Abstract
Cancer cells have a proliferative advantage by utilizing intermediates of aerobic glycolysis (Warburg-effect) for their macromolecule synthesis. Although the exact causes of this Warburg-effect are unclear, high osmotic stress in solid tumor microenvironment is considered as one of the important factors. Oleanolic acid (OA) is known to exert anti-inflammatory and anti-cancer effect. In our current studies, using breast cancer cell lines, we determined the protective role of OA in high salt mediated osmotic stress induced cancer growth. Hypertonic (0.16M NaCl) culture conditions enhanced the cancer cell growth (26%, p<0.05) and aerobic glycolysis as marked by increased glucose consumption (34%, p<0.05) and lactate production (25%, p<0.05) over untreated cells. This effect was associated with increased expression and activity of key rate-limiting enzymes of aerobic glycolysis, namely, hexokinase, pyruvate kinase-typeM2 and lactate dehydrogenase-A. Interestingly, this high salt mediated enhanced expression of aerobic glycolytic enzymes was efficiently reversed by OA along with decreased cancer cell proliferation. In cancer cells, enhanced aerobic glycolysis is associated with decreased mitochondrial activity and mitochondrial-associated caspase activity. As expected, high salt further inhibited the mitochondrial related cytochrome oxidase and caspase-3 activity. However, OA efficiently reversed the high salt mediated inhibition of cytochrome oxidase, caspase activity and pro-apoptotic Bax expression, thus suggesting that OA induced mitochondrial activity and enhanced apoptosis. Taken together, our data indicate that OA efficiently reverses the enhanced Warburg-like metabolism induced by high salt mediated osmotic stress along with potential application of OA in anti-cancer therapy.
Keywords: Oleanolic acid, Breast cancer, Warburg-effect, Glycolysis, Osmotic Stress, Apoptosis
Introduction
An altered metabolism in tumor cell relative to normal cell is considered a generally accepted hallmark of cancer [1]. The original pioneering studies by Otto Warburg in early 1920s and subsequent research for 100 years have demonstrated that, under aerobic conditions, while normal cells completely metabolize glucose to carbon dioxide, in contrast, rapidly dividing cancer cells under similar conditions metabolize glucose incompletely to lactate, despite this process being least efficient in terms of net ATP production [2]. This unique phenomenon was eventually termed as the Warburg-effect or aerobic glycolysis. The altered metabolism has been considered as a consequence of cell proliferation. Further, several of these glycolytic intermediates are important for the synthesis of macromolecules such as lipids, nucleotides amino acids etc, and thus arguing that aerobic glycolysis provides raw materials for cell proliferation [3]. The depletion of metabolic bi-products of aerobic glycolysis for synthesis o cell proliferation machinery also leads to subsequent allosteric effects on rate-limiting glycolytic enzymes [4]. Recent evidence suggests that this Warburg-effect is not limited to cancer cells but is also observed in immune cell activation in lymph nodes [5].
Although the exact causes leading to Warburg-effect are still unclear, inflammatory signaling has been shown to induce this phenomenon [6]. Importantly, chronic inflammation is also a well-known cause for cancer development [7]. Traditionally, high salt (sodium chloride) has been thought to induce inflammatory response through oxidative and osmotic stress, a key feature well-recognized in cardiovascular diseases [8]. Recent epidemiological studies demonstrate that high salt is correlated with increased incidence of cancer [9]. However, a direct role of high salt in cancer cell activation and proliferation is not well-established. Interestingly, studies by Wu et al [10] have demonstrated that high sodium chloride in the culture conditions induced a direct CD4+T cell activation to inflammatory Th17 phenotype, suggesting a direct inflammatory effect of salt. Importantly, an equi-molar mannitol in their studies did not yield similar CD4+T cell activation, thus precluding a potential osmotic stress mechanism but rather a direct salt-induced pro-inflammatory signaling mechanism towards activation of T-cells. As the micro-environment conditions in lymph nodes and solid tumors have similarity with respect lower pH and increased osmolality [11]. However, it is unclear whether this unique micro-environment is a downstream consequence of higher cellular activity in solid tumors and lymph nodes, or if it is an upstream cause leading to higher cellular activity. In our current communication, under in vitro conditions, we studied a direct role of high salt (NaCl) on the Warburg- metabolism in breast cancer cells. Further, we have analyzed the potential protective effect of oleanolic acid (OA) a natural plant triterpenoid small molecule with known anti-inflammatory effect [12] towards abrogation of salt-induced damage on cancer cells.
