Abstract
The purpose of this study was to evaluate two practical interval training protocols on cardiorespiratory fitness, lipids, and body composition in overweight/obese women. Thirty women (mean ± SD; Weight: 88.1 ± 15.9 kg; BMI: 32.0 ± 6.0 kg·m2) were randomly assigned to ten 1-minute high-intensity intervals (90%VO2peak, 1min recovery), or five 2-minute high-intensity intervals (80-100% VO2peak, 1 min recovery), or control. Peak oxygen uptake (VO2peak), peak power output, body composition, and fasting blood lipids were evaluated before and after 3 weeks of training, completed 3 days per week. Results from ANCOVA analyses demonstrated no significant training group differences for any primary variables (p>0.05). When training groups were collapsed, 1MIN and 2MIN resulted in a significant increase in peak power output (∆18.9 ± 8.5 watts; p=0.014) and time to exhaustion (∆55.1 ± 16.4 sec; p=0.001); non-significant increase in VO2peak (∆2.36 ± 1.34 ml·kg−1·min−1; p=0.185); and a significant decrease in fat mass (∆−1.96 ± 0.99kg; p=0.011). Short-term interval exercise training may be effective for decreasing fat mass and improving exercise tolerance in overweight and obese women.
Keywords: percent body fat, peak oxygen consumption, visceral fat, intermittent exercise
Introduction
It is well documented that physical inactivity directly increases the risk of various health parameters including poor cardiorespiratory fitness, adiposity, impaired glucose tolerance, and hypertension, which in combination often contribute to chronic disease (Lee, Blair, & Jackson, 1999). Exercise is growing in acceptance as a method for healthcare professionals to implement for disease prevention. Currently, the American College of Sports Medicine recommends between 150 and 250 minutes of moderate physical activity per week as an effective measure for developing and maintaining health (Donnelly et al., 2009). This recommendation is further published from the Centers for Disease Control and Prevention and from the Committee on Exercise and Cardiac Rehabilitation of the American Heart Association (Haskell et al., 2007). Despite the consensus regarding the importance of physical activity, most adults fail to meet even the minimum physical activity guidelines. Countless studies have shown that lack of time, motivation, and adherence are the most commonly cited reasons for not exercising (Booth, Roberts, & Laye, 2012).
The use of interval-based training programs to stimulate improvements in metabolic and cardiovascular health, requiring less time than traditional exercise have received increased attention in clinical populations (Shiraev & Barclay, 2012). As little as six sessions, over a two week time period, have elicited beneficial cardiometabolic adaptations (Babraj et al., 2009; Little et al., 2011; Little, Safdar, Wilkin, Tarnopolsky, & Gibala, 2010), while other studies suggest a longer training duration is imperative (Fisher et al., 2015; Gillen et al., 2012; Tjonna et al., 2009). Many recent studies have demonstrated shorter duration interval based exercise (30 sec – 4 min) may be advantageous over prolonged training for cardiorespiratory fitness, skeletal muscle oxidative capacity, improved fuel utilization, and a concomitant improvement in insulin sensitivity (Babraj et al., 2009; Cocks et al., 2015; Gremeaux et al., 2012; Kessler, Sisson, & Short, 2012). While it is known that interval training yields rapid adaptations in mitochondrial capacity and insulin sensitivity (Cocks et al., 2015; Fisher et al., 2015; Hood, Little, Tarnopolsky, Myslik, & Gibala, 2011; Little et al., 2011; Little et al., 2010), additional research is warranted to identify the optimal combination of training intensity and volume necessary to induce adaptations in a practical, time-efficient manner in overweight and obese women. Initial evidence suggests that men may have more pronounced benefits compared to women (Gillen et al., 2012); with little data available for women (Gillen, Percival, Ludzki, Tarnopolsky, & Gibala, 2013). It is imperative to find more convenient options for exercise in this population.
Exercise capacity has become a well-established independent predictor for cardiovascular disease (Myers et al., 2002). Recently, sprint interval training, which involves 30 second ‘all out’ supramaximal sprints with a four minute recovery period, has been show to rapidly improve cardiorespiratory fitness in a variety of populations (Tremblay, Simoneau, & Bouchard, 1994; Whyte, Gill, & Cathcart, 2010). The feasibility of higher intensity exercise has been questioned as it has been shown to result in negative feelings toward exercise and poor adherence (Ekkekakis, Hall, & Petruzzello, 2008). Additionally, sprint interval training typically requires specialized equipment to achieve appropriate intensity. Due to the growing body of literature supporting the benefits from interval training, there is a need to identify other feasible protocols that result in similar health benefits and may be potentially completed outside of a laboratory. A few recent investigations have demonstrated support for more submaximal protocols with one minute interval bouts (Boyd, Simpson, Jung, & Gurd, 2013; Hood et al., 2011; Little et al., 2011; Skelly et al., 2014; A. E. Smith-Ryan, Melvin, & Wingfield, 2015), with the majority of data in men. Yet, some data suggest longer (4min) lower intensity intervals; but require more time (Helgerud et al., 2007; Tjonna et al., 2009). Additionally, both protocols evaluated in the current study have been reported to be highly enjoyable in overweight and obese individuals (A.E. Smith-Ryan, 2015). Some evidence demonstrates that beneficial physiological adaptations result when exertion is reduced from ‘all-out’ effort to 60% peak power output (Hood et al., 2011), as well as increasing interval duration to 4 min at 90% heart rate (Tjonna et al., 2009). However, in an overweight/obese population, alterations in interval duration, and intensity, have just recently been explored (Cocks et al., 2015; Fisher et al., 2015; Gillen et al., 2013; Gillen et al., 2014). Identifying the effects of two interval protocols may help provide more feasible options for interval training in a clinical population, specifically for overweight/obese women. Therefore the purpose of this study was to compare the physiological adaptations as a result of two practical higher intensity training protocols in overweight/obese women. The primary aim was to evaluate the effects of high intensity interval training on cardiorespiratory fitness [(VO2peak), time to exhaustion (TTE), peak power output (PPO)] and fasting blood metabolites (glucose, insulin, lipids) before and after a 3 week intervention. The secondary aim was to evaluate the effects on body composition. High intensity training using a 2 minute on 1 minute off protocol (adapted from Smith et al.(2009)) at undulating intensities (2MIN); and a 1 minute high intensity interval training protocol applying a 1 minute on 1 minute off protocol (adapted from Boyd et al.(2013)) at maximal intensity (1MIN) were examined in comparison to a control (CON) group. It was hypothesized that both training protocols would improve cardiorespiratory fitness and body composition.
