Abstract
A comprehensive cannabinoids urine quantification method may improve clinical and forensic results interpretation and is necessary to support our clinical research. A liquid chromatography tandem mass spectrometry quantification method for Δ9-tetrahydrocannabinol (THC), 11-hydroxy-THC (11-OH-THC), 11-nor-9-carboxy-THC (THCCOOH), Δ9-tetrahydrocannabinolic acid (THCAA), cannabinol (CBN), cannabidiol (CBD), cannabigerol (CBG), Δ9-tetrahydrocannabivarin (THCV), 11-nor-9-carboxy-THCV (THCVCOOH), THC-glucuronide (THC-gluc) and THCCOOH-gluc (THCCOOH-gluc) in urine was developed and validated according to the Scientific Working Group on Toxicology guidelines. Sample preparation consisted of disposable pipette extraction (WAX-S) of 200μL urine. Separation was achieved on a Kinetex C18 column using gradient elution with flow rate 0.5 mL/min, mobile phase A (10 mM ammonium acetate in water) and mobile phase B (15% methanol in acetonitrile). Total run time was 14 min. Analytes were monitored in both positive and negative ionization modes by scheduled multiple reaction monitoring. Linear ranges were 0.5–100 μg/L for THC and THCCOOH, 0.5–50 μg/L for 11-OH-THC, CBD, CBN, THCAA and THC-gluc, 1–100 μg/L for CBG, THCV and THCVCOOH and 5–500 μg/L for THCCOOH-gluc (R2>0.99). Analytical biases were 88.3–113.7%, imprecisions 3.3–14.3%, extraction efficiencies 42.4–81.5% and matrix effect −10 to 32.5%. We developed and validated a comprehensive, simple and rapid LC-MS/MS cannabinoid urine method for quantification of 11 cannabinoids and metabolites. This method is being used in a controlled cannabis administration study, investigating urine cannabinoid markers documenting recent cannabis use, chronic frequent smoking or route of drug administration and potentially improving urine cannabinoid result interpretation.
Keywords: cannabinoid, urine, LC-MS/MS, DPX, THC
Introduction
A comprehensive method for multiple cannabinoids quantification may improve urine results interpretation [1–3]. Cannabinoids intake is estimated to be 181.8 million cannabis users worldwide [4]. Cannabis potency also increased from 3–4% to >10% over the last two decades with some regional differences [4–6]. The ability to genetically select strains with high cannabis potency contributed to the dramatic increase in Δ9-tetrahydracannabinol (THC), the primary psychoactive ingredient in cannabis [4, 7].
Several biological specimens are objective indicators of substance abuse including urine, blood, oral fluid and hair, each with different strengths, limitations and detection windows. Blood and oral fluid are often selected for detecting recent drug exposure while hair provides a much longer historical exposure [1, 8]. Drugs appear rapidly in blood and oral fluid with short windows of detection (1–3 days) [1] while drugs typically appear in hair one week after consumption [9, 10] and remain in the hair follicle until the hair is cut providing windows of detection for several weeks, months or even years after consumption [1]. Drug distribution into urine and detection windows fall between those of blood/oral fluid and hair with drug initially appearing in urine within hours of consumption and persisting for days to weeks after consumption [1, 11]. Urine is frequently utilized for clinical and forensic toxicology, workplace, pain management and drug treatment testing programs [1, 8, 12–14].
Urine drug testing generally includes an initial immunoassay screen followed by a more selective confirmation method if the screen is positive. Traditionally, gas chromatography coupled to mass spectrometry (GC-MS) was the method of choice. Improvements in liquid chromatography tandem mass spectrometry (LC-MS/MS) during the last decade led to increases in drug testing by this technique. LC-MS/MS can enable simpler sample preparation compared to GC-MS and avoids derivatization. It is sometimes possible to avoid extraction (solid phase or liquid-liquid) and simply dilute urine [15] or precipitate plasma/blood proteins with acid or solvent before LC-MS/MS analysis [15]. However, these simple preparation approaches can lead to frequent instrument contamination requiring source or mass spectrometer maintenance. In addition, suppression or enhancement of ionization may occur when sample preparation is less extensive. Therefore, we elected to evaluate an approach involving acetonitrile precipitation and a simple one step solid phase extraction with DPX WAX-S tips to reduce instrument contamination and reduce ion suppression/enhancement. Analysis without hydrolysis permits direct measurement of phase two glucuronide cannabinoid metabolites [16].
Cannabinoid excretion is prolonged in chronic frequent cannabis smokers after abstinence initiation [17, 18]. This prolonged excretion confounds distinction of recent cannabis intake, suggesting the importance of identifying new cannabinoid markers for improving interpretation of urine cannabinoid results.
We developed and validated a comprehensive, simple and rapid LC-MS/MS cannabinoid urine method for quantification of THC; its active hydroxylated metabolite 11-hydroxy-THC (11-OH-THC), the further oxidized inactive metabolite, 11-nor-9-carboxy-THC (THCCOOH), cannabis constituents: cannabidiol (CBD) and cannabinol (CBN), and phase 2 glucuronidated metabolites, THCCOOH-glcuronide (THCCOOH-gluc) and THC-glucuronide (THC-gluc). Less commonly monitored cannabinoids also were quantified including the THC precursor, Δ9-tetrahydrocannabinolic acid (THCAA), the plant constituents: cannabigerol (CBG), Δ9-tetrahydrocannabivarin (THCV) and its metabolite 11-nor-9-carboxy-THCV (THCVCOOH). Urine samples collected after controlled cannabis administration will be analyzed via this novel method to determine if THCAA, CBG, THCV, THCVCOOH might assist identifying recent cannabis intake.
Method
Chemicals and supplies
THC, 11-OH-THC, THCCOOH, THCCOOH-gluc, CBD, CBN, d3-THC, d3-11-OH-THC, d9-THCCOOH, d3-CBD, d3-CBN and d3-THCCOOH-gluc (d3-THCCOOH-gluc) were purchased from Cerilliant (Round Rock, TX, USA). CBG was obtained from Restek (Bellefonte, PA, USA), THCAA was from Lipomed (Arlesheim, Switzerland), THCVCOOH and THC-gluc from ElSohly Laboratories (Oxford, MS, USA) and THCV from RTI International (Research Triangle Park, NC, USA). Acetonitrile, ammonium acetate and formic acid (LC-MS grade) were acquired from Sigma Aldrich (St. Louise, MO, USA) and methanol (LC-MS grade) from Fischer Scientific (Fair Lawn, NJ, USA). Water was purified in-house by an ELGA Purelab Ultra Analytic purifier (Siemens Water Technologies, Lowell, MA, USA). WAX-S tips (DPX Labs, Columbia, SC, USA) were used to extract samples, and a Kinetex C18 2.1 × 50 mm, 2.6 μm column combined with a 2.1 × 2 mm guard column of identical phase (Phenomenex Inc., Torrance, CA, USA) accomplished analyte separation.
