Abstract
Context:
The hormonal basis of vasomotor symptoms (VMS) in hypogonadal men is incompletely understood.
Objective:
To determine the contributions of testosterone and estradiol deficiency to VMS in hypogonadal men.
Design:
Two randomized trials were conducted sequentially between September 2004 and April 2011. Controls were recruited separately.
Setting:
A single-site academic medical center.
Participants:
Healthy men ages 20–50, with normal serum testosterone levels.
Intervention:
Cohort 1 (n = 198, 81% completion) received goserelin acetate every 4 weeks to suppress gonadal steroids and were randomized to placebo or 1.25, 2.5, 5, or 10 g of testosterone gel daily for 16 weeks. Cohort 2 (n = 202, 78% completion) received the same regimen as cohort 1 plus anastrozole to block aromatization of testosterone. Controls (n = 37, 89% completion) received placebos for goserelin acetate and testosterone.
Main Outcome Measures:
Incidence of visits with VMS. This was a preplanned secondary analysis.
Results:
VMS were reported at 26% of visits in cohort 1, and 35% of visits in cohort 2 (P = .02), demonstrating an effect of estradiol deficiency. When adjacent estradiol level groups in cohort 1 were compared, the largest difference in VMS incidence was observed between the 5–9.9 and 10–14.9 pg/mL groups (38% vs 16%, P < .001). In cohort 2, the 10-g testosterone group differed significantly from placebo (16% vs 43%, P = .048) after adjustment for small differences in estradiol levels, indicating that high testosterone levels may suppress VMS.
Conclusions:
Estradiol deficiency is the key mediator of VMS in hypogonadal men. At high levels, testosterone may have a suppressive effect.
Vasomotor symptoms (VMS) incidence was studied in healthy men treated with goserelin acetate and testosterone add-back with or without anastrozole. Estradiol deficiency is the key mediator of VMS.
Vasomotor symptoms (VMS), or hot flashes, are a cardinal event of the menopause and are reported by about 75% of postmenopausal women (1). Although less well appreciated, VMS are common in men with severe hypogonadism. For example, approximately 70% of men undergoing androgen deprivation therapy for prostate cancer report hot flashes (1–3). As in women, VMS can disrupt many aspects of a man's life, including sleep, cognitive function, and social life, resulting in a lower sense of wellbeing (2). Although the hormonal basis of VMS, particularly the importance of estrogen withdrawal, has been investigated extensively in women (4–7), the roles of testosterone and estradiol in the pathogenesis of VMS in hypogonadal men are not well characterized. To our knowledge, neither the degree to which gonadal steroid levels must be suppressed nor the specific roles of androgen and estrogen deficiency in the pathogenesis of VMS in men have been reported.
To address these questions, we measured serum testosterone and estradiol levels and characterized the incidence of VMS in healthy male volunteers treated with a GnRH agonist and varying doses of testosterone. Because most circulating estradiol in men is produced by aromatization of testosterone (8–11), we repeated this experiment in another group of men but added an aromatase inhibitor to suppress estradiol synthesis. Using this paradigm, we expected serum testosterone levels to be similar in groups that received concordant testosterone doses, whereas estradiol levels would be discordant because aromatase inhibition suppresses estradiol synthesis dramatically. By comparing the incidence of VMS both within and across the 2 cohorts, we deduced the relative roles of testosterone and estradiol, and estimated the minimal levels needed to prevent VMS.
Materials and Methods
Study subjects
This is a substudy of a physiologic investigation of the consequences of testosterone and estradiol deficiency and their relative roles in various manifestations of male hypogonadism (12). We recruited 3 cohorts, designated as cohort 1 (n = 198), cohort 2 (n = 202), and controls (n = 37). The baseline characteristics of these cohorts have been reported previously (12). Entrance criteria were identical for all 3 cohorts. All men were 20–50 years old, in good general health and had testosterone levels within our laboratory's reference range (270–1070 ng/dL). Additional eligibility criteria have been published previously (12).
Study design and protocol
Subjects in cohorts 1 and 2 received goserelin acetate (Zoladex; AstraZeneca Pharmaceuticals LP) 3.6 mg sc at weeks 0, 4, 8, and 12 and were randomly assigned to 1 of 5 groups that received 0 (placebo), 1.25, 2.5, 5, or 10 g of a topical 1% testosterone gel (AndroGel; AbbVie Pharmaceuticals) daily for 16 weeks. Men in cohort 2 also received anastrozole (Arimidex; AstraZeneca Pharmaceuticals LP) 1 mg orally each day to suppress aromatization of testosterone to estradiol. Controls were recruited separately and received placebos for both goserelin acetate and testosterone. All subjects, including the controls, were blinded to group assignment. Investigators were blinded to assignments for all groups except the controls.
