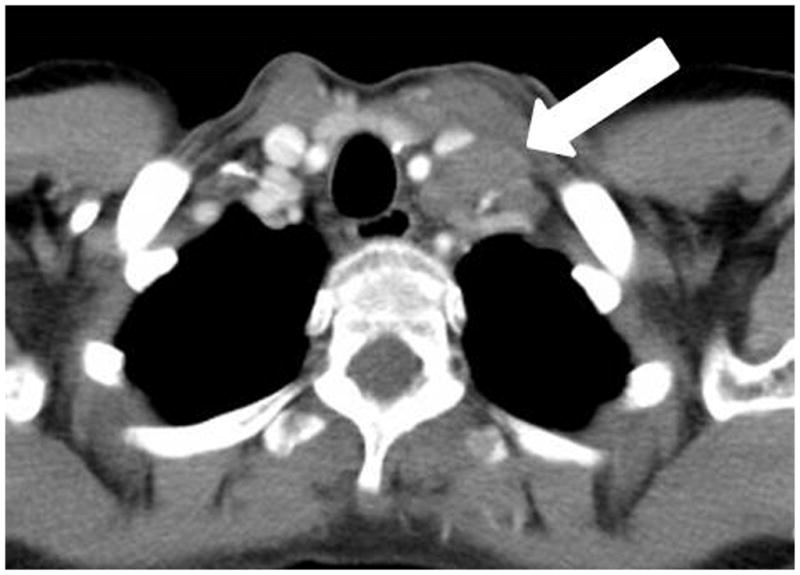
A 44 year-old woman with myasthenia gravis and thymoma type B3, which was surgically resected. Follow-up chest CT after 69 months of surgery demonstrated an enlarged left supraclavicular lymph node, which was histologically confirmed as metastatic thymoma.
Lung parenchymal metastasis was significantly more common in thymic carcinomas than thymomas (p=0.0005; Fig. 3). The presence of haematogenous metastasis (including lung, liver, adrenal, spleen, distant bone, and brain) was also more common in thymic carcinomas (p=0.04; Fig. 4). Otherwise, there was no significant difference in the metastatic locations across WHO subtypes or between thymomas and thymic carcinomas in the 24 patients with metastasis or recurrence.
Fig. 3.
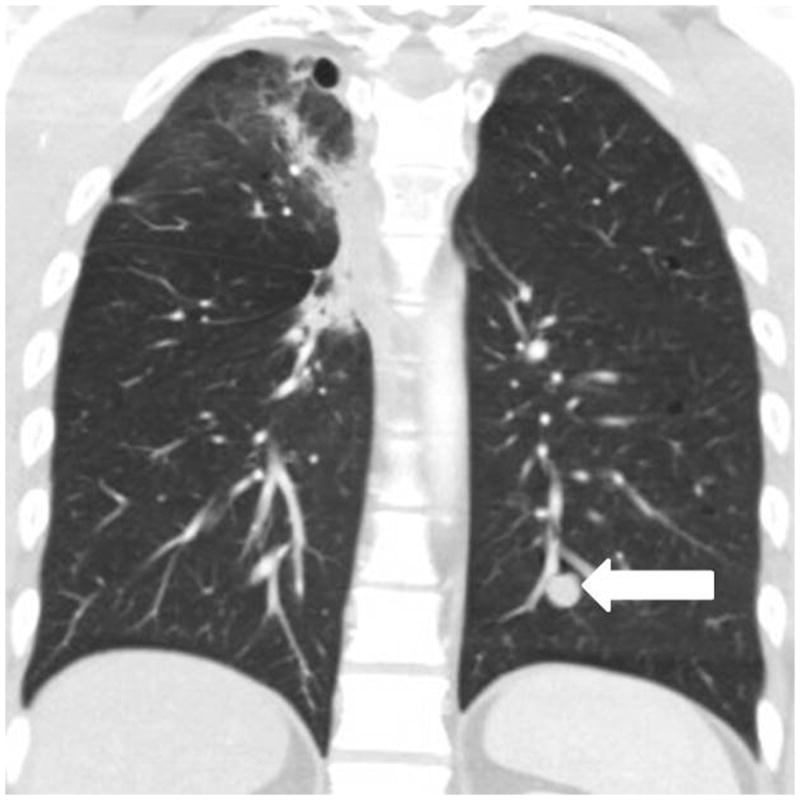
A 33-year-old man with thymic carcinoma, treated with preoperative mediastinal radiation with concurrent cisplatin and etoposide, followed by radical thymectomy. Follow-up chest CT 5.3 months after surgery demonstrated a new nodule in the left lower lobe (arrow), which was subsequently resected and was pathologically confirmed as a metastasis.
