Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a risk factor for lung cancer. This study evaluates alternative measures of COPD based on spirometry and quantitative image analysis to better define a phenotype that predicts lung cancer risk.
Methods
Three-hundred-forty-one lung cancer cases and 752 volunteer controls age 21–89 years participated in a structured interview, standardized CT scan and spirometry. Logistic regression, adjusted for age, race, gender, pack-years, and inspiratory and expiratory total lung volume, was used to estimate the odds of lung cancer associated with FEV1/FVC, percent voxels less than -950 Hounsfield Units on the inspiratory scan (HUI) and percent voxels less than -856 HU on expiratory scan (HUE).
Results
The odds of lung cancer were increased 1.4- to 3.1-fold among those with COPD compared to those without, regardless of assessment method, however, in multivariable modeling, only percent voxels < -856 HUE as a continuous measure of air trapping (OR=1.04; 95% CI (1.03, 1.06)) and FEV1/FVC < 0.70 (OR=1.71; 95% CI (1.21, 2.41)) were independent predictors of lung cancer risk. Nearly 10% of lung cancer cases were negative on all objective measures of COPD.
Conclusion
Measures of air trapping using quantitative imaging, in addition to FEV1/FVC, can identify individuals at high risk of lung cancer and should be considered as supplemental measures at the time of screening for lung cancer.
Impact
Quantitative measures of air trapping based on imaging provide additional information for the identification of high risk groups who might benefit the most from lung cancer screening.
Keywords: lung cancer, risk assessment, CT scans, COPD, quantitative imaging
Introduction
Chronic Obstructive Pulmonary Disease (COPD) and lung cancer share a common risk factor, cigarette smoking, and COPD is associated with a 2- to 4-fold increased risk for lung cancer independent of smoking habits (1–9), even among never smokers (10). Lung cancer risk has been shown to vary with COPD phenotypes, i.e., emphysema and chronic bronchitis (3, 7, 11–15). In a meta-analysis, lung cancer was associated with a previous history of COPD (OR=2.2, 95% CI 1.7–3.0), chronic bronchitis (OR=1.5, 95% CI 1.3–1.8), and emphysema (OR=2.0, 95% CI 1.7–2.4) (6). Most epidemiologic studies of COPD, however, rely on self-report of COPD phenotype and are subject to both recall bias and misclassification.
Prospective studies have evaluated the association between computed tomography (CT) evidence of emphysema and/or spirometry-defined measures of airflow obstruction and risk of lung cancer, reducing the potential for disease misclassification. Some studies report a 2- to 4-fold increased risk of lung cancer in the presence of CT evidence of emphysema, with no or lower risks associated with airflow obstruction (5, 16–18). In a limited number of studies using quantitative image analysis of CTs (qCT), lung cancer risk was not associated with a low attenuation measure of emphysema (9, 19, 20). In the National Lung Screening Trial (NLST), qCT measures of emphysema did not improve lung cancer risk prediction above that associated with self-report of COPD (21). While lung cancer risk was not associated with airway dimensions in the NLST, risk of lung cancer has been shown to increase with decreasing forced expiratory volume in 1 second (FEV1) even in smokers with only minimal declines in FEV1 (15).
COPD is a heterogeneous disease and characteristics driving the association with lung cancer are still unclear. FEV1 alone may not fully explain the complexity of the disease (22). QCT measures of COPD can differentiate between airway-predominant and emphysema-predominant disease and may better distinguish COPD subtypes and improve estimation of lung cancer risk associated with COPD. The INHALE study evaluates alternative measures of COPD based on spirometry, quantitative image analysis more extensive than that evaluated in NLST and radiologist interpretation of low dose CT, and associations with lung cancer risk.
Materials and Methods
Study Participants
The INHALE study began enrollment in May, 2012 and is ongoing. Lung cancer patients (cases) included were enrolled at the Karmanos Cancer Center (KCC) and Henry Ford Health System (HFHS), both in Detroit. Institutional Review Boards at both institutions approved this study, and informed consent was obtained from each participant. Eligible cases were 21–89 years of age at diagnosis, enrolled within 12 months of diagnosis, able to complete the CT scan and spirometry, and never had taken Amiodarone or been diagnosed with bronchiectasis or cystic fibrosis because these conditions obscure the CT images.
The analyses presented were restricted to whites and African Americans enrolled as of July, 2014 and included 341 cases who had completed an interview, 292 (86% of interviewed cases) who had completed the CT scan (288 have been quantitatively analyzed), and 317 (93% of interviewed cases) who completed a spirometry or pulmonary function test (PFT).
