Abstract
The mosquito Aedes aegypti is the primary urban vector for dengue virus (DENV) worldwide. Insight into interactions occurring between host and pathogen is important in understanding what factors contribute to vector competence. However, many of the molecular mechanisms for vector competence remain unknown. Our previous global transcriptional analysis suggested that differential expression of apoptotic proteins is involved in determining refractoriness vs susceptibility to DENV-2 infection in Ae. aegypti females following a DENV-infected blood meal. To determine whether DENV-refractory Ae. aegypti showed more robust apoptosis upon infection, we compared numbers of apoptotic cells from midguts of refractory and susceptible strains and observed increased numbers of apoptotic cells in only the refractory strain upon DENV-2 infection. Thereafter, we manipulated apoptosis through dsRNA interference of the initiator caspase, Aedronc. Unexpectedly, dsAedronc-treated females showed both decreased frequency of disseminated infection and decreased virus titer in infected individuals. Insect caspases have also previously been identified as regulators of the cellular recycling process known as autophagy. We observed activation of autophagy in midgut and fat body tissues following a blood meal, as well as programmed activation of several apoptosis-related genes, including the effector caspase, Casps7. To determine whether autophagy was affected by caspase knockdown, we silenced Aedronc and Casps7, and observed reduced activation of autophagy upon silencing. Our results provide evidence that apoptosis-related genes are also involved in regulating autophagy, and that Aedronc may play an important role in DENV-2 infection success in Ae. aegypti, possibly through its regulation of autophagy.
Keywords: mosquito, Aedes aegypti, dengue virus, apoptosis, programmed cell death, autophagy, caspase, midgut, vector biology, host-pathogen interaction
Graphical abstract
1. Introduction
Dengue fever, typically caused by one of four common serotypes of dengue virus (DENV 1-4), is one of the most medically important arthropod-borne diseases in the world. Much of the persistence and spread of DENV can be attributed to the expansion of its mosquito vectors, Aedes aegypti and Aedes albopictus. Although some recent advances have been made in the development of a vaccine (Thomas & Rothman, 2015), all current candidates are only partially effective (Guzman & Harris, 2015; Sabchareon et al, 2012). Mosquito control, therefore, remains the only viable option for preventing and containing transmission of DENV.
To become transmissible by a mosquito vector, DENV must successfully infect and replicate in the mosquito midgut epithelium, escape into the hemocoel and infect salivary gland tissues (reviewed in Black et al, 2002). Transcriptional analyses of female Ae. aegypti response following DENV-infected blood meals have identified multiple gene networks and pathways that represent potential molecular targets for blocking DENV transmission (Behura et al, 2011, 2014; Bonizzoni et al, 2012a; Chauhan et al, 2012; Colpitts et al, 2011; Sim et al, 2013). Our previous whole transcriptome study indicated that a group of genes associated with apoptosis showed significant coordinated upregulation in a DENV refractory strain in response to DENV-2 infection (Behura et al, 2011). Apoptosis of mosquito salivary gland and midgut tissue has been associated with infection by multiple viruses including, West Nile virus, Sindbis virus (SINV), densovirus and nucleopolyhedrovirus (Kelly et al, 2012; Liu et al, 2011; Roekring & Smith, 2010; Vaidyanathan & Scott, 2006). Progressive salivary gland apoptosis has been correlated with decreased long-term West Nile virus titers in Culex quinquefasciatus (Girard et al, 2007).
Apoptosis is typically activated when stimuli, such as UV light, irradiation or developmental changes, induce activation of cysteine proteases known as caspases, which then cleave downstream proteins leading to cell death. In Ae. aegypti, the initiator caspase, Aedronc (also known as CaspL2), auto-activates upon association to the adaptor protein Ark to form a complex known as the apoptosome. Activated Aedronc then cleaves and activates the effector caspases, Casps7 and Casps8. The Ae. aegypti inhibitor of apoptosis 1 (AeIAP1) binds and inhibits all three of these caspases, preventing spontaneous apoptosis. In addition, the IAP-antagonist protein Michelob_x (Mx) competitively binds to caspases and prevents their inhibition by AeIAP1 (Liu & Clem, 2011; Wang & Clem, 2011).
In other arthropods, some proteins traditionally associated with apoptosis, also regulate the cell recycling mechanism known as autophagy (Baehrecke, 2005; Daish et al, 2004; Hou et al, 2008; Kim et al, 2010; Lee et al, 2003; Maiuri et al, 2007; Martin & Baehrecke, 2004). Autophagy is a highly conserved catabolic process activated in all eukaryotic cells to degrade unused or old organelles and cellular cargo, and is driven by a suite of core autophagy-related (ATG) proteins (Feng et al, 2014; McPhee & Baehrecke, 2009; Yorimitsu & Klionsky, 2005). During macroautophagy, a double membrane vesicle called an autophagosome forms around cytosolic components, which subsequently fuse with lysosomes and degrade its contents (Feng et al, 2014). Under certain conditions in Drosophila, midgut and salivary gland tissues show both high caspase activity and the formation of autophagosomes (Lee & Baehrecke, 2001; Lee et al, 2002; Martin & Baehrecke, 2004; Nezis et al, 2009; Velentzas et al, 2007), suggesting that apoptosis and autophagy may be highly integrated in arthropods. Mosquitoes are known to activate autophagy following vitellogenesis to recover excess yolk protein (Bryant & Raikhel, 2011; Mane-Padros et al, 2012).
Autophagy has been shown to be necessary for dengue infection in mammalian cells. DENV-infected murine cells show increased autophagosome formation, expression of LC3/ATG8 on autophagosomal membranes, and maturation of autophagosomes (Lee et al, 2008; Lee et al, 2013; McLean et al, 2011). Depletion of autophagy can decrease DENV titers over 1000-fold (Lee et al, 2008; Heaton & Randall, 2010; McLean et al, 2011; Mateo et al, 2013). However, whether autophagy functions similarly in invertebrate hosts has not yet been determined.
Here we investigated the role of cell death mechanisms in DENV infection of Ae. aegypti strains known to be refractory and susceptible to DENV infection. We observed increased apoptotic activity only in midguts of refractory strain females upon DENV-2 infection. However, RNAi-induced reduction of Aedronc, that should limit activation of apoptosis, unexpectedly resulted in decreased levels of DENV-2 infection. We also observed that following a naïve blood meal, females showed activated autophagy, as well as parallel upregulation of autophagic and apoptotic genes, including Casps7. RNAi-induced Aedronc reduction attenuated Casps7 upregulation as well as activation of autophagy. Further analysis revealed that Casps7 may also regulate autophagy, suggesting autophagy could play a similar role in DENV-2 infection in mosquitoes as has been demonstrated for humans.
2. Materials and Methods
2.1 Mosquito Rearing and Maintenance
Mosquitoes were reared and maintained according to standard laboratory protocol (Clemons et al, 2010). Similar numbers of larvae (~125) were grown in pans of distilled water and fed a slurry of bovine liver powder ad libitum. Pupae were collected and placed in 250 mL of distilled H20 in 475 mL cups and allowed to emerge as adults in a 19 L plastic container with mesh top. Moyo-R (MR) and Moyo-S (MS) strains used in this study were previously derived from the Moyo-In-Dry strain, collected from Mombasa, Kenya in 1974. These strains were selected for refractoriness (MR) or susceptibility (MS) to the malaria parasite Plasmodium gallinaceum (Thathy et al, 1994), but were subsequently found to also show low frequency (~0.2) and high frequency (~0.54) of DENV-2 dissemination, respectively, when provided an infected blood meal (Schneider et al, 2007).
2.2 Virus Propagation and Blood feeding
Ae. albopictus C6/36 cells were cultured according to protocol in Schneider et al. (2007), with minor modifications. Cells were maintained in 75-cm3 flasks with L-15 media (Lonza, Allendale, NJ) supplemented with 10% fetal bovine serum (FBS), at 28°C without CO2. At ~85% confluency, cells were inoculated with DENV-2 (strain JAM1409, a high passage virus; Deubel et al, 1986) at a multiplicity of infection (MOI) of 0.1 and incubated with L-15 media with 2% FBS at 28°C witho ut CO2. After 7 days, cells were centrifuged at 2500 RPM for 10 min and supernatant was collected for mosquito infections.
Mosquitoes were fed defibrinated sheep blood (Colorado Serum Company) mixed in a 1:1 ratio with either control C6/36 cell supernatant, or DENV-2 JAM1409 supernatant as previously described (Schneider et al, 2007). Briefly, blood feeding was performed using a glass membrane feeder attached to a circulating water bath that warmed blood to 37°C. Sausage casing was used as an artificial memb rane to cover the bottom of the feeder. Mosquitoes were allowed to feed for ~10 minutes before fully engorged females were CO2-anesthetized and separated into 475 mL cups with 12-15 individuals each. Infective blood feeding for MS and MR strain females were always performed concurrently. Mosquitoes were maintained in an environmental chamber according to our standard laboratory protocol (Clemons et al, 2010) and fed a 5% sucrose solution ad libitum.
2.3 Gene knockdown by RNAi
To create dsRNA, total genomic DNA was extracted from two adult MR females using a simple, rapid alkaline method (Rubeck & Dissing, 1998). Primers with the T7 promoter sequence (5’- TAATACGACTCACTATAGGG-3’) added to their 5’ ends were used to amplify regions of Aedronc, Casps7, ATG1, ATG5 and ATG7 genes that showed little or no homology with other genes according to VectorBase (Supp. Table S1). Aedronc is necessary for apoptosis in Ae. aegypti (Liu & Clem, 2011; Wang & Clem, 2011), and a Casps7 homolog was found to regulate autophagy is Drosophila (Hou et al, 2008; Kim et al, 2010). ATG1, ATG5 and ATG7 were chosen based on their known core roles in autophagy in Drosophila (Chang et al, 2010). As a non-targeting control, B-galactosidase gene (B-gal) was amplified from pBluescriptIIKS plasmid (Berois et al, 2012). dsRNA was synthesized and purified from PCR product using the MEGAscript Kit (Ambion) according to manufacturer's protocol.
To deliver dsRNA, 2-3 day old cold-anesthetized adult female mosquitoes were injected in the thorax with ~69 nL of 2.3 μg/μL dsRNA using a micro-injector as previously described (Berois et al, 2012; Sim & Dimopoulos, 2010). Mosquitoes were then maintained in an environmental chamber and fed a 5% sucrose solution ad libitum. Gene knockdown was tested 48 h post injection (HPI) via quantitative PCR (qPCR) on pools of five mosquitoes, as described below. Mosquitoes used for DENV-2 infection studies were blood fed 48 HPI, as described above. Statistical analysis of Aedronc or Casps7 knockdown was performed by Student's t-test to compare dsAedronc- or dsCasps7-treated females, respectively, to dsB-gal treated females.
