Abstract
Objective
Apolipoprotein E (apoE), a major cholesterol carrier in the brain, is associated with a strong risk for Alzheimer’s disease. Compared to the risky APOE4 gene allele, the effects of the protective APOE2 gene allele are vastly understudied, and thus, need to be further clarified.
Methods
We reviewed National Alzheimer’s Coordinating Center (NACC) clinical records and performed preclinical experiments using human apoE-targeted replacement (apoE-TR) mice, which do not show amyloid pathology.
Results
Clinically, the APOE2 allele was associated with less cognitive decline during aging. This effect was also seen in subjects with little amyloid pathology, or after adjusting for Alzheimer’s disease-related pathologies. In animal studies, aged apoE2-TR mice also exhibited preserved memory function in the water maze tests. Regardless, apoE2-TR mice showed similar or greater age-related changes in synaptic loss, neuroinflammation and oxidative stress compared to apoE3- or apoE4-TR mice. Interestingly, apoE concentrations in the cortex, hippocampus, plasma and cerebrospinal fluid (CSF) were positively correlated with memory performance across apoE isoforms, where apoE2-TR mice had higher apoE levels. Moreover, apoE2-TR mice exhibited the lowest levels of cholesterol in the cortex, in spite of higher levels in CSF and plasma. These cholesterol levels were associated with apoE levels and memory performance across apoE isoforms.
Interpretation
APOE2 is associated with less cognitive decline during aging. This can occur independently of age-related synaptic/neuroinflammatory changes and amyloid accumulation. Higher levels of apoE and associated cholesterol metabolism in APOE2 carriers might contribute to this protective effect.
INTRODUCTION
Alzheimer’s disease (AD) is an increasingly prevalent, age-related neurodegenerative disease associated with cognitive decline. Epidemiological studies have shown that the ε4 allele of the apolipoprotein E gene (APOE4) is a strong genetic risk factor for sporadic late-onset AD, while the APOE ε2 allele (APOE2) is a protective factor. Compared to the common APOE ε3 allele (APOE3), one APOE4 allele increases AD risk by 3–4 fold, whereas one APOE2 allele reduces risk by approximately half.1 ApoE forms lipoprotein particles and regulates lipid transport. In the central nervous system, apoE is mainly produced by astrocytes and plays a critical role in supplying cholesterol and other lipids to neurons.2 While apoE isoforms can directly affect the accumulation of amyloid-β (Aβ), a peptide believed to play a central role in AD pathogenesis, increasing evidence has demonstrated that apoE isoforms also contribute to cognitive decline through Aβ-independent pathways by regulating neuronal cholesterol metabolism, synaptic function, and neuroinflammation.1, 2
Several clinical studies have also shown that APOE2 protects against cognitive decline in elderly people not diagnosed with dementia, while APOE4 exacerbates it.3–6 However, such protective effects of APOE2 on cognitive performance have not been fully understood, compared to the deleterious effects of APOE4. Indeed, the number of clinical studies investigating the protective effects of APOE2 on cognitive decline in elderly people are much fewer than those of risky APOE4, likely due to lower frequency (e.g., ~8% of Caucasian population) and relatively milder effect of the APOE2 allele.7 Moreover, despite the general notion that APOE2 is associated with longevity and reduced Aβ neuropathology, controversies still exist regarding the effects of APOE2 on brain activity, synaptic integrity, neuroinflammation, as well as AD neuropathology, making it more difficult to understand how APOE2 protects against age-related cognitive decline and AD.7
Analyses of knock-in mouse models expressing each human apoE isoform under the control of the mouse endogenous promoter (targeted replacement or “TR” mice) have provided valuable knowledge regarding the effects of each apoE isoform on the metabolism of apoE itself, lipids and Aβ as well as synaptic/cognitive function.8–18 However, similar to clinical studies, the number of studies focusing on the effects of apoE2 is far fewer compared to apoE4, and especially, it is scarcely addressed how apoE2 affects cognitive function in these mouse models.
To close the gap in our knowledge regarding APOE2-mediated effects on cognitive function, we reviewed the clinical records of a large number of subjects enrolled by National Alzheimer’s Coordinating Center (NACC). Furthermore, we also assessed spatial memory performance, synaptic and neuroinflammatory changes, and brain lipid metabolism in apoE2-, apoE3- and apoE4-TR mice at different ages to clarify the effects of apoE2 on cognitive function in these animal models in the absence of amyloid pathology.
MATERIALS AND METHODS
Human clinical data
The clinical data at NACC, which were collected by the 34 past and present Alzheimer’s Disease Centers (ADCs) from September 2005 to November 2014 as the longitudinal Uniform Data Set19, were assessed in this study. NACC subjects are regarded as a referral-based or volunteer case series, and the majority of them are consisted of Caucasians (~83% of total subjects) and then black or African Americans (~10%). This cumulative database contains clinical evaluations and neuropathology data when available, of non-demented and demented subjects. To assess the effects of APOE genotype on the risk of cognitive decline during aging, we restricted our analysis to 21,531 subjects who were genotyped for APOE (Table 1). To maintain mutually exclusive groups, 338 subjects who had APOE ε2/ε4 genotype were excluded from all analyses.6, 20 Of the 2,750 subjects who had neuropathology data available, minimal amyloid cases were defined as having “no neocortical neuritic plaques (the Consortium to Establish a Registry for AD (CERAD) neuritic plaque score = 0) and no or sparse diffuse plaques (CERAD diffuse plaque score = 0 or 1)”. In 294 subjects whom only neuritic plaque evaluation was available, minimal amyloid cases were defined as having “no neocortical neuritic plaques (CERAD neuritic plaque score = 0)”. 492 cases were defined as minimal amyloid cases (18.3%, Table 1).
Table 1.
Demographic characteristics of subjects with APOE genotype at NACC database
| E2/E2 | E2/E3 | E3/E3 | E3/E4 | E4/E4 | p-value | |
|---|---|---|---|---|---|---|
| Total No. | 78 | 1893 | 10734 | 6812 | 1440 | — |
| Female No. (%) | 49 (62.8) | 1082 (57.2) | 6119 (57.0) | 3823 (56.1) | 767 (53.2) | 0.06 |
| Age at initial visit, yr | 74.1 ± 10.1 [53–95] | 73.1 ± 11.2 [20–102] | 72.8 ± 10.8 [21–109] | 72.1 ± 9.9 [20–99] | 69.8 ± 8.6 [27–91] | <0.001 |
| Education, yr | 15.4 ± 3.0 [3–20] | 15.1 ± 3.3 [1–25] | 14.9 ± 3.6 [0–28] | 15.0 ± 3.4 [0–30] | 15.2 ± 3.2 [0–30] | 0.58 |
| Visits, No. | 3.4 ± 2.0 [1–9] | 3.8 ± 2.3 [1–10] | 3.6 ± 2.3 [1–10] | 3.4 ± 2.2 [1–10] | 3.2 ± 2.1 [1–10] | <0.001 |
| Follow-up, yr | 3.0 ± 2.5 [0–8] | 2.8 ± 2.5 [0–9] | 3.0 ± 2.6 [0–9] | 2.7 ± 2.5 [0–9] | 2.6 ± 2.3 [0–9] | <0.001 |
| MMSE at last visit | 26.5 ± 4.9 [6–30] | 26.1 ± 5.5 [0–30] | 25.0 ± 6.7 [0–30] | 22.6 ± 7.8 [0–30] | 19.9 ± 8.2 [0–30] | <0.001 |
| Non-demented, include MCI, at last visit (%) | 64 (82.0) | 1321 (69.8) | 6821 (63.6) | 3107 (45.6) | 406 (28.2) | <0.001 |
| Dementia No. at last visit (%) | 14 (18.0) | 572 (30.2) | 3913 (36.4) | 3705 (54.4) | 1034 (71.8) | <0.001 |
| Pathologically assessed No. | 6 | 204 | 1325 | 911 | 231 | — |
| Female No. (%) | 3 (50.0) | 96 (47.1) | 618 (46.6) | 400 (43.9) | 87 (37.7) | 0.12 |
| Age at death, yr, | 83.3 ± 12.5 [67–96] | 83.0 ± 12.4 [44–105] | 81.4 ± 12.2 [31–111] | 79.5 ± 10.5 [33–101] | 77.3 ± 8.6 [49–93] | <0.001 |
| Interval from last visit, mo, | 22.8 ± 17.3 [6–55] | 13.5 ± 12.9 [0–77] | 14.6 ± 15.1 [0–99] | 15.5 ± 15.6 [0–98] | 16.3 ± 15.5 [0–79] | 0.12 |
| Minimal amyloid pathology No. (%) | 5 (83.3) | 87 (42.6) | 352(26.6) | 48 (5.3) | 0 (0) | <0.001 |
For continuous data, values are mean ± SD [range].
p-value are from one way ANOVA (continuous data) or Chi-square test (categorical value).
Animals
Human apoE TR mice expressing human apoE2, apoE3, or apoE4, under the control of the mouse apoE promoter8, 9 on a pure C57BL/6J background were purchased from Taconic. All cohorts from this study were generated from homozygous breeding pairs, group housed without enrichment structures in a specific pathogen-free environment in ventilated cages, and used for experiments according to the standards established by the Mayo Clinic Institutional Animal Care and Use Committee. We tested young and old groups of both sexes (Table 2). Mice were euthanized and tissues were collected within one month of the behavior test for biochemical and histochemical studies.
Table 2.