Controls were identified through distribution of brochures/flyers in physicians’ offices and throughout the community, internet and community newspaper advertisements, recruitment at community centers, health fairs and senior expos, and through current or former smoking patients aligned with HFHS. Controls were 21–89 years of age, able to complete the CT scan and spirometry, carried health insurance (in the event medical follow-up was required based on a clinical finding on the CT or spirometry), and never had taken Amiodarone, had surgical removal of any portion of either lung, or been diagnosed with lung cancer, bronchiectasis or cystic fibrosis.
Of eligible control participants contacted through July, 2014, 752 interviews were completed, 725 (96%) CTs were performed (721 have been quantitatively analyzed), and 746 (99%) completed spirometry. Approximately 11% of potential control participants contacted were ineligible, the majority due to lack of health insurance.
Data Collection
Interviews were conducted by phone or in-person to collect demographics, exposure and smoking history, medical history, medication use, diet, physical activity, and family health history. Smoking data includes duration, number and type of cigarettes smoked, passive smoking exposure, and hookah use. Self-report of physician diagnoses of COPD (including chronic bronchitis, emphysema, and/or COPD) was included in the medical history. Only diagnoses at least one year before the lung cancer diagnosis/interview were included in the analysis. Data were also collected on prescription and over the counter medications taken for these conditions. First-degree family history of cancer (including lung cancer) or COPD, and data for more distant relatives who had been diagnosed with lung cancer, were enumerated.
For COPD phenotyping, participants underwent a low dose, non-contrast chest CT scan and a spirometry or pulmonary function test. Chest CTs were taken at both full inspiration and full expiration under a protocol standardized across scanners as required by the imaging software. CT images were analyzed by board certified radiologists for abnormalities and by VIDA Diagnostics (www.vidadiagnostics.com) for the quantification of COPD phenotype. Emphysema was quantified as the percent voxels less than -950 Hounsfield Units on inspiratory scan (HUI) (qCT emphysema), while air trapping was quantified as the percent voxels less than -856 Hounsfield Units on expiratory scan (HUE) (qCT air trapping). Spirometry was performed by trained technicians using the EasyOne® Plus Spirometer (Medical Technologies, Inc., Andover, MA) in accordance with ATS guidelines. FEV1, FVC, and FEV1/FVC were measured. Board certified pulmonologists at both institutions reviewed the results and classified COPD status.
Measures
Age was recorded as age at diagnosis for cases and age at time of interview for controls. Never smokers were individuals who smoked fewer than 100 cigarettes in their lifetime, while ever smokers included both former and current smokers. Pack-years was calculated by multiplying the number of years smoked by the average number of cigarettes smoked per day divided by 20. Family history of lung cancer was positive if the participant reported at least one first degree relative with a diagnosis of lung cancer. Cancer staging was based on American Joint Committee on Cancer guidelines, 7th edition, and histology was defined according to clinical assessment. FEV1/FVC < 0.70 was considered a diagnosis of COPD based on American Thoracic Society Criteria. COPD severity was classified according to Global Initiative for Chronic Obstructive Lung Disease (GOLD) standards for airflow obstruction(23). Whole lung qCT measurements were evaluated in three ways: dichotomized based on the 90th percentile threshold values among a sample of normal, non-smoking individuals as described in the COPDGene CT Workshop Group study(24) (qCT emphysema was dichotomized at 4.8% while qCT air trapping was dichotomized at 19.5%); above and below the median in controls; and as continuous measures.
Statistical Analysis
Tests of homogeneity between cases and controls were performed using chi-squared tests for categorical variables or t-tests for continuous variables. Cohen’s kappa was used to measure agreement between pairs of measures to determine concordance in cases and control separately. In these analyses, the qCT COPDGene threshold values were used to dichotomize the measures. Logistic regression was used to estimate the odds of lung cancer associated with each of the COPD measures, adjusted for age, race (in the total sample), gender and pack-years, as well as inspiratory and expiratory total lung volume in models with qCT measures. The Breslow-Day test was used to evaluate homogeneity of lung cancer-COPD odds ratios between whites and African Americans. Backward selection was used to obtain the subset of COPD measures that maximized prediction of lung cancer risk. The full model included COPD based on spirometry (FEV1/FVC < 0.70), and percent emphysema and percent air trapping as both continuous and dichotomized threshold values. Covariates were included regardless of statistical significance. The final backward selection model was used to generate predicted probabilities of lung cancer for variously defined COPD phenotype measures, using the mean value for all other covariates in the model. Backward regression was repeated comparing qCT measures in the ‘unaffected’ (non-tumor) lung of cancer cases to the ‘worst’ lung among controls.
