Abstract
Context:
Polycystic ovarian syndrome (PCOS), the most common endocrine disorder of reproductive-aged women, is associated with systemic low-grade inflammation.
Objective:
We propose that increased or altered intrafollicular inflammatory reactions also occur in periovulatory follicles of PCOS patients.
Design:
Gene profiling and quantitative PCR (qPCR) analyses in granulosa-lutein cells (GCs) collected from PCOS and non-PCOS women undergoing in vitro fertilization were compared with serum and follicular fluid (FF) levels of cytokines and chemokines.
Setting:
This was a university-based study.
Patients:
Twenty-one PCOS and 45 control patients were recruited: demographic, hormone, body mass index, and pregnancy outcomes were abstracted from patient data files.
Interventions:
GC cytokine/chemokine mRNAs were identified and analyzed by gene-chip microarrays/qPCR before and after culture with human chorionic gonadotropin, DHT, IL-6, or IL-8; serum/FF cytokine levels were also analyzed.
Main Outcome Measures:
Relative serum/FF cytokine levels and GC cytokine expression before and after culture were compared and related to body mass index.
Results:
The following results were found: 1) PCOS GCs express elevated transcripts encoding cytokines, chemokines, and immune cell markers, 2) based on gene profiling and qPCR analyses, obese PCOS patients define a distinct PCOS disease subtype with the most dramatic increases in proinflammatory and immune-related factors, and 3) human chorionic gonadotropin and DHT increased cytokine production in cultured GCs, whereas cytokines augmented cytokine and vascular genes, indicating that hyperandrogenism/elevated LH and obesity in PCOS women augment intrafollicular cytokine production.
Conclusions:
Intrafollicular androgens and cytokines likely comprise a local regulatory loop that impacts GC expression of cytokines and chemokines and the presence of immune cells; this loop is further enhanced in the obese PCOS subtype.
Gene profiling of granulosa cells collected from PCOS and non-PCOS patients showed enhanced intrafollicular expression of specific cytokines and chemokines, especially in the obese PCOS subtype.
Ovulation of a healthy, mature oocyte is critical for reproductive success. Ovulation is a complex inflammatory-like process that involves follicular production of inflammatory cytokines, induction of prostaglandins, and the recruitment of immune cells, including leukocytes, neutrophils, and macrophages (1–9). Successful ovulation also depends on cumulus cell oocyte complex expansion, a process that is controlled by cytokines and inflammatory molecules. The LH surge and specific downstream signaling cascades initiate all of these events (10).
Ovulation is severely impaired in women with polycystic ovarian syndrome (PCOS) due, in part, to tonic elevation of LH, the absence of an LH surge, and the premature arrest of follicular growth. PCOS is the most common female reproductive endocrine disorder, affecting 6.5%–8% of women (11). The major biochemical hallmark of PCOS is hyperandrogenemia, as defined by the Rotterdam Criteria, and either oligoanovulation or polycystic ovaries, excluding other endocrinopathies (12). Remarkably, PCOS appears to be an evolutionarily conserved disorder (13) that, in our current society, is linked to the following: 1) insulin resistance (14, 15), 2) a proinflammatory condition (14–26), and 3) frequently obesity (14, 16, 27).
Increased ovarian androgen production in PCOS is multifactorial and has been attributed to heightened insulin and LH signaling to thecal cells (16), intrinsically elevated P450c17 enzyme activity (17), and possibly theca cell DENND1A.V2 isoform overexpression (18, 26). Selective insulin resistance at the level of the ovary may also alter theca cell androgen production via IGF-1 signaling in the presence of increased local insulin (19). In primate models, androgen treatment is associated with increased FSH receptor expression along with the up-regulation of IGF-1 and the IGF-1 receptor, yielding a mitogenic effect on small antral follicle formation, similar to that seen in PCOS (20, 21). Hyperinsulinemia appears to impact ovulatory dysfunction by multiple mechanisms, including promoting a generalized inflammatory state characterized by increased systemic levels of C-reactive protein (CRP), central adiposity, and altered adipokine production, all of which may play a role in the PCOS phenotype and be linked to elevated androgens (27) and systemic inflammation (14, 15).
Based on increasing evidence that PCOS is associated with a low-grade systemic proinflammatory state and our preliminary data that granulosa-lutein cell (GC) production of cytokines is markedly increased in PCOS ovulatory follicles, we sought to determine whether GCs from PCOS patients expressed higher levels of selected immune, inflammatory, and vascular-related genes compared with a control cohort and whether this was related to body weight.
Materials and Methods
Patient recruitment, specimen collection, and sample processing
Subjects
Women undergoing planned in vitro fertilization (IVF) were recruited in a prospective manner for participation between July 2013 and June 2015; all provided written informed consent prior to inclusion (Institutional Review Board of Baylor College of Medicine [BCM], Houston, Texas [(IRB Protocol H-27739]). Subjects included 21 PCOS and 45 normoovulatory women undergoing IVF as donors for planned preimplantation genetic screening or for unexplained, male, or tubal factor infertility. Women with PCOS met Rotterdam diagnostic criteria and all had oligoanovulation. Women with endometriosis, a cancer diagnosis, diabetes, or an autoimmune disease were excluded.
Patients received a standard IVF flexible-start antagonist stimulation protocol with exogenous FSH and human menopausal gonadotropins at individualized doses and underwent transvaginal ultrasound-guided follicle aspiration 36 hours after human chorionic gonadotropin (hCG) and/or a GnRH agonist.
Serum
Serum was prepared from blood drawn from fasting subjects on the morning of oocyte retrieval and stored at −80°C until analyzed for cytokines and T by The University of Virginia Ligand Assay Core (Supplemental Table 1).
Follicular fluid
Follicular fluid (FF) from 22 patients at the BCM was obtained from pooled follicular aspirate at oocyte retrieval. FF samples from the CReATe Fertility Center (Toronto, Ontario, Canada) were collected from 20 patients undergoing IVF who gave written informed consent approved by their local institution. Grossly clear FF was collected from a single large follicle on the first puncture of each ovary. All FF samples were centrifuged to remove cells and stored at −80°C for analyses (Supplemental Table 1).
Granulosa-lutein cells
The top portion of the cell pellet collected from pooled FF samples for each BCM patient was aspirated and washed in a 1:1 mixture of DMEM and Ham's mixture F-12 (DMEM F-12; Life Technologies, Thermo Fisher Scientific). The washed cell pellet was resuspended in media, layered over a 50% Percoll:PBS (Percoll; GE Healthcare Life Sciences) solution, and separated from red blood cells with centrifugation. The cellular layer at the Percoll/PBS interface was aspirated and washed in media, removing residual Percoll. If red blood cells were visible in this pellet, it was incubated with a 4:1 ratio of red blood cell lysis buffer (Buffer EL; QIAGEN, QIAGEN Sciences) (modified from Chilvers at al [28]). The final cell pellet was used for protein, RNA, or culture analyses. Cumulus cells (lacking blood cells) were collected by microdissection from oocytes prior to hyaluronidase application.
