Abstract
IgE-dependent mast cell activation is a major effector mechanism underlying the pathology associated with allergic disorders. The most dramatic of these IgE-associated disorders is the fatal anaphylaxis which can occur in some people who have developed IgE antibodies to otherwise innocuous antigens, such as those contained in certain foods and medicines. Why would such a highly “maladaptive” immune response develop in evolution, and be retained to the present day? Host defense against parasites has long been considered the only beneficial function that might be conferred by IgE and mast cells. However, recent studies have provided evidence that, in addition to participating in host resistance to certain parasites, mast cells and IgE are critical components of innate (mast cells) and adaptive (mast cells and IgE) immune responses that can enhance host defense against the toxicity of certain arthropod and animal venoms, including enhancing the survival of mice injected with such venoms. Yet, in some people, developing IgE antibodies to insect or snake venoms puts them at risk for having a potentially fatal anaphylactic reaction upon subsequent exposure to such venoms. Delineating the mechanisms underlying beneficial versus detrimental innate and adaptive immune responses associated with mast cell activation and IgE is likely to enhance our ability to identify potential therapeutic targets in such settings, not only for reducing the pathology associated with allergic disorders but perhaps also for enhancing immune protection against pathogens and animal venoms.
Keywords: Allergy, anaphylaxis, parasites, serine proteases, toxins, venoms
Mast cells and their potential roles in host defense
Mast cells (MCs) are of hematopoietic origin [1, 2]. Distinct from basophils and other granulocytes, mature MCs normally do not circulate in the blood but instead are derived from circulating progenitors that enter, and then complete their differentiation/maturation in, virtually all vascularized tissues [1, 2]. MCs are particularly abundant near cutaneous and mucosal body surfaces, where immune surveillance often first takes place. This tissue distribution has positioned MCs, along with epithelial cells, macrophages, and dendritic cells (DCs), as critical sentinels in sensing microbial invasion and other environment challenges. MCs express a wide spectrum of cell surface receptors, including immunoglobulin Fc receptors (FcRs), pattern recognition receptors (e.g., TLRs), and many other innate receptors (e.g., complement receptors, T1/ST2, CD48), which enable them to respond to endogenous mediators and exogenous stimuli to release potent chemical mediators and growth factors/cytokines/chemokines [1, 3, 4]. MCs are also located in close proximity to blood vessels, lymphatics, nerves, and secretory glands. Therefore, MCs and MC-derived products can potentially influence not only innate and adaptive immune responses but also tissue remodeling and homeostasis [1, 4].
The most powerful trigger for MC activation for mediator release is the crosslinking of high affinity IgE receptors, FcεRIs, by IgE and antigen, leading to the rapid release by degranulation of preformed, granule-associated, biologically potent chemical mediators, followed by the de novo synthesis of lipid-derived mediators, cytokines and chemokines [3, 5-7]. IgE-mediated MC mediator release can cause many of the pathological and clinical features associated with allergic disorders, including fatal anaphylaxis [7-9]. Other adaptive stimuli (e.g., IgG immune complexes) and innate stimuli (e.g., pathogens, pathogen products, or endogenous mediators/cytokines) also can activate MCs [1, 4]. The kinetics, types and amounts of different products that can be produced by activated MCs are determined by the nature of the stimulus (or stimuli) encountered, the presence or absence of co-stimulatory or inhibitory factors, and by the location and phenotype of the MCs [1, 3, 4]. In addition to modulating local inflammation and tissue remodeling, MC granules and mediators can travel to remote sites, such as draining lymph nodes, where they can influence immune responses [10, 11]. Many MC populations have a long life span in tissues and degranulated MCs are able to replenish their granule contents, which allows them to be activated repetitively [12, 13]. Furthermore, MC populations can expand at sites of inflammation via proliferation or progenitor recruitment, and MCs numbers often return roughly to baseline levels once the inflammation has resolved [14].
Distinct from other immune cells, which can “come and go” during inflammatory processes, MCs are long-lived tissue-dwelling cells, a property which permits them to participate in the initiation, progression and resolution of immune responses. Because of the potentially catastrophic effects of extensive and systemic MC activation (as seen in fatal anaphylaxis), it is critical for MC activation to be tightly controlled. In allergy, MC activation can contribute to pathology and tissue dysfunction. In some infections, MC activation is regarded as beneficial, as it can enhance immunity and host defense. However, there also is evidence that excessive MC activation can lead to tissue damage. Potential protective vs. pathological roles of MCs in various bacterial [15], viral [16], and fungal [17] infections recently have been reviewed elsewhere. In this article, we focus on the roles of IgE and MCs in host defense against parasites and review evidence that MCs and IgE-associated Th2 immunity can enhance host defenses against animal venoms.
The role of IgE in parasite immunity
Host responses to intestinal nematode infections are typically characterized by Th2 immunity [18-23], with elevated levels of parasite antigen-specific and non-specific IgE, tissue and blood eosinophilia (and sometimes increased numbers of basophils), and intestinal pathology, including crypt hyperplasia, goblet cell hyperplasia, and mucosal MC (MMC) hyperplasia [18, 19, 23]. Data from epidemiological studies suggest a protective role of IgE antibodies in infections with certain parasites in humans, as the levels of parasite-specific IgE and resistance to infection correlate positively [24-26]. It is thought that IgE antibodies mediate their protective function by interacting with cells that express the high affinity IgE receptor (FcεRI) (such as MCs and basophils [in humans and mice], and possibly DCs and eosinophils [in humans]) or the low affinity IgE receptor (CD23) (including eosinophils, DCs, platelets, macrophages [in humans], and B cells [in both humans and mice]) [5, 27]. Although the features of such immune responses can vary depending on the parasite causing the infection, an increase in IgE levels occurs in many of them, with much of the IgE often not specific for defined parasite antigens [28, 29]. However, the actual contributions of antigen-specific or non-specific IgE antibodies in such settings, and their relevance for parasite clearance, or parasite-induced pathology, is still not fully understood. It is likely that binding of parasite-specific IgE and antigen to FcεRI receptors and the ensuing activation of effector cells, such as MCs and basophils, can result in the production and release of biologically active mediators that favor parasite expulsion [19, 30]. Another mechanism that is thought to contribute to parasite expulsion is antibody-dependent cellular cytotoxicity (ADCC) via IgE and/or IgG receptors [31-33]. Notably, parasite infections are initially characterized by production of large amounts of nonspecific IgE while parasite-specific IgE is detectable only at later stages of primary infections or after multiple infections. Pritchard proposed the hypothesis that the nonspecific IgE represents an advantage for the parasite, rather than the host, by competing with parasite-specific IgE for receptor binding sites, which could interfere with the ability of IgE-mediated immunity to expel the parasites [28, 29]. In support of Pritchard's hypothesis, Watanabe recently showed that administration of non-specific IgE impaired protection against T. spiralis infection in mice [34].
IgE−/− mice represent a valuable model system for studying the potential contribution of IgE to parasite resistance (Table 1). Abnormalities in host responses to Brugia malayi, Heligmosomoides polygyrus, N. brasiliensis, T. spiralis and Schistosoma mansoni have been observed in these mice [35-38], indicating beneficial functions for IgE in these infections. Nevertheless, findings in IgE−/− mice are not always in accord with observations from experiments employing other approaches to manipulate levels of IgE or IgE signaling. For example, anti-IgE treatment decreased worm burden and egg production in Schistosoma mansoni-infected mice, supporting the possibility of detrimental, rather than benificial, roles for IgE in Schistosomiasis [39], whereas IgE−/− mice exhibited increased worm burdens and reduced granulomatous inflammation following primary infection with Schistosoma mansoni [35]. Furthermore, deletion of FcεRI did not alter worm burden (but was associated with increased granuloma volume and liver pathology) [40] and other approaches to alter IgE levels revealed little or no impact on Schistosomiasis in mice (reviewed in [41]). The contribution of IgE to resistance to Schistosomiasis is thought to mediated at least in part via CD23, which can facilitate parasite antigen presentation and subsequent production of protective IgG1 antibodies, whereas FcεRIs on MCs and basophils appear not to be critical for protection against Schistosomiasis [41], indeed, as noted above, they may contribute to granuloma volume and liver pathology [41]. Moreover, the genetic background of the host can influence the contributions of IgE in parasite immunity. For example, it has been shown that IgE-deficient SJA/9 mice were resistant to primary and secondary infections with N. brasiliensis or T. spiralis [42], whereas BALB/c IgE−/− mice were more susceptible to T. spiralis than control mice, exhibited prolonged clearance of the adult worms from the small intestine, and harbored higher numbers of viable T. spiralis larvae in the skeletal muscle after primary infection [37].
Table 1.
Potential contribution of IgE to parasite resistance
| Model | Results | References | ||
|---|---|---|---|---|
| Nematodes | ||||
| Trichinella spiralis | Treatment/ Model | Worms | Muscle Larvae | Reference |
| Anti-IgE treatment in Wistar/Lewis rats | N.D | Increased (2-3 fold) (~week 4) | [177] | |
| IgE and T cell transfer in Albino Oxford rats | Decreased (by ~40%) (24 h after infection) | N.D. | [178] | |
| SJA/9 mice (genetically IgE-deficient mice) | N.D | No effect (week 4 or 9 in primary, week 4 in secondary) | [42] | |
| Transfer of IgE without parasite specificity into (BALB/c × SJL/J) F1, (C57BL/6 × DBA/2) F1, BALB/c, and SJL/J mice | N.D | Increased (~1.5 fold) except in IgE-low responder SJL/J (week 5) | [34] | |
| BALB/c IgE-deficient mice | Increased from days 7-28 | Increased (~2 fold, day 28) | [37] | |
| Trichinella muris | Model | Worms | |
|---|---|---|---|
| C57BL/6 FcRγ-deficient mice | No effect throughout the experiments from days 10-35 | [179] | |
| C57BL/6 μMT mice | Increased at day 35 (worms are expelled by day 35 in wild-type mice) | [180] |
| Angiostrongylus costaricensis | Model | Worms | |
|---|---|---|---|
| SJA/9 mice | No effect on day 45 | [181] |
| Nippostrongylus brasiliensis | Model | Worms | Egg production | |
|---|---|---|---|---|
| BALB/c JHD mice | No effect (day 7 in primary or day 7 in secondary) | No effect (day 7 in primary or day 7 in secondary) | [74] | |
| C57BL/6 AID-deficient mice | No effect (days 6 or 10) | N.D. | [75] | |
| SJA/9 mice | No effect (day 7 or 14 in primary, day 7 in secondary) | N.D. | [42] |
| Heligmosomoides polygyrus | Model | Worms | Egg production | |
|---|---|---|---|---|
| BALB/c JHD mice | No effect (day 14 in primary or day 14 in secondary) | No effect (day 14 in primary or day 14 in secondary) | [74] | |
| BALB/c IgE-deficient mice | No effect (days 14-20 in secondary infection) | N.D. | [182] | |
| C57BL/6 JHD mice | Unable to mount a protective immune response (days 14-20 in secondary infection) | N.D. | ||
| C57BL/6 AID-deficient mice | ||||
| C57BL/6 FcRγ-deficient mice | Partially able to mount a protective immune response (at days 14-20 in the secondary infection, there were <10% the number of worms vs. in the primary infection in wild-type mice vs. 30% in FcRγ-deficient mice) | N.D. |
| Strongyloides venezuelensis | Treatment/ Model | Worms | Egg production | |
|---|---|---|---|---|
| C57BL/6 AID-deficient mice | No effect on day 8 but 3-4 fold higher on day 10 | 9 day delay in clearance | [75] | |
| BALB/c FcεRIα-deficient mice | N.D. | No effect on clearance | ||
| Anti-IgE antibody injection into wild-type mice | No effect | N.D. | ||
| Serum injection from S.v. infected mice (IgE, IgG, or IgE + IgG) into C57BL/6 AID-deficient mice | Lower worm burden on day 7 (~50% reduction after IgE, or IgG injections, 80-90% reduction after IgG + IgE injections) | N.D. | ||
| C57BL/6 FcRγ-deficient mice | No effect on day 8 but increased on day 13 | Increased (days 9-13) | [61] |
| Brugia malayi | Model | Worms | |
|---|---|---|---|
| BALB/c IgE-deficient mice | Increased at peak (2-3 fold, weeks 2 to 6 in primary infection); no effect in secondary infection | [36] | |
| C57BL/6 μMT mice | More microfilariae in blood (3-15 fold, between days 56-93) | [183] | |
| C57BL/6 FcRγ-deficient mice | More microfilariae in blood (8-10 fold on day 60) |
| Brugia pahangi | Model | Worms | |
|---|---|---|---|
| C57BL/6 μMT mice | Unable to mount protective immune response on secondary infection (day 14) | [184] | |
| BALB/c JHD mice | Increased (weeks 3 and 12) |
| Malaria | ||||
|---|---|---|---|---|
| Plasmodium berghei (PbANKA) | Treatment/ Model | Parasitemia | Survival | |
| Anti-IgE treatment in C57BL/6 wild-type mice | Increased (~2 fold on days 6 and 7) | Shorter (at least 2 days) | [104] | |
| C57BL/6 FcεRIα-deficient mice | Lower (between days 18-22) | Higher survival rate after day 15 | [185] | |
| C57BL/6 IgE-deficient mice | N.D. | 3-4 weeks longer | ||
| Other parasites | |||||
|---|---|---|---|---|---|
| Schistosoma mansoni | Treatment/ Model | Worms | Egg production | Granuloma size | |
| 129/terSv IgE-deficient mice | Increased adult worms (by 50% at week 8) | N.D. | Smaller (by ~40% at week 8) | [35] | |
| BALB/c FcεRIα-deficient mice | No effect (week 8) | No effect (eggs in tissues, week 8) | Larger (by ~25% at week 8 ) | [40] | |
| Anti-IgE treatment in BALB/c wild-type mice | Decreased (by ~50% at week 8) | Decreased eggs in liver (by ~80% at week 8); Decreased egg production per worm (by ~60% at week 8) | Smaller (week 8) | [39] | |
| C57BL/6 μMT mice | No effect (week 8) | No effect on eggs in liver or intestine (week 8); lower egg excretion (by ~70% at week 8) | Larger (by 42% at week 8, 50% at week16) | [186] | |
| C57BL/6 FcRγ-deficient mice | N.D. | No effect (week 8) | Larger (by ~20% at week 8, ~50% at week 16) | ||
| SJA/9 mice | No effect (week 8) | No effect (week 8) | N.D | [187] | |
| Shistosoma japonicum | Model | Worms | Granuloma size | |
|---|---|---|---|---|
| SJA/9 mice | No effect (week 8) | Smaller (by 40-50%) on week 8 | [188] |
The roles of mast cells in parasite immunity
Intestinal nematodes
MCs have long been considered major sentinels in host defense against helminth infection [43, 44], and intestinal nematode infections in rodents are widely used to interrogate the contribution of MCs and other Th2 cell-associated immune response to parasite resistance [18, 23]. Depending on the type of parasite, there is evidence that expansion of MMCs can contribute importantly to host defense against certain intestinal nematodes (Table 2). For example, administration of exogenous IL-3 [45-47] or IL-18+IL-2 [48] which can increase numbers of MMCs (among other effects), can accelerate intestinal clearance in mice infected with T. spiralis, S. ratti or S. venezuelensis, whereas anti-SCF or anti-c-Kit treatments prevented MMC expansion and also delayed T. spiralis expulsion [49, 50]. IL-3-deficient mice, which cannot produce an intestinal MMC hyperplasia response, exhibit increased susceptibility to S. venezuelensis infection [51]. IL-4 deficient mice have reduced intestinal MMC hyperplasia after infection with T. spiralis, S. ratti, H. polygyrus or N. brasiliensis (reviewed in [43]). These and other studies (reviewed in [43]) highlight the association of Th2 responses and certain specific cytokines (e.g., SCF, IL-3, IL-4, IL-9, IL-10, IL-18) in the induction of intestinal MMC hyperplasia and enhanced immunity to helminthes.
Table 2.
