Abstract
Context:
Growing evidence challenges the concept that high-density lipoprotein-cholesterol (HDL-C) is cardioprotective after menopause. HDL particle concentration (HDL-P) and cholesterol efflux capacity (CEC) might be better predictors of cardiovascular risk.
Objective:
Quantify alterations in HDL-P and CEC during menopause, correlating those changes with alterations in estradiol (E2) and FSH.
Design:
Longitudinal study of HDL metrics before and after menopause as indexed by the final menstrual period (FMP).
Participants:
Forty-six women, mean baseline age 47.1 years, 33% black, 67% white.
Main Outcomes and Measures:
HDL-P concentration (HDL-PIMA) by calibrated ion mobility analysis (IMA); macrophage CEC with cAMP-stimulated macrophages; ATP-binding cassette transporter A1 (ABCA1)-specific CEC with BHK cells expressing human ABCA1.
Results:
After a median of 2.1 years since FMP, both HDL-C (P = .03) and HDL-PIMA (P = .01) increased, with a selective increase in large HDL-PIMA (P = .01), whereas sizes of medium and small HDL-PIMA were decreased (P < .05). These changes were independent of race, body mass index, and time difference. Macrophage CEC and ABCA1-specific CEC increased after FMP (both P < .001). Greater declines in E2 correlated with larger increases in small HDL-PIMA (P = .01), whereas greater increases in FSH associated with greater reductions in the size of medium HDL-PIMA (P = .04). Macrophage CEC and ABCA1-specific CEC correlated positively with E2 levels only before menopause (P = .04 and .009, respectively).
Conclusions:
Large HDL-PIMA and CEC increased significantly in the early phase of the menopausal transition. Whether patterns of these alterations differ in late postmenopause is unknown. Further exploration is needed to assess that and to determine whether the reported changes in HDL metrics associate with increased cardiovascular risk after menopause.
We studied changes in HDL-P and efflux capacity early in the menopausal transition and correlated those changes with sex hormones alterations. Increases in large HDL-P and efflux capacity were found.
The risk of cardiovascular disease (CVD) increases in postmenopausal women (1, 2). As women transition through menopause, levels of low-density lipoprotein cholesterol (LDL-C), apolipoprotein B (apoB), and total cholesterol (3, 4) rise. In contrast, there is little agreement on postmenopausal changes in high-density lipoprotein cholesterol (HDL-C), which is widely considered cardioprotective. Thus, some studies have detected reductions in HDL-C (4, 5), whereas others have reported increased levels around menopause (3, 6, 7) or no change (2, 8).
Epidemiological and clinical studies demonstrate a robust negative association between HDL-C and CVD risk in the overall population (9, 10). However, the relationship is less clear in postmenopausal women. Lower HDL-C was a better predictor of CVD in such women than other lipid risk factors (11). However, other lines of evidence challenge the idea that HDL-C is cardioprotective in this population (12–16). In one study, progression of carotid intimal medial thickness was associated inversely with HDL-C in men, but was positively associated in women aged 45–60 years (12). In another study, high levels of HDL-C were associated with increased risk of nonfatal stroke and cerebral infarction, but only in women older than age 52 years (14). Moreover, inverse associations of HDL-C level with aortic calcification were reported in premenopausal women, whereas higher HDL-C levels were associated with higher levels of aortic calcification in postmenopausal women (15). More recently, we reported that increases in HDL-C levels over 9 years of follow-up during the menopausal transition were independently associated with greater carotid intimal medial thickness progression (16). Taken together, these findings suggest that HDL-C is not necessarily cardioprotective as women transition through menopause.
Alternatively, HDL-C levels may not capture the proposed cardioprotective effects of HDL as supported by several lines of evidence. For example, interventional trials with drugs that elevate HDL-C by different mechanisms failed to reduce CVD risk in statin-treated subjects (17, 18). Genetic studies suggest that differences in HDL-C levels do not affect CVD risk (19). In contrast, a recent study of P376L variant of scavenger receptor BI (SR-BI) encoded by the gene SCARB1, the hepatic receptor for HDL, demonstrated that the gene increased both HDL-C and CVD risk (20). These observations imply that HDL-C levels do not directly reflect atheroprotection and that new HDL metrics that predict risk need to be developed.
Recent studies demonstrated that cholesterol efflux capacity (CEC)—the ability of serum depleted of LDL and other apoB-containing lipoproteins—to promote cholesterol efflux from cultured macrophages (21) associates strongly and inversely with prevalent and incident CVD, supporting HDL's proposed role in protection against atherogenesis (22, 23). Importantly, HDL-C levels explain only about 33% of the variance in cholesterol efflux capacity (22, 23), suggesting that the level or activity of an HDL species with known cardioprotective function(s) might be a better predictor of CVD risk than HDL-C.
The number of possible candidates for the cardioprotective subspecies is large, because HDL is a heterogeneous collection of particles composed primarily of cholesterol, phospholipid, cholesteryl ester, and apolipoprotein A-I (apoA-I) (24). These particles range in size from 7 to 14 nm, and their content of cholesterol can vary more than 4-fold. Thus, traditional measurements of HDL—HDL-C levels—do not necessarily indicate either the overall abundance of HDL particles or the distribution of the different subspecies (25). This has led investigators to propose that HDL particles' (HDL-P) concentration and size might be better predictors of CVD risk than HDL-C (26). Several studies support this proposal (27, 28), but others do not (29), and the role of HDL-P as a predictor of CVD risk remains unclear. We recently showed that quantifying HDL particle concentration (HDL-PIMA) with calibrated ion mobility analysis (IMA) yields values in excellent agreement with our current understanding of the size distribution and stoichiometry of apoA-I per HDL particle, in contrast with other methods used to determine HDL-P (25).