Instrumentation
Experiments were performed on an 8050 Shimadzu triple quadrupole mass spectrometer with electrospray ionization using scheduled multiple reaction monitoring (MRM). The LC system was a Nexera LC-30 ultra-high pressure liquid chromatograph (Shimadzu Scientific, Columbia, MD, USA). LabSolution LCMS software version 5.72 was used for data acquisition and ASCENT Series 3 software from Indigo BioAutomation (Indianapolis, IN, USA) for data analysis.
Calibrators, quality control (QC) and internal standards
Separate working cannabinoids calibrator and quality control stock solutions were prepared in methanol. Calibrators were prepared at 0.5, 1, 2.5, 5, 10, 25, 50 and 100 μg/L for THC and THCCOOH, at 0.5, 1, 2.5, 5, 10, 25 and 50 μg/L for 11-OH-THC, CBD, CBN, THCAA and THC-gluc, at 1, 2, 5, 10, 20, 50 and 100 μg/L for CBG, THCV and THCVCCOOH and at 5, 10, 25, 50, 100, 250 and 500 μg/L for THCCOOH-gluc after fortifying 20 μL working stock solution into 200 μL blank human urine. Three QC working solutions at low, medium and high concentrations were prepared across the linear dynamic range. QC samples were prepared by adding 20 μL QC working solution to 200 μL blank human urine yielding QC samples at 1.5, 4.5 and 80 μg/L for THC and THCCOOH, at 1.5, 4.5 and 40 μg/L for 11-OH-THC, CBD, CBN, THCAA and THC-gluc, at 3, 9 and 80 μg/L for CBG, THCV and THCVCOOH and at 15, 45 and 400 μg/L for THCCOOH-gluc. The internal standard working solution consisted of 50 μg/L d3-THC, d9-THCCOOH, d3-11-OH-THC, d3-CBD, d3-CBN with 1000 μg/L d3-THCCOOH-gluc in methanol. All stock and working solutions were stored in amber glass vials at −20°C.
Sample preparation
Blank human urine, 200 μL, was fortified with internal standard (d3-THC, d9-THCCOOH, d3-11-OH-THC, d3-CBD, d3-CBN and d3-THCCOOH-gluc), 20 μL methanol and clarified via centrifugation at 15,000g, 4°C for 5 min after adding 500 μL acetonitrile. Supernatant (550 μL) was transferred to a 2.0 mL microcentrifuge tube containing 200 μL 5% aqueous formic acid followed by aspiration 4 times through 1 mL WAX-S tips (DPX Labs, Columbia, SC) containing 20 mg resin and 40 mg salt. 90 μL upper, organic layer was transferred to a clean 1.5 mL microcentrifuge tube containing 210 μL mobile phase A, followed by centrifugation at 15,000g, 4°C for 5 min. The supernatant was transferred to a 200 μL glass insert autosampler vial and 30 μL injected onto the LC-MS/MS instrument.
LC-MS/MS analysis
Chromatographic separation was achieved with a Kinetex C18 column at 40°C. Gradient elution was performed with mobile phase A) 10 mM ammonium acetate in water and B) 15 % methanol in acetonitrile at a flow rate of 0.5 mL/min. The gradient program was 30% B for 0.50 min, to 50% B at 1.0 min, 70.7% B at 8.33 min, 98% B at 9.0 min holding for 3.0 min, re-equilibration to 30% B over 0.10 min and holding for 1.80 min. Total run time was 14 min. Flow was diverted to waste from 0–1 min and for the last 4.5 min of the run. Autosampler and column oven temperatures were 4 and 40°C, respectively. MS/MS data were acquired via electrospray ionization mode (ESI) with scheduled multiple reaction monitoring (MRM) in both positive and negative mode. Quantifier ion and qualifier ion transitions were monitored for each analyte and internal standard. MS/MS parameters were optimized by injecting 5 μL of individual analytes (100 μg/L). Optimized source parameters were nebulizing gas flow 2 L/min, heating gas flow 10 L/min, drying gas flow 6 L/min, interface temperature 350°C, desolvation line temperature 200°C and heat block temperature 450°C. Individual MS/MS settings are displayed in Table 1.
Table 1.
LC-MS/MS parameters for cannabinoids, their metabolites and internal standards in human urine. Bold masses depict quantifier transition.
| Analyte | Q1 mass (amu) | Q3 mass (amu) | Dwell time (ms) | Q1 Pre Bias (V) | Collision Energy (V) | Q3 Pre Bias (V) | Ionization Mode | Retention time (min) |
|---|---|---|---|---|---|---|---|---|
| THCVCOOH | 315.2 | 271.3 | 25 | 20 | 19 | 19 | Negative | 1.6 |
| 315.2 | 163.0 | 25 | 20 | 29 | 11 | |||
|
| ||||||||
| THCCOOH-gluc | 519.4 | 343.3 | 25 | 26 | 25 | 26 | Negative | 1.75 |
| 519.4 | 299.3 | 25 | 26 | 34 | 21 | |||
|
| ||||||||
| d3-THCCOOH-gluc | 522.4 | 346.2 | 25 | 26 | 25 | 25 | Negative | 1.74 |
| 522.4 | 302.2 | 25 | 26 | 35 | 22 | |||
|
| ||||||||
| THC-gluc | 489.1 | 313.3 | 25 | 24 | 30 | 22 | Negative | 1.79 |
| 489.1 | 112.8 | 25 | 24 | 21 | 24 | |||
|
| ||||||||
| THCCOOH | 343.4 | 299.3 | 25 | 17 | 21 | 22 | Negative | 2.24 |
| 343.4 | 245.3 | 25 | 17 | 28 | 18 | |||
|
| ||||||||
| d9-THCCOOH | 352.1 | 308.4 | 25 | 17 | 21 | 22 | Negative | 2.23 |
| 352.1 | 254.2 | 25 | 17 | 30 | 30 | |||
|
| ||||||||
| THCAA | 357.1 | 313.3 | 25 | 17 | 25 | 22 | Negative | 2.76 |
| 357.1 | 245.1 | 25 | 17 | 31 | 17 | |||
|
| ||||||||
| 11-OH-THC | 329.4 | 172.9 | 95 | 16 | 20 | 23 | Negative | 3.84 |
| 329.4 | 172.9 | 95 | 16 | 29 | 30 | |||
|
| ||||||||
| d3-11-OH-THC | 332.1 | 314.1 | 95 | 16 | 21 | 23 | Negative | 3.82 |
| 332.1 | 173.2 | 95 | 16 | 28 | 12 | |||
|
| ||||||||
| THCV | 287.0 | 165.2 | 45 | −20 | −21 | −19 | Positive | 5.24 |
| 287.0 | 231.2 | 45 | −20 | −20 | −15 | |||
|
| ||||||||
| CBD | 313.3 | 245.0 | 45 | 20 | 21 | 26 | Negative | 5.74 |
| 313.3 | 107.0 | 45 | 20 | 38 | 23 | |||
|
| ||||||||
| d3-CBD | 316.1 | 248.1 | 45 | 21 | 22 | 27 | Negative | 5.73 |
| 316.1 | 106.9 | 45 | 21 | 31 | 22 | |||
|
| ||||||||
| CBG | 317.2 | 193.1 | 45 | −23 | −17 | −19 | Positive | 5.9 |
| 317.2 | 123.0 | 45 | −23 | −30 | −19 | |||
|
| ||||||||
| CBN | 309.4 | 279.0 | 45 | 15 | 30 | 29 | Negative | 6.92 |
| 309.4 | 222.2 | 45 | 15 | 46 | 11 | |||
|
| ||||||||
| d3-CBN | 316.1 | 248.1 | 45 | 15 | 31 | 20 | Negative | 6.89 |
| 316.1 | 106.9 | 45 | 15 | 44 | 24 | |||
|
| ||||||||
| THC | 315.2 | 193.1 | 45 | −22 | −23 | −19 | Positive | 7.6 |