2.4 Quantitative PCR
To compare expression of apoptotic genes across time and between MR and MS strains, females were collected before feeding and every 24 h until 72 h PBM. To determine whether strains showed differential activation of apoptotic genes upon DENV-2 infection, we tested 5 pooled whole bodies at 24 and 48 h PBM and 10 pooled midguts at 48 h PBM from DENV-2 and naïve-fed MR and MS. To look at expression changes of autophagy and apoptosis-related genes after a blood meal, adult MR females were fed a naïve blood meal, and collected before feeding and every 12 h until 72 h PBM.
RNA was isolated using TRIzol reagent according to manufacturer's protocol (Invitrogen). RNA samples were DNA-digested using RQ1 DNAse I (Promega) and then cDNA was created using Superscript II Reverse Transcriptase (Invitrogen). Primers were designed for the amplification of apoptosis genes (Aedronc, AeIAP1, Mx), apoptosis-related genes (Aep53, IAP5, IAP9, Aebruce, AeDNR1, Aedebcl, Aebuffy, Casps8, Imp) and autophagy genes (ATG1, ATG4A, ATG5, ATG8, ATG12) (Supp. Table S2). Apoptosis-related genes were chosen based on their potential relationship to autophagy (Hou et al, 2008; Kim et al, 2010; Lee et al, 2003). This cDNA was used as template in real time quantitative PCR (qPCR), using Power SYBR Green One-Step (Applied Biosystems) for quantification. RNA expression was obtained using the ABI 7500 Fast System Sequence Detector System (Applied Biosystems). Delta Ct values (Schmittgen & Livak, 2008) for genes of interest were normalized against the housekeeping gene ribosomal protein S17 (RPS17) (Morlais et al, 2003) or ribosomal protein L8 (RPL8) (Lan & Fallon, 1992). Relative quantification (RQ) values were obtained using the delta-delta CT (ΔΔCT) method.
Statistical analysis for RQ data was performed by either (A) two-way ANOVA to investigate effects of time PBM and strain on RQ of genes, followed by the Tukey honestly significant difference (HSD) test to identify significant pair-wise differences among means, (B) Student's t-test to compare ratios of Aedronc, AeIAP1 and Mx transcript from DENV-fed to control-fed females between strains, or (C) one-way ANOVA to investigate effects of time PBM on expression of autophagy and apoptosis-related genes, followed by the Tukey HSD test to identify significant differences among means in expression relative to 0 h PBM.
2.5 TUNEL Staining
To test for apoptotic activity in response to DENV-2 infection, midguts from mosquitoes fed on control or DENV-2 infected artificial blood meals were dissected out in PBS at 48 h PBM and frozen in PBS at −80°C until staining. Mi dguts were whole mounted onto glass slides and then stained for fragmented DNA using the ApopTag Detection kit (Chemicon, Temecula, CA). Nuclei were stained with DAPI. Slides were immediately viewed using a Leica DM550 B Widefield Microscope and images were captured with a Hamamatsu ORCA-R2 C10600 Digital Camera. Slides were visualized at 40X and 2-3 images were taken from different sections of each midgut. TUNEL-positive cells were counted from each section and calculated as a proportion of the total number of cells visible within the midgut. Statistical analysis comparing midgut proportion of TUNEL-positive cells between groups was performed using a Mann-Whitney U Test.
2.6 RT-PCR
To determine whether DENV-2 blood fed females showed disseminated infection from the midgut, total RNA was isolated from homogenized individual leg tissue of females 14 d PBM using TRIzol reagent. Virus was detected using DENV-specific primers that amplified a 511 bp region of the genome (Lanciotti et al, 1992), and multiplexed with primers for ribosomal protein L8 (RPL8) as a control (Lan & Fallon, 1992). cDNA was amplified using the Superscript III One-Step RT-PCR kit (Invitrogen) according to manufacturer's protocol. The proportion of positive leg samples was obtained for each of three biological replicates. Proportion data was arcsin transformed and then analyzed for significance using Student's t-test.
2.7 Virus Titration
Virus titer was quantified from individual females fed a DENV-2 infected blood meal 14 d PBM, as well as from the artificial blood meals used for feeding, using the TCID50 method (Nawtaisong et al, 2009). To quantify virus in infected mosquitoes, single mosquitoes were ground in 1 mL of Schneider's media (Lonza) on ice and the triturate was then filtered through 0.45 μm filters. 100 μL of filtered triturate was then 10-fold serially diluted in 2% FBS L-15 media (Lonza) in 96-well plates, and then an equal volume of C6/36 cells was added to each well. For quantification of DENV-2 from artificial blood meals used for infection, 100 μL of the blood meal was serially diluted as described above. Cells were incubated for 4-5 days before fixation with 3:1 acetone:PBS solution. DENV was detected using the DENV-2 antibody 2B29A (Santa Cruz Biotechnology) in a 1:100 dilution, followed by 1:100 sheep anti-mouse biotinylated IgG (GE Healthcare) and addition of 1:100 Strepavidin Alexafluor-680 (Invitrogen). Plates were imaged using a LI-COR scanner. Cells showing cytoplasmic fluorescence were scored as positive for infection. The TCID50 was calculated using the Reed-Muench method (Reed & Muench, 1938). The titers of DENV-2 within blood meal ranged from 106.3-107.7 TCID50/mL. Statistical analysis comparing virus titers was performed using a Mann-Whitney U test.
2.8 Western Blots
Adult MR females were injected with dsRNA as above. Due to the expected constant flux of autophagy, females were also co-injected with 10 mM hydroxychloroquine (HCQ) to block degradation of autolysosomes (Barth et al, 2010; Kroemer & Jäättelä, 2005; Rubinsztein et al, 2009). To determine whether programmed autophagy was caspase-dependent in fat body, females were fed a control artificial blood meal as above, and at 36 h PBM five midgut-free carcasses were collected for protein extraction, as most autophagy genes were previously shown to have maximum transcription at this time point (Bryant & Raikhel, 2011). To determine whether DENV affected autophagy, and whether autophagy was caspase-dependent in mosquito midgut, females were fed either a control or DENV-2 infected artificial blood meal as above. To allow sufficient time for virus to replicate (Salazar et al, 2007), 10 midguts were dissected out in 1X PBS at 3 d PBM, and stored in PBS at −80°C until protein extraction. Approximately 14 ng of total protein was size-fractionated on Tris-Glycine gels (Pierce) and then transferred to PDVF membrane. ATG8 was detected using polyclonal rabbit anti-mosquito ATG8 at a 1:1500 dilution (Bryant & Raikhel, 2011), followed by secondary anti-rabbit-HRP antibody (Life Technologies) at 1:2500 dilution. α-tubulin was used as a loading control at a 1:10,000 dilution followed by secondary anti-mouse antibody at a 1:10,000 dilution (Santa Cruz Biotechnology). ATG8 is normally cytoplasmic, but upon activation of autophagy, is conjugated to phosphatidyl ethanolamine (PE) on the autophagosomal membrane through a series of ubiquitination cascades (Klionsky et al, 2012). ImageJ was used for densitometry analysis of a representative western blot comparing the ratio of total ATG8 protein to α-tubulin or ratio of ATG-PE to ATG8. Statistical analysis was performed using either: (A) one-way ANOVA to investigate effects of treatment on ratio of total ATG8 protein to α-tubulin or ratio of ATG-PE to ATG8, followed by the Tukey HSD test to identify significant pair-wise differences relative to dsB-gal-treated samples at 36 h PBM, or (B) Student's t-test.
2.9 Lysotracker Staining
Lysotracker is an acidotropic dye used as a marker for autolysosome formation (Bryant & Raikhel, 2011; Klionsky et al, 2012; Mauvezin et al, 2014). To determine whether autophagosomes were present in individual tissues, midguts and fat body were dissected out individually before blood feeding and 36 h PBM in 1X PBS and then stained with 0.5 μM LysoTracker Red (Invitrogen) for 5 min. DAPI was used as a DNA counterstain. Slides were immediately viewed using a Leica DM550 B Widefield Microscope and images were captured with a Hamamatsu ORCA-R2 C10600 Digital Camera. Slides were visualized at 100-200X.
3. Results
3.1. Refractory mosquitoes show increased midgut epithelial cell death following DENV infection
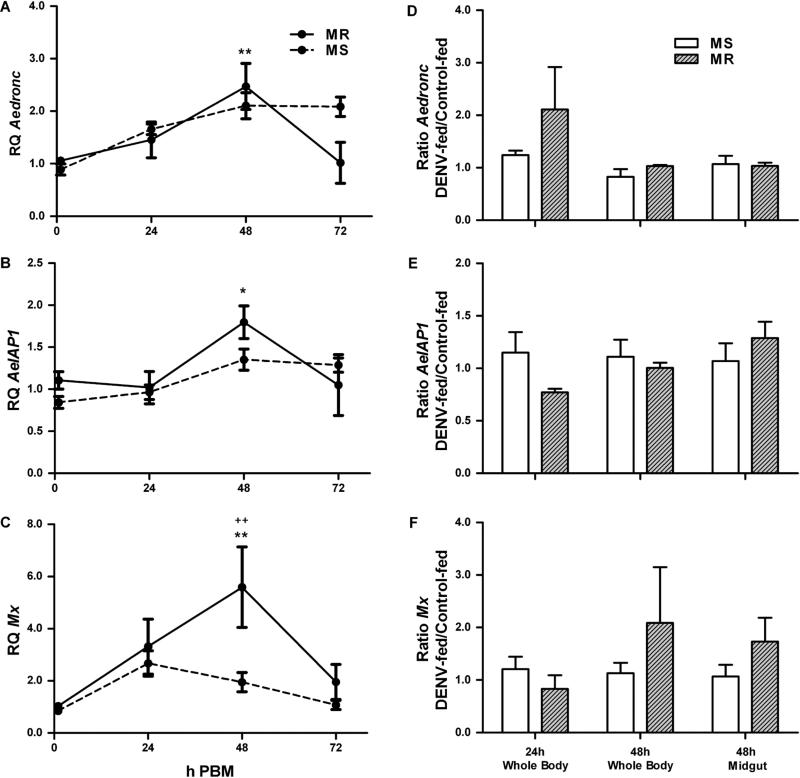
In order to determine transcriptional activity of apoptotic genes following a blood meal, we measured whole body transcriptional activity of Aedronc, AeIAP1 and Mx in MR and MS females 0-72 h following a naïve blood meal. MR females showed a 2.5-fold increase in Aedronc expression at 48 h PBM (p=0.005), which then declined by 72 h (p=0.015) (Fig 1A). In contrast, MS females showed a 2.1-fold, albeit insignificant (p=0.050), increase at this time. MR also showed a significant 1.8-fold increase (p=0.026) of AeIAP1 expression (Fig 1B) by 48 h, while MS did not show a significant (p=0.763) increase. Mx expression was elevated 5.6-fold in the MR strain by 48 h (p=0.002), but only 2.0-fold in the MS strain (p=1.000), compared to 0 h PBM (Fig 1C). At 48 h, MR females had significantly increased Mx compared to MS females (p=0.003). Thus, MR showed upregulation of Aedronc, AeIAP1 and Mx following a blood meal. In addition, at 48 h PBM, Mx was significantly upregulated in MR compared to the MS strain.
