Abstract
Context:
Small studies suggest exogenous estrogen may improve vitamin D status, but the etiology is unclear because women who use hormones may make lifestyle choices that differentially affect vitamin D status.
Objective:
Our objective was to investigate the association between use of hormonal contraception and 25-hydroxy-vitamin D (25(OH)D).
Design:
We used linear regression modeling of cross-sectional data to estimate percent change in season-adjusted serum 25(OH)D with estrogen use after adjustment for other factors.
Setting:
At the enrollment clinic visit (2010–2012) into a cohort study of uterine fibroids, each subject provided a blood sample, had anthropomorphic variables and skin reflectance measured, and answered questionnaires on demographics, dietary and supplement intake, contraceptive use, reproductive and medical history, and behaviors.
Participants:
A total of 1662 African American women, community volunteers, 23–34 years old, living in the Detroit, Michigan, area were included.
Interventions:
None.
Main Outcomes and Measures:
Serum 25(OH)D was measured.
Results:
Serum 25(OH)D concentrations were low (70% <20 ng/ml). Current use of an estrogen-containing contraceptive was associated with a 20% (95% confidence interval: 14–27) increase in 25(OH)D after adjustment. There was no increase in 25(OH)D among participants who had used estrogen in the past, but were not current users, indicating that results were unlikely to be due to unmeasured confounding by factors related to contraceptive choice.
Conclusions:
The increase in 25(OH)D with use of estrogen-containing contraceptives raise mechanistic questions regarding the biological pathways involved, and highlights the need for studies that examine possible endogenous estrogen effects on vitamin D.
In a cohort of young African American women, current use of an estrogen-containing contraceptive was associated with a 20% increase in serum 25(OH)D after adjustment for season and other covariates.
Vitamin D has been associated with numerous reproductive outcomes (1). Findings have been conflicting, however, and the biologic mechanisms have not been established. Although the most biologically active form of vitamin D is 1,25-dihydroxyvitamin D, most studies measure serum 25-hydroxy vitamin D (25(OH)D), which has a longer half-life and is the accepted clinical biomarker of vitamin D status (2). Multiple studies have suggested that use of estrogen, in the form of hormone replacement therapy (3, 4), oral contraception (5–12), or exogenous estrogen broadly (hormone replacement therapy or oral contraception) (13, 14) may be associated with increases in measured 25(OH)D. The existing studies in reproductive-aged women are generally small and vary widely in control of confounders. Importantly, the choice of a specific type of contraceptive may be associated with factors (eg, weight, smoking, use of nutritional supplements, level of exercise) that may also impact concentrations of 25(OH)D. Therefore it remains possible that the observed associations between exogenous estrogen and 25(OH)D are due to factors other than the hormonal exposure itself.
Examining the relationship between exogenous hormones and vitamin D levels is important for understanding normal metabolic pathways, making clinical decisions, and interpreting epidemiologic research on many outcomes associated with vitamin D. Changes in vitamin D resulting from either endogenous or exogenous hormones may have implications for timing of clinical tests for vitamin D deficiency, identification of vulnerable populations, and discovery of possible biologic pathways by which vitamin D might influence health outcomes.
We investigated the relationship between the use of exogenous hormones and measured vitamin D status in a cohort of young African American women from a single area in the Northern United States. Careful attention to modeling of season and a rich set of covariates allowed us to characterize the degree to which current use of estrogen-containing contraception influences measured concentrations of 25(OH)D.
Materials and Methods
The Study of Environment, Lifestyle & Fibroids recruited 1696 black women aged 23–34 from the Detroit, Michigan, area to participate in a prospective study of the risk factors for fibroid incidence and growth. Details of the study have been published previously (15). Recruitment used both targeted media campaigns designed to saturate the community with information about the study and direct mailings of study brochures to age-eligible women who had been patients at Henry Ford Health System, a collaborating institution. Main inclusion criteria included age 23—34 years; self-identification as black, African American, or partly African American; intact uterus; and no prior clinical diagnosis of fibroids. All women provided informed consent. The study was approved by Institutional Review Boards at the National Institute of Environmental Health Sciences and the Henry Ford Health System.
Measurement of 25(OH)D
At enrollment, women attended a clinical visit for an ultrasound examination to screen for fibroids, biological sample collection (including a nonfasting blood sample), and anthropometric measurements. Blood for analysis of 25(OH)D was drawn using a red top vacutainer without additive or preservative and processed. The serum was aliquoted and stored at −80 C within 24 hours (90% within 5 hours of blood draw). Analysis of 25(OH)D was conducted at Heartland Laboratories using the LIAISON 25 OH Vitamin D Total assay, a competitive chemiluminescence immunoassay (16, 17). Samples were analyzed in 41 batches. Each batch contained 46 participant samples, two duplicate blind quality control samples created from pooled serum and one National Institute of Standards and Technology (NIST) level 1 calibration standard (SRM 972). Seven batches also contained the NIST level 1 standard diluted 2- and 3-fold. Based on blinded controls, the intra- and interassay coefficients of variation were 2.9% and 8.6%, respectively. Thirty-two women had a failed blood draw and two women refused the blood draw, leaving 1662 women for analysis.