| 315.2 | 123.0 | 45 | −22 | −34 | −21 | |||
|
| ||||||||
| d3-THC | 318.2 | 196.2 | 45 | −22 | −23 | −12 | Positive | 7.57 |
| 318.2 | 123.0 | 45 | −22 | −35 | −22 | |||
Data analysis
Calibration curves were constructed daily from peak area ratios of analytes to their respective internal standard, with a weighting factor of 1/x2. Calibration curves were from 0.5–100 μg/L for THC and THCCOOH, 0.5–50 μg/L for 11-OH-THC, CBD, CBN, THCAA and THC-gluc, 1–100 μg/L for CBG, THCV and THCVCOOH and 5–500 μg/L for THCCOOH-gluc.
Method validation
Method validation parameters included specificity, sensitivity, linearity, imprecision, extraction efficiency, matrix effect, stability, dilution integrity and carryover. Method validation was conducted according to the Scientific Working Group Toxicology (SWGTOX) guidelines [19].
Specificity
Analyte identification criteria were relative retention time within ±0.05 min of the mean calibrator retention time and transitions of quantifier/qualifier peak area ratios within ±20% of mean calibrator transition ratios. Possible endogenous interferences were evaluated by analyzing ten urine specimens from different individuals. No endogenous interferences were observed if low QCs fulfilled the identification (MRM ratios within ±20% of mean calibrator MRM ratios) and QC quantification criteria (quantified within ±20% of expected concentration) for all ten blank urine lots.
Sensitivity and linearity
Decreasing concentrations of drug fortified in urine were analyzed to establish limit of detection (LOD) and limit of quantification (LOQ) in duplicates on three different occasions. LOD was the lowest concentration with a signal to noise (S/N) ratio of at least 3, peak area with a quantifier/qualifier transition ratio within ±20% and retention time within ±0.05 min. LOQ was defined by LOD criteria in addition to S/N ratio of at least 10 and accuracy of ±20% of target concentration. Linearity was evaluated by least square regression with at least 7 concentrations on five separate days. Calibrators were required to be within ±15% of target except for the lowest calibrator that had to be within ±20% with a correlation coefficient >0.99.
Analytical recovery and imprecision
Intra- and inter-day analytical recovery (bias/accuracy) and imprecision were determined from five replicates at three QC concentrations in five analytical batches (N=25). Analytical recovery was established by comparing mean results to the nominal concentration. Imprecision was expressed by coefficient of variation (CV%).
Extraction efficiency and matrix effect
Determination of extraction efficiency and matrix effect was according to Matuszewski et al. [20]. Extraction efficiency was calculated by dividing mean analyte peak area of sample fortified with analyte and internal standard prior to extraction with the mean analyte peak area of blank sample fortified with analyte and internal standard after extraction (N=12). Matrix effect was determined by dividing mean analyte peak area fortified with analyte and internal standard after extraction with analyte and internal standard spiked in mobile phase (N=12). The value was expressed in percent and subtracted from 100 to represent the signal suppression or enhancement due to presence of matrix. In addition, matrix effect also was evaluated by ten blank urine lots fortified with low or high QC solution and internal standard, and processed with calibrators prepared with a different blank urine lot to verify accurate quantification.
Stability
Analyte stability in urine was evaluated in triplicate at low and high QC concentrations under three conditions: short term stability in room temperature after 21h; 4°C after 72h; and three freeze-thaw cycles at −20°C (N=3). In addition, autosampler stability was determined after 96h at 4°C. Stability was assessed by comparing specimen concentrations with QC specimens prepared on the day of analysis (N=5).
Dilution integrity
Dilution integrity was demonstrated by diluting fortified urine specimens (N=3) containing 200 μg/L of THC, THCCOOH, CBG, THCV and THCVCOOH, 100 μg/L of 11-OH-THC, CBD, CBN, THCAA and THC-gluc and 1000 μg/L of THCCOOH-gluc 1:10 with blank human urine. Internal standard was added and samples extracted as described previously. Dilution integrity was acceptable if specimens quantified ±20% of expected diluted concentrations.
Carryover
Carryover was determined by injecting extracted blank urine (N=3) containing internal standard after a specimen containing analytes at twice the upper limit of quantification (ULOQ). Carryover was considered insignificant if the LOD criteria were not met.
Clinical study
Urine specimens were collected from a healthy cannabis user who provided written informed consent to participate in a National Institute of Drug Abuse (NIDA), Institutional Review Board-approved clinical study investigating cannabinoid pharmacokinetics and novel markers of cannabis intake following a single THC dose (6.9%, w/w; 54 mg total dose) via three different routes of administration. This double blind, randomized and placebo controlled clinical study will include a total of 20 participants and four dosing sessions per participant when completed. During the four randomized sessions participants received combinations of placebo, smoked, vaporized or THC-containing brownies (p.o.). Participants resided in a closed research facility during each session which lasted for up to 72 h after cannabis administration; participants were admitted 15–21 h before cannabis administration. Urine specimens were collected ad libitum upon admission for each session starting at admission until 72 h after cannabis administration. As proof of method applicability, results from urine specimens collected from a single participant for a cannabis vaporizer session are presented in this manuscript. All urine specimens were stored at −20°C prior analysis.