RNA extraction, quantitative PCR, and microarray analyses
Freshly isolated GC pellets were lysed in 500 μL RLT buffer (QIAGEN RNEasy minikit RLT buffer + 1:100 addition of β-mercaptoethanol; QIAGEN Sciences) and stored at −20°C. Total RNA was extracted using the RNeasy minikit; contaminating DNA was eliminated by treatment with deoxyribonuclease I. Total RNA was reverse transcribed and analyzed by PCR as described previously (29). Primers are listed in Supplemental Table 2.
For the microarray analysis, GC RNA quality was confirmed using the Agilent Bioanalyzer 2100 and the NanoDrop ND-1000 spectrophotometer and analyzed by Affymetrix Human Genome U133 Plus 2.0 array gene chip, by the Microarray Core Facility at the BCM. Microarray data were analyzed as previously reported using the Robust Multiarray Averaging function (30) from the Affy package (version 1.5.8) with open-sourced BioConductor software (31). The average fold differences in RNA were imported into Ingenuity Systems and analyzed using the tools provided in the Ingenuity program.
Granulosa-lutein cell cultures
GC viability (Tryptan Blue dye exclusion) was routinely approximately 75%–85%. GCs were resuspended in DMEM F-12 solution supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin (5000 U/mL) plus 1% Fungizone (both from GIBCO-BRL) and seeded into 24-well culture plates (Greiner Bio-One CellStar Plate number 662160) with 5 × 105 cells/well in 0.5 mL media. Cells were cultured for 24 hours and washed to remove serum, and serum-free DMEM F-12 (with 1% antibiotic/antimycotic) was added prior to treatment with either DHT (10 ng/mL; Steraloids, Inc), IL-6 (10 ng/mL), and IL-6 soluble receptor (25 ng/mL; R&D Systems) or IL-8 (10 ng/mL, recombinant human IL-8; BD Pharmingen) for 24 hours. In some experiments, cells were cultured for 5 days in 10% serum-supplemented media to regain LH responsiveness (32) prior to the addition of hCG (0.1 IU/mL; Pregnyl, Organon Special Chemicals) for 24 hours in serum-free media. Cultured cells were lysed in 500 μL RLT buffer (QIAGEN Sciences), with storage at −20°C until RNA extraction. For cultures with IL-8, media samples were saved at −80°C for hormone analysis.
Immunofluorescent staining of cultured GCs
GCs were cultured on glass coverslips, fixed in methanol for 10 minutes at −20°C, blocked in 5% normal goat serum + 5% BSA + 0.3% Triton X-100, and incubated with primary antibodies: CD45/H-230 (1:125; Santa Cruz Biotechnology) and programmed cell death-1 (PD-1) (1:200; biotin-conjugated CD279, number 13–9985-81; eBioscience). Secondary antibodies used were: Alexa Fluor-conjugated streptavidin 568 (red) for PD-1 and Alexa Fluor goat antirabbit 488 (green) for CD45 (both from Invitrogen). Nuclei were stained with 4′,6′-diamino-2-phenylindole at 1:1000 for 5 minutes. Digital images were taken with an AxioPlan2 microscope (Zeiss).
Western blot
GCs were lysed with radioimmunoprecipitation assay buffer (Boston Bioproducts) containing 1:100 complete protease inhibitors (Roche) and 1:100 phosphatase inhibitors (Xpert phosphatase inhibitor cocktail, Novusci; GenDEPOT), supernatants collected by centrifugation, resolved on an 8% polyacrylamide gel via electrophoresis, and protein bands identified with antibodies to CD45 (as listed above) at a 1:500 concentration and β-actin (Cytoskeleton) at a 1:2000 concentration.
Statistics
Data were assessed for normality with a Shapiro-Wilks test. Normally distributed patient data were compared with a two-sample t test. Nonparametric patient data were compared with a Mann-Whitney test. Proportions were expressed as percentages and analyzed with either a Fisher's exact test or Pearson's χ2 test, depending on sample size. Relative mRNA values and immunoassay results were expressed as mean ± SEM and compared as appropriate for data distribution. Paired culture data were analyzed with a Wilcoxon's signed rank test. Significance was assigned for a two-sided value of P < .05. All statistical analyses were performed using StataIC 13 (Statacorp).
Results
Characteristics of the study population
Baseline demographic and clinical data were statistically similar for PCOS and control women with the expected exception of an increased antimullerian hormone (AMH) and number of oocytes obtained in the PCOS patients. IVF outcomes were also similar between the two groups (Supplemental Table 3).
mRNA expression results
To determine whether GCs from PCOS patients expressed higher levels of selected immune, inflammatory, and vascular-related genes compared with a control cohort and whether this was related to body weight, we selected 33 genes related to ovulation, vascular development, and granulosa cell functions for quantitative PCR (qPCR) analyses using control and PCOS GC RNA (Figure 1, A–D). The heat map (Figure 1A) depicts relative qPCR results for these 33 genes (from 49 patients: 32 control and 17 PCOS women). The most differentially regulated genes were a group of proinflammatory cytokines and some proangiogenic genes (yellow boxes) elevated in high-cytokine PCOS patients but low in a small number of low-cytokine PCOS patients. The only phenotypic difference between these two PCOS subgroups was a greater BMI (kilograms per square meter) in the high-cytokine cohort (P = .002 by Mann-Whitney test). BMI and cytokine profiles did not correlate in other PCOS and control samples. The cumulative results displayed by the heat map (Figure 1A) and qPCR data (Figure 1B) document that the expression of several proinflammatory cytokines/chemokines as well as the immune cell marker CD45 is increased significantly in PCOS compared with control GCs as well as in cumulus cells (Supplemental Figure 1).
Figure 1.
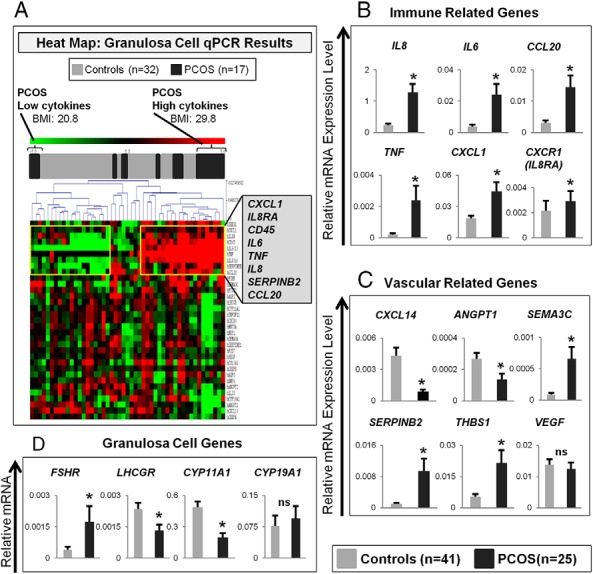
A, Heat map of relative qPCR results. B–D, Relative mRNA expression of genes in luteinized granulosa cell collections of control compared with PCOS women. B, Immune-related genes. C, Vascular-related genes. D, Genes specific to granulosa cells. Statistical comparisons were made with the Mann-Whitney test. *, P < .05. ns, not significant.