Measurement of supplement and contraceptive use
Women brought all prescription and over-the-counter medication as well as any vitamin, mineral, or herbal supplements they had used in the past 24 hours to the clinic visit where the information was recorded (24-hour questionnaire). These products were coded using the Slone Drug Dictionary (Boston University) to determine constituents where possible. Women were also asked directly about use in the 4 weeks before the clinical visit of: contraception (pills, patches, implants, injections, vaginal ring, or hormonal intrauterine device [IUD]), multivitamin, vitamin D supplement, or cod liver oil (4-week questionnaire). In addition, women completed a Block 2005 Food Frequency Questionnaire (FFQ) (18), which was used to calculate both supplement and dietary intake of vitamin D (IU/d) based on the US Department of Agriculture's Food and Nutrient Database for Dietary Studies.
Based on questionnaire responses, we categorized hormonal contraceptive use as current use of an estrogen-containing product (combination contraceptive pill, contraceptive patch or ring), current use of a progestin-only product (hormonal implant or IUD, depot medroxyprogesterone acetate, progestin-only pill), or none (including nonhormonal IUDs). We determined that all women who used estrogen had used preparations with ethinyl estradiol 10—40 mcg except for one who used a preparation with estradiol valerate.
We categorized supplement use based on information from the 24-hour questionnaire, the 4-week questionnaire, and the FFQ. Participants provided information on use of multivitamins (assumed to contain vitamin D at lower doses 400–800 IU), single vitamin D supplements with or without calcium (assumed to contain vitamin D at higher doses >1000 IU), and cod liver oil (assumed to contain vitamin D at higher levels [450 IU per teaspoon] and grouped with single vitamin D supplements). Women could report more than one supplement type. Nonusers were those who reported no multivitamin or single vitamin D supplement use on any of the questionnaires. Supplement users were categorized based on their highest reported intake across questionnaires. We considered women who reported use of any supplement containing vitamin D on the 24-hour questionnaire as regular supplement users, and women who only reported use of any supplement containing vitamin D in the past 4 weeks as irregular users. We categorized dose of supplement as follows: low (≤200 IU/d of vitamin D supplement on the FFQ or irregular use of only one supplement type); intermediate (201–600 IU/d on the FFQ, irregular use of multiple supplement types or regular use of a single multivitamin); or high (>600 IU/d on the FFQ, regular use of multiple supplement types or regular use of a single vitamin D supplement).
Measurement of additional covariates
Telephone and computer-based questionnaires elicited data on a wide range of covariates identified based on a review of the literature. Covariates of interest for vitamin D status included time spent outdoors and vacation time spent in sunny locations during the winter. We created a “time outside” variable that incorporated total hours spent outside between 9 am and 5 pm during warm weather (warm enough to go outside without a jacket or sweater) adjusted (discounted 25–90%) for sun avoidance behaviors and sunscreen use. For data analysis, we transformed time outside to a five-level variable based on quintile cut points; the within-group median number of total hours spent outside ranged from ½ hour/d for the lowest 20% to 4.5 hours/d for the highest 20%. We categorized vacation time in a warm sunny place during the preceding winter as none, a week or less, or more than a week. We used a participant's self-reported frequency and quantity of alcohol drinking during the past year to categorize her alcohol use as low (fewer than 10 drinks a year), moderate (drinking below the threshold (19) for binge or heavy drinking), and heavy (at least six to eight drinks on days when drinking occurred, or four or more drinks on a single occasion at least two to three times per month). We estimated dietary intake of vitamin D from the FFQ (18) and categorized it using quartile cut points.
At the clinic visit, height and weight were measured in duplicate for each woman and used to calculate body mass index (BMI): weight (kg)/height2 (m2). Skin reflectance on the upper inner right arm was measured using a Cortex Technology DSM II Colormeter. The final melanin index, the average of the triplicate measurements, was approximately normally distributed and ranged from 30 to 106.
Statistical analyses
Primary analysis
Measured 25(OH)D levels had a skewed distribution (Figure 1). We log2-transformed 25(OH)D values for data analysis because that transformation normalized the distribution of residuals better than others.
Figure 1.