Recent studies have shown that small HDLs, but not large HDLs, can accept cholesterol exported from cells by the ATP-binding cassette transporter A1 (ABCA1) transporter, the major pathway for removing cholesterol from macrophages (30). Importantly, small HDLs carry much less cholesterol per particle, indicating that HDL-C levels do not necessarily provide useful information on the CEC of HDL subspecies. These observations suggest that both the size and concentration of HDL particles may be important CEC determinants.
Menopause is a crucial period of life when women are subjected to significant alterations in lipid metabolism and sex hormones that likely contribute to an increase in CVD risk (3, 31). To better understand the relationship between HDL and cardiovascular risk in postmenopausal women, we quantified CEC and HDL-PIMA in women from the Pittsburgh site of the Study of Women's Health Across the Nation (SWAN). We also evaluated the relationships between alterations in those two HDL metrics and changes in estradiol (E2) and FSH, two cardinal markers of hormonal changes across menopause. We hypothesized that significant reduction in total and large HDL-P and CEC measures would occur with change in menopausal status from pre- to postmenopause. We previously reported a positive correlation between E2 and size of HDL-P as measured by nuclear magnetic resonance spectroscopy in women at midlife (32); therefore, we hypothesized that reduction in sizes of HDL-P subclasses would occur as well. Finally we hypothesized that these changes would be significantly associated with changes in E2 and FSH
Materials and Methods
Study participants
SWAN is an ongoing, multiethnic, longitudinal investigation of the biological, physical, and psychological changes during the menopausal transition. The study design was previously described (33). In brief, between 1996 and 1997, 3302 women aged 42–52 years were recruited from seven designated sites (Boston, MA; Detroit, MI; Oakland, CA; Los Angeles, CA; Pittsburgh, PA; Chicago, IL; and Newark, NJ). Eligibility criteria for SWAN were: 1) an intact uterus and at least one ovary, 2) not pregnant or breastfeeding, 3) at least one menstrual period within the past 3 months, and 4) no hormone therapy (HT) use within the past 3 months.
The current ancillary SWAN study was conducted at the Pittsburgh site. Inclusion criteria for this study were: 1) available locally stored blood samples from women both before and after menopause and 2) no use of HT or lipid-lowering medications at these two time points. Forty-six women met these criteria and were included in the current study with a total of 92 observations (two per woman). All protocols were approved by the University of Pittsburgh Institutional Review Board, and all the women provided written informed consent before enrollment in SWAN.
Study covariates
Weight and height were measured at each clinic visit. Body mass index (BMI) was calculated as weight/height2. Race/ethnicity was self-reported. Menopausal status was determined annually based on reports about the frequency and regularity of menstrual bleeding and the use of HT as follows: 1) premenopause: monthly bleeding with no perceived change in cycle interval; 2) early perimenopause: monthly bleeding with a perceived change in cycle interval but at least one menstrual period within the past 3 months; 3) late perimenopause: 3 consecutive months of amenorrhea; 4) postmenopause: 12 consecutive months of amenorrhea. Time since menopause was calculated as the difference between the final menstrual period (FMP; the one followed by 12 months of amenorrhea) and the date of the clinical study visit when blood specimens were collected.
Blood samples
When the women were premenopausal, blood was drawn in the morning after a 10–12 hour fast on days 2–5 of a spontaneous menstrual cycle. After menopause, a random fasting sample was taken within 90 days of the annual visit. Blood was kept for up to 1 hour at 4 C until it was separated and then frozen at –80 C. Assays for lipids/apolipoproteins and sex hormones were performed by SWAN laboratories usually within 3 months of collection. Blood specimens stored at the University of Pittsburgh for local hypothesis testing were used to quantify HDL-P concentration and CEC at the University of Washington. Blood specimens stored at the SWAN repository were used to measure lipids/apolipoproteins for 13 observations of those included in the current study with no local samples in storage. Data from the Dallas Heart Study (22) showed a reduction in CEC of plasma stored for 3 to 12 months at a temperature lower than –70 C. A study of 180 plasma samples stored at –70 C for periods of time ranging from 1 to 12 years showed no significant impact of time of storage on macrophage CEC (Ronsein and Heinecke, unpublished data). Other studies with freshly obtained human plasma demonstrated that macrophage CEC, ABCA1-specific CEC, and HDL-PIMA are stable to three freeze-thaw cycles (Ronsein and Heinecke, unpublished data). All specimens used for HDL metrics in the current study were stored at –80 C and had never been thawed.
Lipids and apolipoproteins
Standard laboratory methods were used to determine levels of triglycerides, LDL-C, HDL-C, apoA-1, and apoB. Assays were performed at the Medical Research and Heinz (University of Pittsburgh) laboratories, both certified by the Centers for Disease Control and Prevention. LDL-C was calculated by the Friedewald equation for all women with triglyceride levels below 400 mg/dl (34). Direct LDL-C was measured for those with triglycerides of at least 400 mg/dl.
Quantification of HDL-P size and concentration, using calibrated ion mobility analysis (HDL-PIMA)
The exact procedures for this method have been published (25). In brief, HDL isolated by ultracentrifugation from serum was converted into gas-phase ions by electrospray ionization. The resulting ions (largely neutralized by alpha particles, yielding a small proportion of singly charged cations) were introduced into a differential mobility analyzer. Particles were separated according to their electrophoretic mobility in a strong electromagnetic field and then detected by a particle counter. HDL peak areas were converted into aqueous particle concentrations using a calibration curve constructed with glucose oxidase. Total HDL-PIMA was obtained by summing the concentrations of the small, medium, and large HDL particles (termed S-HDL-PIMA [diameter 7.2–8.2 nm], M-HDL-PIMA [8.2–9.7 nm], and L-HDL-PIMA [9.7–11.8 nm], respectively). The intra-assay and interassay coefficient of variations ranged between 0.7%–20.1% and 1.1%–21%, respectively (25).
