Abstract
Purpose
While major prostate cancer active surveillance (AS) programs recommend repeat testing such as PSA and prostate biopsy, compliance with such testing is unknown. Our objective was to determine whether men in the community receive the same intensity of AS testing as in prospective AS protocols.
Materials and Methods
We performed a retrospective cohort study of men aged ≥66 in the SEER-Medicare database diagnosed with prostate cancer from 2001–2009 who did not receive curative therapy in the year after diagnosis with ≥1 post-diagnosis prostate biopsy. We used multivariable-adjusted Poisson regression to determine the association between frequency of AS testing with patient demographics and clinical features. Among 1349 men with 5 years follow-up, we determined the proportion undergoing testing of the intensity recommended by the Sunnybrook and PRIAS programs (≥14 PSA and ≥2 biopsy), and at Johns Hopkins (≥10 PSA and ≥4 biopsy).
Results
Among 5192 patients undergoing AS, >80% had ≥1 PSA test per year but <13% received biopsy beyond the first 2 years. MRI was rarely used during the study period. On multivariable analysis, recent diagnosis and higher income were associated with higher frequency of surveillance biopsy, while older age and greater comorbidity were associated with fewer biopsies. African American men underwent fewer PSAs but similar numbers of biopsies. During 5 years of AS, only 11.1% and 5.0% met the testing standards of the Sunnybrook/PRIAS and Johns Hopkins programs.
Conclusions
In the community, very few elderly men receive the intensity of AS testing recommended by major prospective AS programs.
Keywords: prostate cancer, active surveillance, expectant management, watchful waiting, treatment
Introduction
Overtreatment of PCa is a significant public health concern, and many men with favorable-risk PCa may be managed with active surveillance (AS). This approach reduces treatment-related morbidity without affecting oncological outcomes and is recommended by several professional societies.1
While use of AS is increasing,2–5 there is little consensus over what AS actually entails. There is significant variation among published AS protocols, which offer starkly different implications for patient burden and healthcare resource use. For example, the Johns Hopkins program recommends prostate specific antigen (PSA) checks every 6 months, annual prostate biopsies until age 75, and, recently, magnetic resonance imaging (MRI) for men with PSA>10 ng/ml.6 By contrast, the Sunnybrook AS program in Toronto includes PSA measurements every 3 months for 2 years, then every 6 months. Confirmatory biopsy is recommended within 1 year of enrollment, with subsequent biopsy every 3–4 years.7 Finally, the Prostate Cancer Research International Active Surveillance (PRIAS) protocol includes PSA every 3 months for 2 years then every 6 months, with biopsies at years 1, 4 and 7.8
Previous studies have shown that most men on AS remain treatment-free at 5 and 10 years when adhering to one of these regimens. Actual adherence and AS effectiveness are important for men with a significant life expectancy given the persistent hazard of PCa progression even at 20 years.7 Conversely, excessive and invasive surveillance testing may unnecessarily increase morbidity for elderly men with significant comorbidity and a low risk of PCa death. There is a knowledge gap regarding AS patterns of care in the community and whether they conform to widely accepted regimens.
Our objective was to determine whether real-world practice patterns in a nationally-representative sample of US PCa patients conform to the intensity of surveillance in published prospective protocols. We hypothesized that men on AS would have ≥2 PSAs per year and 2 biopsies during a 5 year period, representing the minimum frequency of testing in prospective surveillance cohorts.