Results
Characteristics of Study Participants
Three-hundred-forty-one lung cancer cases and 752 controls were included. Cases and controls did not differ significantly according to gender or race, but cases tended to be slightly older, have a greater pack-year history, and were more likely to have a family history of lung cancer (Table 1). While never smoking cases were enrolled, recruitment of never smoking controls has not yet been prioritized. Among cases, the majority of primary lung tumors were adenocarcinoma (53.6%), while other non-small cell histology categories comprise 31.3% of cases; 8.9% were characterized as small cell carcinoma.
Table 1.
Characteristics of study participants.
| Variable | Lung Cancer Cases (n=341) | Controls (n=752) | p-value |
|---|---|---|---|
| Gender (n, %) | |||
| Male | 158 (46.3) | 342 (45.5) | 0.793 |
| Female | 183 (53.7) | 410 (54.5) | |
| Race (n, %) | |||
| White | 190 (55.7) | 388 (51.6) | 0.206 |
| Black | 151 (44.3) | 364 (48.4) | |
| Age (mean, SD) | 63.7 (9.8) | 61.5 (9.3) | 0.001 |
| Family history of lung cancer (n, %) | |||
| No | 259 (76.2) | 640 (85.1) | <0.001 |
| Yes | 81 (23.8) | 112 (14.9) | |
| Smoking status (n, %) | |||
| Never1 | 24 (7.0) | 7 (0.9) | <0.001 |
| Ever | 317 (93.0) | 745 (99.1) | |
| Pack years (smokers only) (mean, SD) | 43.6 (30.4) | 36.2 (25.5) | <0.001 |
| Histology | |||
| Adenocarcinoma | 180 (53.6) | ||
| Squamous cell | 90 (26.8) | ||
| Other NSCLC2 | 15 (4.5) | -- | |
| Small Cell | 30 (8.9) | ||
| Other | 21 (6.2) | ||
| Stage3 | |||
| I | 84 (24.9) | -- | |
| II | 50 (14.8) | -- | -- |
| III | 90 (26.6) | -- | |
| IV | 114 (33.7) | -- | |
Never smokers are defined as having smoked fewer than 100 cigarettes in their lifetime.
Other non-small cell lung cancer (NSCLC) includes types such as adenosquamous and large cell.
Stage is defined according to American Joint Committee on Cancer guidelines.
There were a significantly higher proportion of cases as compared with controls with self-reported COPD and emphysema, radiologist-assessed emphysema, and emphysema and air trapping as measured on qCT (Table 2). Cases also tended to have more severe COPD as measured by GOLD score compared to controls. Both cases and controls under-reported a COPD diagnosis at interview (32.2% and 22.8%, respectively) as compared with COPD diagnosed on spirometry (50.5% and 32.3%, respectively).
Table 2.
Characteristics of study participants by alternative COPD measures.
| Variable | Lung Cancer Cases | Controls | Test of Homogeneity |
|---|---|---|---|
| Self-reported emphysema (n, %)1 | |||
| No | 256 (82.8) | 677 (91.2) | <0.001 |
| Yes | 53 (17.2) | 66 (8.9) | |
| Self-reported COPD (n, %)1 | |||
| No | 211 (67.8) | 572 (77.2) | 0.002 |
| Yes | 100 (32.2) | 169 (22.8) | |
| Emphysema on CT scan (n,%) | |||
| No | 109 (36.9) | 382 (52.6) | <0.001 |
| Yes | 186 (63.1) | 344 (47.4) | |
| GOLD COPD severity (n, %) | |||
| 0 (none) | 157 (49.5) | 505 (67.7) | <0.001 |
| 1 (mild) | 20 (6.3) | 37 (5.0) | |
| 2 (moderate) | 88 (27.8) | 133 (17.8) | |
| 3 (severe) | 43 (13.6) | 63 (8.4) | |
| 4 (very severe) | 9 (2.8) | 8 (1.1) | |
| Emphysema on qCT (median, IQR)2 | 1.8 (3.8) | 1.1 (2.6) | <0.001 |
| Air trapping on qCT (median, SD)3 | 23.5 (19.7) | 16.6 (17.3) | <0.001 |
| Emphysema on qCT2 (threshold value of 4.8%) | |||
| No | 220 (76.4) | 606 (84.6) | 0.002 |
| Yes | 68 (23.6) | 110 (15.4) | |
| Air trapping on qCT3 (threshold value of 19.5%) | |||
| No | 151 (52.4) | 493 (68.7) | <0.001 |
| Yes | 137 (47.6) | 225 (31.3) | |
Self-report of emphysema and COPD (including reports of COPD, chronic bronchitis and/or emphysema) at least one year prior to diagnosis/interview.
Percent of total lung voxels < -950 HU in inspiration across both lungs.
Percent of total lung voxels < -856 HU in expiration across both lungs.