Materials and Methods
Cell cultures
The highly metastatic breast cancer cell line, MDA-MB-231 (HTB-26™), and normalized breast epithelial cell line MCF10A (CRL-10317™) were obtained from the American Type Culture Collection (Manassas, VA) and cultured in RPMI-1640 along with the media supplements at 37 °C in 5% CO2 incubator as recommended by the manufacturer. In all our cell cultures, media were supplemented with TNFα (50ng/mL) and TGFβ (50ng/mL) to promote active and robust cell growth [13]. For drug treatment studies, oleanolic acid (5 μM) in DMSO was used. For high salt studies, sodium chloride (NaCl, Sigma Aldrich, St Louis, MO) was supplemented (0 to 0.16 M) to RPMI-1640 media. The NaCl concentration RPMI-1640 is 0.1 M and thus following NaCl supplementation the final NaCl concentration in culture media was 0.1 to 0.26 M.
MTT assay
The cell viability was assessed by measuring MTT (4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide) assay as previously described [14]. Briefly, following various cell culture conditions, the cells were incubated with 5mg/ml MTT in PBS for 2 hrs and latter lysed with lysis buffer. Detection at 570 nm was performed using EMax Plus spectrophotometer and data analysis was carried out using software provided by the manufacturer (Molecular Devices, Sunnyvale, CA). Viability was calculated as percentage compared to vehicle-control treated cells.
Glucose consumption assay
To measure the glucose uptake was performed using the glucose uptake kit (Cayman Chemicals, Ann Arbor, MI) as per manufacturer's protocol. Briefly, cells were seeded at a density of 5000 cells/well in 96-well plates. Under various experimental conditions, cells were cultured along with 2-NBDG, a fluorescent analog of 2-deoxy glucose. The quantity of glucose consumption of the tested cancer cells was detected by fluorimetric analysis (485/535 nm) according to the manufacturer's instructions using Spectra Max Plus (Molecular Devices, Sunnyvale, CA).
Lactate assay
The production of lactic acid was determined using lactate colorimetric Assay Kit (Sigma Aldrich, St Louis, MO) as the manufacturer's protocols. Briefly, following cell culture and centrifuge isolation cells were mixed with 45 ml of Lactate Assay Buffer. The absorbance at 570 nm was read using a EMax Plus spectrophotometer (Molecular Devices, Sunnyvale, CA) for quantitative analysis.
Western blot
Western blot analysis was performed as described previously [15] on total protein extracted from cell lysates following various experimental condition. All primary and secondary Abs were obtained from Santa Cruz Biotech (Dallas, TX) unless mentioned otherwise. The following specific primary antibodies to hexokinase (~100kDa,sc-374091), pyruvate kinase-M1 (PKM1~60kDa,#7067, Cell Signaling Technology, Danvers,MA), pyruvate kinase-M2 (PKM2~60kDa,#4053, Cell Signaling Technology, Danvers,MA), lactate dehydrogenase-A (LDH-A~51kDa, sc-130327), Peroxisome proliferator-activated receptor gamma coactivator 1-alpha PGC1 (~kDa,sc-13067), Cytochrome-C (~49kDa,sc-514435), Bcl-2 (~29kDa,sc-509), Bax (~23kDa,sc-20067) and β-actin (42kDa, sc-10731) were utilized. The nitrocellulose membranes probed by appropriate secondary antibodies were developed using the chemiluminescence kit (Millipore) and analyzed on using Bio-Rad Universal Hood II (Hercules, CA). Densitometric analysis was done using the software provided by the company.
Pyruvate kinase activity
The pyruvate kinase activity was measured using the PK enzyme activity assay kit (Sigma Aldrich, St Louis, MO) as per manufacturer's protocol. Briefly, cell extracts were prepared and assayed for PK activity using buffers provided by manufacturer. The absorbance at 570 nm was read using a EMax Plus spectrophotometer (Molecular Devices, Sunnyvale, CA). The protein activity was measured using a standard curve developed using the standards provided by manufacturer and activity quantitative expressed as units/mg protein.