Results
Method validation data
Ten urine samples from ten different individuals contained no endogenous interfering peaks documenting specificity. Sensitivity and linearity data are shown in Table 2. The LOD and LOQ were within 0.5–5 g/L, with results for each analyte reported in Table 2. The ULOQ was 100 μg/L for THC, THCCOOH, CBG, THCV and THCVCOOH, 50 μg/L for 11-OH-THC, CBD, CBN, THCAA and THC-gluc, and 500 μg/L for THCCOOH-gluc. All correlation coefficients exceeded 0.99. Analytical recovery and imprecision data are found in Table 3. Intra- and inter-day analytical recoveries were 88.3–113.7 and 90.7–109.8%, respectively. While intra- and inter-day imprecisions were 3.3–14.3 and 4.8–13.5% CV, respectively. Mean extraction efficiency was 42.4–81.5% and mean matrix effect −10.0 to 32.5% (Table 4).
Table 2.
Cannabinoids in human urine: limits of detection (LOD), linear ranges, regression equations and correlation coefficients (N=5).
| Analyte | Internal standard | LOD (μg/L) | Linear Range (μg/L) | Regression equation (y=mx+b) | Correlation Coefficient (R2) |
|---|---|---|---|---|---|
| THCVCOOH | d9-THCCOOH | 1 | 1–100 | y=0.085 (0.001)x + 0.005 (0.02) | 0.9913 – 0.9987 |
| THCCOOH-gluc | d3-THCCOOH-gluc | 5 | 5–500 | y = 0.011 (0.001)x + 0.001 (0.003) | 0.9945 – 0.9991 |
| THC-gluc | d3-THCCOOH-gluc | 0.5 | 0.5–50 | y = 0.01 (0.001)x − 0.0001 (0.0005) | 0.9915 – 0.9980 |
| THCCOOH | d9-THCCOOH | 0.5 | 0.5–100 | y = 0.207 (0.008)x − 0.003 (0.01) | 0.9956 – 0.9985 |
| THCAA | d9-THCCOOH | 0.5 | 0.5–50 | y = 0.378 (0.033)x − 0.007 (0.034) | 0.9919 – 0.9966 |
| 11-OH-THC | d3-11-OH-THC | 0.5 | 0.5–50 | y = 0.183 (0.008)x − 0.009 (0.011) | 0.9944 – 0.9975 |
| THCV | d3-11-OH-THC | 1 | 1–100 | y = 2.006 (0.157)x + 0.127 (0.172) | 0.9927 – 0.9993 |
| CBD | d3-CBD | 0.5 | 0.5–50 | y = 0.255 (0.011)x − 0.01 (0.013) | 0.9936 – 0.9983 |
| CBG | d3-CBN | 1 | 1–100 | y = 0.498 (0.049)x + 0.014 (0.061) | 0.9954 – 0.9987 |
| CBN | d3-CBN | 0.5 | 0.5–50 | y = 0.146 (0.007)x + 0.0001 (0.009) | 0.9965 – 0.9990 |
| THC | d3-THC | 0.5 | 0.5–100 | y = 0.203 (0.006)x + 0.004 (0.009) | 0.9971 – 0.9993 |
Table 3.
Analytical bias and imprecision for cannabinoids in human urine.
| Analytical bias (% expected concentration) | Imprecision (% coefficient of variation) | ||||
|---|---|---|---|---|---|
|
| |||||
| Concentration (μg/L) | Intra-day (mean, N=5) | Inter-day (mean, N=25) | Intra-day (N=5) | Inter-day (N=25) | |
|
| |||||
| THCVCOOH | 3 | 92.7 | 99.3 | 12.7 | 11.6 |
| 9 | 94.3 | 96.4 | 12.2 | 9.0 | |
| 80 | 89.1 | 94.5 | 7.1 | 7.9 | |
|
| |||||
| THCCOOH-gluc | 15 | 97.8 | 99.6 | 12.7 | 8.7 |
| 45 | 98.6 | 101 | 6.4 | 6.5 | |
| 400 | 88.3 | 90.7 | 5.6 | 6.3 | |
|
| |||||
| THC-gluc | 1.5 | 98.8 | 100 | 14.3 | 13.2 |
| 4.5 | 101 | 98.1 | 13.0 | 10.0 | |
| 40 | 93.5 | 92.4 | 8.8 | 9.6 | |
|
| |||||
| THCCOOH | 1.5 | 99.1 | 107 | 12.2 | 9.6 |
| 4.5 | 104 | 106 | 3.3 | 6.6 | |
| 80 | 93.7 | 98.1 | 8.4 | 8.1 | |
|
| |||||
| THCAA | 1.5 | 110 | 104 | 5.2 | 11.2 |
| 4.5 | 98.9 | 100 | 9.1 | 9.9 | |
| 40 | 97.6 | 99.0 | 10.0 | 13.5 | |
|
| |||||
| 11-OH-THC | 1.5 | 110 | 105 | 8.8 | 8.0 |
| 4.5 | 99.9 | 103 | 8.2 | 6.8 | |
| 40 | 98.3 | 98.8 | 10.7 | 7.6 | |
|
| |||||
| THCV | 3.0 | 103 | 102 | 11.6 | 8.5 |
| 9.0 | 102 | 101 | 7.9 | 7.0 | |
| 80 | 99.0 | 97.7 | 11.6 | 7.8 | |
|
| |||||
| CBD | 1.5 | 106 | 106 | 10.4 | 9.8 |
| 4.5 | 112 | 109 | 6.7 | 6.5 | |
| 40 | 100 | 108 | 11.6 | 8.0 | |
|
| |||||
| CBG | 3.0 | 99.6 | 99.8 | 12.0 | 7.6 |
| 9.0 | 102 | 103 | 7.1 | 6.2 | |
| 80 | 102 | 103 | 14.1 | 7.9 | |
|
| |||||
| CBN | 1.5 | 114 | 110 | 6.4 | 5.6 |
| 4.5 | 111 | 110 | 3.8 | 4.8 | |
| 40 | 101 | 106 | 11.2 | 6.5 | |
|
| |||||
| THC | 1.5 | 105 | 104 | 10.0 | 7.7 |
| 4.5 | 108 | 103 | 5.0 | 6.8 | |
| 80 | 96.3 | 99.7 | 11.9 | 7.4 | |
Table 4.