Characteristics of apoE-TR mice used in this study
| E2 | E3 | E4 | p-value | |
|---|---|---|---|---|
| Young cohort No. | 29 | 26 | 26 | — |
| Female No. (%) | 15 (51.7) | 12 (46.2) | 14 (53.8) | 0.857 |
| Water maze tested No. (removed) | 29 (0) | 26 (0) | 26 (0) | — |
| Age at sacrifice, mo | 7.3 ± 0.7 [6.3–7.9] | 7.6 ± 0.2 [7.4–7.8] | 7.2 ± 0.3 [6.6–7.5] | 0.015 |
| Male body weight, gram | 28.7 ± 2.1 [25.9–33.1] | 29.5 ± 2.7 [22.2–33.4] | 30.5 ± 2.8 [25.3–34.9] | 0.217 |
| Female body weight, gram | 24.7 ± 2.5 [21.7–28.9] | 21.9 ± 1.9 [19.4–24.7] | 23.5 ± 1.7 [21.2–27.1] | 0.006 |
| Old cohort No. | 29 | 22 | 29 | — |
| Female No. (%) | 10 (34.5) | 9 (40.9) | 14 (48.3) | 0.565 |
| Water maze tested No. (removed) | 24 (2) | 22 (4) | 19 (2) | 0.584 |
| Age at sacrifice, mo | 22.9 ± 1.0 [21.3–24.7] | 22.7 ± 0.9 [21.7–24.5] | 23.1 ± 0.9 [22.2–25.0] | 0.467 |
| Male body weight, gram | 31.7 ± 2.3 [27–36.9] | 33.2 ± 3.4 [28.6–38.4] | 33.8 ± 2.2 [30.4–37.9] | 0.793 |
| Female body weight, gram | 25.1 ± 3.4 [19.3–30.0] | 25.9 ± 3.8 [22.6–33.7] | 25.5 ± 3.1 [17.8–29.3] | 0.898 |
For continuous data, values are mean ± SD [range].
p-value are from one way ANOVA (continuous data) or Chi-square test (categorical value).
WM = water maze
Water maze test
Hippocampus-dependent learning and memory function was investigated with the water maze test as described previously with some modification.21 The test was conducted in a circular pool filled with water adjusted to ambient temperature. In the hidden platform training, a transparent platform was submerged 1.5 cm below water level. The water tank was located in a test room in which there were many external cues. The position of the cues remained unchanged throughout the water-maze task. A camera fixed on the ceiling of the room was used to track the movement with ANY-maze software (Stoelting). In the hidden-platform training, the mice were given two trials (one session) on the first day and four trials (two sessions) on each of the following four days. The intertrial interval was approximately 10 minutes and the intersession interval was 2 hours. During each trial, the mice were released from four pseudo-randomly assigned starting points and allowed to swim for 60 seconds. After mounting the platform, the animals were allowed to remain there for 10 seconds, and were then placed in a holding cage with a heating lamp until the start of the next trial. If a mouse was unable to find the platform within 60 seconds, it was guided to the platform and allowed to rest on the platform for 10 seconds. Probe tests were performed 24 hours and 72 hours after the last hidden-platform training. In the probe tests, the hidden platform was removed and the animal was released from the opposite quadrant and allowed to swim freely for 60 seconds. Mice that did not swim and remained in the original area during the initial 40 seconds of the first probe test were excluded from further data analysis (~1% of total mice). In the visible-platform test, performed after the last probe test on the same day, the platform was elevated above the water surface. The mice were given three trials with an intertrial interval of 1 hour. All experiments were conducted at approximately the same time each day. For aged groups, a pretraining session was carried out one day before hidden-platform training, in which animals were given 60 seconds of free swimming without the platform, to remove any mice that were unable to swim. The number of excluded mice was similar and not significantly different among apoE isoforms (Table 2).
Tissue harvest and sample preparation
After fasting for 4 hours, mice were anesthetized with xylazine and ketamine (20 and 100 mg/kg, respectively). CSF and blood were collected from the cisterna magna compartment and femoral vein, respectively, according to the previously described method.22 After transcardial perfusion with PBS, the brain was collected, and divided along the sagittal plane, and the right hemisphere was stored at −80°C until biochemical analysis. The left hemisphere was fixed with 4% paraformaldehyde overnight, dropped through 30% sucrose solution overnight, embedded in OCT compound (Tissue-Tek), and stored at −80°C until histological analysis. For biochemical analysis, tissues were homogenized by polytron homogenizer (KINEMATICA) at a ratio of 20 ml/g wet-weight brain in ice-cold TBS or RIPA lysis buffer (Millipore) plus 0.1% SDS, complete protease inhibitors and PhosSTOP phosphatase inhibitors (Roche). Such a detergent-rich lysis buffer has been shown to inhibit apoE oligomerization and fully release the lipid-bound apoE epitopes which if not exposed, may hamper accurate detection of apoE levels by immunoassay.12 After centrifugation at 100,000 g for 1 hour at 4°C, the supernatant was collected and used for biochemical analysis.
ELISA and other biochemical assays
Levels of apoE, postsynaptic density 95 (PSD95), and glial fibrillary acidic protein (GFAP) were determined by ELISAs as previously described.23 Levels of CD11b were determined by ELISA using a mouse monoclonal M1/70 capture antibody (R&D Systems) and biotin-conjugated mouse monoclonal 5C6 antibody (AbD Serotec). Recombinant mouse CD11b proteins (R&D Systems) were used as standards. Levels of tumor necrosis factor α (TNFα) and interleukin-1β (IL1β) were determined by commercial ELISA kit (R&D Systems). Colorimetric quantification was performed on a Synergy HT plate reader (BioTek) using horseradish peroxidase (HRP)-linked streptavidin (Vector) or Poly-HRP 40 streptavidin (Fitzgerald) and 3,3′,5,5′-tetramethylbenzidine substrate (Sigma). Levels of total cholesterol, glutamate, and malondialdehyde (MDA) were determined by enzymatic assays, according to the manufacture’s instructions (Life Technologies or Wako: total cholesterol and glutamate, Oxford Biomedical Research: MDA). Western blot analysis was performed according to previously-described procedures with some modifications.21 In brief, RIPA brain lysate mixed with 4x Laemmli Sample Buffer (BIO-RAD) were run on the NuPAGE electrophoresis system (Life Technologies). Immunoreactive bands were detected and quantified using Odyssey Infrared Imaging system (LI-COR Biosciences). Antibodies against synaptophysin (mouse, Merck), N-methyl-D-aspartate receptor 1 (NMDAR1, rabbit, Merck), vesicular glutamate transporter type 1 (vGlut1, mouse, Merck), glutamic acid decarboxylase 67 (GAD67, mouse, Merck), phospho-tau (AT8, pSer202+Thr205, Thermo Scientific) and glutaminase (rabbit, Abcam) were used.
Quantitative real-time PCR
Total RNA was purified from brain cortical area of female mice by Direct-zol RNA miniprep (Zymo Research), eluted in nuclease-free water (Ambion) and stored at −80°C. Reverse transcription was performed using SuperScript III First-Strand Synthesis System (Life Technologies). Real-time PCR was conducted with Universal SYBR Green Supermix (Bio-Rad) using an iCycler thermocycler (Bio-Rad) to detect levels of the corresponding Sirt3 (NAD-dependent deacetylase sirtuin-3, mitochondrial), mitochondrial transcription factor A (TFAM) and β-actin (Qiagen). The following primers were used: Sirt3 forward primer, GAGCGGCCTCTACAGCAA, and reverse primer, GGAAGTAGTGAGTGACATTGGG. TFAM forward primer, AACACCCAGATGCAAAACTTTCA, and reverse primer, GACTTGGAGTTAGCTGCTCTTT. β-actin forward primer, AGTGTGACGTTGACATCCGTA, and reverse primer, GCCAGAGCAGTAATCTCCTTC (Integrated DNA technologies). The relative levels of expression were quantified and analyzed by Bio-Rad iCycler iQ software (Bio-Rad). Relative mRNA levels were calculated by ΔΔCt method with β-actin used as an internal control for each specific gene amplification.
Shotgun Lipidomics analysis of brain lipids
Shotgun lipidomic analysis of lipids was performed as described previously.24, 25 Lipids were extracted from each dissected brain cortical area of male mice by the modified Bligh and Dyer method. A triple-quadrupole mass spectrometer (Thermo Scientific) equipped with a NanoMate device (Advion) and Xcalibur system was utilized to analyze lipids (Thermo Scientific). All tandem mass spectrometry analyses were automatically acquired by a customized sequence operated under Xcalibur software. Internal standards for quantification of individual molecular species of other lipid classes were added to each brain tissue sample.
Statistical analysis
To assess the risk of cognitive decline during aging in NACC cohort, we used a COX proportional hazard model with sex, years of education, and APOE genotypes as covariates, the date of birth as the time of origin, and the age of onset of cognitive decline as the time of event. We used the NACC variable “NACCAGED” to define the age of onset of cognitive decline, which was determined by clinicians after consulting with medical records, direct observation and subject/informant report. When NACC recorded a different value at multiple visits within the same subjects, we used the record at their last visit (0.43% of total subjects). Subjects whose age of cognitive decline was unknown were eliminated from the analysis, where most of them had already been cognitively impaired at their initial visit (1.06% of total subjects), while others declined during their follow-up visits (0.20% of total subjects). Subjects who did not show any cognitive decline at their last visit were right-censored (33.7% of total subjects). Effects of covariates in each model reported in this study are summarized in Supplementary Table 1–3. Hazard ratio (HR) and 95% confidence interval (CI) for APOE genotypes are reported. To confirm findings from the COX proportional hazard model in subjects with minimal amyloid pathology, a logistic regression model for the presence of cognitive impairment was performed by adjusting for sex, years of education, age at death, and the duration between age at the last cognitive test and age at death. We used “NACCAGED” or another NACC variable “NACCUDSD”, which described cognitive status (Normal cognition, impaired but not mild cognitive impairment (MCI), MCI or dementia), to define the presence/absence of cognitive impairment until their last visit. Effects of APOE genotype on the presence of cognitive impairment trended to be similar in the presence/absence of age at death as a covariant in the model, which acted in the opposite direction to what would be generally expected (Supplementary Table 4&5). Effects of APOE genotype on Clinical Dementia Rating (CDR) sum of boxes, and memory score were analyzed using a multivariable linear regression model adjusting for sex, years of education, age at the last cognitive test, the duration between age at the last cognitive test and age at death, CERAD scores of amyloid plaques (diffuse plaques and neuritic plaques), Braak neurofibrillary tangle stage, and the presence/absence of vascular pathology. Effects of APOE genotype and other covariates were consistent whether only neuritic plaques or both diffuse plaques and neuritic plaques were included as amyloid plaques in multivariable linear regression models reported in this study (Supplementary Table 6&7). Tukey-Kramer test was conducted to assess the difference of adjusted mean of cognitive scores among APOE genotypes. In all analyses, the observed effects of APOE genotype were similar with or without considering the racial/ethnic differences (data not shown).