Subjects were seen every 4 weeks for 16 weeks. At each visit, fasting blood was collected to measure serum testosterone and estradiol levels. Daily diaries were reviewed to assess compliance with the topical gel. VMS occurrence was assessed in 2 ways. First, subjects were asked to keep a diary indicating the number of VMS they experienced each day (Supplemental Figure 1). The description of VMS provided during informed consent is included in the Supplemental Methods. Second, as part of a structured interview at each visit, subjects were asked: “In the last week, how many hot flashes have you experienced?” Results from the diaries and structured interview were highly correlated (r = 0.96). Complete VMS diaries were returned at approximately 40% of visits and used when available. If diaries were not returned, data from the structured interview (completed at every study visit) were used. At each visit, subjects also completed surveys assessing fatigue and several domains of health-related quality of life (Supplemental Results) (13, 14). The study was approved by the Institutional Review Board of Partners HealthCare. All subjects provided written informed consent. This protocol was registered at ClinicalTrials.gov NCT00114114.
Measurements
Serum total testosterone was measured by solid-phase chemiluminescent immunoassay using an automated analyzer (Centaur XP; Siemens). The sensitivity of this assay is 20 ng/dL. Total testosterone was remeasured by liquid chromatography, tandem mass spectroscopy at all time points from 5 randomly selected men in each of the 5 groups in cohort 1 and the controls. The correlation between the testosterone assays was 0.93, and the assays gave very similar results (TRIA = 0.98TLC/MS/MS + 21). Serum estradiol was measured using liquid chromatography, tandem mass spectroscopy. The sensitivity of this assay is 1.25 pg/mL (15).
Statistical analysis
All subjects who returned for at least 1 visit and who reported taking at least 80% of their study medication doses are included in the data analysis. Data were analyzed at the level of the individual visit. Thus, each one of a subject's follow-up visits from weeks 4 to 16 was independently classified based on whether or not he reported any VMS in the preceding 7 days. The serum testosterone and estradiol levels at each visit were used to reflect the hormonal milieu during the preceding 7 days. The proportions of visits in each testosterone dose group (0, 1.25, 2.5, 5, or 10 g daily) at which subjects reported at least 1 VMS event in the last 7 days were compared using logistic models in which the mean proportions and their SEs were estimated with generalized estimating equations that account for the intrasubject correlations of the longitudinal events. Similar analyses were performed when visits were grouped by serum testosterone levels (<100, 100–199, 200–299, 300–499, 500–699, and ≥700 ng/dL) or serum estradiol levels (<5, 5–9.9, 10–14.9, 15–24.9, 25–34.9, or ≥35 pg/mL) at each visit. Data are presented as the mean ± SD unless otherwise noted. All P values are 2-sided. P < .05 are considered significant.
Data interpretation
Relative roles of testosterone and estradiol in VMS
We used 3 approaches to assess our primary goal, to determine whether deficiency of estradiol, testosterone, or both leads to the development of VMS. To ascertain the role of estradiol, we first compared the overall incidence of visits with VMS in men in whom aromatization of testosterone to estradiol was intact (cohort 1) vs the incidence of VMS in men in whom aromatization was suppressed (cohort 2). Administration of identical testosterone doses to men treated with a GnRH agonist alone (cohort 1) or men treated with a GnRH agonist plus anastrozole (cohort 2) should produce dose groups with similar testosterone levels but discordant estradiol levels. Thus, differences between cohorts 1 and 2 should reflect estradiol deficiency. Next, we compared the incidence of visits with VMS between cohorts 1 and 2 at each testosterone dose. Finally, to determine whether testosterone exerts an effect on VMS, we compared the incidence of visits with VMS in the placebo testosterone group with the incidence of VMS in the 1.25-, 2.5-, 5-, and 10-g testosterone groups in cohort 2.
Levels of testosterone and estradiol necessary to prevent VMS
We also used 3 approaches to estimate the levels of testosterone and estradiol below which VMS reporting in cohort 1 began to increase. First, we clustered visits for men in cohort 1 according to their ambient testosterone or estradiol levels as described above, and compared the incidence of visits with VMS in each testosterone or estradiol level group with the controls. Next, we compared the incidence of visits with VMS in adjacent estradiol and testosterone level groups in cohort 1. Finally, we compared the incidence of visits with VMS in men with testosterone or estradiol levels above or below prespecified “breakpoints” (5, 10, or 15 pg/mL for estradiol and 100, 200, and 300 ng/dL for testosterone) in cohort 1.