Potential contribution of mast cells to parasite resistance
| Model | Results | References | ||
|---|---|---|---|---|
| Nematodes | ||||
| Trichinella spiralis | Treatment/Model | Worms | Muscle Larvae | |
| Anti-c-Kit antibody treatment in NIH mice | Increased (3 fold on day 10) | N.D. | [49] | |
| Anti-c-Kit or anti-SCF antibody treatments in NIH mice | Increased (5-15 fold on day 10) after injections of either anti-c-Kit or anti-SCF antibodies | N.D. | [50] | |
| WBB6F1-KitW/W-v mice | Delay in expulsion (at least 7 days) | ~2 fold increase | [80] | |
| WBB6F1-KitW/W-v mice and WCB6F1-MgfSl/Sl-d mice | Increased (KitW/W-v mice: 8-9 fold, MgfSl/Sl-d mice: 3-4 fold) on day 12 | N.D. | [81] | |
| WBB6F1-KitW/W-v mice | 9-13 day delay in expulsion | N.D. | [79] | |
| WBB6F1-KitW/W-v mice | 3-4 fold increase on day 13 | N.D. | [82] | |
| BALB/c mMCP-1-deficient mice | 10-15 fold increase on days 15-16 | Increased on day 30 | [53] | |
| C57BL/6 mMCP-6-deficient mice | No effect from days 4-18 | % necrotic larvae decreased (week 5) | [62] | |
| Trichinella muris | Treatment/ Model | Worms | |
|---|---|---|---|
| Anti-c-Kit antibody treatment in BALB/c or C57BL/6 mice | Lower (~20% on day18) | [179] | |
| C57BL/6-KitW-sh/W-sh mice | Increased (~3 fold on day 21) | [66] |
| Nippostrongylus brasiliensis | Treatment/ Model | Worms | Egg production | |
|---|---|---|---|---|
| MF-1 mMCP-1-deficient mice | Increased on day 8, but no effect on day 10 | No effect | [189] | |
| SCF/anti-SCF antibody injections in Wistar rats | N.D. | SCF: increased; Anti-SCF: decreased | [124] | |
| WBB6F1-KitW/W-v mice | No effect (days 7 or 14) | N.D. | [69] | |
| C57BL/6-KitW-sh/W-sh mice | ~4 day delay in expulsion in primary, but no effect in secondary | N.D. | [68] | |
| BALB/c mMCP-1 deficient mice | N.D. | No effect | [53] | |
| BN-DonryuF1-KitW-s/W-s rats | N.D. | Decreased on day 8 (~25-30%) but no effect on day 11 | [125] | |
| BALB/c IL-3-deficient mice | N.D. | No effect | [190] | |
| IL-3-deficient KitW/W-v mice (mixed background of C57BL/6 & WBB6) | N.D. | No effect |
| Heligmosomoides polygyrus | Worms | Egg production | ||
|---|---|---|---|---|
| WBB6F1-KitW/W-v mice | No effect (weeks 3 or 9) | N.D. | [191] | |
| WBB6F1-KitW/W-v mice and C57BL/6-KitW-sh/W-sh mice | Increased (~2 fold at week 3 in primary; ~10-20 fold in secondary) | Increased (2-3 fold on days 14-18) | [66] |
| Strongyloides venezuelensis | Worms | Egg production | ||
|---|---|---|---|---|
| C57BL/6 and BALB/c IL-3-deficient mice | N.D. | 2-3 day delay in expulsion; increased egg numbers at peak | [51] | |
| WBB6F1-KitW/W-v mice | N.D. | 12 day delay in expulsion; increased egg numbers at peak | ||
| IL-3-deficient KitW/W-v mice (mixed background of C57BL/6 & WBB6) | Increased (~500 fold on days 18 and 19) | 35 day delay in expulsion; increased egg numbers at peak | ||
| WBB6F1-KitW/W-v mice | Increased (day 14) | 7-10 day delay in expulsion | [78] | |
| 129 5-lipoxygenase (5-LO)-deficient mice | Increased (2-3 fold on days 9-14) | Increased (2-3 fold on days 7-14) | [64] | |
| IL-2 + IL-18 injections into C57BL/6 wild-type mice | Rapid expulsion after implantation of adult worms | N.D. | [48] | |
| WBB6F1-KitW/W-v mice | N.D. | 6-7 day delay in expulsion; increased egg numbers at peak | ||
| BALB/c PI3K-deficient mice | Increased (~200-250 fold on day 13) | 11 day delay in expulsion; increased egg numbers at peak | [87] |
| Strongyloides ratti | Worms | Egg production | ||
|---|---|---|---|---|
| IL-3 administration into Wistar rats and C57BL/6 wild type mice | Decreased (IL-3 dose-dependently) | N.D. | [46] | |
| WBB6F1-KitW/W-v mice | Increased larvae (2-3 fold on day 2) and ≥3 day delay in expulsion assessed by counts of larvae | N.D. | [76] | |
| BALB/c Cpa3Cre mice | Increased (by 3-4 fold on day 6) | N.D. | [115] |
| Malaria | ||||
|---|---|---|---|---|
| Plasmodium yoelii | Parasitemia | Survival | ||
| WBB6F1-KitW/W-v mice | Increased on day 3 but no effect thereafter (co-infection with Salmonella typhimurium on day 10) | N.D. | [92] | |
| Plasmodium berghei (PbANKA) | ||||
|---|---|---|---|---|
| C57BL/6-KitW-sh/W-sh mice | No effect | No effect | [185] | |
| WBB6F1-KitW/W-v mice | Increased (~3 fold) | 1-2 weeks shorter | [192] | |
| C57BL/6 Histidine decarboxylase (HDC)-deficient mice | Decreased (after either mosquito bites or injections of infected erythrocytes) | ~13 day longer survival (after either mosquito bites or injections of infected erythrocytes) | [98] | |
| WBB6F1-KitW/W-v mice | Increased (days 7-9) | N.D. | [101] |
| Other parasites | ||||
|---|---|---|---|---|
| Schistosoma japonica | Worms | Granuloma size | ||
| WBB6F1-KitW/W-v mice | No effect (week 7) | Smaller (by 20-40%, week 7) | [193] | |
| Leishmania major | Cutaneous Leishmaniasis | Parasite Burden | ||
|---|---|---|---|---|
| WBB6F1-KitW/W-v mice | No effect on histopathological features (up to day 150) | N.D. | [116] | |
| WBB6F1-KitW/W-v mice and WCB6F1-MgfSl/Sl-d mice | Reduced size of cutaneous lesions in each type of mouse (throughout the experiments) | No effect | [117] | |
| WBB6F1-KitW/W-v mice | Larger size of lesions (weeks 2-10) | Larger number (spleen, weeks 1 & 3; ear, week 4) | [118] | |
| C57BL/6 and BALB/c Cpa3Cre mice | No effect (throughout the experiments) | No effect | [119] |
| Ticks | |||
|---|---|---|---|
| Haemaphyasalis longicornis | Resistance | ||
| WBB6F1-KitW/W-v mice | No resistance on secondary or third infestation | [194] | |
| C57BL/6-KitW-sh/W-sh mice | No resistance on secondary infestation | [109] | |
| Dermacentor variabilis | Resistance | ||
|---|---|---|---|
| WBB6F1-KitW/W-v mice | No resistance on primary or secondary infestation, but acquired resistance was observed on third and fourth infestations | [195] |
Homing of MMCs to the epithelial layer of the gut mucosa is also critical for anti-parasite functions of MCs. Notch 2 signaling appears to play an important role in regulating MMC migration and distribution in gut mucosa and thus can influence anti-parasite immunity in mice infected with S. venezuelensis [52]. The expulsion of T. spiralis is slower in Mcpt1−/− mice despite their higher numbers of MMCs in the submucosa (but not the epithelial layer) of the infected intestines, suggesting an important role for this chymase in the tissue migration/localization of MMCs within the intestines [53].
In addition to changes in the numbers and distribution of MCs at sites of infection, degranulation of MCs can be observed in the vicinity of certain parasites, and activated MCs have the potential to contribute to anti-parasite immunity by multiple mechanisms. MC-derived proteases and other mediators can be toxic to parasites [54], stimulate intestinal smooth muscle contraction (which may hasten worm expulsion) [55-57], and/or enhance mucosal permeability [58, 59]. MC-mediated alterations in intestinal barrier function can lead to enhanced influx of fluid and blood-borne antibodies into the gut lumen, thus contributing to an unfavorable environment for the parasites. MC granule-associated glycosaminoglycans have been shown to prevent adult worm attachment and invasion of intestinal mucosa by S. venezuelensis [60, 61]. MCs and MC-derived cytokines/chemokines/mediators can modulate host-pathogen interactions by influencing the recruitment or function of other innate immune cells, and this could have positive or negative effects on the parasites. For example, IgE-dependent release of mMCP6 from MCs can enhance recruitment of eosinophils [62], which are thought to have effects (such as producing IL-10 at early stages of infection) that favor the growth and survival of parasite larvae in skeletal muscle infected with T. spiralis [63]. However, leukotrienes, particularly, LTB4, can reduce S. venezuelensis worm burden, possibly via the recruitment of certain inflammatory cells [64]. Furthermore, MCs have the potential to shape parasite-associated adaptive immune responses via production of soluble mediators or via cell-cell interactions with DCs and other antigen-presenting cells [4, 44, 65]. Finally, IgE-independent MC degranulation has been shown to enhance early phases of Th2 immune responses following infections with Heligmosomoides polygyrus and Trichuris muris [44, 66].
As discussed above, MC activation is likely to contribute to intestinal worm expulsion, at least in some settings. However, because host-defense mechanisms often employ redundant or partially overlapping elements, it has been challenging to identify conclusive evidence that MCs, and particularly IgE-dependent MC activation, confer survival benefit to the host during parasite infection. In the case of infection with N. brasiliensis, basophils appear to play a more critical role than MCs in host resistance [38, 67, 68]. Studies in MC-deficient WBB6F1-KitW/W-v mice indicate that MCs make little or no contribution to the expulsion of N. brasiliensis during primary infections with this nematode [69], whereas a modest defect in expulsion of N. brasiliensis was observed in MC-deficient C57BL/6-KitW-sh/W-sh mice during primary but not secondary infections [68]. Furthermore, mice deficient in the mouse MC protease mMCP1 (Mcpt1−/− mice) remain competent to clear N. brasiliensis [53]. Depending on the basophil-deficient models used to analyze the responses, basophils either have been shown to promote defense against N. brasiliensis, especially during the secondary infection [67, 68], or to have no role in worm clearance or tissue eosinophilia during primary or secondary infections [70].
One interpretation of these apparently discordant observations is that, under certain conditions (e.g., depending on the animals’ microbiomes or other environmentally-influenced factors), mechanisms that can compensate for a lack of basophils may be engaged that preserve immune resistance to these parasites [71]. Notably, Th2 cytokine-mediated goblet cell hyperplasia and intestinal smooth muscle contraction [72, 73], but neither B cells nor IgE [74, 75], appear to be required for rapid expulsion of N. brasiliensis, and Schwartz et al. reported that IgE-dependent secretion of IL-4/IL-13 by basophils can contribute critically to protective immunity against this parasite [38]. Other studies employing genetically c-kit mutant MC-deficient mice or MC-protease-deficient mice suggest that MCs can contribute to resistance to infections with Strongyloides ratti [76, 77], S. venezuelensis [48, 51, 78], T. spiralis [53, 62, 79-82], Heligmosomoides polygyrus [44, 66] and Trichuris muris [66, 83] (Table 2). Increases in gastrointestinal MCs is a striking common feature of infection with these nematodes, except for Trichuris muris [83].
As in other nematode infections, infection with T. spiralis is associated with IgE production, expansion of intestinal MCs [84], and the release of MC-associated mMCP1 into the circulation [53]. IgE produced during T. spiralis infection is mainly bound to intestinal MCs and intense IgE deposition is found by immunohistochemistry around necrotic cysts. IgE−/− mice with primary T. spiralis infection exhibit attenuated MMC hyperplasia, enhanced numbers of adult worms in the intestine, and larger numbers of viable larvae in the muscle [37], supporting a protective function of IgE in this infection. An important contribution of the MC chymase, mMCP-1, in the clearance of T. spiralis was demonstrated using Mcpt1−/− mice, which exhibited significant delay in the intestinal expulsion of the parasite during primary and challenge infections [53]. The clearance of T. spiralis requires the destruction of larval cysts in infected muscle and the expulsion of adult worms from the intestine. While efficient expulsion of T. spiralis adult worms from the intestine is dependent on mMCP1, mMCP6 (a MC-specific tryptase) can significantly enhance both eosinophil recruitment and the necrosis of larvae in skeletal muscle during chronic infection with this parasite [62].
Strongyloides venezuelensis, a gastrointestinal nematode that infects rodents and is used to model human S. stercoralis infection [85], has a life cycle similar to N. brasiliensis [86]. S. venezuelensis infection induces a predominant Th2 response associated with the production of S. venezuelensis-specific IgE and IgG1 antibodies [75]. While MCs and antibody responses are not essential for host defense against N. brasiliensis, there is evidence that intestinal MCs [48, 51, 78, 87] and humoral responses [75] are critical for the rapid expulsion of S. venezuelensis from the intestines. The importance of parasite-specific antibodies has been demonstrated using IgG/IgE-deficient AID−/− mice which exhibit delayed expulsion of S. venezuelensis and harbor larger numbers of adult worms in their intestines; adoptive transfer of immune sera from S. venezuelensis-infected WT mice restores resistance in these mice [75]. This study, and the finding that FcRγ-deficient mice exhibited delayed expulsion of S. venezuelensis, represent evidence that IgG and IgE antibodies can collaboratively support expulsion of S. venezuelensis [75]. Mice that lacked MCs (KitW/Wv mice) or IL-3 were unable to mount an intestinal mastocytosis and also exhibited delays in S. venezuelensis expulsion [51], while IL-18 treatment both increased MMC numbers and accelerated parasite expulsion [48]. However, IL-18 treatment and transfer of S.v immune sera-derived IgE or IgG failed to promote worm expulsion in KitW/Wv mice, suggesting that antibody-dependent MC activation is required for the rapid expulsion of S. venezuelensis. Taken together, the available evidence indicates that FcRγ-dependent MMC activation can importantly enhance the expulsion of S. venezuelensis from the intestines [88]. In the life cycle of S. venezuelensis, third stage larvae (L3) migrate to the lung, where they induce pulmonary eosinophilia and goblet cell hyperplasia, effects that may influence host defense against S. venezuelensis. S. venezuelensis-derived chitin may stimulate alveolar epithelial cells to produce IL-33, which can activate lung ILC2s to produce IL-5 & IL-13, enhancing the survival of eosinophils in the lungs [85]. MCs [89-91], as well as ILC2s, can be activated by IL-33, but it is not clear whether MCs are involved in this innate phase of the host response to S. venezuelensis.
Vector-borne parasitic diseases
Malaria
Malaria, a mosquito-borne pathogen, is the most deadly parasitic disease in humans [92]. Most malaria-associated mortality is caused by severe anemia and/or cerebral malaria following uncontrolled infection with Plasmodium falciparum [93]. While the involvement of IgE in malaria parasite transmission and pathogenesis remains unclear and is controversial [94], several studies suggest both detrimental and beneficial roles for MCs in malaria (Table 2). It is possible that MCs can influence multiple stages of malaria infection. As MCs are abundant in skin, their reactions to mosquito saliva during blood feeding may have a ‘gate keeper’ effect on the initial stage of malaria transmission. IgE-independent and IgE-dependent (after repeated exposures to mosquito bites) degranulation of dermal MCs triggered by mosquito saliva has been shown to promote aspects of the ensuing inflammatory response, including local recruitment of granulocytes and induction of hyperplasia of draining lymph nodes [94, 95]. On the other hand, dermal MCs activated by mosquito saliva can down–regulate antigen-specific immune responses, probably via their secretion of MIP2 and IL-10 [96]. Guermonprez et al. recently provided evidence, in a mouse model of lethal malaria (infection with Plasmodium berghei ANKA), that MCs can promote disease through the activation of tissue-damaging CD8+T cells [97]. Children with malaria have increased plasma levels of Flt3 ligands, and Guermonprez et al. further showed that, in mice, uric acid crystals derived from Plasmodium berghei ANKA-infected RBCs can trigger MCs to release Flt3, which then can expand a unique class of DCs and favor subsequent activation of pathogenic CD8+T cells [97].
