Abstract
Context:
Retinoic acid receptor responder protein 2 (RARRES2) is a small secreted protein involved in multiple cancers, including adrenocortical carcinoma (ACC). However, discordant tumor and serum RARRES2 levels have been reported in various cancers. The etiology of this discordance is unknown and has not been studied in pair-matched tumor and serum samples.
Objective:
To determine tissue and serum RARRES2 levels in patients with adrenocortical neoplasm and to elucidate the prognostic implications of RARRES2 levels.
Design, Settings, and Patients:
Tissue and serum RARRES2 levels were analyzed. A pair-matched analysis was performed to examine tissue and serum RARRES2 from 51 patients with benign adrenocortical tumors and 18 patients with ACC. Overall survival was analyzed based on RARRES2 expression. A mouse xenograft model was used to determine the source of serum RARRES2.
Results:
Patients with ACC had decreased tumor RARRES2 gene expression (P < .0001) and increased serum RARRES2 levels (P < .005) as compared with patients with benign adrenocortical tumors. Higher serum RARRES2 levels were associated with improved overall survival (P = .0227). A mouse xenograft model demonstrated that higher tissue RARRES2 expression was associated with higher RARRES2 secretion in the serum and that there was an intrinsic mechanism in maintaining serum RARRES2 homeostasis.
Conclusions:
Serum and tissue RARRES2 expression levels are paradoxical in patients with ACC. The elevated RARRES2 in patient serum is unlikely to be secreted from tumor cells. Serum RARRES2 may be used as a novel prognostic marker for ACC.
Serum and tissue RARRES2 expression levels are paradoxical in patients with adrenocortical carcinoma. Serum RARRES2 levels are a prognostic marker for adrenocortical carcinoma.
Incidentally detected adrenal incidentalomas have been increasing in prevalence with the advent of the computed tomography scan (1). These incidentalomas are common compared with adrenocortical carcinoma (ACC), but many patients undergo an adrenalectomy to exclude the diagnosis of ACC. ACC, a rare malignancy with an annual incidence of 0.7–2.0 cases per million people (2–4), has a poor prognosis. The 5-year overall survival rate ranges from 32% to 42% but is heterogeneous (2, 5). Furthermore, even after complete tumor resection, over half of the patients develop recurrent disease. Many patients undergo chemotherapy, which consists of a regimen, including mitotane plus combination chemotherapy with etoposide, doxorubicin, and cisplatin. Unfortunately, this regimen has limited impact on overall survival and low response rates (6, 7). Understanding the mechanism behind disease progression and identifying biomarkers for diagnosis and prognosis are of great importance in selecting patients who may benefit from intervention and/or adjuvant therapy. Furthermore, patients with a poor prognosis may be appropriately counseled and referred for clinical trials.
Retinoic acid receptor responder protein 2 (RARRES2) (also known as chemerin) is a small secreted protein that was first identified as a ligand for the orphan G protein-coupled receptor chemokine-like receptor 1 (8). To date, RARRES2 is mainly recognized for its 2 major roles as a chemoattractant and an adipokine (9). As a chemoattractant, it promotes chemotaxis of chemokine-like receptor 1-expressing immune cells, including immature dendritic cells, macrophages, and natural killer cells, to lymphoid organs and sites of injury (8, 10–12). As an adipokine, it regulates adipogenesis and adipocyte metabolism (13, 14). Several studies have shown the potential involvement of RARRES2 in cancer. RARRES2 is transcriptionally down-regulated in ACC (15–17). Its expression is also reported to be reduced in melanoma, skin squamous cell carcinoma, hepatocellular carcinoma, and in lung, breast, colon, and metastatic prostate cancers (18–20). However, RARRES2 protein levels in peripheral blood were reported to be significantly higher in patients with lung cancer than in healthy volunteers (21). Similarly, increased serum RARRES2 levels were detected in patients with gastric cancer as compared with healthy subjects (22, 23). To our knowledge, no studies have been conducted to determine RARRES2 expression level concomitantly in tissue and serum with pair-matched cancer patient samples. The mechanism of this paradoxical tissue and serum RARRES2 expression is also unknown.
In this study, we performed a pairwise comparison of tissue and serum RARRES2 levels in patients with adrenocortical tumors to determine the diagnostic and prognostic value of RARRES2 as a biomarker, and to elucidate the source of serum RARRES2 levels.