Methods
We performed a retrospective cohort study of men with localized PCa deferring initial curative therapy. Using the Surveillance, Epidemiology and End Results (SEER)-Medicare linked database with hospital, outpatient and physician claims, we identified 427,592 men diagnosed with prostate adenocarcinoma from 2001–2009. SEER is a collection of population-based cancer registries encompassing approximately 30% of the US population.9 We excluded men with advanced/metastatic PCa (≥T3), age <66, or missing diagnosis month and year. We also excluded men without continuous Medicare Parts A and B coverage, those diagnosed on autopsy or who died within 3 months of diagnosis. Using ICD-9-CM codes, we excluded men who received radical prostatectomy, radiation, cryotherapy or androgen deprivation therapy <1 year after diagnosis (Supplemental Table 1a).10
This left 74,992 men undergoing conservative management for PCa from which we excluded n=69,800 men without repeat prostate biopsy during 2 years after diagnosis, leaving a final study population of n=5192 men undergoing AS (Supplemental Figure 1). Compared to all conservatively managed men, the study population meeting our definition for AS was significantly younger and diagnosed more recently. Men were censored at the time of treatment if they received any treatment (prostatectomy, radiation therapy, cryotherapy or hormonal therapy), at death, or otherwise as of December 31, 2011. Mean and median follow-up times were 46.5 and 37.5 months (range, 12.2–133.9).
Our primary endpoint was the proportion of men receiving the testing intensity recommended by the Sunnybrook, PRIAS and Johns Hopkins programs. We also examined MRI use during AS (Supplemental Table 1b), and determined the proportion of men exceeding 10 PSAs or 5 biopsies within 5 years, the maximum intensity recommended by the National Comprehensive Cancer Network (NCCN) guidelines.11
Independent variables of interest were diagnosis year, age (years), race (white, black, other), Elixhauser comorbidity index with the Klabunde modification (0 versus 1+)12, marital status (single/divorced/widowed versus married), median income in the patient’s home zip code (above versus below median), biopsy Gleason score (≤6, >6) and clinical stage (≤T2a, >T2a). PSA data are currently unavailable in SEER so were not included in risk classification or analysis.13
Statistical Analysis
All statistical analyses were performed using SAS version 9.3 (Cary, NC). We used multivariable-adjusted Poisson regression, clustering patients by SEER registry, to determine the association between frequency of surveillance biopsies and independent variables. Natural log of the number of follow-up months was used to offset the varying length of follow-up per patient. This analysis was repeated in the subset of 1391 men with low-risk features (≤cT2a and Gleason ≤6) diagnosed during the last 5 years of the study (2004–2009), who are more similar to contemporary AS patients.