Fig. 4.
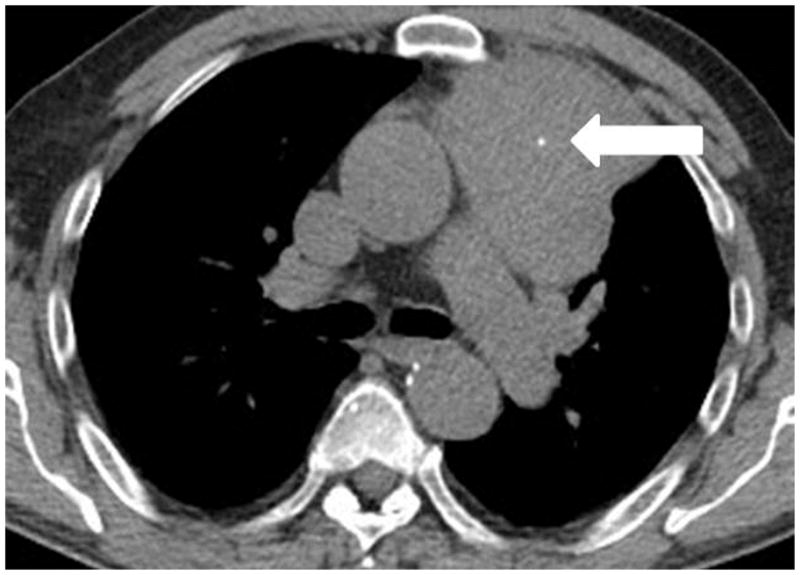
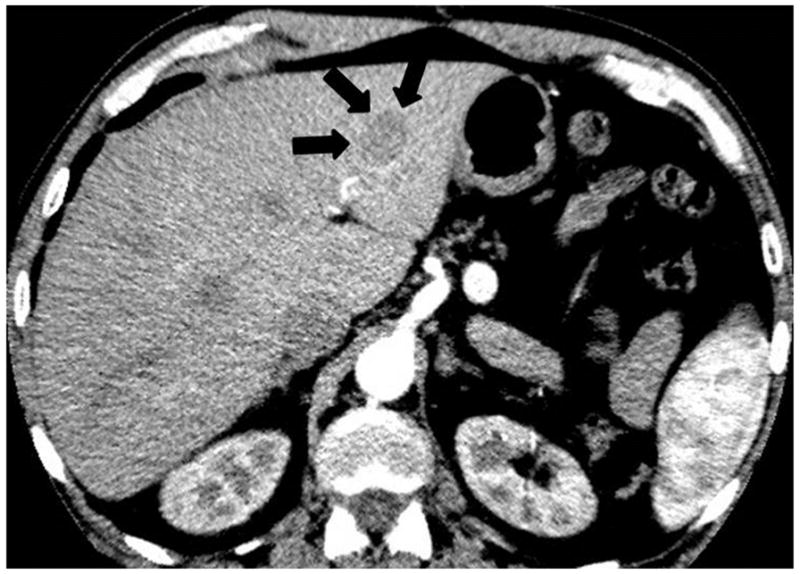
A 64-year-old man with thymic carcinoma. (a) Preoperative CT demonstrated a large anterior mediastinal mass with a lobulate contour abutting the pleura, with a focus of calcification (arrow). The patient underwent radical thymectomy followed by chest radiation with concurrent chemotherapy with two cycles of etoposide and cisplatin. (a) Follow-up chest CT 25 months after surgery demonstrated a new liver lesion in the left lobe (arrows). The liver biopsy was performed and metastasis from thymic carcinoma was confirmed.