Materials and Methods
Human tissue samples
Twenty-one normal adrenal glands were obtained at the time of nephrectomy for organ donation. Fifty-one benign adrenocortical tumor samples and 26 ACC samples (primary or local recurrence) were procured immediately after surgical resection. All tissue samples were snap frozen and stored at −80°C. Tumors were classified as benign when the Weiss criteria scores were less than 3, and there was no evidence of recurrence or metastasis during follow-up (mean follow-up, 3.2 y). Tumors were classified as ACC when the Weiss criteria scores were more than or equal to 3 (Table 1).
Table 1.
Clinical Features of Tissue Samples Used in Tissue RARRES2 Expression Assessment
| Benign Adrenocortical Tumor | ACC | |
|---|---|---|
| Number of patients | 51 | 26 |
| Age (average ± SD) | 47.3 ± 15.6 | 48.0 ± 13.7 |
| Sex (female/male) | 32/19 | 17/9 |
| Type of tumor | Primary | 8 primary, 3 locoregional recurrence without distant metastasis, 15 locoregional recurrence with distant metastasis |
| Syndromea | ||
| Adrenal hypercortisolism | 27 | 11 |
| Subclinical adrenal hypercortisolism | 2 | 0 |
| Primary hyperaldosteronism | 19 | 1 |
| Nonfunctioning | 3 | 14 |
Functional status at initial presentation.
Human serum samples
Twenty-one fasting serum samples were obtained from healthy volunteers. Fifty-three fasting preoperative serum samples were obtained on the day of surgery from patients with benign adrenocortical tumors with Weiss criteria scores of less than 3 and no evidence of recurrence or metastasis during follow-up. Twenty fasting preoperative serum samples were obtained on the day of surgery from patients with primary, locoregional recurrence and distant metastatic ACC with Weiss criteria scores of more than or equal to 3. Serum samples were collected and stored at −80°C (Tables 2 and Table 3). All patients gave written informed consent for sample collection.
Table 2.
Clinical Features of Tissue Samples Used in Serum RARRES2 Expression Assessment
| Healthy Controls | Benign Adrenocortical Tumor | ACC | |
|---|---|---|---|
| Number of patients | 21 | 53 | 20 |
| Age (average ± SD) | 45.8 ± 12.9 | 47.8 ± 15.6 | 50.2 ± 10.5 |
| Sex (female/male) | 10/11 | 32/21 | 12/8 |
| Type of tumor | Primary | 1 primary, 3 locoregional recurrence without distant metastasis, 16 locoregional recurrence with distant metastasis | |
| Syndromea | |||
| Adrenal hypercortisolism | 27 | 6 | |
| Subclinical adrenal hypercortisolism | 2 | 0 | |
| Primary hyperaldosteronism | 21 | 1 | |
| Nonfunctioning | 3 | 13 |
Functional status at initial presentation.
Table 3.
Clinical Characteristics of Patients With ACC for Survival Analysis
| Patient Characteristics | |
|---|---|
| Sex | |
| Male, n (%) | 8 (40%) |
| Female, n (%) | 12 (60%) |
| Age at diagnosis, median (range) | 51 (32–68) |
| Age at operation, median (range) | 55 (34–72) |
| BMI, mean (range) | 26.5 (17.7–35.5) |
| Syndromea | |
| Hyperaldosteronism | 1 |
| Hypercortisolism | 6 |
| Nonfunctional | 13 |
| Extent of disease | |
| Primary | 1 (5%) |
| Locoregional disease, n (%) | 3 (15%) |
| Distant mets, n (%) | 16 (80%) |
| Previous treatment | |
| Surgery, n (%)b | 16 (80%) |
| Chemotherapy, n (%)c | 17 (85%) |
| Ki67 (%)d | |
| <5%, n (%) | 2 (12.5%) |
| >20%, n (%) | 14 (87.5%) |
Functional status at initial presentation.
Previous surgeries include primary resection of ACC, metastectomy, radiofrequency ablations, and debulking procedures.
Patients received chemotherapeutic regimens, including combinations of mitotane, etoposide, doxorubicin, cisplatin, protease inhibitors, abraxane, tariquidar, imatinib, streptozocin, or cetuximab.
Ki67% for patients with available data.
RNA isolation, cDNA synthesis, and TaqMan real-time quantitative PCR
Total RNA was isolated using the RNA/DNA/Protein Purification Plus kit (Norgen Biotek Corp), according to the manufacturer's protocol. The RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems). TaqMan real-time quantitative polymerase chain reaction was performed on 7900HT fast real-time PCR systems (Applied Biosystems). The reaction was prepared by mixing cDNA, TaqMan 2× universal PCR master mix and TaqMan gene expression assays (Applied Biosystems). The gene expression assays used were: RARRES2 (Hs00161209_g1) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Hs99999905_m1).