In animal studies engaging one-way ANOVA (probe test of the water maze, and some biochemical assays), post-hoc Tukey-Kramer test was conducted to assess the effects of each apoE isoform when the main effect of apoE isoform was present (p < 0.05). When the main effect of apoE isoform was present (p < 0.05) in animal studies engaging repeated-measures one-way ANOVA (hidden- & visible-platform training of the water maze), post-hoc Tukey-Kramer test was conducted to assess the effects of each apoE isoform across all training/test if the interaction between apoE isoform and training/test was not significant, or within each training/test if the interaction between apoE isoform and training/test was significant (p < 0.05). To assess the effects of age and apoE isoform together, two-way ANOVA was conducted. Then, post-hoc Tukey-Kramer test was performed to see the effects of each apoE isoform when the main effect of apoE isoforms was significant (p < 0.05), but the interaction between age and apoE isoform was not significant. In the presence of significant interaction between age and apoE isoform (p < 0.05), post-hoc Bonferroni test was conducted to see the effects of age within each apoE isoform, while post-hoc Tukey-Kramer test was conducted to see the effects of each apoE isoform within the old age group. To assess the association between cognitive score and levels of the particular molecules, Pearson correlation test was conducted.
All statistical analyses were performed by JMP (version 10.0.0, SAS Institute Inc.). P-value, F-value and/or effect size (η2 = SSeffect / SStotal (SS = Sum of Squares)) are reported in ANOVA analyses. P-value less than 0.05 was considered significant.
RESULTS
APOE2 is clinically associated with a lower risk of cognitive decline during aging
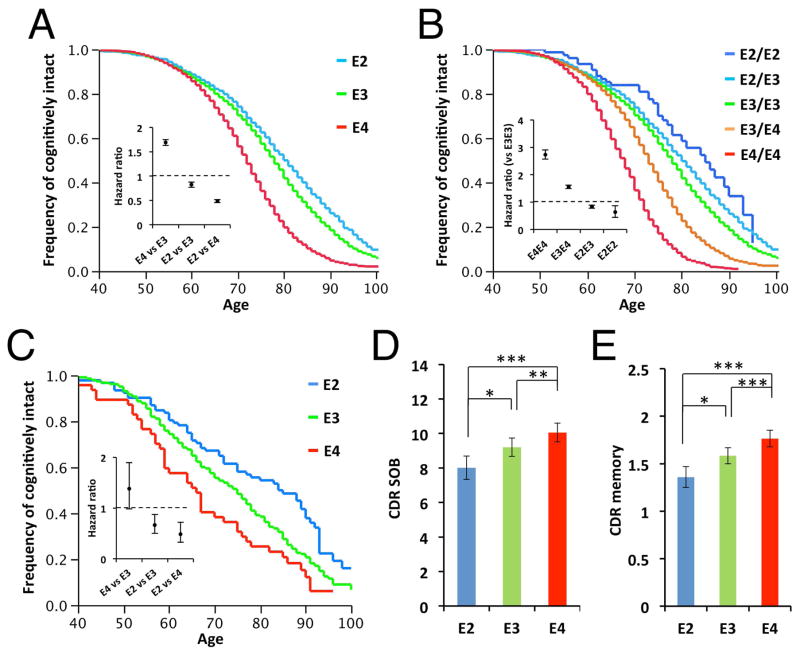
We first analyzed NACC clinical records to investigate how APOE genotypes affect the risk of cognitive decline during aging. As expected from the previous report,26 there was a significant interaction between sex and APOE genotype (p < 0.001): Indeed, these effects were stronger in females than males (HR for APOE4 vs. APOE3 in male = 1.52, 95% CI = 1.45–1.60, p < 0.001; HR for APOE4 vs. APOE3 in female = 1.88, 95% CI = 1.79–1.98, p < 0.001; HR for APOE2 vs. APOE3 in male = 0.86, 95% CI = 0.79–0.95, p = 0.002; HR for APOE2 vs. APOE3 in female = 0.79, 95% CI = 0.72–0.86, p < 0.001). However, as these trends were similar across males and females, we combined effects from both sexes for further analyses. Compared to control APOE3 homozygous subjects, APOE4 accelerated cognitive decline (HR = 1.70, 95% CI = 1.64–1.76, p < 0.001), while APOE2 subjects were protected from cognitive decline (HR = 0.83, 95% CI = 0.77–0.88, p < 0.001) (Fig. 1A & Supplementary Table 1). Of note, there were allele dose-dependent effects; APOE2 and APOE4 homozygous tended to have stronger effects than heterozygous APOE2/APOE3 and APOE3/APOE4 subjects, respectively (Fig. 1B & Supplementary Table 2). Moreover, a retrospective approach using a postmortem cohort in which amyloid pathology was histologically minimal showed that these APOE genotype-dependent effects appeared to be independent of Aβ accumulation. Specifically, APOE2 was protective compared to APOE3 as well as APOE4 (HR for APOE2 vs. APOE3 = 0.68, 95% CI = 0.51–0.89, p = 0.005; HR for APOE2 vs. APOE4 = 0.51, 95% CI = 0.34–0.77, p < 0.001; Fig. 1C & Supplementary Table 3). These trends persisted even when adjusted for difference in subtle amyloid pathology (i.e., negative or sparse diffuse plaques) (data not shown) or in the logistic regression models for the presence of cognitive impairment (Supplementary Table 4&5). To assess whether the amyloid-independent effects of APOE on cognitive decline would be observed irrespective of the absence/presence of dementia, we further assessed the effects of APOE genotype on cognitive decline scores (CDR sum of boxes (SOB), and CDR memory) in all pathologically-assessed subjects by adjusting for amyloid accumulation as well as tau and vascular pathology. As we observed the trend that the effects of APOE2 were more evident in elderly individuals, we restricted subjects whose age was above 70 years old at death. In this multivariable regression analysis, the effect of APOE genotype was significant (effects of APOE genotype on CDR SOB and CER memory: p < 0.001; effects of covariates were also summarized in Supplementary Table 6), and APOE2 carriers were protected from cognitive decline (APOE2 vs. APOE3: p = 0.049; APOE2 vs. APOE4: p < 0.001; Fig. 1D) as well as memory decline (APOE2 vs. APOE3: p = 0.017; APOE2 vs. APOE4: p < 0.001; Fig. 1E), compared to APOE3 and APOE4 carriers. Together, these results show strong association of APOE genotypes with cognitive decline during aging, in particular by demonstrating that APOE2 is associated with a lower risk of cognitive decline, including memory dysfunction, independently of Aβ accumulation.
Fig. 1.
APOE2 eases cognitive decline during aging. (A–C) Survival plot for the onset of cognitive decline in subjects categorized by APOE genotype carrier status (A), and individual APOE allele genotypes (B) are shown (n=20,485). Effects of APOE genotype carrier status on the survival plot for the onset of cognitive decline in subjects with minimal amyloid pathology (n=479) (C). The inset shows the COX regression hazard ratio with 95% confidence interval. E2 represents people with APOE2/APOE2 or APOE2/APOE3 allele, while E4 represents people with APOE4/APOE4 or APOE3/APOE4 allele. (D–E) Effects of APOE genotype on CDR sum of boxes (SOB) score (D), and CDR memory score (E) in elderly subjects (above 70 years old at death, n=2,163) by adjusting for sex, years of education, age at cognitive test, duration between age at cognitive test and age at death, CERAD scores of diffuse plaques and neuritic plaques, Braak stage of neurofibrillary tangles, and presence/absence of vascular pathology. Data are presented as adjusted mean ± standard error of the mean. *p<0.05, **p<0.01, ***p<0.001; compared across apoE isoforms, by Tukey-Kramer test.