Ion mobility analysis data are expressed in terms of particle diameter (nm), which corresponds to the calculated diameter of a singly charged, spherical particle with the same electrophoretic mobility (35).
Cholesterol efflux capacity specific to macrophages or to the ABCA-1
Cholesterol efflux was measured after a 4-hour incubation in medium with 2.8% serum depleted of apoB-containing lipoproteins by polyethylene glycol precipitation (21), using cells radiolabeled with [3H]cholesterol. Macrophage CEC was measured with J774 macrophages (21). Efflux was calculated as the percentage of total [3H]cholesterol (medium plus cells) released into the medium of cells stimulated with a cAMP analog after the value obtained with cells stimulated with medium alone was subtracted. ABCA1-specific CEC was measured with BHK cells expressing mifepristone-inducible human ABCA1 (36). Efflux was calculated as the percentage of total [3H]cholesterol released into the medium of cells stimulated with mifepristone after the value obtained with cells stimulated with medium alone was subtracted. The average interassay coefficient of variations (%) of duplicate macrophage CEC and ABCA1-specific CEC were 2.51% and 1.85%, respectively.
Sex hormones
Hormone levels in EDTA plasma were measured by the University of Michigan Endocrine Laboratory (Automated Chemiluminescence System –180; Bayer Diagnostics Corp.). E2 was measured in duplicate with a modified, off-line ACS-180 (E2–6) immunoassay, and FSH was measured in singlicate with a two-site chemiluminometric immunoassay. The inter- and intra-assay coefficients of variation for E2 were 10.6% and 6.4%, respectively. For FSH, they were 11.4% and 3.8%, respectively. The cycle day of the blood draw was reported as days 2–5 of the menstrual cycle or outside of that period.
Statistical analysis
Because the current study design involved repeated measures of HDL metrics obtained from the same women before menopause and then after menopause, the primary analyses included: 1) a series of Wilcoxon signed-rank tests to assess whether changes in HDL metrics within woman varied by menopausal status and 2) linear mixed effect models to assess whether differences by menopausal status could be explained by race, BMI (time varying covariate), and the time difference between the two assessments. Linear mixed effects models enabled us to account for within-woman correlation, adjust for time-varying covariates, and include a random intercept to represent each woman. To determine whether the association between each HDL-PIMA subclass and each CEC outcome measure varied before as compared to after menopause, an interaction term between menopausal status and the HDL-PIMA metric was added to the linear mixed effects model for the corresponding CEC outcome measure. Log-transformed values were used in the linear mixed effects models so that the resulting variables were approximately normally distributed.
In addition, Spearman and partial Spearman rank correlational analyses were presented to illustrate the relationship between the change in HDL metrics from pre- to postmenopause with the change in hormones and conventional lipid measures from pre- to postmenopause. Change was defined as the subtraction of the premenopausal value from the postmenopausal value for an individual woman on the log-transformed scale. Analyses were performed with SAS (version 9.3; SAS Institute) and R package. All statistical tests were two-sided; P values <.05 were considered significant.
Results
Demographics and lipid risk factors before and after menopause
We studied 46 women (67% white and 33% black) with a median baseline age of 47 years. At the initial evaluation, 41% were premenopausal and 59% were early perimenopausal. At the follow-up visit, all women were postmenopausal, with a median time since FMP of 2.14 years (quartile 1, quartile 3: 1.6, 3.5). As expected, BMI, LDL-C, apoB, and FSH levels were significantly higher after menopause, whereas levels of E2 were lower relative to before menopause. Both HDL-C and apoA-I significantly increased after menopause; the increase in apoA-I (13%) was larger than the increase in HDL-C (5%), Table 1.
Table 1.
Demographic Characteristics and Laboratory Values of Women Before and After Menopause (n = 46)
| Study Variables | Before Menopause | After Menopause | P Valuea |
|---|---|---|---|
| Age, y | 47.0 (45.0, 49.0) | 53.5 (52.0, 55.0) | <.001 |
| BMI, kg/m2 | 28.5 (24.6, 32.1) | 29.7 (25.1, 34.5) | .01 |
| E2, pg/ml | 70.6 (45.5, 116.3) | 15.3 (9.7, 23.0) | <.001 |
| FSH, mIU/ml | 20.5 (12.7, 36.1) | 95.3 (62.5, 138.1) | <.001 |
| apoA-I, mg/dl | 142.5 (126.0, 161.0) | 161.0 (144.0, 175.0) | <.001 |
| HDL-C, mg/dl | 53.0 (44.0, 62.0) | 55.8 (45.0, 68.0) | .01 |
| apoB, mg/dl | 111.0 (89.0, 123.0) | 111.5 (92.0, 139.0) | .02 |
| LDL-C, mg/dl | 117.5 (99.0, 135.0) | 131.0 (113.0, 163.0) | <.001 |
| Triglycerides, mg/dl | 90.0 (69.0126.0) | 112.0 (78.0136.0) | .08 |
| HDL-PIMA, uM/liter | 14.8 (13.3, 16.5) | 15.5 (13.6, 17.4) | .01 |
| S-HDL-PIMA, uM/liter | 4.5 (3.3, 5.7) | 4.8 (3.1, 6.0) | .39 |
| M-HDL-PIMA, uM/liter | 6.8 (5.7, 8.2) | 6.7 (5.7, 8.3) | .87 |
| L-HDL-PIMA, uM/liter | 3.3 (2.1, 4.4) | 3.7 (2.3, 5.0) | .01 |
| Mean size S-HDL-PIMA, nm | 8.19 (8.10, 8.24) | 8.17 (8.09, 8.23) | .01 |
| Mean size M-HDL-PIMA, nm | 9.05 (9.00, 9.09) | 9.00 (8.97, 9.06) | .002 |
| Mean size L-HDL-PIMA, nm | 10.86 (10.77, 10.91) | 10.85 (10.76, 10.94) | .80 |
| ABCA1-specific CEC, %b | 15.0 (13.1, 16.7) | 16.7 (15.3, 18.2) | <.001 |
| Macrophage CEC, %b | 14.5 (13.1, 16.0) | 16.9 (15.7, 17.7) | <.001 |
Results are median and (quartile 1, quartile 3).