Several vascular-related genes, but unexpectedly not VEGF expression, differed in PCOS vs control GCs (Figure 1C). Expression of GC specific genes also differed in the two groups (Figure 1D). There was a positive trend with BMI for higher IL8 and CXCL1 expression (Supplemental Figure 2A), with the positive IL8 and BMI correlation apparent in PCOS but not control patients (Supplemental Figure 2B). When clinical parameters and GC cytokine mRNA expression were compared between women who did and did not conceive with their fresh embryo transfer, only age was a significant clinical parameter for the pregnancy outcome (Supplemental Table 4); only IL6 expression was statistically associated with a pregnancy event for PCOS and control women (Supplemental Figure 2C).
To refine these analyses and to determine more specifically whether BMI impacted the cytokine expression profiles, we ran RNA microarray analyses on samples (three to five RNA pools/sample) from six different groups that were distinct for PCOS status, BMI, and cytokine profile (Figure 2). The three groups with low cytokines are similar whether lean or overweight, with mild differences noted in the PCOS lean. Differences were most dramatic in the overweight PCOS group compared with all others. When the two overweight high-cytokine groups (PCOS vs non-PCOS) are compared alone, there are 695 probes with a greater than 4-fold increase, with most being inflammatory-related genes. The overweight high-cytokine PCOS group shows marked enhancement of inflammatory gene profiles as denoted by gene ontology analyses (Supplemental Data). Thus, PCOS and obesity enhance the follicular cytokine expression.
Figure 2.
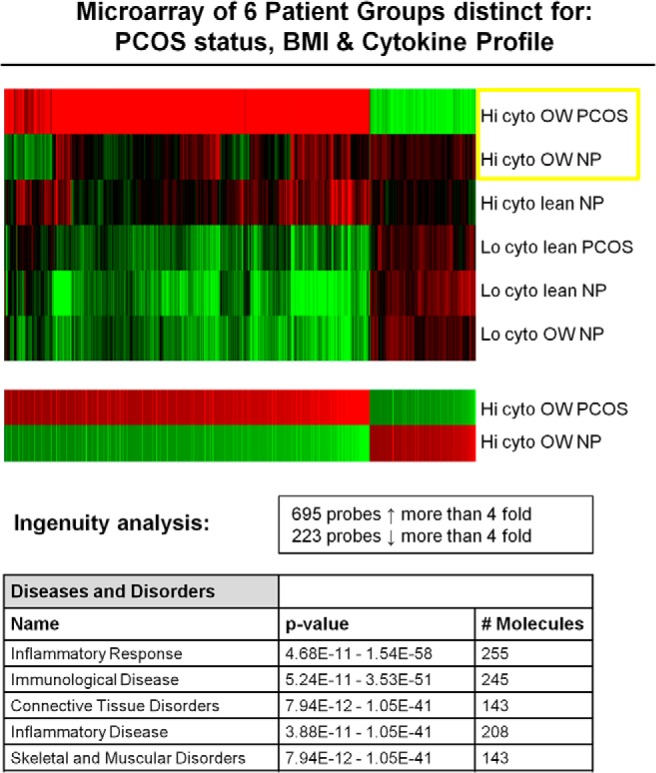
This microarray figure depicts the six groups organized from highest to lowest discordance. The two overweight high-cytokine groups (PCOS vs non-PCOS) are compared separately together in the lower figure, which shows a greater than 4-fold alteration in the expression for 918 gene probes between those two groups. The top five categories of increased gene regulation via the Ingenuity analysis are shown in the lower table. BMI, body mass index; cyto, cytokine; Hi, high; Lo, low; NP, samples from normoovulatory patients; PCOS, samples from patients with PCOS.
CD45 expression is increased in PCOS compared with control GC collections
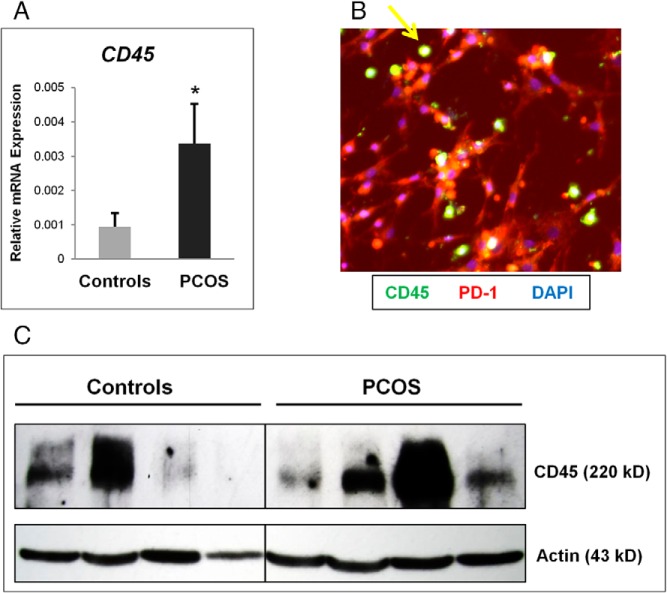
In accordance with the heat map (Figure 1A), the levels of CD45 mRNA (Figure 3A) and protein shown by immunofluorescence (Figure 3B) and Western blot (Figure 3C) were significantly higher in freshly isolated PCOS vs control GCs, indicating that more perifollicular immune cells are associated with the PCOS GCs.
Figure 3.
CD45 expression is increased in PCOS compared with control granulosa cell collections. A, CD45 mRNA expression is increased in granulosa cell collections from the PCOS compared with the control group. *, P < .05. B, Immunofluorescent identification of leukocytes (CD45) in PCOS granulosa cells (PD-1) cultured on coverslips for 5 days. Yellow arrow indicates CD45+ leukocyte cell. C, Western blot of granulosa cell lysate in representative control and PCOS samples, normalized to β-actin levels. Note the marked variation of CD45 protein levels and mRNA expression common in individual patient samples but overall increased in the PCOS group as a whole.
In vitro treatment of cultured GCs
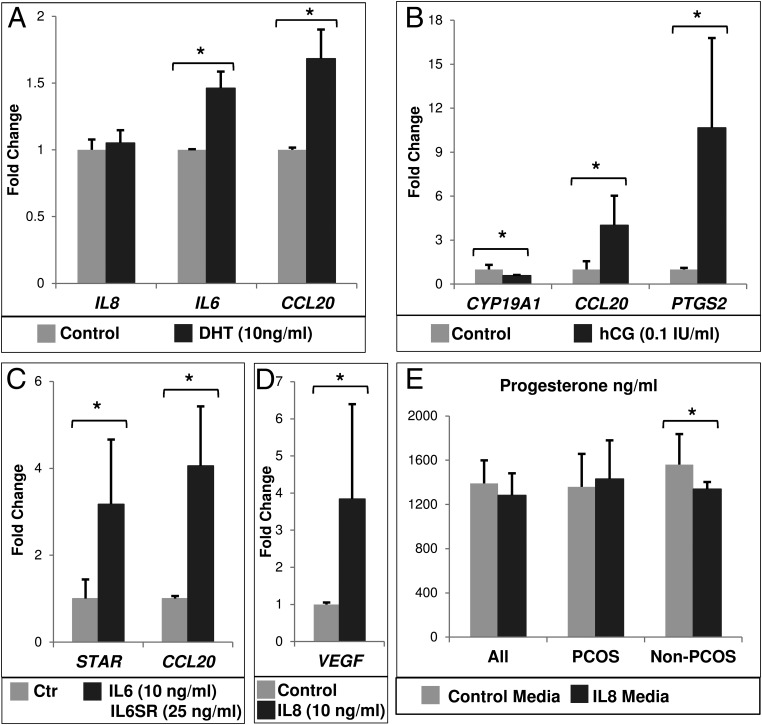
Because the PCOS condition is associated not only with elevated cytokine levels but also with elevated androgens and LH, we analyzed the effects of hormones on cytokine expression and, conversely, of cytokines on GC marker genes in GC cultures. When DHT (a nonaromatizable androgen) was added to GC cultures (n = 12; control n = 11 and PCOS n = 1), it significantly induced IL6 and CCL20 but not IL8 mRNA expression (Figure 4A). When hCG was added to the GC cultures (n = 5: control n = 2; PCOS n = 3) for 24 hours (after 5 d of culture to allow cells to regain LH responsiveness [32]), it induced CCL20 mRNA greater than 4-fold but not IL6 or IL8 expression; as anticipated, CYP19A1 decreased and PTGS2 expression increased (Figure 4B).