Cytochrome activity
Cytochrome C oxidase assay was assessed using the appropriate colorimetric assay kit (BioVision, Inc, Milpitas, CA) performed as per manufacturer's protocol. The absorbance at 550 nm was read using a EMax Plus spectrophotometer (Molecular Devices, Sunnyvale, CA) for quantitative analysis.
Caspase activity
Caspase3 activity was assessed using the appropriate fluorimetric assay kit (Cell Signaling technologies, Danvers, MA) performed as per manufacturer's protocol. The fluorescence measured at 380/460 and was read using Spectra Max Plus (Molecular Devices, Sunnyvale, CA).
Annexin-V flow cytometry
Apoptosis was analyzed using AlexaFluo-488 conjugated-Annexin-V by flow cytometry [16]. The labeling was performed in 1:20 dilution to a 200 μL final volume of cells (1 × 106 cells/mL). The samples were latter analyzed using a FACS Calibur/LSRII flow cytometer (Becton-Dickinson, Franklin Lakes, NJ). Data were analyzed using company provided software. Gates were set according to isotype controls.
Statistical Analysis
Data are expressed as mean ± standard deviation. Statistical differences between means were analyzed using a paired or unpaired Student's t test, or subjected to analysis of variance and post hoc test. A value of P less than 0.05 was considered significant.
Results
Oleanolic Acid reverses high salt mediated enhanced glucose consumption and lactate production in cancer cells
Due to aerobic glycolysis, cancer cells are characterized by increased glucose uptake and lactate production. As inflammatory and osmotic stress is known to upregulate Warburg-effect [17], therefore, we measured the protective effect of oleanolic acid towards reversing the Warburg-glycolysis following high salt mediated osmotic stress. Dose-dependent studies with varying sodium chloride (NaCl - 0.1 to 0.26 M) on breast cancer cell line (MDA-MD-231) and normalized breast epithelial cell line (MCF10A) demonstrated an enhanced (figure 1A-F) cell growth and proliferation at 0.16 M NaCl for 12 - 96 hour time period. Further, treatment with oleanolic acid (OA 5 μM) at 0.16 M NaCl induced a 35% decrease in proliferation on MDA-MB-231 breast cancer cells, while only 20% decrease in proliferation in MCF10A (normalized) breast epithelial cells. Previous studies from other laboratories have demonstrated that OA reduces the Warburg effect in breast cancer cells [18]. In our current study we have further explored the role of oleanolic acid in high salt induced osmotic stress leading to Warburg-like glycolysis effect. Warburg-like aerobic glycolysis in cancer cells leads to enhanced glucose consumption and lactate production. Analysis of glucose consumption by 2-NBDG uptake assay [19] in MDA-MB-231 breast cancer cells (figure 1G) demonstrated enhanced uptake following 0.16 M NaCl treatment (4290±450 RFU) over DMSO treated negative control cells (3180±390 RFU), which was significantly (p<0.05) reduced following treatment with OA (2230±280 RFU). Furthermore, direct analysis of Warburg-like effect for lactate production in MDA-MB-231 breast cancer cells demonstrated that there was a 25.1% increase in lactate production following high salt treatment (as compared to DMSO treated negative control), while there was a 45.4% decrease in lactate production following OA treatment in high-salt induced breast cancer cells. Although a similar trend, a lowered effect on reversal of aerobic glycolysis following OA treatment was observed in normalized MCF10A breast epithelial cells compared to MDA-MB-231 cells. These data suggest that high-salt mediated osmotic stress exaggerated the Warburg-like aerobic glycolysis effect on breast cancer cells which was efficiently reversed by oleanolic acid.
Figure 1. Oleanolic acid suppresses high salt induced aerobic glycolysis in breast cancer cells.
