Abstract
Reducing plasma levels of low-density lipoprotein cholesterol (LDL-C) remains the cornerstone in the primary and secondary prevention of cardiovascular disease. However, lack of efficacy and adverse effects mean that a substantial proportion of patients fail to achieve acceptable LDL-C levels with currently available lipid-lowering drugs. Over the last decade, inhibition of proprotein convertase subtilisin/kexin type 9 (PCSK9) has emerged as a promising therapeutic strategy to reduce residual cardiovascular disease risk. Binding of PCSK9 to the LDL receptor targets the receptor for lysosomal degradation. The recognition that inhibition of PCSK9 increases LDL receptor activity has led to the development of a number of approaches to directly target PCSK9. Numerous monoclonal antibodies against PCSK9 are currently being evaluated in phase 3 trials, involving various patient categories on different background lipid-lowering therapies. Current evidence shows reductions in LDL-C levels of up to 70 % may be achieved with PCSK9 inhibition, independent of background statin therapy. This review examines the most recent evidence and future prospects for the use of PCSK9 inhibitors in the prevention of cardiovascular disease.
Keywords: Low-density lipoprotein, Proprotein convertase subtilisin kexin type 9 (PCSK9), Prevention, Monoclonal antibody, Evolocumab, Alirocumab
Introduction
Atherosclerotic cardiovascular disease (CVD) is the leading cause of mortality worldwide [1]. Atherosclerosis occurs as a consequence of metabolic and inflammatory changes to the arterial wall, which promote the macrophage-mediated intimal deposition of pro-atherogenic low-density lipoprotein cholesterol (LDL-C), contributing to plaque formation, limiting blood flow to vital organs and increasing the risk of atherothrombotic and atheroembolic sequelae. Dyslipidaemia has become an important risk factor to target in both the primary and secondary prevention of CVD. With the advent of statins, which inhibit 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, sustained reductions in LDL-cholesterol have become achievable. Large-scale clinical trials demonstrate that a 40 mg dL−1 (1 mmol L−1) decrease in LDL-C results in a 22 % reduction in adverse cardiovascular events [2, 3]. The overwhelming evidence of the clinical efficacy and cost-effectiveness of statins has led to their establishment as the first-line treatment of dyslipidaemia [4]. However, despite optimal statin therapy, less than half of recurrent cardiovascular events can be prevented. Indeed, satisfactory control of dyslipidaemia is not achieved in certain patients, even with combination lipid-lowering therapy.
Impelled by the need for additional lipid management strategies, recent attention has focused on a new class of agent, proprotein convertase subtilisin/kexin type 9 (PSCK9) inhibitors. These demonstrate much promise, particularly for those unable to take statins, e.g. due to adverse effects or drug–drug interactions [5]. The discovery of PCSK9-based therapies began in 2003, with an astute clinical observation of a French family, which demonstrated features of familial hypercholesterolemia (FH), without mutations in the genes contemporaneously recognised to cause FH; the LDL receptor gene (LDLR, accounting for 95 % of FH defects), or apolipoprotein B gene, encoding the protein that binds to the LDLR (ApoB, accounting for 4 % of FH defects) [6, 7]. These findings led to the identification of two novel missense mutations that increased the activity of a serine protease enzyme, originally called neural apoptosis-regulated convertase 1 (NARC-1) and subsequently renamed proprotein convertase subtilisin/kexin type 9 [8]. This discovery has led to novel therapeutic options in lipid management [9, 10]. The goals of this review are to explore the mechanism of action of PCSK9 inhibitors and their potential to improve cardiovascular outcomes.
The structure and function of PCSK9
Synthesis and structure
PCSK9, found at chromosome 1p32, is 22 kb in length, with 12 exons that encode a 692-amino acid protein [11]. It is a proteinase K-like enzyme, belongs to the secretory subtilase family and is primarily synthesised and secreted by hepatocytes [12, 13]. The synthesis of PCSK9 is up-regulated by sterol-regulatory-element-binding protein-2 (SREBP-2), a transcription factor that regulates PCSK9 expression by binding to the sterol-regulatory element in the promoter region of the gene [14]. SREBP-2 also increases LDL receptor and cholesterol synthesis, via the activation of genes encoding key enzymes involved in cholesterol homeostasis, including HMG-CoA reductase [15]. It is activated by low intracellular cholesterol concentrations. SREBP-2 and PCSK9 expression is suppressed in fasting mice fed a cholesterol-rich diet [16]. Prolonged fasting in animals and humans, however, also causes a decrease in PCSK9 and SREBP-2 activity [17]. In addition, in vivo evidence suggests a possible role for insulin in increasing the expression of PCSK9 [18].
The PCSK protein product is comprised of a N-terminal signal peptide, prodomain, catalytic domain, hinge region, and cysteine-rich C-terminal domain [13, 19]. Following the removal of the signal peptide domain, PCSK9 is synthesised as a ~74 kDa zymogen, which undergoes autocatalytic cleavage in the endoplasmic reticulum and Golgi body, to generate a pro-domain fragment and ~62 kDa mature protein, which remain strongly associated to one another [20–22].
LDL receptor cycling
The first 8 members of the PCSK family, PCSK 1–8, are serine proteases involved in the processing of inactive precursor proteins to generate functional and bioactive peptides, polypeptides and hormones, which play important roles in regulating growth and metabolism [23–25]. In contrast, PCSK9 plays a crucial role in the regulation of LDL receptor recycling [26]. The PCSK9 complex binds to the epidermal growth factor A (EGF-A) domain of the LDL receptor, leading to the lysosomal degradation of the latter and reduced clearance of circulating LDL-C. Extrahepatic actions of PCSK9 include enhancement of chylomicron secretion and regulation of enterocyte cholesterol balance [13]. Moreover, data from experimental models suggest that the role of PCSK9 extends beyond lipid homeostasis; it is implicated as a regulator of glucose metabolism, liver regeneration and susceptibility to hepatitis C virus infection [27–30].
In mouse models, the accumulation of cholesteryl esters in aortic atherosclerotic lesions was markedly reduced by PCSK9 inactivation [31]. Conversely, overexpression of PCSK9 induced an excess burden of atherosclerosis. In LDLR-deficient mice, knockdown or overexpression of PCSK9 had no significant effects on cholesteryl ester accumulation or atheromatous plaque size. This study strongly suggested that the process by which PCSK9 enhances atherosclerosis is primarily mediated by its action on the LDLR [31]. Figure 1 displays normal, physiological LDLR recycling.
Fig. 1.
Normal, physiological LDLR recycling. LDL low density lipoprotein, LDL-C low-density lipoprotein cholesterol, LDLR low density lipoprotein receptor
In humans studies, PCSK9 loss-of-function mutations have been associated with reductions in LDL-C and cardiovascular events [32]. Conversely, those with high levels of PCSK9 have higher level of plasma LDL-C and significantly increased lifetime CVD risk [32]. Gain-of-function mutations on PCSK9 are associated with a severe form of autosomal dominant hypercholesterolemia, phenotypically indistinguishable from FH due to LDL-receptor mutations [32].
Regulation
PCSK9 concentrations demonstrate a diurnal rhythm synchronous to cholesterol synthesis, with changes of ±15 % from the mean value [33]. PCSK9 synthesis also induced by insulin and repressed by glucagon in rodents [18]. In healthy humans, PCSK9 levels are demonstrably reduced with fasting (decreasing 60 % over 36 h), and increase in the post-prandial period, suggesting a similar effect [33–35]. In addition, PCSK9 is positively controlled by the oxysterol-activated liver X receptor (LXR) [18, 36].