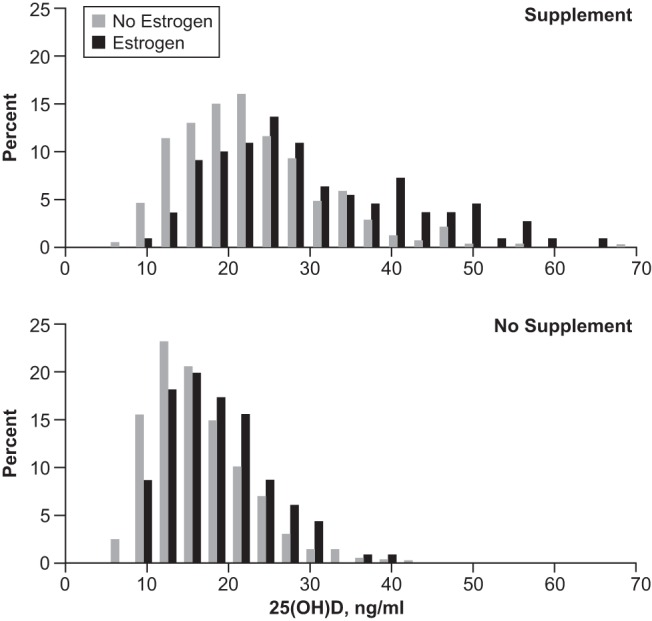
Distribution of measured serum 25(OH)D (ng/ml) among 1662 young African American women from Detroit, Michigan, stratified by vitamin supplement use (top and bottom panels) and use of estrogen-containing contraception at the time of blood sample. Supplement and no estrogen, N = 561; supplement and estrogen, N = 110; no supplement and no estrogen, N = 875; no supplement and estrogen, N = 116.
Serum 25(OH)D levels show marked seasonal variation that was modeled using a cosinor model (20, 21). The time variable in these models, denoted d, was a transformation of the date of blood draw to the number of days from January 1 (ie, January 1–December 31 transformed to day 1 though day 365). Initial linear regression models included sine and cosine of 2πkd/365.25 for k = 1, 2, 3, 4 (ie, the first through fourth harmonics). Using likelihood ratio (LR) tests, we concluded that the first and second harmonics adequately captured the seasonal variation.
To examine the crude seasonally adjusted association between use of an estrogen-containing contraceptive and 25(OH)D, we added a term for current use of an estrogen-containing contraceptive to the seasonal model based on the first two harmonics. Next, we built a full model (described in detail in the following section) that incorporated estrogen use and all other variables, together with selected interaction terms that involved sine and cosine of the first harmonic to allow for possible differences between subgroups in their seasonal patterns. We used a backwards elimination strategy using LR tests that addressed the interactions in the model to form a final parsimonious model from the full model without sacrificing goodness-of-fit.
The backwards elimination approach removed main effects, interaction terms alone (both sine and cosine) or main effects and interactions as a block using a P value threshold of .1. Following the removal of each block, we refit the model, and evaluated goodness-of-fit using LR tests, removing additional variables as needed. Model reduction stopped when goodness-of-fit was no longer consistent with the full model. We used the resulting final multivariable model to determine if exogenous estrogen was associated with serum 25(OH)D after accounting for other important available predictors.
In preliminary analyses, women with very low serum concentrations of 25(OH)D tended to have high residuals from model predicted values. Consequently, we included an indicator for very low vitamin D intake (no supplement use and below the median [100 IU/d] for dietary vitamin D intake) to allow for a different seasonal pattern for this subset of women. Preliminary analyses also indicated that 25(OH)D levels varied little across categories of BMI, alcohol use, and education except for a shift in the highest categories. Therefore we dichotomized BMI, alcohol use, and education (BMI ≥ 35 vs BMI <35, heavy drinking vs all others, college education vs all others). All models were fully nested using N = 1658 women with complete data on all covariates.
The full model for log2 25(OH)D included sine and cosine terms for the first and second harmonics as described previously and hormonal contraceptive use (any estrogen, progesterone only, none [reference]) plus the following variables: vitamin D-containing supplement use (low, intermediate, high, none [reference]), dietary vitamin D intake (lowest quartile [reference]), very low vitamin D intake (yes/no), time spent outside (lowest quintile [reference]), winter vacation to a sunny location (<7 days, ≥7 days, none [reference]), age at enrollment (continuous), college education (yes/no), current smoking (yes/no), parous (yes/no), heavy alcohol use (yes/no), BMI ≥35 (yes/no), and melanin (continuous on a 10-point increment scale). In addition, the full model included interaction terms with the first harmonic sine and cosine variables to allow for different seasonal patterns among women within the separate categories of supplement use, very low vitamin D intake, time spent outside, winter vacation in a sunny location, heavy alcohol use, current smoking, and BMI higher than 35. Finally, it also included an interaction between the first harmonic and the continuous measure of melanin.