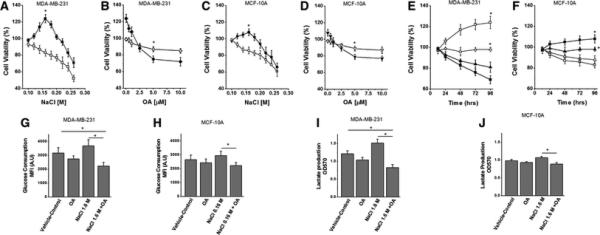
(A) Dose titration of salt (NaCl - 0.1 to 0.26 M) on MDA-MB-231, highly invasive epithelial breast cancer cell line; (■) without oleanolic acid (5 μM), (□) with oleanolic acid (5 μM); (*) statistical significance at 0.16 M NaCl used in further experiments. (B) Dose titration of oleanolic acid (OA – 0 to 10 μM) on MDA-MB-231, highly invasive epithelial breast cancer cell line; (◇) with regular NaCl (0.1 M), (◆) with high NaCl (0.16 M); (*) statistical significance at 5 μM used in further experiments. (C) Dose titration of salt (NaCl - 0.1 to 0.26 M) on MCF10A, normalized epithelial breast cancer cell line; (●) without oleanolic acid (5 μM), (○) with oleanolic acid (5 μM); (*) statistical significance at 0.16 M NaCl used in further experiments. (D) Dose titration of oleanolic acid (OA – 0 to 10 μM) on MCF10A, highly invasive epithelial breast cancer cell line; (unfilled▼) with regular NaCl (0.1 M), (filled▼) with high NaCl (0.16 M); (*) statistical significance at 5 μM used in further experiments. Cell viability analysis using MTT assay for 12-96 hours on MDA-MB-231 (E) and MCF10A (F) cell lines; (Δ) with vehicle-control (negative control) DMSO treatment; (▲) with OA (5 μM); (□) with 0.16M NaCl; (■)with 0.16M NaCl+OA (5 μM). (G-H)Glucose consumption assay in MDA-MB-231(G) and MCF10A (H) cell lines under various culture conditions. (G-H) Lactate production analysis in MDA-MB-231(I) and MCF10A (J) cell lines under various culture conditions. All data represented as mean values ± SEM from four independent experiments. (*) p-value <0.05.
Oleanolic acid inhibits high salt induced glycolytic enzymes
Enhanced expression of rate-limiting glycolytic enzymes Hexokinase, lactate dehydrogenase (LDH) and pyruvate kinase (PK) have been shown to play an important role in drug-resistance following breast cancer chemotherapy [20]. Therefore, we have determined the effect of high salt mediated osmotic stress on expression of key glycolytic enzymes and potential protective effect of oleanolic acid under the above conditions. As shown in figure 2A, 0.1 M NaCl induced significant over expression of the enzymes hexokinase, PKM2 and LDHA in MDA-MB-231 breast cancer cells which was efficiently reversed by OA. Further, analysis of PK activity by specific enzyme activity assay demonstrated a 58.7% decreased PK activity following OA treatment in high-salt mediated osmotic stress induced breast cancer cells. While similar pattern was observed in MCF10A normalized breast epithelial cells, the expression patterns were not statistically significant. These data indicate that high salt mediated stress induces over-expression of pro-cancerous glycolytic enzymes, which was significantly inhibited by natural plant triterpenoid, oleanolic acid, suggesting its protective effect on breast cancer cells.
Figure 2. Oleanolic acid suppresses rate-limiting enzymes in aerobic glycolysis.
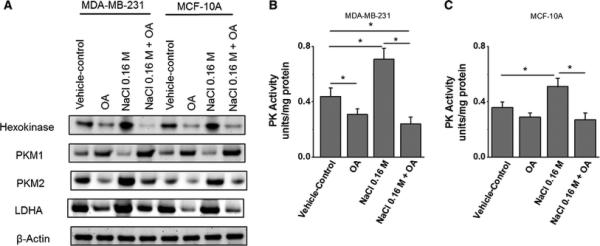
(A) Western blot analysis of the expression of key rate-limiting enzymes in aerobic glycolysis, hexokinase, PKM1, PKM2, LDH-A following high salt (0.16M NaCl) and OA (5 μM) in MDA-MB-231 and MCF10A cell lines. As it can be noted PKM1 and PKM2 demonstrated an opposite expression pattern and β-actin is used as loading control. Quantitative densitometric analysis (not shown) was performed for data-analysis. (B-C) Pyruvate kinase (PK) enzymatic activity is MDA-MB-231 (B) and MCF10A (C) cell lines following various culture conditions. Enzyme activity presented as units per mg total protein. All data represented as mean values ± SEM from four independent experiments. (*) p-value <0.05.