Results
Baseline characteristics and protocol completion
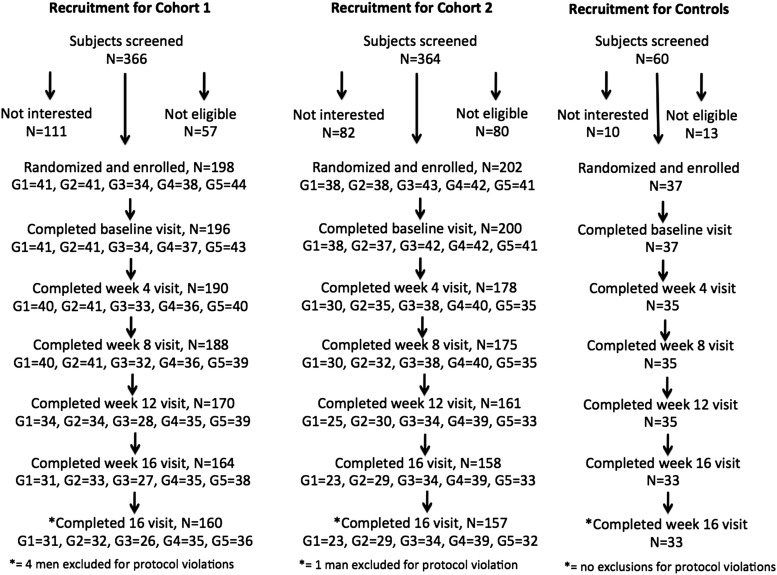
Baseline characteristics were similar among groups and across cohorts (Table 1). Recruitment and progression through the protocol is shown in Figure 1. Sixteen men (6 in cohort 1 and 10 in cohort 2) listed VMS as a reason for discontinuing the protocol. Follow-up data were available in 13 of these 16 men. None of the controls discontinued the protocol due to VMS.
Table 1.
Baseline Characteristics of Subjects in Cohort 1 (Upper Row of Each Pair), Cohort 2 (Lower Row of Each Pair), and Controls
| Testosterone Dose |
Control (n = 37) | |||||
|---|---|---|---|---|---|---|
| Group 1 0 g/d (n = 41) (n = 38) | Group 2 1.25 g/d (n = 41) (n = 38) | Group 3 2.5 g/d (n = 34) (n = 43) | Group 4 5 g/d (n = 38) (n = 42) | Group 5 10 g/d (n = 44) (n = 41) | ||
| Age (y) | 32 ± 9 | 34 ± 7 | 32 ± 8 | 34 ± 8 | 33 ± 8 | 28 ± 14 |
| 34 ± 7 | 33 ± 7 | 33 ± 7 | 33 ± 6 | 34 ± 6 | ||
| Height | 179 ± 6a | 177 ± 6 | 176 ± 6 | 173 ± 7a | 177 ± 8 | 177 ± 7 |
| 175 ± 6 | 177 ± 6 | 177 ± 8 | 176 ± 7 | 177 ± 6 | ||
| Weight (kg) | 84 ± 14 | 84 ± 14 | 78 ± 15 | 78 ± 14a | 85 ± 18 | 83 ± 18 |
| 84 ± 15 | 87 ± 17 | 83 ± 14 | 87 ± 15 | 83 ± 12 | ||
| BMI (kg/m2) | 26 ± 4 | 27 ± 4 | 25 ± 4 | 26 ± 4 | 27 ± 5 | 26 ± 5 |
| 27 ± 5 | 28 ± 5 | 26 ± 4 | 28 ± 5 | 26 ± 4 | ||
| Testosterone (ng/dL) | 510 ± 160 | 506 ± 154 | 574 ± 125 | 506 ± 138 | 529 ± 140 | 558 ± 200 |
| 511 ± 181 | 548 ± 189 | 512 ± 159 | 514 ± 176 | 517 ± 151 | ||
| Estradiol (pg/mL) | 27 ± 8a | 27 ± 8a | 32 ± 10 | 27 ± 8 | 29 ± 9 | 27 ± 9 |
| 32 ± 10 | 32 ± 10 | 30 ± 13 | 30 ± 10 | 27 ± 9 | ||
Data are expressed as the mean ± SD. To convert testosterone to nmol/L, multiply by 0.03467. Modified and reproduced with permission from Finkelstein et al (12).
Significant difference vs cohort 2, P < .05.
Figure 1.
Recruitment, enrollment, and progression through the study protocol for men in cohort 1 (GnRH agonist plus testosterone, n = 198), cohort 2 (GnRH agonist plus testosterone plus anastrozole, n = 202), and controls (placebo GnRH agonist plus placebo testosterone, n = 37). Four men in cohort 1 and 1 in cohort 2 were excluded for protocol violations. In cohort 1, 8 men completed only their baseline visit, 2 discontinued the study at week 4, and 24 men discontinued between weeks 8 and 16. In cohort 2, 24 men completed only their baseline visit, 3 discontinued the study at week 4, and 17 men discontinued between weeks 8 and 16. Three men in cohort 1 and 1 man in cohort 2 did not have estradiol levels measured at any visit and were excluded from the analysis of VMS and estradiol levels. Modified and reproduced with permission from Finkelstein et al (12).