Methods
Participants
Participants were recruited from an urban area in the Southeastern United States of America. All procedures were approved by the University’s Biomedical Review Board (Approval: 12-1026) and were completed in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Following email and telephone screening, 44 women completed an in-person eligibility visit in which they responded to a health history questionnaire to screen for the following: age 18 to 55 years, overweight (BMI > 25 kg·m2); <10lb body weight change within the last three months; sedentary (did not meet American College of Sports Medicine exercise recommendations; no limitations to physical activity (cardiovascular, musculoskeletal; and no use of prescription medication that may have influence metabolism or weight loss). A routine medical screening, electrocardiogram, and physician clearance were used to assess subjects for inclusion. Individuals from this initial group were excluded if they reported: i) actual BMI was ≤ 25 kg·m2; ii) a history of hypertension or metabolic renal, hepatic, autoimmune, or neurological disease; iii) currently participating in high-intensity exercise; iv) resting blood pressure was above safety cutoff (> 140/90 mmHg); electrocardiogram demonstrated an irregularity; v) personal physician did not approve participation; or vi) participant decided it was too great of a time commitment. A final group of 32 females met the eligibility requirements. Written informed consent was obtained from all individual participants included in the study. The authors confirm that all ongoing and related trials for this intervention are registered (#NCT 02444377). Data on the men has been previously published and separated due to heterogeneity (A. E. Smith-Ryan et al., 2015). All CONSORT procedures and guidelines were followed.
Experimental Design
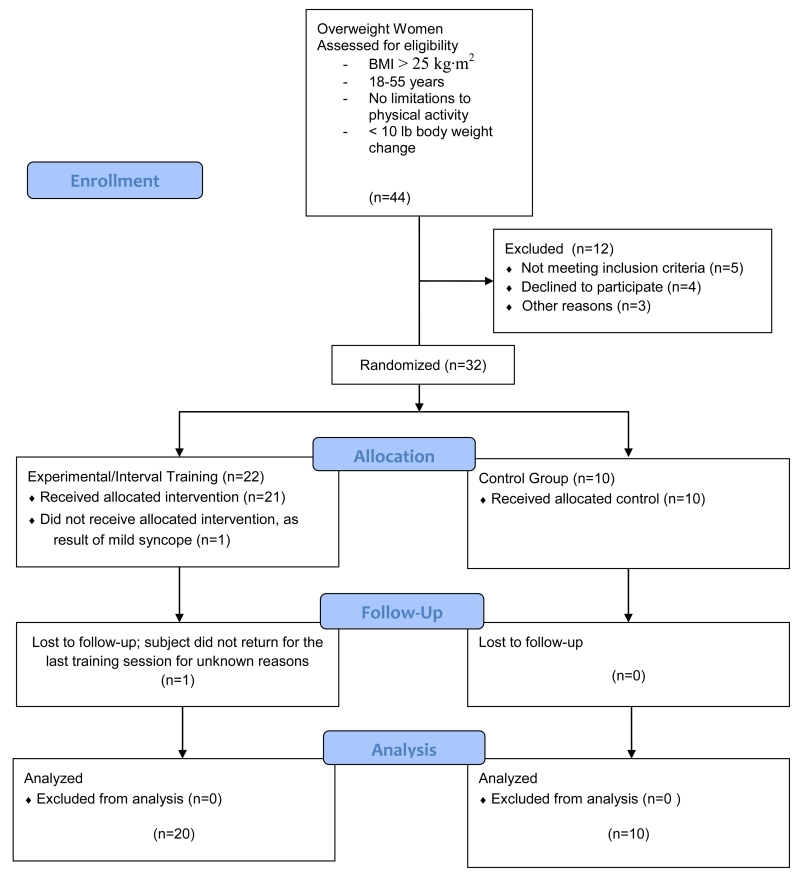
Following enrollment, 32 female participants were randomized by the PI (AESR) (August 2012-May 2014), using block random allocation software into three groups in accordance with the CONSORT guidelines (Figure 1): 2MIN group (n=11), 1MIN group (n=11), and control group, which performed no training (control; n=10). Retention of subjects was high, despite the intensity and frequency of training: one subject dropped out after the pre-VO2peak test (due to mild syncope), and one subject dropped out for no known reason after completing 8/9 training sessions, yielding a final evaluable group of 30 women (Table 1). Prior to the start of the 3 week training program, and in the week immediately following the cessation of the training program, participants visited the laboratory in a fasted condition on two occasions to complete all baseline body composition, cardiorespiratory, and blood metabolite tests (Figure 2). The same assessments were completed post 3 weeks of training, between 48-144 hours of the last training session.
Figure 1.
Consort guidelines flow diagram for enrollment, exclusion, and randomization.
Table 1.
Anthropometric variables of 1MIN, 2MIN, and control groups at baseline. Mean ± SD. There were no significant baseline differences.
| Age (yrs) | Height (cm) | Body Mass (kg) | BMI (kg/m2) | %BodyFat | Vo2peak (ml·kg·min−1) |
|
|---|---|---|---|---|---|---|
| 1MIN (n=11) | 33.2 ± 12.8 | 164.5 ± 4.8 | 91.5 ± 14.4 | 33.9 ± 6.1 | 38.2 ± 5.6 | 23.2 ±5.9 |
| 2MIN (n=10) | 33.6 ± 11.6 | 170.0 ± 4.8 | 82.8 ± 12.2 | 28.6 ± 3.7 | 37.9 ± 3.7 | 25.0 ± 5.8 |
| Control (n=9) | 35.2 ± 11.4 | 163.2 ± 3.9 | 90.3 ± 21.0 | 33.7 ± 6.7 | 37.9 ± 7.0 | 24.6 ± 8.9 |
|
| ||||||
| n=30 | 33.9 ± 11.6 | 166.0 ± 5.3 | 88.1 ± 15.9 | 32.0 ± 6.0 | 38.0 ± 5.3 | 24.3 ± 6.8 |
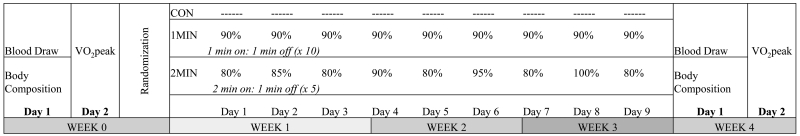
Figure 2.
Experimental design.
Body composition and blood variables were measured in the morning, after an eight hour fast, at a similar time of day (± 2 hours) at pre and post-testing. Cardiorespiratory fitness (VO2peak) was assessed following cessation of exercise for at least 24 hours. Participants were randomized into respective training groups (1MIN, 2MIN) or no training (CON), for three weeks.
Measurements
Height was measured to the nearest 0.5 cm using a calibrated stadiometer; body mass was measured using a calibrated clinical scale to the nearest 0.01 kg with participants wearing only Spandex shorts or a tight-fitting bathing suit. Serum blood samples were drawn at the University Hospital. Samples were separated and processed by McLendon Clinical Laboratories (Chapel Hill, NC). All samples were analyzed using established enzymatic assays for fasting blood glucose (GLU), total cholesterol (TC), triglycerides (TG), high-density lipoproteins (HDL) and insulin. Low-density (LDL) and very low-density lipoprotein cholesterol (VLDL) were calculated using Friedwald’s equations [LDL = TC-TG/2.2; VLDL = TC-(LDL+HDL)].