Wilcoxon signed-rank test.
% cell-associated radiolabeled cholesterol.
HDL-PIMA, macrophage CEC, and ABCA1-specific CEC increase from pre- to postmenopause
Both HDL-PIMA (the total number of HDL particles) and L-HDL-PIMA (the number of large HDL particles) increased significantly (by 5% and 12%, respectively), after menopause, Table 1. We also detected significant increases in macrophage CEC and ABCA1-specific CEC (17% and 11%, respectively). The mean sizes of S-HDL-PIMA and M-HDL-PIMA decreased significantly after menopause, but the changes were small (0.2% and 0.6%), Table 1.
Correlations of changes in HDL-C, HDL-PIMA, and CEC with changes in lipids and apolipoproteins over menopause
Increases in HDL-C from pre- to postmenopause significantly and positively correlated with simultaneous increases in HDL-PIMA, M-HDL-PIMA, and L-HDL-PIMA, and with the mean size of L-HDL-PIMA, Table 2. The same patterns were observed for apoA-I. Increases in LDL-C and, to a lesser degree, apoB significantly and negatively correlated with the changes in mean sizes of M-HDL-PIMA and L-HDL-PIMA. Increases in triglyceride levels significantly and positively correlated with changes in S-HDL-PIMA but negatively with changes in L-HDL-PIMA and mean sizes of M-HDL-PIMA and L-HDL-PIMA. There was not a significant correlation between changes in triglyceride and changes in HDL-PIMA, which could be explained by the opposite correlations of triglycerides changes with changes in S-HDL-PIMA and L-HDL-PIMA that could cancel each other out.
Table 2.
Spearman Correlations Between the Within-Woman Changes in HDL Metrics and the Within-Woman Changes in Lipids/Apolipoproteins
| Change in | HDL-C | ApoA-I | LDL-C | ApoB | Triglyceridesa |
|---|---|---|---|---|---|
| HDL-C | 1 | 0.64*** | −0.03 | −0.04 | −0.37** |
| HDL-PIMAa | 0.46** | 0.39** | −0.11 | 0.05 | −0.18 |
| S-HDL-PIMAa | −0.26 | −0.09 | 0.01 | 0.05 | 0.33* |
| M-HDL-PIMAa | 0.46** | 0.37* | −0.09 | −0.03 | −0.26 |
| L-HDL-PIMAa | 0.67*** | 0.48*** | 0.06 | 0.11 | −0.31* |
| Mean size, S-HDL-PIMAa | −0.07 | −0.04 | −0.03 | −0.12 | −0.07 |
| Mean size, M-HDL-PIMAa | 0.28 | 0.24 | −0.35* | −0.22 | −0.42** |
| Mean size, L-HDL-PIMAa | 0.39** | 0.18 | −0.29* | −0.31* | −0.37* |
| ABCA1-specific CECa | 0.15 | 0.29* | 0.02 | −0.04 | 0.07 |
| Macrophage CECa | 0.10 | 0.15 | −0.07 | −0.17 | 0.08 |
P < .05;
P ≤ .01;
P < .001.
Differences between log-transformed values before and after menopause.
The only established lipid risk factor that correlated with changes in ABCA1-specific efflux was changes in apoA-I; no lipid factors correlated with changes in macrophage CEC, Table 2.
Associations of HDL-PIMA and CEC with menopausal status after adjustments for other potential confounders from linear mixed effect models
The significant increases in HDL-PIMA and L-HDL-PIMA from pre- to postmenopause and the significant decreases in the sizes of S- and M-HDL-PIMA were not attenuated by adjusting for race, BMI, or time between the two assessments. The increases in macrophage and ABCA1-specific CEC also remained highly significant in the adjusted models (Supplemental Table).
Medium and large HDL subspecies correlate strongly with macrophage CEC and ABCA1-specific CEC
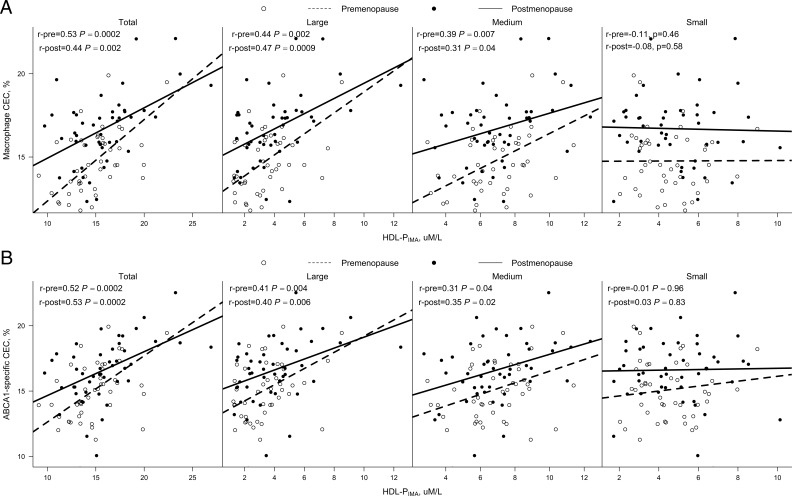
To determine how well the various HDL subspecies promote cholesterol efflux, we examined the relationships between CEC and those of S-HDL-PIMA, M-HDL-PIMA, and L-HDL-PIMA in samples stratified by menopausal status (Figure 1). Macrophage CEC and ABCA1-specific CEC correlated with the concentrations of total HDL-PIMA, which appeared to be driven by their correlations with both M-HDL-PIMA and L-HDL-PIMA both before and after menopause. Interestingly, S-HDL-PIMA did not correlate with macrophage CEC or ABCA1-specific CEC either before or after menopause.