We reported model-based estimates and 95% confidence intervals (CIs) for the percent difference in serum 25(OH)D associated with a 1 unit change in the predictor using the formula 100(2β − 1).
Sensitivity analyses
We conducted five sensitivity analyses.
Given the numerous lifestyle choices that may impact the choice of contraceptive, unmeasured lifestyle factors may be acting as confounders of the observed association. To evaluate this possibility, we examined the association between past (but not current) use of estrogen-containing contraceptives and 25(OH)D. This strategy was based on our observation that intermittent use of hormonal contraception was common, which supports the assumption of this analysis: past and current users of estrogen share unmeasured lifestyle factors related to contraceptive choice. For this analysis, we categorized women as current users of estrogen, past (but not current) users of estrogen, or never users of estrogen. The two indicator variables for current use of estrogen and past use of estrogen replaced the single “use of estrogen” variable in the final model (never users of estrogen became the reference).
The dose of ethinyl estradiol in the current contraception was available for most women (N = 20 missing). To explore dose-response, we used a model that included indicator variables for current ethinyl estradiol dose (0 [reference], 10 or 15 mcg, 20 or 25 mcg, 30 mcg, 35 mcg).
To examine the possibility that the effect of exogenous estrogen was different for women using supplements that included vitamin D, we constructed interaction terms between use of exogenous estrogen and the three levels of supplement use.
To examine the influence of the source of data for supplement use we reran the final model using supplement use defined solely by the FFQ or by the 24-hour/4-week questionnaires.
The samples for 25(OH)D were sent for assay in two shipments several months apart. To assess whether this temporal separation in assays influenced results, we reran the final model excluding samples from the smaller shipment (N = 307).
Results
Serum measurements were available for 1662 participants. Overall concentrations of 25(OH)D were low (median, 15.7 ng/ml), well below the 20 ng/ml level recommended by the Institute of Medicine (2). Use of any supplement containing vitamin D was common (40%); however, supplement use varied with factors such as education, smoking, melanin, and time spent outdoors (Table 1).
Table 1.
Demographic Characteristics, Median 25(OH)D ng/ml and Vitamin Supplement Use: Study of Environment Lifestyle & Fibroids Participants 2010–2012
| N (%) | Median 25(OH)D ng/ml | Percent Using Supplementa | |
|---|---|---|---|
| Overall | 1662 (100) | 15.7 | 40 |
| Age | |||
| 23–26 | 511 (31) | 14.9 | 35 |
| 27–30 | 571 (34) | 16.0 | 41 |
| 30+ | 580 (35) | 15.9 | 44 |
| Education levelb | |||
| <HS/GED | 367 (22) | 14.1 | 28 |
| Some beyond HS | 832 (50) | 15.1 | 37 |
| Bachelors/masters/doctorate | 462 (28) | 18.1 | 56 |
| Smoking | |||
| Nonsmoker | 1219 (73) | 16.1 | 44 |
| Former | 123 (7) | 16.0 | 38 |
| Current | 320 (19) | 13.9 | 26 |
| Alcohol usec | |||
| None | 490 (29) | 15.4 | 38 |
| Moderate | 843 (51) | 16.5 | 45 |
| Heavy | 329 (20) | 14.2 | 34 |
| BMI (kg/m2)d | |||
| <25 | 329 (20) | 18.0 | 41 |
| 25–29 | 345 (21) | 16.4 | 41 |
| 30–34 | 318 (19) | 17.3 | 45 |
| 35+ | 670 (40) | 13.9 | 37 |
| Parity | |||
| Nulliparous | 651 (39) | 15.1 | 45 |
| Parous | 1011 (61) | 15.9 | 38 |
| Use of hormonal contraceptionb | |||
| None | 1193 (72) | 15.0 | 39 |
| Any estrogen | 226 (14) | 19.4 | 49 |
| Progestin alone | 241 (15) | 16.3 | 39 |
| Dietary vitamin De | |||
| 39 IU/d | 378 (23) | 13.7 | 37 |
| 78 IU/d | 383 (23) | 15.2 | 39 |
| 129 IU/d | 481 (29) | 16.1 | 43 |
| 246 IU/d | 420 (25) | 17.1 | 43 |
| Categorized melanin indexf | |||
| 1 | 430 (26) | 17.5 | 46 |
| 2 | 390 (23) | 15.3 | 45 |
| 3 | 444 (27) | 15.7 | 39 |
| 4 | 398 (24) | 14.6 | 31 |
| Sunny vacation previous winter | |||
| None | 1311 (79) | 15.6 | 40 |
| ≤7 days | 246 (15) | 15.7 | 42 |
| >7 days | 105 (6) | 16.5 | 42 |
| Time outsideb,g | |||
| Half hour/day | 326 (20) | 16.1 | 49 |
| 1 h/day | 332 (20) | 16.2 | 39 |
| 2 h/day | 349 (21) | 16.0 | 44 |
| 3 h/day | 321 (19) | 15.7 | 41 |
| 4.5 h/day | 333 (20) | 13.9 | 28 |
| Season of blood draw | |||
| Winter | 347 (21) | 13.4 | 38 |
| Spring | 388 (23) | 14.8 | 41 |
| Summer | 495 (30) | 18.9 | 41 |
| Fall | 432 (26) | 14.6 | 41 |
Abbreviations: BMI, body mass index; HS/GED, high school/general equivalency degree; 25(OH)D, 25-hydroxy-vitamin D.