Fig 1. Apoptotic gene expression following a DENV-infected blood meal.
(A-C) MS (susceptible strain) and MR (refractory strain) females 4-5 days post eclosion were fed a naïve artificial blood meal and qPCR was performed on RNA extracted from whole mosquitoes collected at 0, 24, 48, and 72 h post blood meal (PBM). Expression of (A) Aedronc, (B) AeIAP1, and (C) Mx was analyzed. Expression is represented as Relative Quantitation (RQ) compared to samples collected before a blood meal. Apoptosis genes showed significant transcriptional upregulation following blood meal compared to females 0 h PBM. Each data point represents mean expression (n=3 replicates; pools of 5 whole mosquitoes per replicate). (D-F) 2-3 day old adult MS and MR females were fed either control or DENV-2 JAM1409-supplemented artificial blood meals. At 24 and 48 h PBM, either whole mosquitoes or midguts were collected and tested for expression of (D) Aedronc, (E) AeIAP1, or (F) Mx transcript. Data represent ratio of transcript of DENV-2-fed to control-fed females (n=3-5 replicates; individual replicates represent the mean of 5 pooled whole females or 10 pooled midguts). Error bars represent standard errors of means. Statistical comparisons were performed using (A-C) two-way ANOVA followed by the Tukey HSD test to identify significant PBM differences relative to 0 h PBM or (D-F) Student's t-test to compare ratios of transcript of DENV-2-fed to control-fed females between MS and MR. *p<0.05; **p<0.01 within strain comparison between 0 h PBM and time point. ++p<0.01 between strain comparison.
To determine whether apoptotic gene expression was changed upon DENV-2 infection, we measured whole body transcription levels of Aedronc, AeIAP1 and Mx in MS and MR females following a naïve or DENV-2-infected blood meal. We did not find any statistically significant differences in the expression of Aedronc, AeIAP1 or Mx due to infection (Fig 1E-F; Supp. Table S3). However, at 24 h PBM Aedronc was elevated in DENV-fed MR compared to DENV-fed MS (Fig 1D), and at 48 h, Mx was ~2.0-fold greater in DENV-fed MR compared to DENV-fed MS, but highly variable between replicates (Fig 1F). We also looked at transcripts of Aedronc, AeIAP1 and Mx in the midguts of MS and MR mosquitoes at 48 h PBM (Fig 1D-F). Again, there were no statistically significant differences for Aedronc, AeIAP1 or Mx upon infection or between strains. However, Mx again showed slightly higher expression in infected MR midguts (Fig 1F), as observed previously in whole bodies, which may suggest elevated apoptotic activation in refractory mosquitoes in response to DENV.
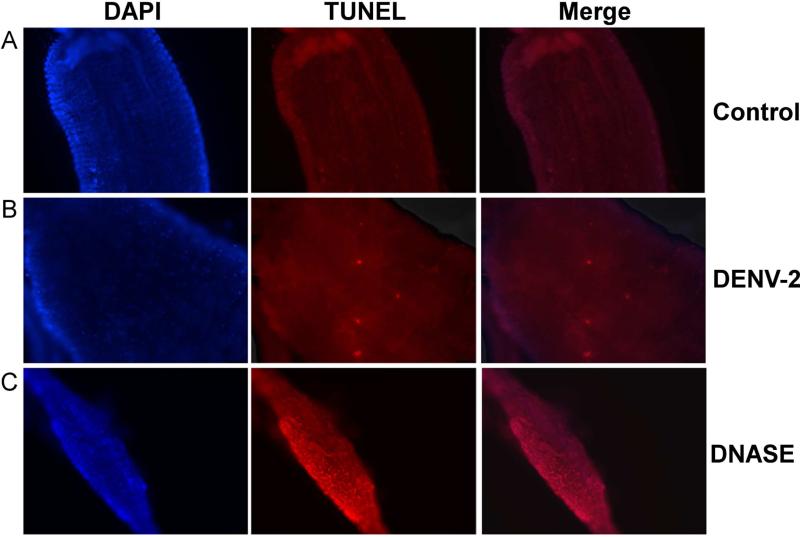
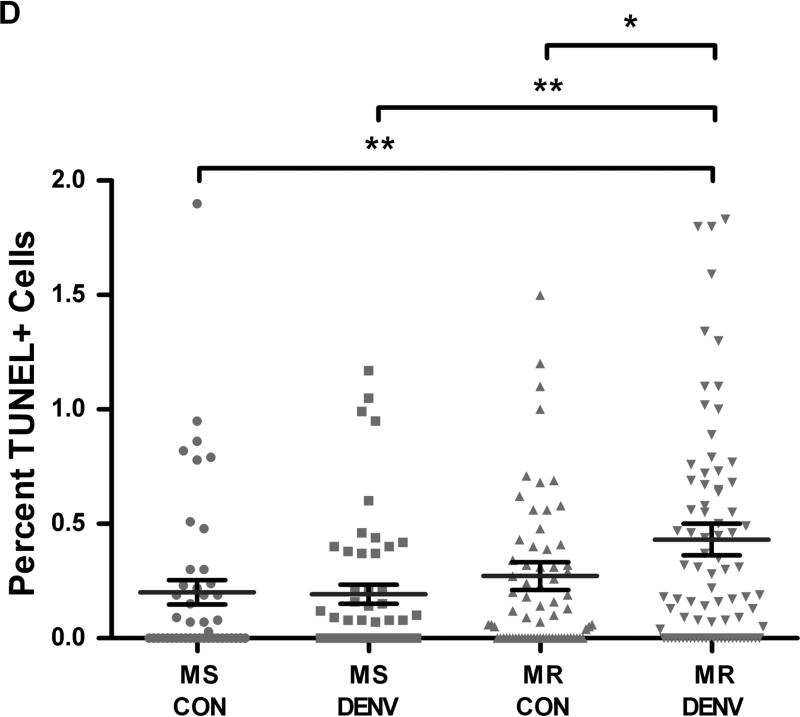
We then assessed apoptotic activity in midgut cells from MS and MR 48 h PBM by TUNEL staining. We observed a basal level of apoptosis occurring in the midguts of naïve-fed females in both strains, with no significant differences between strains (p=0.167). Upon challenging MS and MR with DENV-2, MS midguts did not show differences in TUNEL-positive cells compared to control midguts. However, DENV-2 infected MR midguts showed a significant increase from 0.26% to 0.45% (p=0.0388) of cells being TUNEL-positive compared to control-fed MR midguts (Fig 2A-C). In addition, DENV-2 infected MR midguts also reflected a significantly higher percentage of TUNEL-positive cells than those from control or DENV-2 infected MS midguts (p=0.0055; p=0.0074, respectively) (Fig 2D). We did not observe any obvious bias for anterior or posterior localization of apoptotic foci under any of the conditions tested. Thus, refractory mosquitoes showed significantly more apoptotic activity in midgut tissue upon DENV-2 exposure across all comparisons.
Fig 2. DENV-2-infected midguts from refractory strain (MR) of Ae. aegypti show increased apoptotic cells.
MR and MS females 4-5 days post eclosion were fed either (A) control or (B) DENV-2 JAM1409-supplemented artificial blood meals. At 48 h PBM midguts were dissected out and nuclei were stained with DAPI (blue) and apoptotic cells were stained by TUNEL (red). (C) As a positive control, midguts were incubated with DNAse for 30 min and then stained for TUNEL. Depicted images are of MR mosquitoes. Magnification at 40X. (D) Apoptotic nuclei were quantified as the proportion of the total number of cells in each midgut section (n=3 replicates; 16-28 sections per independent replicate). Statistical analysis of percent midgut cells staining TUNEL+ between groups was performed using Mann-Whitney U test. *p<0.05; **p<0.01.
3.2. Knockdown of Aedronc decreases DENV infection
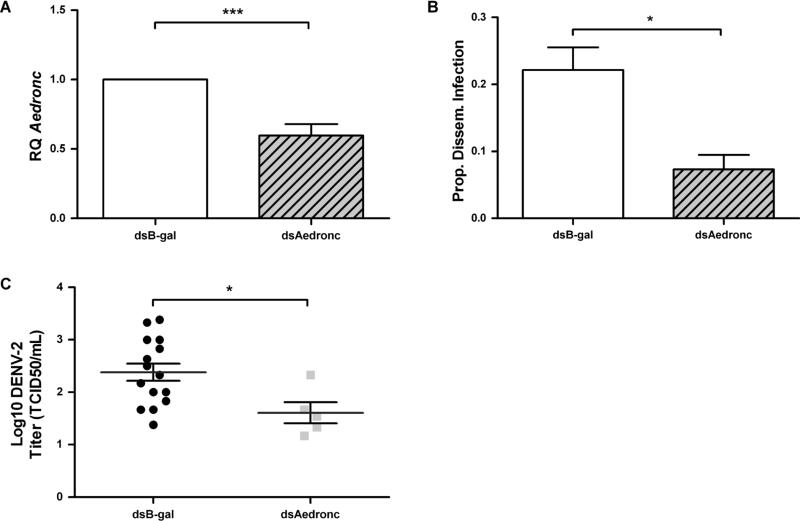
To determine whether apoptosis contributed to DENV susceptibility, we knocked down Aedronc in MR females. Females injected with dsAedronc showed ~40% reduction in Aedronc transcript by 48 HPI (Fig 3A). Knockdown of Aedronc significantly decreased the percentage of mosquitoes showing disseminated infection from 22.1% to 7.3% (p=0.0236) (Fig 3B). Mosquitoes showing disseminated infection and reduced for Aedronc also showed a half-log reduction in DENV titer from TCID50 102.4 to 101.6 (p=0.0204) (Fig 3C). Thus, Aedronc knockdowns resulted in both decreased dissemination frequencies and virus titer in those individuals that were infected. In combination, these experiments show that Aedronc is important for DENV-2 infection and may play a positive role in the ability of the virus to replicate in or infect cells.
Fig 3. Aedronc knockdown decreases the proportion of mosquitoes infected and DENV-2 titer among infected individuals.