Subset analysis was also performed in men with 5 years of follow-up to determine the total number of tests received and how many fulfilled the testing combination recommended by major AS protocols, with data censored at exactly ≥5 years (e.g. over 5 years Sunnybrook and PRIAS require 14 PSAs and 2 follow-up biopsies, while Johns Hopkins requires 10 PSAs and ≥4 biopsies).8 We performed a subset analysis of men diagnosed under age 70, since the Johns Hopkins program discontinues biopsies at age 7514 These calculations were also repeated in low-risk men diagnosed during the last 5 years of the study.
Results
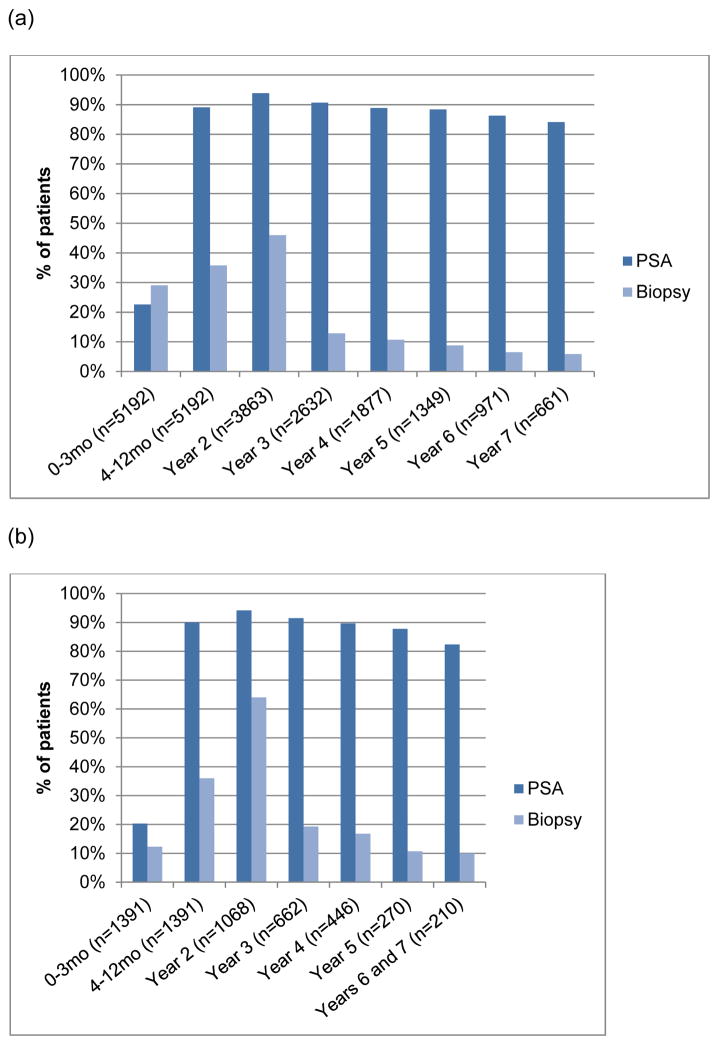
Our cohort contains 5192 men diagnosed with localized PCa from 2001–2009 and undergoing AS. The median age was 73.5, 9.7% were African American, and 76.8% had a comorbidity score of 0 (Table 1).The vast majority of men had at least one PSA per year (83.3–93.9% depending on the year). The proportion of patients undergoing surveillance biopsies declined over time (Figure 1a). Biopsy was performed in 29.1% during months 0–3, 35.8% in months 4–12, and 46.0% in year 2, with a median of 217 days until the first repeat biopsy (range, 32–730). By year 3, this dropped to 12.9% and by year 7, only 5.9% of men still on AS underwent biopsy. In the contemporary low-risk men (Figure 1b), >82% had at least 1 PSA test per year (ranging from 82–94% depending on the year). In this subset, 12.3% had re-biopsy within 1–3 months, 36% at 4–12 months, and 64% in year 2, with <19% having a biopsy during any subsequent year. MRI was rarely used during the study period (≤4% during any given year, Supplemental Table 2).
Table 1.
Demographics of the study population
| Overall population (n=5192) # (%) | Contemporary low- risk (n=1391) # (%) | |
|---|---|---|
|
| ||
| Age | ||
| Median | 73.5 | 72.2 |
| <70 | 1518 (29.2%) | 502 (36.1%) |
| 70–79 | 2907 (56.0%) | 782 (56.2%) |
| 80+ | 767 (14.8%) | 107 (7.7%) |
|
| ||
| Race | ||
| Caucasian | 4324 (83.3%) | 1179 (84.8%) |
| Black | 505 (9.7%) | 126 (9.1%) |
| Other/missing | 363 (7.0%) | 86 (6.2%) |
|
| ||
| Elixhauser comorbidity index | ||
| 0 | 3986 (76.8%) | 1067 (76.7%) |
| 1+ | 1206 (23.2%) | 324 (23.3%) |
|
| ||
| Median income in zip code (quartiles) | ||
| Below median | 2500 (48.2%) | 588 (42.3%) |
| Above median | 2563 (49.4%) | 762 (54.8%) |
| Missing | 129 (2.5%) | 41 (3.0%) |
|
| ||
| Married | ||
| Yes | 3365 (64.8%) | 946 (68.0%) |
| No | 1021 (19.7%) | 223 (16.0%) |
| Missing | 806 (15.5%) | 222 (16.0%) |
|
| ||
| Diagnosis year | ||
| 2001 | 533 (10.3%) | -- |
| 2002 | 526 (10.1%) | -- |
| 2003 | 513 (9.9%) | -- |
| 2004 | 489 (9.4%) | 158 (11.4%) |
| 2005 | 515 (9.9%) | 169 (12.2%) |
| 2006 | 597 (11.5%) | 222 (16.0%) |
| 2007 | 607 (11.7%) | 250 (18.0%) |
| 2008 | 650 (12.5%) | 250 (18.0%) |
| 2009 | 762 (14.7%) | 342 (24.6%) |
|
| ||
| Clinical stage (since 2004) | ||
| ≤T2a | 2188 (60.4%) | 1391 (100%) |
| ≥T2b | 1432 (39.6%) | -- |
|
| ||
| Gleason score (since 2004) | ||
| ≤6 | 2023 (55.9%) | 1391 (100%) |
| >6 | 1331 (36.8%) | -- |
| Missing | 266 (7.3%) | -- |
Figure 1.