Local recurrence at the site of the primary tumour after surgical resection was noted only in patients with thymomas (type B3 in one, B2 in two, B1 in one, and AB in one patient), and not in patients with thymic carcinoma (p=0.05). In five patients who developed local recurrence at the site of the primary tumour after surgical resection, four patients also developed metastasis away from the primary site, while one patient had local recurrence alone without metastasis. Among the five patients whose primary tumour was surgically unresectable, four patients developed metastasis away from the primary site, while one patient did not develop metastasis.
The time between surgery and the first scan demonstrating metastasis or recurrence was assessed in 74 patients with detailed WHO classification available, classified into thymic carcinoma, high-risk thymoma (B3 and B2), and low-risk thymoma (B1, AB, A). Three patients were excluded from this analysis because of lack of detailed WHO classification information. The time to metastasis was shortest in thymic carcinoma, followed by high-risk thymoma, and longest in low-risk thymoma (median time in months to metastasis or recurrence: 3.6, 68.8, and not reached), respectively; log-rank p<0.001). When five patients with metastasis on the preoperative scan, which persisted on the postoperative imaging (all having thymic carcinoma) were excluded from the analysis, the median time to the first non-baseline metastasis or recurrence was 25.1 months in thymic carcinoma, which was significantly shorter than high-risk and low-risk thymomas (log-rank p=0.0015; Fig. 5).
Fig. 5.
Time from the surgery date to the first scan demonstrating metastasis of recurrence in 69 patients with the detailed WHO classifications after excluding five patients whose metastasis persisted since baseline. The patients were classified into thymic carcinoma, high-risk thymoma (B3 and B2), and low-risk thymoma (B1, AB, A). The median time in months to metastasis or recurrence was 25.1 months, 68.8 months, and not reached, respectively (log-rank p=0.0015).
The median number of individual episodes of the development of metastasis or recurrence among the 24 patients, counting all sites and lesions noted on the same scan as one episode, was 2.5 episodes per patients, ranging from 1–7 episodes. The number of episodes did not differ significantly between thymic carcinoma and thymomas (p=0.39).
DISCUSSION
The present study demonstrated that 31% of patients with thymomas or thymic carcinomas had metastasis or recurrence, and a half of these patients developed three or more episodes of metastasis or recurrence during their disease course. Metastasis or recurrence was more frequently noted in thymic carcinomas than thymomas, and in high-risk thymomas than in low-risk thymomas. Haematogenous metastasis, notably lung metastasis, was more frequent in thymic carcinomas. Time to metastasis or recurrence after surgery was shortest in thymic carcinomas, followed by high-risk thymomas, and was longest in low-risk thymomas. The study provides a detailed description of the pattern of metastasis or recurrence in thymomas and thymic carcinomas over the entire time course of follow-up, which can serve as a guide for optimising follow-up assessment for these tumours. To the authors’ knowledge, this is the first report describing the metastasis and recurrence patterns in thymomas and thymic carcinomas based on the systematic review of the longitudinal imaging data, and the patterns according to WHO classifications of the tumour histology.
Metastasis or recurrence was noted in approximately one-third of the patients in the present cohort (24/77; 31%), which is consistent with that of the prior reports29. In the study of Blumberg et al.,30 tumour recurred in 25 patients (29%) among 86 thymoma patients who had complete resection. In a recent cohort study of thymic carcinoma patients from the European Society of Thoracic Surgeon Database, the tumour recurrence rate was 28% (54/140) among those who underwent complete resection31.
Patients with metastasis or recurrence were significantly younger at the time of surgery, which was also consistent with the prior observations where younger age was indicative of invasiveness in thymomas14, 32. In a study by Marom et al.2 of 99 thymoma patients, younger age was associated with advanced Masaoka stages (stage III/IV)14. Another recent study of thymoma using a worldwide database demonstrated that type A and AB thymomas occur significantly more often in older age groups, which contributes to the explanation of the present findings32. Metastasis or recurrence occurred more frequently in thymic carcinomas than thymomas. Metastasis or recurrence occurred more frequently in high-risk thymomas (B2 and B3) than low-risk thymomas (A, AB, and B1). The results support the previously reported observations indicating more aggressive behaviours of thymic carcinomas compared to thymomas, as well as the association between oncological behaviour and the WHO histological classifications of thymic tumours7, 21, 32.