Oleanolic acid exerts protective effect by inducing mitochondrial activity following high salt stimulation
Stress induced Warburg-like metabolic mechanisms have been shown to down-regulate the mitochondrial activity. Peroxisome proliferator-activated receptor gamma co-activator-1α (PGC1) is a critical regulator of mitochondrial biosynthesis and cytochrome C (CytC) is a crucial component of electron transport chain on mitochondrial membrane [21]. We have tested PGC1 and CytC expression and activity to determine the interplay of high salt and oleanolic acid on mitochondria-mediated metabolic activity. As shown in figure 3A, 0.16 M NaCl inhibited expression of PGC1 (2.1 fold) and CytC (1.8 fold) expression in MDA-MB-231 breast cancer cells. Further, treatment of OA enhanced the expression of PGC1 and CytC in high-salt mediated stress cells, thus suggesting the potential cancer cell death induction by OA. Cytochrome C is the only water soluble component of electron transport chain and the release of Cytochrome C is known to activate caspase pathway which is involved in cell death phenomenon. Analysis of cytochrome and caspase activity (figure 3B-C) demonstrated that high salt treatment reduced both cytochrome and caspase activity in MDA-MB-231 and MCF-10A cells, which was significantly reversed by oleanolic acid treatment. These data strongly indicate that oleanolic acid can induce cell death in cancer cells and thus may have potential anti-cancer effect.
Figure 3. Oleanolic acid induces mitochondrial activity in breast cancer cells.
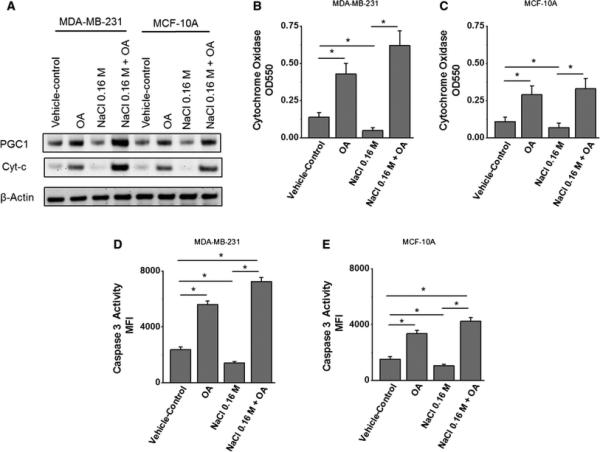
(A) Western blot analysis of the expression of key mitochondrial activity associated enzymes, peroxisome proliferator-activated receptor gamma co-activator-1α PGC1 and cytochrome-C (CytC) following high salt (0.16M NaCl) and OA (5 μM) in MDA-MB-231 and MCF10A cell lines. (B-C) Colorimetric-based cytochrome oxidase enzymatic activity is MDA-MB-231 (B) and MCF10A (C) cell lines following various culture conditions. (D-E) Fluorimetric-based cytochrome oxidase enzymatic activity is MDA-MB-231 (D) and MCF10A (E) cell lines following various culture conditions. All data represented as mean values ± SEM from four independent experiments. (*) p-value <0.05.
Oleanolic acid induces apoptosis and protection from cancer growth
As our previous studies (figure 3) have demonstrated enhanced caspase activity following OA treatment to high salt co-treated cells, we studied for the potential apoptotic effect of OA in these conditions. To specifically study the apoptotic effect of OA, we analyzed for the expression of anti-apoptotic protein, bcl-2, and pro-apoptotic Bax protein [22]. As shown in figure 4A, high salt treatment to MDA-MB-231 breast cancer cells induced expression of anti-apoptotic Bcl-2, while there was minimal change in expression of pro-apoptotic Bax. This effect was reversed by OA which enhanced expression of pro-apoptotic Bax protein and thus altering the Bcl-2/Bax balance. Annexin-V a known binding molecule with phosphotidyl-serine is used as an early marker for apoptosis. Flow cytometry analysis (figure 4B-C) demonstrated reduced annexin-V binding when MDA-MB-231 breast cancer cells were treated with high salt as demonstrated by annexin-V (an apoptosis marker) flow cytometry with an increase in annexin-V staining from 5.3±2.2% (under high salt treatment) to 46.4±7.1% following oleanolic acid treatment under similar high salt condition on MDA-MB-231 breast cancer cell line. Taken together, these data suggest that oleanolic acid exerts protective effect by upregulating the apoptosis in breast cancer cells.