MCs and histamine have been implicated in the severity of malaria [94] as well as in increased intestinal permeability associated with malaria infection [92]. Severe malaria is correlated with higher levels of histamine in the blood and tissues and inhibition of histamine signaling confers protection against severe malaria in mice [98]. VEGF also is increased and has been implicated in cerebral malaria. Malaria parasite antigen(s) from lysates of P. falciparum (FCR-3 strain)-infected human erythrocytes has been shown to induce VEGF production from a human MC line (HMC1) [99], suggesting another potential detrimental effect of MCs in cerebral malaria. Patients infected with P. falciparum are prone to co-infection with intestinal bacteria and are at a higher risk of developing bacteremia and invasive bacteria disease, suggesting that malaria can impair intestinal barrier function. Chau et al. showed that P. yoelii-infected mice developed L-arginine deficiency, which was associated with intestinal mastocytosis, elevated levels of histamine, enhanced epithelial permeability, and increased bacterial translocation in the gut [100]. Nevertheless, beneficial contributions of MCs in malaria have been suggested based on experiments in c-kit mutant MC-deficient mice. Furuta et al. showed that engraftment with TNF+/+, but not −/−, MCs reduced parasitemia and mortality in KitW/Wv mice after infection with P. berghei ANKA, suggesting that MC-derived TNF can enhance resistance [101] (although TNF has been implicated in both malaria protection and pathogenesis [102, 103]). Furuta et al. further showed that MCs can produce TNF in response to the binding of FcεRI/IgE or TLR4 to malaria parasite-derived peroxiredoxin [101, 104].
These studies collectively indicate that, in malaria, MCs can be activated by multiple signals, including exogenous stimuli from disease-vectors (e.g., mosquito saliva) and parasite products (e.g., peroxiredoxin), as well as by endogenous mediators (e.g., uric acid crystal) that are generated during infection. Moreover, MC responses to those stimuli via either innate (e.g., TLR4-dependent) or adaptive (e.g., FcεRI-dependent) mechanisms can have effects which may contribute either to parasite resistance or to pathology associated with the infection. It is possible that the outcome of the infection in individual hosts may reflect the net balance between protective vs. pathological consequences of IgE-dependent and IgE-independent MC activation in this setting, which in turn might be influenced by the species of parasite, the genetic background of the hosts, and other factors that modulate MC phenotype and other aspects of the immune response in that subject.
Ixodid ticks
MCs have also been implicated in acquired immunity to the feeding of larval Ixodid ticks, which can transmit to their hosts a wide range of pathogens, including the agents of Rocky Mountain spotted fever (Rickettsia rickettsia), Q fever (Coxiella burnetii), tularemia (Francisella tularensis), granulocytic ehrlichiosis (Ehrlichia ewingii), monocytotropic ehrlichiosis (Ehrlichia chaffeensis), and others [105]. However, as discussed above, there can be substantial redundancy or overlap in the functions of various elements of host defense, and the relative importance of MCs vs. basophils in host resistance to the feeding of such ticks may depend importantly on the species of tick and species of host. In mice, there is evidence that basophils, more than MCs, can enhance resistance to secondary infestations with larval Ixodid Dermacentor variabilis ticks [106]. In guinea pigs, treatment with a rabbit anti-basophil serum (which markedly depleted basophils in the blood and tissues) essentially abrogated the ability of animals subjected to a prior primary infestation with larval Ixodid Amblyomma americanum ticks to exhibit resistance to the feeding of such larval ticks during a secondary infestation [107].
Studies in MC-deficient mice [108] and in mice genetically deficient in basophils [109] show that both MCs and basophils, as well as IgE, can contribute to acquired immunity to the feeding of Haemaphysalis longicornis ticks in mice. MC deficient KitW/Wv mice exhibited a defect in resistance against Haemaphysalis longicornis tick infestation. Matsuta et al. showed that active immune serum, but not heat-inactivated immune serum, from tick-infested mice was able to transfer resistance to MC-engrafted KitW/Wv mice, but not to MC-deficient KitW/Wv mice, suggesting an essential role of IgE and MCs [108]. This study raised the possibility that, in mediating resistance to the feeding of larval Ixodid ticks, MCs provide function in mice similar to those of basophils in guinea pigs [107]. A subsequent study by Wada et al. using genetically engineered basophil-deficient mice [109] confirmed the inability of MC-deficient KitWsh/Wsh mice to exhibit acquired resistance, but also showed that basophils were required to establish acquired resistance against feeding of Haemaphysalis longicornis ticks. Furthermore, basophil recruitment to tick feeding sites during the secondary infestation was demonstrated by immunohistochemistry using anti-mMCP8 (basophil-specific marker) [109]. Adoptive transfer of MCs from FcRγ—deficient mice was able to restore resistance in MC-deficient mice, however basophil transfer from FcRγ deficient mice did not restore resistance in basophil-deficient mice. These findings indicate that antibodies, probably IgE antibodies, play an essential role to acquired resistance for these ticks via interacting with Fc receptors on basophils rather than MCs. However, mice deficient in either MCs or basophils are eventually able to clear the tick larvae, albeit more slowly than in WT mice.
Taken together, work in helminthic infections, vector-borne parasitic diseases, and Ixodid ticks suggests that there is redundancy in the protective mechanisms which can help to guard against the invasion or (for ticks and probably also other exoparasites) the feeding of these pathogens, the detrimental effects of the pathology they induce, or their ultimate clearance by the host. Because basophils and MCs may have overlapping or complementary functions as effectors of adaptive immune responses that interfere with tick feeding or infection with intestinal parasites, it would be interesting to evaluate these parasite infection of tick feeding models in mice that lack both cell types.
Challenges in defining the roles of MCs in intestinal helminth and Leishmania major infections
c-kit mutant MC-deficient mice have been widely used in the past three decades to investigate in vivo functions of MCs [110, 111], but it has been difficult to characterize definitely the MCs’ roles in intestinal parasite infection using these mice. The delay in intestinal worm clearance observed in c-kit mutant MC-deficient mice may not be fully explained by their lack of intestinal MCs because these mice also have abnormal gut motility due to their deficiency in the interstitial cells of Cajal (ICC) network [112], as well as other abnormalities, some of them affecting elements of immune responses in addition to MCs [110, 111, 113]. It is also difficult to use “MC-knock-in” mice (i.e., genetically MC-deficient mice engrafted with WT or genetically-altered in vitro-derived cultured MCs [110, 111]) to confirm the contributions of MCs to mucosal immunity because in vitro-derived cultured MCs generated in IL-3-containing medium do not correctly engraft into the intestinal mucosa after intravenous injection into c-kit mutant mice [52, 77]. Engraftment of the MMC compartment can be achieved and anti-helminth immunity can be repaired by bone marrow transplantation [44, 76, 77, 114] in c-kit mutant mice, but this approach also replaces with donor-derived WT cells other hematopoietic cell lineages in c-kit mutant mice. PI3K−/− mice, which virtually lack gastrointestinal MMCs, exhibited increased susceptibility to S. venezuelensis infection. In order to engraft intestinal MMCs into these mice, Fukao et al. injected intravenously, on days 3, 5, 7 after S. venezuelensis infection, cultured MCs that had or had not been primed with IL-4+IL-10; despite achieving similar anatomical distributions in the intestines of the recipient mice, only the IL-4+IL-10-primed MCs restored anti-parasite resistance in the PI3k−/− mice [87]. Sakata-Yanagimoto et al. found that Notch2 signaling is required both for the normal appearance of MMCs in the small intestinal epithelium and the rapid expulsion of S. venezuelensis in a primary infection [52]. They also found that engraftment of either Notch2-deficient or wild type bone marrow-derived cultured MCs did not restore anti-parasite immunity in KitW-sh/W-sh mice after S. venezuelensis infection, which they speculated might reflect the abnormal anatomical distribution of the MCs in the intestines of the recipient KitW-sh/W-sh mice [52]. Such studies highlight the potential importance of anatomical location, as well as numbers, of intestinal MCs in resistance to infection with some intestinal nematodes.
Given the concerns about c-kit related but MC-independent defects in c-kit mutant mice (reviewed in [110, 111, 113]), it is notable that the contribution of MCs to the immune response to S. ratti infection has recently been confirmed using c-kit independent, MC-deficient mice. Blankenhaus et al. [115] reported that BALB/c-Cpa3Cre/+ mice, in which MCs are depleted due to Cre mediated-cytotoxicity, exhibited an increased parasite burden in the small intestine at day 6 after S. ratti inoculation. This finding is consistent with previous observations in WBB6F1-KitW/W-v mice which exhibited increased larval counts and a delay in worm expulsion during a primary infection with S. ratti. The impaired host response to a primary infection with S. ratti in WBB6F1-KitW/W-v mice can be corrected by bone marrow transplantation, but not by short-term engraftment of adoptively-transferred MCs, probably because of the incomplete restoration of the MMC compartment by the adoptive transfer of in vitro-derived cultured mature MCs (as discussed above) [76, 77]. Blankenhaus et al. also provided evidence that IL-9-mediated MC activation is a key mechanism mediating S. ratti repulsion, a process that is suppressed by Foxp3+ Treg cells in a non-redundant manner in the BALB/c, but not the C57BL/6, strain [115].
Findings in various MC-deficient models do not always yield consistent conclusions about the contributions of MCs to parasite resistance, perhaps because of differences in the other phenotypic abnormalities which occur in c-kit mutant vs. alternative types of MC-deficient mice and/or differences in experimental design or related to animal facilities (e.g., differences in mouse microbiomes) (reviewed in [110, 111, 113]). For example, studies in WBB6F1-KitW/W-v mice suggested that MCs make no contribution [116], had effects that increased skin lesion size [117], or enhanced protection against Leishmania major [118]. Upon infection with L. major, Th1 cells in resistant mice activate macrophages, which is thought to promote destruction of the parasite whereas Th2 cells in susceptible mice inhibit macrophages and thereby favor the systemic dissemination of the pathogens. Studies in c-kit-independent MC-deficient mice that are either resistant (Th1 prone C57BL/6-Cpa3Cre/+) or susceptible (Th2 prone BALB/c-Cpa3Cre/+) to Leishmaniasis detected no role for MCs in the development of lesion size, parasite burden, cytokine production or immune responses in cutaneous leishmaniasis in mice [119].
Another challenge in the analysis of the roles of IgE and MCs (and basophils) in parasite immunity using experimental models in mice is the potential difficulty of extending the results directly to humans. For example, humans exhibit a broader distribution of FcεRI among hematopoietic cells types than is observed in WT mice [5, 27]. FcεRIs are primarily restricted to MCs and basophils in mice under baseline conditions, whereas FcεRIs are also expressed in human eosinophils and platelets, which have been reported to be able to participate in parasite killing by IgE-mediated ADCC, a mechanism of defense against parasites that has not yet been reported in rodents [31-33, 120]. Moreover, there are potentially important differences in the phenotype of MC populations in mice and humans [121].
Potential negative functions of MCs during parasite infections
While MC activation can enhance host defense in some settings, MC mediators also can potentially cause pathology. For example, KitW/Wv mice are slower in T. spiralis parasite expulsion but also exhibit reduced Th2 responses, milder intestinal pathology/inflammation, less mucosal tissue damage, and attenuated body weight loss after infection (however, these effects are not necessarily due to their MC deficiency) [82, 114]. Mcpt1−/− mice have reduced immunity against T. spiralis infection, but also exhibit less intestinal pathology/inflammation. mMCP1 released by MCs upon T. spiralis infection can potentially degrade occludin and other tight junction proteins, compromising intestinal barrier function and thereby promoting intestinal inflammation and pathology [82]. Based on studies in C57BL/6J WT mice, it has been proposed that MCs may contribute to the epithelial hyper-permeability associated with reductions in tight junction proteins (occludin and zonula occludens 1) that is observed after infection with either S. venezuelensis or N. brasiliensis, and that this may enhance both systemic absorption of endotoxin through the gut and IgG flux into intestine lumen [122].
In some settings, MCs can even have effects which promote parasite-induced immunosppression. In a mouse model of chronic Schistosomiasis elicited by repetitive skin infection, depletion of MCs in Mcpt5iDTR mice by DT injection reduced skin IL-10 levels, but increased MHC-II+ cells, suggesting a possible role for MCs in down-regulating parasite immunity [123]. Furthermore, some effects of MCs in parasite infections might favor the parasite. As noted above, anti-SCF treatment diminished intestinal MMC hyperplasia in rats infected with N. brasiliensis or T. spiralis, but such anti-SCF treatment decreased parasite egg production during N. brasiliensis infection [124]. These findings were in accord with results from prior work reporting that, during a primary infection with N. brasiliensis, c-kit mutant MC-deficient Ws/Ws rats exhibited significantly less egg output in the feces at day 8 of infection than did the corresponding wild type rats [125]. This result raised the possibility that some effects of SCF and/or MCs (perhaps MC-dependent enhancement of local vascular permeability at sites of parasite infection), actually favored parasite fecundity in this setting. However, neither the anti-SCF treatment nor the mutations in Ws/Ws rats exclusively affected MCs, so neither of these studies proved that the positive effects on parasite fecundity observed in animals with reduced numbers of MCs necessarily reflected an effect of MCs on the infection.
One hypothesis that is consistent with the divergent findings from studies investigating whether MCs and IgE can influence parasite immunity is that, depending on the setting, IgE and MCs either can have net effects favoring the host or can have net effects that favor the parasite. In light of the long-term co-evolution of parasites and their hosts, with each attempting to probe weaknesses in or to co-opt the defense mechanisms of the other, it should not be surprising that the engagement of MCs and IgE during host/parasite interactions may not always produce results that favor the host. When viewed from the perspective that vertebrates have been co-evolving with parasites for millions of years, and that the parasites’ objectives may better be served by a viable rather than dead host, it is not surprising that, depending on the parasites and the particular setting, effector mechanisms such as IgE and MCs may be exploited by the parasties to their own advantage. For example, by eliciting a Th2 response that could result in IgE-dependent MC activation and release of vasoactive mediators in response to parasite antigens at sites of parasite infection, the parasite could thereby regulate local blood flow and vascular permeability in ways that can help to enhance the parasite's ability to derive nutrition from the host.
The good and bad sides of IgE-associated Th2 immune response
IgE-associated Th2 immune responses occur both in allergic disorders and in helminth infections [126, 127]. However, because most allergens do not pose a direct threat to the non-sensitized host, Th2 immune responses resulting in the production of antigen-specific IgE antibodies to such allergens are widely regarded as “misdirected/maladaptive” immune responses [128, 129]. Although most allergens are intrinsically innocuous, certain allergenic proteins possess enzymatic activity (for instance, the papaya protein papain or the major allergen from house dust mite Der p 1 have protease activity), and exposure to such proteins can impair the integrity of epithelial barriers in the skin, the lung, and possibly also the gastrointestinal tract [130-133]. In this regard, such allergens can be considered to be potentially harmful and therefore an appropriate target for a “protective” immune response [134]. In contrast to such potentially harmful substances, intrinsically toxic molecules, such as components of animal venoms, represent an obvious danger for the host, and IgE-associated allergic reactions against a variety of venoms have been reported [135-140]. Some of them, for instance those against components of the venoms of hymenoptera like the honeybee or the yellow jacket, have a high prevalence [141], whereas fewer cases of allergic reactions to components of snake or jellyfish venoms, have been reported [136, 142], perhaps because of lower rates of exposure to such toxins.