Among the preoperative imaging features, pleural nodule and infiltration of the surrounding fat were associated with metastasis or recurrence, which is consistent with the prior report by Jeong et al.1,8 Calcification was also associated with metastasis or recurrence, which may be due to its association with type B tumours and invasiveness17, 18. Although a lobulate contour was not associated with metastasis or recurrence in the present study, as opposed to the findings by Jeong et al.,8 this may be due to a small number of patients with available preoperative imaging in the present cohort. The results again highlight the importance of preoperative imaging features as a factor that is associated with the subsequent development of metastasis and recurrence.
The most common location for metastasis or recurrence was the pleura (71%), followed by the lung (38%) and thoracic nodes (38%). Frequent pleural involvement was expected and was as described in previous reports21, 33; however, no difference was noted for the frequency of pleural involvement between thymomas and thymic carcinomas. Indeed, 60% (6/10) of thymic carcinoma patients with metastasis had pleural involvement, indicating it is a common pattern of spread in this group as well. This finding is similar to the recent report of Ruffini et al.,31 which noted 24 cases of locoregional (including 12 pleural) recurrence among 47 thymic carcinoma patients, although the frequency of pleural involvement was higher in the present cohort. Lung metastasis was significantly more common in thymic carcinomas than in thymomas, as were the other sites of haematogenous spread, which supports the observations by Huang et al.21 in their prior study comparing thymomas and thymic carcinomas. Thoracic nodal involvement was noted in supraclavicular, para-oesophageal, retrocrural, pericardial, and axillary nodes; however, no other mediastinal or hilar nodes were involved, which contrasts the patterns of spread in other types of thoracic malignancy such as lung cancer. This distinct pattern of nodal spread, which has not been described in detail, adds additional useful information to the imaging assessment of thymic tumours in their initial assessment and during follow-up.
Abdominopelvic metastasis was noted in 50% of the patients with metastasis or recurrence, with no significant difference between thymomas and thymic carcinomas, indicating the need for attention to abdominal surveillance during follow-up in these patients. Liver metastasis was most common, noted in 50% (5/10) of thymic carcinoma patients and 20% (3/14) of thymoma patients who had metastasis or recurrence, without a statistically significant difference between the two groups, which is somewhat in contrast to the prior observations. Huang et al. reported that the sites of disease progression were distant in thymic carcinomas whereas they were overwhelmingly intrathoracic in thymomas. The difference could be, in part, because the present data included all sites of involvement throughout the course of the study, rather than the sites at the time of the first episode of metastasis or progression. In fact, pleural and pericardial involvement was seen as the first sites of involvement in 15 of the 24 patients (63%) in the present cohort. Furthermore, the estimate of 25th percentile (25% of the patients having metastasis or recurrence) was 45 months for thoracic involvement compared to 174.5 months for extrathoracic involvement, suggesting that thoracic sites are involved sooner, while abdominal sites were involved later during the disease course. The present study was designed to demonstrate the site of metastasis throughout the course of follow-up, to provide information to clinicians and radiologists who may be involved in the diagnosis and management of these patients at any time point during the disease course. Local recurrence was noted only in thymoma patients, indicating the need for close assessment of the postoperative bed in these patients.
The number of episodes of metastasis or recurrence in individual patients with metastasis or recurrence was up to seven times (median: 2.5 episodes). Seven of the 24 patients (29%) had five or more episodes during the follow-up, further indicating the recurrent nature of the disease. Most of the previous clinical studies focused on the clinical outcome, i.e., overall survival and progression-free survival, and thus emphasised the recurrence patterns at the time of tumour progression, rather than the involvement during the entire course of disease. One of the motivations for the present study was to document the persistent potential for recurrence of thymic tumours during the prolonged disease course.