Enzyme-linked immunosorbent assay
Human Chemerin Quantikine ELISA kit (limit of quantification, 78 pg/mL) or mouse Chemerin Quantikine ELISA kit (limit of quantification, 47 pg/mL) (R&D Systems) were used to measure human or mouse RARRES2 levels in human and mouse serum samples. All samples were measured in duplicate at 1:100 dilutions.
Cell lines and animals
Human ACC cell lines NCI-H295R (H295R) were purchased from ATCC and authenticated by short-tandem repeat profiling. Cells were cultured in DMEM supplemented with 1% insulin transferrin selenium and 2.5% NuSerum I (Corning) and maintained in a standard humidified incubator with 5% CO2 at 37°C. RARRES2-overexpression plasmid was generated by cloning RARRES2 cDNA from pCMV-XL5-RARRES2 (Origene) into the pcDNA4/Myc-His A vector (Life Technologies) to generate pcDNA4-RARRES2 so that only the untagged protein was expressed. Polyclonal stable cell lines overexpressing RARRES2 or an empty vector were generated by transfecting H295R cells with BglII (New England Biolabs) linearized pcDNA4-RARRES2 plasmid or the pcDNA4/Myc-His A vector, followed by selection with 1-μg/mL Zeocin (Life Technologies). Immunodeficient NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ (NOD scid-γ) mice were purchased from The Jackson Laboratory and were maintained and bred under the guidelines of the National Institutes of Health Animal Research Advisory Committee. Mice littermates that were 3–4 months old were randomly assigned to 2 groups with 7 mice in each group. Each mouse received sc flank injections bilaterally with 100 μL of a 1:1 mixture of either medium or 4 × 106 cells with Matrigel (Corning, Inc). Tumor sizes were measured and recorded once a week. Mice were euthanized when tumor sizes reached 2 cm in diameter. Whole-blood samples were collected twice: before injection from the facial vein and immediately after euthanization by cardiac puncture. Serum was obtained by centrifugation of blood samples for 15 minutes at 4000 rpm.
Western blot analysis
Total protein lysates were subjected to SDS-PAGE gel electrophoresis, transferred to polyvinylidene difluoride membranes, and immunostained with primary antibodies overnight at 4°C. The primary antibodies used were the RARRES2 antibody (1:1000, ab72965; Abcam) and GAPDH antibody (1:5000, sc-47724; Santa Cruz Biotechnology, Inc). The secondary antibody used was goat antimouse IgG-horseradish peroxidase (1:5000, sc-2005; Santa Cruz Biotechnology, Inc). Proteins were detected with SuperSignal West Pico or West Femto Chemiluminescent Substrate (Thermo Fisher Scientific).
Statistical analysis
Mann Whitney tests were used to compare RARRES2 levels between different groups. The primary outcome variable analyzed was overall survival. Overall survival was defined as the time from preoperative blood samples drawn to disease-related mortality or last follow-up, which was censored for those patients who underwent repeated abdominal surgery. The association between human serum RARRES2 levels and survival was assessed using the Kaplan-Meier method, with statistical differences determined by log-rank test. P < .05 was considered statistically significant. All calculations were performed using GraphPad Software 6.0.
Results
RARRES2 gene expression level is down-regulated in ACC tissue samples but is not associated with prognosis
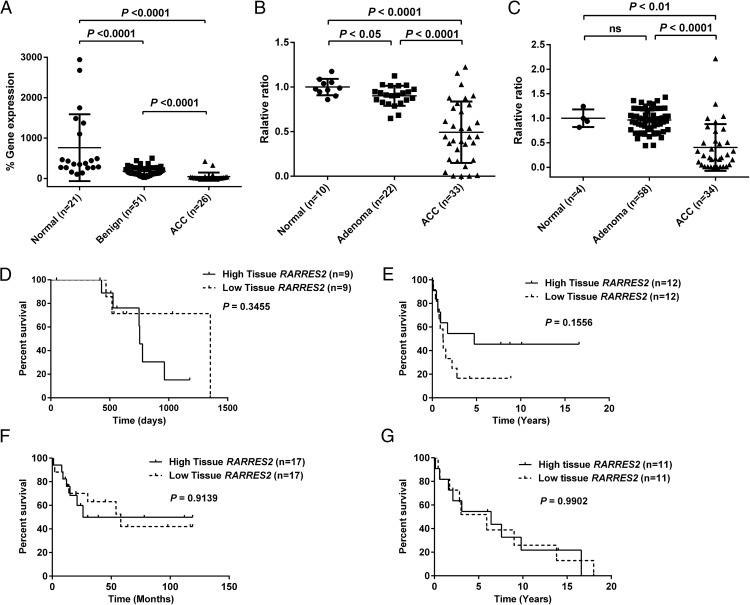
RARRES2 gene expression levels were measured in 21 normal adrenocortical tissues, 51 benign adrenocortical tumors and 26 ACC samples. Significant down-regulation of RARRES2 expression was observed in ACC samples when compared with benign or normal samples (P < .0001 in both comparisons); it was also observed in benign samples when compared with normal adrenocortical tissues (P < .0001) (Figure 1A). These results are consistent with those from our previous genome-wide gene expression study in another independent cohort, in which RARRES2 was down-regulated in primary ACC samples (n = 5) as compared with benign adrenocortical tissues (n = 74) (15). Similar results were also obtained when we analyzed 2 publicly available adrenocortical tissue databases (NCBI-GEO, accession number GSE10927; and EMBL-EBI, accession number E-TABM-311) (Figure 1, B and C) (24, 25). To determine whether tissue RARRES2 expression is associated with prognosis, we analyzed whether RARRES2 expression levels in ACC tissue samples were associated with patient overall survival in our current patient cohort and patient cohorts depicted in GSE10927, E-TABM-311, and Demeure et al (26). No significant association was found between tissue RARRES2 expression and patient overall survival (Figure 1, D–G).