Refractory (MR) females 2-3 days post eclosion were injected with either dsAedronc or dsB-gal and: (A) tested for knockdown of Aedronc by qRT-PCR 48 h post injection (HPI). Expression is represented as Relative Quantitation (RQ) compared to dsB-gal-treated females (n=5 replicates; 5 pooled females per replicate). (B) 48 HPI, females were provided DENV-2 JAM1409-supplemented artificial blood meals. Mosquitoes were collected at 14 d PBM and tested for disseminated infection by RT-PCR of DENV RNA from leg tissue (n=3 replicates; no. infected females/total tested per replicate: B-gal: 7/30; 12/44; 9/57; Aedronc: 4/38; 3/36; 1/32). (C) Mosquitoes were homogenized in cell media, and infectious titer was measured by TCID50 assay (n=2 replicates). Error bars represent standard errors of means from biological replicates. Statistical analyses were performed using (A-B) Student's t-test or (C) Mann-Whitney U test. *p<0.05; ***p<0.001.
3.3. Autophagy is upregulated following blood feeding
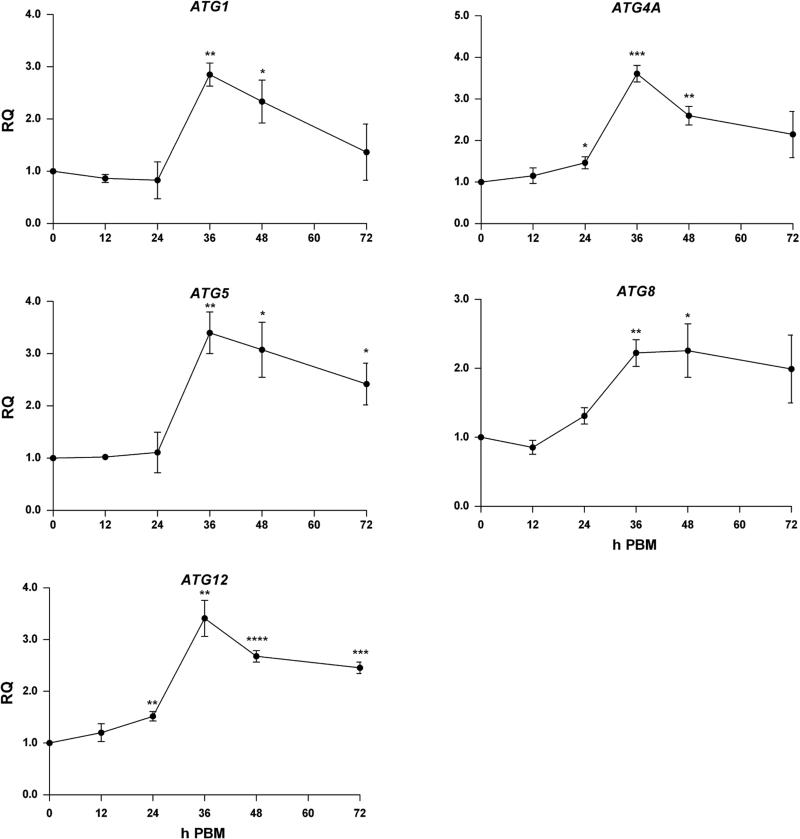
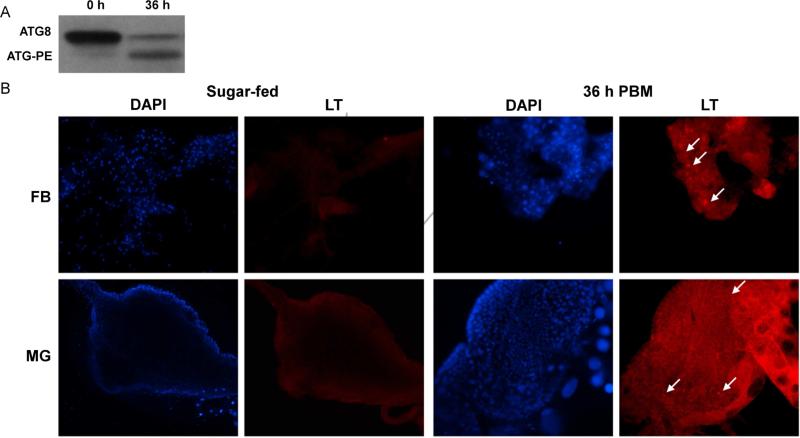
To analyze the possibility that apoptosis genes are involved with regulation of autophagy in Ae. aegypti, we assessed whether programmed autophagy occurs post blood meal. Transcriptional activity of the ATG genes, ATG1, ATG4A, ATG5, ATG8 and ATG12 was elevated in whole MR females following a naïve blood meal compared to sugar fed mosquitoes (Fig 4). Each gene showed statistically significant increases in expression following a blood meal, and we observed significant differences in expression between time points as well (Supp. Table S4). Expression of ATG1, ATG4A, ATG5 and ATG12 showed the greatest increases in expression at 36 h, with relative expression 2.9-3.6-fold higher (p=0.0011; p=0.0002; p=0.0038; p=0.0023) than that of sugar-fed mosquitoes. ATG8 peaked at 48 h with 2.3-fold greater expression (p=0.0314) than sugar-fed mosquitoes. To confirm autophagy is activated 36 h PBM, we performed western blotting of ATG8. We observed that females showed non-lipidated ATG8 prior to blood feeding. However at 36 h PBM, we observed an increase in ATG8-PE and concordant reduction in non-lipidated ATG8 (Fig 5A), thus indicating activation of autophagy. To visualize where autophagic vesicles occurred, we stained fat body and midgut tissues with the acidotropic dye, Lysotracker Red. Here, we found that both fat body and midgut tissue were devoid of Lysotracker staining before feeding, but showed bright puncta at 36 h PBM, further indicating activation of autophagy at that time. (Fig 5B). Taken together, these data confirm that autophagy is active at 36 h PBM in naïve-fed MR females.
Fig 4. Autophagy genes are upregulated following a blood meal.
MR females 4-5 days post eclosion were fed a naïve artificial blood meal and qPCR was performed on RNA extracted from whole mosquitoes collected at 0, 12, 24, 36, 48, and 72 h PBM. Expression of the autophagy genes ATG1, ATG4A, ATG5, ATG8 and ATG12 was analyzed. Expression is represented as Relative Quantitation (RQ) compared to samples collected before a blood meal. Autophagy genes showed significant upregulation following blood meal compared to females fed 0 h PBM. Each data point represents mean expression (n=3 replicates; pools of 5 whole mosquitoes per replicate). Error bars represent standard error of means. Statistical comparisons were performed using one-way ANOVA followed by the Tukey HSD test to identify significant PBM differences relative to 0 h PBM. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001
Fig 5. ATG8-PE conjugation and Lysotraker Red staining indicate autophagosome biogenesis following a blood meal.
(A) MR females 4-5 days post eclosion were fed a naïve artificial blood meal. At 0 and 36 h PBM, pools of 5 whole females were tested for presence of the autophagy marker ATG8. ATG8 is cytoplasmic, but becomes conjugated to phosphatidyl-ethanolamine (PE) on the autophagosomal membrane during autophagy. (B) Female fat body and midgut tissues from mosquitoes fed only on sugar water and from mosquitoes 36 h PBM were stained with DAPI and Lysotracker Red. White arrows point to puncta representative of autophagosomes. Images are at 100X magnification.
3.4. Apoptosis-related genes are activated during autophagy
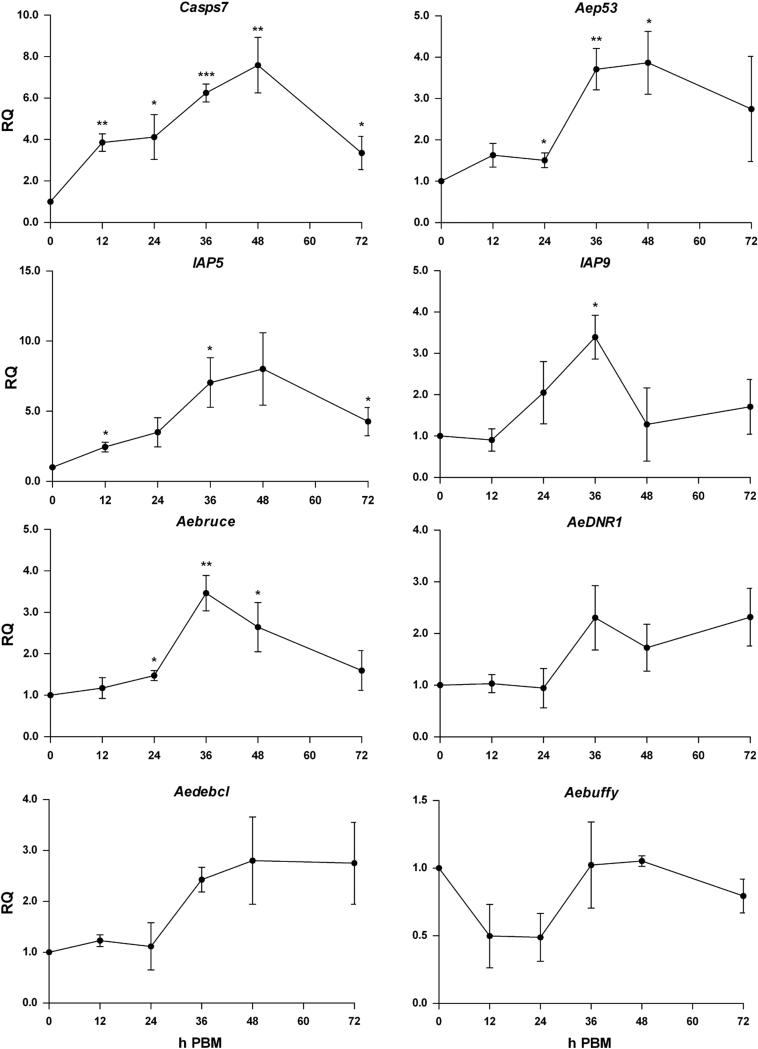
To determine whether Casps7 could be involved with autophagy, we assessed its expression following a naïve blood meal. Expression increased following a blood meal starting at 12 h PBM, and continuing to rise to 7.6-fold until 48 h PBM (p=0.0079). By 72 h PBM, transcript levels had begun to decline (Fig 6). We then monitored expression of other apoptosis-related genes post blood meal. Aep53, IAP5, IAP9, and Aebruce were significantly upregulated following a naïve blood meal, and had similar temporal profiles that coincided with the activation of autophagy (Fig 6; Supp. Table S5). Expression was increased at least 3.4-fold following a blood meal, suggesting that apoptosis-related proteins could be associated with other physiological processes related to blood feeding. AeDNR1, Aedebcl and Aebuffy, did not show statistically significant differences in expression, but AeDNR1 and Aedbcl showed patterns of upregulation by 36 h PBM, and Aebuffy showed a pattern of downregulation from 12-24 h PBM. Expression of Casps8 and Imp was unchanged (data not shown). Thus several apoptosis-related genes show expression profiles paralleling programmed autophagy after blood feeding.