Thymic carcinomas had the shortest time to metastasis or recurrence, with a median of 3.6 months including patients with metastasis persisted since baseline and 25.1 months after excluding these five patients, followed by high-risk thymomas with a median of 68.8 months. Although differences in time to metastasis or recurrence across different tumour subtypes were consistent with the prior reports21, 29–31, 34, the actual length of time observed in the present and prior studies are somewhat different from each other. A prior study by Huang et al. comprising 23 thymic carcinoma patients, reported a median time from surgery to progression (defined as the occurrence of any treatment failure) of 9 months, while a more recent study of 229 thymic carcinoma patients from the European cohort reported the freedom from recurrence rate of 43% at 10 years and a cumulative incidence of recurrence of 32% at 10 years21, 29, 32. In terms of thymomas, the median time from surgery to progression was 20 months in a study of Huang et al. with 97 thymoma patients, while another study of 81 thymoma patients by Sandri et al.33 reported a mean interval from surgery to recurrence of 86.5 months21, 29. Although these studies define and measure the length of time slightly differently, a wide spectrum of reported results indicate significant variations of tumoural behaviour depending on clinical, pathological, and other factors, which further emphasises the importance of the ongoing efforts by the ITMIG to create a large database across the globe15. The results of the present study in terms of time to metastasis or recurrence fall within the spectrum of previously reported values, although it should be noted that the present cohort is from a single tertiary-care centre with expertise and resources in advanced cancer treatment, thus possibly attracting higher-risk patients. Given the present observations and the results of the study by Sandri et al.33, follow-up imaging for high-risk tumours may need to be longer than the previously suggested length of 3 years29.
The new era of multicentre, multidisciplinary, large database approaches has brought exciting new opportunities to address many important issues and challenges in thymic tumours. Several important clinical observations focusing on treatment and prognosis have already been published15, 31–33, 35. Advances in molecular and genomic investigations are also being applied to further contribute to the in-depth understanding of these tumours and the development of novel therapy36–41. Imaging plays a key role in the diagnosis and follow-up in patients with thymic tumours, and therefore, need to advance in parallel with these clinical and translational efforts. With this in mind, more than 800 scans in 77 patients were reviewed in the present study, which attempted to provide a basis for future radiological investigations of this entity in larger cohorts. It is the authors’ hope that the radiological approach and findings described in the present study can, at least in part, contribute to the development of a unified strategy for the management and follow-up of patients with thymic tumours.
The limitations of the study include its retrospective design with a relatively small number of patients from a single institution. Imaging studies were performed as part of the standard clinical care, without a predefined interval. Not all patients had preoperative scans, which was likely due to the practice of the tertiary-care centre; however, the imaging characteristics of preoperative studies have been extensively studied, and therefore, were not the main purpose of the present study. Not all the sites of metastasis or recurrence were histopathologically confirmed; however, the previously reported strategy and criteria were applied to define the positive findings25–27. Due to these limitations with a retrospective nature, the study did not investigate the association with overall survival or progression-free survival, which has been extensively studied in the clinical literature and was beyond the scope of this radiological study. The results of the present study should be interpreted in the context of the study design.
In conclusion, the patterns of metastasis and recurrence of thymomas and thymic carcinomas were distinctively different across histological subtypes. Metastasis and recurrence were more common with shorter length of time after surgery in thymic carcinomas, followed by high-risk thymomas. Median time to metastasis or recurrence was more than 5 years after surgery in high-risk thymomas, indicating the need for long-term follow-up for patients with these tumours. The present study provides the radiological observations of the patterns of metastasis and recurrence which may serve as a guide for future studies in larger cohorts and prospective design, to further optimise the management and follow-up of patients with thymomas and thymic carcinomas.
Highlights.
Patterns of metastasis of thymic tumors were different across histologic subtypes
Metastasis/recurrence occurred sooner in thymic carcinoma and high-risk thymoma
Hematogenous metastasis was more frequent in thymic carcinomas
The results may help to optimize radiologic follow-up of thymic epithelial tumors
Acknowledgments
Disclosure
Khandelwal, Araki, Ramaiya: Nothing to disclose
Sholl: Scientific advisory board for Genentech
Hatabu: Research support from Canon Inc, Toshiba Medical systems, AZE Inc., Konica-Minolta inc.; Consultant to Toshiba Medical systems
Nishino: Research grant to the institution from Merck Investigator Studies Program, Canon Inc.; Consultant to Bristol-Myers Squibb, WorldCareClinical, and Toshiba Medical systems.