Use of any supplement containing vitamin D based on self-report at clinic visit and report on food frequency questionnaire.
Missing: education (n = 1), contraception (n = 2; unable to determine hormonal or nonhormonal intrauterine device), time outside (n = 1).
Self-reported alcohol use in the past year. Heavy use meets criteria for heavy or binge use.
Calculated for measured height and weight at time of blood draw. n = 13 women with BMI <18.5 kg/m2 included with <25 kg/m2.
Within-group median dietary vitamin D intake (IU/d) from food sources alone as calculated by the Food Frequency Questionnaire.
Categorized using quartile cut points.
Time spent outdoors in warm/sunny months. Total hours per week adjusted for self-reported sun avoidance behaviors and sun screen use were used to determine quintile cut points. Rounded median unadjusted hours/day are reported for each group.
Participants using an estrogen-containing contraceptive at baseline had an elevated median 25(OH)D (19.4 ng/ml); however, they were also more likely to be using a supplement (49%). Distribution of 25(OH)D stratified by both supplement use and estrogen-containing contraceptive use (Figure 1) showed an overall increase in 25(OH)D among users of estrogen-containing contraceptives among both supplement users and nonusers. In a model only adjusted for season, use of an estrogen containing contraceptive was associated with a 30% increase (95% CI: 22%, 38%) in 25(OH)D.
The full model included 43 variables (Supplemental Table 1) with an R2 of 0.37. After model reduction 19 variables remained (Supplemental Table 1). The final model had an R2 of 0.36. Percent change in serum concentrations of 25(OH)D for use of an estrogen containing contraceptive and use of supplement (to provide context for the magnitude of increase) are shown in Table 2. Use of an estrogen containing contraceptive was associated with a 20% higher 25(OH)D (95% CI, 14–27), which was greater in magnitude than the low-dose supplement (14% difference; 95% CI, 8–21). Only the use of a high or intermediate dose of supplement was associated with larger percent differences, 77% difference (95% CI, 60–95) and 46% difference (95% CI, 38–54), respectively.
Table 2.
Percent Change in 25-Hydroxy Vitamin D Concentrations (ng/ml) for Use of an Estrogen-Containing Contraception and Use of Vitamin D-Containing Supplement
| % Differencea | 95% Lower Limit | 95% Upper Limit | |
|---|---|---|---|
| Use of estrogen-containing contraceptionb | 20.0 | 13.8 | 26.5 |
| Use of supplementc | |||
| Low dosed | 14.2 | 7.8 | 20.9 |
| Intermediate dosee | 46.0 | 38.2 | 54.2 |
| High dosef | 76.6 | 59.7 | 95.2 |
Adjusted model includes all variables in Table 2 plus sine/cosine for first and second harmonics, current BMI >35 kg/m2, smoking with interactions with the first harmonic, indicator for very low D intake with interactions with first harmonic, college education, interaction between smoking and first harmonic, continuous melanin, and quartiles of dietary vitamin D intake and parity. We provide β estimates for all variables (Supplemental Table 1).
Estrogen containing contraception includes: combination birth control pills, contraceptive patch, or vaginal ring.
Supplement use based on highest reported dose/frequency on Food Frequency Questionnaire, or questionnaire at time of blood draw about use within either last 24 h (regular use) or past 4 weeks (irregular use).
Low-dose supplement: vitamin D content < = 200 IU/d or self-report of using single supplement irregularly.
Intermediate-dose supplement: vitamin D content 201–600 IU/d or using multiple supplements irregularly or a single supplement regularly.
High-dose supplement: vitamin D content 601+ IU/day or using multiple supplements daily.
In a sensitivity analysis that classified women as current, past, or never users of estrogen-containing contraception, current users (N = 226) showed a percent difference similar to that from the main analysis (19% difference; 95% CI, 12–26), whereas past users (N = 1020) had a small negative association (−1% difference, 95% CI: −5 to 3) consistent with the null.