Fig 6. Upregulation of apoptosis-related genes post blood meal coincides with autophagy activation.
MR females 4-5 days post eclosion were fed a naïve artificial blood meal and qPCR was performed on RNA extracted from whole mosquitoes collected at 0, 12, 24, 36, 48, and 72 h PBM. Expression of multiple apoptosis-related genes were analyzed, including Casps7, Aep53, IAP5, IAP9, Aebruce, AeDNR1, Aedebcl, and Aebuffy. Expression is represented as Relative Quantitation (RQ) compared to samples collected before a blood meal. Apoptosis-related genes showed significant transcriptional upregulation following blood meal compared to females 0 h PBM. Each data point represents mean expression (n=3 replicates; pools of 5 whole mosquitoes per replicate). Error bars represent standard error of means. Statistical comparisons were performed using oneway ANOVA followed by the Tukey HSD test to identify significant PBM differences relative to 0 h PBM. *p<0.05; **p<0.01; ***p<0.001.
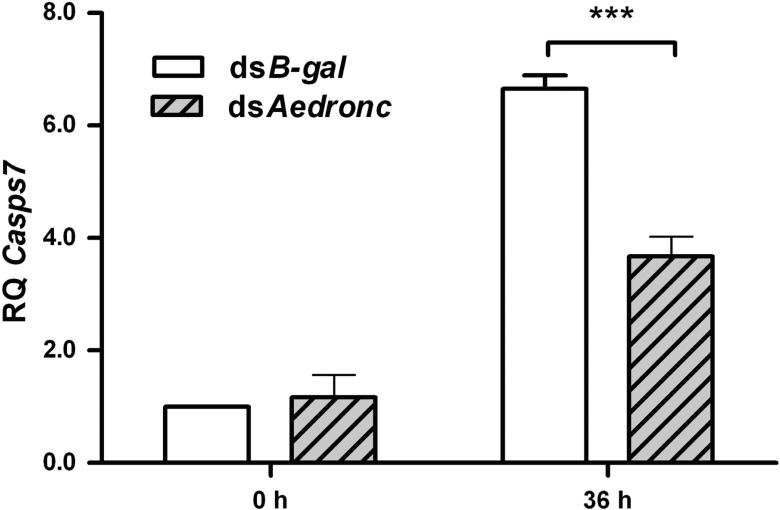
3.5. Aedronc and Casps7 genes regulate autophagy
To determine whether Casps7 expression was affected by Aedronc knockdown in MR females, we analyzed Casps7 transcription following dsAedronc treatment (Fig 7). Aedronc knockdown did not affect transcript levels of Casps7 before a blood meal. However, at 36 h PBM, we observed a significant reduction in Casps7 expression following Aedronc reduction (p=0.0089) that represented ~60% of the transcription levels found in dsB-gal treated individuals. Thus, Aedronc may affect transcriptional activation of Casps7 during blood feeding. We assessed expression of several ATG genes at 36 h PBM (ATG1, ATG4A, ATG5, ATG8 and ATG12) post Aedronc-reduction, but did not find evidence that Aedronc knockdown attenuated transcriptional activation for any of these genes (data not shown).
Fig 7. Aedronc regulates Casps7 transcription following a blood meal.
MR females 2-3 days post eclosion were injected with either dsAedronc or dsB-gal, and then fed a naïve artificial blood meal 48 HPI. Females were collected at 0 and 36 h PBM. Total RNA was extracted and transcript from Casps7 was quantified via qRTPCR. Aedronc knockdown decreased Casps7 transcript following a blood meal, but not before. Expression is represented as Relative Quantitation (RQ) compared to dsB-gal-treated females. Each data point represents mean expression (n=3-5 replicates; pools of 5 whole mosquitoes per replicate). Error bars represent standard error of means. Statistical comparisons were performed using Student's t-test. *** p<0.001.
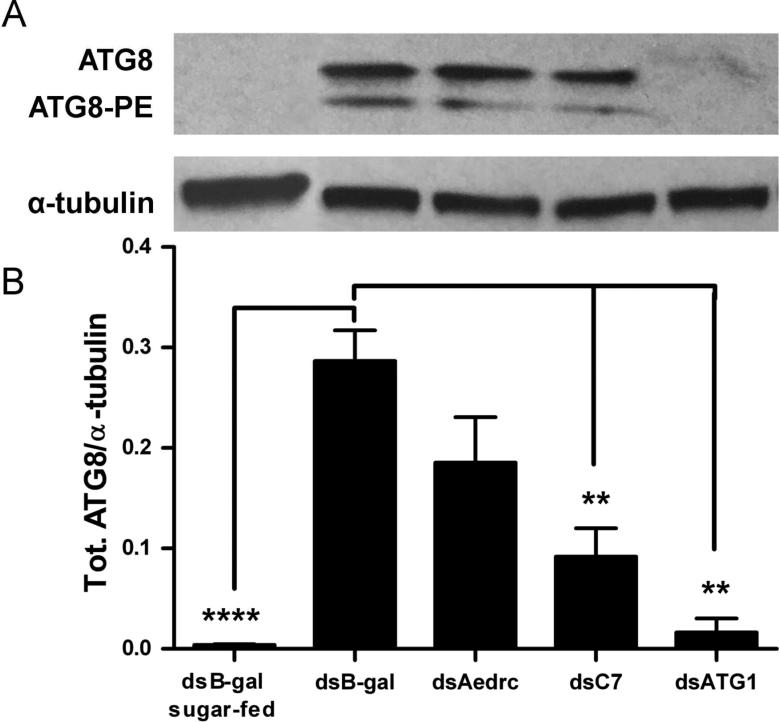
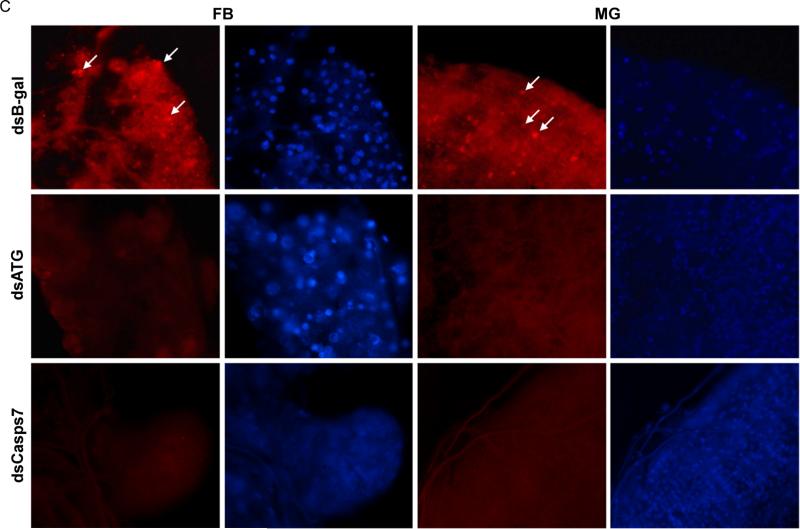
We then assessed the effect of Aedronc and Casps7 reduction on autophagy in midgut-free carcasses of MR females. Naïve blood-fed dsB-gal females showed significantly higher levels of total ATG8 at 36 h PBM compared to dsB-gal females fed only on sucrose solution (p<0.0001) (Fig 8A-B), indicating that ATG8 is induced upon blood feeding. dsAedronc-treated females showed a small but non-significant (p=0.077) reduction in total ATG8. However, midgut-free carcasses from dsCasps7-treated females showed a significant reduction of total ATG8 compared to dsB-gal-treated females (p=0.005). ATG1-silenced females showed near absence of total ATG8 (p=0.001), suggesting ATG1 is necessary for the production or stabilization of ATG8 in fat body. We did not observe differences in the ratios of ATG8-PE/ATG8 across treatments (not shown). We also assayed for differences in Lysotracker staining both in fat body and midgut tissue. Midguts and fat body from dsB-gal-treated females showed consistently bright puncta. Upon dsRNA-mediated depletion of ATG1, ATG5 and ATG7, in parallel with depletion of Casps7, both tissues showed absence of Lysotracker puncta (Fig. 8C). These experiments suggest that in midgut-free carcasses, Casps7 is important for autophagy, but Aedronc may have a more limited role.
Fig 8. Casps7 regulates autophagy activation in fat body.
(A) MR females 2-3 days post eclosion were injected with dsAedronc, dsCasps7, dsATG1 or dsB-gal, and then fed a naïve artificial blood meal 48 HPI. 36 h PBM, midguts were dissected out and remaining bodies without midguts were tested for ATG8 by western blot. (B) Densitometry analysis was used to quantify the ratio of total ATG8 to α-tubulin (n=3-4 replicates; 5 pooled bodies without midguts per replicate). Error bars represent standard error of means. Statistical comparisons were performed using oneway ANOVA followed by the Tukey HSD test to identify significant differences compared to dsB-gal-treated sample 36 h PBM. ** p<0.01; **** p<0.0001. (C) Females were treated with either dsCasps7, a cocktail of dsATG1, dsATG5 and dsATG7 (dsATG), or dsB-gal and then fed a naïve blood meal as above. 36 h PBM, fat body and midguts were dissected out and stained with DAPI and Lysotracker Red. Images represent magnification at 200X.