Aged APOE2 mice preserves spatial memory function
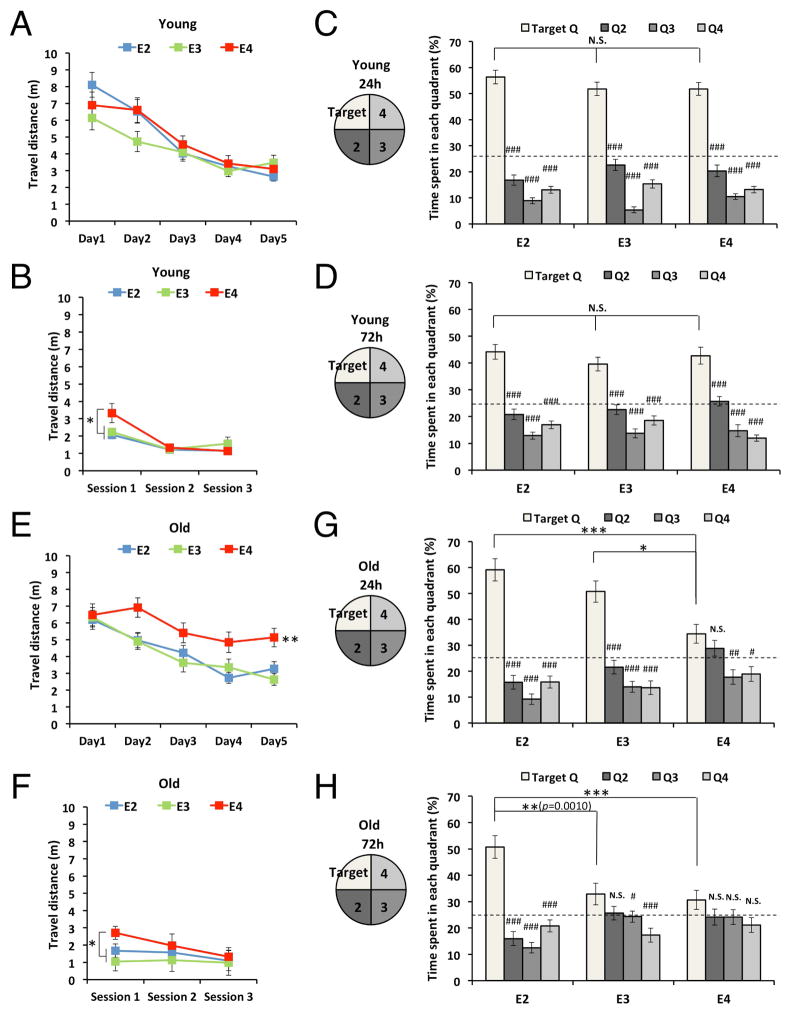
To further investigate the effects of APOE genotype on cognitive function, we employed knock-in animal models that express each human apoE isoform under the control of mouse endogenous promoter known as apoE-targeted replacement (apoE-TR) (Table 2). These mice do not have detectable Aβ deposition (data not shown). Spatial memory function at a young age was assessed by the water maze test, a commonly used test for rodent spatial learning and memory. During the hidden-platform training, all young apoE-TR mice exhibited robust learning, as shown in the distance traveled to reach the hidden platform, but no differences were observed among the three apoE isoforms (effects of training: F = 33.2, p < 0.001; effects of APOE genotype: F = 1.3, p = 0.293; APOE genotype x Training: F = 1.8, p = 0.085; Fig. 2A). Likewise, in the visible platform test, all young groups performed well, indicating that no differences existed in visual functions. However, apoE4-TR mice performed poorly at the first phase of the visible platform test, perhaps due to emotional disturbances in these mice (effects of test: F = 31.3, p < 0.001; effects of APOE genotype: F = 2.3, p = 0.109; APOE genotype x Test: F = 3.5, p = 0.009; Fig. 2B). In probe tests performed at 24 hours and 72 hours after the last hidden-platform training, all groups of young mice stayed longer in the target quadrant compared to other quadrants (p < 0.001). Also, no differences existed in the latencies to reach the target quadrant when compared across apoE isoforms (Fig. 2C & D). Thus, all young groups showed good memory retention in the probe tests.
Fig. 2.
Aged apoE2-TR mice possess preserved spatial learning memory. (A–D) Spatial learning and memory assessed by the water maze test in young apoE-TR mice. Distance traveled to platform in hidden-platform training (A) and visible-platform test (B), time spent in each quadrant at the first probe test (24 hours after the last hidden-platform training, C) and the second probe test (72 hours after the last hidden-platform training, D) are shown (n = 24–29/group). (E–H) Spatial learning and memory assessed by the water maze test in old apoE-TR mice. Distance traveled to platform in hidden-platform training (E) and visible-platform test (F), time spent in each quadrant at the first probe test (24 hours after the last hidden-platform training, G) and the second probe test (72 hours after the last hidden-platform training, H) are shown (n = 17–22/ group). Data are presented as mean ± standard error of the mean. “Target” indicates the area where the platform was constantly located in the hidden-platform test. *p<0.05, **p<0.01, ***p<0.001; compared across apoE isoforms, or #p<0.05, ##p<0.01, ###p<0.001; compared with the target quadrant within each apoE isoform, by repeated-measures one-way ANOVA (hidden- & visible-platform training) or one-way ANOVA (probe test) with post-hoc Tukey-Kramer test. N.S.; not significant
In contrast to the memory function in young mice, old apoE-TR mice showed marked differences in spatial learning and memory. At old age, apoE4-TR mice showed significantly impaired learning ability (p < 0.01) compared to apoE2- and apoE3-TR mice (effects of training: F = 23.8, p < 0.001; effects of APOE genotype: F = 6.2, p = 0.004; APOE genotype x Training: F = 1.5, p = 0.177; Fig. 2E). Similar to young mice, old apoE4-TR mice performed poorly at the initial session of the visible platform test, while all groups performed well at the later sessions of this test (effects of training: F = 9.7, p < 0.001; effects of APOE genotype: F = 3.4, p = 0.041; APOE genotype x Training: F = 3.0, p = 0.023; Fig. 2F), indicating that no differences exist in visual function. During the first probe test at 24 hours, poor memory performance was observed in apoE4-TR mice: their time spent in the target quadrant was comparable to a non-target quadrant and was also significantly less than that of apoE2-TR and apoE3-TR mice (Fig. 2G). During the second probe test at 72 hours, only apoE2-TR mice showed good memory retention: they stayed longer in the target quadrant compared to all other non-target quadrants (p < 0.001) as well as compared to apoE3-TR and apoE4-TR mice (Fig. 2H). Importantly, while this performance in the second probe test by old apoE2-TR mice was comparable with young mice, both apoE3- and apoE4-TR showed poor performance at old age, as shown in the weak or non-significant difference between their time spent in the target quadrant and non-target quadrants (Fig. 2H). Such poor performance was also confirmed by the direct comparison between young and old mice. When data was stratified by gender, we still observed similar effects of apoE2 and apoE4 in both sexes, though the effects of apoE2 were more evident in female mice than male mice (data not shown). These results using animal models are generally consistent with human clinical data, indicating that APOE2 genotype is protective against, whereas APOE4 genotype accelerates, cognitive decline during aging compared to APOE3 genotype.
Age- and apoE-dependent synaptic, neuroinflammatory, and oxidative changes
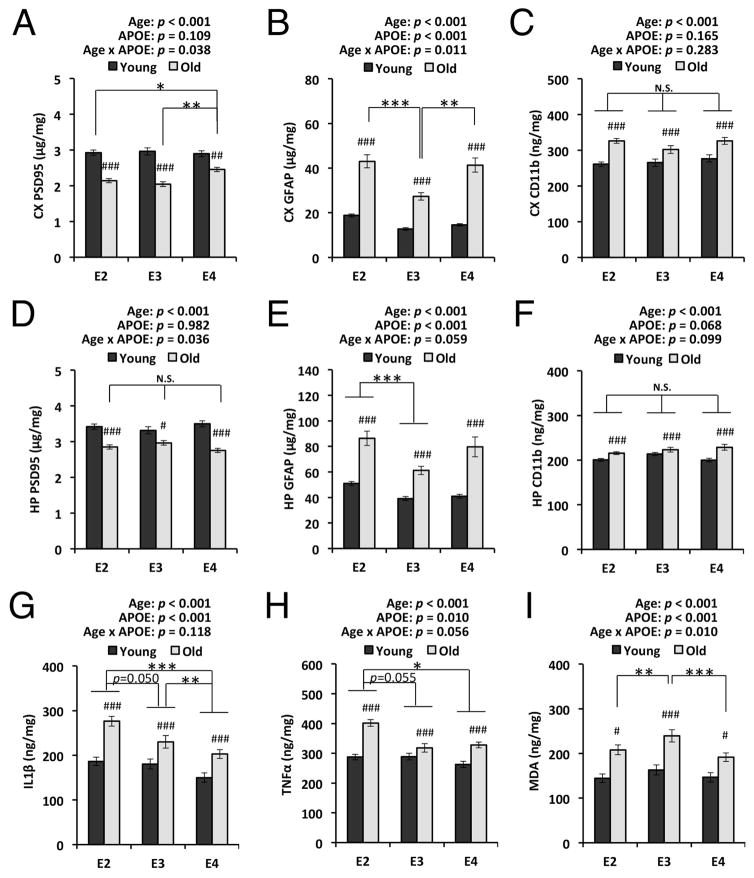
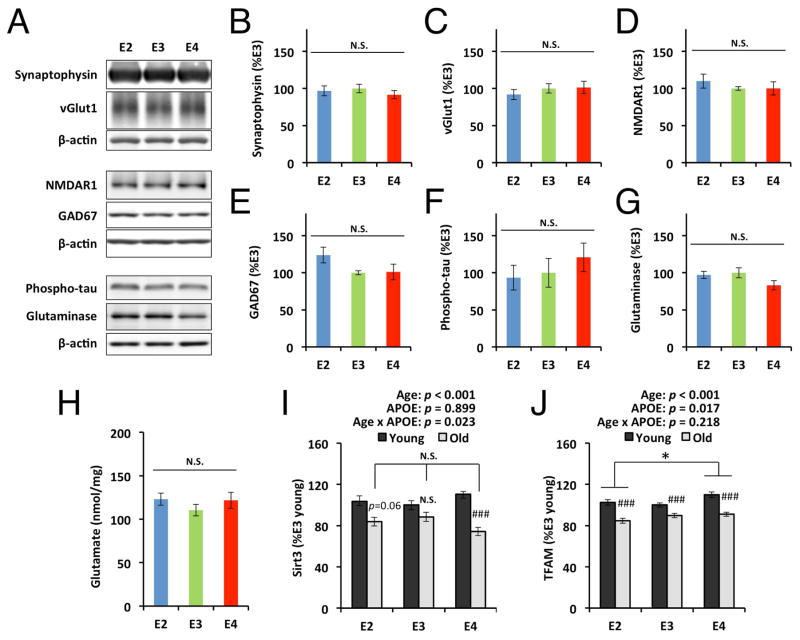