Each dose of ethinyl estradiol (10 or 15 mcg, 20 or 25 mcg, 30 mcg, 35 mcg) had a similar percent difference (range, 19.9–21.1%) compared to no use of estrogen-containing contraception.
Classification of supplement dose using solely the FFQ or the 24-hour/4-week questionnaire resulted in estimates for the estrogen effect that were nearly identical to those in our primary analysis (data not shown).
The interaction between estrogen and supplement use was not statistically significant, indicating that the estrogen association remained constant across levels of supplement use. Finally, the primary findings were robust when we excluded observations from the smaller shipment of 25(OH)D samples (results not shown).
Discussion
Previous studies have suggested an increase in serum 25(OH)D among women using exogenous estrogen (3–14). Most previous studies of premenopausal women were small (fewer than 150 women) (5–10) and/or were unable to account for supplement use behavior that may vary with contraceptive use—covariation that is evident in our study. Using detailed information from 1662 young African American women in the Detroit area, we provide strong evidence to support the hypothesis that exogenous estrogen use increases serum 25(OH)D. Other factors included in the model showed effects in expected directions supporting the validity of our data. For example, increased BMI was associated with a decrease in the concentration of 25(OH)D as reported in other studies (10, 14, 21) (Supplemental Table).
In our model, supplement use was the strongest predictor of vitamin D levels. Although use of supplements varied by type of contraceptive, the final adjusted model identified an independent 20% increase in 25(OH)D among women using estrogen-containing contraceptives. The 20% increase we observed is well within the range (5–40%) reported in prior studies of pre- and postmenopausal women (3–7).
The mechanism for this increase is not well-understood. In addition to elevations in 25(OH)D, exogenous estrogen has been associated with increases in the vitamin D binding protein (VDBP) (7, 22–24), the active metabolite 1,25(OH)2 D (7, 22–25), and the metabolically inactive 24,25(OH) vitamin D (3). VDBP is synthesized in the liver and may respond in a similar fashion to other estrogen-sensitive hepatic proteins (thyroid binding globulin, SHBG, and corticosteroid binding globulin) (26–28). It has been suggested that increases in VDBP may increase the half-life of 25(OH)D (29). However, the ratio of circulating VDBP to 25(OH)D is very high (1000:1) (30), and whether inducing physiologic changes in VDBP can influence 25(OH)D has not been directly tested.
Our models accounted for only about a third of the observed variance in serum 25(OH)D concentrations, similar to previous studies where the primary goal was to predict serum 25(OH)D based on covariates available in epidemiologic studies (4, 9, 10, 31). Recognized inaccuracies in the calculation of vitamin D from dietary sources (32, 33) likely diminished the explanatory power of all these models. In ours, the inability to precisely quantify the dose and consistency of supplement use also contributed. Genetic variants can influence 25(OH)D (34), but genotype data were unavailable in all these studies. It is also likely that some predictors of vitamin D status remain unidentified. Importantly, however, even with relatively high-quality data on many of the known predictors of vitamin D, predictions of new observations (for instance at a different time point, or for participants with missing serum measures) based on all such models would be imprecise. This imprecision has implications for epidemiologic studies. Prediction of past, present, or future concentrations of 25(OH)D to address missing data will result in substantial error. Measured vitamin D status at each time point of interest is crucial particularly in young populations where individuals may frequently change important behaviors such as supplement use, dietary preferences, contraceptive use, or leisure time activities.
The assay used to measure 25(OH)D in this study tends to underestimate 25(OH)D when compared to the gold standard of liquid chromatography-tandem mass spectrometry (35). Our internal quality control results based on known NIST standards suggest that the LIASION assay underestimated 25(OH)D by 2–5 ng/ml across the range of values measured. The assay orders the individuals appropriately regarding their 25(OH)D, however, so continuous values from this assay are useful in epidemiologic studies.
Despite possible underestimation by our assay, the women in this study would still be regarded as having low concentrations of 25(OH)D. This result is not surprising because vitamin D deficiency is common across the United States with 42% of all women below the 20 ng/ml threshold, and a higher prevalence of deficiency (80%) among non-Hispanic black women (36). Low vitamin D levels have been associated with reproductive outcomes including reduced fertility, preeclampsia, gestational diabetes, and poor fetal growth (reviewed in Luk (1)). In reproductive age women planning pregnancy, clinical testing of vitamin D levels should take into account recent contraceptive use. Women who discontinue estrogen-containing contraception to start a pregnancy attempt may be at risk of falling 25(OH) D during the important preconception and early gestation period.
The observed association between estrogen-containing contraceptive use and 25(OH)D levels could result in confounding bias even in vitamin D studies involving nonreproductive outcomes (reviewed in (37)). For example, a recent study (38) found that the observed association between 25(OH)D and C-reactive protein was due solely to confounding by contraceptive use. Careful documentation of exogenous hormone use will be important to any study examining the role of vitamin D and health or disease in women.