Figure 4. Oleanolic acid induces apoptosis in breast cancer cells.
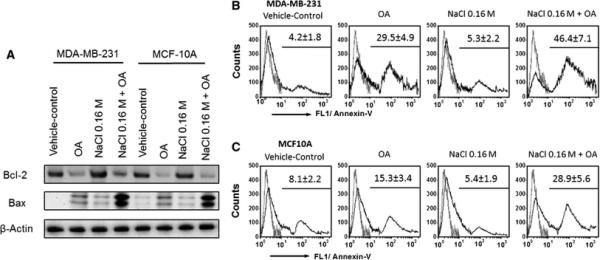
(A) Western blot analysis of the expression of key apoptosis associated enzymes, Bcl-2 (anti-apoptosis) and Bax (proapoptosis) following high salt (0.16M NaCl) and OA (5 μM) in MDA-MB-231 and MCF10A cell lines. (B-C) Flow cytometry based annexin-V binding assay to determine apoptosis in MDA-MB-231 (B) and MCF10(C) cell lines following various culture conditions. All data represented as mean values ± SEM from four independent experiments. (*) p-value <0.05.
Discussion
Oleanolic acid (OA), a pentacyclic triterpenoid, is a natural plant compound documented to possess a wide variety of bioactivities including anti-inflammatory, hepato- and nephrotoxicity protection, and antitumor property [23]. However, the exact effect of OA on the metabolic pathway in cancer cells under various oxidative and inflammatory stress conditions are unclear. Recent studies by Liu et al [18] have suggested that OA suppresses Warburg effect in cancer cells. In our current communication, we further support these finding by providing evidence the OA exerts protective effect from high-salt mediated osmotic stress on breast cancer cells.
Osmotic stress is known to trigger cancer by its ability to induce proliferation through growth factor signaling [24]. Osmotic stress as induced by hyertonic saline and mannitol has shown to induce glucose transport and lactic acidosis suggesting their role in inducing Warburg effect in cancer cells [25]. In line with this evidence, our studies with high salt in the external environment as induced Warburg-like effect of increase glucose consumption and lactic acid production (figure 1) specifically in breast cancer cell line and not in normalized breast epithelial cells. In normal cells, the metabolism of glucose to lactate (by aerobic glycolysis) generates only 2 ATPs per molecule of glucose, and oxidative phosphorylation generates up to 36 ATPs upon complete oxidation of one glucose molecule [2]. However, rapidly proliferating cells such as cancers switch to aerobic glycolysis as these cell derive important metabolic requirements such as synthesis of fatty acids, amino acid and nucleic acids from intermediates in glycolysis.
Understanding of this aberrant phenomenon of aerobic glycolysis in cancer cells has led to novel tumor therapeutic strategies targeted at switching back to mitochondrial oxidative phosphorylation and there by leading to cancer cell death [26]. Several compounds have been reported to suppress the growth of tumor cells by affecting aerobic glycolysis [26]. Three key rate limiting enzymes in aerobic glycolysis are: hexokinase, pyruvate kinase (PK) and lactate dehydrogenase (LDH) [20]. Interestingly, hexokinase is thought to bind to VDAC site and prevent activation of pro-apoptotic Bax [27]. In our current studies high salt in the external culture media induced the expression and activity of these rate-limiting enzymes (figure 2). Importantly, OA has efficiently decreased the expression of these three aerobic glycolysis associated enzymes thus strongly suggesting a protective role of this compound in cancers. Previously a specific small molecule inhibitor of hexokinase, 2-deoxyglucose (2-DG) has demonstrated promising chemotherapeutic molecule in combination radiation therapy to induce DNA based anti-tumor treatment approach [28]. Similar combination therapeutic strategies with specific inhibition of PK and LDH are under various phases of clinical trials. As our studies demonstrate that OA exerts down-regulation of these three enzymes, this compound has a promising potential in cancer therapy.