Figure 1.
The tissue RARRES2 gene expression level is down-regulated in ACC but is not associated with patient prognosis. A, TaqMan real-time quantitative PCR reveals down-regulation of the RARRES2 gene expression level in our cohort of ACC samples as compared with benign adrenocortical tumors. Human RARRES2 expression is presented as % gene expression (= 2 −Δ(Ct RARRES2 − Ct GAPDH) × 100). B, RARRES2 expression as analyzed in NCBI-GEO database GSE10927. Data are presented as relative ratio to normal tissue. C, RARRES2 expression as analyzed in European Bioinformatics Institute database E-TABM-311. Data are presented as relative ratio to normal tissue. The tumor RARRES2 gene expression level is not associated with prognosis in 4 independent datasets, including our current cohort (D), patient cohort reported in GSE10927 (E), patient cohort reported in E-TABM-311 (F), and patient cohort reported in Demeure et al (26) (G). High and low RARRES2 groups were defined by median tissue gene expression. ns, not significant.
Serum RARRES2 protein level is elevated in patients with ACC and is associated with prognosis
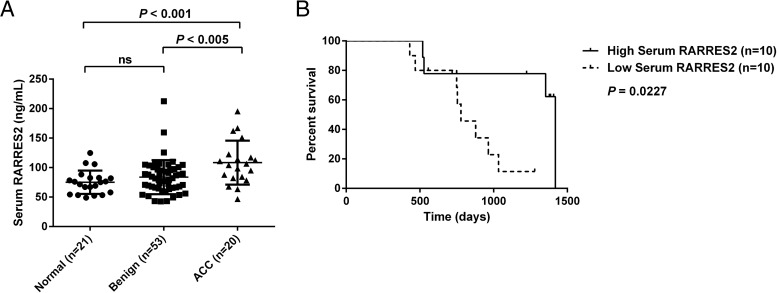
Serum RARRES2 protein levels were measured in serum samples collected from 21 healthy volunteers, 53 patients with benign adrenocortical tumors, and 20 patients with ACC. Among these, serum samples from 51 patients with benign tumors and 18 patients with ACC were paired with the tissues analyzed for RARRES2 gene expression. Serum RARRES2 levels were significantly elevated in patients with ACC compared with patients with benign tumors (P < .005) and healthy controls (P < .001), whereas no significant difference was observed between healthy controls and patients with benign tumors (Figure 2A). Serum RARRES2 levels were not significantly different based on tumor functional status, disease burden, gender, or maximum standardized uptake value, as determined by fluorodeoxyglucose positron emission tomography/computed tomography (data not shown). However, survival analysis based on median serum RARRES2 levels (105 ng/mL) showed that higher RARRES2 serum levels were associated with longer overall survival (P = .0227; median survival of 1419 days for high serum RARRES2 group vs 778 days for low-serum RARRES2 group; median follow-up, 2.3 y) (Figure 2B).
Figure 2.
The serum RARRES2 level is up-regulated in patients with ACC and is associated with prognosis. A, The serum RARRES2 level was significantly elevated in patients with ACC as compared with healthy controls and patients with benign adrenocortical tumors. B, Kaplan-Meier analysis for the serum RARRES2 level showed a significant difference in survival based on serum RARRES2 levels (P = .0227). The median survival for the high serum RARRES2 group is 1419 days, and the median survival for the low-serum RARRES2 group is 778 days. The median follow-up is 2.3 years. High and low RARRES2 groups were defined by median serum RARRES2 level. ns, not significant.