Lung Cancer Risk Estimates by Individual Alternative Measures of COPD
In the total sample, lung cancer risk was significantly and consistently associated with each of the COPD measures (Table 3). The odds of lung cancer were increased approximately 1.4- to 3.1-fold among those with COPD compared to those without, regardless of assessment method. Associations were also consistent within and between each racial group. Among whites, the ORs indicate a significant 2- to 3.3-fold increase in lung cancer risk associated with most measures of COPD, with the exception of self-reported COPD (OR=1.18; 95% CI 0.78, 1.77) and radiologist-evaluated emphysema (OR=1.42; 95% CI 0.95, 2.12). Among African Americans, odds of lung cancer were increased 2- to 3.6-fold associated with COPD measures. ORs were not significantly different between whites and African Americans for any of the COPD measures.
Table 3.
Association of lung cancer risk with alternative measures of COPD
| Measure | Total Sample | White | African American | pheterogeneity2 |
|---|---|---|---|---|
|
| ||||
| OR1 (95%CI) | OR1 (95%CI) | OR1 (95% CI) | ||
| Self-reported emphysema3 | 1.87 (1.25, 2.79) | 1.84 (1.13, 3.02) | 2.24 (1.11, 4.53) | 0.76 |
| Self-reported COPD3 | 1.43 (1.05, 1.94) | 1.18 (0.78, 1.77) | 2.04 (1.27, 3.28) | 0.12 |
| Spirometry (FEV1/FVC < 0.70) | 1.98 (1.50, 2.61) | 1.96 (1.34, 2.88) | 1.97 (1.31, 2.95) | 0.76 |
| Emphysema on CT based on radiologist read | 1.80 (1.35, 2.41) | 1.42 (0.95, 2.12) | 2.40 (1.55, 3.72) | 0.06 |
| Emphysema by qCT-950insp (>4.8% threshold)4 | 2.66 (1.80, 3.95) | 2.56 (1.51, 4.34) | 2.92 (1.59, 5.33) | 0.54 |
| Emphysema by qCT-950insp (> median) | 3.06 (2.16, 4.34) | 2.62 (1.64–4.20) | 3.64 (2.13–6.22) | 0.19 |
| Air trapping by qCT-856exp (>19.5% threshold)5 | 2.82 (1.95, 4.09) | 3.32 (1.98, 5.60) | 2.38 (1.40, 4.06) | 0.35 |
| Air trapping by qCT-856exp (> median) | 2.71 (1.88, 3.90) | 3.03 (1.84–5.00) | 2.32 (1.35–3.99) | 0.36 |
Odds ratios adjusted for age, race (in total sample), gender, pack years; total lung volume was an additional covariate in the qCT
Test of homogeneity of odds ratios across races.
Self-report of emphysema and COPD (including reports of COPD, chronic bronchitis and/or emphysema) at least one year prior to diagnosis/interview.
Emphysema based on a threshold of 4.8% voxels < -950 HU in inspiratory scans across both lungs as described in COPDGene CT Workshop Group (24).
Air trapping based on a threshold of 19.5% voxels < -856 HU in expiratory scans across both lungs as described in COPDGene CT Workshop Group (24).
Concordance and Joint Effects of COPD Measures on Lung Cancer Risk
The two clinically well-defined and consistently reported measures, COPD defined by spirometry and the COPDGene CT Workshop Group study(24) thresholds for qCT emphysema and air trapping, were used to evaluate concordance between measures. Forty-seven percent of cases and 64% of controls had no evidence of either spirometry-based COPD or qCT emphysema (percent voxels < -950 HUI < 4.8%) (Table 4). Thirty-five percent of cases and 25% of controls were discordant for the two measures, the majority of which had spirometry-defined COPD but no qCT emphysema. Nineteen percent and 11% of the cases and controls, respectively, had positive results on both measures.
Table 4.
Multivariable modeling of the association of lung cancer risk with joint of measures of COPD.
| Measures | Nlung cancer cases | Ncontrols | Contrast | OR1 (95% CI) | p- value |
|---|---|---|---|---|---|
| Model 1 | |||||
| FEV1/FVC < 0.70/Emphysema by qCT-950insp (>4.8% threshold)2 | 131 | 454 | Ref (−/−) | -- | -- |
| 85 | 149 | +/− vs. −/− | 2.05 (1.44, 2.92) | <0.001 | |
| 12 | 29 | −/+ vs. −/− | 2.99 (1.39, 6.40) | 0.005 | |
| 52 | 81 | +/+ vs. −/− | 3.15 (1.96, 5.07) | <0.001 | |
| Model 2 | |||||
| FEV1/FVC < 0.70/Air trapping by qCT-856exp (>19.5% threshold)3 | 99 | 391 | Ref (−/−) | -- | -- |
| 49 | 99 | +/− vs. −/− | 2.08 (1.36, 3.20) | 0.001 | |
| 43 | 93 | −/+ vs. −/− | 2.42 (1.46, 3.99) | 0.001 | |
| 89 | 132 | +/+ vs. −/− | 3.95 (2.48, 6.28) | <0.001 | |
Odds ratios are adjusted for age, race, gender, pack years and total lung volume (inspiratory and expiratory).