PCSK9 circulates in plasma in three main forms [37]. When secreted, PCSK9 exists as a monomer, but can self-associate into di- and trimeric complexes, facilitated by the catalytic domain. It is present in free and protein-bound forms in human plasma, with 40 % of circulating PCSK9 exclusively associated with LDL [16]. LDL-bound PCSK9 has diminished LDL receptor-binding activity. It has been proposed that this is a regulatory mechanism, by which higher plasma concentrations of LDL results in a greater proportion of LDL-bound PCSK9, thereby inhibiting PCSK9-mediated degradation of the LDL receptor [16]. In vitro evidence suggests that self-associated di-/trimers have enhanced LDL receptor-binding and degrading activity, compared with the monomer form [38]. PCSK9 also circulates as a 55 kDa furin-cleaved inactive fragment, resulting from the cleavage of the 62 kDa protein: mutations in the mature PCSK9 protein have been associated with increased or decreased susceptibility to furin cleavage, leading PCSK9 loss-of-function and gain-of-function phenotypes [22].
Mechanism of action
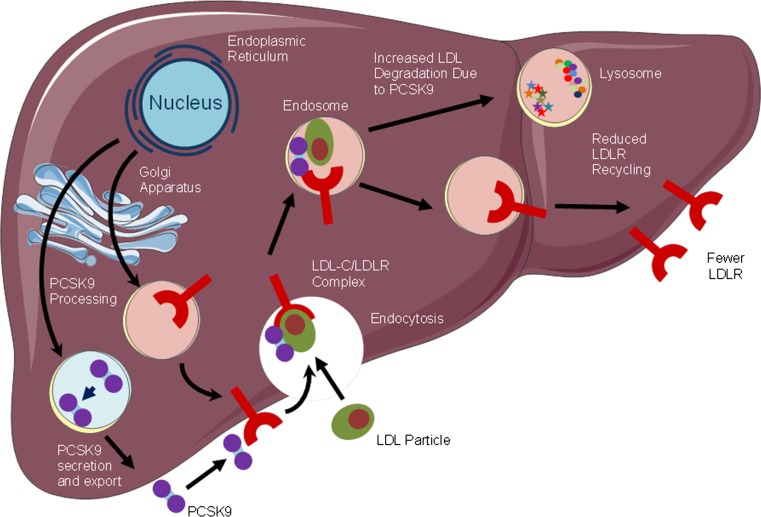
PCSK9 acts primarily as a soluble protein, targeting degradation of the membrane-bound LDLR by extracellular binding via rerouting to the lysosomal pathway [39]. At the molecular level, PCSK9 blocks the LDLR in an extended (open) conformation. This is achieved when the catalytic domain of PCSK9 (aa153–421) and the EGF-A domain of LDLR (aa314–355) bind [40]. This failure of the receptor to adopt a closed conformation results in a slowed recycling to the plasma membrane and subsequent degradation. LDL-receptors—like PCSK9—are particularly abundant in the liver, the primary organ responsible for clearance of plasma LDL. As the number of LDL-receptors on the surface of liver cells determines the rate of LDL removal from the bloodstream, PCSK9 presented an appealing target to beneficially modulate lipid homeostasis. Figure 2 illustrates the mechanism of action of PCSK9.
Fig. 2.
Mechanism of action of PCSK9. LDL low density lipoprotein, LDL-C low-density lipoprotein cholesterol, LDLR low density lipoprotein receptor, PCSK9 proprotein convertase subtilisin/kexin type 9
Impelled by promising pre-clinical evidence, the clinical development of therapeutic inhibitors of PCSK9 has progressed rapidly, with promising results reported from phase 2 and 3 clinical studies, in statin-intolerant and familial hypercholesterolemia patients, with sub-optimal LDL-C levels.
PCSK9 inhibitors
Inhibition strategies
Several strategies have been proposed for targeting PCSK9. Messenger RNA (mRNA) knockdown approaches, which include the use of PCSK9 antisense oligonucleotides, have been evaluated in animal models. Antisense oligonucleotides administered to mice reduced PCSK9 expression by >90 % and lowered plasma cholesterol levels by 53 % [41, 42]. A single intravenous injection of PCSK9 RNA interference (RNAi) delivered in lipidoid nanoparticles to cynomolgus monkeys reduced plasma PCSK9 and LDL-C levels (by 70 and 56 %, respectively) [43]. However, the use of monoclonal antibodies (mAb), which interfere with the interaction of the PCSK9 catalytic domain and LDLR, is particularly promising [44]. In nonhuman primates, intravenous infusion of mAb1 (3 mg kg−1), which is specific for the catalytic domain of PCSK9, resulted in marked (80 %) reduction in plasma LDL-C [45].
PCSK inhibition may yield non-LDL-lowering, pleiotropic effects. High levels of lipoprotein(a) are an independent predictor of cardiovascular mortality, even in statin-treated patients with low LDL-C [46]. PCSK9 inhibitors reduce lipoprotein(a) by approximately 30 %. Such an effect is not observed with statin- or ezetimibe-mediated upregulation of LDL receptor activity (as lipoprotein(a) is not cleared by LDLR-dependent mechanisms, and is mainly regulated by hepatic secretion) [47]. Thus, PCSK9 inhibition as a therapeutic strategy has theoretical advantages beyond LDL-C lowering, raising the possibility that cardiovascular outcomes may be additionally favourable. Figure 3 displays the mechanism of action of PCSK9 mAb, in the presence of a statin.
Fig. 3.
Mechanism of action of PCSK9 mAb in presence of a statin. LDL low density lipoprotein, LDL-C low-density lipoprotein cholesterol, LDLR low density lipoprotein receptor, PCSK9 proprotein convertase subtilisin/kexin type 9
In clinical studies, three monoclonal antibodies have demonstrated significant promise: evolocumab (AMG-145), alirocumab (SAR236553/REGN727) and bococizumab; the latter of which is in the early stages of development. Table 1 lists PCSK9 inhibitors in development.
Table 1.
| PCSK9 Inhibitors undergoing preclinical and clinical evaluation
| Pharmaceutical company | Drug class | Agent | Phase |
|---|---|---|---|
| Sanofi/Regeneron | Human mAb | Alicocumab (SAR236553/REGN727) | 3 |
| Amgen | Human mAb | Evolocumab (AMG 145) | 3 |
| Pfizer/Rinat | mAb | Bococizumab (RN316) | 3 |
| Novartis | mAb | LGT-209 | 2 |
| Roche/Genetech | mAb | RG7652 | 2 |
| Alnylam Pharmaceuticals/The Medicines Company | siRNA oligonucleotide | ALN-PCS02 | 1 |
| Bristol-Myers Squibb/Adnexus | Monobody | BMS-962476 | 1 |
| Idera Pharmaceuticals | Antisense Oligonucleotide | TBD | PC |
| Merck | mAb | 1D05-IgG2 | PC |
| Schering-Plough | Mimetic peptides | LDL EGF-AB peptide fragment | PC |
mAb monoclonal antibody, PC pre-clinical, siRNA small interfering ribonucleic acid
Evolocumab
Evolocumab in primary hypercholesterolemia
Evolocumab is a fully human monoclonal antibody inhibitor of PCSK9. In the Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL-C in Patients Currently Not Receiving Drug Therapy for Easing Lipid Levels (MENDEL) trial, 406 patients with hypercholesterolaemia and statin intolerance were randomly assigned to evolocumab 70, 105 and 140 mg every 2 weeks; evolocumab 280, 350 and 420 mg every 4 weeks; placebo every 2 weeks or every 4 weeks, or ezetimibe once-daily. Evolocumab reduced LDL-C concentrations in all dose groups, with the maximal effect for the regimen of 140 mg every 2 weeks (~51 %) and no reported treatment-related adverse events [48].