Mean extraction efficiencies, matrix effects and analyte stability at room temperature, 4 and −20°C for cannabinoids in human urine.
| Analyte | Extraction efficiency (%, N=12) | Matrix effect (% of signal suppressed, N=12) | RT 21h (% difference, N=3) | 4°C 72h (% difference, N=3) | 3 freeze-thaw cycles (% difference, N=3) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | Low | High | Low | High | Low | High | |
| THCVCOOH | 81.5 | 73.6 | 9.2 | 9.1 | −1.7 | 2.0 | −1.3 | 3.1 | −6.7 | 0.0 |
| THCCOOH-gluc | 63.4 | 57.9 | 0.3 | 10.2 | −20.3 | −20.3 | 2.8 | 2.5 | 3.7 | 3.1 |
| THC-gluc | 72.0 | 67.6 | 32.0 | 32.5 | −15.4 | −4.1 | −3.0 | −1.5 | −0.1 | 0.0 |
| THCCOOH | 73.9 | 69.4 | 0.0 | 9.0 | 1.3 | 5.1 | 6.2 | 2.8 | −1.3 | −1.1 |
| THCAA | 42.4 | 42.5 | −10.0 | −6.0 | −55.4 | −57.1 | −15.8 | 5.2 | 3.7 | −7.7 |
| 11-OH-THC | 73.6 | 71.1 | 7.2 | 11.3 | −4.2 | −5.3 | −3.1 | 2.9 | 3.5 | −2.2 |
| THCV | 68.9 | 65.8 | 1.9 | 9.0 | −52.7 | −41.8 | −14.9 | −2.5 | −10.6 | −10.1 |
| CBD | 73.6 | 68.7 | 2.0 | 11.5 | −59.4 | −56.6 | −2.0 | −8.9 | 0.7 | −9.4 |
| CBG | 69.5 | 66.9 | 1.4 | 6.5 | −62.6 | −62.7 | −9.3 | −14.7 | −10.1 | −14.7 |
| CBN | 54.7 | 52.3 | 1.1 | 10.7 | −55.2 | −57.1 | −0.8 | −5.4 | −3.9 | −6.9 |
| THC | 58.4 | 55.7 | 0.6 | 6.4 | −55.7 | −58.3 | −16.4 | −8.8 | −5.0 | −7.2 |
After 21h at room temperature, THC, CBD, CBN, THCV and THCAA concentrations decreased 42–63%. Concentrations for all other analytes were within ±21.3%. All analytes were stable refrigerated for 72h at 4°C with concentrations within ±16.4%. After three freeze-thaw cycles, concentrations were within ±14.7% (Table 4). Autosampler stability was ±16.8% for all analytes except for THCAA, which decreased 40%. Dilution integrity (1:10 with blank urine) was within ±19.9% of target concentration for all analytes. No carryover was observed for cannabinoids after injecting negative specimens after samples containing twice the ULOQ. Negative specimens did not contain analyte above the LOD.
Proof of method
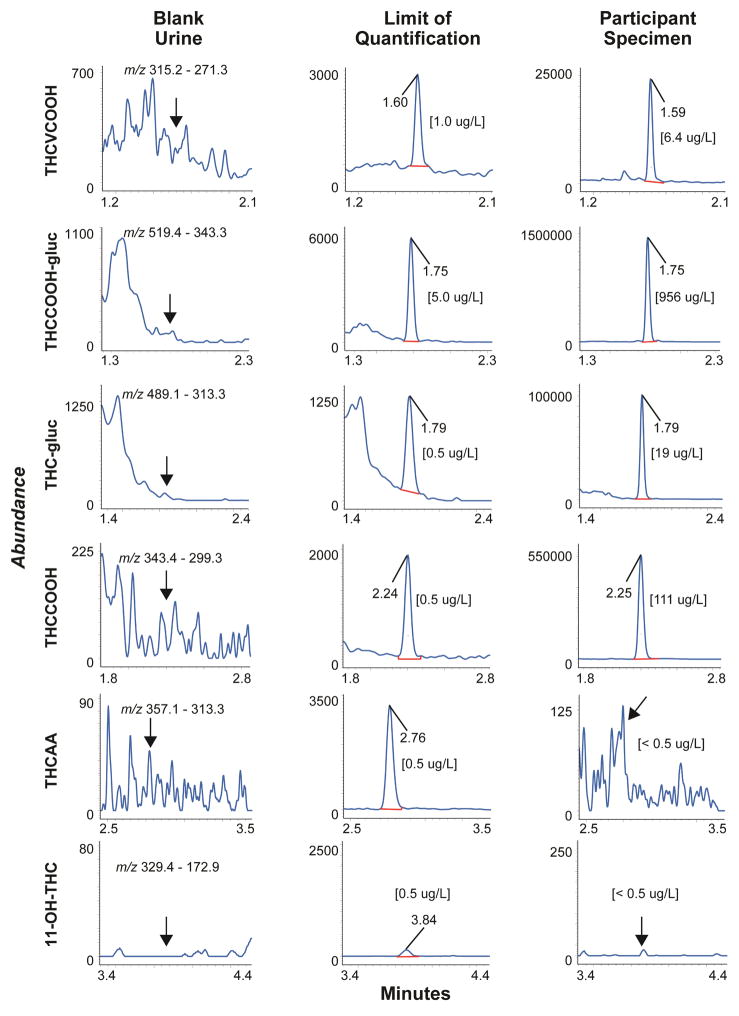
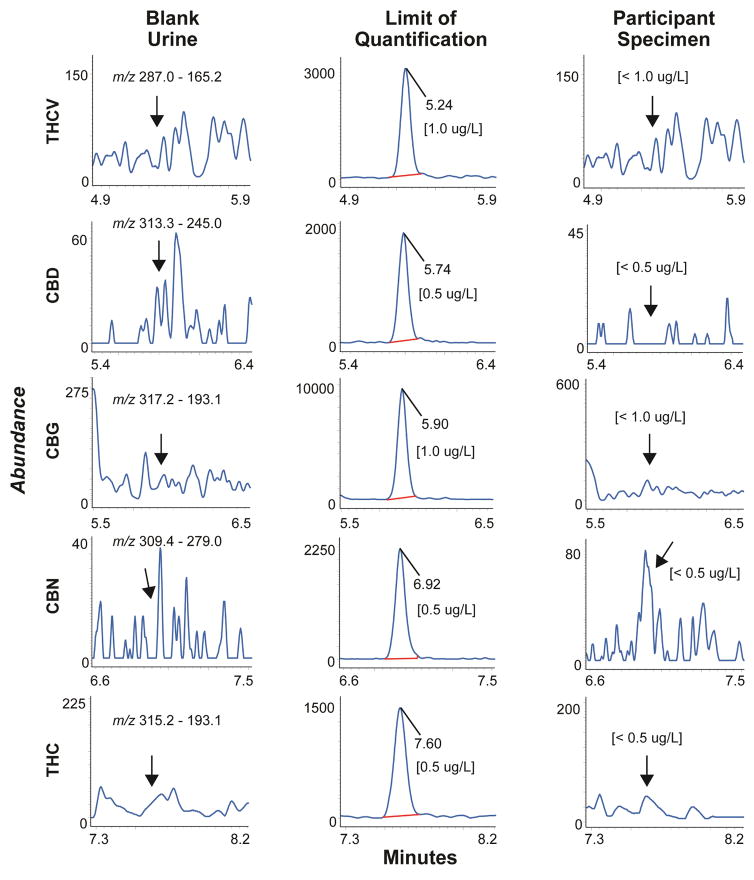
Chromatograms from a blank urine specimen, a blank urine specimen fortified with analytes at the LOQ and a urine specimen from a study participant 20 h after inhalation of 6.9% THC are shown in Figures 1 and 2.