Emphysema based on a threshold of 4.8% voxels < -950 HU in inspiratory scans across both lungs as described in COPDGene CT Workshop Group (24).
Air trapping based on a threshold of 19.5% voxels < -856 HU in expiratory scans across both lungs as described in COPDGene CT Workshop Group (24).
Thirty-five percent of the cases and 55% of the controls had no evidence of either COPD on spirometry or qCT air trapping (percent voxels < -856 HUE < 19.5%)(Table 4). Similar percentages of cases (33%) and controls (27%) were discordant for the two measures and proportionality tests were not significant in the two discordant groups. Thirty-two percent and 19% of the cases and controls, respectively, had positive results on both measures. Overall, 28.7% of cases were negative for these three objective COPD measures (FEV1/FVC ≥ 0.70, percent voxels < -856 HUE ≥ 19.5%, percent voxels < -950 HUI ≥ 4.8%). There was minimal agreement between self-report of COPD or emphysema and these objective measures in both cases (Cohen’s kappa: 0.18–0.28) and controls (Cohen’s kappa: 0.13–0.21)(Supplemental Figure 1).
Since concordance between objective COPD measures was low to moderate, lung cancer risk was evaluated for associations with joint measures of COPD (Table 4). Individuals with FEV1/FVC < 0.70 but without qCT evidence of emphysema or air trapping were at two-fold increased risk of lung cancer. Risk was slightly higher with qCT evidence of emphysema or air trapping in the absence of spirometry findings (OR=2.99; 95% CI (1.39, 6.40) and OR=2.42; 95% CI (1.46, 3.99), respectively). Positive findings on both spirometry and either one of the qCT measures (emphysema or air trapping) were associated with statistically significant risk estimates 3.15 and 3.95, respectively.
Independent Effects of COPD Measures on Lung Cancer Risk
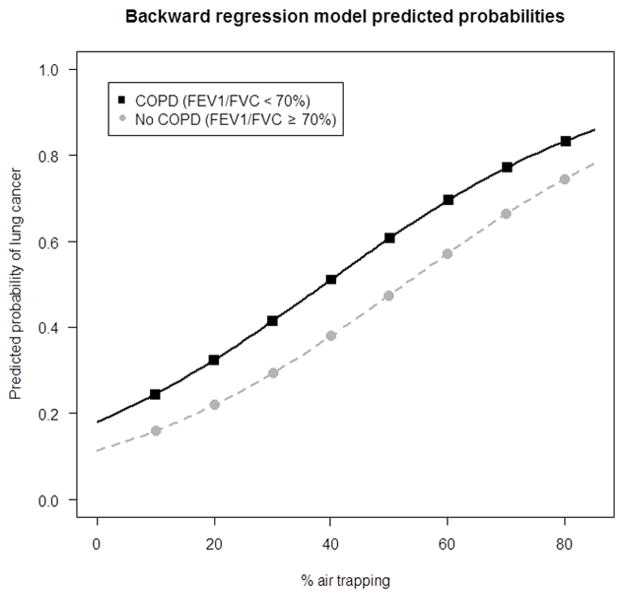
Using backward regression modeling to identify those COPD features most significantly associated with lung cancer, both qCT air trapping (continuous) and FEV1/FVC < 0.70 were independent and statistically significant predictors of lung cancer risk adjusting for covariates (Table 5). Odds of lung cancer increased by 4% for each 1% increase in qCT air trapping (p < 0.001), while the odds of lung cancer were 71% higher among those with FEV1/FVC < 0.70 as compared to individuals with FEV1/FVC ≥ 0.70 (p = 0.002). Predicted probabilities ranged from 0.16 (for 10% qCT air trapping and FEV1/FVC ≥ 0.70) to 0.51 (for 40% qCT air trapping and FEV1/FVC < 0.70) (Figure 1). Similar results were obtained when analyses were based on measures from the unaffected lung in cases compared with the ‘worst’ lung in controls (Table 5).
Table 5.
Multivariable modeling of the association of lung cancer risk with measures of COPD across both lungs and comparing measures in the unaffected lung in cases to the worst lung in controls.
| Measures across both lungs | Unaffected lung in lung cancer cases and worst lung in controls | |||
|---|---|---|---|---|
|
| ||||
| Measure | OR1 (95% CI) | p-value | OR1 (95% CI) | p-value |
| Percent air trapping2 | 1.04 (1.03–1.06) | <0.001 | 1.03 (1.01–1.04) | <0.001 |
| FEV1/FVC < 0.70 | 1.71 (1.21– 2.41) | 0.002 | 1.81 (1.29–2.55) | 0.001 |
The following covariates were included in the model regardless of statistical significance: age, race, gender, pack years, and total lung volume (inspiratory and expiratory).