Footnotes
Author Contributions
Authors are required to identify the contributions for which they are responsible. The author responsible for the integrity of the entire study should be identified. Please list the following phrases and beside each indicate the name(s) of the author(s) to whom they apply:
- guarantor of integrity of the entire study: Nishino
- study concepts and design: Nishino, Khandelwal, Sholl, Araki, Ramaiya, Hatabu
- literature research: Nishino, Khandelwal
- clinical studies: Nishino, Khandelwal, Sholl, Araki, Ramaiya, Hatabu
- experimental studies / data analysis: Nishino, Khandelwal
- statistical analysis: Nishino
- manuscript preparation: Nishino, Khandelwal
- manuscript editing: Nishino, Khandelwal, Sholl, Araki, Ramaiya, Hatabu
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nishino M, Ashiku SK, Kocher ON, Thurer RL, Boiselle PM, Hatabu H. The thymus: a comprehensive review. RadioGraphics. 2006;26:335–348. doi: 10.1148/rg.262045213. [DOI] [PubMed] [Google Scholar]
- 2.Marom EM. Advances in thymoma imaging. J Thorac Imaging. 2013;28:69–80. doi: 10.1097/RTI.0b013e31828609a0. quiz 81–63. [DOI] [PubMed] [Google Scholar]
- 3.Marom EM. Imaging thymoma. J Thorac Oncol. 2010;5:S296–303. doi: 10.1097/JTO.0b013e3181f209ca. [DOI] [PubMed] [Google Scholar]
- 4.Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymomas with special reference to their clinical stages. Cancer. 1981;48:2485–2492. doi: 10.1002/1097-0142(19811201)48:11<2485::aid-cncr2820481123>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Huang J, Detterbeck FC, Wang Z, Loehrer PJ., Sr Standard outcome measures for thymic malignancies. J Thorac Oncol. 2010;5:2017–2023. doi: 10.1097/JTO.0b013e3181f13682. [DOI] [PubMed] [Google Scholar]
- 6.Park MS, Chung KY, Kim KD, et al. Prognosis of thymic epithelial tumours according to the new World Health Organization histologic classification. Ann Thorac Surg. 2004;78:992–997. doi: 10.1016/j.athoracsur.2004.03.097. discussion 997–998. [DOI] [PubMed] [Google Scholar]
- 7.Okumura M, Ohta M, Tateyama H, et al. The World Health Organization histologic classification system reflects the oncologic behavior of thymoma: a clinical study of 273 patients. Cancer. 2002;94:624–632. doi: 10.1002/cncr.10226. [DOI] [PubMed] [Google Scholar]
- 8.Jeong YJ, Lee KS, Kim J, Shim YM, Han J, Kwon OJ. Does CT of thymic epithelial tumours enable us to differentiate histologic subtypes and predict prognosis? AJR Am J Roentgenol. 2004;183:283–289. doi: 10.2214/ajr.183.2.1830283. [DOI] [PubMed] [Google Scholar]
- 9.Verghese ET, den Bakker MA, Campbell A, et al. Interobserver variation in the classification of thymic tumours—a multicentre study using the WHO classification system. Histopathology. 2008;53:218–223. doi: 10.1111/j.1365-2559.2008.03088.x. [DOI] [PubMed] [Google Scholar]
- 10.Rosado-de-Christenson ML, Strollo DC, Marom EM. Imaging of thymic epithelial neoplasms. Hematol Oncol Clin North Am. 2008;22:409–431. doi: 10.1016/j.hoc.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa K, Asamura H, Matsuno Y, et al. Thymoma: a clinicopathologic study based on the new World Health Organization classification. J Thorac Cardiovasc Surg. 2003;126:1134–1140. doi: 10.1016/s0022-5223(03)00798-0. [DOI] [PubMed] [Google Scholar]
- 12.Moran CA, Weissferdt A, Kalhor N, et al. Thymomas I: a clinicopathologic correlation of 250 cases with emphasis on the World Health Organization schema. Am J Clin Pathol. 2012;137:444–450. doi: 10.1309/AJCP76KEGWQKWOKA. [DOI] [PubMed] [Google Scholar]
- 13.Moran CA, Walsh G, Suster S, Kaiser L. Thymomas II: a clinicopathologic correlation of 250 cases with a proposed staging system with emphasis on pathologic assessment. Am J Clin Pathol. 2012;137:451–461. doi: 10.1309/AJCP36ALGUZWOSEA. [DOI] [PubMed] [Google Scholar]