Testosterone and estradiol levels
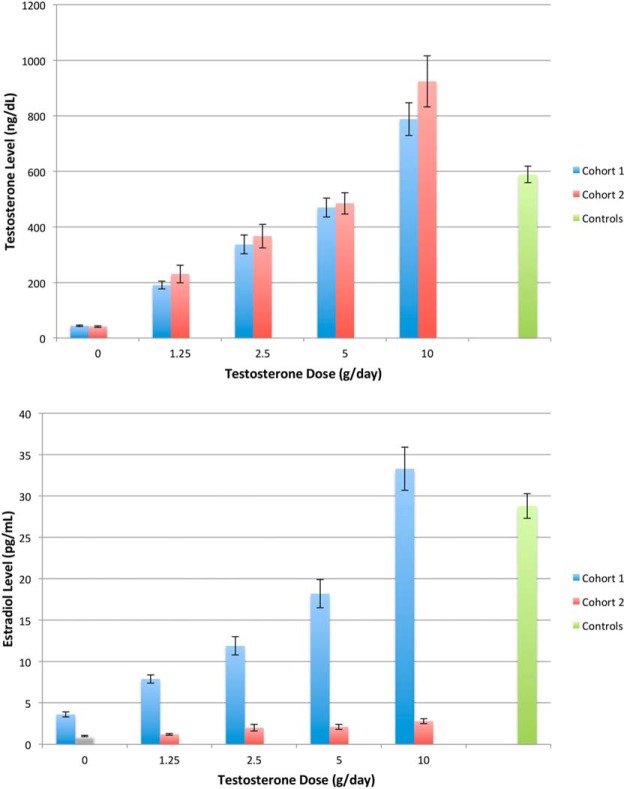
In cohort 1, mean serum testosterone levels from weeks 4 through 16 were 44 ± 13, 191 ± 78, 337 ± 173, 470 ± 201, and 788 ± 353 ng/dL, and mean estradiol levels were 3.6 ± 1.4, 7.9 ± 2.9, 11.9 ± 5.7, 18.2 ± 10.2, and 33.3 ± 15.3 pg/mL in men receiving goserelin acetate and 0, 1.25, 2.5, 5, or 10 g of testosterone gel daily (Figure 2). In cohort 2, mean testosterone levels were 41 ± 13, 231 ± 171, 367 ± 248, 485 ± 240, and 924 ± 521 ng/dL, and mean estradiol levels were 1.0 ± 0.4, 1.2 ± 0.4, 2.0 ± 2.3, 2.1 ± 1.9, and 2.8 ± 1.8 pg/mL. Mean serum testosterone and estradiol levels in the controls from weeks 0 through 16 were 589 ± 171 ng/dL and 28.8 ± 8.6 pg/mL. Within each testosterone dose group in cohorts 1 and 2, mean serum testosterone and estradiol levels on therapy were similar in the men who completed the protocol and those who discontinued the protocol prematurely (data not shown).
Figure 2.
Mean serum testosterone (upper panel) and estradiol (lower panel) levels in men treated with a GnRH agonist plus 0, 1.25, 2.5, 5, or 10 g of testosterone gel daily (cohort 1, n = 198, blue bars) or in men treated with the same regimen plus anastrozole (cohort 2, n = 202, red bars). Controls (green bars) received placebos for both the GnRH agonist and testosterone gel. Error bars are ± SE. Modified and reproduced with permission from Finkelstein et al (12).
Relative roles of testosterone and estradiol deficiency in the development of VMS
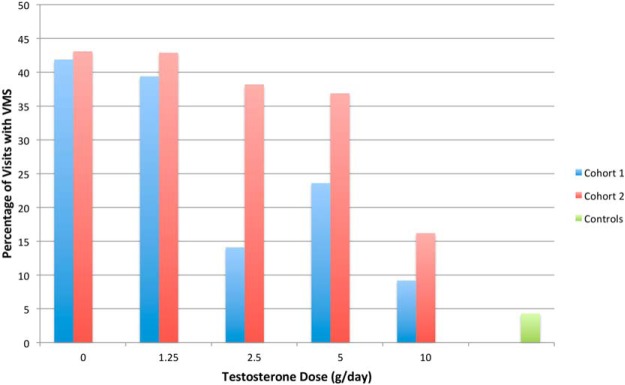
VMS were reported at 26% of visits in cohort 1 and at 35% of visits in cohort 2 (P = .02), demonstrating an effect of estradiol deficiency. Controls reported VMS at 4% of visits. Although the percentage of visits at which subjects reported VMS was higher in all testosterone dose groups in cohort 2 than in cohort 1, only the difference between the 2.5 g daily groups was statistically significant (14% in cohort 1 vs 38% in cohort 2, P = .003) (Figure 3). Sixty-four percent of men in cohort 1 who received placebo testosterone reported experiencing VMS on at least 1 occasion.
Figure 3.