Figure 4.
Relative mRNA expression in granulosa cells cultured in vitro with the indicated treatment for 24 hours in serum-free media. For A–D, data are shown as fold change (mean ± SEM) relative to control (vehicle only) group, which is equivalent to 1. For A–E, data were analyzed by a Wilcoxon's signed rank test. *, P < .05. n, number of separate culture experiments with different patient samples. A, DHT treatment (n = 12). B, hCG treatment for 24 hours in serum-free media after 5 days of culture (n = 5). C, Treatment with IL-6 and IL-6 soluble receptor (IL6SR) (n = 9). D, IL-8 treatment (n = 9). E, Progesterone production as measured in spent media from IL-8-treated granulosa cells in culture (n = 10). Progesterone levels in control were compared with IL-8-treated wells and were further stratified by clinical grouping. Whereas cells from PCOS women (n = 4) demonstrated a small, nonsignificant increase in progesterone production, those from control women (n = 6) showed a significant decrease in progesterone production.
IL-6 and its soluble receptor induced expression of CCL20 and STAR (n = 9: control n = 7; PCOS n = 2) (Figure 4C), whereas IL-8 significantly increased VEGF mRNA (n = 9: control n = 5; PCOS n = 4) (Figure 4D). IL-8 also stimulated a small but nonsignificant increase in progesterone from PCOS GCs (n = 4) but reduced progesterone production in non-PCOS GCs (n = 6) (Figure 4E). Transcripts for LHCGR, CYP11A1, STAR, or CYP19A1 did not differ between the IL-8-treated and control groups, even when stratified by PCOS status (data not shown).
When the GC culture data were analyzed based on control or PCOS cells, the resultant small n lost significance, but the directional trend in every case was similar to that in the undivided group (Supplemental Figure 3).
Follicular fluid and serum results
To determine whether changes in cytokine, chemokine, and immune cell gene expression profiles were related to levels of these factors in FF, a total of 42 FF and 28 serum samples from the BCM (Houston, TX) and from the CReATe Fertility Center (Toronto, Canada) were analyzed. Paired FF and serum samples were available for 22 patients; and 20 BCM serum samples were also linked to mRNA data from GCs of the same patient (Table 1).
Table 1.
Comparison of Serum and FF Immunoassay Results: Stratified by Study Group and Also by BMI, Controls Versus PCOS and Lean (BMI < 25) Versus Overweight (BMI ≥ 25 kg/m2)
| Controls (n = 26) | PCOS (n = 22) | P Value | Lean (n = 31) | Overweight (n = 17) | P Value | |
|---|---|---|---|---|---|---|
| Age, y | 31.8 ± 6.4 | 30.4 ± 4.3 | NSa | 31.7 ± 6.0 | 30.4 ± 4.9 | NSa |
| BMI, kg/m2 | 23.8 ± 5.3 | 25.9 ± 4.9 | NSa | 21.8 ± 2.1 | 30.1 ± 4.7 | na |
| Serum AMH, ng/mL | 3.0 (1.0, 3.8) | 6.4 (4.6, 8.2) | <.00001b | 3.8 (1.9, 5.1) | 4.5 (2.8, 6.4) | NSb |
| HOMA-IR | 1.4 ± 0.93 | 1.4 ± 0.95 | NSb | 0.9 ± 0.49 | 2.0 ± 0.95 | .006b |
| Serum CRP, mg/L | 3.4 (1.7, 6.6) | 2.5 (1.5, 10.1) | NSb | 2.1 (1.3, 6.2) | 4.7 (2.2, 8.8) | NSb |
| Serum TNFα, pg/mL | 0.87 ± 0.11 | 0.88 ± 0.14 | NSa | 0.87 ± 0.13 | 0.88 ± 0.14 | NSa |
| Serum IL-6, pg/mL | 1.56 ± 1.16 | 1.40 ± 0.73 | NSb | 1.31 ± 1.03 | 1.66 ± 0.69 | .046b |
| Serum T, ng/dL | 36.0 (21.6, 60.8) | 55.0 (38.2, 98.0) | NSb | 35.5 (21.6, 51.4) | 66.7 (42.5, 102.6) | .02b |
| FAI | 0.70 (0.58, 0.90) | 2.11 (1.23, 2.77) | .009b | 0.70 (0.58, 1.01) | 2.11 (1.33. 3.01) | .003b |
| FF IL-6, pg/mL | 6.4 (5.0, 10.8) | 8.4 (6.7, 24.2) | .07b | 7.3 (5.1, 12.4) | 8.4 (6.4, 13.0) | NSb |
| FF CRP, mg/L | 1.36 (0.53, 3.64) | 1.90 (0.57, 4.12) | NSb | 1.25 (0.56, 2.98) | 3.97 (0.56, 7.94) | NSb |
| FF TNFα, pg/mL | 0.72 ± 0.16 | 0.64 ± 0.13 | NSa | 0.70 ± 0.15 | 0.58 ± 0.04 | NSa |
| FF IL-8, pg/mL | 118.0 (46.3, 177.7) | 122.3 (94.3, 165.6) | NSb | 114.4 (66.0, 163.4) | 130.1 (81.3, 221.3) | NSb |
| FF T, ng/dL | 648 (425, 922) | 523 (467, 674) | NSb | 515 (413, 816) | 726 (523, 979) | .08b |
| FF Estradiol, pg/mL | 210 070 (81 300; 370 205) | 148 305 (73 464; 293 540) | NSb | 145 045 (73 324; 348 735) | 293 540 (148 305; 370 205) | NSb |
Abbreviations: AMH, antimullerian hormone; BMI, body mass index; na, not applicable; NS, nonsignificant. Data are shown as mean ± SD or median (interquartile range).
Comparison with a two-sample t test.
Comparison with a Mann-Whitney test.