Proportion of men receiving a PSA test and biopsy during each year of active surveillance in the (a) overall study population (n=5192) and (b) contemporary low-risk subset (n=1391).
On multivariable analysis, PSAs during AS were more frequent in men with stage ≥T2a and Gleason>6 (Table 2a). Surveillance biopsies were less frequently performed with increasing age, comorbidity, and other/missing race; whereas, higher income, and more recent year of diagnosis predicted greater frequency of biopsy. In the contemporary low-risk group, higher income was associated with a trend toward more PSA testing (Table 2b). Biopsies were less frequent with older age and greater comorbidity, but were more frequent during later years. Factors associated with more MRIs included higher income, more recent diagnosis and Gleason>6 (Supplemental Table 3). Age >80 and higher comorbidity score significantly predicted fewer MRIs.
Table 2.
Multivariable analysis to predict number of PSA tests and biopsies during active surveillance in the (a) overall population (n=5192) and (b) the contemporary low-risk subset (n=1391).
| (a)
| ||
|---|---|---|
| Variable | PSA OR (95% CI), p-value |
Biopsy OR (95% CI), p-value |
|
| ||
| Age | ||
| <70 | 1 (ref.) | 1 (ref.) |
| 70–79 | 1.0 (0.96 – 1.04), 0.86 | 0.91 (0.87 – 0.95), <0.0001 |
| 80+ | 0.99 (0.91 – 1.07), 0.80 | 0.86 (0.82 – 0.90), <0.0001 |
|
| ||
| Race | ||
| White | 1 (ref.) | 1 (ref.) |
| Black | 0.93 (0.84 – 1.03), 0.17 | 0.97 (0.91 – 1.02), 0.25 |
| Other/Missing | 0.92 (0.81 – 1.04), 0.17 | 0.94 (0.89 – 0.99), 0.02 |
|
| ||
| Charlson comorbidity index | ||
| 0 | 1 (ref.) | 1 (ref.) |
| 1+ | 0.97 (0.93 – 1.01), 0.18 | 0.93 (0.90 – 0.97), 0.002 |
|
| ||
| Income level | ||
| Below median | 0 (ref.) | 1 (ref.) |
| Above Median | 1.01 (0.99 – 1.03), 0.21 | 1.01 (1.00 – 1.02), 0.02 |
|
| ||
| Married | 0.99 (0.92 – 1.06), 0.76 | 0.96 (0.90 – 1.02), 0.21 |
|
| ||
| Diagnosis year | 1.01 (0.99 – 1.03), 0.19 | 1.13 (1.11 – 1.14), <.0001 |
|
| ||
| Clinical stage | ||
|
| ||
| ≤T2a | 1 (ref.) | 1 (ref.) |
| >T2a | 1.09 (1.05 – 1.13), <.0001 | 0.99 (0.94 – 1.04), 0.71 |
|
| ||
| Gleason score | ||
|
| ||
| ≤6 | 1 (ref.) | 1 (ref.) |
| >6 | 1.37 (1.27 – 1.48), <.0001 | 0.96 (0.92 – 1.00), 0.05 |
| (b)
| ||
|---|---|---|
| Variable | PSA OR (95% CI), p-value |
Biopsy OR (95% CI), p-value |
|
| ||
| Age | ||
| <70 | 1 (ref.) | 1 (ref.) |
| 70–79 | 1.02 (0.95 – 1.08), 0.52 | 0.88 (0.81 – 0.94), 0.0006 |
| 80+ | 0.97 (0.91 – 1.04), 0.35 | 0.74 (0.69 – 0.80), <.0001 |
|
| ||
| Race | ||
| White | 1 (ref.) | 1 (ref.) |
| Black | 1.00 (0.91 – 1.10), 0.99 | 1.03 (0.94 – 1.14), 0.58 |