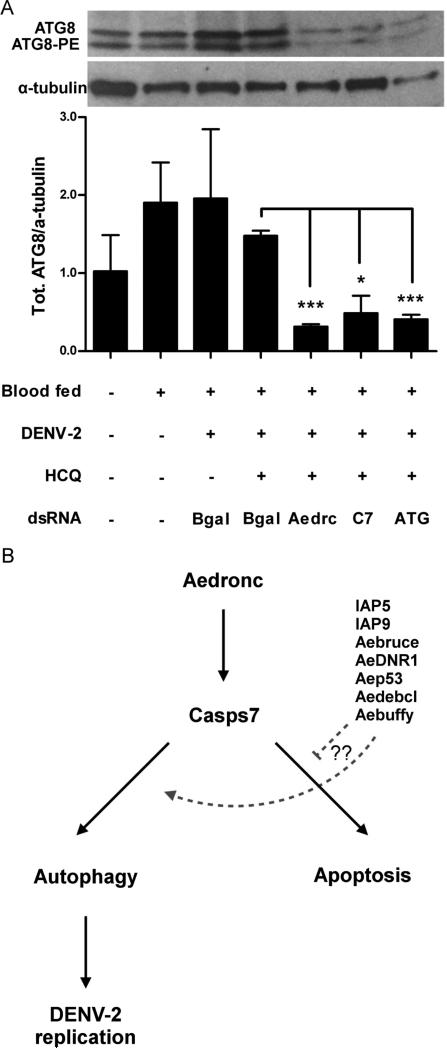
To determine whether Aedronc or Casps7 is involved in regulating autophagy in the midgut and whether autophagy is affected by DENV-2 infection, we assessed midgut tissue from MR females fed a DENV-2 infected blood meal. Females fed a control naïve blood meal showed a small, but non-significant (p=0.245), 1.9-fold increase in total ATG8 compared to females fed only on sucrose solution (Fig 9A). When compared to the increase of total ATG8 observed in midgut-free carcasses between sugar- and blood-fed females (Fig 8B), this suggests that there is a basal level of autophagy occurring in midgut tissue. Females challenged with DENV-2 did not show significant differences compared to those fed naïve blood. Total ATG8 was significantly reduced in DENV-2 fed females following knockdown of Aedronc (p=0.009), Casps7 (p=0.0133) or triple depletion of ATG1, ATG5, and ATG7 compared to DENV-2 fed females treated with dsB-gal (p=0.0003) (Fig 9A). Thus, in midgut tissue, both Aedronc and Casps7 are necessary for autophagy. We did not observe differences in the ratios of ATG8-PE/ATG8 across treatments (not shown). Taken together, these experiments suggest that Aedronc and Casps7 may act as positive regulators of autophagy, albeit with tissue-specific differences. While Casps7 knockdown appears to be essential for autophagy activation in both fat body and midgut, Aedronc may primarily affect autophagy regulation in midgut tissue.
Fig 9. Aedronc and Casps7 knockdown reduces autophagy in midgut.
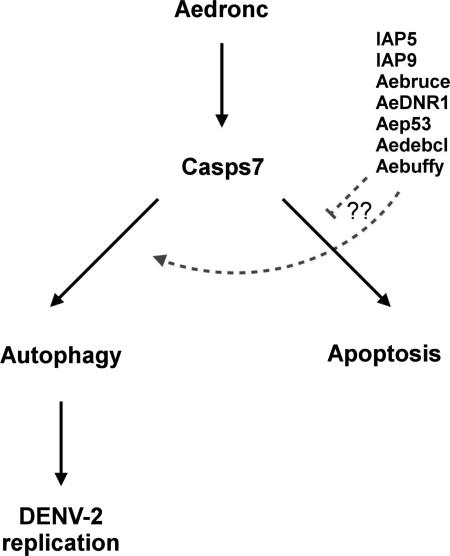
(A) MR females 2-3 days post eclosion were co-injected with hydroxychloroquine (HCQ), and dsB-gal, dsAedronc, dsCasps7 or mixture of dsATG1, dsATG5 and dsATG7 (ATG). 48 HPI, mosquitoes were fed either a control or DENV-2 infected artificial blood meal. At 3 d PBM, midguts were dissected out and analyzed for ATG8 by western blot. Densitometry analysis was used to quantify the ratio of total ATG8 to α-tubulin. (n=3-4 replicates; 10 pooled midguts per replicate). Total ATG8/α-tubulin was reduced in midguts of mosquitoes co-injected with HCQ and dsAedronc, dsCasps7 or dsATG compared to midguts from those treated with HCQ and dsB-gal. Data represent means of replicates. Error bars represent standard error of means. Statistical comparisons were performed using Student's t-test of ratios of total ATG8/α-tubulin between groups * p<0.05; *** p<0.001. (B) Model for caspase control of autophagy. Following a blood meal, Aedronc is needed for transcriptional activation of or stability of Casps7 transcript. Casps7 can activate apoptosis or autophagy, but is guided to activate autophagy following a blood meal, possibly with the help of other apoptosis-related proteins. Autophagy may be associated with DENV-2 infection of midgut cells wherein it attenuates the apoptotic program and increases infection.
4. Discussion
4.1 Apoptosis in DENV infection
In this study we found that knockdown of Aedronc decreased DENV-2 infection in our refractory Ae. aegypti strain. In contrast, Ocampo et al (2013) previously reported that silencing Aedronc increased mosquito susceptibility to DENV. There are several possible explanations for this discrepancy. However, this simply may reflect that activation of apoptosis is complex and can be triggered through multiple pathways depending on experimental conditions such as MOI and tissue type (Klomporn et al, 2011; McLean et al, 2011; Pattanakitsukul et al, 2007; Theparrit, et al, 2013; Zargar et al, 2011). We note that in Ocampo et al both the range of titers of DENV used for infection and corresponding oral susceptibility seen with their refractory and susceptible strains were higher than those used in our study. Further, Ocampo et al primarily used immunofluorescence of head and midgut tissue to detect virus, while our study focused on detection of DENV RNA from leg tissue and virus titer. Therefore, it can be difficult to directly compare results across studies.
Another report showed that silencing Aedronc reduced the intensity of SINV infection in Ae. aegypti midgut and salivary glands, similar to our findings (Wang et al, 2012). SINV is well known to attenuate apoptosis in insects, and the finding that silencing this gene decreased infection suggests caspases may be multi-functional. However, a subsequent report by the same group (O'Neill et al, 2015) showed that although recombinant SINV-induced overexpression of Drosophila Rpr did initially induce strong apoptosis, they observed strong selection for null mutation viruses and an associated reduction in apoptosis. Results from these reports may be explained by difference in experimental approach and the genes targeted. O'Neill et al (2015) focuses on upstream activation of apoptosis by massive overexpression of Rpr, while the study by Wang et al (2012) utilized knockdown of AeIAP1 and Aedronc. Downstream apoptotic effectors, such as Aedronc and Casps7 could operate within both processes dependent on context, while upregulation of Mx may favor a pro-apoptotic outcome.
From our study it is unclear whether apoptosis or just caspase function is actually important for infection. Apoptosis is activated in response to infection with all four serotypes of DENV in human cells, through both intrinsic and extrinsic pathways (Klomporn et al, 2011; Liao et al, 2010; Nasirudeen et al, 2008; Umareddy et al, 2007). Furthermore, DENV has mechanisms to attenuate the apoptotic program (Lee et al, 2005; Li et al, 2012, McLean et al, 2011), suggesting apoptosis could also limit infection in the mosquito. Consistent with these studies, we observed increased apoptosis in MR midguts following a DENV-infected blood meal. However, we also observed programmed autophagy at this time. In addition, we and others (Bonizzoni et al, 2012a; Puglise et al, 2015) observed upregulation of several apoptosis-related genes, some of which control autophagy in insects (Accorsi et al, 2015; Denton et al, 2012b). While these genes may indeed be activated in response to pathogen infection, our finding that Aedronc knockdown reduces infection suggests that apoptosis-related genes may have additional roles. Furthermore, we found that both pro- (Casps7, Aedronc, Mx, Aedbcl) and anti-apoptotic (AeIAP1, IAP5, IAP9, Aebruce) genes were upregulated PBM, complicating the interpretation of how apoptosis, autophagy, or interaction between the two processes influences infection. In conjunction with other studies, it is clear that apoptosis is a complex process whose proteins may also function non-apoptotically depending on context.
4.2 Caspase control of autophagy
Autophagy is generally considered a pro-survival mechanism, while apoptosis is considered pro-death. However, in certain circumstances, cell death genes have been shown to have non-apoptotic functions, including the activation of autophagy (Kuranaga 2012; Denton et al, 2012a). In insects, autophagy occurs in tissues undergoing degradation during morphogenesis (Berry & Baehrecke, 2007; Lee et al, 2002; Rusten et al, 2004), where autophagic vesicles have been associated with type II programmed cell death, or autophagic cell death (Kroemer & Levine, 2008). Genetic screens in D. melanogaster show that pro-apoptotic proteins positively regulate autophagy, while IAPs act as negative regulators (Hou et al, 2008; Kim et al, 2010). Our data indicate that Aedronc, Casps7 and several other apoptosis-related genes are transcriptionally active during programmed autophagy in Ae. aegypti, including Casps7, Aep53 and several IAP orthologs (IAP5, IAP9, Aebruce). This complements and confirms other transcription studies where autophagic and IAP genes were upregulated in other strains of Ae. aegypti following blood feeding (Bonizzonni et al, 2011; Bonizzoni et al, 2012b; Puglise et al, 2015). In this study, we present a model where caspases are involved with the control of programmed autophagy post meal meal (Fig 9B). Initiator caspase Aedronc controls transcription or stability of effector caspase Casps7. Casps7 can promote either apoptosis or autophagy, possibly with the guidance of other apoptosis-related proteins. The mechanism by which Casps7 could activate autophagy is still unknown. In D. melanogaster, a Casps7 ortholog promotes autophagy by inhibiting translocation of the autophagy inhibitor, ATP, during oogenesis (DeVorkin et al, 2014). Differences in activity of the pro- and cleaved forms of effector caspase may promote autophagy without inducing apoptosis. Interestingly, Kim et al (2010) found that in D. melanogaster full length Casps7 promoted autophagy, whereas expression of the truncated version induced apoptosis. Thus, the subactivation of caspases can lead to substrate cleavage without the induction of apoptosis.
In summary, we present evidence that the initiator caspase Aedronc is important for DENV infection in Ae. aegypti, possibly through its regulation of autophagy. Autophagy has previously been implicated in the replication and infection of DENV in mammalian cells. Inhibition of autophagy in murine systems can reduce DENV infection by over 1000-fold (Lee et al, 2008; Heaton & Randall, 2010; McLean et al, 2011; Mateo et al, 2013). In addition, stimulation of autophagy in epithelial cells by DENV NS4A protein protects them against DENV-induced apoptosis (McLean et al, 2011). Autophagy also promotes DENV infection by altering lipid metabolism and enhancing maturation of infectious particles (Heaton & Randall, 2010; Panyasrivit et al, 2009; Mateo et al, 2013), and it is possible that cellular components freed by autophagy could be important for the rearrangement of internal membrane structures that act as sites of replication (Junjhon et al, 2014). While these DENV-autophagy connections have not yet been tested in Ae. aegypti, we previously found transcriptional upregulation of autophagy genes ATG4A, Aedebcl and Casps7 in MS strain and not MR strain following DENV infection (Behura, S.K. & Severson, D.W., unpublished data; Chauhan et al, 2012). Given the importance that programmed autophagy plays in mosquito physiology following vitellogenesis and the infection of murine hosts, it is probable that this process is important in infection of vector hosts as well, but has yet to be determined. Understanding such host factors is necessary in order to develop novel methods of blocking transmission of vector-borne diseases.
Supplementary Material
Highlights.
DENV-refractory, but not susceptible mosquitoes show increased apoptosis of midgut tissue upon DENV-2 infection.
Unexpectedly, silencing the initiator caspase Aedronc decreases DENV-2 dissemination and infectious titer in Aedes aegypti.
Programmed autophagy is activated following a blood meal, and several apoptosis-related genes show upregulation paralleling this process.