Serum free androgen index (FAI) was elevated in both lean and overweight PCOS and overweight non-PCOS women compared with lean women (Supplemental Figure 4 [D] and Table 1) and was significantly correlated with BMI, FF CRP, and FF testosterone (FF T) levels (data not shown) and the relative expression of IL8 mRNA in that patient's associated GCs (Supplemental Figure 4 [A]). Although there was also a trend for increased GC IL8 expression with insulin resistance (homeostatic model assessment of insulin resistance [HOMA-IR]) levels, there were no other significant correlations between serum parameters and GC cytokine mRNA expression. Serum IL-8 levels were below detection (<31.2 pg/mL) showing that FF IL-8 is derived from the follicles and not from systemic sources. CYP19A1 mRNA expression was significantly negatively correlated with FAI, follicular fluid testosterone, and HOMA-IR (Supplemental Figure 4 [B] and Table 1).
When PCOS vs non-PCOS serum and FF samples were compared, only PCOS AMH and FAI levels were elevated; PCOS FF IL-6 concentrations likely would achieve significance with more samples (P = .07) (Table 1). Because some FF samples were pooled, the chance of finding a difference between groups likely decreased. When data analysis was stratified only by BMI status, the overweight group demonstrated significantly higher serum IL-6, T, FAI, and HOMA-IR levels compared with the lean group (Table 1; Supplemental Figure 4 [D]); FF T levels may achieve significance in the overweight cohort with more samples (P = .08). Serum IL-6 levels divided by both diagnosis and weight categories are shown in Supplemental Figure 4 [C]. There was no relationship between FF IL-6 levels and serum IL-6 levels.
Discussion
PCOS is a complex endocrine disease linked to elevated systemic levels of LH, androgens, and cytokines suggestive of a proinflammatory state and insulin resistance. However, the roles of these components at the ovarian level remain understudied, due, in part, to limited tissue availability and the diverse PCOS patient phenotype. In this prospective analysis of PCOS and control patients, we show the following: 1) GCs from PCOS patients express elevated transcripts encoding cytokines, chemokines, and immune cell markers; 2) obese PCOS patients define a distinct PCOS disease subtype; and 3) intrafollicular androgens and cytokines likely comprise a local regulatory loop that impacts GC expression of cytokines and chemokines and the presence of immune cells.
Specifically, our microarray analyses demonstrate that the obese PCOS patient represents a distinct phenotype with a dramatically increased perifollicular immune/cytokine response. That BMI and cytokine profiles were not linked in the control women and that several lean PCOS women exhibited a high cytokine profile suggests that the PCOS condition itself defines a propensity for a proinflammatory perifollicular milieu, which is then exponentially amplified by the PCOS obese condition. The PCOS-associated increase in perifollicular inflammatory cytokines and immune cells is not explained by systemic inflammation alone; rather, it is related to elevated follicular levels of androgens, LH signaling, and consequent increases in IL-6, IL-8, CCL20, and other factors such that the women with the highest cytokine profile all had PCOS and all were overweight.
Our analyses of the cytokine/chemokine/immune landscape in PCOS vs control GCs extend previous gene profiling analyses of ovarian stroma, granulosa cells (33, 34), and cumulus cells (35), suggesting that there are unique differences in expression profiles in these cell types specific to PCOS, as also seen in nonluteinized granulosa cells from small follicles (≤10 mm) aspirated for in vitro oocyte maturation cycles (33). We document that the PCOS GC expression of specific cytokines and chemokines after IVF stimulation differs from that of normoovulatory control women. These data contrast with the study of Jasper and Norman (36) in which unstimulated FF and cell cultures showed no difference in TNF and IL-1 presence between normal and PCOS ovaries and from Wu et al (37), who found no difference between stimulated groups, ie, PCOS vs age- and BMI-matched control women. Although the reasons for the different results between Wu's study and our study are not entirely clear, we used a prospective approach for patient recruitment, had a larger patient cohort, applied a different cell isolation procedure, and used different gene profiling analyses. It is probable that the IVF stimulation itself alters FF and GC cytokine levels and may engender a difference in results between groups, but this in itself reveals important physiological differences between control and PCOS conditions.
Although cells obtained from IVF follicular aspirations are predominantly GCs after cell isolation and washing, they may contain up to 15%–30% leukocytes (38, 39). Although data on the presence and types of immune cells in follicular aspirates of PCOS patients remain controversial due to limited studies (33, 37, 40, 41), our novel observations indicate that PCOS GCs contained higher relative CD45 mRNA and protein than control GCs, suggesting that more CD45+ immune cells are recruited (40) or less likely that there is greater immune cell activation (42). GCs depleted of leukocytes were not analyzed due to the small number of cells obtained. Although we cannot accurately describe the contribution of each cell type, the marked difference in the proinflammatory gene expression between control and PCOS GCs is striking. This is clinically relevant and likely reflects heightened GC cytokine expression and greater immune cell presence as well as the cellular and paracrine interactions between these cells in their in vivo periovulatory niche.
Whereas cytokines and immune cells are essential for normal ovulation and corpus luteum formation (8, 9, 43, 44), their abundance or dysregulation may play a role in the heightened vascular response to ovarian stimulation, which is more common in PCOS and is associated with altered expression of proangiogenic genes in the granulosa cells of women with PCOS (33, 34, 45). The potent neutrophil chemoattractant, IL-8, is secreted both constitutively and by induction with hCG (46) from GCs and ovarian stromal cells in vitro (47–50) and can interact with angiogenic factors in vitro (48), activate vascular endothelial growth factor (VEGF) receptor pathways (51), and modify GC steroidogenesis (52). Our results show that GC IL8 mRNA is increased in PCOS women and is highly related to an increased BMI specifically in our PCOS subcohort, supporting the positive correlation between IL8 mRNA and FAI and a positive trend between IL8 mRNA and HOMA-IR in those patients for whom we analyzed serum samples. Because serum IL-8 was below the threshold of detection, our results suggest that the GC IL8 mRNA-BMI relationship is associated with other systemic or follicular factors that can impact ovarian IL-8 production. Although our in vitro GC cultures demonstrated IL-8 induction of VEGF mRNA, overall PCOS GC VEGF expression did not differ from controls, perhaps because cells obtained prior to ovulation do not represent the local VEGF production a few days after ovulation when luteal VEGF serum levels peak and clinical ovarian hyperstimulation syndrome (OHSS) becomes apparent (53) or that the ovarian stimulation regimen was controlled and OHSS was obviated. Although in cultured bovine granulosa cell IL-8 may decrease FSH-stimulated estradiol production and increase LH-stimulated progesterone production (52), we did not find a change in aromatase expression, and changes in progesterone were minor.
IL-6 is an important regulator of cumulus cell-oocyte complex expansion (54) and improves murine oocyte quality and the pup live birth rate when used with oocyte in vitro maturation protocols (29). IL-6 is higher in the FF of PCOS patients (55) (shown herein), is associated with improved odds for life birth after fresh embryo transfer (as shown herein), and is also associated with positive pregnancy outcomes (37, 54, 56, 57) that may be related to the ability of IL-6 to enhance steroidogenesis in an immune cell-dependent manner (54), perhaps by increasing STAR (as shown herein). Although some controversy remains (58) as to the role of IL-6 in promoting pregnancy outcome, increased IL-6 in PCOS women is unlikely to be detrimental to reproductive outcomes; rather, it may be beneficial. Only when markedly elevated does FF IL-6 appear to be a harbinger of OHSS, increased VEGF expression, and vascular permeability (51, 59–62). Moreover, in accordance with a recent meta-analysis (63), serum IL-6 levels in our cohort were significantly associated with an increased BMI but not with PCOS as a group. Thus, serum IL-6 unlikely impacts ovarian function because there is no correlation between serum IL-6 and FF IL-6 levels.