| Other/Missing | 0.91 (0.78 – 1.05), 0.20 | 0.93 (0.86 – 1.01), 0.10 |
|
| ||
| Charlson comorbidity index | ||
| 0 | 1 (ref.) | 1 (ref.) |
| 1+ | 0.99 (0.92 – 1.07), 0.85 | 0.89 (0.84 – 0.94), <.0001 |
|
| ||
| Income level | ||
| Below Median | 1 (ref.) | 1 (ref.) |
| Above Median | 1.02 (1.00 – 1.05), 0.06 | 1.01 (1.00 – 1.03), 0.09 |
|
| ||
| Married | 1.04 (0.94 – 1.15), 0.47 | 1.00 (0.92 – 1.09), 0.92 |
|
| ||
| Diagnosis year | 1.01 (0.99 – 1.03), 0.46 | 1.12 (1.10 – 1.14), <.0001 |
Among 1349 men with exactly 5 years of follow-up (Table 3), only 34% had two surveillance biopsies, 14.5% had 3, and 6.6% had ≥4. The majority had ≥1 PSA test per year (90.6%) and 12.4% had ≥1 MRI. Only 150 (11.1%) men met the Sunnybrook/PRIAS recommendations of ≥14 PSAs and 2 biopsies, and 68 (5.0%) fulfilled the Johns Hopkins recommendation ≥10 PSAs and ≥4 biopsies during 5 years. Among men <70 at diagnosis, 33 of 402 (8.2%) fulfilled the Johns Hopkins testing protocol. Compared with the maximum frequency of testing suggested by the NCCN guidelines, 798 (59.2%) men had >10 PSAs and 28 (2.1%) had ≥5 biopsies during a 5 year period.
Table 3.
Total number of tests received during 5 years of follow-up for prostate cancer patients on active surveillance
| Total tests received during 5 years of active surveillance | # (%) of all men n=1349 |
# (%) of low risk n=258 |
|---|---|---|
| Median PSA tests (IQR) | 11 (8) | 10 (5) |
| ≥5 PSA tests (every year) | 1222 (90.6%) | 242 (93.8%) |
| ≥10 PSA tests (every 6 mos for 5 years) | 798 (59.2%) | 152 (58.9%) |
| ≥20 PSA tests (every 3 mos for 5 years) | 138 (10.2%) | 18 (7.0%) |
| Median MRI (IQR) | 0 (0) | 0 (0) |
| ≥1 MRI | 167 (12.4%) | 31 (12.0%) |
| ≥2 MRI | 48 (4.7%) | 31 (12.0%) |
| ≥3 MRI | 48 (4.7%) | 11 (4.3%) |
| Median biopsies after diagnosis* | 1 (1) | 1 (1) |
| ≥2 biopsies after diagnosis* | 458 (34.0%) | 119 (46.1%) |
| ≥3 biopsies after diagnosis* | 196 (14.5%) | 53 (20.5%) |
| ≥4 biopsies after diagnosis* | 89 (6.6%) | 27 (10.5%) |
| Combo: ≥10 PSA tests and ≥2 biopsies | 296 (21.9%) | 83 (32.2%) |
| Combo (Prostate Cancer Research International Active Surveillance study 8): ≥14 PSA and ≥2 biopsies | 150 (11.1%) | 34 (13.2%) |
| Combo (Hopkins14): ≥10 PSA tests and ≥4 biopsies | 68 (5.0%) | 23 (8.9%) |
All men were required to have one biopsy within 2 years after diagnosis for inclusion in the study, so 2 biopsies means 1 additional biopsy beyond the necessary biopsy for inclusion to the cohort. These categories are not mutually exclusive (i.e. men who had ≥4 biopsies after diagnosis are also counted as having ≥3 and ≥2 biopsies.