Several studies have confirmed that osmotic stress down-regulates apoptosis while inhibition of aerobic glycolysis upregulates apoptosis [29]. In line with these studies, our data confirm that OA inhibits osmotic stress mediated upregulation of anti-apoptosis to induce cell death. Recent studies in hematological cancers have demonstrated that aerobic glycolysis suppresses p53 activity to induce protection from apoptosis [30]. Further as mitochondrial activity is down-regulated under aerobic glycolysis conditions it is suggested that mitochondrial associated Bcl-2 apoptotic regulators and caspase proteins are not released to induce cell death. Our studies demonstrate that high salt which induces aerobic glycolysis has down-regulated mitochondrial activity associated caspases and pro-apoptotic proteins. While OA reversed this effect by enhancing the mitochondrial activity and also efficiently inducing release of mitochondrial associated caspases and pro-apoptotic Bax proteins and potentially leading to cell death and thereby protection from cancer. However, our current study is limited to high salt induced osmotic stress, while other non-ionic osmotic stress inducers such as mannitol need further study.
In conclusion, our results indicate that high salt mediated osmotic stress induces Warburg-like effect which is more pronounced in breast cancer cells over normal cells. OA reverses this aerobic glycolysis by inducing mitochondrial activity and apoptosis there by leading to cell death. We think that OA, a natural plant compound, offers as a novel therapeutic agent as potential future combinatorial agent with current cancer chemo- and radiation therapy to further enhance the efficiency of anti-cancer treatment.
Acknowledgement
This work was supported by NIH-5U54CA163066. The authors thank Department of Biological Sciences, Tennessee State University for the kind support on this project.
Footnotes
Conflict of Interest: All authors have no conflict of interest.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Maldonado EN, Lemasters JJ. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion. 2014;19(Pt A):78–84. doi: 10.1016/j.mito.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324(5930):1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lincet H, Icard P. How do glycolytic enzymes favour cancer cell proliferation by nonmetabolic functions? Oncogene. 2015;34(29):3751–3759. doi: 10.1038/onc.2014.320. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R. Bringing Warburg to lymphocytes. Nat Rev Immunol. 2015;15(10):598. doi: 10.1038/nri3918. [DOI] [PubMed] [Google Scholar]
- 6.Wen H, Ting JP, O'Neill LA. A role for the NLRP3 inflammasome in metabolic diseases--did Warburg miss inflammation? Nat Immunol. 2012;13(4):352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colotta F, Allavena P, Sica A, et al. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30(7):1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 8.Azak A, Huddam B, Gonen N, et al. Salt intake is associated with inflammation in chronic heart failure. Int Cardiovasc Res J. 2014;8(3):89–93. [PMC free article] [PubMed] [Google Scholar]
- 9.Tsugane S, Sasazuki S, Kobayashi M, et al. Salt and salted food intake and subsequent risk of gastric cancer among middle-aged Japanese men and women. Br J Cancer. 2004;90(1):128–134. doi: 10.1038/sj.bjc.6601511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C, Yosef N, Thalhamer T, et al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature. 2013;496(7446):513–517. doi: 10.1038/nature11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng GM, To KK. Adverse Cell Culture Conditions Mimicking the Tumor Microenvironment Upregulate ABCG2 to Mediate Multidrug Resistance and a More Malignant Phenotype. 2012 doi: 10.5402/2012/746025. ISRN Oncol, 2012(746025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee W, Yang EJ, Ku SK, et al. Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo. Inflammation. 2013;36(1):94–102. doi: 10.1007/s10753-012-9523-9. [DOI] [PubMed] [Google Scholar]