MENDEL-2 evaluated the efficacy, safety and tolerability of evolocumab compared with placebo and oral ezetimibe in 614 patients with hypercholesterolemia (LDL-C 100–190 mg dL−1 or 2.6–4.9 mmol L−1) [49]. Patients 18–80 years of age with Framingham risk scores ≥10 % were randomised to one of six groups; (i) oral placebo and sub-cutaneous (SC) placebo fortnightly; (ii) oral placebo and SC placebo monthly; (iii) ezetimibe and SC placebo fortnightly; (iv) ezetimibe and SC placebo monthly; (v) oral placebo and evolocumab 140 mg fortnightly; or (vi) oral placebo and evolocumab 420 mg monthly. Evolocumab treatment produced greatest reductions in LDL-C from baseline, by 55–57 % more than placebo and 38–40 % more than ezetimibe (both p < 0.001).
In the LDL-C Assessment With PCSK9 monoclonal Antibody Inhibition Combined With Statin therapy (LAPLACE TIMI-57), 631 patients with hypercholesterolemia on statins were randomised to different regimens of evolocumab, with varying dosages and intervals of administration: 70 mg, 105 mg, and 140 mg or matching placebo every 2 weeks; or 280 mg, 350 mg, and 420 mg or matching placebo every 4 weeks [50]. At week 12, the mean LDL-C concentration reduction was dose-dependent, ranging from 41.8 to 66.1 % every 2 weeks, and from 41.8 to 50.3 % every 4 weeks [51]. The LAPLACE-2 trial assessed the response to addition of evolocumab (140 mg every 2 weeks or 420 mg monthly) vs. placebo, to moderate- or high-intensity statin therapy in 1896 patients with hyperlipidaemia [52]. The trial observed that evolocumab reduced plasma LDL-C concentrations by 66–75 %, vs. placebo, at the mean of weeks 10 and 12. Evolocumab added to statin therapy resulted in additional LDL-C lowering.
More recently, the Durable Effect of PCSK9 Antibody CompARed wiTh placEbo Study (DESCARTES) placed patients into one of four background lipid-lowering strategies (dietary changes alone, dietary changes plus atorvastatin 10 mg, dietary changes plus atorvastatin 80 mg, and dietary changes plus atorvastatin 80 mg and ezetimibe 10 mg) based on their LDL-C levels and cardiovascular risk [53]. Individuals with LDL-C ≥75 mg/dl were randomised to receive monthly SC evolocumab 420 mg or placebo. The mean reduction in LDL-C from baseline in the evolocumab group was 57.0 ± 2.1 % (p < 0.001 vs. placebo). The mean reduction was 55.7 ± 4.2 % among patients who underwent dietary changes alone, 61.6 ± 2.6 % among those who received 10 mg of atorvastatin, 56.8 ± 5.3 % among those who received 80 mg of atorvastatin, and 48.5 ± 5.2 % among those who received a combination of 80 mg of atorvastatin and 10 mg of ezetimibe (p < 0.001 for all comparisons). Evolocumab treatment also significantly reduced levels of apolipoprotein B, lipoprotein(a) and triglycerides.
Evolocumab in familial hypercholesterolemia
In the Reduction of LDL-C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) trial, 167 patients with heterozygous FH (HeFH) and poorly-controlled LDL-C (≥2.6 mmol L−1 or 100 mg dL−1) despite maximally-tolerated statin therapy, were randomised 1:1:1 to receive evolocumab 350 mg, 420 mg or matched placebo, every four weeks. A substantial reduction in LDL-C was observed (43 % for 350 mg vs. 55 % for 420 mg) in addition to that due to high-intensity statin therapy [54]. RUTHERFORD-2 subsequently evaluated evolocumab in combination with other lipid-lowering therapies in patients with HeFH [55]. In total, 331 HeFH patients unable to achieve target LDL-C (defined as per RUTHERFORD) despite maximally-tolerated statin alone, or in combination with ezetimibe, were randomised 2:1 to receive evolocumab 140 mg every 2 weeks, evolocumab 420 mg monthly, or matched placebo, for 12 weeks. Based on the Simon Broome criteria, 80 % of participants had definite FH; 20 % had probable FH. All patients received a statin; two-thirds received ezetimibe. Both schedules demonstrated significant reduction in mean LDL-C at week 12 (59.2 % for 140 mg every 2 weeks vs. 61.3 % for 420 mg monthly; both p < 0.0001).
Classically, homozygous FH (HoFH) patients were thought to have dual null mutations, conferring no LDL receptor activity, and thus would not be expected to respond to PCSK9 inhibition (which is LDL receptor-dependent). Indeed, a small proportion of FH patients are true genetic homozygotes, with identical null or loss-of-function mutations in both alleles of the affected gene. However, advanced genetic profiling has demonstrated that most patients with homozygous loss-of-function mutations are actually compound heterozygotes, with different receptor mutations. As such, HoFH patients may be phenotypically stratified using fibroblast culture; those with <2 % of LDL uptake are receptor negative; those with 2–25 % are receptor defective, compared to wild-type controls [56]. Thus, patients with HoFH may still have a degree of functional LDL receptor activity, which is associated with severity of LDL cholesterol elevation, and may be modulated via PCSK9 inhibition. Indeed, in the recent Trial Evaluating PCSK9 Antibody in Subjects With LDL Receptor Abnormalities (TESLA) Part B study, 50 patients with HoFH, on stable lipid-lowering therapy and not on lipoprotein apheresis, received evolocumab 420 mg monthly, in addition to statin therapy and other lipid-lowering medications [57]. Indeed, TESLA demonstrated that in the Evolocumab-treated HoFH patients, LDL-C was reduced by 31 % from baseline at week 12 compared with placebo (p < 0.0001); no serious adverse side effects were noted.
Evolocumab in statin-intolerant patients
With infrequent reports of adverse effects, PCSK9 inhibitors have been heralded as a potentially effective alternative treatment option for those who are statin-intolerant. Muscle-related side effects (MRSE) are the commonest reason given for discontinuation of statins. Worldwide, the incidence of myopathy is 1.5–5 % of statin-treated patients, although this is highly-dependent on the definition used [58]. One study found that mild-to-moderate muscular symptoms occurred more frequently in patients treated with high-dose statins in clinical practice (in 10.5 %), compared to randomised trials [59]. However, another reported that most patients discontinuing statins due to MRSE that are re-challenged demonstrate good tolerance long-term [60]. Despite the uncertainties regarding the true incidence of MRSE, there is a clear clinical need for alternative therapies in patients at high cardiovascular risk, with more severe degrees of myotoxicity [58].