Among low-risk patients with 5-years follow-up, 93.8% had ≥1 PSA per year, 12.0% had ≥1 MRI, and 46.1% had at least one additional surveillance biopsy. In this subset, 34 (13.2%) and 23 (8.9%) men met the testing standards used by the PRIAS and Johns Hopkins programs, respectively. Among 95 low risk men age <70 at diagnosis with 5 years of follow-up, 15 (15.8%) fulfilled the Johns Hopkins protocol.
Discussion
Our study shows that AS is not very "active" in US PCa patients age >65. Although most men on AS had ≥1 PSA per year, only a minority underwent re-biopsy after year two. During a 5 year period, only 11.1% and 5.0% received testing commensurate with the PRIAS and Johns Hopkins protocols, respectively. These data suggest that real-world practice for elderly PCa patients across the US differs substantially from follow-up protocols used in published prospective AS series.
Overall, the issue of compliance with follow-up testing during AS has received little attention. Bul et al. recently reported updated data from PRIAS with a median follow-up of 1.6 years.8 75.6% men remained on AS, 21.1% had active treatment, 0.8% switched to WW, and 1.7% were lost to follow-up. Compliance with the first surveillance biopsy was 81%. In the Johns Hopkins AS program, the median time spent on AS free from curative intervention was 8.5 years.15 Through 12 years of follow-up, overall biopsy compliance was 89% (ranging from 79 to 100% each year).14 By contrast, in our real-world population, only 5.0% of men adhered to these standards, receiving the 10 PSA tests and 5 biopsies required by Johns Hopkins over 5 years.
Compliance with AS has had mixed results in other populations. A small pilot study of 27 inner-city men followed for 1 year reported 82% adherence with the testing protocol (PSA every 3 months, annual DRE and repeat biopsy).16 By contrast, at a US veterans hospital, only 24 of 45 men (53.3%) complied with the protocol-recommended biopsy at 1 year.17 Both reports had short follow-up so long-term adherence with AS in these settings is unknown.
Finally, Ritchey et al. examined quality of care indicators among 13,876 men with early stage PCa from 2000–2001 without initial definitive treatment.18 Although this was a mixed population including WW and hormonal therapy, they identified compliance with follow-up testing as a major area for quality improvement. More recently, Chamie et al. examined quality of care during the first 2 years after diagnosis in SEER-Medicare from 2004–2007.19 Only 166 of 3656 conservatively managed patients (4.5%) fulfilled their arbitrarily-defined AS quality indicator: ≥4 PSAs, 4 office visits, and repeat biopsy within 2 years after diagnosis.
Our results including 5192 men with follow-up through 2011 suggest that surveillance testing except PSA is uncommon beyond the first two years after diagnosis and very few men actually receive the testing intensity recommended by major prospective AS programs. The underlying reason for this low level of testing utilization is unknown. Perhaps this is because the association between intensity of follow-up testing and disease-specific outcomes is unknown. On one hand, elderly men have more aggressive disease and higher rates of reclassification on AS biopsies.20 Conversely, the long natural history of PCa and competing risk for mortality, less intense follow-up in elderly men may not affect oncologic outcomes and may even be favorable by reducing the risks of invasive follow-up testing such as biopsy.21 If there is no difference in outcome between intense surveillance and little surveillance or even WW in certain patient populations, then we should do less. Meanwhile, it is possible that younger healthy men with a long life expectancy may benefit from more intensive follow-up testing to identify progressive disease within the window of curability. Clinical trials like the Study of Active Monitoring in Sweden22 and modeling studies will help address these unanswered questions.