Percent of visits from weeks 4, 8, 12, and 16, at which men in cohort 1 (blue bars) and cohort 2 (red bars) reported any VMS when grouped according to their randomly assigned testosterone dose. A visit was considered to be positive for VMS if the participant reported any VMS in the 7 days before each study visit. The incidence of visits with VMS differed significantly between the 2.5-g testosterone groups. Number of visits with VMS over total visits (cohort 1, cohort 2) in each testosterone dose group: 67/160, 50/116 for 0 g; 63/160, and 60/140 for 1.25 g; 18/128 and 58/152 for 2.5 g; 34/144 and 59/160 for 5 g; 14/152 and 22/136 for 10 g; and 6/140 for controls.
Within cohort 2, the percentage of visits with VMS was significantly greater in the placebo testosterone group (43%) than in the 2.5 g (38%, P = .01 vs placebo) and 10 g (16%, P < .001 vs placebo) groups, and tended to be higher in the group that received 5 g of testosterone daily (37%; P = .054 vs placebo). When the differences between testosterone dose groups within cohort 2 were adjusted for the small differences in estradiol levels, only the 10-g group remained significantly different from placebo (placebo vs 10 g, P = .048; placebo vs 5 g, P = .70; placebo vs 2.5 g, P = .08; placebo vs 1.25 g, P = .44), indicating that high levels of testosterone are needed to suppress VMS.
Relationship of VMS to estradiol and testosterone levels
When visits were grouped by ambient estradiol levels, the incidence of VMS in cohort 1 was significantly greater than in controls until estradiol levels rose above 25 pg/mL. When adjacent estradiol level groups were compared, a significant difference in the incidence of visits with VMS was observed between the 5–9.9 and 10–14.9 pg/mL groups (38% vs 16%, P < .001) (Figure 4A). When visits in cohort 1 were grouped by ambient testosterone levels, the incidence of VMS at every level group except for 500–699 ng/dL differed from the controls (Figure 4B). When adjacent testosterone level groups were compared, a significant difference was observed between the less than 100 and 100–199 ng/dL groups (49% vs 26%, P < .001) and the 300–499 and 500–699 ng/dL groups (20% vs 6%, P = .006).
Figure 4.
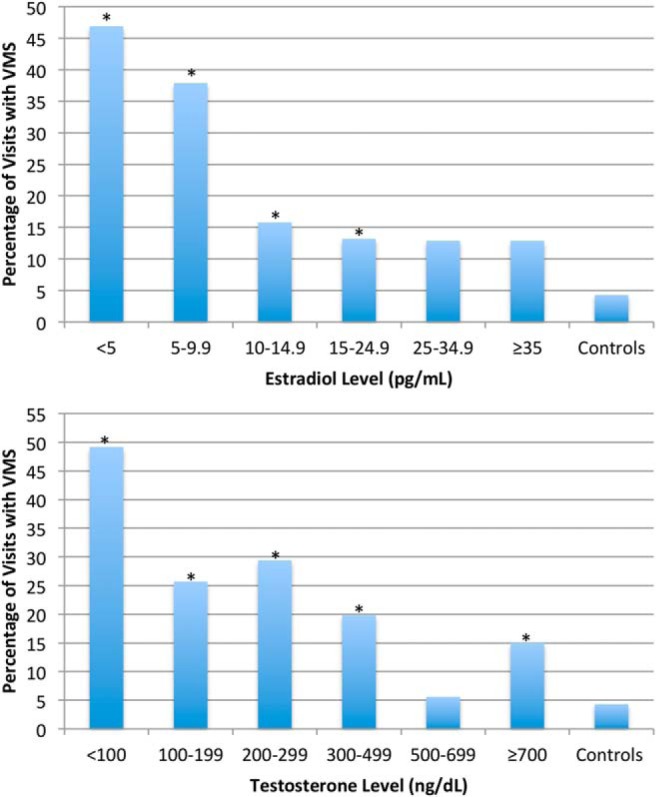
Percent of visits at which men in cohort 1 reported VMS according to their mean serum estradiol (upper panel) and testosterone (lower panel) levels from weeks 4 to 16. The percentage of visits with incident VMS in the controls is also shown. The incidence of VMS decreased significantly as estradiol levels rose from 5–9.9 to 10–14.9 pg/mL. For testosterone, the incidence of VMS decreased significantly as serum levels rose from less than 100 to 100–199 ng/dL and again from 300–499 to 500–699 ng/dL. Groups with an asterisk differed significantly from the controls. For each estradiol level group, number of visits with VMS over total visits: 82/175 for les than 5 pg/mL, 67/177 for 5–9.9 pg/mL, 15/95 for 10–14.9 pg/mL, 15/114 for 15–24.9 pg/mL, 8/62 for 25–34.9 pg/mL, 9/70 for more than or equal to 35 pg/mL, and 6/140 for controls. For each testosterone level group, number of visits with VMS over total visits: 97/197 for less than 100 ng/dL, 26/101 for 100–199 ng/dL, 30/102 for 200–299 ng/dL, 23/116 for 300–499 ng/dL, 4/72 for 500–699 ng/dL, and 16/107 for more than or equal to 700 ng/dL.