Cardiorespiratory Measurements
Before and after training, participants performed a continuous graded exercise test on an electronically braked cycle ergometer (Corival 400, Lode, Groningen, The Netherlands) to determine maximal oxygen consumption (VO2peak). Pedal cadence was maintained at 70 rpm, while the power output was initially set at 50 watts (W) for a five minute warm-up, and increased by 1 W every 3 seconds until the participant could no longer maintain the power output (cadence dropped below 50 rpm). For each test, the time at which the cadence dropped below 50 rpm was recorded as the time to exhaustion and the highest power output obtained was recorded as peak power output. Respiratory gases were monitored and continuously analyzed with open-circuit spirometry using a calibrated metabolic cart (True One 2400®, Parvo-Medics, Inc., Provo, UT). Data was averaged over 15-second intervals, with the highest 15-second oxygen consumption and heart rate values identified as the VO2peak and ventilatory threshold. Heart rate was monitored continuously during exercise by a heart rate monitor (Polar FS1, Polar Electro Inc. Lake Success, NY). Test-retest reliability for the VO2peak protocol demonstrated reliable between-day testing with an intraclass correlation coefficient (ICC) of 0.98 and standard error of the measurement (SEM) of 1.74 ml·kg−1·min−1.
Body Composition Measurement
All body composition measurements were performed at the same time of day, following an 8 hour fast (water intake was allowed up to 1 hr prior to testing). Using a criterion four-compartment model, fat mass (FM), lean mass (LM), and percent body fat (%FAT) were estimated using as described by Wang et al.(2002):
Where BV is total body volume, TBW is total body water, Mo is total body bone mineral, and BM is body mass. Multi-compartment models are known to be the most sensitive and valid methods of body composition, compared to single 2- or 3-compartment approaches. Dual-energy X-ray absorptiometry (Discovery W, Hologic, Bedford, MA) was used to estimate total bone mineral content using the device’s default software (Apex Software V3.3). BMC was converted to Mo using the following equation: Mo = total body bone mineral content × 1.0436. Air-displacement plethysmography (BodPod®; Life Measurement, Inc.; Concord, CA) was used to determine body mass and body volume. Prior to each test, the device was calibrated according to the manufacturer’s instructions. Participants entered the chamber in tight fitting clothing (i.e. compression shorts, sports bra, or swimsuit) and swimming cap, wearing the exact same clothing for baseline and post-measurements. Thoracic lung volume was estimated at pre-testing and subsequently used for post-testing assessments. Previous reports have shown that predicted lung volumes are not significantly different than measured volumes, even in obese subjects (Demerath et al., 2002; McCrory, Mole, Gomez, Dewey, & Bernauer, 1998). Bioimpedance spectroscopywere used to estimate total body water (SFB7, ImpediMed, Queensland, Australia). Total body water estimates were taken while the participant lay supine on a table with their arms and legs separated by ≥ 30˚. Electrodes were placed at the distal ends of the participant’s right hand and food. Prior to analysis, each participant’s height, body weight, age, and sex were entered into the bioimpedance spectroscopy device. The average of two trials within ± 0.05 liters was used to represent each participant’s total body water.
Test-retest reliability of the four compartment equation was measured in 14 overweight women and produced an ICC of 0.98 and SEM of 0.65kg; 0.61kg, and 0.60% for FM, LM, and %FAT, respectively.
Ultrasonography was used to determine abdominal wall fat thickness, using a wide-band linear array transducer (GE 12L-RS, Logiq-e B-mode, GE Healthcare, Wisconsin, USA), performed by the same technician (AESR). While the subject was in the supine position, the transducer was placed approximately 1 cm from the umbilicus. Abdominal fat thickness was measured as the distance between the internal surface of the rectus abdominis muscle and the preperitoneal organ line (Naboush & Hamdy, 2013; Sogabe, Okahisa, Hibino, & Yamanoi, 2012). Test-retest reliability from our lab resulted in an ICC of 0.87 and SEM of 3.2 cm.
High-Intensity Interval Training
All training was performed on an electronically braked cycle ergometer (Corival 400, Lode, Gronigen, The Netherlands), trained one-on-one under the supervision of trained research staff. Participants were required to train three times a week, with no more than two training sessions back to back. Training schedule was set to the participants’ preference (days of the week and time of day). Participants were randomly assigned to one of three groups. Respective groups consisted of: 1MIN high intensity intervals: 10 repetitions of 1 minute bouts with 1 minute rest periods at 90% of the power output obtained during VO2peak (total of 10 minutes of cycling) modified from: (Boyd et al., 2013); 2MIN high intensity intervals: 5 bouts of 2 minutes cycling with 1 minute recovery utilizing undulating intensities (80-100% VO2peak) [modified from: (Smith et al., 2009) (Figure 2)] (total of 10 minutes of cycling), or no exercise at all (control). Heart rate and ratings of perceived exertion were used and tracked to monitor intensity. The research staff set the power output at their respective intensities for each work bout, and each training session under a highly controlled setting. Adherence to the training was 100%, with all participants completing all three training days per week, for a total of 9 training session.
Statistical Analysis
Differences between groups (1MIN vs. 2MIN vs. control) from baseline to week three were analyzed using an analysis of covariance (ANCOVA) with baseline scores used as the covariate. Due to the additional aim of evaluating the effects of short-term interval training in this population, a follow-up analysis was completed using an ANCOVA when there were no treatment effects; training groups were collapsed (interval) compared to control. The homogeneity of regression assumption for ANCOVA was met for all variables. All post-hoc comparisons were Bonferroni adjusted. Descriptive statistics are presented as mean ± standard deviation. Raw values and adjusted change scores (week 3 minus baseline) for all dependent variables are displayed. Effect sizes (ηp2) and 95% confidence intervals (CI) are also presented. All statistical procedures were performed using SPSS (version 20.0, SPSS Inc., Chicago, IL). Power calculations were completed using nQuery + nTerim 2.0 (Statistical Solutions, Boston, MA) using data from similar interval interventions (Talanian, Galloway, Heigenhauser, Bonen, & Spriet, 2007; Tjonna et al., 2009) in order to evaluate change scores. For powering our study we assumed a meaningful difference of 2.0 ml·kg·min−1 from VO2peak. We further assumed a standard deviation of 2.5 ml·kg·min−1 at each pre- and post-testing time point; with attrition not greater than 10%. Under these assumptions, having 30 evaluable subjects, would provide at least 80% power for an ANCOVA (assuming a 0.85 correlation between measurements which implies a standard deviation for change scores of 1.37) at the 0.05 level.
Results
Of the initial 32 subjects enrolled, 30 were evaluated in the analysis. There were no significant baseline differences between 1MIN (n=11), 2MIN (n=10), and control (n=9) groups (Table 1). No adverse events were reported during study participation.