Limitations of our study include the use of SEER-Medicare data, which only includes men aged ≥65 in SEER regions. The median age at PCa diagnosis in the US is 66 years. Although most prospective surveillance protocols do not specify age-tailored follow-up, it is nevertheless possible that men <65 years may undergo more intensive testing than observed in this population. Also, SEER-Medicare does not differentiate AS from WW, so we used the absence of treatment within 1 year and ≥1 biopsy within 2 years post-diagnosis to define the cohort. However, the true intention of the patients and physicians is unknown leading to possible misclassification. Some on AS may not have had a follow-up biopsy at all, and their inclusion would further reduce the proportion receiving follow-up biopsies. The results of surveillance testing are unknown, and it is possible for example that stable PSA values over time or finding no cancer on follow-up biopsy may have led to less frequent testing thereafter. Some elderly men may have converted to WW during follow-up. Although not used frequently during the study period, increasing use of MRI may also impact the number of biopsies performed. Also, PSA values are currently unavailable for analysis in SEER-Medicare,13 limiting our risk classification and precluding its inclusion in the analysis. Finally, men in our study were only followed through 2011. Although major AS programs were already ongoing in North America, mainstream adoption of conservative management increased dramatically since 2010.5 Therefore, this study reflects the early adoption period of AS in the US. While later year of diagnosis was significantly associated with the frequency of imaging and biopsy, these numbers may be expected to change in the future, particularly with expanding use of MRI.23
Strengths of our data include a large US sample of men diagnosed during a 9 year period with up to 11 years of follow-up, allowing an evaluation of real-world AS utilization patterns across a long interval. Men with HMO enrollment or non-continuous coverage were excluded to avoid incomplete capture of testing, and subset analysis of men with 5 years of follow-up allowed us to calculate the total number of tests received during a standardized interval.
In conclusion, US men age >65 traditionally did not receive follow-up testing at levels consistent with standards of major prospective AS protocols. While most men receive ≥1 PSA per year, biopsies are performed infrequently after the first 2 years even though numerous protocols recommend otherwise. Since long-term data on AS outcomes are based on structured protocols with a greater intensity of testing, patients should be counseled on the possibility that less frequent monitoring could result in a greater likelihood of missing the window of curability should their disease progress or become reclassified as more aggressive. Additional studies are needed to determine the association of surveillance intensity with oncologic outcomes and quality of life in order to optimize AS for patients and encourage its broad adoption.
Supplementary Material
Acknowledgments
We would like to thank Sasha DeWitt for her input on the study.
Funding: This work was supported by the Laura & Issac Perlmutter NYU Cancer Center (to SL and DVM); the Louis Feil Charitable Lead Trust (to SL and DM); and the National Cancer Institute at the National Institutes of Health (Award Number K07CA178258 to SL). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AS
active surveillance
- PCa
prostate cancer
- PSA
prostate specific antigen
- MRI
magnetic resonance imaging
- NCCN
National Comprehensive Cancer Network
- PRIAS
Prostate Cancer Research International Active Surveillance
- WW
watchful waiting
Footnotes
Previous presentations: Abstract presented at the American Urological Association 2015 meeting
Disclosures: SL reports an ad board for Bayer. HL reports the following disclosures: MedReviews (co-owner), Watson (Speaker Bureau), Serenity (consultant/investor), Thera Coat (Consultant), Sonacare (Investor), Biozeus (consultant). DM is a consultant for Castlight health, LLC and for US FDA
References