In conclusion, we found that use of an estrogen-containing contraceptive was associated with increased level of serum 25(OH)D among young African American women. This finding has clinical implications when counseling women who are planning to conceive and identifying women who may be at risk of deficiency. There are also implications for studies of vitamin D and any health outcome where exogenous estrogen use may be an important confounder. Further work is needed to elucidate the biological pathways responsible for the observed association and to investigate endogenous estrogen effects including how 25(OH)D levels may vary with life course changes such as onset of puberty, pregnancy, and menopause. Finally, we, like others, had limited ability to adequately explain vitamin D variation based solely on regression models. To the extent possible, epidemiologic studies should measure 25(OH)D concentrations at the time points of interest for the hypothesis being tested.
Acknowledgments
This work was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- CI
- confidence interval
- FFQ
- Block 2005 Food Frequency Questionnaire
- IUD
- intrauterine device
- LR
- likelihood ratio
- NIST
- National Institute of Standards and Technology
- 25(OH)D
- 25-hydroxy-vitamin D
- VDBP
- vitamin D binding protein.
References
- 1. Luk J, Torrealday S, Neal Perry G, Pal L. Relevance of vitamin D in reproduction. Hum Reprod(Oxford, England). 2012;27:3015–3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Institute of Medicine Food and Drug Board. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press; 2010. [Google Scholar]
- 3. Bansal N, Katz R, de Boer IH, et al. Influence of estrogen therapy on calcium, phosphorus, and other regulatory hormones in postmenopausal women: the MESA study. J Clin Endocrinol Metab. 2013;98:4890–4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Millen AE, Wactawski-Wende J, Pettinger M, et al. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: the Women's Health Initiative Calcium plus Vitamin D clinical trial. Am J Clin Nutr. 2010;91:1324–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Harris SS, Dawson Hughes B. The association of oral contraceptive use with plasma 25-hydroxyvitamin D levels. J Am Coll Nutr. 1998;17:282–284. [DOI] [PubMed] [Google Scholar]
- 6. Thane CW, Bates CJ, Prentice A. Oral contraceptives and nutritional status in adolescent British girls. Nutr Res. 2002;22:449–462. [Google Scholar]
- 7. Moller UK, Streym S, Jensen LT, et al. Increased plasma concentrations of vitamin D metabolites and vitamin D binding protein in women using hormonal contraceptives: a cross-sectional study. Nutrients. 2013;5:3470–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wolman R, Wyon MA, Koutedakis Y, Nevill AM, Eastell R, Allen N. Vitamin D status in professional ballet dancers: winter vs. summer. J Sci Med Sport. 2013;16:388–391. [DOI] [PubMed] [Google Scholar]
- 9. Hedlund L, Brembeck P, Olausson H. Determinants of vitamin D Status in Fair-skinned women of childbearing age at northern latitudes. PLoS One. 2013;8:e60864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gagnon C, Baillargeon JP, Desmarais G, Fink GD. Prevalence and predictors of vitamin D insufficiency in women of reproductive age living in northern latitude. Eur J Endocrinol. 2010;163:819–824. [DOI] [PubMed] [Google Scholar]
- 11. Garcia-Bailo B, Karmali M, Badawi A, El-Sohemy A. Plasma 25-hydroxyvitamin D, hormonal contraceptive use, and cardiometabolic disease risk in an ethnically diverse population of young adults. J Am Coll Nutr. 2013;32:296–306. [DOI] [PubMed] [Google Scholar]
- 12. Nesby-O'Dell S, Scanlon KS, Cogswell ME, et al. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–192. [DOI] [PubMed] [Google Scholar]
- 13. Sowers MR, Wallace RB, Hollis BW, Lemke JH. Parameters related to 25-OH-D levels in a population-based study of women. Am J Clin Nutr. 1986;43:621–628. [DOI] [PubMed] [Google Scholar]
- 14. Ritterhouse LL, Lu R, Shah HB, et al. Vitamin d deficiency in a multiethnic healthy control cohort and altered immune response in vitamin D deficient European-American healthy controls. PLoS One. 2014;9:e94500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baird DD, Harmon QE, Upson K, et al. A Prospective, ultrasound-based study to evaluate risk factors for uterine fibroid incidence and growth: methods and results of recruitment. J Womens Health. 2015;24:907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wagner D, Hanwell HE, Vieth R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D. Clin Biochem. 2009;42:1549–1556. [DOI] [PubMed] [Google Scholar]