Figure 1.
Spearman correlations of macrophage CEC and ABCA1-specific CEC with HDL-PIMA stratified by menopausal status. Macrophage CEC (A) and ABCA1 CEC (B) of serum HDL (apoB-depleted serum) was monitored using [3H]cholesterol-loaded J774 macrophages or BHK cells expressing human ABCA1 in women before and after menopause (n = 46). Results are percentages of radiolabel present in the medium after a 4-hour incubation with cells. Lines of best fit were calculated by least squares maximum likelihood linear regression. Pre, premenopause; post, postmenopause.
Positive associations between total HDL-PIMA and macrophage CEC differ by menopausal status
The positive associations between macrophage CEC and total HDL-PIMA (Figure 1) were significantly smaller after (r = 0.44, P = .002) than before menopause (r = 0.53, P = .0002), which was documented by an interaction between the two variables that was independent of race, BMI, and time difference between the two assessments (interaction P value = .028). Other associations between HDL subspecies and CEC measures did not vary by menopausal status.
Changes in S-HDL-PIMA, L-HDL-PIMA and mean size of M-HDL-PIMA, but not changes in HDL-PIMA and CEC, correlate with changes in E2 and FSH levels
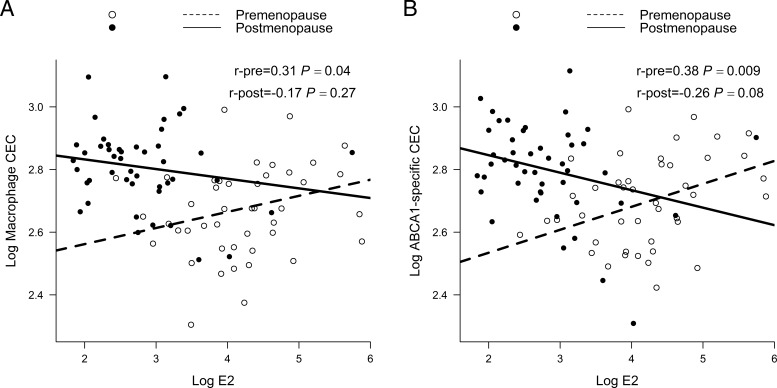
Independent of time difference between the two assessments, race and BMI, a bigger decline in E2 was significantly correlated with a larger increase in S-HDL-PIMA and a larger decline in L-HDL-PIMA, whereas a greater rise in FSH was significantly correlated with a bigger reduction in size of M-HDL-PIMA. Changes in macrophage and ABCA1-specific CEC did not correlate with changes in E2 and FSH, Table 3. Interestingly, both macrophage CEC and ABCA1-specific CEC moderately and positively correlated with E2 levels before menopause (r = 0.31 and 0.38, P = .04 and .009, respectively) but not after menopause, Figure 2.
Table 3.
Spearman Correlations Between the Within-Woman Changes in Sex Hormones and the Within-Woman Changes in HDL Metrics
| E2a |
FSHa |
|||
|---|---|---|---|---|
| Unadjusted | Adjustedb | Unadjusted | Adjustedb | |
| Total HDL-PIMAa | 0.25 | 0.17 | −0.06 | −0.09 |
| S-HDL-PIMAa | −0.29* | −0.32* | 0.01 | 0.06 |
| M-HDL-PIMAa | 0.24 | 0.25 | 0.04 | −0.05 |
| L-HDL-PIMAa | 0.36* | 0.30 | 0.05 | 0.01 |
| Mean size, S-HDL-PIMAa | −0.10 | −0.08 | −0.04 | −0.10 |
| Mean size, M-HDL-PIMAa | −0.06 | −0.05 | −0.21 | −0.31* |
| Mean size, L-HDL-PIMAa | 0.18 | 0.22 | −0.18 | −0.29 |
| ABCA1-specific CECa | 0.17 | 0.22 | −0.02 | −0.03 |
| Macrophage CECa | 0.22 | 0.21 | 0.01 | 0.04 |
P < .05; **P ≤ .01; ***P < .001.
Differences between log-transformed values before and after menopause.
Adjusted for time difference between the two assessments, BMI, race, and cycle day of blood draw at the baseline visit.
Figure 2.
Spearman correlations of macrophage CEC and ABCA1-specific CEC with E2 stratified by menopausal status. Macrophage CEC (A) and ABCA1 CEC (B) of serum HDL (apoB-depleted serum) were quantified as described in the Figure 1 legend. Log transformation was used for better visualization given the highly skewed distribution for E2. Lines of best fit were calculated by least squares maximum likelihood linear regression. Pre, premenopause; post, postmenopause.
Discussion
We found that HDL-PIMA and L-HDL-PIMA increased after menopause, whereas sizes of M-HDL-PIMA and S-HDL-PIMA decreased. Those changes were independent of race, BMI, and time difference between the two assessments. We also found that changes in E2 levels correlated negatively with changes in S-HDL-PIMA and positively with changes in L-HDL-PIMA whereas changes in FSH levels correlated negatively with changes in the mean size of M-HDL-PIMA.