Knockdown of Aedronc and the effector caspase, Casps7, reduces programmed autophagy in midgut tissues.
Acknowledgements
We thank Alexander Raikhel and Vladimir Kokoza for generously providing mosquito ATG8 antibody for our experiments. We also thank Diane Lovin and Susanta Behura for assistance with virus titration and data analysis.
Abbreviations
- DENV
dengue virus
- SINV
Sindbis virus
- MS
Moyo-S
- MR
Moyo-R
- IAP
Inhibitor of apoptosis
- Mx
Michelob_x
- ATG
autophagy-related protein
- HPI
hours post injection
- PBM
post blood meal
- RH
relative humidity
- DENV
dengue virus
- MOI
multiplicity of infection
- qPCR
quantitative PCR
- RPS17
ribosomal protein S17
- RPL8
Ribosomal protein L8
- B-gal
B-galactosidase
- HCQ
hydroxychloroquine
- FBS
fetal bovine serum
- PE
Phosphatidyl ethanolamine
- RQ
relative quantitation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Matthew W. Eng, Eck Institute for Global Health and Department of Biological Sciences, University of Notre Dame, Notre Dame, IN 46556
Madeleine N. van Zuylen, Eck Institute for Global Health and Department of Biological Sciences, University of Notre Dame, Notre Dame, IN 46556
David W. Severson, Eck Institute for Global Health and Department of Biological Sciences, University of Notre Dame, Notre Dame, IN 46556
References
- Accorsi A, Zibaee A, Malagoli D. The multifaceted activity of insect caspases. J. Insect Physiol. 2015;76:17–23. doi: 10.1016/j.jinsphys.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Baehrecke EH. Autophagy: dual roles in life and death? Nat. Rev. Mol. Cell Biol. 2005;6:505–10. doi: 10.1038/nrm1666. [DOI] [PubMed] [Google Scholar]
- Barth S, Glick D, MacLeod KF. Autophagy: assays and artifacts. J Pathol. 2010;221:117–124. doi: 10.1002/path.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behura SK, Gomez-Machorro C, Harker BW, deBruyn B, Lovin DD, Hemme RR, Mori A, Romero-Severson J, Severson DW. Global cross-talk of genes of the mosquito Aedes aegypti in response to dengue virus infection. PLoS Negl. Trop. Dis. 2011;5:e1385. doi: 10.1371/journal.pntd.0001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behura SK, Gomez-Machorro C, deBruyn B, Lovin DD, Harker BW, Romero-Severson J, Mori A, Severson DW. Influence of mosquito genotype on transcriptional response to dengue virus infection. Funct. Integr. Genomics. 2014;14:581–589. doi: 10.1007/s10142-014-0376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berois M, Romero-Severson J, Severson DW. RNAi knock-downs support roles for the mucin-like (AeIMUC1) gene and short-chain dehydrogenase/reductase (SDR) gene in Aedes aegypti susceptibility to Plasmodium gallinaceum. Med. Vet. Entomol. 2012;26:112–5. doi: 10.1111/j.1365-2915.2011.00965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell. 2007;131:1137–48. doi: 10.1016/j.cell.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black WC, IV, Bennett KE, Gorrochótegui-Escalante N, Barillas-Mury CV, Fernández-Salas I, Muñoz MDL, Farfán-Alé JA, Olson KE, Beaty BJ. Flavivirus susceptibility in Aedes aegypti. Arch. Med. Res. 2002;33:379–388. doi: 10.1016/s0188-4409(02)00373-9. [DOI] [PubMed] [Google Scholar]
- Bonizzoni M, Dunn WA, Campbell CL, Olson KE, Dimon MT, Marinotti O, James AA. RNA-seq analyses of blood-induced changes in gene expression in the mosquito vector species, Aedes aegypti. BMC Genomics. 2011;12:82. doi: 10.1186/1471-2164-12-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzoni M, Dunn WA, Campbell CL, Olson KE, Marinotti O, James AA. Complex modulation of the Aedes aegypti transcriptome in response to dengue virus infection. PLoS One. 2012a;7:e50512. doi: 10.1371/journal.pone.0050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzoni M, Dunn WA, Campbell CL, Olson KE, Marinotti O, James AA. Strain variation in the transcriptome of the dengue fever vector, Aedes aegypti. G3 (Bethesda) 2012b;2:103–14. doi: 10.1534/g3.111.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant B, Raikhel AS. Programmed autophagy in the fat body of Aedes aegypti is required to maintain egg maturation cycles. PLoS One. 2011;6:e25502. doi: 10.1371/journal.pone.0025502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y-Y, Neufeld TP. Autophagy takes flight in Drosophila. FEBS Lett. 2010;584:1342–1349. doi: 10.1016/j.febslet.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan C. Comparative expression profiles of midgut genes in dengue virus refractory and susceptible Aedes aegypti across critical period for virus infection. PLoS One. 2012;7:e47350. doi: 10.1371/journal.pone.0047350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemons A, Mori A, Haugen M, Severson DW, Duman-Scheel M. Culturing and egg collection of Aedes aegypti. Cold Spring Harb. Protoc. 20102010:pdb.prot5507. doi: 10.1101/pdb.prot5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colpitts TM, Cox J, Vanlandingham DL, Feitosa FM, Cheng G, Kurscheid S, Wang P, Krishnan MN, Higgs S, Fikrig E. Alterations in the Aedes aegypti transcriptome during infection with west nile, dengue and yellow fever viruses. PLoS Pathog. 2011;7:e1002189. doi: 10.1371/journal.ppat.1002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daish TJ, Mills K, Kumar S. Drosophila caspase DRONC is required for specific developmental cell death pathways and stress-induced apoptosis. Dev. Cell. 2004;7:909–15. doi: 10.1016/j.devcel.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Denton D, Chang T-K, Nicolson S, Shravage B, Simin R, Baehrecke EH, Kumar S. Relationship between growth arrest and autophagy in midgut programmed cell death in Drosophila. Cell Death Differ. 2012a;19:1299–307. doi: 10.1038/cdd.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denton D, Nicolson S, Kumar S. Cell death by autophagy: facts and apparent artefacts. Cell Death Differ. 2012b;19:87–95. doi: 10.1038/cdd.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deubel V, Kinney RM, Trent DW. Nucleotide sequence and deduced amino acid sequence of the structural proteins of dengue type 2 virus, Jamaica genotype. Virol. 1986;155:365–377. doi: 10.1016/0042-6822(86)90200-x. [DOI] [PubMed] [Google Scholar]
- DeVorkin L, Go NE, Hou Y-CC, Moradian A, Morin GB, Gorski SM. The Drosophila effector caspase Dcp-1 regulates mitochondrial dynamics and autophagic flux via SesB. J. Cell Biol. 2014;205:477–92. doi: 10.1083/jcb.201303144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y, He D, Yao Z, Klionsky DJ. The machinery of macroautophagy. Cell Res. 2014;24:24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard YA, Schneider BS, McGee CE, Wen J, Han VC, Popov V, Mason PW, Higgs S. Salivary gland morphology and virus transmission during long-term cytopathologic West Nile Virus infection in Culex mosquitoes. Am. J. Trop. Med. Hyg. 2007;76:118–128. [PubMed] [Google Scholar]
- Guzman MG, Harris E. Dengue. Lancet. 2015;385:453–465. doi: 10.1016/S0140-6736(14)60572-9. [DOI] [PubMed] [Google Scholar]
- Heaton NS, Randall G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe. 2010;8:422–32. doi: 10.1016/j.chom.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y-CC, Chittaranjan S, Barbosa SG, McCall K, Gorski SM. Effector caspase Dcp-1 and IAP protein Bruce regulate starvation-induced autophagy during Drosophila melanogaster oogenesis. J. Cell Biol. 2008;182:1127–39. doi: 10.1083/jcb.200712091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junjhon J, Pennington JG, Edwards TJ, Perera R, Lanman J, Kuhn RJ. Ultrastructural characterization and three-dimensional architecture of replication sites in dengue virus-infected mosquito cells. J. Virol. 2014;88:4687–97. doi: 10.1128/JVI.00118-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly EM, Moon DC, Bowers DF. Apoptosis in mosquito salivary glands: Sindbis virus-associated and tissue homeostasis. J. Gen. Virol. 2012;93:2419–2424. doi: 10.1099/vir.0.042846-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-I, Ryu T, Lee J, Heo Y-S, Ahnn J, Lee S-J, Yoo O. A genetic screen for modifiers of Drosophila caspase Dcp-1 reveals caspase involvement in autophagy and novel caspase-related genes. BMC Cell Biol. 2010;11:9. doi: 10.1186/1471-2121-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klomporn P, Panyasrivanit M, Wikan N, Smith DR. Dengue infection of monocytic cells activates ER stress pathways, but apoptosis is induced through both extrinsic and intrinsic pathways. Virology. 2011;409:189–97. doi: 10.1016/j.virol.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Jäättelä M. Lysosomes and autophagy in cell death control. Nat. Rev. Cancer. 2005;5:886–897. doi: 10.1038/nrc1738. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuranaga E. Beyond apoptosis: caspase regulatory mechanisms and functions in vivo. Genes Cells. 2012;17:83–97. doi: 10.1111/j.1365-2443.2011.01579.x. [DOI] [PubMed] [Google Scholar]
- Lan Q, Fallon a M. Sequence analysis of a mosquito ribosomal protein rpL8 gene and its upstream regulatory region. Insect Mol. Biol. 1992;1:71–80. doi: 10.1111/j.1365-2583.1993.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Calisher CH, Gubler DJ, Chang G, Vorndamt AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 1992;30:545–51. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Baehrecke EH. Steroid regulation of autophagic programmed cell death during development. Development. 2001;128:1443–55. doi: 10.1242/dev.128.8.1443. [DOI] [PubMed] [Google Scholar]
- Lee C-Y, Cooksey BA, Baehrecke EH. Steroid regulation of midgut cell death during Drosophila development. Dev. Biol. 2002;250:101–111. doi: 10.1006/dbio.2002.0784. [DOI] [PubMed] [Google Scholar]
- Lee C-Y, Clough EA, Yellon P, Teslovich TM, Stephan DA, Baehrecke EH. Genome-wide analyses of steroid- and radiation-triggered programmed cell death in Drosophila. Curr. Biol. 2003;13:350–7. doi: 10.1016/s0960-9822(03)00085-x. [DOI] [PubMed] [Google Scholar]