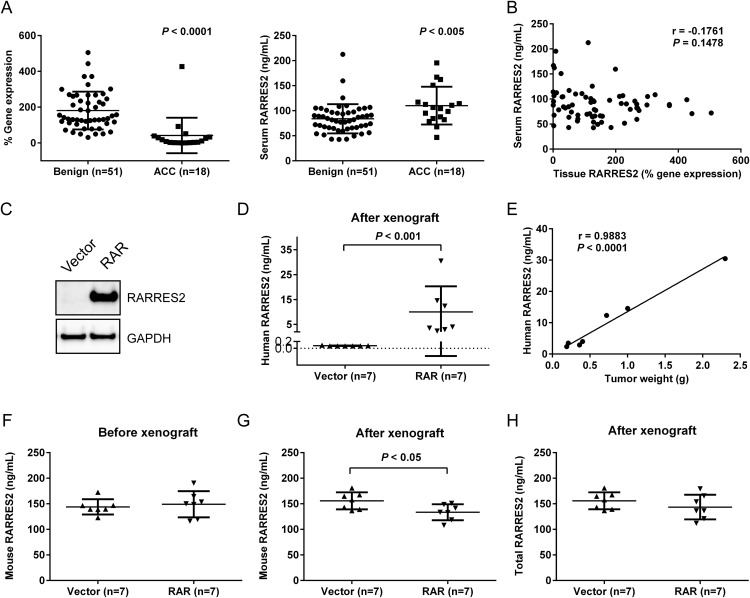
Elevated serum RARRES2 levels are not from tumor cell secretion
Given the low tissue RARRES2 levels yet high serum RARRES2 levels in patients with ACC, we sought to understand the reason for this apparent paradox. Pair-matched tissue and serum samples from 51 patients with benign adrenocortical tumors and 18 patients with ACC were analyzed for RARRES2 expression, which confirmed the existence of this paradox within the same patient cohort (Figure 3A). However, no significant correlation between tissue and serum RARRES2 levels was observed (r = −0.1761, P = .1478) (Figure 3B). To determine whether the elevated serum RARRES2 levels came from active secretion of RARRES2 from ACC tissues, we conducted a mouse xenograft experiment. Immunodeficient NOD scid-γ mice were randomly assigned to 2 groups: one group received injections of H295R cells stably transfected with a plasmid expressing RARRES2 (RAR), and the second group received injections of H295R cells stably transfected with an empty vector (Figure 3C). The mice were monitored for 8 weeks before reaching the endpoint of the study. All xenografts developed into tumors except for 1 xenograft in the RARRES2 group. In this experimental model, we can distinguish the origin of RARRES2 in the serum: human RARRES2 should come from the xenografted human cells expressing human RARRES2, whereas mouse RARRES2 should come from the mouse host environment. As expected, the human RARRES2 level in the serum was high in the RAR group after the xenograft experiment (Figure 3D). The amount of secreted human RARRES2 was significantly correlated with tumor weight (r = 0.9883, P < .0001), indicating that more cells with RARRES2 expression led to increased RARRES2 secretion and detection in the serum (Figure 3E). The vector group members with minimal basal human RARRES2 expression developed tumors with minimal detection of human RARRES2 in serum (Figure 3D). Before the xenograft experiment, no significant difference in mouse serum RARRES2 levels was observed between the groups (Figure 3F). After the xenograft experiment, mouse serum RARRES2 levels in the RAR group significantly decreased compared with the vector group (Figure 3G), but total serum RARRES2 levels remained constant in both groups (Figure 3H).
Figure 3.
Elevated serum RARRES2 levels observed in patients do not come from tumors expressing low RARRES2. A, Pair-matched analysis for tissue RARRES2 (left) and serum RARRES2 (right) levels from 51 patients with benign adrenocortical tumors and 18 patients with ACC. B, Tissue RARRES2 expression has no significant correlation with the serum RARRES2 level from the same patients. r, correlation coefficient. Pair-matched tissue and serum RARRES2 levels from 51 benign adrenocortical tumors and 18 ACC samples were examined. % gene expression = 2 −Δ(Ct RARRES2 − Ct GAPDH) × 100. C, Representative Western blot showing the difference in the RARRES2 protein level in stable H295R cells overexpressing RARRES2 (RAR) as compared with stable H295R cells overexpressing an empty vector. The stable cell lines were used in mouse xenografts. D, The human RARRES2 level increased significantly in mouse serum when the mice were xenografted with stable RARRES2 overexpressing H295R cells. E, The human RARRES2 level in mouse serum correlated significantly with tumor weight. F, Mouse serum RARRES2 levels before the xenograft experiment. No significant difference was observed. G, Mouse serum RARRES2 levels after the xenograft experiment. Mice xenografted with RARRES2-overexpressing ACC cells showed significantly decreased mouse RARRES2 levels. H, Total (mouse and human) RARRES2 levels in mouse serum after the xenograft experiment. No significant difference was observed between the groups.