Cardiorespiratory Fitness
There was no significant treatment effect (p=0.291; ηp2=0.094,CI:[ −0.89-4.35]) for VO2peak when analyzed for 2MIN (2.97 ± 1.95 ml·kg−1·min−1), 1MIN (1.80 ± 1.86 ml·kg−1·min−1), and CON (0.61 ± 2.18 ml·kg−1·min−1) (Table 2). Time to exhaustion demonstrated a significant interaction (p=0.001; ηp2=0.447); there was a significant increase in time to exhaustion in 2MIN (p=0.001) and 1MIN (p=0.001) groups both training groups compared to the control group; (Table 2). There was a significant interaction for peak power output (p=0.014; ηp2=0.290), with significant increases for 2MIN (p=0.015) and 1MIN (p=0.043) compared to control.
Table 2.
Changes from pre- to post-training are reported for cardiorespiratory fitness: peak oxygen consumptions (VO2peak) time to exhaustion (TTE) and peak power output (PPO); body composition: fat mass (FM), percent body fat (%BF), lean mass (LM), abdominal fat thickness (AFT); and blood metabolites for 1MIN, 2MIN, and control groups. Values are mean change ± standard deviation (SD).
| SIT | HIT | CON | P Value | ||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Pre | Post | Pre | Post | Pre | Post | ||
| Vo2peak (ml·kg−1·min−1) | 23.18 ± 5.95 | 25.03 ± 6.05 | 25.01 ± 5.75 | 27.25 ± 6.02 | 23.64 ± 9.27 | 24.64 ± 8.93 | 0.291 |
| TTE (sec) | 760.36 ± 88.47 | 810.33 ± 93.23* | 771.09 ± 65.50 | 828.96 ± 77.14* | 734.63 ± 157.21 | 719.88 ± 155.80 | 0.001 |
| PPO (W) | 179.25 ± 9.52 | 196.5 ± 10.44* | 175.20 ± 10.42 | 196.00 ± 11.43* | 149.86 ± 12.46 | 148.14 ± 13.67 | 0.014 |
| FM (kg) | 35.53 ± 10.07 | 33.39 ± 9.26 | 31.71 ± 7.54 | 31.00 ± 6.81 | 35.16 ± 13.52 | 36.16 ± 12.00 | 0.330 |
| %BF (%) | 38.19 ± 5.60 | 36.01 ± 5.00 | 37.91 ± 3.70 | 36.39 ± 3.12 | 37.92 ± 6.96 | 37.93 ± 5.80 | 0.185 |
| LM (kg) | 56.01 ± 5.66 | 57.92 ± 5.68 | 51.13 ± 5.48 | 53.53 ± 5.84 | 55.07 ± 9.66 | 57.03 ± 7.83 | 0.358 |
| AFT (cm) | 24.50 ± 12.44 | 13.89 ± 9.83 | 21.99 ± 10.47 | 13.90 ± 5.08 | 20.36 ± 8.51 | 16.91 ± 9.36 | 0.597 |
|
| |||||||
| Glucose (mmol/L) | 84.09 ± 8.78 | 84.82 ± 6.87 | 88.82 ± 9.61 | 88.60 ± 7.00 | 89.00 ± 9.96 | 89.50 ± 9.62 | 0.780 |
| Cholesterol (mg/dL) | 190.18 ± 42.07 | 193.36 ± 38.58 | 177.64 ± 30.31 | 173.0 ± 40.00 | 161.00 ± 24.05 | 161.875 ± 26.40 | 0.441 |
| Triglycerides (mg/dL) | 102.73 ± 48.18 | 108.82 ± 50.06 | 86.18 ± 36.98 | 97.70 ± 61.84 | 94.88 ± 49.11 | 96.13 ± 51.82 | 0.728 |
| HDL (mg/dL) | 59.36 ± 14.83 | 58.55 ± 15.06 | 62.64 ± 9.41 | 63.50 ± 9.65 | 48.50 ± 8.91 | 51.00 ± 9.37 | 0.633 |
| LDL (mg/dL) | 110.27 ± 30.67 | 113.18 ± 27.49 | 96.73 ± 28.61 | 90.00 ± 33.49 | 93.50 ± 24.65 | 91.63 ± 24.39 | 0.134 |
| Insulin (IU/L) | 16.34 ± 11.07 | 14.82 ± 9.71 | 13.43 ± 9.29 | 12.41 ± 6.98 | 9.73 ± 3.80 | 9.04 ± 2.73 | 0.563 |
Values are mean ± SD.
P-value is the interaction, from the ANCOVA
indicates a significant change from baseline
When collapsed across training groups (Table 3), there was no significant difference between groups for VO2peak (p=0.313). In contrast, the interval groups together elicited an increase in time to exhaustion of 55.12 (± 16.34 sec) compared to a decrease in the control group (−15.42 ± 16.34 sec) (p=0.001; CI:[38.23-101.01]). There was also a significant treatment effect for peak power output (p=0.004; ηp2=0.280, CI:[7.78-30.94]).
Table 3.
Cardiorespiratory fitness (VO2peak; ml·kg·min−1; TTF; sec; PPO; W), body composition (FM; kg; %BF; LM: kg; AFT, cm), and blood metabolites (mg/dL) and insulin (IU/L) collapsed across training groups (INT) versus control.
| INT | CONTROL | 1P Value | |
|---|---|---|---|
|
|
|||
| Change | Change | ||
|
|
|||
| Vo2peak | 2.36 ± 1.34 | 0.62 ± 2.16 | 0.313 |
| TTE | 55.12 ± 16.35* | -15.42 ± 26.56 | 0.001 |
| PPO | 18.86 ± 13.65* | -1.71 ± 12.84 | 0.004 |
| FM | −1.96 ± 0.99* | −0.49 ± 1.62 | 0.011 |
| %BF | −2.11 ± 0.88* | −0.49 ± 1.43 | 0.059 |
| LM | 1.43 ± 1.80 | 1.17 ± 1.30 | 0.730 |
| AFT | −11.29 ± 18.35 | −3.44 ± 9.21 | 0.321 |
|
| |||
| Glucose | −0.13 ± 2.27 | −1.23 ± 3.75 | 0.600 |
| Cholesterol | 0.91 ± 8.00 | 0.12 ± 13.27 | 0.921 |
| Triglycerides | −9.00 ± 12.80 | −1.25 ± 20.60 | 0.528 |
| HDL | 0.21 ± 2.44 | 1.70 ± 4.20 | 0.559 |
| LDL | −2.18 ± 6.20 | −2.42 ± 10.20 | 0.968 |
| Insulin | 0.26 ± 1.41 | 1.45 ± 2.25 | 0.384 |
Values are mean ± SD.
1P values are for the differences between the two groups, INT vs. CON
significant treatment effect p<0.05 via ANCOVA (post values are significantly different from each other, covarying for pre values).
Heart rate (HR) and rating of perceived exertion (RPE) decreased significantly from the end of the first training session to the end of the last interval session (p<0.05) for 2MIN (HR: −6.7 bpm; RPE: −2.1 a.u.) and for 1MIN (HR: −7.1 bpm; RPE: −2.3 bpm). Average peak HR across all sessions for 2MIN and 1MIN were 165.2 bpm and 168.7 bpm, respectively. For RPE, average peak values for all for all 2MIN and 1MIN sessions were 14.9 a.u. and 14.1 a.u., respectively. There was no significant difference for average wattage between groups for training W (p=0.153) for 1MIN (161 ± 33W) compared to 2MIN (158 ± 20W).