In 1991, Margie Profet proposed her provocative “toxin hypothesis”, by postulating that acute allergic reactions, manifested as immediately occurring signs and symptoms in response to allergen exposure (such as sneezing, coughing, vomiting or diarrhea), evolved as a defense mechanism allowing the sensitized host to respond promptly to, and to expel, neutralize and/or avoid, noxious substances which might be indicative of potentially life-threatening situations [143]. She noted that most allergens are either themselves toxins (e.g., venoms) or could originate from sources that can contain toxic chemical substances (e.g. tree nuts or sea foods) and thereby might function as carriers of such toxins [143]. The immune response would then be initiated not only against the dangerous toxic compound itself (e.g., venom components), but also against associated proteins in the toxin-containing food [143]. In a mechanism termed “sensing by proxy” by Palm et al. in their recent update of the “toxin hypothesis” [144], a strong pre-emptive effector immune reaction would then be initiated upon subsequent sensing of proteins that are associated with or bound to toxins, even in the absence of the actually harmful (toxic) agent. This hypothesis could explain why potentially dangerous allergic immune responses can be generated against seemingly harmless proteins [143, 144]. A similar idea was raised by James Stebbings, Jr. 17 years before Profet's paper was published. Stebbings hypothesized that “a major function of the immediate hypersensitivity reactions has been the protection of terrestrial vertebrates from the bites of, or invasion by, arthropods” [145]. Such reactions not only could help the host to resist the pathological effects of toxins contained in arthropod saliva, but also, by inducing the bitten host to scratch or otherwise attempt to remove the arthropod, could help to reduce the transmission of arthropod-borne infections. However, until recently, Profet's “toxin hypothesis” was largely ignored by the scientific community [144]; Stebbings’ paper was mentioned even less in the literature. Evidence that supports Stebbing's and Profet's hypotheses has recently been uncovered by experimental data showing that key effector elements of allergic diseases also can contribute to defense against arthropod and animal venoms [146-148].
Evidence from animal studies
The idea that key elements of what is now sometimes called the “allergy module of immunity” (specifically, IgE and MCs) could be beneficial in host defense against components of venoms was suggested in 1965 and 1971 by the results of two studies led by R.D. Higginbotham. Higginbotham showed that subcutaneous injection of Russell's viper venom or the honeybee venom induced degranulation of skin MCs in mice [149, 150]. Mast cell granules contain large amounts of heparin, a highly anionic compound that can bind to certain cationic venom components. Higginbotham showed that the toxicity of such venoms was significantly reduced if the venom was mixed with heparin prior to its injection into mice. These findings provided evidence that MCs can be activated by venoms in vivo and that subsequently released heparin could contribute to host defense by neutralizing toxic venom components [149, 150]. However, since animals that genetically lack MCs were not described until many years after Higginbotham's studies [110, 111], the importance of MCs in conferring protection against venom-induced toxicity could not be tested directly.
More than 40 years after Higginbotham's discoveries, our group used genetically MC-deficient mice to provide direct experimental evidence that MCs can contribute importantly to innate resistance against the venoms of certain snakes, including the Israeli mole viper, the Western diamondback rattle snake and the Southern Copperhead snake [151], as well as the venoms of the Gila monster [152], two species of scorpions [152], and the European honeybee [146, 151]. Experiments using pharmacological inhibitors of MC-associated proteases and engraftment of MC-deficient animals with MCs treated with shRNA to knock down their levels of carboxypeptidase A3 (CPA-3) indicated that MC-mediated resistance against the venom of the Israeli mole viper and one of its important toxins, sarafotoxin 6b, was largely dependent on release of the MC-associated CPA-3 [151]. Elegant genetic and biochemical studies by Hans-Reimer Rodewald and colleagues showed that CPA-3 is indeed the critical MC-derived protease that detoxifies sarafotoxin 6b, by removing its C-terminal tryptophan [153]. Studies by our lab [151, 152] and others [153] with various mice genetically deficient in MCs or MC-specific proteases revealed that, depending on the species of venomous animal, either CPA-3 [151] or the chymase mMCP4 [152] can reduce the toxicity of either whole venoms [151, 152] or individual toxic components of such venoms [151-153]. Because these same MC-derived proteases also can degrade certain endogenous peptides that are structurally related to toxins in certain venoms, such as endothelin 1 (which is similar to sarafotoxin 6b) and vasoactive intestinal polypeptide (which is similar to helodermin in Gila monster venom), we have suggested that an additional function of such MC-derived proteases is to limit the pathology observed when high levels of such endogenous peptides are produced during diseases or at sites of inflammatory or immune responses (reviewed in [154]).
It seems plausible that the ability of MCs to enhance host resistance to environmental toxins by responding to them by releasing proteases which can degrade such compounds is an ancient property of MCs. Tunicates, which arose in evolution before the development of classical adaptive immunity [155], contain cells called test cells which are present in large numbers beneath surfaces exposed to the environment and which share morphological, ultrastructural and functional features of vertebrate MCs [156, 157]. Such features include containing abundant cytoplasmic granules that contain heparin, histamine and serine proteases, and being able to degranulate in response to compounds such as compound 48/80 [156, 157]. If the MC lineage (represented in tunicates by test cells) learned long ago to respond to and de-toxify certain dangerous compounds, then is there evidence that this host defense function of MCs can be enhanced by their ability to respond to such toxins via mechanisms dependent on adaptive immunity and specifically those involving IgE? Given that the aggregation of FcεRI-bound IgE by specific antigen is among the strongest of MC activation stimuli [3, 5, 6, 27], it is tempting to speculate that venom-specific IgE antibodies could increase the detoxifying potential of MCs by permitting them to be activated more rapidly and extensively than would be the case upon the host's first exposure to that venom. However, reports about the development of IgE antibodies to components of snake, honeybee, jellyfish and scorpion venoms have mainly called attention to the risk of anaphylaxis in subjects containing IgE against such venom components [135-137, 140, 141].
The possibility that anti-venom IgE antibodies might confer benefit in host responses to venoms has only recently been investigated [146-148]. Complementary studies of Ruslan Medzhitov's group [147] and our lab [146, 148] have provided experimental evidence that IgE antibodies can play a beneficial role in acquired host defense against whole honeybee venom (BV) [146], Russell's viper venom (RVV) [148], or an allergenic component of honeybee venom, bee venom phospholipase A2 (bvPLA2) [147]. This work recently has been reviewed in detail [153]. We will comment here on some of the important results and implications of these studies.
Palm et al. focused mainly on the characterization of the acquired immune response against bvPLA2 [147], which constitutes ~12% of total BV protein and is one of the major BV allergens [158, 159]. They showed that repeated intraperitoneal immunization with bvPLA2 could increase the resistance of wild type animals to challenge with a near lethal dose of bvPLA2, as assessed by changes in body temperature. This resistance was significantly reduced when the immunized mice were deficient in B cells or in FcεRIα (the IgE-binding component of the high affinity IgE receptor FcεRI [27]), providing evidence that this protective immune response was antibody-mediated, most likely by IgE antibodies. However, bvPLA2-immunized MyD88-deficient mice acquired a similar degree of resistance against bvPLA2 challenge as did control mice, suggesting that additional pathways independent of MyD88 and IL-33 signaling (which they implicated in the initial Th2 cell-associated response to bvPLA2, also can contribute to the protective type 2 antibody response against bvPLA2 [147].
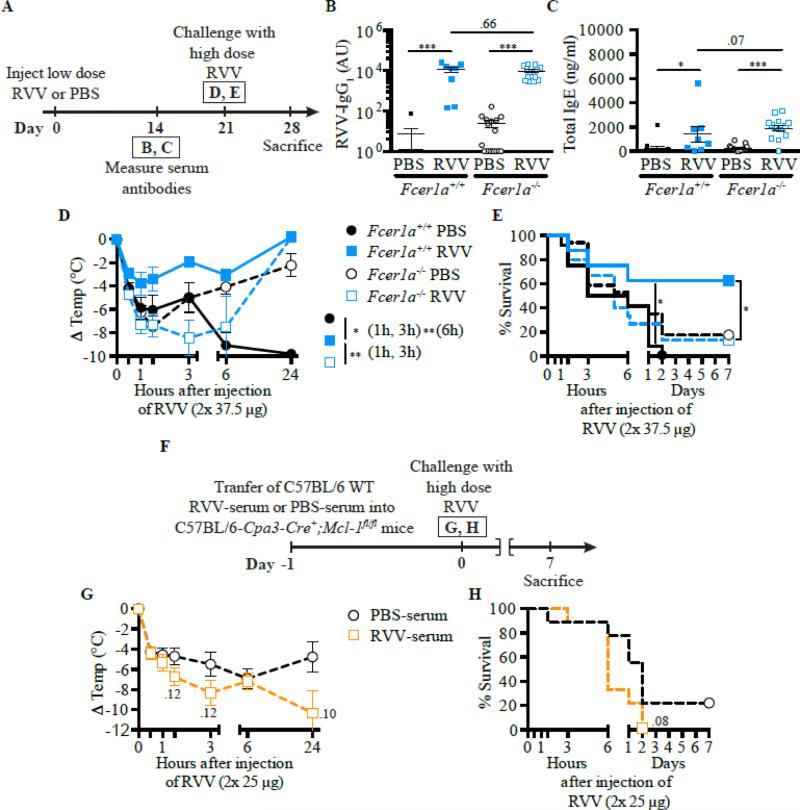
Our studies focused on the immune response against whole venoms [146, 148]. Consistent with the study of Palm et al., we observed that wild type mice immunized subcutaneously with a sub-lethal amount of BV developed BV-specific Th2 cells and BV-specific IgG1 and IgE antibodies. Mice immunized with BV were significantly more resistant to challenge with a potentially lethal dose of that venom, as assessed by body temperature and survival of the mice. Several lines of evidence support the critical contribution of IgE antibodies and FcεRI to the acquired resistance and enhanced survival of BV-immunized mice, including data from serum transfer studies. For example, IgE-depleted immune sera failed to confer enhanced resistance to BV to naïve WT mice, and immunization with BV did not enhance acquired resistance to BV in mice that can't produce IgE (IgE−/− mice) or respond to IgE via the FcεRI, (i.e., FcεRIγ−/− and FcεRIα−/− mice) [146]. By, contrast, IgE−/− mice were capable of passively acquiring enhanced resistance to BV-induced morbidity and mortality when injected with BV immune serum from wild type mice [146]. Passive immunization experiments using C57BL/6 Kitw-sh/w-sh mice (these mice lack MCs but have increased levels of basophils [160]) and C57BL/6 Cpa3-Cre; Mcl-1fl/fl mice (these animals are markedly deficient in MCs, despite having normal c-kit, and have reduced levels of basophils [161]) provided evidence that MCs, but not basophils, likely contribute to protective IgE-mediated immunity against BV [146].
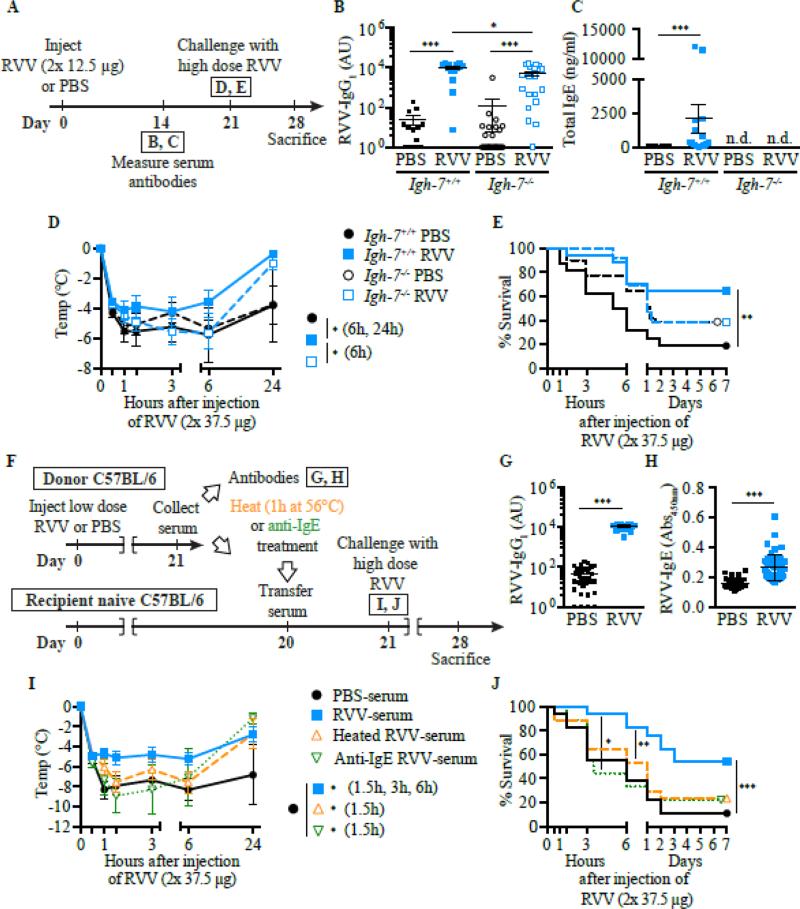
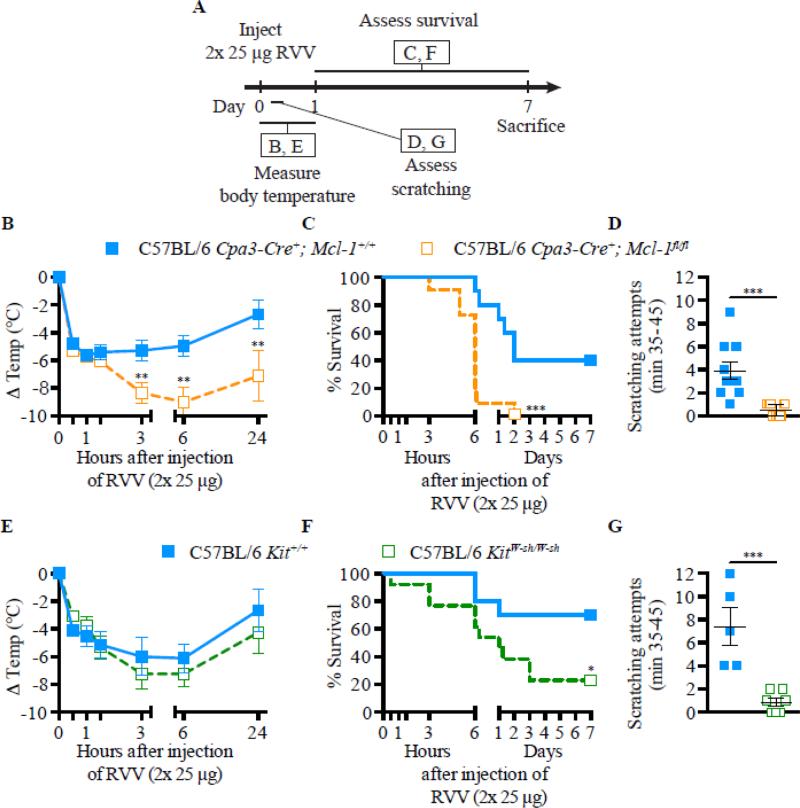
Subsequently, we found that the acquired enhanced resistance to RVV observed in mice that had developed type 2 immune responses to that snake venom also was highly dependent on IgE (Fig. 1) and FcεRIα (Fig. 2 A-F), could be effectively transferred by immune serum into control mice (Fig. 1 F-J), but not into C57BL/6-Cpa3-Cre;Mcl-1fl/fl mice that are markedly deficient in MCs and have a substantial reduction in numbers of basophils (Fig. 2 F-H) [148]. Notably, two different types of genetically MC-deficient mice (i.e., Kitw-sh/w-sh mice and Cpa3-Cre; Mcl-1fl/fl mice) also exhibited significantly diminished innate resistance to the toxicity and lethality of RVV (Fig. 3 A-C, E & F), supporting Higginbotham's hypothesis that MCs can contribute to enhanced innate resistance to this venom [149]. Compared to the corresponding MC-sufficient mice, such naïve MC-deficient mice also exhibited many fewer attempts to scratch sites of RVV injection (Fig. 3 D & G). The latter finding supports the idea proposed by both Stebbings [145] and Profet [143] that elements of allergic responses, in this case, MCs, can confer benefit to hosts experiencing attacks by arthropods [145] or other sources of toxins [143] by altering the host's behavior in ways that would help to eliminate, or at least permit the host to become aware of, the threat. Finally, we found that pre-sensitization with anti-DNP IgE (but not with the same amounts of anti-DNP IgG1 or IgG2b, DNP-specific IgG isotypes with the capacity to activate effector cells via FcγR receptors) significantly increased the resistance of mice to challenge with a potentially lethal amount of RVV admixed with a small amount of DNP-HSA (a 1:75 ratio by weight of DNP-HSA to RVV) [148]. These findings show that local tissue responses mediated by IgE and antigen can enhance host resistance to RVV even when the antigen eliciting MC activation at such sites is not a native constituent of the venom and constitutes a small amount of the injected material. This result is consistent with the general idea that the host needs only to generate an IgE response against a limited number of the components of a complex venom (perhaps as few as one component) in order to manifest enhanced acquired resistance to the morbidity and mortality induce by that venom.