Figure 1.
Multiple reaction monitoring ion chromatograms for THCVCOOH, THCCOOH-gluc, THC-gluc, THCCOOH, THCAA and 11-OH-THC in urine showing blank urine, limits of quantification and an authentic urine specimen collected from a participant 20 h after a vaporized 54 mg THC dose. All analytes were less than limits of quantification for blank urine extracts.
Figure 2.
Multiple reaction monitoring ion chromatograms for THCV, CBD, CBG, CBN and THC in urine showing blank urine, limits of quantification and an authentic urine specimen collected from a participant 20 h after a vaporized 54 mg THC dose. All analytes were less than limits of quantification for blank urine extracts.
This urine cannabinoid method is being used for samples collected in a controlled cannabis administration study following ad libitum inhalation over 10 min of 6.9% vaporized THC (54 mg) (Figures 1, 2 and Table 5). Four analytes were quantified in urine; THCVCOOH, THC-gluc, THCCOOH-gluc and THCCOOH. Concentrations ranged from <1–9.2 μg/L THCVCOOH, 8.6–65.8 μg/L THC-gluc, 655–1780 μg/L THCCOOH-gluc, and 5.3–217.7 μg/L THCCOOH (Table 5). Urine concentrations are not creatinine normalized. THC, 11-OH-THC, THCAA, THCV, CBD, CBG and CBN did not exceed LOQ in any urine specimen.
Table 5.
Cannabinoids concentrations in urine collected from a participant after vaporizing a single 54 mg THC dose ad libitum over 10 minutes. Urine concentrations are not creatinine normalized.
| Time after vaporized cannabis (h) | THCVCOOH (μg/L) | THC-gluc (μg/L) | THCCOOH-gluc (μg/L) | THCCOOH (μg/L) |
|---|---|---|---|---|
| −17.0 | 2.2 | 65.8 | 1069 | 51.4 |
| −11.4 | 1.9 | 14.7 | 1075 | 41.4 |
| −10.5 | 3.3 | 13.0 | 791 | 69.5 |
| −3.0 | 5.1 | 26.4 | 1780 | 91.8 |
| 7.7 | 1.0 | 8.6 | 716 | 14.5 |
| 9.5 | 1.6 | 12.8 | 921 | 22.4 |
| 11.8 | <LOQa | 14.0 | 969 | 11.3 |
| 20.0 | 6.4 | 19.0 | 956 | 111 |
| 26.8 | <LOQa | 17.7 | 1223 | 16.2 |
| 32.5 | <LOQa | 13.1 | 981 | 20.5 |
| 34.6 | <LOQa | 10.9 | 655 | 5.3 |
| 36.2 | <LOQa | 17.9 | 851 | 18.9 |
| 44.0 | 6.9 | 23.4 | 781 | 164 |
| 55.1 | <LOQa | 19.3 | 1083 | 10.0 |
| 58.6 | <LOQa | 17.4 | 1058 | 11.6 |
| 61.3 | 3.3 | 14.7 | 820 | 63.6 |
| 68.0 | 9.2 | 29.2 | 1543 | 218 |
THCVCOOH was less than limit of quantification (1.0 μg/L)
Discussion
We report a validated LC-MS/MS method for simultaneously quantifying eleven cannabinoids and metabolites in urine. This novel LC-MS/MS method employs an efficient and rapid disposable pipette tip extraction procedure without hydrolysis. The method was designed for analysis of urine specimens collected in a controlled cannabis administration study to investigate new urinary markers for recent cannabis intake.
Our laboratory’s previous urine method was updated with four new cannabinoids and metabolites, simplified sample preparation and a shorter run time [2]. To our knowledge, there is no method that simultaneously detects CBG, THCAA, THCV and THCVCOOH. The minor plant cannabinoid CBG was previously quantified in oral fluid [21] and identified in urine [22]. The THC precursor, THCAA, was evaluated as a marker for differentiating illicit cannabis intake from licit THC pharmacotherapy [23]. THCAA concentrations were monitored in plasma and whole blood [24], blood serum and urine [25] and oral fluid [26, 27]. THCAA also was demonstrated in hair after cannabis consumption, passive exposure and external contamination [28, 29]. The plant constituent THCV was recently administered in a controlled study investigating if THCV inhibits THC effects [30], as it was suggested that THCV is a receptor neutral antagonist [31]. THCV and its metabolite THCVCOOH also were targeted for distinguishing intake of illicit cannabis and Marinol, synthetic THC indicated for treatment of weight loss and nausea [32]. This new urine cannabinoid method would be highly useful for simultaneous quantification of all of these cannabinoid analytes in these different clinical applications, and for our goal of finding a urinary cannabinoid marker that may assist in identifying recent cannabis intake.
Disposable pipette tip extraction offers advantages over traditional solid phase or liquid-liquid extraction procedures. The WAX-S tip contains a small amount of loosely packed solid phase sorbent between two filters. This design decreases solvent consumption and minimizes required sample volume. Since the flow is bidirectional, sample and solvent easily flow in and out of the tip reducing the time required for extraction. Also, no conditioning or washing steps are required. DPX procedures were utilized for THC in whole blood and THCCOOH in urine [33, 34]. In only 200 μL urine, we simultaneously quantified 11 cannabinoids at low μg/L concentrations within clinically relevant linear ranges. The method was highly reproducible, with analytical bias <113.7% and imprecision <14.3% for low, mid and high QC concentrations for all analytes.
Ellison reported an extraction efficiency of 57.2% for THCCOOH [34]. In our previous published urine cannabinoid method using supporting-liquid extraction (SLE), extraction efficiency was 34–73% [2]. With WAX-S tips, we further improved extraction efficiency to 42.4–81.5% for our entire 11 cannabinoid analyte panel. The lowest extraction efficiency was for the new analyte THCAA. Despite this low recovery, we achieved the required sensitivity and met all LOQ requirements. In addition, our previous method contained only 4 deuterated internal standards, while d3-THCCOOH-gluc and d3-CBN are now incorporated, yielding a total of six deuterated internal standards for the 11 analytes (Table 2). Commercially available deuterated internal standards were not available for all analytes; deuterated internal standards were assigned based on similar retention time, recovery and matrix effect and their effect on calibrator and QC accuracy. Matrix effects ranged from −10% to +32%. High signal enhancement was observed for THC-gluc, but this analyte did not have a matched deuterated internal standard. d3-THCCOOH-gluc was selected as the internal standard.