- 14.Marom EM, Milito MA, Moran CA, et al. Computed tomography findings predicting invasiveness of thymoma. J Thorac Oncol. 2011;6:1274–1281. doi: 10.1097/JTO.0b013e31821c4203. [DOI] [PubMed] [Google Scholar]
- 15.Huang J, Ahmad U, Antonicelli A, et al. Development of the international thymic malignancy interest group international database: an unprecedented resource for the study of a rare group of tumours. J Thorac Oncol. 2014;9:1573–1578. doi: 10.1097/JTO.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 16.Yanagawa M, Tomiyama N. Prediction of thymoma histology and stage by radiographic criteria. Thorac Surg Clin. 2011;21:1–12. v. doi: 10.1016/j.thorsurg.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Tomiyama N, Muller NL, Ellis SJ, et al. Invasive and noninvasive thymoma: distinctive CT features. J Comput Assist Tomogr. 2001;25:388–393. doi: 10.1097/00004728-200105000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Tomiyama N, Johkoh T, Mihara N, et al. Using the World Health Organization classification of thymic epithelial neoplasms to describe CT findings. AJR Am J Roentgenol. 2002;179:881–886. doi: 10.2214/ajr.179.4.1790881. [DOI] [PubMed] [Google Scholar]
- 19.Sadohara J, Fujimoto K, Muller NL, et al. Thymic epithelial tumours: comparison of CT and MR imaging findings of low-risk thymomas, high-risk thymomas, and thymic carcinomas. Eur J Radiol. 2006;60:70–79. doi: 10.1016/j.ejrad.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Benveniste MF, Moran CA, Mawlawi O, et al. FDG PET-CT aids in the preoperative assessment of patients with newly diagnosed thymic epithelial malignancies. J Thorac Oncol. 2013;8:502–510. doi: 10.1097/JTO.0b013e3182835549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang J, Rizk NP, Travis WD, et al. Comparison of patterns of relapse in thymic carcinoma and thymoma. J Thorac Cardiovasc Surg. 2009;138:26–31. doi: 10.1016/j.jtcvs.2009.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Araki T, Sholl LM, Gerbaudo VH, Hatabu H, Nishino M. Imaging characteristics of pathologically proven thymic hyperplasia: identifying features that can differentiate true from lymphoid hyperplasia. AJR Am J Roentgenol. 2014;202:471–478. doi: 10.2214/AJR.13.11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Araki T, Sholl LM, Gerbaudo VH, Hatabu H, Nishino M. Thymic measurements in pathologically proven normal thymus and thymic hyperplasia: intraobserver and interobserver variabilities. Acad Radiol. 2014;21:733–742. doi: 10.1016/j.acra.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araki T, Sholl LM, Gerbaudo VH, Hatabu H, Nishino M. Intrathymic cyst: clinical and radiological features in surgically resected cases. Clin Radiol. 2014;69:732–738. doi: 10.1016/j.crad.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinjamoori AH, Jagannathan JP, Shinagare AB, et al. Atypical metastases from prostate cancer: 10-year experience at a single institution. AJR Am J Roentgenol. 2012;199:367–372. doi: 10.2214/AJR.11.7533. [DOI] [PubMed] [Google Scholar]
- 26.Shinagare AB, Ramaiya NH, Jagannathan JP, Fennessy FM, Taplin ME, Van den Abbeele AD. Metastatic pattern of bladder cancer: correlation with the characteristics of the primary tumour. AJR Am J Roentgenol. 2011;196:117–122. doi: 10.2214/AJR.10.5036. [DOI] [PubMed] [Google Scholar]
- 27.Shinagare AB, Fennessy FM, Ramaiya NH, Jagannathan JP, Taplin ME, Van den Abbeele AD. Urothelial cancers of the upper urinary tract: metastatic pattern and its correlation with tumour histopathology and location. J Comput Assist Tomogr. 2011;35:217–222. doi: 10.1097/RCT.0b013e31820d7a37. [DOI] [PubMed] [Google Scholar]