Fig 1.
IgE can contribute to acquired resistance to RVV. A Outline of experiments with IgE-deficient (Igh-7−/−) and control (Igh-7+/+) C57BL/6 mice (B-E). B and C, Serum RVV-specific IgG1 (B) and total IgE (C). D and E, Body temperature (D) and survival (E). F, Outline of serum transfer experiments in C57BL/6 mice (G-J). G and H, Serum RVV-specific IgG1 (G) and total IgE (H). I and J, Body temperature (I) and survival (J). Data were pooled from 3-4 experiments (n= 9-25/group). In B, C, G and H, data are shown as individual values and mean±SEM. P values were determined as follows: Mann-Whitney test (B, C, G, and H), Student t test (D and I) and Mantel-Cox test (E and J). *P<0.05, ** P<0.01, and *** P<0.001. n.d., Not detectable. This is a reproduction of Fig. 3 from Starkl P, Marichal T, Gaudenzio N, Reber LL, Sibilano R, Tsai M, Galli SJ. IgE antibodies, FcεRIα and IgE-mediated local anaphylaxis can limit snake venom toxicity. J Allergy Clin Immunol, 2016; 137: 246-57 (ref. [148]), reprinted with the permission of the publisher, Elsevier.
Fig 2.
FcεRIα and FcεRIα-bearing cells can contribute to acquired resistance to RVV. A, Outline of experiments with Fcer1a−/− and control (Fcer1a+/+) C57BL/6 mice (panels B-E). B and C, Serum RVV-specific IgG1 (B) and total IgE (C) levels. D and E, Body temperature (D) and survival (E). F, Outline of serum transfer experiments involving MC-deficient C57BL/6 mice (G and H). G and H, Body temperature (G) and survival (H). Data were pooled from 3 experiments (n=9-17/group). In B and C, data are shown as individual values and mean±SEM. P values were determined as follows: Mann-Whitney test (B and C); Student t test (D and G); Mantel-Cox test (E and H). *P<0.05, ** P<0.01, and *** P<0.001. n.d., Not detectable. This is a reproduction of Fig. 4 from Starkl P, Marichal T, Gaudenzio N, Reber LL, Sibilano R, Tsai M, Galli SJ. IgE antibodies, FcεRIα and IgE-mediated local anaphylaxis can limit snake venom toxicity. J Allergy Clin Immunol, 2016; 137: 246-57 (ref. [148]), reprinted with the permission of the publisher, Elsevier.
Fig 3.
MCs can contribute to innate resistance and behavioral responses to RVV. Experimental outline (A), body temperature (B and E), survival (C and F), and scratching attempts (D and G) of MC-deficient Cpa3-Cre+; Mcl-1fl/fl (B-D) and KitW-sh/W-sh (E-G) mice and corresponding control mice after RVV injection. In D and G, data are shown as individual values and mean±SEM. P values were determined as follows: Student t test (B, D, E, and G) and Mantel-Cox test (C and F). Data were pooled from 2-4 experiments (n=5-21/group). *P<0.05, ** P<0.01, and *** P<0.001. This is a reproduction of Fig. 2 from Starkl P, Marichal T, Gaudenzio N, Reber LL, Sibilano R, Tsai M, Galli SJ. IgE antibodies, FcεRIα and IgE-mediated local anaphylaxis can limit snake venom toxicity. J Allergy Clin Immunol, 2016; 137: 246-57 (ref. [148]), reprinted with the permission of the publisher, Elsevier.
The extent to which Th2 cell and IgE-associated adaptive immune responses can enhance resistance to other venoms or different types of environmental toxins remains to be determined. Similarly, it is not clear why, in some species or individuals, exposure to the same venom or venom component may induce either a protective Th2 cell-associated and IgE- and FcεRI-dependent adaptive immune response (as shown in mouse studies [146-148]) or a catastrophic and potentially fatal reaction (i.e., anaphylaxis). This question is of high interest for basic and clinical research.
Potential roles of IgE in host defense against venoms in humans
Tissue injury, irrespective of the causative agent, seems to be a major trigger of Th2 immune responses in mammals, including humans [21, 127]. Such Th2 responses, which occur in many different mammalian species (including humans, mice, cats, and dogs [162]), may serve a general function in host defense, including as a protective mechanism against venom toxins and other dangerous noxious substances, as well as in the setting of acquired immunity to helminths [143, 144] and perhaps other pathogens [15-17]. Most animal venoms are complex mixtures of biologically active amines, peptides and enzymes and often have neurotoxic and/or hemotoxic activity [163]. However, many venoms also contain compounds that cause tissue damage. The tissue damage induced by venoms could therefore generate the key danger signals sensed by the immune system that initiates type 2 immunity and directs development of IgE antibodies. For instance, the intrinsic Th2 adjuvant effect of BV seems to be related, at least in part, to the cytolytic BV component melittin [158, 164, 165]. Remarkably, many venoms contain one or more isoforms of PLA2 [163]. The key enzymatic activity of PLA2 is the hydrolysis of membrane phospholipids and the generation of arachidonic acid and lysophospholipids [166]. Such lysophospholipids can integrate into the phospholipid bilayer membrane of mammalian cells and result in cell lysis [166], and Palm et al. showed that subcutaneous injection of OVA together with either bvPLA2 or lysophospholipids can induce the generation of OVA-specific Th2 cells [147].
In humans, it is possible that, in most individuals, the tissue damage induced by the venom contained in a single honey bee sting can be sufficient to induce a Th2 response which may be beneficial, rather than detrimental, and which could increase tissue resistance to subsequent BV challenge. Indeed, although many humans have been stung by bees or wasps at some point in their life and many of such individuals exhibit IgE antibodies to BV or wasp venom, only a very small proportion develop clinically detrimental immune responses upon subsequent exposure to such venoms (anaphylaxis represents the most extreme form of such adverse reactions) [167]. And in two studies, of 525 [168] or 220 [169] subjects, patients with Hymenoptera venom allergy and high levels of total IgE (≥ 100 kU/l [168] or ≥250 kU/l [169]) were significantly less likely to develop grade III reactions to venom (i.e., those associated with full shock [168] or with bronchoconstriction, emesis, anaphylactic shock or loss of consciousness [169]) than were those with lower levels of total IgE. In one of the studies, serum levels of venom-specific IgE also correlated inversely to the clinical reaction grades, but this trend did not achieve statistical significance (P = 0.083) [169].
While there might be a variety of reasons for the occurrence of such associations beyond just levels of total and venom-specific IgE [168, 169], these findings support the conclusion that the connection in humans between the development of IgE responses to venom and serious IgE-induced pathology upon a subsequent exposure to venom is complex. Moreover, Th2 responses are subject to substantial immune regulation, which in turn can diminish the pathology induced by exposure to the inducing antigen. For example, beekeepers, who are frequently exposed to bee venom, can exhibit high levels of BV-specific IgG and IgE, associated, in some of these individuals, with the danger of anaphylaxis [170]. However, in many beekeepers, exposure to multiple bee stings during the beekeeping season induces the development of BV-specific, IL-10-producing, inducible type 1 T regulatory (TR1) cells, which suppress T cell responses to BV in vitro and which, in vivo, may contribute to the observed reduction in cutaneous late phase responses to bee stings which occur as the beekeeping season progresses [171]. Mechanisms of antigen-induced, regulatory T cell-dependent immune tolerance also are thought to contribute to the success of venom specific immunotherapy in patients with hymenoptera venom allergy [172].
Given the findings of our group [146] and of Palm et al. [147], it is tempting to speculate that IgE-dependent enhanced resistance to the toxicity of BV or bvPLA2 may represent an initial phase of a beneficial adaptive immune response to BV which, in most individuals frequently exposed to the venom, then can be supplemented or supplanted by T regulatory cell-dependent immune tolerance to BV, one important function of which is to restrain the development of overly excessive, and therefore potentially dangerous, IgE responses to BV. In this scenario, we propose that anaphylaxis (as observed in a small fraction of people with IgE antibodies to BV) represents only the most extreme and maladaptive end of a spectrum of acquired Th2 immunity to venom, and that in most individuals appropriately regulated Th2 immune responses can actually enhance resistance, rather than susceptibility, to venoms. The reasons why certain unfortunate individuals exhibit severe IgE-dependent responses to venom (including anaphylaxis) may be complex, perhaps reflecting a combination of genetic and environmental factors which inordinately enhance (and/or diminish negative regulation of) IgE-dependent effector responses [149, 173, 174]. In fact, one can speculate that the dangerous potential of allergic Th2 responses in some individuals may represent the price paid to maintain, for the species, the benefits of IgE-associated Th2 immune protection.
Conclusions
While there is no doubt that mast cells and IgE can contribute to pathology and mortality in the setting of allergic diseases, this can't be the main evolutionary driver for the development of either mast cells or IgE. As a result, there has been a long standing interest in identifying beneficial roles for mast cells and IgE as well as a suspicion that, in light of the risk incurred when mast cells are extensively activated, the beneficial roles of mast cells and IgE may be substantial. In this article, we have reviewed evidence that, in some settings, mast cells and IgE can contribute to host defense against certain parasites, particularly helminths. However, in other settings mast cells and/or IgE may have net effects that contribute to the pathology associated with such infections, and/or that favor the survival or fecundity of the parasite. Given the long periods of co-evolution between parasites and their vertebrate hosts, and given that it not in the parasite's interest quickly to kill its host, it is not surprising that the relationship between elements of the host's innate and immune defenses and various parasites can be quite complex.
In addition to their role in responses to parasites, mast cells and IgE also can have prominent roles in innate and adaptive immune responses to venoms. In some unfortunate people who have developed IgE to components of venom, subsequent exposure to tiny amounts of that venom can induce anaphylaxis – a dramatically maladaptive response. However, most people who have IgE antibodies to components of venom have no history of anaphylactic reactions to such venoms. In mice, it is now clear that mast cells can be critically important components of innate responses that enhance resistance to the morbidity and mortality induced by diverse reptile or arthropod venoms, and that this reflects the ability of mast cells to release proteases that can degrade and detoxify key components of the venoms. In the case of honey bee venom and Russell's viper venom, IgE, and probably mast cells, also can significantly increase the resistance of mice to the pathology and mortality associated with exposure to otherwise lethal amounts of such venom.
There are multiple other mechanisms of host defense against venoms besides mast cells and IgE, and the roles of mast cells and IgE in host responses to venoms have been evaluated for the venoms of only a small number of venomous species. However, the findings obtained to date in mice support the hypotheses of Stebbings and Profet that components of “allergic defenses”, in this case mast cells and IgE, can be critical components of defenses against both arthropods and venomous animals. Given the long period of co-evolution of vertebrates with venomous reptiles (estimated by the fossil record to be ~200M years [175]) and the much longer existence during evolution of venomous arthropods [176], we propose that enhancing innate and adaptive immune responses that increase resistance to venoms may be ancient and fundamental beneficial roles of mast cells and IgE. And while anaphylaxis is always maladaptive if it is induced by small amounts of venom that otherwise would produce little pathology, the extensive systemic IgE-dependent activation of mast cells (resulting in the systemic release of venom-destroying proteases) may be beneficial to animals envenomated with potentially lethal amounts of venom. In that context even anaphylaxis can be adaptive, assuming of course that the envenomated animal survives the episode.
Acknowledgement
(Acknowledgments of people, grants, funds, etc. should be placed in a separate section on the title page. The names of funding organizations should be written in full.)
We thank the past and current members of the Galli lab and the many collaborators who have made important contributions to the work reviewed herein. The work reviewed herein was supported by grants to S.J.G. from the National Institutes of Health (e.g., R37 AI23990, R01 CA072074, R01 AR067145, and U19 AI104209) and the National Science Foundation, and from several other funding sources, including the Department of Pathology at Stanford University. P.S. was supported by a Max Kade Fellowship of the Max Kade Foundation and the Austrian Academy of Sciences, a Schroedinger Fellowship of the Austrian Science Fund (FWF): J3399-B21, and a Marie Curie Fellowship of the European Commission (H2020-MSCA-IF-2014), 655153. T.M. was supported by a Marie Curie International Outgoing Fellowship for Career Development: European Union's Seventh Framework Programme (FP7-PEOPLE-2011-IOF), 299954, and a “Charge de recherches” fellowship of the Belgian National Fund for Scientific Research (F.R.S-FNRS).