Of particular relevance, our studies confirm the recent report that hCG induces CCL20 expression in GCs as shown by Al-Alem et al (39) and provides the novel observation that IL-6 as well as DHT also increases the expression of the chemokine CCL20 mRNA in cultured GCs. Thus, hCG induction of IL-8 and IL-6 combined with IL-6 and DHT induction of CCL20 appears to comprise a positive feed-forward loop of further chemokine induction in ovulatory follicles to enhance the immune cell presence in the local area (Figure 5). That hCG, DHT, and IL-6 induce CCL20 provide a potentially important physiological link between high levels of androgens in PCOS and elevated cytokines via leukocyte recruitment during ovulation. Whether there is a direct effect of androgens on immune cells or granulosa cells or this occurs via the induction of IL-6 remains to be determined; it is known that IL-6 can induce CCL20 in other cell types via the Janus kinase/signal transducer and activator of transcription pathway (64). The fact that both IL-6 and CCL20 mRNA are increased by DHT exposure in vitro may explain how elevated local androgens could increase the presence of these cytokines (and others through an enhanced leukocyte recruitment process) in PCOS compared with control women, as shown in our study.
Figure 5.
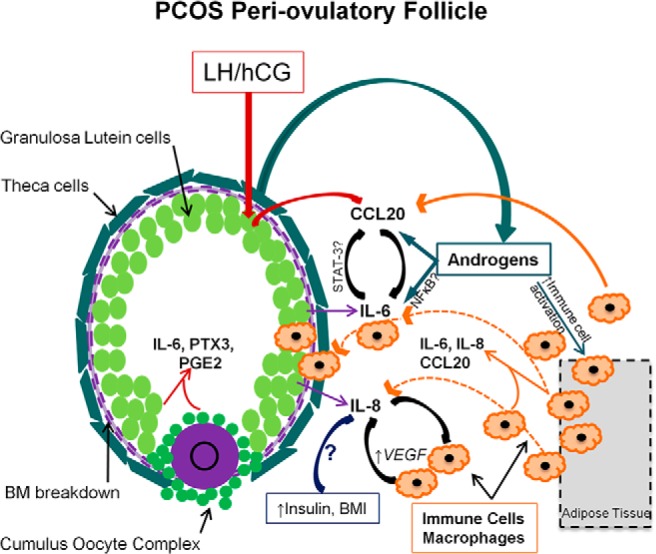
Working model of enhanced cytokine production in the PCOS periovulatory follicle. Cytokines and chemokines are secreted by GCs and are physiological components of the ovulatory response. Elevated local androgens in the PCOS follicle may increase IL6 and CCL20 expression, which can then enhance immune cell activation and recruitment to the area. These recruited immune cells then further contribute to the production of local inflammatory molecules, possibly affecting both angiogenic and steroidogenic pathways. IL8 may be increased by systemic factors associated with elevated body mass index and insulin levels and may also participate in a synergistic-positive feedback loop with immune cells in the production of VEGF and other proangiogenic factors. BM, basement membrane.
Summary
That specific cytokine and proangiogenic transcripts are increased in PCOS GCs and cumulus cells is likely both a cause and an effect of more local immune cells in the PCOS periovulatory follicle, as determined by an increased CD45 mRNA and protein. This finding is independent of the number of oocytes obtained but is related, in part, to PCOS adiposity. Whereas cytokines appear to be critical regulators of periovulatory events and the inflammatory mechanisms required for ovulation (1, 5), their elevated expression likely has implications for oocyte quality and embryo development (for which there is both positive and negative data) (65, 66) as well as early corpus luteum formation and in IVF the risk of OHSS.
Acknowledgments
Present address for J.E.A.: Division of Reproductive Endocrinology and Infertility, San Antonio Military Medical Center, 3551 Roger Brooke Drive, San Antonio, TX 78234. E-mail: jaye.e.adams.mil@mail.mil.
This work was supported by National Institutes of Health Grants NIH-HD-16229 and NIH-HD-07495 and Baylor College of Medicine departmental obstetrics/gynecology small grant funding. This work was supported by the Department of Molecular and Cellular Biology, Department of Obstetrics and Gynecology/Division of Reproductive Endocrinology and Infertility, Genomic and RNA Profiling Core, Clinical Scientist Training Program, University of Virginia, Center for Research in Reproduction, Ligand Assay and Analysis Core; Eunice Kennedy Shriver National Institute of Child Health and Human Development (Specialized Cooperative Centers Program in Reproduction and Infertility Research) Grant U54-HD028934, and Fertility Specialists of Houston (Houston, Texas).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BCM
- Baylor College of Medicine
- CRP
- C-reactive protein
- FAI
- free androgen index
- FF
- follicular fluid
- GC
- granulosa-lutein cell
- hCG
- human chorionic gonadotropin
- HOMA-IR
- homeostatic model assessment of insulin resistance
- IVF
- in vitro fertilization
- OHSS
- ovarian hyperstimulation syndrome
- PCOS
- polycystic ovarian syndrome
- qPCR
- quantitative PCR
- VEGF
- vascular endothelial growth factor.