The Goal Achievement After Utilizing an Anti-PCSK9 Antibody in Statin Intolerant Subjects (GAUSS) study aimed to establish whether there was an advantage to evolocumab over ezetimibe in this context [61]. In the GAUSS trial, 160 patients with statin intolerance were randomised to 5 groups: evolocumab alone at 280, 350, 420 mg, evolocumab at 420 mg with 10 mg ezetimibe once-daily, or 10 mg ezetimibe plus placebo once-daily. Statin intolerance was defined as the inability to tolerate at least one statin at any dose, or an increase in dose, because of intolerable myalgia (muscle pain, soreness, weakness, or cramps) or myopathy (myalgia plus elevated creatine kinase) and having symptom improvement or resolution with statin discontinuation. The administration of evolocumab was significantly associated with a reduction in LDL-C levels, ranging from 40 to 65 %, with good tolerability; myalgia was reported in: 7.4 % receiving evolocumab alone, 20 % receiving the evolocumab and ezetimibe combination, and 3.1 % receiving ezetimibe and placebo [61]. GAUSS-2 assessed statin-intolerant hyperlipidaemic patients [62, 63]. Intolerance was defined as inability to tolerate any dose, or increase the dose above the smallest tablet strength, because of intolerable muscle-related side effects. Evolocumab (140 mg every 2 weeks or 420 mg monthly) reduced LDL-C from baseline by 53 and 56 % respectively, when compared with ezetimibe (37–39 % reduction from baseline; p < 0.001). MRSE occurred in 12 % of evolocumab-treated patients vs. 23 % of ezetimibe-treated patients.
Evolocumab and cardiovascular outcomes
In the OSLER (Open Label Study of Long Term Evaluation Against LDL-C) trial, 4465 patients were randomised to receive either evolocumab 420 mg monthly, or 140 mg every two weeks, and followed up for a median of 11.1 months. The results demonstrated that evolocumab reduced the plasma concentration of LDL cholesterol by 61 %, from a median of 120 mg dL−1 (3.1 mmol L−1) to 48 mg dL−1 (1.2 mmol L−1; p < 0.001). The rate of a composite cardiovascular endpoint (defined as death, acute coronary syndrome, heart failure, stroke or a transient ischaemic attack) at 1 year was reduced from 2.18 % in the standard-therapy group to 0.95 % in the evolocumab group [Hazard Ratio (HR) 0.47; 95 % confidence interval (95 % CI) 0.28–0.78; p = 0.003). A large proportion of these patients were receiving statin therapy at baseline (69.7 % of evolocumab-treated patients vs. 70.9 % of those receiving placebo), though no conclusions are drawn regarding the efficacy of evolocumab over and above statin therapy. Table 2 displays phase 2 studies evaluating evolocumab.
Table 2.
Completed phase II trials of evolocumab
| Author, trial name (reference) | Year | Comparator | Study group | n | Evolocumab dose(s) | Percentage change vs. placebo group | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LCL-C | HDL-C | Non-HDL | TG | ApoB | Lp(a) | ||||||
| Giugliano et al., LAPLACE-TIMI 57 [50] | 2012 | Statin ± ezetimibe | LDL-C > 85 mg mL−1 on-treatment | 236 | 70 mg, 105 mg, 140 mg two-weekly | −41.8 to −66.1 | 6.6 to 8.1 | −38.4 to −61.4 | −18.1 to −33.7 | −34.7 to −56.4 | NA |
| 238 | 280 mg, 350 mg, 420 mg four-weekly | −41.8 to −50.3 | 1.6 to 5.5 | −37.8 to −47.6 | −13.4 to −19.4 | −34.4 to −42.0 | NA | ||||
| Koren et al., MENDEL [48] | 2012 | Placebo only | 100 ≤ LDL-C < 189 mg dL−1 | 135 | 70 mg, 105 mg, 140 mg two-weekly | −37.3 to −47.2 | 4.2 to 10.2 | −35.1 to −45.2 | −7.4 to −12.0 | −32.3 to −44.2 | −11.1 to −29.3 |
| 136 | 280 mg, 350 mg, 420 mg four-weekly | −43.6 to −52.5 | 3.3 to −5.8 | −37.7 to −47.1 | −1.7 to −5.3 | −33.2 to −42.5 | −21.6 to −29.2 | ||||
| Raal et al., RUTHERFORD [54] | 2012 | Statin ± ezetimibe | HeFH; LDL-C ≥ 100 mg dL−1 on-treatment | 111 | 350 mg, 420 mg four-weekly | −43.8 to −55.2 | 6.8 to 7.8 | −41.8 to −53.5 | −15.0 to −19.9 | −34.8 to −46.2 | −23.1 to 31.5 |
| Sullivan et al., GAUSS [61] | 2012 | Statin, ezetimibe or other agent | Statin intolerance, LDL-C ≥ 100 mg dL−1 | 95 | 280 mg to 420 mg four-weekly | −26.0 to −35.9 | 6.6 to 8.5 | 24.8 to −33.6 | −8.7 to −13.8 | −21.4 to −29.9 | −12.4 −18.0 |
| 30 | 420 mg every four-weekly | −47.3 | 13.1 | −44.8 | −4.0 | −36.9 | −21.2 | ||||
| Hirayama et al., YUKAWA-1 [85] | 2014 | Statin ± ezetimibe | High CVD risk, LDL-C ≥ 116 mg dL−1 | 101 | 70 mg to 140 mg two-weekly | −52.9 to −68.9 | 4.4 to 9.1 | −49.5 to −62.6 | −14.3 to −16.6 | −46.8 to −60.7 | −41.5 to 50.6 |
| 104 | 280 mg to 420 mg four-weekly | −58.2 to −63.9 | 13.2 to 16.3 | −53.5 to −58.1 | −17.1 to −20.2 | −47.4 to −53.4 | −32.3 to −39.6 | ||||
Values in table represent percentage (%) change in lipid parameters
ApoB apolipoprotein B, HDL-C high-density lipoprotein cholesterol, HeFH heterozygous familial hypercholesterolaemia, LDL-C low-density lipoprotein cholesterol, Lp(a) lipoprotein (a), mg milligram, n number, NA not available, TG triglycerides
See main text for full explanation of trial abbreviations. To convert stated LDL-C values from mg dL−1 to mmol L−1 divide presented value by 38.67
Alirocumab
Effect of alirocumab in primary hypercholesterolemia
Alirocumab is a fully human monoclonal antibody to PCSK9. Phase II trials demonstrated that as monotherapy, alirocumab can reduce LDL-C as much as intensive statin treatment [64]. The phase III, double-blind, double-dummy ODYSSEY-MONO trial evaluated the safety and efficacy of alirocumab as monotherapy in comparison with ezetimibe, over 24 weeks in patients with primary hypercholesterolemia and moderate cardiovascular risk, not otherwise receiving statins or other lipid-lowering therapy [65]. A total of 103 patients with LDL-C 2.6–4.9 mmol L−1 (100–190 mg dL−1), and 1–5 % 10-year risk of fatal cardiovascular events (estimated via the Systematic COronary Risk Evaluation [SCORE] tool) were randomised to receive either ezetimibe 10 mg or alirocumab, with the aim to achieve target HDL-C using the minimum effective dose of anti-PCSK9 antibody. Alirocumab was initially self-administered at a dose of 75 mg every 2 weeks, and up-titrated to 150 mg if LDL-C at week 8 was >1.8 mmol L−1 (70 mg dL−1). Mean LDL-C reductions of 47 % with alirocumab vs. 16 % with ezetimibe were observed (intention-to-treat analysis; p < 0.0001; 54 vs. 17 %, on-treatment analysis; p < 0.0001). Prior to up-titration, alirocumab 75 mg every 2 weeks reduced LDL-C by 53 %, indicating low-dose alirocumab is sufficient to provide 50 % LDL-C reduction in the majority of patients.