References
- 1.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–86. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurish MF, Austen KF. Developmental origin and functional specialization of mast cell subsets. Immunity. 2012;37:25–33. doi: 10.1016/j.immuni.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annual review of immunology. 2005;23:749–86. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- 4.Abraham SN, St John AL. Mast cell-orchestrated immunity to pathogens. Nat Rev Immunol. 2010;10:440–52. doi: 10.1038/nri2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–72. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 6.Rivera J, Fierro NA, Olivera A, Suzuki R. New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol. 2008;98:85–120. doi: 10.1016/S0065-2776(08)00403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkelman FD. Anaphylaxis: lessons from mouse models. J Allergy Clin Immunol. 2007;120:506–15. doi: 10.1016/j.jaci.2007.07.033. quiz 16-7. [DOI] [PubMed] [Google Scholar]
- 9.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–35. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLachlan JB, Hart JP, Pizzo SV, Shelburne CP, Staats HF, Gunn MD, Abraham SN. Mast cell-derived tumor necrosis factor induces hypertrophy of draining lymph nodes during infection. Nat Immunol. 2003;4:1199–205. doi: 10.1038/ni1005. [DOI] [PubMed] [Google Scholar]
- 11.Kunder CA, St John AL, Li G, Leong KW, Berwin B, Staats HF, Abraham SN. Mast cell-derived particles deliver peripheral signals to remote lymph nodes. J Exp Med. 2009;206:2455–67. doi: 10.1084/jem.20090805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burwen SJ. Recycling of mast cells following degranulation in vitro: an ultrastructural study. Tissue Cell. 1982;14:125–34. doi: 10.1016/0040-8166(82)90012-x. [DOI] [PubMed] [Google Scholar]
- 13.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–79. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 14.Friend DS, Gurish MF, Austen KF, Hunt J, Stevens RL. Senescent jejunal mast cells and eosinophils in the mouse preferentially translocate to the spleen and draining lymph node, respectively, during the recovery phase of helminth infection. J Immunol. 2000;165:344–52. doi: 10.4049/jimmunol.165.1.344. [DOI] [PubMed] [Google Scholar]
- 15.Johnzon CF, Ronnberg E, Pejler G. The Role of Mast Cells in Bacterial Infection. Am J Pathol. 2016;186:4–14. doi: 10.1016/j.ajpath.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Graham AC, Temple RM, Obar JJ. Mast cells and influenza a virus: association with allergic responses and beyond. Front Immunol. 2015;6:238. doi: 10.3389/fimmu.2015.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saluja R, Metz M, Maurer M. Role and relevance of mast cells in fungal infections. Front Immunol. 2012;3:146. doi: 10.3389/fimmu.2012.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–33. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 19.Anthony RM, Rutitzky LI, Urban JF, Jr., Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–87. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everts B, Smits HH, Hokke CH, Yazdanbakhsh M. Helminths and dendritic cells: sensing and regulating via pattern recognition receptors, Th2 and Treg responses. Eur J Immunol. 2010;40:1525–37. doi: 10.1002/eji.200940109. [DOI] [PubMed] [Google Scholar]
- 21.Allen JE, Wynn TA. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris N, Gause WC. To B or not to B: B cells and the Th2-type immune response to helminths. Trends Immunol. 2011;32:80–8. doi: 10.1016/j.it.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grencis RK, Humphreys NE, Bancroft AJ. Immunity to gastrointestinal nematodes: mechanisms and myths. Immunol Rev. 2014;260:183–205. doi: 10.1111/imr.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hagan P, Blumenthal UJ, Dunn D, Simpson AJ, Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature. 1991;349:243–5. doi: 10.1038/349243a0. [DOI] [PubMed] [Google Scholar]
- 25.Rihet P, Demeure CE, Bourgois A, Prata A, Dessein AJ. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. European journal of immunology. 1991;21:2679–86. doi: 10.1002/eji.1830211106. [DOI] [PubMed] [Google Scholar]
- 26.Faulkner H, Turner J, Kamgno J, Pion SD, Boussinesq M, Bradley JE. Age- and infection intensity-dependent cytokine and antibody production in human trichuriasis: the importance of IgE. The Journal of infectious diseases. 2002;185:665–72. doi: 10.1086/339005. [DOI] [PubMed] [Google Scholar]
- 27.Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nature reviews. Immunology. 2008;8:205–17. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- 28.Pritchard DI. Immunity to helminths: is too much IgE parasite--rather than host-protective? Parasite immunology. 1993;15:5–9. doi: 10.1111/j.1365-3024.1993.tb00566.x. [DOI] [PubMed] [Google Scholar]
- 29.Pritchard DI, Hewitt C, Moqbel R. The relationship between immunological responsiveness controlled by T-helper 2 lymphocytes and infections with parasitic helminths. Parasitology. 1997;115(Suppl):S33–44. doi: 10.1017/s0031182097001996. [DOI] [PubMed] [Google Scholar]
- 30.Fitzsimmons CM, Falcone FH, Dunne DW. Helminth Allergens, Parasite-Specific IgE, and Its Protective Role in Human Immunity. Front Immunol. 2014;5:61. doi: 10.3389/fimmu.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joseph M, Auriault C, Capron A, Vorng H, Viens P. A new function for platelets: IgE-dependent killing of schistosomes. Nature. 1983;303:810–2. doi: 10.1038/303810a0. [DOI] [PubMed] [Google Scholar]
- 32.Capron M, Capron A. Immunoglobulin E and effector cells in schistosomiasis. Science. 1994;264:1876–7. doi: 10.1126/science.8009216. [DOI] [PubMed] [Google Scholar]
- 33.Gounni AS, Lamkhioued B, Ochiai K, Tanaka Y, Delaporte E, Capron A, Kinet JP, Capron M. High-affinity IgE receptor on eosinophils is involved in defence against parasites. Nature. 1994;367:183–6. doi: 10.1038/367183a0. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe N. Impaired protection against Trichinella spiralis in mice with high levels of IgE. Parasitol Int. 2014;63:332–6. doi: 10.1016/j.parint.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 35.King CL, Xianli J, Malhotra I, Liu S, Mahmoud AA, Oettgen HC. Mice with a targeted deletion of the IgE gene have increased worm burdens and reduced granulomatous inflammation following primary infection with Schistosoma mansoni. J Immunol. 1997;158:294–300. [PubMed] [Google Scholar]
- 36.Spencer LA, Porte P, Zetoff C, Rajan TV. Mice genetically deficient in immunoglobulin E are more permissive hosts than wild-type mice to a primary, but not secondary, infection with the filarial nematode Brugia malayi. Infection and immunity. 2003;71:2462–7. doi: 10.1128/IAI.71.5.2462-2467.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gurish MF, Bryce PJ, Tao H, Kisselgof AB, Thornton EM, Miller HR, Friend DS, Oettgen HC. IgE enhances parasite clearance and regulates mast cell responses in mice infected with Trichinella spiralis. J Immunol. 2004;172:1139–45. doi: 10.4049/jimmunol.172.2.1139. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz C, Turqueti-Neves A, Hartmann S, Yu P, Nimmerjahn F, Voehringer D. Basophil-mediated protection against gastrointestinal helminths requires IgE-induced cytokine secretion. Proc Natl Acad Sci U S A. 2014;111:E5169–77. doi: 10.1073/pnas.1412663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amiri P, Haak-Frendscho M, Robbins K, McKerrow JH, Stewart T, Jardieu P. Anti-immunoglobulin E treatment decreases worm burden and egg production in Schistosoma mansoni-infected normal and interferon gamma knockout mice. The Journal of experimental medicine. 1994;180:43–51. doi: 10.1084/jem.180.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jankovic D, Kullberg MC, Dombrowicz D, Barbieri S, Caspar P, Wynn TA, Paul WE, Cheever AW, Kinet JP, Sher A. Fc epsilonRI-deficient mice infected with Schistosoma mansoni mount normal Th2-type responses while displaying enhanced liver pathology. Journal of immunology. 1997;159:1868–75. [PubMed] [Google Scholar]
- 41.Burton OT, Oettgen HC. Beyond immediate hypersensitivity: evolving roles for IgE antibodies in immune homeostasis and allergic diseases. Immunol Rev. 2011;242:128–43. doi: 10.1111/j.1600-065X.2011.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Watanabe N, Katakura K, Kobayashi A, Okumura K, Ovary Z. Protective immunity and eosinophilia in IgE-deficient SJA/9 mice infected with Nippostrongylus brasiliensis and Trichinella spiralis. Proc Natl Acad Sci U S A. 1988;85:4460–2. doi: 10.1073/pnas.85.12.4460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pennock JL, Grencis RK. The mast cell and gut nematodes: damage and defence. Chem Immunol Allergy. 2006;90:128–40. doi: 10.1159/000088885. [DOI] [PubMed] [Google Scholar]
- 44.Hepworth MR, Maurer M, Hartmann S. Regulation of type 2 immunity to helminths by mast cells. Gut Microbes. 2012;3:476–81. doi: 10.4161/gmic.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abe T, Nawa Y. Worm expulsion and mucosal mast cell response induced by repetitive IL-3 administration in Strongyloides ratti-infected nude mice. Immunology. 1988;63:181–5. [PMC free article] [PubMed] [Google Scholar]
- 46.Abe T, Sugaya H, Ishida K, Khan WI, Tasdemir I, Yoshimura K. Intestinal protection against Strongyloides ratti and mastocytosis induced by administration of interleukin-3 in mice. Immunology. 1993;80:116–21. [PMC free article] [PubMed] [Google Scholar]
- 47.Korenaga M, Abe T, Hashiguchi Y. Injection of recombinant interleukin 3 hastens worm expulsion in mice infected with Trichinella spiralis. Parasitol Res. 1996;82:108–13. doi: 10.1007/s004360050079. [DOI] [PubMed] [Google Scholar]
- 48.Sasaki Y, Yoshimoto T, Maruyama H, Tegoshi T, Ohta N, Arizono N, Nakanishi K. IL-18 with IL-2 protects against Strongyloides venezuelensis infection by activating mucosal mast cell-dependent type 2 innate immunity. J Exp Med. 2005;202:607–16. doi: 10.1084/jem.20042202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grencis RK, Else KJ, Huntley JF, Nishikawa SI. The in vivo role of stem cell factor (c-kit ligand) on mastocytosis and host protective immunity to the intestinal nematode Trichinella spiralis in mice. Parasite Immunol. 1993;15:55–9. doi: 10.1111/j.1365-3024.1993.tb00572.x. [DOI] [PubMed] [Google Scholar]
- 50.Donaldson LE, Schmitt E, Huntley JF, Newlands GF, Grencis RK. A critical role for stem cell factor and c-kit in host protective immunity to an intestinal helminth. Int Immunol. 1996;8:559–67. doi: 10.1093/intimm/8.4.559. [DOI] [PubMed] [Google Scholar]
- 51.Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, Nawa Y, Dranoff G, Galli SJ. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–3. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 52.Sakata-Yanagimoto M, Sakai T, Miyake Y, Saito TI, Maruyama H, Morishita Y, Nakagami-Yamaguchi E, Kumano K, Yagita H, Fukayama M, Ogawa S, Kurokawa M, Yasutomo K, Chiba S. Notch2 signaling is required for proper mast cell distribution and mucosal immunity in the intestine. Blood. 2011;117:128–34. doi: 10.1182/blood-2010-07-289611. [DOI] [PubMed] [Google Scholar]
- 53.Knight PA, Wright SH, Lawrence CE, Paterson YY, Miller HR. Delayed expulsion of the nematode Trichinella spiralis in mice lacking the mucosal mast cell-specific granule chymase, mouse mast cell protease-1. J Exp Med. 2000;192:1849–56. doi: 10.1084/jem.192.12.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKean PG, Pritchard DI. The action of a mast cell protease on the cuticular collagens of Necator americanus. Parasite Immunol. 1989;11:293–7. doi: 10.1111/j.1365-3024.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 55.Vermillion DL, Ernst PB, Scicchitano R, Collins SM. Antigen-induced contraction of jejunal smooth muscle in the sensitized rat. Am J Physiol. 1988;255:G701–8. doi: 10.1152/ajpgi.1988.255.6.G701. [DOI] [PubMed] [Google Scholar]
- 56.Marzio L, Blennerhassett P, Vermillion D, Chiverton S, Collins S. Distribution of mast cells in intestinal muscle of nematode-sensitized rats. Am J Physiol. 1992;262:G477–82. doi: 10.1152/ajpgi.1992.262.3.G477. [DOI] [PubMed] [Google Scholar]
- 57.Bertaccini G, Morini G, Coruzzi G. Different mechanisms are responsible for the contractile effects of histaminergic compounds on isolated intestinal smooth muscle cells. J Physiol Paris. 1997;91:199–202. doi: 10.1016/s0928-4257(97)89484-3. [DOI] [PubMed] [Google Scholar]
- 58.Madden KB, Whitman L, Sullivan C, Gause WC, Urban JF, Jr., Katona IM, Finkelman FD, Shea-Donohue T. Role of STAT6 and mast cells in IL-4- and IL-13-induced alterations in murine intestinal epithelial cell function. J Immunol. 2002;169:4417–22. doi: 10.4049/jimmunol.169.8.4417. [DOI] [PubMed] [Google Scholar]
- 59.McDermott JR, Bartram RE, Knight PA, Miller HR, Garrod DR, Grencis RK. Mast cells disrupt epithelial barrier function during enteric nematode infection. Proc Natl Acad Sci U S A. 2003;100:7761–6. doi: 10.1073/pnas.1231488100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maruyama H, Yabu Y, Yoshida A, Nawa Y, Ohta N. A role of mast cell glycosaminoglycans for the immunological expulsion of intestinal nematode, Strongyloides venezuelensis. J Immunol. 2000;164:3749–54. doi: 10.4049/jimmunol.164.7.3749. [DOI] [PubMed] [Google Scholar]
- 61.Onah DN, Nawa Y. Mucosal mast cell-derived chondroitin sulphate levels in and worm expulsion from FcRgamma-knockout mice following oral challenge with Strongyloides venezuelensis. J Vet Sci. 2004;5:221–6. [PubMed] [Google Scholar]
- 62.Shin K, Watts GF, Oettgen HC, Friend DS, Pemberton AD, Gurish MF, Lee DM. Mouse mast cell tryptase mMCP-6 is a critical link between adaptive and innate immunity in the chronic phase of Trichinella spiralis infection. J Immunol. 2008;180:4885–91. doi: 10.4049/jimmunol.180.7.4885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang L, Gebreselassie NG, Gagliardo LF, Ruyechan MC, Lee NA, Lee JJ, Appleton JA. Eosinophil-derived IL-10 supports chronic nematode infection. J Immunol. 2014;193:4178–87. doi: 10.4049/jimmunol.1400852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Machado ER, Ueta MT, Lourenco EV, Anibal FF, Sorgi CA, Soares EG, Roque-Barreira MC, Medeiros AI, Faccioli LH. Leukotrienes play a role in the control of parasite burden in murine strongyloidiasis. J Immunol. 2005;175:3892–9. doi: 10.4049/jimmunol.175.6.3892. [DOI] [PubMed] [Google Scholar]
- 65.Vukman KV, Lalor R, Aldridge A, O'Neill SM. Mast cells: new therapeutic target in helminth immune modulation. Parasite Immunol. 2016;38:45–52. doi: 10.1111/pim.12295. [DOI] [PubMed] [Google Scholar]
- 66.Hepworth MR, Danilowicz-Luebert E, Rausch S, Metz M, Klotz C, Maurer M, Hartmann S. Mast cells orchestrate type 2 immunity to helminths through regulation of tissue-derived cytokines. Proc Natl Acad Sci U S A. 2012;109:6644–9. doi: 10.1073/pnas.1112268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–74. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 68.Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol. 2010;184:344–50. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- 69.Uber CL, Roth RL, Levy DA. Expulsion of Nippostrongylus brasiliensis by mice deficient in mast cells. Nature. 1980;287:226–8. doi: 10.1038/287226a0. [DOI] [PubMed] [Google Scholar]
- 70.Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, Allen CD, Locksley RM. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–35. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Voehringer D. Protective and pathological roles of mast cells and basophils. Nature reviews. Immunology. 2013;13:362–75. doi: 10.1038/nri3427. [DOI] [PubMed] [Google Scholar]