- 13.Platt D, Amara S, Mehta T, et al. Violacein inhibits matrix metalloproteinase mediated CXCR4 expression: potential anti-tumor effect in cancer invasion and metastasis. Biochem Biophys Res Commun. 2014;455(1-2):107–112. doi: 10.1016/j.bbrc.2014.10.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiriveedhi V, Banan B, Deepti S, et al. Role of defensins in the pathogenesis of chronic lung allograft rejection. Hum Immunol. 2014;75(4):370–377. doi: 10.1016/j.humimm.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tiriveedhi V, Gelman AE, Mohanakumar T. HIF-1alpha signaling by airway epithelial cell K-alpha1-tubulin: role in fibrosis and chronic rejection of human lung allografts. Cell Immunol. 2012;273(1):59–66. doi: 10.1016/j.cellimm.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiriveedhi V, Takenaka M, Ramachandran S, et al. T regulatory cells play a significant role in modulating MHC class I antibody-induced obliterative airway disease. Am J Transplant. 2012;12(10):2663–2674. doi: 10.1111/j.1600-6143.2012.04191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radmaneshfar E, Kaloriti D, Gustin MC, et al. From START to FINISH: the influence of osmotic stress on the cell cycle. PLoS One. 2013;8(7):e68067. doi: 10.1371/journal.pone.0068067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu J, Wu N, Ma L, et al. Oleanolic acid suppresses aerobic glycolysis in cancer cells by switching pyruvate kinase type M isoforms. PLoS One. 2014;9(3):e91606. doi: 10.1371/journal.pone.0091606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang TB, Zhao Y, Tong ZX, et al. Inhibition of glucose-transporter 1 (GLUT-1) expression reversed Warburg effect in gastric cancer cell MKN45. Int J Clin Exp Med. 2015;8(2):2423–2428. [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4:e532. doi: 10.1038/cddis.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaughan RA, Garcia-Smith R, Dorsey J, et al. Tumor necrosis factor alpha induces Warburg-like metabolism and is reversed by anti-inflammatory curcumin in breast epithelial cells. Int J Cancer. 2013;133(10):2504–2510. doi: 10.1002/ijc.28264. [DOI] [PubMed] [Google Scholar]
- 22.Arora R, Schmitt D, Karanam B, et al. Inhibition of the Warburg effect with a natural compound reveals a novel measurement for determining the metastatic potential of breast cancers. Oncotarget. 2015;6(2):662–678. doi: 10.18632/oncotarget.2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ndlovu BC, Daniels WM, Mabandla MV. Oleanolic Acid enhances the beneficial effects of preconditioning on PC12 cells. Parkinsons Dis. 2014;2014:929854. doi: 10.1155/2014/929854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer OM, Hart S, Gschwind A, et al. Oxidative and osmotic stress signaling in tumor cells is mediated by ADAM proteases and heparin-binding epidermal growth factor. Mol Cell Biol. 2004;24(12):5172–5183. doi: 10.1128/MCB.24.12.5172-5183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Epstein T, Xu L, Gillies RJ, et al. Separation of metabolic supply and demand: aerobic glycolysis as a normal physiological response to fluctuating energetic demands in the membrane. Cancer Metab. 2014;2:7. doi: 10.1186/2049-3002-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee M, Yoon JH. Metabolic interplay between glycolysis and mitochondrial oxidation: The reverse Warburg effect and its therapeutic implication. World J Biol Chem. 2015;6(3):148–161. doi: 10.4331/wjbc.v6.i3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korsmeyer SJ, Wei MC, Saito M, et al. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7(12):1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 28.Maher JC, Krishan A, Lampidis TJ. Greater cell cycle inhibition and cytotoxicity induced by 2-deoxy-D-glucose in tumor cells treated under hypoxic vs aerobic conditions. Cancer Chemother Pharmacol. 2004;53(2):116–122. doi: 10.1007/s00280-003-0724-7. [DOI] [PubMed] [Google Scholar]
- 29.Dmitrieva NI, Michea LF, Rocha GM, et al. Cell cycle delay and apoptosis in response to osmotic stress. Comp Biochem Physiol A Mol Integr Physiol. 2001;130(3):411–420. doi: 10.1016/s1095-6433(01)00439-1. [DOI] [PubMed] [Google Scholar]
- 30.Mason EF, Zhao Y, Goraksha-Hicks P, et al. Aerobic glycolysis suppresses p53 activity to provide selective protection from apoptosis upon loss of growth signals or inhibition of BCR-Abl. Cancer Res. 2011;70(20):8066–8076. doi: 10.1158/0008-5472.CAN-10-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]