The ODYSSEY-COMBO trials evaluated the efficacy of alirocumab in addition to maximally-tolerated daily statin therapy vs. ezetimibe, in patients with hypercholesterolemia and additional CVD risk factors [66, 67]. In ODYSSEY-COMBO II, alirocumab lowered LDL-C levels significantly more than ezetimibe, at both week 24 (50.6 vs. 20.7 % respectively; p < 0.0001) and 52 (49.5 vs. 18.3 % respectively; p < 0.001). In addition, more alirocumab-treated than ezetimibe-treated patients achieved target LDL-C levels (≤1.8 mmol L−1, ≤70 mg dL−1) by week 24 (77 vs. 45.6 %; p < 0.0001). The ODYSSEY-OPTIONS studies demonstrated that the addition of alirocumab to statin regimens produced significantly greater LDL-C reductions than the addition of ezetimibe, doubling of statin dose, or switch to high-potency agent such as rosuvastatin [68, 69].
Alirocumab in familial hypercholesterolemia
The ODYSSEY programme (comprising ODYSSEY-FH I, II, HIGH FH and LONG-TERM trials) assessed the efficacy and safety of alirocumab in HeFH subjects with LDL-C treatment targets dependent on cardiovascular risk status: <2.6 mmol L−1 (<100 mg dL−1) in patients without documented CVD and <1.8 mmol L−1 (<70 mg dL−1) in patients with prior CVD [70–72]. Alirocumab administered at 75 or 150 mg every 2 weeks reduced LDL-C by 48.8 % in FH I and 48.7 % in FH II, respectively, from baseline to week 24, compared with an increase in the placebo arms (9.1 % in FH I; 2.8 % in FH II, respectively; p < 0.0001 for all comparisons). By week 24, more alirocumab-treated patients reached LDL-C treatment goals vs. placebo-treated patients (72.2 vs. 2.4 % in FH I, and 81.4 vs. 11.3 % in FH II; both p < 0.0001). The mean achieved LDL-C levels in alirocumab-treated patients were 1.92 mmol L−1 (74.3 mg dL−1; FH I) and 1.70 mmol L−1 (65.9 mg dL−1; FH II) at week 52. ODYSSEY LONG-TERM assessed the long-term safety and tolerability of alirocumab in 2341 patients with either: (i) HeFH, with or without manifestations of CVD, or (ii) primary hypercholesterolemia and coronary artery disease, with LDL-C inadequately controlled despite maximally-tolerated lipid-modifying pharmacotherapy (44 % received a high-intensity statin at recruitment) [73]. At 24 weeks, mean LDL-C was reduced from baseline by 61 % for alirocumab-treated patients, and increased by 0.8 % for patients treated with placebo (p < 0.0001). LDL-C goals of ≥50 % reduction from baseline were attained in alirocumab-treated patients vs. placebo (76 % vs. 2 % p < 0.0001 for LDL-C <2.6 mmol L−1 or <100 mg dL−1 and 81 vs. 9 %; p < 0.0001 for LDL-C < 1.8 mmol L−1 or < 70 mg dL−1). Most recently, these results have been reported to be maintained up to 78 weeks of treatment, with good tolerance [74]. Table 3 displays phase 2 studies evaluating alirocumab.
Table 3.
Completed phase II trials of alirocumab
| Author (reference) | Year | Comparator | Study group | n | Evolocumab dose(s) | Percentage change vs. placebo group | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LCL-C | HDL-C | Non-HDL | TG | ApoB | Lp(a) | ||||||
| McKenney et al. [78] | 2012 | Atorvastatin 10, 20 or 40 mg | LCL-C ≥ 100 mg dL−1 on-treatment | 92 | 50 mg, 100 mg, 150 mg two-weekly | −34.5 to −67.3 | 5.1 to 7.7 | −31.4 to −60.3 | −15.2 to −28.6 | −29.5 to −58.3 | −13.3 to −28.6 |
| 200 mg, 300 mg four-weekly | −38.1 to −42.6 | 7.3 to 9.5 | −35.2 to −38.5 | −18.1 to −20.5 | −30.9 to −35.3 | −7.9 to −16.7 | |||||
| Roth et al. [77] | 2012 | Atorvastatin 10 or 80 mg | LCL-C ≥ 100 mg dL−1 on-treatment | 60 | 150 mg two-weekly | −48.9 | 6.2 | −36.0 | 7.9 | −42.4 | −32.0 |
| 150 mg four-weekly | −55.9 | 9.4 | −41.6 | −12.8 | −46.0 | −28.2 | |||||
| Stein et al. [64] | 2012 | Statin ± ezetimibe | HeFH; LCL-C ≥ 100 mg dL−1 on-treatment | 31 | 150 mg two-weekly | −18.2 to −31.9 | 4.3 to 7.8 | −15.5 to −27.6 | −6.2 to 5.6 | −14.5 to −22.0 | −3.54 to −11.4 |
| 150 mg, 200 mg, 300 mg four-weekly | −57.3 | 10.1 | −46.6 | −5.7 | −43.8 | −19.47 | |||||
Values in table represent percentage (%) change in lipid parameters
ApoB apolipoprotein B, HDL-C high-density lipoprotein cholesterol, HeFH heterozygous familial hypercholesterolaemia, LDL-C low-density lipoprotein cholesterol, Lp(a) lipoprotein (a), mg milligram, n number, NA not available, TG triglycerides. See main text for full explanation of trial abbreviations
To convert stated LDL-C values from mg dL−1 to mmol L−1 divide presented value by 38.67
The safety of PCSK9 inhibition
So far, the clinical experience with monoclonal antibodies directed toward PCSK9 suggests that they are safe and well-tolerated, with no major safety issues and no evidence of serious drug-related adverse events [75]. The most common adverse events were nasopharyngitis, upper respiratory tract infections, influenza-like symptoms and back pain; injection site reactions were infrequent (<2 and <4 % of alirocumab and ezetimibe-treated patients, respectively) [76]. Isolated reports of adverse effects include: generalised pruritus after the first dose of alirocumab [64], delayed hypersensitivity-type reaction with rash, 12 days following the second injection of alirocumab [77], and a case of cutaneous leucocytoclastic vasculitis reported 9 days after initiation of alirocumab [78]. All of these patients responded well to withdrawal of the trial drug. Regarding completed phase III trials, in GAUSS-2, MRSE occurred in 12 % of evolocumab-treated, and 23 % of ezetimibe-treated patients [62]; in LAPLACE-2, adverse events were reported in 36, 40, and 39 % of evolocumab-, ezetimibe- and placebo-treated patients, respectively [52]. None of the evolocumab-treated patients developed serious adverse reactions. However, elevations in creatine kinase (CK) of 3–10 times the upper limit of normal have been reported in a total of 12 study drug-treated, and 4 placebo-treated patients. No deaths due to serious adverse events have been reported in PCSK9 clinical trials to date. Table 4 displays selected phase 3 studies of anti-PCSK9 mAbs.