Air trapping as a continuous measure, quantified as the percent voxels < -856 HU on expiratory scans.
Figure 1.
Predicted Probabilities of lung cancer based on measures of FEV1/FVC and total lung air trapping as measured on qCT as the % voxels < −856 HU on expiratory scans.
Discussion
Lung cancer and COPD are responsible for over 300,000 deaths each year in the United States. These diseases share a strong risk factor in smoking and COPD increases risk of lung cancer even after adjusting for the effects of smoking. The 2- to 4-fold increased risk of lung cancer associated with a history of COPD has been consistently reported(1–9). Untangling the disease processes and identifying characteristics of COPD patients at highest risk of developing lung cancer require a more detailed evaluation of COPD phenotypes. Previous associations between COPD and lung cancer have relied on self-report, emphysema as reported by radiologist, spirometry, and more recently, emphysema and airway measures based on quantitative analysis of CT images. Each of these approaches comes with limitations including reporting bias, observer error, operator error and expense. We evaluated alternative measures of COPD, based on spirometry, qCT measures of emphysema and air trapping, and radiologist interpretation of low dose CT, and their associations with lung cancer risk.
Distinct groups of individuals could be identified based on joint occurrence of COPD subtypes defined by FEV1/FVC, and qCT measures of emphysema and air trapping. Twenty-five to thirty percent of participants were discordant for FEV1/FVC-defined COPD and one of the qCT measures. This type of distinction was also reported in a population of current and ex-smokers involved in a lung screening program, where 17% of subjects had obstruction based on FEV1/FVC and no evidence of emphysema based on radiologist evaluation of the CT, 15% had no FEV1/FVC-defined obstruction but had evidence of emphysema based on radiologist evaluation of the CT, and 27% had findings of both (25).
COPD subtype was differentially associated with lung cancer risk; spirometry and air trapping independently predicted lung cancer risk after adjustment for age, race, sex, pack-years of smoking, and total lung volume. History of COPD was under-reported on interview in this population when compared to other measures, and while providing some information with regard to risk prediction, was not included in the risk model once spirometry and air trapping measures were included.
The quantitative measure of emphysema was not independently associated with lung cancer risk. These findings are consistent with recent studies suggesting that CT measures of emphysema are not associated with lung cancer independently of other COPD measures (21). In a meta-analysis including 7 studies, Smith et al. (9) report a 3-fold (95% CI 2.71, 4.51) increased risk of lung cancer associated with visually detected emphysema, but a non-significant 1.16-fold (95% CI 0.48, 2.81) increased risk of lung cancer with qCT emphysema using a 5% low attenuation volume threshold. Using a threshold of 4.8% low attenuation volume or a continuous measure, we found similar associations between qCT emphysema and lung cancer risk, which were no longer statistically significant once adjusted for FEV1/FVC and qCT air trapping.
The use of qCT allows for an automated assessment of the presence of emphysema, limiting inter-observer differences, but might miss emphysema subtypes. The threshold value used was based on COPDGene data. This threshold may not be appropriate in all populations; in our population it appears to be a conservative measure of emphysema. The use of low attenuation measures as an aggregate across both lungs also might not fully define subtypes of emphysema that are more strongly associated with lung cancer risk. Hohberger et al. (26) report that malignant nodules occur more often in regions containing more severe emphysema based on radiologist scoring. A more detailed investigation into regional variation in qCT scores and alternative qCT measures is warranted.
The COPDGene study provided evidence that air trapping is strongly correlated to FEV1/FVC and provides an independent measure of airflow obstruction (27). We could not identify a published study evaluating air trapping in relation to lung cancer risk. Using the COPDGene threshold, the median, or the continuous measure, qCT air trapping was strongly associated with lung cancer risk in our study, independent of FEV1/FVC. This finding held when comparing both whole lung values and unaffected/worst values between cases and controls. Mets et al. (28) suggest that repeat measures of air trapping based on percent voxels < -856 HUE vary in individuals with no or mild COPD. That was a very small study and reported variation of ±10 to ±15% between scans repeated six weeks apart. It is difficult to determine how this type of measurement error affects estimates of lung cancer risk.