Urinary cannabinoid stability is a well-known concern. Contributing factors for decreasing urine concentrations are oxidative degradation, fungal and bacteria growth and storage conditions such as tube selection, pH and temperature [1–3, 35]. All analytes were stable in urine, quantifying within ±20% of expected low and high QC concentrations, when stored refrigerated or frozen. At room temperature for 21 h, THCAA, THCV, CBD, CBN and THC concentrations decreased 42–63%; all other analytes were stable with concentrations within ±21.3% of expected concentrations (Table 4). Our stability results are consistent with studies employing authentic urine specimens showing frozen storage at −20°C is recommended for accurate analysis of urine specimens during urine cannabinoid testing [3].
Conclusion
We present a novel urine cannabinoid method for simultaneous quantification of a comprehensive panel of 11 cannabinoids. With disposable pipette tip extraction, rapid sample preparation achieved the required sensitivity and selectivity in a small 200 μL urine sample. This analytical method is utilized for quantifying cannabinoids in urine specimens obtained in our controlled cannabis administration study following smoked, inhaled and oral 6.9% THC. Prolonged cannabinoids excretion in urine of frequent cannabis users confounds distinguishing recent cannabis intake from prolonged excretion [11, 17, 18]. We will employ the current method during our current clinical study to test our hypothesis that monitoring unconjugated minor cannabinoids and/or their unconjugated metabolites may assist distinguishing recent intake from prolonged cannabinoids excretion. We desired to simultaneously monitor free and conjugated forms of all our analytes to maximize the utility of our method; however, only THC-gluc and THCCOOH-gluc standards were commercially available when we developed this method. This analytical method enables investigation of urine cannabinoid markers for documenting recent cannabis use, chronic frequent smoking or route of drug administration, and may potentially improve urine cannabinoid result interpretation.
Acknowledgments
This research was funded by the Intramural Research Program (IRP) of the National Institute on Drug Abuse, National Institutes of Health.
Abbreviations
- THC
Δ9-Tetrahydrocannabinol
- 11-OH-THC
11-hydroxy-THC
- THCCOOH
11-nor-9-carboxy-THC
- THCAA
Δ9-tetrahydrocannabinolic acid
- CBN
cannabinol
- CBD
cannabidiol
- CBG
cannabigerol
- THCV
Δ9-tetrahydrocannabivarin
- THCVCOOH
11-nor-9-carboxy-THCV
- THC-gluc
THC-glucuronide
- THCCOOH-gluc
THCCOOH-gluc
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Musshoff F, Madea B. Review of biologic matrices (urine, blood, hair) as indicators of recent or ongoing cannabis use. Ther Drug Monit. 2006;28(2):155–63. doi: 10.1097/01.ftd.0000197091.07807.22. [DOI] [PubMed] [Google Scholar]
- 2.Scheidweiler KB, Desrosiers NA, Huestis MA. Simultaneous quantification of free and glucuronidated cannabinoids in human urine by liquid chromatography tandem mass spectrometry. Clin Chim Acta. 2012;413(23–24):1839–47. doi: 10.1016/j.cca.2012.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Desrosiers NA, Lee D, Scheidweiler KB, Concheiro-Guisan M, Gorelick DA, Huestis MA. In vitro stability of free and glucuronidated cannabinoids in urine following controlled smoked cannabis. Anal Bioanal Chem. 2014;406(3):785–92. doi: 10.1007/s00216-013-7524-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United Nations Office on Drugs and Crime. [Accessed 17 Jun 2016];World Drug Report 2015. 2015 https://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf.
- 5.ElSohly MA, Mehmedic Z, Foster S, Gon C, Chandra S, Church JC. Changes in cannabis potency over the last 2 decades (1995–2014): analysis of current data in the United States. Biol Psychiatry. 2016;79(7):613–9. doi: 10.1016/j.biopsych.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cressey D. The cannabis experiment. Nature. 2015;524(7565):280–3. doi: 10.1038/524280a. [DOI] [PubMed] [Google Scholar]
- 7.European Monitoring Centre for Drugs and Drug Addiction. [Accessed 17 Jun 2016];EMCDDA highlights new health risks as products and patterns of use change. 2016 http://www.emcdda.europa.eu/news/2016/6/european-drug-report-2016-highlights.
- 8.Hartman RL, Huestis MA. Cannabis effects on driving skills. Clin Chem. 2013;59(3):478–92. doi: 10.1373/clinchem.2012.194381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheidweiler KB, Cone EJ, Moolchan ET, Huestis MA. Dose-related distribution of codeine, cocaine, and metabolites into human hair following controlled oral codeine and subcutaneous cocaine administration. J Pharmacol Exp Ther. 2005;313(2):909–15. doi: 10.1124/jpet.104.082388. [DOI] [PubMed] [Google Scholar]
- 10.Polettini A, Cone EJ, Gorelick DA, Huestis MA. Incorporation of methamphetamine and amphetamine in human hair following controlled oral methamphetamine administration. Anal Chim Acta. 2012;726:35–43. doi: 10.1016/j.aca.2012.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwilke EW, Gullberg RG, Darwin WD, Chiang CN, Cadet JL, Gorelick DA, et al. Differentiating new cannabis use from residual urinary cannabinoid excretion in chronic, daily cannabis users. Addiction. 2011;106(3):499–506. doi: 10.1111/j.1360-0443.2010.03228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meyer MR. Trends in analyzing emerging drugs of abuse--from seized samples to body samples. Anal Bioanal Chem. 2014;406(25):6105–10. doi: 10.1007/s00216-014-8082-3. [DOI] [PubMed] [Google Scholar]
- 13.Reisfield GM, Wasan AD, Jamison RN. The prevalence and significance of cannabis use in patients prescribed chronic opioid therapy: a review of the extant literature. Pain Med. 2009;10(8):1434–41. doi: 10.1111/j.1526-4637.2009.00726.x. [DOI] [PubMed] [Google Scholar]
- 14.Macdonald S, Hall W, Roman P, Stockwell T, Coghlan M, Nesvaag S. Testing for cannabis in the work-place: a review of the evidence. Addiction. 2010;105(3):408–16. doi: 10.1111/j.1360-0443.2009.02808.x. [DOI] [PubMed] [Google Scholar]