Table 4.
Selected phase III clinical trials evaluating alirocumab and evolocumab
| Author, trial name (reference) | Year | n | Agent | Population and study design | FU (w) | Percentage change vs. placebo group | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LDL-C | ApoB | Non-HDL-C | TG | HDL-C | Lp(a) | ||||||
| Farnier et al., ODYSSEY MONO [65] | 2014 | 103 | Alirocumab | Patients with hypercholesterolemia on no statins vs. ezetimibe | 24 | −31.6 | −25.8 | −25.5 | −1.2 | 4.4 | −4.4 |
| Kereiakes et al., ODYSSEY COMBO I [66] | 2015 | 311 | Alirocumab | Patients with hypercholesterolemia not adequately controlled and high CVD risk | 24 | −45.9 | −35.8 | −37.5 | −0.6 | 7.3 | −14.6 |
| Colhoun et al., ODYSSEY COMBO II [67] | 2015 | 707 | Alirocumab | Patients with hypercholesterolemia not adequately controlled and high CVD risk | 24 | −29.7 | −22.4 | −22.9 | −0.3 | 8.1 | −21.7 |
| Robinson et al., ODYSSEY LONG TERM [73] | 2015 | 2341 | Alirocumab | Patients with hypercholesterolemia not adequately controlled and high CVD risk | 24 | −61.9 | −54.0 | −52.3 | −17.3 | 4.6 | −25.6 |
| Blom et al., DESCARTES [53] | 2014 | 901 | Evolocumab | Patients with hyperlipidaemia had four-weekly 420 mg evolocumab in addition to diet alone, diet and atorvastatin or to diet plus atorvastatin plus ezetimibe | 52 | −57.0 | −44.2 | −50.3 | −11.5 | 5.4 | −22.4 |
| Robinson et al., LAPLACE-2 [52] | 2014 | 2067 | Evolocumab | Patients with hyperlipidaemia had either 140 mg fortnightly or 420 mg every 4 weeks evolocumab added to statin therapy compared with ezetimibe | 12 | −59.2 to −70.6 | −47.0 | −54.9 | −9.3 to −31.4 | 3.2 to 9.8 | −19.8 to −36.5 |
| Stroes et al., GAUSS-2 [62] | 2014 | 307 | Evolocumab | Patients with statin intolerance given 140 mg fortnightly or 420 mg every 4 weeks evolocumab and were compared to those on ezetimibe | 12 | −68.8 to −69.7 | −32.9 | NR | NR | 3.6 to 4.8 | −25.3 to −27.9 |
| Koren et al., MENDEL-2 [49] | 2014 | 614 | Evolocumab | Patients with hypercholesterolemia on no statins 140 mg fortnightly or 420 mg every 4 weeks evolocumab and were compared to those on ezetimibe | 12 | −54.8 to −57.1 | −47.8 | −49.8 to −51.2 | −6.2 to −17.7 | 5.9 to 9.3 | −17.8 to −20.4 |
| Raal et al., RUTHERFORD-2 [55] | 2015 | 329 | Evolocumab | Patients with heterozygous FH given 140 mg fortnightly or 420 mg every 4 weeks | 12 | −59.2 to −61.3 | −49.1 | −54.8 to −55.0 | −11.6 to −19.6 | 9.1 to 9.2 | −28.2 to −31.6 |
| Sabatine et al., OSLER-2 [79] | 2015 | 4465 | Evolocumab | Hypercholesterolemia or mixed dyslipidaemia who had participated in the previous OSLER study | 12 | −61.0 | −47.3 | −52.0 | −12.6 | 7.0 | −25.5 |
| Raal et al., TESLA Part B [57] | 2015 | 49 | Evolocumab | Patients with homozygous FH not on apheresis were given 420 mg every 4 weeks of evolocumab | 12 | −30.9 | −23.1 | NR | 0.3 | −0.1 | −11.8 |
Values in table represent percentage (%) change in lipid parameters.
ApoB apolipoprotein B, FU follow-up, HDL-C high-density lipoprotein cholesterol, HeFH heterozygous familial hypercholesterolaemia, LDL-C low-density lipoprotein cholesterol, Lp(a) lipoprotein (a), mg milligram, n number, NA not available, TG triglycerides
See main text for full explanation of trial abbreviations
One putative concern regarding this new class of cholesterol-lowering drugs is the potential for hypocholesterolaemia-associated adverse effects, such as cognitive impairment. Indeed, even allowing for the technical difficulties of accurate LDL-C measurement at severely low levels, many subjects in phase 2 trials reached very low concentrations of LDL-C. Among those treated with alirocumab (150 mg every 2 weeks, for 12 weeks) in addition to atorvastatin, mean LDL-C was only 0.88 ± 0.41 mmol L−1 (34 ± 16 mg dL−1) [78]. Preliminary analyses have not shown any evidence of a treatment-related increase in cognitive impairment [79]. Some studies have suggested an increased risk of haemorrhagic stroke at lower cholesterol concentrations [80]. The identification of rare patients with double loss of function (LOF) mutations in the PCSK9 gene provides some reassurance, however. Such individuals, who have very low plasma PCSK9 and LDL-cholesterol concentrations, appear healthy and without cardiovascular or neurocognitive impairment [81]. In addition, plasma LDL-C does not relate directly to the intracellular cholesterol concentrations involved in physiological functions (e.g. synthesis of hormones and vitamins) and thus, such concerns may be misplaced.
Future directions
Impelled by the growing evidence-base regarding the safety and efficacy of monoclonal PCSK9 inhibitors, considerable momentum has accumulated in the translation of this novel pharmacotherapeutic paradigm to clinical practice. However, there is still a need to evaluate whether PCSK9 inhibition yields benefits on cardiovascular endpoints, for patients with primary hypercholesterolemia. Indeed, three large phase III programmes with anti-PCSK9 monoclonal antibodies are currently ongoing to offer definitive insights into their utilisation in preventing cardiovascular events and improving clinical outcome: the PROFICIO and FOURIER programmes evaluating evolocumab, and the ODYSSEY programme evaluating alirocumab. A list of currently ongoing clinical studies is presented in Table 5. These trials are due to report in 2017–2018, and will surely offer greater insights into the safety and efficacy of PCSK9 inhibition, particularly with regard to effects over and above statin therapy.
Table 5.