Body Composition
Comparisons of all three groups demonstrated no statistically significant interactions or treatment effects for body composition, suggesting there is no difference in training approach, 1:1 vs. 2:1, in the current population.
Although there was no treatment effect for individual training groups (p=0.33; ηp2=0.085), fat mass decreased by 1.83 kg (±1.48kg) for 2MIN and 2.07 kg (±1.40kg) for 1MIN, compared to 0.50 kg (±1.65kg) for control (Table 2). There was also no significant change in abdominal fat thickness (p=0.597) for any group.
When groups were collapsed, compared to the control group, there was a significant main effect for treatment (p=0.011, CI:[−1.78-1.47]) (Table 3); the collapsed interval training groups yielded a reduction in FM by 1.96 kg (±0.99) from three weeks of training. Lean mass demonstrated no significant change (Tables 2 and 3). Percent body fat yielded no treatment effect (p=0.185; ηp2=0.126, CI:[−1.70-1.37]) between all three groups. Additionally, there were no significant treatment effects, but training demonstrated a 11.29 cm (±18.4cm) decrease in abdominal fat thickness, compared to a 3.44 cm (±9.21cm) decrease in the control group (p=0.321, CI: [−14.86-8.38]).
Blood Values
The effects of 2MIN and 1MIN on fasting blood glucose and blood lipids are summarized in Table 2. All mean baseline blood values analyzed, glucose, total cholesterol, triglycerides, HDL, LDL, were considered normal at baseline. There were no significant treatment effects for any measured blood metabolites (p=0.134-0.728) (Table 3).
Discussion
The present study demonstrates that high-intensity interval training is an effective exercise strategy for improving time to exhaustion, peak power output, and fat mass in overweight/obese women. Previous data supports the use of interval training exercise to improve peak power output and fat mass; Gillen et al. (Gillen et al., 2013) previously demonstrated significant improvements in percent body fat and mitochondrial capacity in overweight women using a similar 60 second protocol. Similarly, there were no significant effects on insulin sensitivity (Gillen et al., 2013). Although not significant, nine sessions of 10 total min constant-load interval training, over three weeks in the current study, resulted in similar changes in cardiorespiratory fitness and fat mass reported from previous more chronic (up to 15 weeks) training protocols (Fisher et al., 2015; Helgerud et al., 2007; Heydari, Freund, & Boutcher, 2012; Nybo et al., 2010; Tjonna et al., 2009). In contrast, previous evaluations utilizing a similar 1MIN protocol have resulted in significant improvements in oxygen consumption and insulin sensitivity in healthy men and women and type II diabetics (Hood et al., 2011; Skelly et al., 2014). The lack of effect in insulin and glucose sensitivity may be a result of a lack of dietary intervention (Kessler et al., 2012), or may also be due to the normal blood values at baseline. Benefits for cardiorespiratory fitness in this population may require additional weeks of training. Collectively, the magnitude of the change for both training groups were not different from each other, suggesting either 10 sets of 1 min bouts, or 5 sets of 2 min bouts may provide an equal benefits model for rapid improvements in cardiovascular health and potential changes in fat mass.
Cardiorespiratory Fitness
Maximal exercise capacity has been shown to be the most significant predictor of increased risk of mortality, more than any other clinical variable, in patients with at least one cardiovascular risk factor (Myers et al., 2002). Cardiorespiratory fitness is a modifiable risk factor, with improvements linked directly with reduced mortality and improved health status (McAuley et al., 2012). High intensity exercise has been repeatedly shown to rapidly augment VO2peak in a variety of populations (Gibala, Little, Macdonald, & Hawley, 2012). The present study demonstrated a non-significant average 2.4 ml·kg·min−1(~8%) increase in VO2peak with nine exercise sessions. The magnitude of this change is slightly lower than an average 5.0 ml·kg·min−1 reported by Gillen et al. (Gillen et al., 2013)despite a similar protocol in overweight women. However, Gillen et al. (Gillen et al., 2013) employed six weeks of training. The improvements in the current study were also lower than other studies using a similar 60 second protocol in sedentary men (Esfandiari, Sasson, & Goodman, 2014) and overweight/obese men (Fisher et al., 2015; Whyte et al., 2010). Thus, while cardiorespiratory fitness improved, in order to reach ‘significance’ a longer duration of training may be imperative. Improvements in VO2peak as a result of high-intensity interval training have been largely attributed to increases in mitochondrial biogenesis via upregulation of peroxisome prolifterator-activated receptor γ coactivator (PGC)-1α (Hood et al., 2011). Although this was not measured in the current study, growing research demonstrates fluctuations in ATP turnover during the start and stop nature of interval training, may activate PGC-1α signaling pathway (Daussin et al., 2008). High intensity exercise has also been shown to improve stroke volume and cardiac output, thereby improving oxygen delivery (Esfandiari et al., 2014). The present study did not show statistical significance in VO2peak, but may have practical relevance, as the changes were in line with previous, longer duration studies. The lack of significance may be a result of differences in the interval protocol or population used (men vs. women; overweight/obese). Additionally, the variability among subjects was higher than anticipated. The present study also demonstrated significant increases in time to exhaustion and peak power output, further supporting increases in cardiorespiratory fitness following 3 weeks of interval training.
Body Composition
Similar to previous short duration (2-6 weeks) high-intensity training programs, there was no significant effect on body weight. However, both 1MIN and 2MIN groups demonstrated a significant loss in fat mass, in comparison to the control group. The 2.0 kg loss in fat mass from the present study is greater than compared to Gillen et al. (Gillen et al., 2013) utilizing a similar 1MIN protocol, resulting in an average 0.6 kg loss. The 2.0 kg loss in the current study is similar to other, more chronic interventions. Heydari et al.(2012) and Dunn et al.(2009) reported an average 2.3 kg loss in fat mass following 12 weeks of high intensity training. Trapp et al.(2008) and Tjonna et al.(2009) and also reported about a 2.5 kg loss in fat mass after 15 and 16-weeks of high-intensity training, respectively. It has been suggested that longer duration work bouts (>30 sec) have resulted in greater fat loss (Trapp et al., 2008). In the present study, there was no difference in fat loss between 1MIN and 2MIN groups. Additionally, hypotheses exist regarding a greater initial fat amount may correspond to a greater fat loss (Boutcher, 2011), which would also support why the present study demonstrated greater effects compared to Gillen et al. (2013) using just an overweight population. With a largely obese cohort in the current study, the losses in fat may be attributed to greater baseline fat levels. Mechanistically, high-intensity training may stimulate greater increases in energy expenditure over a similar given amount of work, compared to low-intensity training (Skelly et al., 2014). Although the present study did not evaluate energy expenditure or lipid metabolism, the decrease in fat mass in women, without dietary intervention, may be a result of these metabolic changes. Magnified catecholamine response during exercise, and throughout recovery, likely plays a role in augmented energy expenditure (De Feo, 2013). In theory, maximal fat oxidation (Astorino, Schubert, Palumbo, Stirling, & McMillan, 2013) and elevated energy expenditure (Skelly et al., 2014)from interval training may augment energy expenditure and ultimately yield improvements in body composition over an accumulated time. In contrast, the current protocol in men resulted in no changes in fat mass, but increases in lean mass (A. E. Smith-Ryan et al., 2015); Scalzo et al.(2014) has reported muscle protein synthesis may be more pronounced in men, which is supported by results from this data set.