Discussion
In the present study, tissue and serum RARRES2 levels were examined in healthy controls and patients with benign and malignant adrenocortical tumors. A pair-matched analysis was performed for tissue and serum RARRES2 levels from patients with benign adrenocortical tumors and ACC. High serum levels of RARRES2 was found to indicate improved survival. To our knowledge, this is the first study to examine tissue and serum RARRES2 levels concomitantly in the same cohort for any cancer.
Our data show a paradoxical phenomenon where patients with ACC have lower RARRES2 in the tumor tissue but higher RARRES2 in the serum as compared with patients with benign tumors and healthy controls. These findings are consistent with published reports in patients with lung cancer (20, 21), although paired tumor tissue and serum analysis for RARRES2 was not conducted in these studies. Using an immunodeficient mouse model xenografted with human ACC cells, we showed that higher tumor RARRES2 levels correlated with higher RARRES2 secretion into the serum. These results provide evidence against the possibility that ACCs with low tissue RARRES2 expression will have high RARRES2 secretion into the serum. Therefore, high serum RARRES2 levels may be coming from the host environment instead of tumor cells. In fact, in our study, we showed that when tumors were actively secreting RARRES2 into the host environment, host-originated RARRES2 decreased accordingly so that total serum RARRES2 level remained unchanged. This suggests that there might be an intrinsic mechanism in maintaining homeostasis in the serum RARRES2 level. Thus, it is possible that in patients with ACC which have low RARRES2 tissue expression hence less RARRES2 secretion into the serum, the host environment will release more RARRES2 into the serum in order to keep the balance. The source of serum RARRES2 is unknown. RARRES2 was shown to be strongly expressed in pancreas, liver and adrenal gland (27), which may account for the paradoxical findings. It has been reported that RARRES2 is strongly expressed in the white adipose tissue and that the circulating RARRES2 level is associated with key features of obesity and metabolic syndrome, such as body mass index (BMI), plasma triglycerides, and blood pressure (13, 14). However, it is unlikely that the serum RARRES2 elevation observed in patients with ACC was caused by obesity, as no studies have suggested a link between ACC and obesity. Furthermore, there was no association between BMI and serum RARRES2 levels in our cohort, and the BMI was not significantly different between patients with benign adrenocortical tumors and ACC (data not shown). Research has also suggested that inflammation can stimulate RARRES2 release from adipose tissues into the serum (28). The proinflammatory cytokine TNFα has been shown to stimulate RARRES2 release from adipocytes, whereas anti-TNFα monoclonal antibody therapy can reduce RARRES2 secretion in rheumatoid arthritis and chronic plaque psoriasis (29, 30). However, because ACC is not known to be an immunogenic cancer, further investigations are required to determine the cellular source of RARRES2 in the serum of patients with ACC.
An analysis of serum RARRES2 levels determined that these levels were a prognostic marker of overall survival in patients with ACC. Higher levels of serum RARRES2 were associated with better survival rates. When examining this cohort in detail, most patients had recurrent ACC. These patients with recurrent ACC could be prognostically stratified based on serum RARRES2 levels when deciding on therapeutic management. Erdogan et al (31) have shown that in reoperative surgery for recurrent ACC, patients with a disease-free interval of greater than 6 months and microscopic and gross complete resection had longer survival rates. Thus, patients with high levels of RARRES2 in combination with long disease-free intervals may be ideal candidates for surgical resection if their disease is resectable. Patients with lower levels may benefit from chemotherapy or clinical trials.