- Lee C, Liao C, Lin Y. Flavivirus activates phosphatidylinositol 3-kinase signaling to block caspase-dependent apoptotic cell death at the early stage of virus infection. J. Virol. 2005;79:8388–8399. doi: 10.1128/JVI.79.13.8388-8399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-R, Lei H-Y, Liu M-T, Wang J-R, Chen S-H, Jiang-Shieh Y-F, Lin Y-S, Yeh T-M, Liu C-C, Liu H-S. Autophagic machinery activated by dengue virus enhances virus replication. Virology. 2008;374:240–8. doi: 10.1016/j.virol.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-R, Hu H-Y, Kuo S-H, Lei H-Y, Lin Y-S, Yeh T-M, Liu C-C, Liu H-S. Dengue virus infection induces autophagy: an in vivo study. J. Biomed. Sci. 2013;20:65. doi: 10.1186/1423-0127-20-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Huang R, Liao W, Chen Z, Zhang S, Huang R. Dengue virus utilizes calcium modulating cyclophilin-binding ligand to subvert apoptosis. Biochem. Biophys. Res. Commun. 2012;418:622–7. doi: 10.1016/j.bbrc.2012.01.050. [DOI] [PubMed] [Google Scholar]
- Liao H, Xu J, Huang J. FasL / Fas pathway is involved in dengue virus induced apoptosis of the vascular endothelial cells. 2010;1399:1392–1399. doi: 10.1002/jmv.21815. [DOI] [PubMed] [Google Scholar]
- Liu B, Becnel JJ, Zhang Y, Zhou L. Induction of reaper ortholog mx in mosquito midgut cells following baculovirus infection. Cell Death Differ. 2011;18:1337–45. doi: 10.1038/cdd.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Clem RJ. Defining the core apoptosis pathway in the mosquito disease vector Aedes aegypti: the roles of iap1, ark, dronc, and effector caspases. Apoptosis. 2011;16:105–13. doi: 10.1007/s10495-010-0558-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- Mane-Padros D, Cruz J, Cheng A, Raikhel AS. A critical role of the nuclear receptor HR3 in regulation of gonadotrophic cycles of the mosquito Aedes aegypti. PLoS One. 2012;7:e45019. doi: 10.1371/journal.pone.0045019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DN, Baehrecke EH. Caspases function in autophagic programmed cell death in Drosophila. Development. 2004;131:275–284. doi: 10.1242/dev.00933. [DOI] [PubMed] [Google Scholar]
- Mateo R, Nagamine CM, Spagnolo J, Méndez E, Rahe M, Gale M, Yuan J, Kirkegaard K. Inhibition of cellular autophagy deranges dengue virion maturation. J. Virol. 2013;87:1312–21. doi: 10.1128/JVI.02177-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvezin C, Ayala C, Braden CR, Kim J, Neufeld TP. Assays to monitor autophagy in Drosophila. Methods. 2014;68:134–139. doi: 10.1016/j.ymeth.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean JE, Wudzinska A, Datan E, Quaglino D, Zakeri Z. Flavivirus NS4A-induced autophagy protects cells against death and enhances virus replication. J. Biol. Chem. 2011;286:22147–59. doi: 10.1074/jbc.M110.192500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPhee CK, Baehrecke EH. Autophagy in Drosophila melanogaster. Biochim. Biophys. Acta. 2009;1793:1452–1460. doi: 10.1016/j.bbamcr.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morlais I, Mori A, Schneider JR, Severson DW. A targeted approach to the identification of candidate genes determining susceptibility to Plasmodium gallinaceum in Aedes aegypti. Mol. Genet. Genomics. 2003;269:753–64. doi: 10.1007/s00438-003-0882-7. [DOI] [PubMed] [Google Scholar]
- Nasirudeen AM, Wang L, Liu DX. Induction of p53-dependent and mitochondria-mediated cell death pathway by dengue virus infection of human and animal cells. Microbes Infect. 2008;10:1124–32. doi: 10.1016/j.micinf.2008.06.005. [DOI] [PubMed] [Google Scholar]
- Nawtaisong P, Keith J, Fraser T, Balaraman V, Kolokoltsov A, Davey RA, Higgs S, Mohammed A, Rongsriyam Y, Komalamisra N, Fraser MJ. Effective suppression of dengue fever virus in mosquito cell cultures using retroviral transduction of hammerhead ribozymes targeting the viral genome. Virol. J. 2009;6:73. doi: 10.1186/1743-422X-6-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nezis IP, Lamark T, Velentzas AD, Rusten TE, Bjørkøy G, Johansen T, Papassideri IS, Stravopodis DJ, Margaritis LH, Stenmark H, Brech A. Cell death during Drosophila melanogaster early oogenesis is mediated through autophagy. Autophagy. 2009;5:298–302. doi: 10.4161/auto.5.3.7454. [DOI] [PubMed] [Google Scholar]
- Ocampo CB, Caicedo PA, Jaramillo G, Ursic Bedoya R, Baron O, Serrato IM, Cooper DM, Lowenberger C. Differential expression of apoptosis related genes in selected strains of Aedes aegypti with different susceptibilities to dengue virus. PLoS One. 2013;8:e61187. doi: 10.1371/journal.pone.0061187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill K, Olson BJSC, Huang N, Unis D, Clem RJ. Rapid selection against arbovirus-induced apoptosis during infection of a mosquito vector. Proc. Natl. Acad. Sci. 2015;112:E1152–E1161. doi: 10.1073/pnas.1424469112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyasrivanit M, Khakpoor A, Wikan N, Smith DR. Co-localization of constituents of the dengue virus translation and replication machinery with amphisomes. J. Gen. Virol. 2009;90:448–456. doi: 10.1099/vir.0.005355-0. [DOI] [PubMed] [Google Scholar]
- Pattanakitsakul S, Rungrojcharoenkit K, Kanlaya R, Sinchaikul S, Noisakran S, Chen S-T, Malasit P, Thongboonkerd V. Proteomic analysis of host responses in HepG2 cells during dengue virus infection research articles. J. Proteome Res. 2007;6:4592–4600. doi: 10.1021/pr070366b. [DOI] [PubMed] [Google Scholar]
- Puglise JM, Estep AS, Becnel JJ. Expression profiles and RNAi silencing of Inhibitor of Apoptosis transcripts in Aedes, Anopheles, and Culex mosquitoes ( Diptera : Culicidae ) J. Med. Entomol. 2015;0:1–11. doi: 10.1093/jme/tjv191. [DOI] [PubMed] [Google Scholar]
- Reed LJ, Muench H. A simple method of estimating fifty percent endpoints. Am. J. Trop. Med. Hyg. 1938;27:493–497. [Google Scholar]
- Roekring S, Smith DR. Induction of apoptosis in densovirus infected Aedes aegypti mosquitoes. J. Invert. Pathol. 2010;104:239–241. doi: 10.1016/j.jip.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Rubeck L, Dissing J. Rapid, simple alkaline extraction of human genomic DNA from whole blood, buccal epithelial cells, semen and forensic stains for PCR. Biotechniques. 1998;25:588–90. 592. doi: 10.2144/98254bm09. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, Cuervo AM, Ravikumar B, Sarkar S, Korolchuk VI, Kaushik S, Klionsky DJ. search of an “autophagomometer”. Autophagy. 2009;5:585–589. doi: 10.4161/auto.5.5.8823. [DOI] [PubMed] [Google Scholar]
- Rusten TE, Lindmo K, Juhász G, Sass M, Seglen PO, Brech A, Stenmark H. Programmed autophagy in the Drosophila fat body is induced by ecdysone through regulation of the PI3K pathway. Dev. Cell. 2004;7:179–192. doi: 10.1016/j.devcel.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: A randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- Salazar MI, Richardson JH, Sanchez-Vargas I, Olson KE, Beaty BJ. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007;7:9. doi: 10.1186/1471-2180-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schneider JR, Mori A, Romero-Severson J, Chadee DD, Severson DW. Investigations of dengue-2 susceptibility and body size among Aedes aegypti populations. Med. Vet. Entomol. 2007;21:370–6. doi: 10.1111/j.1365-2915.2007.00699.x. [DOI] [PubMed] [Google Scholar]
- Sim S, Dimopoulos G. Dengue virus inhibits immune responses in Aedes aegypti cells. PLoS One. 2010;5:e10678. doi: 10.1371/journal.pone.0010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim S, Jupatanakul N, Ramirez JL, Kang S, Romero-Vivas CM, Mohammed H, Dimopoulos G. Transcriptomic profiling of diverse Aedes aegypti strains reveals increased basal-level immune activation in dengue virus-refractory populations and identifies novel virus-vector molecular interactions. PLoS Negl. Trop. Dis. 2013;7:e2295. doi: 10.1371/journal.pntd.0002295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thathy V, Severson DW, Christensen BM. Reinterpretation of the genetics of susceptibility of Aedes aegypti to Plasmodium gallinaceum. J. Parasitol. 1994;80:705–12. [PubMed] [Google Scholar]
- Thepparit C, Khakpoor A, Khongwichit S, Wikan N, Fongsaran C, Chingsuwanrote P, Panraksa P, Smith DR. Dengue 2 infection of HepG2 liver cells results in endoplasmic reticulum stress and induction of multiple pathways of cell death. BMC Res. Notes. 2013;6:372. doi: 10.1186/1756-0500-6-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SJ, Rothman AL. Trials and tribulations on the path to developing a dengue vaccine. Vaccine. 2015;33:D24–D31. doi: 10.1016/j.vaccine.2015.05.095. [DOI] [PubMed] [Google Scholar]
- Umareddy I, Pluquet O, Wang QY, Vasudevan SG, Chevet E, Gu F. Dengue virus serotype infection specifies the activation of the unfolded protein response. Virol. J. 2007;4:91. doi: 10.1186/1743-422X-4-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan R, Scott TW. Apoptosis in mosquito midgut epithelia associated with West Nile virus infection. Apoptosis. 2006;11:1643–51. doi: 10.1007/s10495-006-8783-y. [DOI] [PubMed] [Google Scholar]
- Velentzas AD, Nezis IP, Stravopodis DJ, Papassideri IS, Margaritis LH. Apoptosis and autophagy function cooperatively for the efficacious execution of programmed nurse cell death during Drosophila virilis oogenesis. Autophagy. 2007;3:130–132. doi: 10.4161/auto.3582. [DOI] [PubMed] [Google Scholar]
- Wang H, Clem RJ. The role of IAP antagonist proteins in the core apoptosis pathway of the mosquito disease vector Aedes aegypti. Apoptosis. 2011;16:235–48. doi: 10.1007/s10495-011-0575-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Gort T, Boyle DL, Clem RJ. Effects of manipulating apoptosis on Sindbis virus infection of Aedes aegypti mosquitoes. J. Virol. 2012;86:6546–54. doi: 10.1128/JVI.00125-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12:1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargar S, Wani T. a., Jain SK. Morphological changes in vero cells postinfection with dengue virus type-2. Microsc. Res. Tech. 2011;74:314–319. doi: 10.1002/jemt.20908. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.