The non-significant decrease in abdominal fat thickness in the present study, with a mean reduction of 11 cm extend the findings of Heydari et al.(2012), demonstrating a reduction in abdominal fat thickness after 8-12 weeks of high-intensity training. Additionally, Irving et al.(2008) reported a significant 25 cm reduction in visceral thickness fat following high-intensity, but not low-intensity running exercise in obese women. Due to the strong implications of abdominal fat and visceral fat and increased cardiovascular disease risk (Kuk et al., 2006), the impact of interval training after three weeks, may have positive health implications. The high catecholamine release from interval training may be an underlying mechanism for targeting abdominal fat, as well as a larger number of β-adrenergic receptors concentrated within visceral and abdominal thickness fat, compared to subcutaneous fat (Kuk et al., 2006). The variability of the sample was relatively high, which likely contributed to the lack of statistical significance. However, the change in visceral fat may be worthwhile to explore in future longer duration studies. Long term data evaluating visceral fat re-gain and maintenance following cessation of a high-intensity interval training protocol is also needed.
Lipids
The use of high-intensity exercise as prescription for improving insulin sensitivity and glucose tolerance has gained increasing support. As the prevalence of diabesity rises and physical activity falls due to time burdens, high-intensity interval training has shown potential benefits (Nybo et al., 2010). While the present study was unable to stimulate significant positive changes in blood lipids and insulin sensitivity, recent interventions, with similar protocols have demonstrated significant benefits. Hood et al. (2011) reported an improvement (35%) in insulin sensitivity in previously sedentary adults, following a two-week cycling protocol of 10 bouts of 1 min on 1 min off at 60% peak power. Whyte et al. (2010) also demonstrated an improvement in insulin sensitivity (25%) and fat oxidation following two weeks of sprint interval training (6 × 30 sec). Our previously published results in men, also demonstrated significantly positive results on insulin sensitivity (A. E. Smith-Ryan et al., 2015). In comparison, insulin concentrations in the current study improved by 46% following three weeks of training, which is similar to responses reported following more chronic endurance training (Haskell et al., 2007). The lack of significance is likely due to the individual variability of the sample. Additionally, the normal results at baseline, despite other risk factors, may have influenced the lack of change. Previous short training intervention studies have also failed to report a significant influence on insulin and blood lipids with very little volume (8-30sec work bouts) (Heydari et al., 2012; Nybo et al., 2010) . Although not significant, the higher intensity protocol in the current study (10 × 1min at 90%) stimulated a greater influence on insulin concentrations (Table 2).
Limitations
We acknowledge that the current results are limited by a relatively small inter-treatment sample size and sample variability. Despite this, we were able to detect significant effects when training groups were collapsed. The differences from baseline to post-testing, for both 1MIN and 2MIN groups, represent changes that are clinically relevant and beyond the measurement error. Evaluating changes against a steady-state control group would have also strengthened the study, but a fair amount of data exists comparing the two training strategies. .Additionally, it is possible that estrogen may influence exercise performance and blood metabolites (Hackney & Smith-Ryan, 2012). Although we did not measure estrogen concentrations, we did complete testing at weeks 0 and 4, which allowed for testing to occur in similar weeks of the menstrual cycle. Lastly, while nutrition was not a primary aim of the study, it is possible that despite encouragement to maintain typical habits, dietary changes may have had an influence.
Conclusions
The use of repeated all-out sprint interval training, has highlighted the potential rapid health benefits from high-intensity exercise. There is some concern that increasing the duration of the bout, and subsequently reducing intensity, may minimize the health and body composition effects. However, this strategy may improve adherence and feasibility to a variety of populations. The present study demonstrates that 10 minutes of high-intensity work, of either 1 minute or 2 minute intervals, is effective for improving time to exhaustion, peak power output, and fat mass in overweight and obese women. High-intensity interval training may elicit similar benefits to traditional aerobic training with a significantly lower volume and in a shorter period of time (Burgomaster et al., 2008; Nybo et al., 2010), which may be helpful to address the mental and physical challenges associated with initiating an exercise program. A primary strength of our study is that we examined a cohort comprised exclusively of women, in comparison to the majority of high intensity interval data evaluating mixed samples, or men only. To date the longest interval training study that we are aware of, spans 16 weeks (Lunt et al., 2014). More chronic evaluations of high-intensity interval training should be examined in this population. Additionally, the combined effects of interval exercise and dietary modification as a means for weight loss should be evaluated.
Acknowledgements
We would like to thank Mark Weaver, PhD for his statistical review. This study was funded by the Nutrition Obesity Research Center (P30DK056350). The project described was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant 1KL2TR001109. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
We declare no conflicts of interest.