One interesting result from our study is that serum RARRES2 levels were higher in patients with ACC as compared with patients with benign lesions or healthy controls; however, higher serum RARRES2 levels in patients with ACC were associated with better overall survival. We believe that 2 major factors might contribute to this seemingly contradictory phenomenon. First, the higher serum RARRES2 level in patients with ACC does not come from tumors but from the host environment. Second, RARRES2 can act as a chemoattractant and recruit immune cells to site of inflammation (8, 10–12). In fact, in melanomas, it was shown that RARRES2 can suppress tumor growth by recruiting natural killer cells to exhibit antitumor defense (20). Thus, it is possible that ACC tissues express less RARRES2 as a mean of immune evasion (32), whereas the host environment can counteract this attempt by producing more RARRES2 in the serum. As a result, serum RARRES2 levels are higher in patients with ACC; and higher RARRES2 levels, possibly resulting from better host response, are associated with better overall survival.
Because ACC is a rare malignancy (2, 4), the small sample size is a major limitation of this study. Further studies with larger patient cohorts, including more patients with primary ACC, would be beneficial in determining the value of serum RARRES2 as a diagnostic marker for differentiating between benign and malignant adrenocortical tumors. Whether this data can be generalized to patients with primary adrenal tumors without metastatic disease or patients undergoing initial surgery without metastatic disease needs to be determined and is an exciting avenue to pursue in light of the current findings. It would also be interesting to know whether serum RARRES2 levels change with disease progression and or response to treatment on serial blood samples. Unfortunately, we did not have serial serum samples to test this hypothesis but this would be interesting to evaluate in future studies.
In summary, we found that patients with ACC have low RARRES2 gene expression in the tumor but high RARRES2 protein levels in the serum. Serum level, but not tissue RARRES2 level, can serve as a potential prognostic marker for ACC. Our data suggests that the elevated serum RARRES2 level does not originate from ACC tumor tissue. Understanding the mechanism behind the discordant tissue and serum RARRES2 expression, and identifying the source of serum RARRES2 and discovering the physiological role of RARRES2 in ACC will provide more insight into to the role of RARRES2 in ACC.
Acknowledgments
This work was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health (1ZIABC01127506).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACC
- adrenocortical carcinoma
- BMI
- body mass index
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- RARRES2
- retinoic acid receptor responder protein 2.
References
- 1. Toniato A, Merante-Boschin I, Opocher G, Pelizzo MR, Schiavi F, Ballotta E. Surgical versus conservative management for subclinical Cushing syndrome in adrenal incidentalomas: a prospective randomized study. Ann Surg. 2009;249:388–391. [DOI] [PubMed] [Google Scholar]
- 2. Bilimoria KY, Shen WT, Elaraj D, et al. Adrenocortical carcinoma in the United States: treatment utilization and prognostic factors. Cancer. 2008;113:3130–3136. [DOI] [PubMed] [Google Scholar]
- 3. Kamenický P, Droumaguet C, Salenave S, et al. Mitotane, metyrapone, and ketoconazole combination therapy as an alternative to rescue adrenalectomy for severe ACTH-dependent Cushing's syndrome. J Clin Endocrinol Metab. 2011;96:2796–2804. [DOI] [PubMed] [Google Scholar]
- 4. Fassnacht M, Libé R, Kroiss M, Allolio B. Adrenocortical carcinoma: a clinician's update. Nat Rev Endocrinol. 2011;7:323–335. [DOI] [PubMed] [Google Scholar]
- 5. Kerkhofs TM, Verhoeven RH, Van der Zwan JM, et al. Adrenocortical carcinoma: a population-based study on incidence and survival in the Netherlands since 1993. Eur J Cancer. 2013;49:2579–2586. [DOI] [PubMed] [Google Scholar]
- 6. Fassnacht M, Allolio B. Clinical management of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2009;23:273–289. [DOI] [PubMed] [Google Scholar]
- 7. Fassnacht M, Terzolo M, Allolio B, et al. Combination chemotherapy in advanced adrenocortical carcinoma. N Engl J Med. 2012;366:2189–2197. [DOI] [PubMed] [Google Scholar]
- 8. Wittamer V, Franssen JD, Vulcano M, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab. 2010;21:660–667. [DOI] [PubMed] [Google Scholar]
- 10. Wittamer V, Bondue B, Guillabert A, Vassart G, Parmentier M, Communi D. Neutrophil-mediated maturation of chemerin: a link between innate and adaptive immunity. J Immunol. 2005;175:487–493. [DOI] [PubMed] [Google Scholar]