- 72.Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr. Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunol Rev. 2004;201:139–55. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- 73.Hasnain SZ, Evans CM, Roy M, Gallagher AL, Kindrachuk KN, Barron L, Dickey BF, Wilson MS, Wynn TA, Grencis RK, Thornton DJ. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med. 2011;208:893–900. doi: 10.1084/jem.20102057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu Q, Kreider T, Bowdridge S, Liu Z, Song Y, Gaydo AG, Urban JF, Jr., Gause WC. B cells have distinct roles in host protection against different nematode parasites. J Immunol. 2010;184:5213–23. doi: 10.4049/jimmunol.0902879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matsumoto M, Sasaki Y, Yasuda K, Takai T, Muramatsu M, Yoshimoto T, Nakanishi K. IgG and IgE collaboratively accelerate expulsion of Strongyloides venezuelensis in a primary infection. Infection and immunity. 2013;81:2518–27. doi: 10.1128/IAI.00285-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nawa Y, Kiyota M, Korenaga M, Kotani M. Defective protective capacity of W/Wv mice against Strongyloides ratti infection and its reconstitution with bone marrow cells. Parasite Immunol. 1985;7:429–38. doi: 10.1111/j.1365-3024.1985.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 77.Abe T, Nawa Y. Localization of mucosal mast cells in W/Wv mice after reconstitution with bone marrow cells or cultured mast cells, and its relation to the protective capacity to Strongyloides ratti infection. Parasite Immunol. 1987;9:477–85. doi: 10.1111/j.1365-3024.1987.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 78.Khan AI, Horii Y, Tiuria R, Sato Y, Nawa Y. Mucosal mast cells and the expulsive mechanisms of mice against Strongyloides venezuelensis. Int J Parasitol. 1993;23:551–5. doi: 10.1016/0020-7519(93)90159-v. [DOI] [PubMed] [Google Scholar]
- 79.Ha TY, Reed ND, Crowle PK. Delayed expulsion of adult Trichinella spiralis by mast cell-deficient W/Wv mice. Infect Immun. 1983;41:445–7. doi: 10.1128/iai.41.1.445-447.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alizadeh H, Murrell KD. The intestinal mast cell response to Trichinella spiralis infection in mast cell-deficient w/wv mice. J Parasitol. 1984;70:767–73. [PubMed] [Google Scholar]
- 81.Oku Y, Itayama H, Kamiya M. Expulsion of Trichinella spiralis from the intestine of W/Wv mice reconstituted with haematopoietic and lymphopoietic cells and origin of mucosal mast cells. Immunology. 1984;53:337–44. [PMC free article] [PubMed] [Google Scholar]
- 82.Lawrence CE, Paterson YY, Wright SH, Knight PA, Miller HR. Mouse mast cell protease-1 is required for the enteropathy induced by gastrointestinal helminth infection in the mouse. Gastroenterology. 2004;127:155–65. doi: 10.1053/j.gastro.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 83.Koyama K, Ito Y. Mucosal mast cell responses are not required for protection against infection with the murine nematode parasite Trichuris muris. Parasite Immunol. 2000;22:13–20. doi: 10.1046/j.1365-3024.2000.00270.x. [DOI] [PubMed] [Google Scholar]
- 84.Watanabe N, Bruschi F, Korenaga M. IgE: a question of protective immunity in Trichinella spiralis infection. Trends Parasitol. 2005;21:175–8. doi: 10.1016/j.pt.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 85.Yasuda K, Matsumoto M, Nakanishi K. Importance of Both Innate Immunity and Acquired Immunity for Rapid Expulsion of S. venezuelensis. Front Immunol. 2014;5:118. doi: 10.3389/fimmu.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Silveira MR, Nunes KP, Cara DC, Souza DG, Correa A, Jr., Teixeira MM, Negrao-Correa D. Infection with Strongyloides venezuelensis induces transient airway eosinophilic inflammation, an increase in immunoglobulin E, and hyperresponsiveness in rats. Infect Immun. 2002;70:6263–72. doi: 10.1128/IAI.70.11.6263-6272.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fukao T, Yamada T, Tanabe M, Terauchi Y, Ota T, Takayama T, Asano T, Takeuchi T, Kadowaki T, Hata Ji J, Koyasu S. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat Immunol. 2002;3:295–304. doi: 10.1038/ni768. [DOI] [PubMed] [Google Scholar]
- 88.Onah DN, Uchiyama F, Nagakui Y, Ono M, Takai T, Nawa Y. Mucosal defense against gastrointestinal nematodes: responses of mucosal mast cells and mouse mast cell protease 1 during primary strongyloides venezuelensis infection in FcRgamma-knockout mice. Infect Immun. 2000;68:4968–71. doi: 10.1128/iai.68.9.4968-4971.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ho LH, Ohno T, Oboki K, Kajiwara N, Suto H, Iikura M, Okayama Y, Akira S, Saito H, Galli SJ, Nakae S. IL-33 induces IL-13 production by mouse mast cells independently of IgE FcepsilonRI signals. J Leukoc Biol. 2007;82:1481–90. doi: 10.1189/jlb.0407200. [DOI] [PubMed] [Google Scholar]
- 90.Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, Saito H, Galli SJ, Nakae S. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Invest. 2007;87:971–8. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 91.Saluja R, Khan M, Church MK, Maurer M. The role of IL-33 and mast cells in allergy and inflammation. Clin Transl Allergy. 2015;5:33. doi: 10.1186/s13601-015-0076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Potts RA, Tiffany CM, Pakpour N, Lokken KL, Tiffany CR, Cheung K, Tsolis RM, Luckhart S. Mast cells and histamine alter intestinal permeability during malaria parasite infection. Immunobiology. 2016;221:468–74. doi: 10.1016/j.imbio.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Engwerda CR, Kumar R. Mast cells fuel the fire of malaria immunopathology. Nat Med. 2013;19:672–4. doi: 10.1038/nm.3227. [DOI] [PubMed] [Google Scholar]
- 94.Mecheri S. Contribution of allergic inflammatory response to the pathogenesis of malaria disease. Biochim Biophys Acta. 2012;1822:49–56. doi: 10.1016/j.bbadis.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 95.Demeure CE, Brahimi K, Hacini F, Marchand F, Peronet R, Huerre M, St-Mezard P, Nicolas JF, Brey P, Delespesse G, Mecheri S. Anopheles mosquito bites activate cutaneous mast cells leading to a local inflammatory response and lymph node hyperplasia. Journal of immunology. 2005;174:3932–40. doi: 10.4049/jimmunol.174.7.3932. [DOI] [PubMed] [Google Scholar]
- 96.Depinay N, Hacini F, Beghdadi W, Peronet R, Mecheri S. Mast cell-dependent down-regulation of antigen-specific immune responses by mosquito bites. Journal of immunology. 2006;176:4141–6. doi: 10.4049/jimmunol.176.7.4141. [DOI] [PubMed] [Google Scholar]
- 97.Guermonprez P, Helft J, Claser C, Deroubaix S, Karanje H, Gazumyan A, Darasse-Jeze G, Telerman SB, Breton G, Schreiber HA, Frias-Staheli N, Billerbeck E, Dorner M, Rice CM, Ploss A, Klein F, Swiecki M, Colonna M, Kamphorst AO, Meredith M, Niec R, Takacs C, Mikhail F, Hari A, Bosque D, Eisenreich T, Merad M, Shi Y, Ginhoux F, Renia L, Urban BC, Nussenzweig MC. Inflammatory Flt3l is essential to mobilize dendritic cells and for T cell responses during Plasmodium infection. Nat Med. 2013;19:730–8. doi: 10.1038/nm.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Beghdadi W, Porcherie A, Schneider BS, Dubayle D, Peronet R, Huerre M, Watanabe T, Ohtsu H, Louis J, Mecheri S. Inhibition of histamine-mediated signaling confers significant protection against severe malaria in mouse models of disease. J Exp Med. 2008;205:395–408. doi: 10.1084/jem.20071548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Furuta T, Kimura M, Watanabe N. Elevated levels of vascular endothelial growth factor (VEGF) and soluble vascular endothelial growth factor receptor (VEGFR)-2 in human malaria. Am J Trop Med Hyg. 2010;82:136–9. doi: 10.4269/ajtmh.2010.09-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chau JY, Tiffany CM, Nimishakavi S, Lawrence JA, Pakpour N, Mooney JP, Lokken KL, Caughey GH, Tsolis RM, Luckhart S. Malaria-associated L-arginine deficiency induces mast cell-associated disruption to intestinal barrier defenses against nontyphoidal Salmonella bacteremia. Infect Immun. 2013;81:3515–26. doi: 10.1128/IAI.00380-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Furuta T, Kikuchi T, Iwakura Y, Watanabe N. Protective roles of mast cells and mast cell-derived TNF in murine malaria. J Immunol. 2006;177:3294–302. doi: 10.4049/jimmunol.177.5.3294. [DOI] [PubMed] [Google Scholar]
- 102.Korner H, McMorran B, Schluter D, Fromm P. The role of TNF in parasitic diseases: still more questions than answers. Int J Parasitol. 2010;40:879–88. doi: 10.1016/j.ijpara.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 103.Gichohi-Wainaina WN, Melse-Boonstra A, Feskens EJ, Demir AY, Veenemans J, Verhoef H. Tumour necrosis factor allele variants and their association with the occurrence and severity of malaria in African children: a longitudinal study. Malar J. 2015;14:249. doi: 10.1186/s12936-015-0767-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Furuta T, Imajo-Ohmi S, Fukuda H, Kano S, Miyake K, Watanabe N. Mast cell-mediated immune responses through IgE antibody and Toll-like receptor 4 by malarial peroxiredoxin. Eur J Immunol. 2008;38:1341–50. doi: 10.1002/eji.200738059. [DOI] [PubMed] [Google Scholar]
- 105.Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol. 2003;48:307–37. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- 106.Steeves EB, Allen JR. Basophils in skin reactions of mast cell-deficient mice infested with Dermacentor variabilis. Int J Parasitol. 1990;20:655–67. doi: 10.1016/0020-7519(90)90124-6. [DOI] [PubMed] [Google Scholar]
- 107.Brown SJ, Galli SJ, Gleich GJ, Askenase PW. Ablation of immunity to Amblyomma americanum by anti-basophil serum: cooperation between basophils and eosinophils in expression of immunity to ectoparasites (ticks) in guinea pigs. Journal of immunology. 1982;129:790–6. [PubMed] [Google Scholar]
- 108.Matsuda H, Watanabe N, Kiso Y, Hirota S, Ushio H, Kannan Y, Azuma M, Koyama H, Kitamura Y. Necessity of IgE antibodies and mast cells for manifestation of resistance against larval Haemaphysalis longicornis ticks in mice. J Immunol. 1990;144:259–62. [PubMed] [Google Scholar]
- 109.Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, Obata K, Ishikawa R, Yoshikawa S, Mukai K, Kawano Y, Minegishi Y, Yokozeki H, Watanabe N, Karasuyama H. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest. 2010;120:2867–75. doi: 10.1172/JCI42680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Reber LL, Marichal T, Galli SJ. New models for analyzing mast cell functions in vivo. Trends Immunol. 2012;33:613–25. doi: 10.1016/j.it.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Galli SJ, Tsai M, Marichal T, Tchougounova E, Reber LL, Pejler G. Approaches for analyzing the roles of mast cells and their proteases in vivo. Adv Immunol. 2015;126:45–127. doi: 10.1016/bs.ai.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–9. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- 113.Rodewald HR, Feyerabend TB. Widespread immunological functions of mast cells: fact or fiction? Immunity. 2012;37:13–24. doi: 10.1016/j.immuni.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 114.Vallance BA, Blennerhassett PA, Huizinga JD, Collins SM. Mast cell-independent impairment of host defense and muscle contraction in T. spiralis-infected W/W(V) mice. Am J Physiol Gastrointest Liver Physiol. 2001;280:G640–8. doi: 10.1152/ajpgi.2001.280.4.G640. [DOI] [PubMed] [Google Scholar]
- 115.Blankenhaus B, Reitz M, Brenz Y, Eschbach ML, Hartmann W, Haben I, Sparwasser T, Huehn J, Kuhl A, Feyerabend TB, Rodewald HR, Breloer M. Foxp3(+) regulatory T cells delay expulsion of intestinal nematodes by suppression of IL-9-driven mast cell activation in BALB/c but not in C57BL/6 mice. PLoS Pathog. 2014;10:e1003913. doi: 10.1371/journal.ppat.1003913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Katakura K, Saito S, Hamada A, Matsuda H, Watanabe N. Cutaneous leishmaniasis in mast cell-deficient W/Wv mice. Infect Immun. 1993;61:2242–4. doi: 10.1128/iai.61.5.2242-2244.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wershil BK, Theodos CM, Galli SJ, Titus RG. Mast cells augment lesion size and persistence during experimental Leishmania major infection in the mouse. J Immunol. 1994;152:4563–71. [PubMed] [Google Scholar]
- 118.Maurer M, Lopez Kostka S, Siebenhaar F, Moelle K, Metz M, Knop J, von Stebut E. Skin mast cells control T cell-dependent host defense in Leishmania major infections. FASEB J. 2006;20:2460–7. doi: 10.1096/fj.06-5860com. [DOI] [PubMed] [Google Scholar]
- 119.Paul C, Wolff S, Zapf T, Raifer H, Feyerabend TB, Bollig N, Camara B, Trier C, Schleicher U, Rodewald HR, Lohoff M. Mast cells have no impact on cutaneous leishmaniasis severity and related Th2 differentiation in resistant and susceptible mice. Eur J Immunol. 2016;46:114–21. doi: 10.1002/eji.201545613. [DOI] [PubMed] [Google Scholar]
- 120.Dunne DW, Butterworth AE, Fulford AJ, Kariuki HC, Langley JG, Ouma JH, Capron A, Pierce RJ, Sturrock RF. Immunity after treatment of human schistosomiasis: association between IgE antibodies to adult worm antigens and resistance to reinfection. European journal of immunology. 1992;22:1483–94. doi: 10.1002/eji.1830220622. [DOI] [PubMed] [Google Scholar]
- 121.Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat Rev Immunol. 2007;7:93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- 122.Farid AS, Jimi F, Inagaki-Ohara K, Horii Y. Increased intestinal endotoxin absorption during enteric nematode but not protozoal infections through a mast cell-mediated mechanism. Shock. 2008;29:709–16. doi: 10.1097/shk.0b013e31815c3f36. [DOI] [PubMed] [Google Scholar]
- 123.Prendergast CT, Sanin DE, Mountford AP. CD4 T-cell hypo-responsiveness induced by schistosome larvae is not dependent upon eosinophils but may involve connective tissue mast cells. Parasite Immunol. 2016;38:81–92. doi: 10.1111/pim.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Newlands GF, Miller HR, MacKellar A, Galli SJ. Stem cell factor contributes to intestinal mucosal mast cell hyperplasia in rats infected with Nippostrongylus brasiliensis or Trichinella spiralis, but anti-stem cell factor treatment decreases parasite egg production during N brasiliensis infection. Blood. 1995;86:1968–76. [PubMed] [Google Scholar]
- 125.Arizono N, Kasugai T, Yamada M, Okada M, Morimoto M, Tei H, Newlands GF, Miller HR, Kitamura Y. Infection of Nippostrongylus brasiliensis induces development of mucosal-type but not connective tissue-type mast cells in genetically mast cell-deficient Ws/Ws rats. Blood. 1993;81:2572–8. [PubMed] [Google Scholar]
- 126.Fitzsimmons CM, Dunne DW. Survival of the fittest: allergology or parasitology? Trends Parasitol. 2009;25:447–51. doi: 10.1016/j.pt.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 127.Pulendran B, Artis D. New paradigms in type 2 immunity. Science. 2012;337:431–5. doi: 10.1126/science.1221064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8:218–30. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 129.Artis D, Maizels RM, Finkelman FD. Forum: Immunology: Allergy challenged. Nature. 2012;484:458–9. doi: 10.1038/484458a. [DOI] [PubMed] [Google Scholar]
- 130.Wan H, Winton HL, Soeller C, Taylor GW, Gruenert DC, Thompson PJ, Cannell MB, Stewart GA, Garrod DR, Robinson C. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2001;31:279–94. doi: 10.1046/j.1365-2222.2001.00970.x. [DOI] [PubMed] [Google Scholar]
- 131.Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. The Journal of allergy and clinical immunology. 2004;114:997–1008. doi: 10.1016/j.jaci.2004.07.060. quiz 9. [DOI] [PubMed] [Google Scholar]