References
- 1. Norman RJ, Brannstrom M. Cytokines in the ovary: pathophysiology and potential for pharmacological intervention. Pharmacol Ther. 1996;69(3):219–236. [DOI] [PubMed] [Google Scholar]
- 2. Hellberg P, Thomsen P, Janson PO, Brannstrom M. Leukocyte supplementation increases the luteinizing hormone-induced ovulation rate in the in vitro-perfused rat ovary. Biol Reprod. 1991;44(5):791–797. [DOI] [PubMed] [Google Scholar]
- 3. Brannstrom M, Bonello N, Wang LJ, Norman RJ. Effects of tumour necrosis factor α (TNF α) on ovulation in the rat ovary. Reprod Fertil Dev. 1995;7(1):67–73. [DOI] [PubMed] [Google Scholar]
- 4. Brannstrom M, Bonello N, Norman RJ, Robertson SA. Reduction of ovulation rate in the rat by administration of a neutrophil-depleting monoclonal antibody. J Reprod Immunol. 1995;29(3):265–270. [DOI] [PubMed] [Google Scholar]
- 5. Smolikova K, Mlynarcikova A, Scsukova S. Role of interleukins in the regulation of ovarian functions. Endocr Regul. 2012;46(4):237–253. [DOI] [PubMed] [Google Scholar]
- 6. Brannstrom M, Pascoe V, Norman RJ, McClure N. Localization of leukocyte subsets in the follicle wall and in the corpus luteum throughout the human menstrual cycle. Fertil Steril. 1994;61(3):488–495. [PubMed] [Google Scholar]
- 7. Best CL, Pudney J, Welch WR, Burger N, Hill JA. Localization and characterization of white blood cell populations within the human ovary throughout the menstrual cycle and menopause. Hum Reprod. 1996;11(4):790–797. [DOI] [PubMed] [Google Scholar]
- 8. Shirasuna K, Shimizu T, Matsui M, Miyamoto A. Emerging roles of immune cells in luteal angiogenesis. Reprod Fertil Dev. 2013;25(2):351–361. [DOI] [PubMed] [Google Scholar]
- 9. Care AS, Diener KR, Jasper MJ, Brown HM, Ingman WV, Robertson SA. Macrophages regulate corpus luteum development during embryo implantation in mice. J Clin Invest. 2013;123(8):3472–3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richards J, Liu Z, Shimada M. In: Plant TM, Zeleznki AJ, eds. Knobl and Neill's Physiology of Reproduction. 4th ed Chap 22. Ovulation. Boston: Elsevier; 2015:997–1016. [Google Scholar]
- 11. Adams J, Polson DW, Franks S. Prevalence of polycystic ovaries in women with anovulation and idiopathic hirsutism. Br Med J (Clin Res Ed). 1986;293(6543):355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carmina E, Chu MC, Longo RA, Rini GB, Lobo RA. Phenotypic variation in hyperandrogenic women influences the findings of abnormal metabolic and cardiovascular risk parameters. J Clin Endocrinol Metab. 2005;90(5):2545–2549. [DOI] [PubMed] [Google Scholar]
- 13. Azziz R, Dumesic DA, Goodarzi MO. Polycystic ovary syndrome: an ancient disorder? Fertil Steril. 2011;95(5):1544–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gonzalez F. Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids. 2012;77(4):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gonzalez F, Sia CL, Shepard MK, Rote NS, Minium J. Inflammation in response to glucose ingestion is independent of excess abdominal adiposity in normal-weight women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2012;97(11):4071–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dumesic DA, Richards JS. Ontogeny of the ovary in polycystic ovary syndrome. Fertil Steril. 2013;100(1):23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang LH, Rodriguez H, Ohno S, Miller WL. Serine phosphorylation of human P450c17 increases 17,20-lyase activity: implications for adrenarche and the polycystic ovary syndrome. Proc Natl Acad Sci USA. 1995;92(23):10619–10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McAllister JM, Legro RS, Modi BP, Strauss JF., 3rd Functional genomics of PCOS: from GWAS to molecular mechanisms. Trends Endocrinol Metab. 2015;26(3):118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu XK, Zhou SY, Liu JX, et al. Selective ovary resistance to insulin signaling in women with polycystic ovary syndrome. Fertil Steril. 2003;80(4):954–965. [DOI] [PubMed] [Google Scholar]
- 20. Weil SJ, Vendola K, Zhou J, et al. Androgen receptor gene expression in the primate ovary: cellular localization, regulation, and functional correlations. J Clin Endocrinol Metab. 1998;83(7):2479–2485. [DOI] [PubMed] [Google Scholar]
- 21. Weil S, Vendola K, Zhou J, Bondy CA. Androgen and follicle-stimulating hormone interactions in primate ovarian follicle development. J Clin Endocrinol Metab. 1999;84(8):2951–2956. [DOI] [PubMed] [Google Scholar]
- 22. Diamanti-Kandarakis E, Paterakis T, Kandarakis HA. Indices of low-grade inflammation in polycystic ovary syndrome. Ann NY Acad Sci. 2006;1092:175–186. [DOI] [PubMed] [Google Scholar]
- 23. Piotrowski PC RI, Kwintkiewicz J, Duleba AJ. Oxidative stress induces expression of CYP11A, CYP17, STAR, and 3bHSD in rat theca-interstitial cells. J Soc Gynecol Invest. 2005;12(suppl 2):319A. [Google Scholar]
- 24. Spaczynski RZ, Arici A, Duleba AJ. Tumor necrosis factor-α stimulates proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1999;61(4):993–998. [DOI] [PubMed] [Google Scholar]
- 25. Rui L, Aguirre V, Kim JK, et al. Insulin/IGF-1 and TNF-α stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest. 2001;107(2):181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McAllister JM, Modi B, Miller BA, et al. Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci USA. 2014;111(15):E1519–E1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diamanti-Kandarakis E, Dunaif A. Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev. 2012;33(6):981–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chilvers RA, Bodenburg YH, Denner LA, Urban RJ. Development of a novel protocol for isolation and purification of human granulosa cells. J Assist Reprod Genet. 2012;29(6):547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu Z, de Matos DG, Fan HY, Shimada M, Palmer S, Richards JS. Interleukin-6: an autocrine regulator of the mouse cumulus cell-oocyte complex expansion process. Endocrinology. 2009;150(7):3360–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Irizarry RA, Hobbs B, Collin F, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4(2):249–264. [DOI] [PubMed] [Google Scholar]
- 31. Gentleman RC, Carey VJ, Bates DM, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ophir L, Yung Y, Maman E, et al. Establishment and validation of a model for non-luteinized human mural granulosa cell culture. Mol Cell Endocrinol. 2014;384(1–2):165–174. [DOI] [PubMed] [Google Scholar]
- 33. Schmidt J, Weijdegard B, Mikkelsen AL, Lindenberg S, Nilsson L, Brannstrom M. Differential expression of inflammation-related genes in the ovarian stroma and granulosa cells of PCOS women. Mol Hum Reprod. 2014;20(1):49–58. [DOI] [PubMed] [Google Scholar]
- 34. Kaur S, Archer KJ, Devi MG, Kriplani A, Strauss JF, 3rd, Singh R. Differential gene expression in granulosa cells from polycystic ovary syndrome patients with and without insulin resistance: identification of susceptibility gene sets through network analysis. J Clin Endocrinol Metab. 2012;97(10):E2016–E2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kenigsberg S, Bentov Y, Chalifa-Caspi V, Potashnik G, Ofir R, Birk OS. Gene expression microarray profiles of cumulus cells in lean and overweight-obese polycystic ovary syndrome patients. Mol Hum Reprod. 2009;15(2):89–103. [DOI] [PubMed] [Google Scholar]