To address the mechanisms underlying the effects of apoE isoforms on cognitive function in these apoE-TR mice, we assessed the age-and apoE isoform-dependent changes in markers of synaptic integrity, neuroinflammation, and oxidative stress. We hypothesized that apoE2-TR mice might show less age-dependent changes in molecules involved in memory function while apoE4-TR mice exacerbate them, or, alternatively, apoE isoform-dependent differences at old age might help with maintaining memory function in apoE2-TR mice. Levels of PSD95, a marker of post-synaptic terminals, were significantly decreased in the cortex and hippocampus of old mice, compared to young mice, regardless of apoE isoforms (p < 0.001). Although a weak interaction between age and apoE isoform was observed in cortical PSD95 levels (cortical PSD95: p = 0.038, hippocampal PSD95: p = 0.036), there was no tendency to support the apoE-isoform dependent effects on memory function at old age (Fig. 3A&D). Levels of an astrocyte marker GFAP and a microglia marker CD11b generally showed age-dependent increases in the cortex of all mice regardless of apoE isoforms (p < 0.001). A significant interaction (p = 0.011) between age and apoE isoform observed in GFAP levels that were generally higher in aged apoE2- and apoE4-TR mice than apoE3-TR mice (Fig. 3B), while CD11b levels were not changed (Fig. 3C). Hippocampal GFAP and CD11b levels also showed similar age-dependent increases regardless of apoE isoforms (p < 0.001), and GFAP levels were higher in apoE2-TR mice compared to apoE3-TR mice (Fig. 3E&F). IL1β and TNFα levels were consistently increased in the cortex of aged apoE-TR mice (p < 0.001), but there were no significant interactions between age and apoE isoform. Moreover, the levels of these proinflammatory cytokines tended to be increased in apoE2-TR mice, compared to those in apoE3-TR and apoE4-TR mice (effects of apoE isoform on IL1β: F = 13.3, p < 0.001, η2 = 0.11; effects of apoE isoform on TNFα: F = 4.7, p = 0.010, η2 = 0.05; Fig. 3G&H). Levels of malodialdehyde (MDA), an indicator of lipid peroxidation, were also increased in aged mice compared to young mice, irrespective of apoE isoforms. While a significant interaction existed between age and apoE isoform (p = 0.010), MDA levels in apoE2-TR and apoE4-TR mice were lower than that of apoE3-TR mice at old age (Fig. 3I). We also determined the levels of hippocampal synaptophysin, a marker of pre-synaptic terminals, and specific synaptic proteins, vGlut1, NMDAR1, and GAD67 across apoE isoforms at old age by Western blotting, but did not find difference in the levels of these proteins (Fig. 4A–E). Furthermore, we measured the levels of hyper-phosphorylated tau, glutamine, and glutaminase, all of which have been implicated in cognitive function, and found that apoE isoform did not have specific effects on the levels of these molecules (Fig. 4A&F–H). Interestingly, expression of Sirt3 and TFAM, regulators of mitochondrial oxidative stress, were generally decreased in aged mice across all isoforms (TFAM: p < 0.001), though apoE3-TR mice did not have a obvious age-dependent decrease in Sirt3 levels (Fig. 4I&J). Taken together, these results suggest that memory function preservation in apoE2-TR mice was not mediated by age- and apoE-dependent changes of synaptic integrity, neuroinflammation and oxidative stress.
Fig. 3.
Age- and apoE isoform-dependent synaptic/neuroinflammatory/oxidative changes. PSD95 levels assessed by ELISA are compared between young and old mice with each apoE isoform or across apoE isoforms at old age in cortical area (CX, A) and hippocampal area (HP, D) (n=22–29/group). Levels of cortical GFAP (C), hippocampal GFAP (E), cortical CD11b (D), hippocampal CD11b (F), cortical IL1β (G), and cortical TNFα (H) assessed by ELISA are compared between young and old mice with each apoE isoform or across apoE isoforms at old age (n=22–29/group). (I) MDA levels assessed by TBARS assay are compared between young and old mice with each apoE isoform or across apoE isoforms at old age in cortical area (n=19–22/group). Data are presented as mean ± standard error of the mean. #p<0.05, ##p<0.01, ###p<0.001; compared with young mice, or *p<0.05, **p<0.01, ***p<0.001; compared across apoE isoforms at old age, by two-way ANOVA with post-hoc test (see Materials and Methods). P-values above each graph represent the main effects of age and apoE isoform, and the interaction between age and apoE isoform. N.S.; not significant
Fig. 4.
ApoE isoform-dependent changes of specific synaptic markers, molecules related to cognitive function, and regulators of mitochondrial oxidative stress. Densitometric analysis of Western blots (A) of levels of (B) synaptophysin, (C) vGlut1, (D) NMDAR1, (E) GAD67, (F) phosphorylated tau, and (G) glutaminase, in hippocampus are compared among aged apoE-TR mice by normalizing with β-actin levels (n=6/group, 3 male plus 3 female/each group). (H) Levels of cortical glutamate, assessed by enzymatic assay, are compared among aged apoE-TR mice (n=17–22/group). Levels of Sirt3 mRNA (I), and TFAM mRNA (J) assessed by real-time PCR are compared between young and old mice with each apoE isoform in the cortical area (n=5/group, all female). Data are presented as mean ± standard error of the mean. #p<0.05, ##p<0.01, ###p<0.001; compared with young mice, or *p<0.05; compared across apoE isoforms at old age, by two-way ANOVA with post-hoc test (see Materials and Methods; I&J) or one-way ANOVA with post-hoc Tukey-Kramer test (B–H). P-values above each graph (I&J) represent the main effects of age and apoE isoform, and the interaction between age and apoE isoform. N.S.; not significant
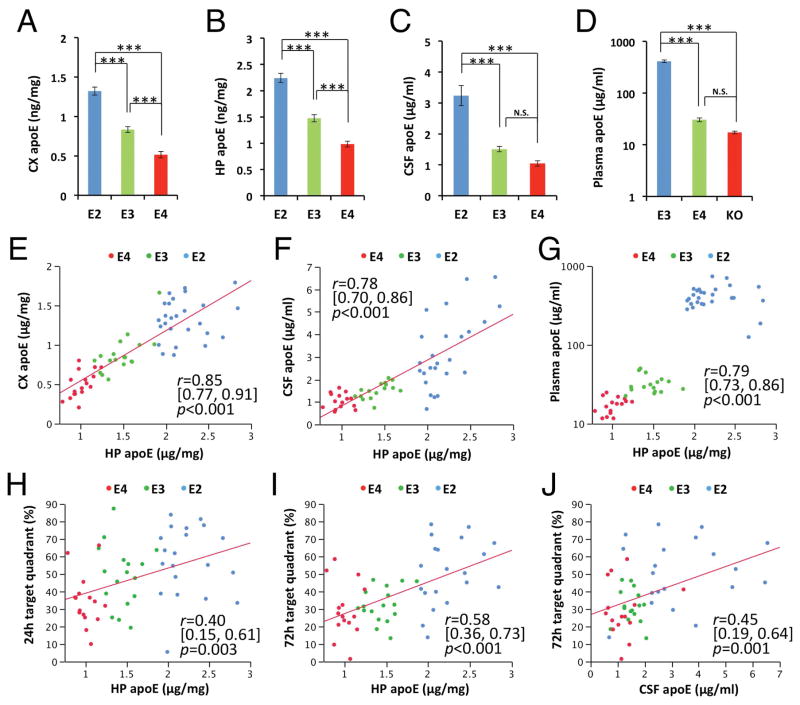
Correlation of brain apoE levels with memory function in aged apoE-TR mice
When apoE levels in cortex, hippocampus, CSF and plasma from aged apoE-TR mice were assessed by ELISA, they tended to be significantly higher in apoE2-TR mice, followed by apoE3-TR, and then apoE4-TR mice (effects of apoE isoform on apoE levels in cortex: F = 65.7, p < 0.001, η2 = 0.69; in hippocampus: F = 256.3, p < 0.001, η2 = 0.87; in CSF: F = 13.0, p < 0.001, η2 = 0.32; in plasma: F = 137.5, p < 0.001, η2 = 0.83; Fig. 5A–D), consistent with a previous report.12 While apoE levels in cortex, hippocampus, CSF and plasma were correlated with one another in aged apoE-TR mice across apoE isoforms (Fig. 5E–G), apoE levels in each compartment positively associated with memory retention during the water maze probe test (Fig. 5H–J & data not shown). Of note, these significant, consistent associations of apoE with memory score were observed only in aged mice but not young mice. On the other hand, associations of these memory scores with markers of synaptic integrity, neuroinflammation and oxidative stress measured across all individual mice were relatively weaker than that of apoE levels (data not shown). Thus, these results imply that higher apoE levels associated with apoE2 are beneficial for better memory performances at old age independently of synaptic integrity, neuroinflammation and oxidative stress.
Fig. 5.