- 15.Dams R, Huestis MA, Lambert WE, Murphy CM. Matrix effect in bio-analysis of illicit drugs with LC-MS/MS: influence of ionization type, sample preparation, and biofluid. J Am Soc Mass Spectrom. 2003;14(11):1290–4. doi: 10.1016/S1044-0305(03)00574-9. [DOI] [PubMed] [Google Scholar]
- 16.Mazur A, Lichti CF, Prather PL, Zielinska AK, Bratton SM, Gallus-Zawada A, et al. Characterization of human hepatic and extrahepatic UDP-glucuronosyltransferase enzymes involved in the metabolism of classic cannabinoids. Drug Metab Dispos. 2009;37(7):1496–504. doi: 10.1124/dmd.109.026898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lowe RH, Abraham TT, Darwin WD, Herning R, Cadet JL, Huestis MA. Extended urinary Delta9-tetrahydrocannabinol excretion in chronic cannabis users precludes use as a biomarker of new drug exposure. Drug Alcohol Depend. 2009;105(1–2):24–32. doi: 10.1016/j.drugalcdep.2009.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goodwin RS, Darwin WD, Chiang CN, Shih M, Li SH, Huestis MA. Urinary elimination of 11-nor-9-carboxy-delta9-tetrahydrocannnabinol in cannabis users during continuously monitored abstinence. J Anal Toxicol. 2008;32(8):562–9. doi: 10.1093/jat/32.8.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scientific Working Group for Forensic T. Scientific Working Group for Forensic Toxicology (SWGTOX) standard practices for method validation in forensic toxicology. J Anal Toxicol. 2013;37(7):452–74. doi: 10.1093/jat/bkt054. [DOI] [PubMed] [Google Scholar]
- 20.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75(13):3019–30. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 21.Desrosiers NA, Scheidweiler KB, Huestis MA. Quantification of six cannabinoids and metabolites in oral fluid by liquid chromatography-tandem mass spectrometry. Drug Test Anal. 2015;7(8):684–94. doi: 10.1002/dta.1753. [DOI] [PubMed] [Google Scholar]
- 22.Hidvegi E, Somogyi GP. Detection of cannabigerol and its presumptive metabolite in human urine after Cannabis consumption. Pharmazie. 2010;65(6):408–11. [PubMed] [Google Scholar]
- 23.Radunz L, Westphal F, Maser E, Rochholz G. THCVA-A - a new additional marker for illegal cannabis consumption. Forensic Sci Int. 2012;215(1–3):171–4. doi: 10.1016/j.forsciint.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Raikos N, Schmid H, Nussbaumer S, Ambach L, Lanz S, Langin A, et al. Determination of Delta9-tetrahydrocannabinolic acid A (Delta9-THCA-A) in whole blood and plasma by LC-MS/MS and application in authentic samples from drivers suspected of driving under the influence of cannabis. Forensic Sci Int. 2014;243:130–6. doi: 10.1016/j.forsciint.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 25.Jung J, Kempf J, Mahler H, Weinmann W. Detection of Delta9-tetrahydrocannabinolic acid A in human urine and blood serum by LC-MS/MS. J Mass Spectrom. 2007;42(3):354–60. doi: 10.1002/jms.1167. [DOI] [PubMed] [Google Scholar]
- 26.Moore C, Rana S, Coulter C. Simultaneous identification of 2-carboxy-tetrahydrocannabinol, tetrahydrocannabinol, cannabinol and cannabidiol in oral fluid. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;852(1–2):459–64. doi: 10.1016/j.jchromb.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 27.Fabritius M, Chtioui H, Battistella G, Annoni JM, Dao K, Favrat B, et al. Comparison of cannabinoid concentrations in oral fluid and whole blood between occasional and regular cannabis smokers prior to and after smoking a cannabis joint. Anal Bioanal Chem. 2013;405(30):9791–803. doi: 10.1007/s00216-013-7412-1. [DOI] [PubMed] [Google Scholar]
- 28.Moosmann B, Roth N, Auwarter V. Hair analysis for THCA-A, THC and CBN after passive in vivo exposure to marijuana smoke. Drug Test Anal. 2014;6(1–2):119–25. doi: 10.1002/dta.1474. [DOI] [PubMed] [Google Scholar]
- 29.Moosmann B, Roth N, Auwarter V. Hair analysis for Delta(9) -tetrahydrocannabinolic acid A (THCA-A) and Delta(9) -tetrahydrocannabinol (THC) after handling cannabis plant material. Drug Test Anal. 2016;8(1):128–32. doi: 10.1002/dta.1830. [DOI] [PubMed] [Google Scholar]
- 30.Englund A, Atakan Z, Kralj A, Tunstall N, Murray R, Morrison P. The effect of five day dosing with THCV on THC-induced cognitive, psychological and physiological effects in healthy male human volunteers: A placebo-controlled, double-blind, crossover pilot trial. J Psychopharmacol. 2016;30(2):140–51. doi: 10.1177/0269881115615104. [DOI] [PubMed] [Google Scholar]
- 31.Wargent ET, Zaibi MS, Silvestri C, Hislop DC, Stocker CJ, Stott CG, et al. The cannabinoid Delta(9)-tetrahydrocannabivarin (THCV) ameliorates insulin sensitivity in two mouse models of obesity. Nutr Diabetes. 2013;3:e68. doi: 10.1038/nutd.2013.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.ElSohly MA, deWit H, Wachtel SR, Feng S, Murphy TP. Delta9-tetrahydrocannabivarin as a marker for the ingestion of marijuana versus Marinol: results of a clinical study. J Anal Toxicol. 2001;25(7):565–71. doi: 10.1093/jat/25.7.565. [DOI] [PubMed] [Google Scholar]
- 33.Schroeder JL, Marinetti LJ, Smith RK, Brewer WE, Clelland BL, Morgan SL. The analysis of delta9-tetrahydrocannabinol and metabolite in whole blood and 11-nor-delta9-tetrahydrocannabinol-9-carboxylic acid in urine using disposable pipette extraction with confirmation and quantification by gas chromatography-mass spectrometry. J Anal Toxicol. 2008;32(8):659–66. doi: 10.1093/jat/32.8.659. [DOI] [PubMed] [Google Scholar]
- 34.Ellison ST, Brewer WE, Morgan SL. Comprehensive analysis of drugs of abuse in urine using disposable pipette extraction. J Anal Toxicol. 2009;33(7):356–65. doi: 10.1093/jat/33.7.356. [DOI] [PubMed] [Google Scholar]
- 35.Wei B, Wang L, Blount BC. Analysis of cannabinoids and their metabolites in human urine. Anal Chem. 2015;87(20):10183–7. doi: 10.1021/acs.analchem.5b02603. [DOI] [PMC free article] [PubMed] [Google Scholar]