Evaluation of testosterone and estradiol breakpoints
The incidence of VMS was significantly greater at visits with testosterone levels below 100 (47% vs 25%), 200 (43% vs 22%), or 300 ng/dL (41% vs 18%) than at visits with testosterone levels above these values (P < .001 for all comparisons). Similarly, the incidence of VMS was significantly greater at visits with estradiol levels below 5 (40% vs 18%), 10 (40% vs 11%), and 15 pg/mL (37% vs 10%) than at visits with estradiol levels above these values (P < .001 for all comparisons).
Discussion
In this study, we used hormonal manipulations so that subgroups of men in cohorts 1 and 2 would have concordant levels of testosterone and discordant estradiol levels as serum testosterone levels ranged from severely hypogonadal to mildly supraphysiologic. As expected, serum estradiol levels increased progressively as the testosterone dose increased in cohort 1 but remained low in cohort 2 because those men also received anastrozole. The incidence of VMS was greater when anastrozole was added to the treatment regimen (cohort 2) than when aromatization of testosterone to estradiol was intact (cohort 1). Notably, the incidence of VMS was elevated even in men whose serum testosterone levels were above the physiologic range if estradiol levels were below 10 pg/mL (Figure 3). This finding provides clear evidence that estrogen deficiency plays a pivotal role in the pathogenesis of VMS in men. Because of concerns that administering estradiol to GnRH agonist-treated men might cause undesirable feminizing side effects such as gynecomastia, we did not include a group of men with isolated testosterone deficiency and normal or high estradiol levels. We did, however, have men who received a GnRH agonist plus testosterone and anastrozole (cohort 2), creating a hormonal milieu of isolated androgen exposure. After adjustment for the small increase in estradiol levels observed in those men as testosterone doses increased, only the 10 g daily group had a significantly lower incidence of VMS than the placebo group, suggesting that only high doses of testosterone reduce the likelihood that estrogen deficiency will cause VMS. When data from cohort 1 were analyzed by ambient hormone levels, VMS incidence approached the controls' rate at testosterone levels above 500 ng/dL. Thus, our data demonstrate that estradiol deficiency is the primary stimulus for VMS in men and that high levels of testosterone reduce VMS.
It is widely believed that declining estradiol levels play an important role in the pathogenesis of VMS in perimenopausal and postmenopausal women (4–7, 16, 17) and can even lead to VMS in premenopausal women during the late luteal phase of the menstrual cycle (18). Similar to men, 69% of healthy premenopausal women treated with a GnRH agonist report VMS (19). It has been proposed that declining estrogen levels narrow the thermoneutral zone between core body temperatures that trigger sweating and shivering (20, 21). Although the brain region responsible for VMS remains uncertain, recent studies suggest that signaling via the neurokinin 3 receptor and arcuate kisspeptin, neurokinin B, and dynorphin neurons projecting to preoptic thermoregulatory areas may explain the link between estrogen deficiency and VMS (22, 23).
Few studies have examined the role of gonadal steroids in the pathogenesis of VMS in men. One study reported that VMS occur less frequently in men with advanced prostate cancer treated with a selective estrogen receptor modulator with intrinsic estrogen receptor-α agonist activity than in men treated with a long-acting GnRH agonist (24), suggesting that estrogen reduces the risk of developing VMS in men. In surgically postmenopausal women, adding low-dose testosterone or methyltestosterone to a regimen of estrogen replacement was no more effective in preventing VMS than was treatment with estrogen alone (25, 26). In our study, testosterone was unable to suppress the development of VMS unless given in doses that produced supraphysiologic levels. Thus, it appears that physiologic levels of androgens do not play a major role in regulation of VMS in men or women.
Recent evidence has shown that estrogen deficiency is responsible for several of the cardinal manifestations of male hypogonadism, including high-turnover bone loss (27–30), accumulation of body fat (12), and surprisingly, loss of libido and erectile function (12). The current study demonstrates that VMS represent another key manifestation of male hypogonadism that is largely related to estrogen deficiency rather than to androgen deficiency.
Unexpectedly, the incidence of VMS was higher in men receiving a GnRH agonist and testosterone add-back despite being given in doses sufficient to produce mean serum testosterone or estradiol levels similar to those of the controls. This finding, along with some other anomalies in the data, probably reflects the fact that gonadal steroid levels fluctuate far more in men given a GnRH agonist and testosterone add-back than in men achieving the same levels via normal endogenous secretion (31). We assessed VMS over a 7-day period and only measured gonadal steroids on the last day of that interval. Thus, levels may have been sufficiently low to cause VMS at one point during that 7-day period but could have been normal on the day of their visit. Although the basis for this increased variability is not known, it is likely related to variability in absorption or compliance with the testosterone gel and/or the absence of the normal feedback regulation of the hypothalamic-pituitary-testicular axis.