This is the first study to report an association between qCT air trapping and lung cancer independent of FEV1/FVC; qCT emphysema did not independently predict lung cancer risk. We also show no racial variation in lung cancer-COPD phenotype associations. There are some limitations to this work. FEV1/FVC and CT testing did not always occur on the same day. Given the case-control design of this study, cases typically had their CT around the time of diagnosis. We evaluated whether time between diagnosis and CT in the cases resulted in differing distributions of qCT measures and no differences were detected. This suggests that measures of COPD are relatively unchanged within short time windows and that the CT accurately reflects the COPD status of the cases. We also evaluated the potential for the lung in which the cancer occurred to have altered qCT measures by analyzing data from the unaffected lung in the cases compared with the ‘worst’ lung in controls; the results did not change.
The findings presented suggest that quantitative imaging to more specifically phenotype COPD, in addition to FEV1/FVC, can aid in the identification of high risk groups. It is unclear whether a combination of air trapping as identified on CT plus FEV1/FVC < 0.70 represents a distinct clinical subtype of COPD related to lung cancer risk. We also identified a relatively large subset of lung cancer cases (29%) without positive findings on three objective measures of COPD. Understanding the shared and distinct biologic pathways underlying COPD and lung cancer has implications for the identification of high risk groups of lung cancer screening and prevention, as well as providing insight into diagnostic and treatment strategies.
Supplementary Material
Acknowledgments
Financial Support: This research was supported by NIH grants/contracts R01CA141769 (A.G. Schwartz, S. Pandolfi, A.O. Soubani, C. Neslund-Dudas, A.A. Ardisana, M.J. Flynn, T. Song, D.L. Spizarny, P.A. Kvale, R.A. Chapman, S.M. Gadgeel), P30CA022453 (A.G. Schwartz), HHSN261201300011I (A.G. Schwartz), and the Herrick Foundation (A.G. Schwartz).
Footnotes
Conflict of Interest Disclosures: For all of the authors, no conflict of interest disclosures were declared.
References
- 1.Mannino DM, Aguayo SM, Petty TL, Redd SC. Low lung function and incident lung cancer in the United States: data From the First National Health and Nutrition Examination Survey follow-up. Archives of internal medicine. 2003;163:1475–80. doi: 10.1001/archinte.163.12.1475. [DOI] [PubMed] [Google Scholar]
- 2.Purdue MP, Gold L, Jarvholm B, Alavanja MC, Ward MH, Vermeulen R. Impaired lung function and lung cancer incidence in a cohort of Swedish construction workers. Thorax. 2007;62:51–6. doi: 10.1136/thx.2006.064196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schabath MB, Delclos GL, Martynowicz MM, Greisinger AJ, Lu C, Wu X, et al. Opposing effects of emphysema, hay fever, and select genetic variants on lung cancer risk. Am J Epidemiol. 2005;161:412–22. doi: 10.1093/aje/kwi063. [DOI] [PubMed] [Google Scholar]
- 4.Littman AJ, Thornquist MD, White E, Jackson LA, Goodman GE, Vaughan TL. Prior lung disease and risk of lung cancer in a large prospective study. Cancer Causes Control. 2004;15:819–27. doi: 10.1023/B:CACO.0000043432.71626.45. [DOI] [PubMed] [Google Scholar]
- 5.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, et al. Association of Radiographic Emphysema and Airflow Obstruction with Lung Cancer. American journal of respiratory and critical care medicine. 2008;178:738–44. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner DR, McLaughlin JR, Hung RJ. Previous lung diseases and lung cancer risk: a systematic review and meta-analysis. PLoS One. 2011;6:e17479. doi: 10.1371/journal.pone.0017479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz AG, Cote ML, Wenzlaff AS, Van Dyke A, Chen W, Ruckdeschel JC, et al. Chronic obstructive lung diseases and risk of non-small cell lung cancer in women. J Thorac Oncol. 2009;4:291–9. doi: 10.1097/JTO.0b013e3181951cd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takiguchi Y, Sekine I, Iwasawa S, Kurimoto R, Tatsumi K. Chronic obstructive pulmonary disease as a risk factor for lung cancer. World journal of clinical oncology. 2014;5:660–6. doi: 10.5306/wjco.v5.i4.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith BM, Pinto L, Ezer N, Sverzellati N, Muro S, Schwartzman K. Emphysema detected on computed tomography and risk of lung cancer: a systematic review and meta-analysis. Lung Cancer. 2012;77:58–63. doi: 10.1016/j.lungcan.2012.02.019. [DOI] [PubMed] [Google Scholar]