- 36. Jasper M, Norman RJ. Immunoactive interleukin-1β and tumour necrosis factor-α in thecal, stromal and granulosa cell cultures from normal and polycystic ovaries. Hum Reprod. 1995;10(6):1352–1354. [DOI] [PubMed] [Google Scholar]
- 37. Wu R, Fujii S, Ryan NK, et al. Ovarian leukocyte distribution and cytokine/chemokine mRNA expression in follicular fluid cells in women with polycystic ovary syndrome. Hum Reprod. 2007;22(2):527–535. [DOI] [PubMed] [Google Scholar]
- 38. Ferrero H, Delgado-Rosas F, Garcia-Pascual CM, et al. Efficiency and purity provided by the existing methods for the isolation of luteinized granulosa cells: a comparative study. Hum Reprod. 2012;27(6):1781–1789. [DOI] [PubMed] [Google Scholar]
- 39. Al-Alem L, Puttabyatappa M, Rosewell K, et al. Chemokine ligand 20: a signal for leukocyte recruitment during human ovulation? Endocrinology. 2015;156(9):3358–3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xiong YL, Liang XY, Yang X, Li Y, Wei LN. Low-grade chronic inflammation in the peripheral blood and ovaries of women with polycystic ovarian syndrome. Eur J Obstet Gynecol Reprod Biol. 2011;159(1):148–150. [DOI] [PubMed] [Google Scholar]
- 41. Gallinelli A, Ciaccio I, Giannella L, Salvatori M, Marsella T, Volpe A. Correlations between concentrations of interleukin-12 and interleukin-13 and lymphocyte subsets in the follicular fluid of women with and without polycystic ovary syndrome. Fertil Steril. 2003;79(6):1365–1372. [DOI] [PubMed] [Google Scholar]
- 42. Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–137. [DOI] [PubMed] [Google Scholar]
- 43. Hernandez-Gonzalez I, Gonzalez-Robayna I, et al. Gene expression profiles of cumulus cell oocyte complexes during ovulation reveal cumulus cells express neuronal and immune-related genes: does this expand their role in the ovulation process? Mol Endocrinol. 2006;20(6):1300–1321. [DOI] [PubMed] [Google Scholar]
- 44. Wu R, Van der Hoek KH, Ryan NK, Norman RJ, Robker RL. Macrophage contributions to ovarian function. Hum Reprod Update. 2004;10(2):119–133. [DOI] [PubMed] [Google Scholar]
- 45. Lebbe M, Woodruff TK. Involvement of androgens in ovarian health and disease. Mol Hum Reprod. 2013;19(12):828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arici A, Oral E, Bukulmez O, Buradagunta S, Engin O, Olive DL. Interleukin-8 expression and modulation in human preovulatory follicles and ovarian cells. Endocrinology. 1996;137(9):3762–3769. [DOI] [PubMed] [Google Scholar]
- 47. Polec A, Tanbo T, Fedorcsak P. Cellular interaction regulates interleukin-8 secretion by granulosa-lutein cells and monocytes/macrophages. Am J Reprod Immunol. 2009;61(1):85–94. [DOI] [PubMed] [Google Scholar]
- 48. Polec A, Raki M, Abyholm T, Tanbo TG, Fedorcsak P. Interaction between granulosa-lutein cells and monocytes regulates secretion of angiogenic factors in vitro. Hum Reprod. 2011;26(10):2819–2829. [DOI] [PubMed] [Google Scholar]
- 49. Runesson E, Bostrom EK, Janson PO, Brannstrom M. The human preovulatory follicle is a source of the chemotactic cytokine interleukin-8. Mol Hum Reprod. 1996;2(4):245–250. [DOI] [PubMed] [Google Scholar]
- 50. Runesson E, Ivarsson K, Janson PO, Brannstrom M. Gonadotropin- and cytokine-regulated expression of the chemokine interleukin 8 in the human preovulatory follicle of the menstrual cycle. J Clin Endocrinol Metab. 2000;85(11):4387–4395. [DOI] [PubMed] [Google Scholar]
- 51. Chen SU, Chou CH, Lin CW, et al. Signal mechanisms of vascular endothelial growth factor and interleukin-8 in ovarian hyperstimulation syndrome: dopamine targets their common pathways. Hum Reprod. 2010;25(3):757–767. [DOI] [PubMed] [Google Scholar]
- 52. Shimizu T, Kaji A, Murayama C, et al. Effects of interleukin-8 on estradiol and progesterone production by bovine granulosa cells from large follicles and progesterone production by luteinizing granulosa cells in culture. Cytokine. 2012;57(1):175–181. [DOI] [PubMed] [Google Scholar]
- 53. Malamitsi-Puchner A, Sarandakou A, Tziotis J, Stavreus-Evers A, Tzonou A, Landgren BM. Circulating angiogenic factors during periovulation and the luteal phase of normal menstrual cycles. Fertil Steril. 2004;81(5):1322–1327. [DOI] [PubMed] [Google Scholar]
- 54. Machelon V, Emilie D, Lefevre A, Nome F, Durand-Gasselin I, Testart J. Interleukin-6 biosynthesis in human preovulatory follicles: some of its potential roles at ovulation. J Clin Endocrinol Metab. 1994;79(2):633–642. [DOI] [PubMed] [Google Scholar]
- 55. Amato G, Conte M, Mazziotti G, et al. Serum and follicular fluid cytokines in polycystic ovary syndrome during stimulated cycles. Obstet Gynecol. 2003;101(6):1177–1182. [DOI] [PubMed] [Google Scholar]
- 56. Kawasaki F, Kawano Y, Kosay Hasan Z, Narahara H, Miyakawa I. The clinical role of interleukin-6 and interleukin-6 soluble receptor in human follicular fluids. Clin Exp Med. 2003;3(1):27–31. [DOI] [PubMed] [Google Scholar]
- 57. Bedaiwy M, Shahin AY, AbulHassan AM, et al. Differential expression of follicular fluid cytokines: relationship to subsequent pregnancy in IVF cycles. Reprod Biomed Online. 2007;15(3):321–325. [DOI] [PubMed] [Google Scholar]
- 58. Altun T, Jindal S, Greenseid K, Shu J, Pal L. Low follicular fluid IL-6 levels in IVF patients are associated with increased likelihood of clinical pregnancy. J Assist Reprod Genet. 2011;28(3):245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chen CD, Chen HF, Lu HF, Chen SU, Ho HN, Yang YS. Value of serum and follicular fluid cytokine profile in the prediction of moderate to severe ovarian hyperstimulation syndrome. Hum Reprod. 2000;15(5):1037–1042. [DOI] [PubMed] [Google Scholar]
- 60. Friedlander MA, Loret de Mola JR, Goldfarb JM. Elevated levels of interleukin-6 in ascites and serum from women with ovarian hyperstimulation syndrome. Fertil Steril. 1993;60(5):826–833. [DOI] [PubMed] [Google Scholar]
- 61. Geva E, Lessing JB, Lerner-Geva L, Azem F, Yovel I, Amit A. Elevated levels of interleukin-6 in the follicular fluid at the time of oocyte retrieval for in vitro fertilization may predict the development of early-form ovarian hyperstimulation syndrome. Fertil Steril. 1997;68(1):133–137. [DOI] [PubMed] [Google Scholar]
- 62. Wei LH, Chou CH, Chen MW, et al. The role of IL-6 trans-signaling in vascular leakage: implications for ovarian hyperstimulation syndrome in a murine model. J Clin Endocrinol Metab. 2013;98(3):E472–E484. [DOI] [PubMed] [Google Scholar]
- 63. Escobar-Morreale HF, Luque-Ramirez M, Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril. 2011;95(3):1048–1058.e1–e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Meares GP, Ma X, Qin H, Benveniste EN. Regulation of CCL20 expression in astrocytes by IL-6 and IL-17. Glia. 2012;60(5):771–781. [DOI] [PubMed] [Google Scholar]
- 65. McCormick B, Thomas M, Maxwell R, Williams D, Aubuchon M. Effects of polycystic ovarian syndrome on in vitro fertilization-embryo transfer outcomes are influenced by body mass index. Fertil Steril. 2008;90(6):2304–2309. [DOI] [PubMed] [Google Scholar]
- 66. Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2011;17(1):17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]