ApoE levels and correlation with memory function in aged apoE-TR mice. (A–D) ApoE levels in cortical areas (CX, A), hippocampus (HP, B), CSF (C) and plasma (D) assessed by ELISA are compared among apoE-TR mice at old age (n=15–27/group). Data are presented as mean ± standard error of the mean. ApoE levels in hippocampus are plotted against apoE levels in cortex (E), CSF (F) and plasma (G). ApoE levels in hippocampus (H&I) and CSF (J) are plotted against the percent of time spent in target quadrant at the probe test 24 hours (H) or 72 hours (I&J) after the last hidden-platform training of the water maze test. *p<0.05, **p<0.01, ***p<0.001; by one-way ANOVA with post-hoc Tukey-Kramer test (A–D). Correlation coefficient (r), 95% CI of r, and p-value were acquired by Pearson correlation test (E–J). N.S.; not significant
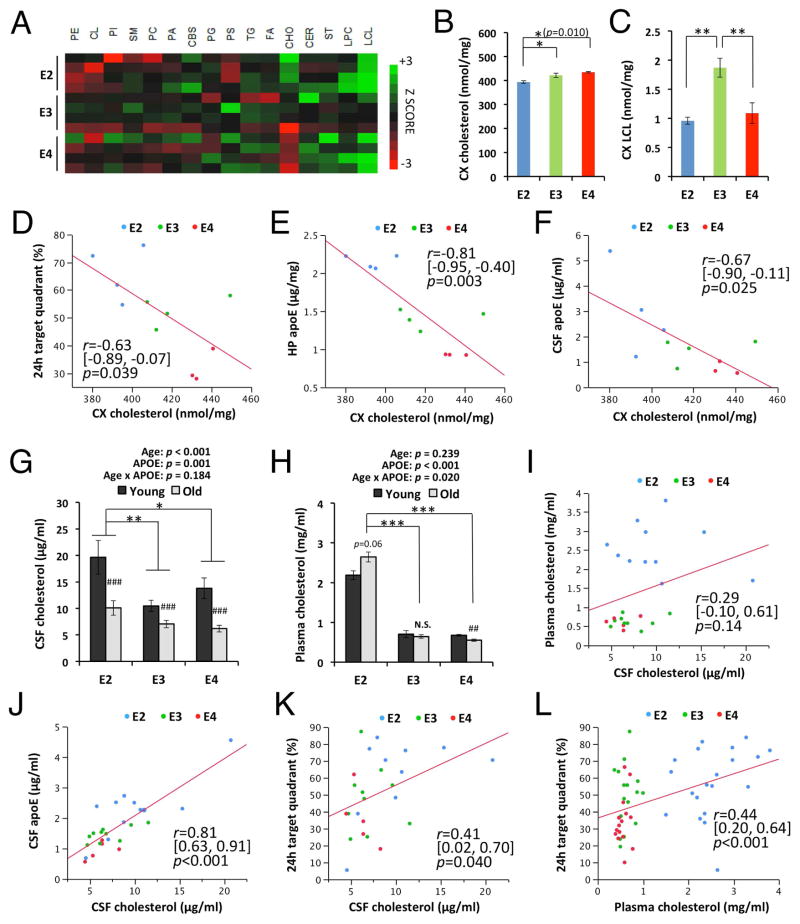
Lower cortical cholesterol levels correlate with better memory function in aged apoE-TR mice
As apoE is a major apolipoprotein regulating brain metabolism of cholesterol and other lipids, we also assessed potential changes in the levels of cholesterol and other lipids in the cortex of these mice at old age by shotgun lipidomic analysis (Fig. 6A). Although we did not detect differences in the levels of most classes of lipids, levels of cholesterols and lyso-cardiolipin showed significant changes (effects of apoE isoform on cholesterol: F = 8.7, p = 0.009, η2 = 0.69; effects of apoE isoform on lyso-cardiolipin: F = 11.8, p = 0.003, η2 = 0.72; Fig. 6B&C). Interestingly, cholesterol levels were significantly lower in apoE2-TR mice and showed inverse associations with memory scores in the water maze test (Fig. 6D). Of note, cortical cholesterol levels negatively correlated with apoE levels in brain (r = −0.81, p = 0.009; Fig. 6E) as well as CSF (r = −0.67, p = 0.025; Fig. 6F), suggesting that higher apoE levels are involved in lowering cholesterol levels in the brains of apoE2-TR mice. We also analyzed cholesterol levels in CSF and plasma from young and old apoE-TR mice. Interestingly, CSF cholesterol levels were decreased in old mice (p < 0.001), suggesting that cholesterol metabolism in the brain was altered in an age-dependent manner. However, CSF cholesterol levels tended to be higher in apoE2-TR mice, compared to apoE3- and apoE4-TR mice, which was opposite of the trend in cortical cholesterol levels (Fig. 6G). Plasma cholesterol levels showed similar trends of increase in apoE2-TR mice, and a significant interaction existed between age and apoE isoform (p = 0.020), where cholesterol levels were decreased in aged apoE4-TR mice, but tended to be increased in aged apoE2-TR mice, compared to young mice (Fig. 6H). While CSF cholesterol did not correlate with plasma cholesterol levels (Fig. 6I), a strong positive association between apoE and cholesterol levels in CSF was observed (r = 0.81, p < 0.001; Fig. 6J), indicating that cholesterol metabolism in CSF was significantly regulated by apoE. Interestingly, CSF cholesterol was mildly but significantly associated with the water maze memory score (Fig. 6K). Plasma cholesterol was also associated with memory score (Fig. 6L). Taken together, these results indicate that cholesterol metabolism linked to apoE metabolism might also contribute to the apoE isoform-dependent differences in cognitive decline during aging.
Fig. 6.
Cholesterol levels correlated with memory and apoE levels in aged mice. (A) Lipidomics analysis of individual lipid levels in the cortex of aged apoE-TR mice. (B–C) Levels of cholesterol (B) and lyso-cardiolipin (C) in cortex (CX) are compared among apoE-TR mice at old age (n=4/group, except for cholesterol level, in which n=4/apoE2- & apoE3-TR mice, n=3/apoE4-TR mice). (D–F) Cholesterol levels in cortex are plotted against the percent of time spent in target quadrant at the probe test 24 hours after the last hidden-platform training (D), and apoE levels in the hippocampus (HP, E) and CSF (F). (G–H) Cholesterol levels in CSF (G) and plasma (H) are compared between young and old mice with each apoE isoform, or across apoE isoforms at old age (n=5–11/group for CSF cholesterol, n=16–27/group for plasma cholesterol). (I–L) CSF cholesterol levels (I–K) or plasma cholesterol levels (L) are plotted against, (I) plasma cholesterol levels, (J) CSF apoE levels, and (K&L) percent of time spent in target quadrant at the probe test 24 hours after the last hidden-platform training. Data are presented as mean ± standard error of the mean. PE = phosphatidylethanolamine, CL = cardiolipin, PI = phosphatidylinositol, SM = sphingomyelin, PC = phosphatidylcholine, PA = phosphatidic acids, CBS = cerebroside, PG = phosphatidylglycerol, PS = phosphatidylserine, TG = triglycerides, FA = fatty acid, CHO = cholesterol, CER = ceramide, ST = sulfatide, LPC = lyso-phosphatidylcholine, LCL = lyso-cardiolipin. ##p<0.01, ###p<0.001; compared with young mice, or *p<0.05, **p<0.01, ***p<0.001; compared across apoE isoforms at old age, by two-way ANOVA with post-hoc test (see Materials and Methods; G&H) ) or one-way ANOVA with post-hoc Tukey-Kramer test (B&C). Correlation coefficient (r), 95%CI of r, and p-value were acquired by Pearson correlation test (D–F & I–L). N.S.; not significant
DISCUSSION
Based on our analyses of NACC clinical records and apoE-TR mouse models, we provide evidence that APOE2 can protect against cognitive decline during aging independently of Aβ accumulation. Animal studies further showed that this effect is also independent of age-related synaptic and neuroinflammatory changes. Interestingly, the levels of both apoE and cholesterol in mouse brains were associated with memory function across apoE isoforms at old age. These results provide important implications for how APOE2 protects against age-related cognitive decline as well as AD, in which apoE-cholesterol metabolism plays a central role.
Consistent with previous studies3–6, our results indicate that the effects of APOE genotype on cognitive function become apparent in an age-dependent manner. Such APOE genotype effects include those against memory function deficits,3, 4, 6 whereas these effects may not be remarkable until middle or early elderly age.27, 28 We also demonstrated the protective effects of homozygous APOE2 alleles, which are susceptible to type III hyperlipoproteinemia, while previous studies missed assessing it probably due to the low frequency of APOE2 allele.7, 29 More importantly, we found that effects of APOE genotype on cognitive decline were observed in subjects with little amyloid pathology. Recently, by reviewing NACC records, Serrano-Pozo et al., concluded that APOE2 effects on easing cognitive decline in AD patients were mediated by reducing AD neuropathology. Such alternative conclusion from their study might be derived from differences in subjects (pure AD or MCI subjects, age at death over 50 years old, and not including cognitively intact subjects (CDR SOB = 0)) or statistical models (treating AD neuropathology as simple binary variables in the linear regression for the mediation analysis).30 As we also observed APOE2 protective effects in cognitively impaired subjects, its effects on cognition are unlikely driven by cognitively normal individuals (Supplementary Table 8&9). Though few clinical studies had formerly investigated the relationship between AD neuropathology and cognitive decline mediated by APOE2, Berlau et al. observed that APOE2 was associated with intact cognition despite increased AD neuropathology in the oldest old subjects, and an amyloid imaging study performed by Kantarci et al., reported similar protective effects in APOE2 carriers with amyloid accumulation.20, 31. Together with our findings, these results indicate that APOE2 has AD neuropathology-independent protective effects on cognitive decline during aging, perhaps in addition to its dependent effects.
To our knowledge, this is the first report demonstrating the protective effects of APOE2 on cognitive decline in animal models. Although a recent study by Siegel et al. assessed the effects of APOE2 on age-related cognitive decline, including memory function, in old apoE-TR mice, they did not observe significant differences.16 One possibility is that old mice employed in their study span a broad age spectrum (14 – 22 months old). Moreover, we found prominent APOE genotype-dependent effects in the delayed probe test, but few studies have examined this. In fact, a study by Andrews-Zwilling et al. observed that impairment of memory retention in apoE4-TR mice at 16 months old appeared only in delayed probe test.15
It remains to be clarified how potential changes in synaptic/neuronal integrity or inflammation associated with APOE2 could contribute to cognitive function or AD risk. Brain imaging studies using functional MRI showed cognitively normal healthy carriers of APOE2 had the same direction of changes in brain activity at rest or during tasks as APOE4 carriers compared to APOE3 carriers, and similar effects were seen on white matter integrity,32, 33 although Suri et al. recently reported contradictory results.34 Young apoE-TR mice also displayed reduced long-term potentiation (LTP) and shorter dendritic length in apoE2-TR, which are comparable to apoE4-TR mice.17, 18 Further, similar to the proinflammatory function associated with APOE4, APOE2 is also associated with promoting inflammatory responses.35–37 We also observed synaptic changes across apoE isoforms, which were not associated with cognitive performance, and increased GFAP and proinflammatory cytokine levels in apoE2-TR mice. Although Love et al. observed small but significantly higher levels of PSD95 levels in the superior temporal cortex from normal individuals with APOE2 (17.6% mean difference, 95% CI = 1.5 – 33.4, p = 0.03, adjusted to APOE3 controls),38 we did not observe such difference in the cortex and hippocampus of apoE-TR mice. Importantly, the memory performance in aged apoE2-TR mice was significantly preserved, despite age-dependent synaptic loss and neuroinflammatory changes. While preserved memory function in elderly APOE2 carriers was also shown in several clinical studies,4, 6 age-associated neurodegenerative changes can occur in elderly subjects regardless of retained memory function.20, 31, 39 Taken together, our results suggest the protective effects of APOE2 on memory decline are independent of both age- and apoE isoform-dependent changes of synaptic/neuronal integrity and neuroinflammation.