- 10.Turner MC, Chen Y, Krewski D, Calle EE, Thun MJ. Chronic obstructive pulmonary disease is associated with lung cancer mortality in a prospective study of never smokers. American journal of respiratory and critical care medicine. 2007;176:285–90. doi: 10.1164/rccm.200612-1792OC. [DOI] [PubMed] [Google Scholar]
- 11.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The Lung Health Study. Jama. 1994;272:1497–505. [PubMed] [Google Scholar]
- 12.McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62:411–5. doi: 10.1136/thx.2006.072348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brownson RC, Alavanja MC, Caporaso N, Berger E, Chang JC. Family history of cancer and risk of lung cancer in lifetime non-smokers and long-term ex-smokers. International journal of epidemiology. 1997;26:256–63. doi: 10.1093/ije/26.2.256. [DOI] [PubMed] [Google Scholar]
- 14.Mayne ST, Buenconsejo J, Janerich DT. Familial cancer history and lung cancer risk in United States nonsmoking men and women. Cancer Epidemiol Biomarkers Prev. 1999;8:1065–9. [PubMed] [Google Scholar]
- 15.Wasswa-Kintu S, Gan WQ, Man SF, Pare PD, Sin DD. Relationship between reduced forced expiratory volume in one second and the risk of lung cancer: a systematic review and meta-analysis. Thorax. 2005;60:570–5. doi: 10.1136/thx.2004.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Torres JP, Bastarrika G, Wisnivesky JP, Alcaide AB, Campo A, Seijo LM, et al. Assessing the relationship between lung cancer risk and emphysema detected on low-dose CT of the chest. Chest. 2007;132:1932–8. doi: 10.1378/chest.07-1490. [DOI] [PubMed] [Google Scholar]
- 17.Ueda K, Jinbo M, Li TS, Yagi T, Suga K, Hamano K. Computed tomography-diagnosed emphysema, not airway obstruction, is associated with the prognostic outcome of early-stage lung cancer. Clin Cancer Res. 2006;12:6730–6. doi: 10.1158/1078-0432.CCR-06-1196. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Swensen SJ, Karabekmez LG, Marks RS, Stoddard SM, Jiang R, et al. Effect of emphysema on lung cancer risk in smokers: a computed tomography-based assessment. Cancer Prev Res (Phila) 2011;4:43–50. doi: 10.1158/1940-6207.CAPR-10-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maldonado F, Bartholmai BJ, Swensen SJ, Midthun DE, Decker PA, Jett JR. Are Airflow Obstruction and Radiographic Emphysema Risk Factors for Lung Cancer? A Nested Case-control Study Using Quantitative Emphysema Analysis. Chest. 2010;138:1295–302. doi: 10.1378/chest.09-2567. [DOI] [PubMed] [Google Scholar]
- 20.Wilson DO, Leader JK, Fuhrman CR, Reilly JJ, Sciurba FC, Weissfeld JL. Quantitative computed tomography analysis, airflow obstruction, and lung cancer in the pittsburgh lung screening study. J Thorac Oncol. 2011;6:1200–5. doi: 10.1097/JTO.0b013e318219aa93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gierada DS, Guniganti P, Newman BJ, Dransfield MT, Kvale PA, Lynch DA, et al. Quantitative CT assessment of emphysema and airways in relation to lung cancer risk. Radiology. 2011;261:950–9. doi: 10.1148/radiol.11110542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han MK, Agusti A, Calverley PM, Celli BR, Criner G, Curtis JL, et al. Chronic obstructive pulmonary disease phenotypes: the future of COPD. American journal of respiratory and critical care medicine. 2010;182:598–604. doi: 10.1164/rccm.200912-1843CC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respiratory care. 2001;46:798–825. [PubMed] [Google Scholar]
- 24.Group COCW, Barr RG, Berkowitz EA, Bigazzi F, Bode F, Bon J, et al. A combined pulmonary-radiology workshop for visual evaluation of COPD: study design, chest CT findings and concordance with quantitative evaluation. Copd. 2012;9:151–9. doi: 10.3109/15412555.2012.654923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balkan A, Bulut Y, Fuhrman CR, Fisher SN, Wilson DO, Weissfeld JL, et al. COPD phenotypes in a lung cancer screening population. The clinical respiratory journal. 2014;10:48–53. doi: 10.1111/crj.12180. [DOI] [PubMed] [Google Scholar]
- 26.Hohberger LA, Schroeder DR, Bartholmai BJ, Yang P, Wendt CH, Bitterman PB, et al. Correlation of regional emphysema and lung cancer: a lung tissue research consortium-based study. J Thorac Oncol. 2014;9:639–45. doi: 10.1097/JTO.0000000000000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schroeder JD, McKenzie AS, Zach JA, Wilson CG, Curran-Everett D, Stinson DS, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol. 2013;201:W460–70. doi: 10.2214/AJR.12.10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mets OM, Isgum I, Mol CP, Gietema HA, Zanen P, Prokop M, et al. Variation in quantitative CT air trapping in heavy smokers on repeat CT examinations. Eur Radiol. 2012;22:2710–7. doi: 10.1007/s00330-012-2526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.