Our study has other limitations. Prospective diaries were used to estimate VMS occurrence at only 40% of visits. However, diary and recall data were highly correlated. Although diaries are better than recall, they are less sensitive than electronic event marking and sternal skin conductance for detecting VMS (32). Thus, we probably underestimated the true incidence of VMS, although self-report should accurately estimate the incidence of VMS that are noticed by subjects. In addition to probable underreporting of mild VMS, false positive reporting also likely occurred, as evidenced by the reporting of VMS at 4.3% of visits (in 11% of men) in the controls. We repeated our analyses requiring that subjects report more than 1 or more than 4 hot flashes to be considered as experiencing VMS, but the overall trends for both analyses did not change (data not shown). Finally, to minimize the risk of gynecomastia, we did not include a group of men who received only a GnRH agonist and estradiol. Thus, in both cohorts 1 and 2, low testosterone levels were almost always accompanied by low estradiol levels, so that changes observed in men with low testosterone levels could be due to androgen deficiency alone, estrogen deficiency alone, or both.
In summary, we created a series of dose-response relationships to determine the relative roles of testosterone and estradiol in the pathogenesis of VMS in men. We found that estradiol is the primary hormonal regulator of male VMS. These data suggest that aromatizable androgens may have advantages over nonaromatizable androgens in the management of symptomatic male hypogonadism, and add to the growing body of evidence that estrogen deficiency should be considered as a discrete hormone deficiency syndrome in men.
Acknowledgments
We thank Dr Alex Linker, Ms Christine Kim, Dr Jonathan Youngner, Dr Christopher Hahn, Dr Andrew Servais, Dr Nicholas Perros, Ms Kendra Wulczyn, and Ms Sarah Hirsch for their dedicated administration of the study protocol and assistance with data management; the staff of the Mallinckrodt General Clinical Research Center for their care of the subjects; Dr Robert M. Neer and Dr Henry M. Kronenberg for their scientific guidance; and Mrs Deborah A. Fitzgerald for her administrative support. AndroGel was provided by AbbVie Pharmaceuticals. Zoladex and Arimidex were provided by AstraZeneca Pharmaceuticals LP. Neither Zoladex nor Arimidex are licensed for use in this setting.
This work was supported by National Institutes of Health Grants R01 AG030545, K24 DK02759, RR-1066, and R01MH082922 and by an investigator-initiated grant from AbbVie Pharmaceuticals.
Disclosure Summary: H.J. receives grant support from Merck and consults for Mitsubishi Tanabe, Noven, Merck, NeRRe Therapeutics, and SAGE Therapeutics. All other authors have nothing to disclose.
Current address for A.P.T.: University of Washington, Seattle, WA 98195. Current address for H.L.: Massachusetts General Hospital, Boston, MA 02114. Current address for M.L.W.: University of Pennsylvania, Philadelphia, PA 19104. Current address for H.J.: Brigham and Women's Hospital, Boston, MA 02115.
Footnotes
- VMS
- vasomotor symptom
References
- 1. Shanafelt TD, Barton DL, Adjei AA, Loprinzi CL. Pathophysiology and treatment of hot flashes. Mayo Clin Proc. 2002;77:1207–1218. [DOI] [PubMed] [Google Scholar]
- 2. Karling P, Hammar M, Varenhorst E. Prevalence and duration of hot flushes after surgical or medical castration in men with prostatic carcinoma. J Urol. 1994;152:1170–1173. [DOI] [PubMed] [Google Scholar]
- 3. Schow DA, Renfer LG, Rozanski TA, Thompson IM. Prevalence of hot flushes during and after neoadjuvant hormonal therapy for localized prostate cancer. South Med J. 1998;91:855–857. [DOI] [PubMed] [Google Scholar]
- 4. Gadomska H, Barcz E, Cyganek A, Leocmach Y, Chadha-Boreham H, Marianowski L. Efficacy and tolerability of low-dose transdermal estrogen (Oesclim) in the treatment of menopausal symptoms. Curr Med Res Opin. 2002;18:97–102. [DOI] [PubMed] [Google Scholar]
- 5. Nelson HD. Commonly used types of postmenopausal estrogen for treatment of hot flashes: scientific review. JAMA. 2004;291:1610–1620. [DOI] [PubMed] [Google Scholar]
- 6. Shulman LP, Yankov V, Uhl K. Safety and efficacy of a continuous once-a-week 17β-estradiol/levonorgestrel transdermal system and its effects on vasomotor symptoms and endometrial safety in postmenopausal women: the results of two multicenter, double-blind, randomized, controlled trials. Menopause. 2002;9:195–207. [DOI] [PubMed] [Google Scholar]
- 7. Simon JA, Bouchard C, Waldbaum A, Utian W, Zborowski J, Snabes MC. Low dose of transdermal estradiol gel for treatment of symptomatic postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2007;109:588–596. [DOI] [PubMed] [Google Scholar]