- 11. Vermi W, Riboldi E, Wittamer V, et al. Role of ChemR23 in directing the migration of myeloid and plasmacytoid dendritic cells to lymphoid organs and inflamed skin. J Exp Med. 2005;201:509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parolini S, Santoro A, Marcenaro E, et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood. 2007;109:3625–3632. [DOI] [PubMed] [Google Scholar]
- 13. Goralski KB, McCarthy TC, Hanniman EA, et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–28188. [DOI] [PubMed] [Google Scholar]
- 14. Bozaoglu K, Bolton K, McMillan J, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–4694. [DOI] [PubMed] [Google Scholar]
- 15. Rechache NS, Wang Y, Stevenson HS, et al. DNA methylation profiling identifies global methylation differences and markers of adrenocortical tumors. J Clin Endocrinol Metab. 2012;97:E1004–E1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Velázquez-Fernández D, Laurell C, Geli J, et al. Expression profiling of adrenocortical neoplasms suggests a molecular signature of malignancy. Surgery. 2005;138:1087–1094. [DOI] [PubMed] [Google Scholar]
- 17. Fernandez-Ranvier GG, Weng J, Yeh RF, et al. Identification of biomarkers of adrenocortical carcinoma using genomewide gene expression profiling. Arch Surg. 2008;143:841–846; discussion 846. [DOI] [PubMed] [Google Scholar]
- 18. Zheng Y, Luo S, Wang G, et al. Downregulation of tazarotene induced gene-2 (TIG2) in skin squamous cell carcinoma. Eur J Dermatol. 2008;18:638–641. [DOI] [PubMed] [Google Scholar]
- 19. Lin W, Chen YL, Jiang L, Chen JK. Reduced expression of chemerin is associated with a poor prognosis and a lowed infiltration of both dendritic cells and natural killer cells in human hepatocellular carcinoma. Clin Lab. 2011;57:879–885. [PubMed] [Google Scholar]
- 20. Pachynski RK, Zabel BA, Kohrt HE, et al. The chemoattractant chemerin suppresses melanoma by recruiting natural killer cell antitumor defenses. J Exp Med. 2012;209:1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Qu X, Han L, Wang S, et al. [Detection of chemerin and it's clinical significance in peripheral blood of patients with lung cancer.]. Zhongguo Fei Ai Za Zhi. 2009;12:1174–1177. [DOI] [PubMed] [Google Scholar]
- 22. Wang C, Wu WK, Liu X, et al. Increased serum chemerin level promotes cellular invasiveness in gastric cancer: a clinical and experimental study. Peptides. 2014;51:131–138. [DOI] [PubMed] [Google Scholar]
- 23. Zhang J, Jin HC, Zhu AK, Ying RC, Wei W, Zhang FJ. Prognostic significance of plasma chemerin levels in patients with gastric cancer. Peptides. 2014;61C:7–11. [DOI] [PubMed] [Google Scholar]
- 24. Giordano TJ, Kuick R, Else T, et al. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res. 2009;15:668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Reyniès A, Assié G, Rickman DS, et al. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J Clin Oncol. 2009;27:1108–1115. [DOI] [PubMed] [Google Scholar]
- 26. Demeure MJ, Coan KE, Grant CS, et al. PTTG1 overexpression in adrenocortical cancer is associated with poor survival and represents a potential therapeutic target. Surgery. 2013;154:1405–1416; discussion 1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chamberland JP, Berman RL, Aronis KN, Mantzoros CS. Chemerin is expressed mainly in pancreas and liver, is regulated by energy deprivation, and lacks day/night variation in humans. Eur J Endocrinol. 2013;169:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Catalán V, Gómez-Ambrosi J, Rodríguez A, et al. Increased levels of chemerin and its receptor, chemokine-like receptor-1, in obesity are related to inflammation: tumor necrosis factor-α stimulates mRNA levels of chemerin in visceral adipocytes from obese patients. Surg Obes Relat Dis. 2013;9:306–314. [DOI] [PubMed] [Google Scholar]
- 29. Herenius MM, Oliveira AS, Wijbrandts CA, Gerlag DM, Tak PP, Lebre MC. Anti-TNF therapy reduces serum levels of chemerin in rheumatoid arthritis: a new mechanism by which anti-TNF might reduce inflammation. PLoS One. 2013;8:e57802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gisondi P, Lora V, Bonauguri C, Russo A, Lippi G, Girolomoni G. Serum chemerin is increased in patients with chronic plaque psoriasis and normalizes following treatment with infliximab. Br J Dermatol. 2013;168:749–755. [DOI] [PubMed] [Google Scholar]
- 31. Erdogan I, Deutschbein T, Jurowich C, et al. The role of surgery in the management of recurrent adrenocortical carcinoma. J Clin Endocrinol Metab. 2013;98:181–191. [DOI] [PubMed] [Google Scholar]
- 32. Vinay DS, Ryan EP, Pawelec G, et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin Cancer Biol. 2015;35(suppl):S185–S198. [DOI] [PubMed] [Google Scholar]