References
- Astorino TA, Schubert MM, Palumbo E, Stirling D, McMillan DW. Effect of two doses of interval training on maximal fat oxidation in sedentary women. Medicine and science in sports and exercise. 2013;45(10):1878–1886. doi: 10.1249/MSS.0b013e3182936261. doi: 10.1249/MSS.0b013e3182936261. [DOI] [PubMed] [Google Scholar]
- Babraj JA, Vollaard NB, Keast C, Guppy FM, Cottrell G, Timmons JA. Extremely short duration high intensity interval training substantially improves insulin action in young healthy males. Bmc Endocrine Disorders. 2009;9:3. doi: 10.1186/1472-6823-9-3. doi: 10.1186/1472-6823-9-31472-6823-9-3 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Comprehensive Physiology. 2012;2(2):1143–1211. doi: 10.1002/cphy.c110025. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutcher SH. High-intensity intermittent exercise and fat loss. Journal of Obesity. 2011;2011:868305. doi: 10.1155/2011/868305. doi: 10.1155/2011/868305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JC, Simpson CA, Jung ME, Gurd BJ. Reducing the intensity and volume of interval training diminishes cardiovascular adaptation but not mitochondrial biogenesis in overweight/obese men. PLoS One. 2013;8(7):e68091. doi: 10.1371/journal.pone.0068091. doi: 10.1371/journal.pone.0068091PONE-D-13-09077 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. Journal of Physiology. 2008;586(1):151–160. doi: 10.1113/jphysiol.2007.142109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks M, Shaw CS, Shepherd SO, Fisher JP, Ranasinghe A, Barker TA, Wagenmakers AJ. Sprint interval and moderate-intensity continuous training have equal benefits on aerobic capacity, insulin sensitivity, muscle capillarisation and endothelial eNOS/NAD(P)Hoxidase protein ratio in obese men. Journal of Physiology. 2015 doi: 10.1113/jphysiol.2014.285254. doi: 10.1113/jphysiol.2014.285254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daussin FN, Zoll J, Dufour SP, Ponsot E, Lonsdorfer-Wolf E, Doutreleau S, Richard R. Effect of interval versus continuous training on cardiorespiratory and mitochondrial functions: relationship to aerobic performance improvements in sedentary subjects. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2008;295(1):R264–272. doi: 10.1152/ajpregu.00875.2007. doi: 10.1152/ajpregu.00875.200700875.2007 [pii] [DOI] [PubMed] [Google Scholar]
- De Feo P. Is high-intensity exercise better than moderate intensity exercise for weight loss? Nutrition, Metabolism & Cardiovascular Diseases. 2013;23:1037–1042. doi: 10.1016/j.numecd.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Demerath EW, Guo SS, Chumlea WC, Towne B, Roche AF, Siervogel RM. Comparison of percent body fat estimates using air displacement plethysmography and hydrodensitometry in adults and children. International journal of obesity and related metabolic disorders. 2002;26(3):389–397. doi: 10.1038/sj.ijo.0801898. doi: 10.1038/sj.ijo.0801898. [DOI] [PubMed] [Google Scholar]
- Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Medicine and science in sports and exercise. 2009;41(2):459–471. doi: 10.1249/MSS.0b013e3181949333. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- Dunn SL. PhD Dissertation. University of New South Wales; 2009. Effects of exercise and dietary intervention on metabolic syndrome markers of inactive premenopausal women. [Google Scholar]
- Ekkekakis P, Hall EE, Petruzzello SJ. The relationship between exercise intensity and affective responses demystified: to crack the 40-year-old nut, replace the 40-year-old nutcracker! Annals of Behavioral Medicine. 2008;35(2):136–149. doi: 10.1007/s12160-008-9025-z. doi: 10.1007/s12160-008-9025-z. [DOI] [PubMed] [Google Scholar]
- Esfandiari S, Sasson Z, Goodman JM. Short-term high-intensity interval and continuous moderate-intensity training improve maximal aerobic power and diastolic filling during exercise. European journal of applied physiology. 2014;114(2):331–343. doi: 10.1007/s00421-013-2773-x. doi: 10.1007/s00421-013-2773-x. [DOI] [PubMed] [Google Scholar]
- Fisher G, Brown AW, Bohan Brown MM, Alcorn A, Noles C, Winwood L, Allison DB. High Intensity Interval- vs Moderate Intensity- Training for Improving Cardiometabolic Health in Overweight or Obese Males: A Randomized Controlled Trial. PLoS One. 2015;10(10):e0138853. doi: 10.1371/journal.pone.0138853. doi: 10.1371/journal.pone.0138853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, Little JP, Macdonald MJ, Hawley JA. Physiological adaptations to low-volume, high-intensity interval training in health and disease. The Journal of physiology. 2012;590(Pt 5):1077–1084. doi: 10.1113/jphysiol.2011.224725. doi: jphysiol.2011.224725 [pii]10.1113/jphysiol.2011.224725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillen JB, Little JP, Punthakee Z, Tarnopolsky MA, Riddell MC, Gibala MJ. Acute high-intensity interval exercise reduces the postprandial glucose response and prevalence of hyperglycaemia in patients with type 2 diabetes. Diabetes, Obesity and Metabolism. 2012 doi: 10.1111/j.1463-1326.2012.01564.x. doi: 10.1111/j.1463-1326.2012.01564.x. [DOI] [PubMed] [Google Scholar]
- Gillen JB, Percival ME, Ludzki A, Tarnopolsky MA, Gibala MJ. Interval training in the fed or fasted state improves body composition and muscle oxidative capacity in overweight women. Obesity (Silver Spring) 2013;21(11):2249–2255. doi: 10.1002/oby.20379. doi: 10.1002/oby.20379. [DOI] [PubMed] [Google Scholar]
- Gillen JB, Percival ME, Skelly LE, Martin BJ, Tan RB, Tarnopolsky MA, Gibala MJ. Three minutes of all-out intermittent exercise per week increases skeletal muscle oxidative capacity and improves cardiometabolic health. PLoS One. 2014;9(11):e111489. doi: 10.1371/journal.pone.0111489. doi: 10.1371/journal.pone.0111489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremeaux V, Drigny J, Nigam A, Juneau M, Guilbeault V, Latour E, Gayda M. Long-term lifestyle intervention with optimized high-intensity interval training improves body composition, cardiometabolic risk, and exercise parameters in patients with abdominal obesity. American Journal of Physical Medicine and Rehabilitation. 2012;91(11):941–950. doi: 10.1097/PHM.0b013e3182643ce0. doi: 10.1097/PHM.0b013e3182643ce0. [DOI] [PubMed] [Google Scholar]
- Hackney A, Smith-Ryan A. Methodological Considerations in Exericse Endocrinology. In: Constantini N, Hackney A, editors. Endocrinology of Physical Activity and Sport: Second Edition. Springer; New York: 2012. pp. 1–19. [Google Scholar]
- Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Bauman A. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. doi: CIRCULATIONAHA.107.185649 [pii]10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- Helgerud J, Hoydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Hoff J. Aerobic high-intensity intervals improve VO2max more than moderate training. Medicine and science in sports and exercise. 2007;39(4):665–671. doi: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- Heydari M, Freund J, Boutcher SH. The effect of high-intensity intermittent exercise on body composition of overweight young males. Journal of Obesity. 2012;2012:480467. doi: 10.1155/2012/480467. doi: 10.1155/2012/480467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood MS, Little JP, Tarnopolsky MA, Myslik F, Gibala MJ. Low-volume interval training improves muscle oxidative capacity in sedentary adults. Medicine and science in sports and exercise. 2011;43(10):1849–1856. doi: 10.1249/MSS.0b013e3182199834. doi: 10.1249/MSS.0b013e3182199834. [DOI] [PubMed] [Google Scholar]