- 132.Donnelly S, Dalton JP, Loukas A. Proteases in helminth- and allergen-induced inflammatory responses. Chemical immunology and allergy. 2006;90:45–64. doi: 10.1159/000088880. [DOI] [PubMed] [Google Scholar]
- 133.Jeong SK, Kim HJ, Youm JK, Ahn SK, Choi EH, Sohn MH, Kim KE, Hong JH, Shin DM, Lee SH. Mite and cockroach allergens activate protease-activated receptor 2 and delay epidermal permeability barrier recovery. The Journal of investigative dermatology. 2008;128:1930–9. doi: 10.1038/jid.2008.13. [DOI] [PubMed] [Google Scholar]
- 134.Chapman MD, Wunschmann S, Pomes A. Proteases as Th2 adjuvants. Current allergy and asthma reports. 2007;7:363–7. doi: 10.1007/s11882-007-0055-6. [DOI] [PubMed] [Google Scholar]
- 135.Jarisch R, Yman L, Boltz A, Sandor I, Janitsch A. IgE antibodies to bee venom, phospholipase A, melittin and wasp venom. Clin Allergy. 1979;9:535–41. doi: 10.1111/j.1365-2222.1979.tb02518.x. [DOI] [PubMed] [Google Scholar]
- 136.Wadee AA, Rabson AR. Development of specific IgE antibodies after repeated exposure to snake venom. J Allergy Clin Immunol. 1987;80:695–8. doi: 10.1016/0091-6749(87)90289-2. [DOI] [PubMed] [Google Scholar]
- 137.Leynadier F, Hassani Y, Chabane MH, Benguedda AC, Abbadi MC, Guerin L. Allergic reactions to North African scorpion venom evaluated by skin test and specific IgE. The Journal of allergy and clinical immunology. 1997;99:851–3. doi: 10.1016/s0091-6749(97)80022-x. [DOI] [PubMed] [Google Scholar]
- 138.Annila I. Bee venom allergy. Clin Exp Allergy. 2000;30:1682–7. doi: 10.1046/j.1365-2222.2000.00885.x. [DOI] [PubMed] [Google Scholar]
- 139.Hoffman DR. Ant venoms. Current opinion in allergy and clinical immunology. 2010;10:342–6. doi: 10.1097/ACI.0b013e328339f325. [DOI] [PubMed] [Google Scholar]
- 140.Tibballs J, Yanagihara AA, Turner HC, Winkel K. Immunological and toxinological responses to jellyfish stings. Inflammation & allergy drug targets. 2011;10:438–46. doi: 10.2174/187152811797200650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bilo BM, Bonifazi F. Epidemiology of insect-venom anaphylaxis. Current opinion in allergy and clinical immunology. 2008;8:330–7. doi: 10.1097/ACI.0b013e32830638c5. [DOI] [PubMed] [Google Scholar]
- 142.Togias AG, Burnett JW, Kagey-Sobotka A, Lichtenstein LM. Anaphylaxis after contact with a jellyfish. The Journal of allergy and clinical immunology. 1985;75:672–5. doi: 10.1016/0091-6749(85)90092-2. [DOI] [PubMed] [Google Scholar]
- 143.Profet M. The function of allergy: immunological defense against toxins. Q Rev Biol. 1991;66:23–62. doi: 10.1086/417049. [DOI] [PubMed] [Google Scholar]
- 144.Palm NW, Rosenstein RK, Medzhitov R. Allergic host defences. Nature. 2012;484:465–72. doi: 10.1038/nature11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stebbings JH., Jr. Immediate hypersensitivity: a defense against arthropods? Perspectives in biology and medicine. 1974;17:233–9. doi: 10.1353/pbm.1974.0027. [DOI] [PubMed] [Google Scholar]
- 146.Marichal T, Starkl P, Reber LL, Kalesnikoff J, Oettgen HC, Tsai M, Metz M, Galli SJ. A beneficial role for immunoglobulin E in host defense against honeybee venom. Immunity. 2013;39:963–75. doi: 10.1016/j.immuni.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Palm NW, Rosenstein RK, Yu S, Schenten DD, Florsheim E, Medzhitov R. Bee venom phospholipase A2 induces a primary type 2 response that is dependent on the receptor ST2 and confers protective immunity. Immunity. 2013;39:976–85. doi: 10.1016/j.immuni.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Starkl P, Marichal T, Gaudenzio N, Reber LL, Sibilano R, Tsai M, Galli SJ. IgE antibodies, FcepsilonRIalpha, and IgE-mediated local anaphylaxis can limit snake venom toxicity. J Allergy Clin Immunol. 2016;137:246–57. e11. doi: 10.1016/j.jaci.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Higginbotham RD. Mast cells and local resistance to Russell's viper venom. J Immunol. 1965;95:867–75. [PubMed] [Google Scholar]
- 150.Higginbotham RD, Karnella S. The significance of the mast cell response to bee venom. J Immunol. 1971;106:233–40. [PubMed] [Google Scholar]
- 151.Metz M, Piliponsky AM, Chen CC, Lammel V, Abrink M, Pejler G, Tsai M, Galli SJ. Mast cells can enhance resistance to snake and honeybee venoms. Science. 2006;313:526–30. doi: 10.1126/science.1128877. [DOI] [PubMed] [Google Scholar]
- 152.Akahoshi M, Song CH, Piliponsky AM, Metz M, Guzzetta A, Abrink M, Schlenner SM, Feyerabend TB, Rodewald HR, Pejler G, Tsai M, Galli SJ. Mast cell chymase reduces the toxicity of Gila monster venom, scorpion venom, and vasoactive intestinal polypeptide in mice. J Clin Invest. 2011;121:4180–91. doi: 10.1172/JCI46139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Schneider LA, Schlenner SM, Feyerabend TB, Wunderlin M, Rodewald HR. Molecular mechanism of mast cell mediated innate defense against endothelin and snake venom sarafotoxin. J Exp Med. 2007;204:2629–39. doi: 10.1084/jem.20071262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Galli SJ, Starkl P, Marichal T, Tsai M. Mast cells and IgE in defense against venoms: Possible “good side” of allergy? Allergol Int. 2016;65:3–15. doi: 10.1016/j.alit.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 155.Laird DJ, De Tomaso AW, Cooper MD, Weissman IL. 50 million years of chordate evolution: seeking the origins of adaptive immunity. Proc Natl Acad Sci U S A. 2000;97:6924–6. doi: 10.1073/pnas.97.13.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cavalcante MC, de Andrade LR, Du Bocage Santos-Pinto C, Straus AH, Takahashi HK, Allodi S, Pavao MS. Colocalization of heparin and histamine in the intracellular granules of test cells from the invertebrate Styela plicata (Chordata-Tunicata). J Struct Biol. 2002;137:313–21. doi: 10.1016/s1047-8477(02)00007-2. [DOI] [PubMed] [Google Scholar]
- 157.Wong GW, Zhuo L, Kimata K, Lam BK, Satoh N, Stevens RL. Ancient origin of mast cells. Biochem Biophys Res Commun. 2014;451:314–8. doi: 10.1016/j.bbrc.2014.07.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Habermann E. Bee and wasp venoms. Science. 1972;177:314–22. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 159.Bilo BM, Rueff F, Mosbech H, Bonifazi F, Oude-Elberink JN. Diagnosis of Hymenoptera venom allergy. Allergy. 2005;60:1339–49. doi: 10.1111/j.1398-9995.2005.00963.x. [DOI] [PubMed] [Google Scholar]
- 160.Grimbaldeston MA, Chen CC, Piliponsky AM, Tsai M, Tam SY, Galli SJ. Mast cell-deficient W-sash c-kit mutant Kit W-sh/W-sh mice as a model for investigating mast cell biology in vivo. The American journal of pathology. 2005;167:835–48. doi: 10.1016/S0002-9440(10)62055-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lilla JN, Chen CC, Mukai K, BenBarak MJ, Franco CB, Kalesnikoff J, Yu M, Tsai M, Piliponsky AM, Galli SJ. Reduced mast cell and basophil numbers and function in Cpa3-Cre; Mcl-1fl/fl mice. Blood. 2011;118:6930–8. doi: 10.1182/blood-2011-03-343962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fitzgerald KT, Flood AA. Hymenoptera stings. Clin Tech Small Anim Pract. 2006;21:194–204. doi: 10.1053/j.ctsap.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 163.Fry BG, Roelants K, Champagne DE, Scheib H, Tyndall JD, King GF, Nevalainen TJ, Norman JA, Lewis RJ, Norton RS, Renjifo C, de la Vega RC. The toxicogenomic multiverse: convergent recruitment of proteins into animal venoms. Annual review of genomics and human genetics. 2009;10:483–511. doi: 10.1146/annurev.genom.9.081307.164356. [DOI] [PubMed] [Google Scholar]
- 164.Cavagnol RM. The pharmacological effects of hymenoptera venoms. Annu Rev Pharmacol Toxicol. 1977;17:479–98. doi: 10.1146/annurev.pa.17.040177.002403. [DOI] [PubMed] [Google Scholar]
- 165.Palm NW, Medzhitov R. Role of the inflammasome in defense against venoms. Proc Natl Acad Sci U S A. 2013;110:1809–14. doi: 10.1073/pnas.1221476110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dennis EA, Cao J, Hsu YH, Magrioti V, Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chemical reviews. 2011;111:6130–85. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Antonicelli L, Bilo MB, Bonifazi F. Epidemiology of Hymenoptera allergy. Curr Opin Allergy Clin Immunol. 2002;2:341–6. doi: 10.1097/00130832-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 168.Przybilla B, Ring J, Grieshammer B. Association of features of atopy and diagnostic parameters in hymenoptera venom allergy. Allergy. 1991;46:570–6. doi: 10.1111/j.1398-9995.1991.tb00625.x. [DOI] [PubMed] [Google Scholar]
- 169.Sturm GJ, Heinemann A, Schuster C, Wiednig M, Groselj-Strele A, Sturm EM, Aberer W. Influence of total IgE levels on the severity of sting reactions in Hymenoptera venom allergy. Allergy. 2007;62:884–9. 4180–91. doi: 10.1111/j.1398-9995.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- 170.Muller UR. Bee venom allergy in beekeepers and their family members. Curr Opin Allergy Clin Immunol. 2005;5:343–7. doi: 10.1097/01.all.0000173783.42906.95. [DOI] [PubMed] [Google Scholar]
- 171.Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. J Exp Med. 2008;205:2887–98. doi: 10.1084/jem.20080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ozdemir C, Kucuksezer UC, Akdis M, Akdis CA. Mechanisms of immunotherapy to wasp and bee venom. Clin Exp Allergy. 2011;41:1226–34. doi: 10.1111/j.1365-2222.2011.03812.x. [DOI] [PubMed] [Google Scholar]
- 173.Holgate ST. Genetic and environmental interaction in allergy and asthma. J Allergy Clin Immunol. 1999;104:1139–46. doi: 10.1016/S0091-6749(99)70005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Holloway JW, Yang IA, Holgate ST. Genetics of allergic disease. J Allergy Clin Immunol. 2010;125:S81–94. doi: 10.1016/j.jaci.2009.10.071. [DOI] [PubMed] [Google Scholar]
- 175.Fry BG, Vidal N, Norman JA, Vonk FJ, Scheib H, Ramjan SF, Kuruppu S, Fung K, Hedges SB, Richardson MK, Hodgson WC, Ignjatovic V, Summerhayes R, Kochva E. Early evolution of the venom system in lizards and snakes. Nature. 2006;439:584–8. doi: 10.1038/nature04328. [DOI] [PubMed] [Google Scholar]
- 176.Giribet G, Edgecombe GD. Reevaluating the arthropod tree of life. Annu Rev Entomol. 2012;57:167–86. doi: 10.1146/annurev-ento-120710-100659. [DOI] [PubMed] [Google Scholar]
- 177.Dessein AJ, Parker WL, James SL, David JR. IgE antibody and resistance to infection. I. Selective suppression of the IgE antibody response in rats diminishes the resistance and the eosinophil response to Trichinella spiralis infection. The Journal of experimental medicine. 1981;153:423–36. doi: 10.1084/jem.153.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ahmad A, Wang CH, Bell RG. A role for IgE in intestinal immunity. Expression of rapid expulsion of Trichinella spiralis in rats transfused with IgE and thoracic duct lymphocytes. J Immunol. 1991;146:3563–70. [PubMed] [Google Scholar]
- 179.Betts CJ, Else KJ. Mast cells, eosinophils and antibody-mediated cellular cytotoxicity are not critical in resistance to Trichuris muris. Parasite Immunol. 1999;21:45–52. doi: 10.1046/j.1365-3024.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- 180.Blackwell NM, Else KJ. B cells and antibodies are required for resistance to the parasitic gastrointestinal nematode Trichuris muris. Infection and immunity. 2001;69:3860–8. doi: 10.1128/IAI.69.6.3860-3868.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Watanabe N, Ishiwata K, Kaneko S, Oku Y, Kamiya M, Katakura K. Immune defense and eosinophilia in congenitally IgE-deficient SJA/9 mice infected with Angiostrongylus costaricensis. Parasitology research. 1993;79:431–4. doi: 10.1007/BF00931835. [DOI] [PubMed] [Google Scholar]
- 182.McCoy KD, Stoel M, Stettler R, Merky P, Fink K, Senn BM, Schaer C, Massacand J, Odermatt B, Oettgen HC, Zinkernagel RM, Bos NA, Hengartner H, Macpherson AJ, Harris NL. Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell host & microbe. 2008;4:362–73. doi: 10.1016/j.chom.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 183.Gray CA, Lawrence RA. A role for antibody and Fc receptor in the clearance of Brugia malayi microfilariae. European journal of immunology. 2002;32:1114–20. doi: 10.1002/1521-4141(200204)32:4<1114::AID-IMMU1114>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 184.Paciorkowski N, Shultz LD, Rajan TV. Primed peritoneal B lymphocytes are sufficient to transfer protection against Brugia pahangi infection in mice. Infection and immunity. 2003;71:1370–8. doi: 10.1128/IAI.71.3.1370-1378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Porcherie A, Mathieu C, Peronet R, Schneider E, Claver J, Commere PH, Kiefer-Biasizzo H, Karasuyama H, Milon G, Dy M, Kinet JP, Louis J, Blank U, Mecheri S. Critical role of the neutrophil-associated high-affinity receptor for IgE in the pathogenesis of experimental cerebral malaria. The Journal of experimental medicine. 2011;208:2225–36. doi: 10.1084/jem.20110845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jankovic D, Cheever AW, Kullberg MC, Wynn TA, Yap G, Caspar P, Lewis FA, Clynes R, Ravetch JV, Sher A. CD4+ T cell-mediated granulomatous pathology in schistosomiasis is downregulated by a B cell-dependent mechanism requiring Fc receptor signaling. The Journal of experimental medicine. 1998;187:619–29. doi: 10.1084/jem.187.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.El Ridi R, Ozaki T, Kamiya H. Schistosoma mansoni infection in IgE-producing and IgE-deficient mice. The Journal of parasitology. 1998;84:171–4. [PubMed] [Google Scholar]
- 188.Owhashi M, Nawa Y, Watanabe N. Granulomatous response in selective IgE-deficient SJA/9 mice infected with Schistosoma japonicum. International archives of allergy and applied immunology. 1989;90:310–2. doi: 10.1159/000235044. [DOI] [PubMed] [Google Scholar]
- 189.Wastling JM, Knight P, Ure J, Wright S, Thornton EM, Scudamore CL, Mason J, Smith A, Miller HR. Histochemical and ultrastructural modification of mucosal mast cell granules in parasitized mice lacking the beta-chymase, mouse mast cell protease-1. Am J Pathol. 1998;153:491–504. doi: 10.1016/s0002-9440(10)65592-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kimura K, Song CH, Rastogi A, Dranoff G, Galli SJ, Lantz CS. Interleukin-3 and c-Kit/stem cell factor are required for normal eosinophil responses in mice infected with Strongyloides venezuelensis. Lab Invest. 2006;86:987–96. doi: 10.1038/labinvest.3700458. [DOI] [PubMed] [Google Scholar]
- 191.Hashimoto K, Uchikawa R, Tegoshi T, Takeda K, Yamada M, Arizono N. Immunity-mediated regulation of fecundity in the nematode Heligmosomoides polygyrus--the potential role of mast cells. Parasitology. 2010;137:881–7. doi: 10.1017/S0031182009991673. [DOI] [PubMed] [Google Scholar]
- 192.Asami M, Owhashi M, Abe T, Nawa Y. Susceptibility of multipotent haemopoietic stem cell deficient W/Wv mice to Plasmodium berghei-infection. Immunol Cell Biol. 1991;69(Pt 5):355–60. doi: 10.1038/icb.1991.51. [DOI] [PubMed] [Google Scholar]
- 193.Owhashi M, Horii Y, Ikeda T, Maruyama H, Abe T, Nawa Y. Importance of mast-cell-derived eosinophil chemotactic factor A on granuloma formation in murine Schistosomiasis japonica: evaluation using mast-cell-deficient W/WV mice. Int Arch Allergy Appl Immunol. 1990;92:64–8. doi: 10.1159/000235226. [DOI] [PubMed] [Google Scholar]
- 194.Matsuda H, Fukui K, Kiso Y, Kitamura Y. Inability of genetically mast cell-deficient W/Wv mice to acquire resistance against larval Haemaphysalis longicornis ticks. J Parasitol. 1985;71:443–8. [PubMed] [Google Scholar]
- 195.denHollander N, Allen JR. Dermacentor variabilis: resistance to ticks acquired by mast cell-deficient and other strains of mice. Exp Parasitol. 1985;59:169–79. doi: 10.1016/0014-4894(85)90069-4. [DOI] [PubMed] [Google Scholar]