In addition, we showed that macrophage CEC and ABCA1-specific CEC were significantly higher after menopause independent of race, BMI, and time difference between the two assessments. Interestingly, macrophage CEC and ABCA1 CEC correlated with E2 levels before but not after menopause, suggesting a weaker role of endogenous estradiol on CEC after menopause. Although the current study design lacking a premenopausal control group may not allow us to state that the reported changes were ultimately because of menopause, our findings suggest that changes in sex hormones during the menopausal transition could be potential modulators of CEC and the sizes and concentrations of HDL subspecies.
A key issue is whether the increases in HDL-PIMA, L-HDL-PIMA, macrophage CEC, and ABCA1-specific CEC contribute to the increased risk of CVD observed in postmenopausal women. This possibility seems counterintuitive, given that macrophage CEC and ABCA1-specific CEC are impaired in both incident and prevalent CVD (22, 23). Moreover, we have shown that macrophage CEC is reduced in subjects with coronary artery endothelial dysfunction (37). HDL-PIMA is also decreased in subjects with established carotid atherosclerosis (38).Thus, impaired CEC and lower levels of HDL-PIMA could contribute to CVD risk. In contrast, large population studies have shown that subjects with SCARB1 P376L, found to be associated with complete loss of function of SR-BI, have an increased risk of CHD (20). Interestingly, these subjects have marked increases in large HDL particles, apoA-I, and HDL-C.
Studies in genetically engineered animals demonstrate that loss of SCARB1 impairs hepatic uptake of HDL cholesterol, increases levels of HDL-C, and induces a marked accumulation of large HDL particles (39). Importantly, reverse cholesterol transport from macrophages is impaired in these animals (40). In contrast, overexpression of SCARB1 in the liver promotes reverse cholesterol transport from macrophages and enhances the delivery of HDL-derived cholesterol in the feces (40). Reverse cholesterol transport from macrophages is a key step in preventing macrophage cholesterol accumulation, and atherosclerosis development is markedly accelerated in hypercholesterolemic mice that lack SCARB1 (39). In the current study, we also reported increases in HDL-C, apoA-I, and large HDL particles in women after their final menstrual period, a time coincident with CVD risk increase (1, 2). Although we reported increases in CEC after menopause, the increase in macrophage CEC as related to the increase in HDL-PIMA was higher before than after menopause, suggesting that the menopausal transition may impair HDL particles' ability to promote CEC irrespective of the increases in HDL-C and large HDL particles. In future studies, it will be important to determine whether estrogen and other hormones altered by menopause regulate hepatic expression of SCARB1, if this associates with elevated levels of HDL-C and large HDL particles, and if elevated levels of HDL-C and large HDL particles predict increased CVD risk.
In a longitudinal results from the SWAN study, we found a transient increase in HDL-C level around the onset of menopause (3, 7) followed by a reduction 1 year after FMP (3). It is important to note that we studied women relatively early in menopause in this study. Our finding of higher L-HDL-PIMA in this subset of women who were within 1–5 years of their FMP at follow-up agrees with this pattern. Whether the reported increases in total HDL-PIMA continues after menopause and are driven mainly by a parallel increase in L-HDL-PIMA (rather than by increases in other HDL subclasses) are unclear and should be evaluated in future longitudinal studies.
To our knowledge, associations between sex hormones and the concentrations and sizes of HDL particles, as measured by the validated calibrated IMA assay (25), have not been evaluated over menopause before. Irrespective of the methods used to quantify HDL particles, evidence supports relevant associations between sex hormones and HDL metrics. Among 120 participants from the Pittsburgh Healthy Women Study, serum E2 were positively associated with large HDL subclasses, quantified as levels of HDL2-C. Additionally, a decline in E2 between the perimenopause and postmenopause was accompanied by a reduction in HDL2-C (41). Furthermore, we previously assessed cross-sectional associations of nuclear magnetic resonance spectrometry lipoprotein particle concentrations and sizes with endogenous sex hormones in a sample of 120 midlife women from the Pittsburgh site of SWAN (32). A positive correlation between E2 levels and size of HDL-P was independent of age, race, and other potential covariates. The findings of independent correlations between greater rises in FSH, a cardinal marker of menopause, and bigger reduction in size of M-HDL-PIMA along with findings for E2 in the current study are in line with the evidence given previously and suggest an independent impact of menopause and related sex hormonal alterations on HDL metrics.
Only one other clinical study has performed a cross-sectional analysis to evaluate associations between CEC and menopause. The subjects were 29 premenopausal women (age <52 years, E2 >0.15 pmol/ml) and 31 postmenopausal women (age >52 years, E2 <0.15 pmol/ml) (42). That study did not find any significant differences in CEC between the two groups. Additionally, no significant association was found between E2 levels and CEC. However, the cross-sectional nature of the study and its potential misclassification of premenopausal status (which used age and a very low E2 cut rather than bleeding status) may contribute to their negative results. In future studies, it will be important to assess the longitudinal associations of the changes in E2 and CEC over the menopausal transition in larger numbers of subjects.
Strengths of our study include the use of validated assays to assess CEC and HDL particle concentrations in a mixed ethnic group of white and black women and being the first longitudinal available data to comprehensively describe new metrics of HDL during the menopausal transition. Limitations include our modest sample size, the short time between the final menstrual period and evaluation, and the absence of a group of women measured twice but did not change in menopausal status. These aspects of the study limited our ability to assess changes in HDL subclasses and function at later stages of the menopausal transition. We also did not evaluate other proposed antiatherosclerotic properties of HDL (eg, antioxidant capacity, HDL paraoxonase-1). However, no large clinical studies have yet shown that these metrics predict CVD status or risk in humans. Future studies should comprehensively assess how changes in HDL metrics impact risk of CVD in postmenopausal women and whether these alterations could be contributing factors in the unexpected recent findings that higher HDL-C may not be consistently beneficial in postmenopausal women.