- 28.Marom EM, Rosado-de-Christenson ML, Bruzzi JF, Hara M, Sonett JR, Ketai L. Standard report terms for chest computed tomography reports of anterior mediastinal masses suspicious for thymoma. J Thorac Oncol. 2011;6:S1717–1723. doi: 10.1097/JTO.0b013e31821e8cd6. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Detterbeck FC, Wang Z, Loehrer PJ., Sr Standard outcome measures for thymic malignancies. J Thorac Oncol. 2011;6:S1691–1697. doi: 10.1097/JTO.0b013e3182254ac1. [DOI] [PubMed] [Google Scholar]
- 30.Blumberg D, Port JL, Weksler B, et al. Thymoma: a multivariate analysis of factors predicting survival. Ann Thorac Surg. 1995;60:908–913. doi: 10.1016/0003-4975(95)00669-c. discussion 914. [DOI] [PubMed] [Google Scholar]
- 31.Ruffini E, Detterbeck F, Van Raemdonck D, et al. Thymic carcinoma: a cohort study of patients from the European society of thoracic surgeons database. J Thorac Oncol. 2014;9:541–548. doi: 10.1097/JTO.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 32.Weis CA, Yao X, Deng Y, et al. The impact of thymoma histotype on prognosis in a worldwide database. J Thorac Oncol. 2015;10:367–372. doi: 10.1097/JTO.0000000000000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandri A, Cusumano G, Lococo F, et al. Long-term results after treatment for recurrent thymoma: a multicenter analysis. J Thorac Oncol. 2014;9:1796–1804. doi: 10.1097/JTO.0000000000000370. [DOI] [PubMed] [Google Scholar]
- 34.Safieddine N, Liu G, Cuningham K, et al. Prognostic factors for cure, recurrence and long-term survival after surgical resection of thymoma. J Thorac Oncol. 2014;9:1018–1022. doi: 10.1097/JTO.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 35.Ahmad U, Yao X, Detterbeck F, et al. Thymic carcinoma outcomes and prognosis: results of an international analysis. J Thorac Cardiovasc Surg. 2015;149:95–100. 101 e101–102. doi: 10.1016/j.jtcvs.2014.09.124. [DOI] [PubMed] [Google Scholar]
- 36.Padda SK, Riess JW, Schwartz EJ, et al. Diffuse high intensity PD-L1 staining in thymic epithelial tumours. J Thorac Oncol. 2015 Mar;10(3):500–8. doi: 10.1097/JTO.0000000000000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Girard N. Thymic tumours: adopting an orphan thoracic tumour as a model of personalized medicine. J Thorac Oncol. 2014;9:1737–1739. doi: 10.1097/JTO.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 38.Wang Y, Thomas A, Lau C, et al. Mutations of epigenetic regulatory genes are common in thymic carcinomas. Sci Rep. 2014;4:7336. doi: 10.1038/srep07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lattanzio R, La Sorda R, Facciolo F, et al. Thymic epithelial tumours express vascular endothelial growth factors and their receptors as potential targets of antiangiogenic therapy: a tissue micro array-based multicenter study. Lung Cancer. 2014;85:191–196. doi: 10.1016/j.lungcan.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Omatsu M, Kunimura T, Mikogami T, et al. Immunohistochemical analysis of thymic carcinoma focusing on the possibility of molecular targeted and hormonal therapies. Gen Thorac Cardiovasc Surg. 2012;60:803–810. doi: 10.1007/s11748-012-0160-x. [DOI] [PubMed] [Google Scholar]
- 41.Aisner SC, Dahlberg S, Hameed MR, et al. Epidermal growth factor receptor, C-kit, and Her2/neu immunostaining in advanced or recurrent thymic epithelial neoplasms staged according to the 2004 World Health Organization in patients treated with octreotide and prednisone: an Eastern Cooperative Oncology Group study. J Thorac Oncol. 2010;5:885–892. doi: 10.1097/JTO.0b013e3181d86a30. [DOI] [PMC free article] [PubMed] [Google Scholar]