- 1.National Comprehensive Cancer Network. [Accessed January 25, 2016];Clinical Practice Guidelines: Prostate Cancer Treatment. 2016 http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 2.Womble PR, Montie JE, Ye Z, et al. Contemporary Use of Initial Active Surveillance Among Men in Michigan with Low-risk Prostate Cancer. Eur Urol. 2015;67:44. doi: 10.1016/j.eururo.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 3.Loeb S, Berglund A, Stattin P. Population Based Study of Use and Determinants of Active Surveillance and Watchful Waiting for Low and Intermediate Risk Prostate Cancer. The Journal of urology. 2013 doi: 10.1016/j.juro.2013.05.054. [DOI] [PubMed] [Google Scholar]
- 4.Weerakoon M, Papa N, Lawrentschuk N, et al. The current use of active surveillance in an Australian cohort of men: a pattern of care analysis from the Victorian Prostate Cancer Registry. BJU Int. 2015;115(Suppl 5):50. doi: 10.1111/bju.13049. [DOI] [PubMed] [Google Scholar]
- 5.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA. 2015;314:80. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 6.Carter HB, Walsh PC, Landis P, et al. Expectant management of nonpalpable prostate cancer with curative intent: preliminary results. J Urol. 2002;167:1231. [PubMed] [Google Scholar]
- 7.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 8.Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. European urology. 2013;63:597. doi: 10.1016/j.eururo.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 9. [Accessed February 8, 2016]; http://seer.cancer.gov/about/overview.html.
- 10.Filson CP, Schroeck FR, Ye Z, et al. Variation in use of active surveillance among men undergoing expectant treatment for early stage prostate cancer. J Urol. 2014;192:75. doi: 10.1016/j.juro.2014.01.105. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. [Accessed June 13, 2014];Clinical Practice Guidelines: Prostate Cancer. 2014 http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- 12.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 13.Furlow B. US National Cancer Institute investigates PSA coding errors. Lancet Oncol. 2015 doi: 10.1016/S1470-2045(15)70196-8. [DOI] [PubMed] [Google Scholar]
- 14.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 15.Tosoian JJ, Mamawala M, Epstein JI, et al. Intermediate and Longer-Term Outcomes From a Prospective Active-Surveillance Program for Favorable-Risk Prostate Cancer. J Clin Oncol. 2015 doi: 10.1200/JCO.2015.62.5764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anai S, Nakamura K, Chang MN, et al. The feasibility of expectant management with inner-city men with newly diagnosed localized prostate cancer. J Health Care Poor Underserved. 2008;19:164. doi: 10.1353/hpu.2008.0024. [DOI] [PubMed] [Google Scholar]
- 17.Lee EK, Baack J, Penn H, et al. Active surveillance for prostate cancer in a veteran population. The Canadian journal of urology. 2010;17:5429. [PubMed] [Google Scholar]
- 18.Ritchey J, Gay EG, Spencer BA, et al. Assessment of the quality of medical care among patients with early stage prostate cancer undergoing expectant management in the United States. J Urol. 2012;188:769. doi: 10.1016/j.juro.2012.04.106. [DOI] [PubMed] [Google Scholar]
- 19.Chamie K, Williams SB, Hershman DL, et al. Population-based assessment of determining predictors for quality of prostate cancer surveillance. Cancer. 2015;121:4150. doi: 10.1002/cncr.29574. [DOI] [PubMed] [Google Scholar]
- 20.GLOBOCAN. [Accessed September 20, 2015];Prostate Cancer Fact Sheet. 2012 http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- 21.Eggener S. How active should active surveillance be? BJU Int. 2015;115:176. doi: 10.1111/bju.12859. [DOI] [PubMed] [Google Scholar]
- 22.Bratt O, Carlsson S, Holmberg E, et al. The Study of Active Monitoring in Sweden (SAMS): a randomized study comparing two different follow-up schedules for active surveillance of low-risk prostate cancer. Scand J Urol. 2013;47:347. doi: 10.3109/21681805.2013.813962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fascelli M, George AK, Frye T, et al. The role of MRI in active surveillance for prostate cancer. Curr Urol Rep. 2015;16:42. doi: 10.1007/s11934-015-0507-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.