In conclusion, we found HDL-PIMA, L-HDL-PIMA, macrophage CEC, and ABCA1-specific CEC increased after menopause. Additionally, changes in E2 and FSH correlated with changes in both size and concentrations of HDL-PIMA independent of time difference between the two assessments, BMI and race, suggesting a potential role of menopause and menopause-related changes in sex hormones on quality metrics of HDL. Because a variation in SCARB1 that increases HDL-C, large HDL particles, and surprisingly CVD risk (20), it will be important to determine the factors in these subjects that associate with CVD risk, and examine whether similar factors are linked to CVD risk in postmenopausal women.
Acknowledgments
The Study of Women's Health Across the Nation (SWAN) has grant support from the NIH, Department of Health and Human Services, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR), and the NIH Office of Research on Women's Health (ORWH) (Grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, the SWAN Repository [U01AG017719]).
This work was supported by grants from the American Heart Association (15MCPRP25350002), the Central Research Development Fund at the University of Pittsburgh, and the Diabetes Research Center, University of Washington (National Institutes of Health [NIH] P30 DK017047). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or the NIH.
Clinical Centers: University of Michigan, Ann Arbor, MI: Siobán Harlow, principal investigator (PI) 2011–present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA: Joel Finkelstein, PI 1999–present, Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL: Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser: Ellen Gold, PI; University of California, Los Angeles: Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY: Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry, New Jersey Medical School, Newark: Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA: Karen Matthews, PI. NIH Program Office: National Institute on Aging, Bethesda, MD: Winifred Rossi, 2012–present; Sherry Sherman, 1994–2012; Marcia Ory, 1994–2001; National Institute of Nursing Research, Bethesda, MD: Program Officers.
Central laboratory: University of Michigan, AnnArbor: Daniel McConnell (Central Ligand Assay Satellite Services). SWAN repository: University of Michigan, Ann Arbor: Siobán Harlow, 2013–present; Dan McConnell, 2011–2013; MaryFran Sowers, 2000–2011.
Coordinating center: University of Pittsburgh, Pittsburgh, PA: Maria Mori Brooks, PI 2012–present; KimSutton-Tyrrell, PI 2001–2012; NewEngland Research Institutes, Watertown, MA: Sonja McKinlay, PI 1995–2001. Steering Committee: Susan Johnson, current chair. Chris Gallagher, former chair. The authors thank the study staff at each site and all the women who participated in SWAN.
Disclosure Summary: M.M.B.: research grant, Gilead Sciences, Inc; T.J.O.: advisory board, Eli Lilly; J.W.H.: named as a coinventor on patents from the US Patent Office on the use of HDL markers to predict the risk of cardiovascular disease: consultant for Merck, Amgen, and Pacific Biomarkers.
Footnotes
- ABCA1
- ATP-binding cassette transporter A1
- apoA-I
- apolipoprotein A-I
- apoB
- apolipoprotein B
- BMI
- body mass index
- CEC
- cholesterol efflux capacity
- CVD
- cardiovascular disease
- E2
- estradiol
- FMP
- final menstrual period
- HDL-C
- high-density lipoprotein cholesterol
- HDL-P
- HDL particle concentration
- HDL-PIMA
- HDL-P concentration via calibrated ion mobility analysis
- HT
- hormone therapy
- IMA
- ion mobility analysis
- LDL-C
- low-density lipoprotein cholesterol
- L-HDL-PIMA
- concentration of large HDL particles
- M-HDL-PIMA
- concentration of medium HDL particles
- SCARB1
- the gene encoding the scavenger receptor class BI (SR-BI)
- S-HDL-PIMA
- concentration of small HDL particles
- SWAN
- Study of Women's Health Across the Nation.
References
- 1. Mozaffarian D, Benjamin EJ, Go AS, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. [DOI] [PubMed] [Google Scholar]
- 2. Kannel WB, Hjortland MC, McNamara PM, Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976;85:447–452. [DOI] [PubMed] [Google Scholar]
- 3. Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54:2366–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Li Z, McNamara JR, Fruchart JC, et al. Effects of gender and menopausal status on plasma lipoprotein subspecies and particle sizes. J Lipid Res. 1996;37:1886–1896. [PubMed] [Google Scholar]
- 5. Stevenson JC, Crook D, Godsland IF. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis. 1993;98:83–90. [DOI] [PubMed] [Google Scholar]
- 6. Anderson KM, Wilson PW, Garrison RJ, Castelli WP. Longitudinal and secular trends in lipoprotein cholesterol measurements in a general population sample. The Framingham Offspring Study. Atherosclerosis. 1987;68:59–66. [DOI] [PubMed] [Google Scholar]
- 7. Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. Lipid changes during the menopause transition in relation to age and weight: the Study of Women's Health Across the Nation. Am J Epidemiol. 2009;169:1352–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fukami K, Koike K, Hirota K, Yoshikawa H, Miyake A. Perimenopausal changes in serum lipids and lipoproteins: a 7-year longitudinal study. Maturitas. 1995;22:193–197. [DOI] [PubMed] [Google Scholar]
- 9. Matthews KA, Wing RR, Kuller LH, Meilahn EN, Plantinga P. Influence of the perimenopause on cardiovascular risk factors and symptoms of middle-aged healthy women. Arch Intern Med. 1994;154:2349–2355. [PubMed] [Google Scholar]
- 10. Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels: the Framingham Study. JAMA. 1986;256:2835–2838. [PubMed] [Google Scholar]
- 11. Bass KM, Newschaffer CJ, Klag MJ, Bush TL. Plasma lipoprotein levels as predictors of cardiovascular death in women. Arch Intern Med. 1993;153:2209–2216. [PubMed] [Google Scholar]
- 12. Fan AZ, Dwyer JH. Sex differences in the relation of HDL cholesterol to progression of carotid intima-media thickness: the Los Angeles Atherosclerosis Study. Atherosclerosis. 2007;195:e191–e196. [DOI] [PubMed] [Google Scholar]