Major ongoing clinical studies of PCSK9 inhibitors
| Title | Description | Study identifier |
|---|---|---|
| Trial assessing efficacy, safety and tolerability of PCSK9 inhibition in paediatric subjects with genetic LDL disorders | 10–17 year olds with outcomes focused on cardiovascular risk | NCT02392559 |
| Effects of selective inhibition of cholesterol absorption with ezetimibe on intestinal cholesterol homeostasis in dyslipidemic men with insulin-resistance—a pilot study | Aged 18–60 and has metabolic syndrome | NCT01849068 |
| Evaluating PCSK9 binding antibody influence on cognitive health in high cardiovascular risk subjects | Testing spatial working memory in those aged 40 to 85 taking evolocumab | NCT02207634 |
| Further cardiovascular outcomes research with PCSK9 inhibition in subjects with elevated risk | 5 year cardiovascular death, myocardial infarction, hospitalization for unstable angina, stroke, or coronary revascularization | NCT01764633 |
| The evaluation of bococizumab (PF-04950615; RN316) in reducing the occurrence of major cardiovascular events in high risk subjects | Effect of bococizumab on number of Cardiovascular Events | NCT01975389 |
| A phase 1 study of an investigational drug, ALN-PCSSC, in subjects with elevated low density lipoprotein cholesterol (LDL-C) | Safety | NCT02314442 |
| A 2-part, phase 1, single and multiple ascending dose study to assess the safety, pharmacokinetics, and pharmacodynamics of CAT-2054 in healthy subjects | Frequency and severity of adverse events | NCT02374047 |
| Open label study of long term evaluation against LDL-C trial-2 | Incidence of adverse events | NCT01854918 |
| ODYSSEY outcomes: evaluation of cardiovascular outcomes after an acute coronary syndrome during treatment with alirocumab SAR236553 (REGN727) | To evaluate the effect of alirocumab on any adverse cardiovascular event | NCT01663402 |
In July 2015, the United States Food and Drug Administration (FDA) approved alirocumab as a second-line treatment, for adults with HeFH, and those with proven CVD with hypercholesterolemia refractory to diet modification and maximally-tolerated statin therapy. One month later, the FDA similarly approved evolocumab for clinical usage. These approvals were conditional on the subsequent completion of planned phase III trials to determine efficacy in primary hypercholesterolemia. Both agents have also recently received marketing authorisation by the European Medicines Agency.
Pharmacogenetic considerations
Although the PCSK9 locus is polymorphic, evidence has not yet emerged to suggest that routine genetic testing would predict responsiveness to PCSK9 inhibition, in patients with primary hypercholesterolaemia. In patients with HoFH, there exists evidence of differential response to PCSK9 inhibition, dependent on the specific underlying causative gene mutation(s). Evolocumab was demonstrably effective in lowering LDL-C, only in patients with residual LDL receptor function (the receptor-defective phenotype; 2–25 % function), but not receptor negative patients [54, 55, 57]. Stratification of FH patients, via fibroblast culture or pharmacogenetic testing (which many candidates may have underwent as part of FH diagnosis), may allow personalised prediction of responsiveness to PCSK9 inhibition.
Pharmacoeconomic considerations
Since the approval of these agents by regulatory bodies, the uptake of PCSK9 inhibitors in US clinical practice has in large, been slow. This may be explained by several key factors. Priced over $14,000 per year before discounts ($14,100 for evolocumab, $14,600 for alirocumab), and with a paucity of definitive data regarding improvements in cardiovascular outcomes, insurers have been reluctant to fund the available PCSK9 inhibitors. Recent pharmacoeconomic analysis by the US Institute for Clinical and Economic Review, calculated the overall price, best representing the potential benefits to patients, would be between $3615 and 4811—a 67 % discount on the current list price [82]. However, when compared to apheresis (the current best-available, alternative treatment, following statin and second-line medical therapy for uncontrolled hypercholesterolaemia), which costs approximately $8000 per month ($96,000 per year), the price of evolocumab and alirocumab appear more attractive. Schulman et al. estimate that in a typical insurance pool, if 5 % of the estimated 27 % of US adults 40–64 years of age who have hypercholesterolaemia were eligible for a PCSK9 inhibitor, annual premiums would increase by approximately $124 per person; taxpayers would face the burden of similar increases in the cost of the Medicare Part D program [83].
Conclusions
A quarter century after approval of the first statin in 1987, reduction of LDL-C remains the best-validated treatment strategy in preventing cardiovascular disease. PCSK9 is a promising molecular target to reduce levels of LDL-C and other atherogenic lipoproteins, below levels achievable with statins. However, uncertainties remain regarding the long-term impact of therapeutic reduction of plasma LDL-C to very low concentrations (<1 mmol L−1). Additionally, the increased risk of progression to diabetes seen with high-intensity statin treatment might also occur with PCSK9 inhibition, possibly resulting from the intracellular accumulation of lipids in insulin-secreting pancreatic beta cells [84]. However, more data are needed from large trials to exclude important emergent adverse effects of PCSK9 inhibitors. Although self-administered injections might not appear attractive for lifelong treatment, this route of administration may be acceptable to high-risk patients, unable to tolerate statins, or who need to achieve more stringent LDL-C targets. There seems little doubt that the advent of therapeutic PCSK9 inhibition heralds a change to the future of lipid management.
Compliance with ethical standards
Conflict of interest
None.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s11239-016-1373-0.
References
- 1.Mendis S, Puska P, Norrving B. Global atlas on cardiovascular disease prevention and control. Geneva: World Health Organization; 2011. [Google Scholar]
- 2.Cholesterol Treatment Trialists C, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cholesterol Treatment Trialists C, Mihaylova B, Emberson J, Blackwell L, Keech A, Simes J, et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koo BK. Statin for the primary prevention of cardiovascular disease in patients with diabetes mellitus. Diabetes Metab J. 2014;38(1):32–34. doi: 10.4093/dmj.2014.38.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corrao G, Conti V, Merlino L, Catapano AL, Mancia G. Results of a retrospective database analysis of adherence to statin therapy and risk of nonfatal ischemic heart disease in daily clinical practice in Italy. Clin Ther. 2010;32(2):300–310. doi: 10.1016/j.clinthera.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Abifadel M, Varret M, Rabès J-P, Allard D, Ouguerram K, Devillers M, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34(2):154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 7.Graham CA, McIlhatton BP, Kirk CW, Beattie ED, Lyttle K, Hart P, et al. Genetic screening protocol for familial hypercholesterolemia which includes splicing defects gives an improved mutation detection rate. Atherosclerosis. 2005;182(2):331–340. doi: 10.1016/j.atherosclerosis.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 8.Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci. 2003;100(3):928–933. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambert G, Sjouke B, Choque B, Kastelein JJP, Hovingh GK. The PCSK9 decade thematic review series: new lipid and lipoprotein targets for the treatment of cardiometabolic diseases. J Lipid Res. 2012;53(12):2515–2524. doi: 10.1194/jlr.R026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vogel RA. PCSK9 inhibition: the next statin? J Am Coll Cardiol. 2012;59(25):2354–2355. doi: 10.1016/j.jacc.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 11.Artenstein AW, Opal SM. Proprotein convertases in health and disease. N Engl J Med. 2011;365(26):2507–2518. doi: 10.1056/NEJMra1106700. [DOI] [PubMed] [Google Scholar]