- 8. Ferrini RL, Barrett-Connor E. Sex hormones and age: a cross-sectional study of testosterone and estradiol and their bioavailable fractions in community-dwelling men. Am J Epidemiol. 1998;147:750–754. [DOI] [PubMed] [Google Scholar]
- 9. Khosla S, Melton LJ, 3rd, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83:2266–2274. [DOI] [PubMed] [Google Scholar]
- 10. Longcope C, Kato T, Horton R. Conversion of blood androgens to estrogens in normal adult men and women. J Clin Invest. 1969;48:2191–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van den Beld AW, de Jong FH, Grobbee DE, Pols HA, Lamberts SW. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle strength, bone density, and body composition in elderly men. J Clin Endocrinol Metab. 2000;85:3276–3282. [DOI] [PubMed] [Google Scholar]
- 12. Finkelstein JS, Lee H, Burnett-Bowie SA, et al. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369:1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. [DOI] [PubMed] [Google Scholar]
- 14. Cleary PD, Morrissey G, Oster G. Health-related quality of life in patients with advanced prostate cancer: a multinational perspective. Qual Life Res. 1995;4:207–220. [DOI] [PubMed] [Google Scholar]
- 15. Khosla S, Amin S, Singh RJ, Atkinson EJ, Melton LJ, 3rd, Riggs BL. Comparison of sex steroid measurements in men by immunoassay versus mass spectroscopy and relationships with cortical and trabecular volumetric bone mineral density. Osteoporos Int. 2008;19:1465–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Freedland SJ, Eastham J, Shore N. Androgen deprivation therapy and estrogen deficiency induced adverse effects in the treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2009;12:333–338. [DOI] [PubMed] [Google Scholar]
- 17. Wibowo E, Schellhammer P, Wassersug RJ. Role of estrogen in normal male function: clinical implications for patients with prostate cancer on androgen deprivation therapy. J Urol. 2011;185:17–23. [DOI] [PubMed] [Google Scholar]
- 18. Hahn PM, Wong J, Reid RL. Menopausal-like hot flashes reported in women of reproductive age. Fertil Steril. 1998;70:913–918. [DOI] [PubMed] [Google Scholar]
- 19. Joffe H, Crawford S, Economou N, et al. A gonadotropin-releasing hormone agonist model demonstrates that nocturnal hot flashes interrupt objective sleep. Sleep. 2013;36:1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Freedman RR, Blacker CM. Estrogen raises the sweating threshold in postmenopausal women with hot flashes. Fertil Steril. 2002;77:487–490. [DOI] [PubMed] [Google Scholar]
- 21. Freedman RR, Krell W. Reduced thermoregulatory null zone in postmenopausal women with hot flashes. Am J Obstet Gynecol. 1999;181:66–70. [DOI] [PubMed] [Google Scholar]
- 22. Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jayasena CN, Comninos AN, Stefanopoulou E, et al. Neurokinin B administration induces hot flushes in women. Sci Rep. 2015;5:8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu EY, Getzenberg RH, Coss CC, et al. Selective estrogen receptor α agonist GTx-758 decreases testosterone with reduced side effects of androgen deprivation therapy in men with advanced prostate cancer. Eur Urol. 2015;67:334–341. [DOI] [PubMed] [Google Scholar]
- 25. Shifren JL, Braunstein GD, Simon JA, et al. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343:682–688. [DOI] [PubMed] [Google Scholar]
- 26. Watts NB, Notelovitz M, Timmons MC, Addison WA, Wiita B, Downey LJ. Comparison of oral estrogens and estrogens plus androgen on bone mineral density, menopausal symptoms, and lipid-lipoprotein profiles in surgical menopause. Obstet Gynecol. 1995;85:529–537. [DOI] [PubMed] [Google Scholar]
- 27. Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J Clin Invest. 2000;106:1553–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Khosla S, Melton LJ, 3rd, Riggs BL. Estrogens and bone health in men. Calcif Tissue Int. 2001;69:189–192. [DOI] [PubMed] [Google Scholar]
- 29. Leder BZ, LeBlanc KM, Schoenfeld DA, Eastell R, Finkelstein JS. Differential effects of androgens and estrogens on bone turnover in normal men. J Clin Endocrinol Metab. 2003;88:204–210. [DOI] [PubMed] [Google Scholar]
- 30. Finkelstein JS, Lee H, Leder BZ, et al. Gonadal steroid-dependent effects on bone turnover and bone mineral density in men. J Clin Invest. 2016;126:1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moore AF, Lee H, Linker AH, et al. Dose-response relationships and variability of testosterone levels in severely hypogonadal men treated with a testosterone gel. Abstract presented at the 88th Annual Meeting of The Endocrine Society, Boston, MA, 2006. [Google Scholar]
- 32. Hanisch LJ, Palmer SC, Marcus SC, Hantsoo L, Vaughn DJ, Coyne JC. Comparison of objective and patient-reported hot flash measures in men with prostate cancer. J Support Oncol. 2009;7:131–135. [PubMed] [Google Scholar]