- 13. Keidar S, Bogner I, Gamliel-Lazarovich A, Leiba R, Fuhrman B, Kouperberg E. High plasma high-density lipoprotein levels, very low cardiovascular risk profile, and subclinical carotid atherosclerosis in postmenopausal women. J Clin Lipidol. 2009;3:345–350. [DOI] [PubMed] [Google Scholar]
- 14. Bots ML, Elwood PC, Nikitin Y, et al. Total and HDL cholesterol and risk of stroke. EUROSTROKE: a collaborative study among research centres in Europe. J Epidemiol Community Health. 2002;56:i19–i24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Woodard GA, Brooks MM, Barinas-Mitchell E, Mackey RH, Matthews KA, Sutton-Tyrrell K. Lipids, menopause, and early atherosclerosis in Study of Women's Health Across the Nation Heart women. Menopause. 2011;18:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. El Khoudary SR, Wang L, Brooks MM, Thurston RC, Derby D, Matthews KA. Increases in high density lipoprotein-cholesterol levels are associated with greater intima-media thickness progression over the menopausal transition: the Study of Women's Health Across the Nation (SWAN). Journal of Clinical Lipidology. In press. [Google Scholar]
- 17. Kingwell BA, Chapman MJ, Kontush A, Miller NE. HDL-targeted therapies: progress, failures and future. Nat Rev Drug Discov. 2014;13:445–464. [DOI] [PubMed] [Google Scholar]
- 18. AIM-HIGH Investigators, Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 19. Voight BF, Peloso GM, Orho-Melander M, et al. Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet. 2012;380:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zanoni P, Khetarpal SA, Larach DB, et al. , CHD Exome+ Consortium; CARDIoGRAM Exome Consortium; Global Lipids Genetics Consortium. Rare variant in scavenger receptor BI raises HDL cholesterol and increases risk of coronary heart disease. Science. 2016;351:1166–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH. The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol. 2010;30:796–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rohatgi A, Khera A, Berry JD, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med. 2014;371:2383–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Khera AV, Cuchel M, de la Llera-Moya M, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah AS, Tan L, Long JL, Davidson WS. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J Lipid Res. 2013;54:2575–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hutchins PM, Ronsein GE, Monette JS, et al. Quantification of HDL particle concentration by calibrated ion mobility analysis. Clin Chem. 2014;60:1393–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. [DOI] [PubMed] [Google Scholar]
- 27. Mackey RH, Greenland P, Goff DC, Jr., Lloyd-Jones D, Sibley CT, Mora S. High-density lipoprotein cholesterol and particle concentrations, carotid atherosclerosis, and coronary events: MESA (multi-ethnic study of atherosclerosis). J Am Coll Cardiol. 2012;60:508–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mora S, Otvos JD, Rifai N, Rosenson RS, Buring JE, Ridker PM. Lipoprotein particle profiles by nuclear magnetic resonance compared with standard lipids and apolipoproteins in predicting incident cardiovascular disease in women. Circulation. 2009;119:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Du XM, Kim MJ, Hou L, et al. HDL particle size is a critical determinant of ABCA1-mediated macrophage cellular cholesterol export. Circ Res. 2015;116:1133–1142. [DOI] [PubMed] [Google Scholar]
- 31. El Khoudary SR, Santoro N, Chen HY, et al. Trajectories of estradiol and follicle-stimulating hormone over the menopause transition and early markers of atherosclerosis after menopause. Eur J Prev Cardiol. 2016;23:694–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. El Khoudary SR, Brooks MM, Thurston RC, Matthews KA. Lipoprotein subclasses and endogenous sex hormones in women at midlife. J Lipid Res. 2014;55:1498–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sowers M, Crawford S, Sternfed B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, eds. Menopause: Biology and Pathology. New York, NY: Academic Press; 2000:175–188. [Google Scholar]
- 34. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 35. Guha S, Li M, Tarlov MJ, Zachariah MR. Electrospray-differential mobility analysis of bionanoparticles. Trends Biotechnol. 2012;30:291–300. [DOI] [PubMed] [Google Scholar]
- 36. Bergt C, Pennathur S, Fu X, et al. The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc Natl Acad Sci U S A. 2004;101:13032–13037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Monette JS, Hutchins PM, Ronsein GE, et al. Patients with coronary endothelial dysfunction have impaired cholesterol efflux capacity and reduced HDL particle concentration. Circulation Research. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ronsein GE, Hutchins PM, Isquith D, Vaisar T, Zhao XQ, Heinecke JW. Niacin therapy increases high-density lipoprotein particles and total cholesterol efflux capacity but not ABCA1-specific cholesterol efflux in statin-treated subjects. Arterioscler Thromb Vasc Biol. 2016;36:404–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rigotti A, Trigatti BL, Penman M, Rayburn H, Herz J, Krieger M. A targeted mutation in the murine gene encoding the high density lipoprotein (HDL) receptor scavenger receptor class B type I reveals its key role in HDL metabolism. Proc Natl Acad Sci U S A. 1997;94:12610–12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest. 2005;115:2870–2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kuller LH, Gutai JP, Meilahn E, Matthews KA, Plantinga P. Relationship of endogenous sex steroid hormones to lipids and apoproteins in postmenopausal women. Arteriosclerosis. 1990;10:1058–1066. [DOI] [PubMed] [Google Scholar]
- 42. Badeau RM, Metso J, Kovanen PT, Lee-Rueckert M, Tikkanen MJ, Jauhiainen M. The impact of gender and serum estradiol levels on HDL-mediated reverse cholesterol transport. Eur J Clin Invest. 2013;43:317–323. [DOI] [PubMed] [Google Scholar]