- Irving BA, Davis CK, Brock DW, Weltman JY, Swift D, Barrett EJ, Weltman A. Effect of exercise training intensity on abdominal visceral fat and body composition. Medicine and science in sports and exercise. 2008;40(11):1863–1872. doi: 10.1249/MSS.0b013e3181801d40. doi: 10.1249/MSS.0b013e3181801d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler HS, Sisson SB, Short KR. The potential for high-intensity interval training to reduce cardiometabolic disease risk. Sports Medicine. 2012;42(6):489–509. doi: 10.2165/11630910-000000000-00000. doi: 10.2165/11630910-000000000-000002 [pii] [DOI] [PubMed] [Google Scholar]
- Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring) 2006;14(2):336–341. doi: 10.1038/oby.2006.43. doi: 14/2/336 [pii]10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. American Journal of Clinical Nutrition. 1999;69(3):373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- Little JP, Gillen JB, Percival ME, Safdar A, Tarnopolsky MA, Punthakee Z, Gibala MJ. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. Journal of applied physiology. 2011;111(6):1554–1560. doi: 10.1152/japplphysiol.00921.2011. doi: japplphysiol.00921.2011 [pii]10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. The Journal of physiology. 2010;588(Pt 6):1011–1022. doi: 10.1113/jphysiol.2009.181743. doi: 10.1113/jphysiol.2009.181743jphysiol.2009.181743 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt H, Draper N, Marshall HC, Logan FJ, Hamlin MJ, Shearman JP, Frampton CM. High intensity interval training in a real world setting: a randomized controlled feasibility study in overweight inactive adults, measuring change in maximal oxygen uptake. PLoS One. 2014;9(1):e83256. doi: 10.1371/journal.pone.0083256. doi: 10.1371/journal.pone.0083256PONE-D-13-12272 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley PA, Artero EG, Sui X, Lee DC, Church TS, Lavie CJ, Blair SN. The obesity paradox, cardiorespiratory fitness, and coronary heart disease. Mayo Clinic Proceedings. 2012;87(5):443–451. doi: 10.1016/j.mayocp.2012.01.013. doi: 10.1016/j.mayocp.2012.01.013S0025-6196(12)00266-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory MA, Mole PA, Gomez TD, Dewey KG, Bernauer EM. Body composition by air-displacement plethysmography by using predicted and measured thoracic gas volumes. Journal of Applied Physiology. 1998;84(4):1475–1479. doi: 10.1152/jappl.1998.84.4.1475. [DOI] [PubMed] [Google Scholar]
- Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. New England Journal of Medicine. 2002;346(11):793–801. doi: 10.1056/NEJMoa011858. doi: 10.1056/NEJMoa011858346/11/793 [pii] [DOI] [PubMed] [Google Scholar]
- Naboush A, Hamdy O. Measuring visceral and hepatic fat in clinical practice and clinical research. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2013;19(4):587–589. doi: 10.4158/EP12331.OR. doi: 10.4158/EP12331.OR. [DOI] [PubMed] [Google Scholar]
- Nybo L, Sundstrup E, Jakobsen MD, Mohr M, Hornstrup T, Simonsen L, Krustrup P. High-intensity training versus traditional exercise interventions for promoting health. Medicine and science in sports and exercise. 2010;42(10):1951–1958. doi: 10.1249/MSS.0b013e3181d99203. doi: 10.1249/MSS.0b013e3181d99203. [DOI] [PubMed] [Google Scholar]
- Scalzo RL, Peltonen GL, Binns SE, Shankaran M, Giordano GR, Hartley DA, Miller BF. Greater muscle protein synthesis and mitochondrial biogenesis in males compared with females during sprint interval training. The FASEB Journal. 2014;28(6):2705–2714. doi: 10.1096/fj.13-246595. doi: 10.1096/fj.13-246595fj.13-246595 [pii] [DOI] [PubMed] [Google Scholar]
- Shiraev T, Barclay G. Evidence based exercise - clinical benefits of high intensity interval training. Austrailian Family Physician. 2012;41(12):960–962. [PubMed] [Google Scholar]
- Skelly LE, Andrews PC, Gillen JB, Martin BJ, Percival ME, Gibala MJ. High-intensity interval exercise induces 24-h energy expenditure similar to traditional endurance exercise despite reduced time commitment. Applied Physiology, Nutrition and Metabolism. 2014;39(7):845–848. doi: 10.1139/apnm-2013-0562. doi: 10.1139/apnm-2013-0562. [DOI] [PubMed] [Google Scholar]
- Smith-Ryan AE. Enjoyment of high-intensity interval training in an overweight/obese cohort: A short report. Physiological Measurement. 2015 doi: 10.1111/cpf.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Ryan AE, Melvin MN, Wingfield HL. High-intensity interval training: Modulating interval duration in overweight/obese men. The Physcian and Sports Medicine. 2015;43(2):107–113. doi: 10.1080/00913847.2015.1037231. doi: 10.1080/00913847.2015.1037231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AE, Walter AA, Graef JL, Kendall KL, Moon JR, Lockwood CM, Stout JR. Effects of beta-alanine supplementation and high-intensity interval training on endurance performance and body composition in men; a double-blind trial. Journal of the International Society of Sports Nutrition. 2009;6:5. doi: 10.1186/1550-2783-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogabe M, Okahisa T, Hibino S, Yamanoi A. Usefulness of differentiating metabolic syndrome into visceral fat type and subcutaneous fat type using ultrasonography in Japanese males. Journal of gastroenterology. 2012;47(3):293–299. doi: 10.1007/s00535-011-0489-4. doi: 10.1007/s00535-011-0489-4. [DOI] [PubMed] [Google Scholar]
- Talanian JL, Galloway SD, Heigenhauser GJ, Bonen A, Spriet LL. Two weeks of high-intensity aerobic interval training increases the capacity for fat oxidation during exercise in women. Journal of applied physiology. 2007;102(4):1439–1447. doi: 10.1152/japplphysiol.01098.2006. [DOI] [PubMed] [Google Scholar]
- Tjonna AE, Stolen TO, Bye A, Volden M, Slordahl SA, Odegard R, Wisloff U. Aerobic interval training reduces cardiovascular risk factors more than a multitreatment approach in overweight adolescents. Clinical Science (Lond) 2009;116(4):317–326. doi: 10.1042/CS20080249. doi: 10.1042/CS20080249CS20080249 [pii] [DOI] [PubMed] [Google Scholar]
- Trapp EG, Chisholm DJ, Freund J, Boutcher SH. The effects of high-intensity intermittent exercise training on fat loss and fasting insulin levels of young women. International Journal of Obesity (Lond) 2008;32(4):684–691. doi: 10.1038/sj.ijo.0803781. doi: 10.1038/sj.ijo.08037810803781 [pii] [DOI] [PubMed] [Google Scholar]
- Tremblay A, Simoneau JA, Bouchard C. Impact of exercise intensity on body fatness and skeletal muscle metabolism. Metabolism. 1994;43(7):814–818. doi: 10.1016/0026-0495(94)90259-3. doi: 0026-0495(94)90259-3 [pii] [DOI] [PubMed] [Google Scholar]
- Wang Z, Pi-Sunyer FX, Kotler DP, Wielopolski L, Withers RT, Pierson RN, Jr., Heymsfield SB. Multicomponent methods: evaluation of new and traditional soft tissue mineral models by in vivo neutron activation analysis. The American journal of clinical nutrition. 2002;76(5):968–974. doi: 10.1093/ajcn/76.5.968. [DOI] [PubMed] [Google Scholar]
- Whyte LJ, Gill JM, Cathcart AJ. Effect of 2 weeks of sprint interval training on health-related outcomes in sedentary overweight/obese men. Metabolism. 2010;59(10):1421–1428. doi: 10.1016/j.metabol.2010.01.002. doi: 10.1016/j.metabol.2010.01.002S0026-0495(10)00017-X [pii] [DOI] [PubMed] [Google Scholar]