- 17. Ersfeld DL, Rao DS, Body JJ, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON automated analyzer. Clin Biochem. 2004;37:867–874. [DOI] [PubMed] [Google Scholar]
- 18. Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124:453–469. [DOI] [PubMed] [Google Scholar]
- 19. National Institute on Alcohol Abuse and Alcoholism NIH. Drinking Levels Defined. http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- 20. Wang Y, Jacobs EJ, McCullough ML, et al. Comparing methods for accounting for seasonal variability in a biomarker when only a single sample is available: insights from simulations based on serum 25-hydroxyvitamin d. Am J Epidemiol. 2009;170:88–94. [DOI] [PubMed] [Google Scholar]
- 21. Sachs MC, Shoben A, Levin GP, et al. Estimating mean annual 25-hydroxyvitamin D concentrations from single measurements: the Multi-Ethnic Study of Atherosclerosis. Am J Clin Nutr. 2013;97:1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dick IM, Prince RL, Kelly JJ, Ho KK. Oestrogen effects on calcitriol levels in post-menopausal women: a comparison of oral versus transdermal administration. Clin Endocrinol. 1995;43:219–224. [DOI] [PubMed] [Google Scholar]
- 23. Bouillon R, Van Baelen H, De Moor P. 25-hydroxyvitamin D and its binding protein in maternal and cord serum. J Clin Endocrinol Metab. 1977;45:679–684. [DOI] [PubMed] [Google Scholar]
- 24. Aarskog D, Aksnes L, Markestad T, RÃ̧dland O. Effect of estrogen on vitamin D metabolism in tall girls. J Clin EndocrinolMetab. 1983;57:1155–1158. [DOI] [PubMed] [Google Scholar]
- 25. Gallagher JC, Riggs BL, Deluca HF. Effect of estrogen on calcium absorption and serum vitamin D metabolites in postmenopausal osteoporosis. J Clin Endocrinol Metab. 1980;51:1359–1364. [DOI] [PubMed] [Google Scholar]
- 26. Laurell CB, Rannevik G. A comparison of plasma protein changes induced by danazol, pregnancy, and estrogens. J Clin Endocrinol Metab. 1979;49:719–725. [DOI] [PubMed] [Google Scholar]
- 27. Sitruk-Ware RL, Menard J, Rad M, et al. Comparison of the impact of vaginal and oral administration of combined hormonal contraceptives on hepatic proteins sensitive to estrogen. Contraception. 2007;75:430–437. [DOI] [PubMed] [Google Scholar]
- 28. Wiegratz I, Kutschera E, Lee JH, et al. Effect of four different oral contraceptives on various sex hormones and serum-binding globulins. Contraception. 2003;67:25–32. [DOI] [PubMed] [Google Scholar]
- 29. Aloia J, Mikhail M, Dhaliwal R, et al. Free 25(OH)D and the vitamin D paradox in African Americans. J Clin Endocrinol Metab. 2015;100:3356–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bouillon R. The vitamin D binding protein DBP. Feldman DJ, Wesley JP, Adams J, eds. Vitamin D. 3rd ed San Diego, CA: Academic Press; 2011;57–72. [Google Scholar]
- 31. Bjorn Jensen C, Thorne-Lyman AL, Vadgard Hansen L, et al. Development and validation of a vitamin D status prediction model in Danish pregnant women: a study of the Danish National Birth Cohort. PLoS One. 2013;8:e53059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor CL, Patterson KY, Roseland JM, et al. Including food 25-hydroxyvitamin D in intake estimates may reduce the discrepancy between dietary and serum measures of vitamin D status. J Nutr. 2014;144:654–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patterson KY, Phillips KM, Horst RL, et al. Vitamin D content and variability in fluid milks from a US Department of Agriculture nationwide sampling to update values in the National Nutrient Database for Standard Reference. J Dairy Sci. 2010;93:5082–5090. [DOI] [PubMed] [Google Scholar]
- 34. Wang TJ, Zhang F, Richards JB, et al. D. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010;376:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farrell CJ, Martin S, McWhinney B, Straub I, Williams P, Herrmann M. State-of-the-art vitamin D assays: a comparison of automated immunoassays with liquid chromatography-tandem mass spectrometry methods. Clin Chem. 2012;58:531–542. [DOI] [PubMed] [Google Scholar]
- 36. Forrest KY, Stuhldreher WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Research (New York, NY). 2011;31:48–54. [DOI] [PubMed] [Google Scholar]
- 37. Pludowski P, Holick MF, Pilz S, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun Rev. 2013;12:976–989. [DOI] [PubMed] [Google Scholar]
- 38. Garcia-Bailo B, Josse AR, Jamnik J, Badawi A, El-Sohemy A. Positive association between 25-hydroxyvitamin D and C-reactive protein is confounded by hormonal contraceptive use. J Womens Health. 2013;22:417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]