Recent clinical studies, including a meta-analysis, showed that lower apoE levels in plasma were associated with increased risk of developing AD.40, 41 Though controversies exist, another clinical study showed a negative correlation of CSF apoE levels with the increased risk of AD and its related pathology.42 Our animal study clearly presents significant positive correlations between spatial learning memory and apoE levels in plasma, CSF and brain tissues from aged apoE-TR mice across all isoforms, although we could not detect such significant correlations when analyzed within each individual isoform (data not shown), likely because of small sample numbers and experimental limitations to assess mouse memory function. Indeed, a major obstacle in animal cognitive tasks is the within-group variation in the test performance, which the procedure of experiment itself can affect.43, 44 Alternatively, apoE levels might not fully represent the levels of cholesterol-lipidated forms of apoE, although apoE and cholesterol levels in CSF are positively correlated.45 Such possibility is corroborated by several studies indicating that the lipidation status of apoE has a greater impact on apoE function than apoE levels per se.46, 47 Thus, the levels of lipidated-apoE in brain soluble or CSF fraction might have more clear correlation with memory scores even within each APOE genotype.
While cholesterol is a critical component of the brain, in particular in neuronal membranes, it remains to be clarified how cholesterol contributes to cognitive function and cognitive decline during aging. In vitro experiments have shown that the cholesterol supply to neurons through astrocyte-produced apoE is essential for synaptogenesis.48 Though controversies exist, brain cholesterol is thought to be reduced in an age-dependent manner, suggesting that cognitive decline during aging is accompanied with brain cholesterol loss.49, 50 Adverse events associated with the use of cholesterol lowering statins on cognitive function have also been shown in elderly people.51 However, there is conflicting evidence showing that reduction of cholesterol levels in aged neurons can increase signaling potency through receptor clustering at lipid rafts, and promote cell survival under stress conditions.49, 52 In addition, increases in cholesterol in neurons also promote amyloid-β accumulation and AD-related abnormal pathologies.53, 54 Interestingly, Oikawa et al. demonstrated that cholesterol levels in cortical synapses were decreased in APOE2 carriers without amyloid pathology, compared to APOE3 carriers in aged non-demented individuals.55 We have also found the reduced cortical cholesterol levels in apoE2-TR mice compared to apoE3- and apoE4-TR mice, although cholesterol levels in CSF and plasma were significantly increased in apoE2-TR mice consistent with their apoE levels. These results suggest that intracellular and extracellular cholesterol levels are oppositely regulated by apoE levels and/or isoforms; higher apoE levels associated with APOE2 in extracellular space may lead to more abundant cholesterol loading to apoE2/lipoprotein particles, resulting in less cell-associated cholesterol but more extracellular cholesterol, which might be in equilibrium with CSF cholesterol. As CSF cholesterol levels were decreased at old age (Fig. 6G), such cholesterol turnover required for maintaining cognitive function might be disturbed during aging. Of note, apoE-cholesterol uptake into cells through the LRP1/HSPG pathway, presumably a major pathway in neurons, is not different among apoE isoforms, although apoE2 uptake via the LDLR pathway is substantially decreased.2, 29 Thus, further studies investigating how APOE2 uniquely affects cholesterol levels in specific compartments, such as myelin sheets, neuronal synapses or glial cells might provide additional insight into how APOE2-associated cholesterol metabolism contributes to maintaining cognitive function during aging.
A potential limitation of our study, in particular our animal experiments, is that we did not utilize multiple corrections for analyzing across several molecules or multiple correlations. Importantly, however, the P-values in most of our results that were used to draw the main conclusion were generally very strong (p < 0.001) Regarding brain/CSF cholesterol levels and their relationship with memory scores where P-values were not strong (Fig. 6), these findings might need to be cautiously interpreted, and further study would be warranted to verify our observations.
In summary, our results provide direct evidence that apoE2 can protect against cognitive decline during aging independently of age-related synaptic and neuroinflammatory changes or Aβ accumulation. Moreover, our results suggest that this protective effect might be mediated through regulating apoE-cholesterol metabolism. In terms of therapeutic implication, it warrants additional investigation into whether targeting the apoE-cholesterol pathway by pharmaceutical treatments can prevent cognitive decline during aging independently of Aβ accumulation,56 and how such effects are further influenced by APOE genotype.
Supplementary Material
Acknowledgments
We thank Ms. Sarah E. Monsell for helping with analyzing NACC clinical records, Mr. Michael S. Penuliar for helping behavior experiments, Drs. Mary Jo Ladu and Leon M. Tai for providing apoE ELISA protocol and reagents, Dr. Miao Wang for helping with the lipidomic study, Ms. Caroline Stetler and Ms. Mary D. Davis for careful reading of this manuscript, and Drs. Yu Yamazaki, Masaya Tachibana and other Bu laboratory members for kind help and discussions. This research was supported by grants from the NIH (R01AG035355, R01AG027924, R01AG046205, RF1AG051504, P01NS074969, and P50AG016574) and the Alzheimer’s Association and Cure Alzheimer’s Fund (to G.B.); a NIRG from the Alzheimer’s Association (to T.K.); and fellowship supports from the Japan Heart Foundation, Naito Foundation and BrightFocus Foundation (to M.S.). The NACC database is funded by NIA/NIH Grant U01 AG016976. The NACC contributors are described in the Supplementary Table 10.
Footnotes
Author Contributions
M.S., T.K., J.D.F., and G.B. contributed to the concept and study design. M.S., T.K., L.Y., D.L., M.S., Y.F., L.P., J.L.F., X.H., J.D.F., and G.B., contributed to data acquisition and analysis. M.S. contributed to drafting the manuscript and figures. All authors edited and reviewed the final manuscript.
Conflicts of Interest
Nothing to report.
References
- 1.Liu CC, Kanekiyo T, Xu H, et al. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013 Feb;9(2):106–118. doi: 10.1038/nrneurol.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10(5):333–344. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyman BT, Gomez-Isla T, Briggs M, et al. Apolipoprotein E and cognitive change in an elderly population. Ann Neurol. 1996;40(1):55–66. doi: 10.1002/ana.410400111. [DOI] [PubMed] [Google Scholar]
- 4.Helkala EL, Koivisto K, Hanninen T, et al. Memory functions in human subjects with different apolipoprotein E phenotypes during a 3-year population-based follow-up study. Neurosci Lett. 1996 Feb 9;204(3):177–180. doi: 10.1016/0304-3940(96)12348-x. [DOI] [PubMed] [Google Scholar]
- 5.Staehelin HB, Perrig-Chiello P, Mitrache C, et al. Apolipoprotein E genotypes and cognitive functions in healthy elderly persons. Acta Neurol Scand. 1999 Jul;100(1):53–60. doi: 10.1111/j.1600-0404.1999.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RS, Bienias JL, Berry-Kravis E, et al. The apolipoprotein E epsilon 2 allele and decline in episodic memory. J Neurol Neurosurg Psychiatry. 2002 Dec;73(6):672–677. doi: 10.1136/jnnp.73.6.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suri S, Heise V, Trachtenberg AJ, et al. The forgotten APOE allele: a review of the evidence and suggested mechanisms for the protective effect of APOE varepsilon2. Neurosci Biobehav Rev. 2013 Dec;37(10 Pt 2):2878–2886. doi: 10.1016/j.neubiorev.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan PM, Mezdour H, Quarfordt SH, et al. Type III hyperlipoproteinemia and spontaneous atherosclerosis in mice resulting from gene replacement of mouse Apoe with human Apoe*2. J Clin Invest. 1998 Jul 1;102(1):130–135. doi: 10.1172/JCI2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knouff C, Hinsdale ME, Mezdour H, et al. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J Clin Invest. 1999;103(11):1579–1586. doi: 10.1172/JCI6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Wilson WA, Moore SD, et al. Human apoE4-targeted replacement mice display synaptic deficits in the absence of neuropathology. Neurobiol Dis. 2005;18(2):390–398. doi: 10.1016/j.nbd.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Bour A, Grootendorst J, Vogel E, et al. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav Brain Res. 2008;193(2):174–182. doi: 10.1016/j.bbr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Riddell DR, Zhou H, Atchison K, et al. Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels. J Neurosci. 2008;28(45):11445–11453. doi: 10.1523/JNEUROSCI.1972-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bales KR, Liu F, Wu S, et al. Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice. J Neurosci. 2009;29(21):6771–6779. doi: 10.1523/JNEUROSCI.0887-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castellano JM, Kim J, Stewart FR, et al. Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance. Sci Transl Med. 2011;3(89):3002156. doi: 10.1126/scitranslmed.3002156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews-Zwilling Y, Bien-Ly N, Xu Q, et al. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci. 2010;30(41):13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel JA, Haley GE, Raber J. Apolipoprotein E isoform-dependent effects on anxiety and cognition in female TR mice. Neurobiol Aging. 2012;33(2):345–358. doi: 10.1016/j.neurobiolaging.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trommer BL, Shah C, Yun SH, et al. ApoE isoform affects LTP in human targeted replacement mice. Neuroreport. 2004;15(17):2655–2658. doi: 10.1097/00001756-200412030-00020. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez GA, Burns MP, Weeber EJ, et al. Young APOE4 targeted replacement mice exhibit poor spatial learning and memory, with reduced dendritic spine density in the medial entorhinal cortex. Learn Mem. 2013;20(5):256–266. doi: 10.1101/lm.030031.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): the neuropsychologic test battery. Alzheimer Dis Assoc Disord. 2009;23(2):91–101. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berlau DJ, Corrada MM, Head E, et al. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology. 2009;72(9):829–834. doi: 10.1212/01.wnl.0000343853.00346.a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeda S, Sato N, Uchio-Yamada K, et al. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc Natl Acad Sci U S A. 2010;107(15):7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DeMattos RB, Bales KR, Parsadanian M, et al. Plaque-associated disruption of CSF and plasma amyloid-beta (Abeta) equilibrium in a mouse model of Alzheimer’s disease. J Neurochem. 2002;81(2):229–236. doi: 10.1046/j.1471-4159.2002.00889.x. [DOI] [PubMed] [Google Scholar]
- 23.Shinohara M, Fujioka S, Murray ME, et al. Regional distribution of synaptic markers and APP correlate with distinct clinicopathological features in sporadic and familial Alzheimer’s disease. Brain. 2014;137(Pt 5):1533–1549. doi: 10.1093/brain/awu046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang K, Cheng H, Gross RW, et al. Automated lipid identification and quantification by multidimensional mass spectrometry-based shotgun lipidomics. Anal Chem. 2009;81(11):4356–4368. doi: 10.1021/ac900241u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang K, Han X. Accurate quantification of lipid species by electrospray ionization mass spectrometry - Meet a key challenge in lipidomics. Metabolites. 2011;1(1):21–40. doi: 10.3390/metabo1010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altmann A, Tian L, Henderson VW, et al. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75(4):563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunce D, Bielak AA, Anstey KJ, et al. APOE genotype and cognitive change in young, middle-aged, and older adults living in the community. J Gerontol A Biol Sci Med Sci. 2014;69(4):379–386. doi: 10.1093/gerona/glt103. [DOI] [PubMed] [Google Scholar]
- 28.Jorm AF, Mather KA, Butterworth P, et al. APOE genotype and cognitive functioning in a large age-stratified population sample. Neuropsychology. 2007;21(1):1–8. doi: 10.1037/0894-4105.21.1.1. [DOI] [PubMed] [Google Scholar]
- 29.Mahley RW, Huang Y, Rall SC. Pathogenesis of type III hyperlipoproteinemia (dysbetalipoproteinemia): questions, quandaries, and paradoxes. Journal of Lipid Research. 1999 Nov 1;40(11):1933–1949. [PubMed] [Google Scholar]
- 30.Serrano-Pozo A, Qian J, Monsell SE, et al. APOEepsilon2 is associated with milder clinical and pathological Alzheimer disease. Ann Neurol. 2015;77(6):917–929. doi: 10.1002/ana.24369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kantarci K, Lowe V, Przybelski SA, et al. APOE modifies the association between Abeta load and cognition in cognitively normal older adults. Neurology. 2012;78(4):232–240. doi: 10.1212/WNL.0b013e31824365ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trachtenberg AJ, Filippini N, Cheeseman J, et al. The effects of APOE on brain activity do not simply reflect the risk of Alzheimer’s disease. Neurobiol Aging. 2012;33(3):12. doi: 10.1016/j.neurobiolaging.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Westlye LT, Reinvang I, Rootwelt H, et al. Effects of APOE on brain white matter microstructure in healthy adults. Neurology. 2012;79(19):1961–1969. doi: 10.1212/WNL.0b013e3182735c9c. [DOI] [PubMed] [Google Scholar]
- 34.Suri S, Mackay CE, Kelly ME, et al. Reduced cerebrovascular reactivity in young adults carrying the APOE epsilon4 allele. Alzheimers Dement. 2015;11(6):648–657. doi: 10.1016/j.jalz.2014.05.1755. [DOI] [PubMed] [Google Scholar]
- 35.Maezawa I, Maeda N, Montine TJ, et al. Apolipoprotein E-specific innate immune response in astrocytes from targeted replacement mice. J Neuroinflammation. 2006;3:10. doi: 10.1186/1742-2094-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsoi LM, Wong KY, Liu YM, et al. Apoprotein E isoform-dependent expression and secretion of pro-inflammatory cytokines TNF-alpha and IL-6 in macrophages. Arch Biochem Biophys. 2007;460(1):33–40. doi: 10.1016/j.abb.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 37.Kuhel DG, Konaniah ES, Basford JE, et al. Apolipoprotein E2 accentuates postprandial inflammation and diet-induced obesity to promote hyperinsulinemia in mice. Diabetes. 2013;62(2):382–391. doi: 10.2337/db12-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Love S, Siew LK, Dawbarn D, et al. Premorbid effects of APOE on synaptic proteins in human temporal neocortex. Neurobiol Aging. 2006;27(6):797–803. doi: 10.1016/j.neurobiolaging.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Fjell AM, McEvoy L, Holland D, et al. Brain changes in older adults at very low risk for Alzheimer’s disease. J Neurosci. 2013;33(19):8237–8242. doi: 10.1523/JNEUROSCI.5506-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rasmussen KL, Tybjaerg-Hansen A, Nordestgaard BG, et al. Plasma levels of apolipoprotein E and risk of dementia in the general population. Ann Neurol. 2015;77(2):301–311. doi: 10.1002/ana.24326. [DOI] [PubMed] [Google Scholar]
- 41.Wang C, Yu JT, Wang HF, et al. Meta-analysis of peripheral blood apolipoprotein E levels in Alzheimer’s disease. PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0089041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cruchaga C, Kauwe JSK, Nowotny P, et al. Cerebrospinal fluid APOE levels: an endophenotype for genetic studies for Alzheimer’s disease. Human Molecular Genetics. 2012 Oct 15;21(20):4558–4571. doi: 10.1093/hmg/dds296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolfer DP, Stagljar-Bozicevic M, Errington ML, et al. Spatial Memory and Learning in Transgenic Mice: Fact or Artifact? News Physiol Sci. 1998;13:118–123. doi: 10.1152/physiologyonline.1998.13.3.118. [DOI] [PubMed] [Google Scholar]
- 44.Lathe R. The individuality of mice. Genes Brain Behav. 2004;3(6):317–327. doi: 10.1111/j.1601-183X.2004.00083.x. [DOI] [PubMed] [Google Scholar]
- 45.Vuletic S, Kennedy H, Albers JJ, et al. Cerebrospinal fluid apolipoprotein E and phospholipid transfer protein activity are reduced in multiple sclerosis; relationships with the brain MRI and CSF lipid variables. Mult Scler Relat Disord. 2014;3(4):533–541. doi: 10.1016/j.msard.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koldamova R, Fitz NF, Lefterov I. The role of ATP-binding cassette transporter A1 in Alzheimer’s disease and neurodegeneration. Biochim Biophys Acta. 2010;8:824–830. doi: 10.1016/j.bbalip.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirsch-Reinshagen V, Burgess BL, Wellington CL. Why lipids are important for Alzheimer disease? Mol Cell Biochem. 2009;326(1–2):121–129. doi: 10.1007/s11010-008-0012-2. [DOI] [PubMed] [Google Scholar]
- 48.Mauch DH, Nagler K, Schumacher S, et al. CNS synaptogenesis promoted by glia-derived cholesterol. Science. 2001;294(5545):1354–1357. doi: 10.1126/science.294.5545.1354. [DOI] [PubMed] [Google Scholar]
- 49.Ledesma MD, Martin MG, Dotti CG. Lipid changes in the aged brain: effect on synaptic function and neuronal survival. Prog Lipid Res. 2012;51(1):23–35. doi: 10.1016/j.plipres.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 50.van Vliet P. Cholesterol and late-life cognitive decline. J Alzheimers Dis. 2012;30(2):2011–111028. doi: 10.3233/JAD-2011-111028. [DOI] [PubMed] [Google Scholar]
- 51.Shinohara M, Sato N, Shimamura M, et al. Possible modification of Alzheimer’s disease by statins in midlife: interactions with genetic and non-genetic risk factors. Front Aging Neurosci. 2014;6(71) doi: 10.3389/fnagi.2014.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sodero AO, Trovò L, Iannilli F, et al. Regulation of tyrosine kinase B activity by the Cyp46/cholesterol loss pathway in mature hippocampal neurons: relevance for neuronal survival under stress and in aging. Journal of Neurochemistry. 2011;116(5):747–755. doi: 10.1111/j.1471-4159.2010.07079.x. [DOI] [PubMed] [Google Scholar]
- 53.Marquer C, Laine J, Dauphinot L, et al. Increasing membrane cholesterol of neurons in culture recapitulates Alzheimer’s disease early phenotypes. Mol Neurodegener. 2014;9(60):1750–1326. doi: 10.1186/1750-1326-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Paolo G, Kim TW. Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci. 2011;12(5):284–296. doi: 10.1038/nrn3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oikawa N, Hatsuta H, Murayama S, et al. Influence of APOE genotype and the presence of Alzheimer’s pathology on synaptic membrane lipids of human brains. J Neurosci Res. 2014;92(5):641–650. doi: 10.1002/jnr.23341. [DOI] [PubMed] [Google Scholar]
- 56.Cramer PE, Cirrito JR, Wesson DW, et al. ApoE-directed therapeutics rapidly clear beta-amyloid and reverse deficits in AD mouse models. Science. 2012;335(6075):1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.