- 12.Maxwell KN, Breslow JL. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci USA. 2004;101(18):7100–7105. doi: 10.1073/pnas.0402133101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discovery. 2012;11(5):367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 14.Jeong HJ, Lee H-S, Kim K-S, Kim Y-K, Yoon D, Park SW. Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatory element binding protein-2. J Lipid Res. 2008;49(2):399–409. doi: 10.1194/jlr.M700443-JLR200. [DOI] [PubMed] [Google Scholar]
- 15.Goldstein JL, Brown MS. History of discovery: the LDL receptor. Arterioscler Thromb Vasc Biol. 2009;29(4):431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kosenko T, Golder M, Leblond G, Weng W, Lagace TA. Low density lipoprotein binds to proprotein convertase subtilisin/kexin type-9 (PCSK9) in human plasma and inhibits PCSK9-mediated low density lipoprotein receptor degradation. J Biol Chem. 2013;288(12):8279–8288. doi: 10.1074/jbc.M112.421370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Browning JD, Horton JD. Fasting reduces plasma proprotein convertase, subtilisin/kexin type 9 and cholesterol biosynthesis in humans. J Lipid Res. 2010;51(11):3359–3363. doi: 10.1194/jlr.P009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costet P, Cariou B, Lambert G, Lalanne F, Lardeux B, Jarnoux A-L, et al. Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. J Biol Chem. 2006;281(10):6211–6218. doi: 10.1074/jbc.M508582200. [DOI] [PubMed] [Google Scholar]
- 19.Benjannet S, Rhainds D, Essalmani R, Mayne J, Wickham L, Jin W, et al. NARC-1/PCSK9 and its natural mutants zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem. 2004;279(47):48865–48875. doi: 10.1074/jbc.M409699200. [DOI] [PubMed] [Google Scholar]
- 20.Park SW, Moon Y-A, Horton JD. Post-transcriptional regulation of low density lipoprotein receptor protein by proprotein convertase subtilisin/kexin type 9a in mouse liver. J Biol Chem. 2004;279(48):50630–50638. doi: 10.1074/jbc.M410077200. [DOI] [PubMed] [Google Scholar]
- 21.Nassoury N, Blasiole DA, Tebon Oler A, Benjannet S, Hamelin J, Poupon V, et al. The cellular trafficking of the secretory proprotein convertase PCSK9 and its dependence on the LDLR. Traffic. 2007;8(6):718–732. doi: 10.1111/j.1600-0854.2007.00562.x. [DOI] [PubMed] [Google Scholar]
- 22.Lambert G. Unravelling the functional significance of PCSK9. Current opinion in lipidology. 2007;18(3):304–309. doi: 10.1097/MOL.0b013e3281338531. [DOI] [PubMed] [Google Scholar]
- 23.Turpeinen H, Ortutay Z, Pesu M. Genetics of the first seven proprotein convertase enzymes in health and disease. Current genomics. 2013;14(7):453. doi: 10.2174/1389202911314050010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desai NR, Kohli P, Giugliano RP, O’Donoghue ML, Somaratne R, Zhou J, et al. AMG145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: an analysis from the LDL-C Assessment with Proprotein Convertase Subtilisin Kexin Type 9 Monoclonal Antibody Inhibition Combined with Statin Therapy (LAPLACE)-Thrombolysis in Myocardial Infarction (TIMI) 57 trial. Circulation. 2013;128(9):962–969. doi: 10.1161/CIRCULATIONAHA.113.001969. [DOI] [PubMed] [Google Scholar]
- 25.Couture F, D’Anjou F, Day R. On the cutting edge of proprotein convertase pharmacology: from molecular concepts to clinical applications. Biomol Concepts. 2011;2(5):421–438. doi: 10.1515/bmc.2011.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cariou B, Le May C, Costet P. Clinical aspects of PCSK9. Atherosclerosis. 2011;216(2):258–265. doi: 10.1016/j.atherosclerosis.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Levy E, Ouadda ABD, Spahis S, Sane AT, Garofalo C, Grenier É, et al. PCSK9 plays a significant role in cholesterol homeostasis and lipid transport in intestinal epithelial cells. Atherosclerosis. 2013;227(2):297–306. doi: 10.1016/j.atherosclerosis.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Farnier M. PCSK9: from discovery to therapeutic applications. Archives of cardiovascular diseases. 2014;107(1):58–66. doi: 10.1016/j.acvd.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 29.Farnier M. PCSK9 inhibitors. Curr Opin Lipidol. 2013;24(3):251–258. doi: 10.1097/MOL.0b013e3283613a3d. [DOI] [PubMed] [Google Scholar]
- 30.Bridge SH, Sheridan DA, Felmlee DJ, Crossey MM, Fenwick FI, Lanyon CV, et al. PCSK9, apolipoprotein E and lipoviral particles in chronic hepatitis C genotype 3: evidence for genotype-specific regulation of lipoprotein metabolism. J Hepatol. 2015;62(4):763–770. doi: 10.1016/j.jhep.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 31.Denis M, Marcinkiewicz J, Zaid A, Gauthier D, Poirier S, Lazure C, et al. Gene inactivation of proprotein convertase subtilisin/kexin type 9 reduces atherosclerosis in mice. Circulation. 2012;125(7):894–901. doi: 10.1161/CIRCULATIONAHA.111.057406. [DOI] [PubMed] [Google Scholar]
- 32.Cohen JC, Boerwinkle E, Mosley TH, Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354(12):1264–1272. doi: 10.1056/NEJMoa054013. [DOI] [PubMed] [Google Scholar]
- 33.Persson L, Cao G, Ståhle L, Sjöberg BG, Troutt JS, Konrad RJ, et al. Circulating proprotein convertase subtilisin kexin type 9 has a diurnal rhythm synchronous with cholesterol synthesis and is reduced by fasting in humans. Arterioscler Thromb Vasc Biol. 2010;30(12):2666–2672. doi: 10.1161/ATVBAHA.110.214130. [DOI] [PubMed] [Google Scholar]
- 34.Browning JD, Horton JD. Fasting reduces plasma proprotein convertase, subtilisin/kexin type 9 and cholesterol biosynthesis in humans. J Lipid Res. 2010;51(11):3359–3363. doi: 10.1194/jlr.P009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persson L, Cao G, Stahle L, Sjoberg BG, Troutt JS, Konrad RJ, et al. Circulating proprotein convertase subtilisin kexin type 9 has a diurnal rhythm synchronous with cholesterol synthesis and is reduced by fasting in humans. Arterioscler Thromb Vasc Biol. 2010;30(12):2666–2672. doi: 10.1161/ATVBAHA.110.214130. [DOI] [PubMed] [Google Scholar]
- 36.Maxwell KN, Soccio RE, Duncan EM, Sehayek E, Breslow JL. Novel putative SREBP and LXR target genes identified by microarray analysis in liver of cholesterol-fed mice. J Lipid Res. 2003;44(11):2109–2119. doi: 10.1194/jlr.M300203-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Tavori H, Giunzioni I, Linton MF, Fazio S. Loss of plasma proprotein convertase subtilisin/kexin 9 (PCSK9) after lipoprotein apheresis. Circ Res. 2013;113(12):1290–1295. doi: 10.1161/CIRCRESAHA.113.302655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan D, Yancey PG, Qiu S, Ding L, Weeber EJ, Linton MF, et al. Self-association of human PCSK9 correlates with its LDLR-degrading activity. Biochemistry. 2008;47(6):1631–1639. doi: 10.1021/bi7016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci. 2007;32(2):71–77. doi: 10.1016/j.tibs.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leren TP. Sorting an LDL receptor with bound PCSK9 to intracellular degradation. Atherosclerosis. 2014;237(1):76–81. doi: 10.1016/j.atherosclerosis.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 41.Graham MJ, Lemonidis KM, Whipple CP, Subramaniam A, Monia BP, Crooke ST, et al. Antisense inhibition of proprotein convertase subtilisin/kexin type 9 reduces serum LDL in hyperlipidemic mice. J Lipid Res. 2007;48(4):763–767. doi: 10.1194/jlr.C600025-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Lindholm MW, Elmen J, Fisker N, Hansen HF, Persson R, Moller MR, et al. PCSK9 LNA antisense oligonucleotides induce sustained reduction of LDL cholesterol in nonhuman primates. Mol Ther. 2012;20(2):376–381. doi: 10.1038/mt.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frank-Kamenetsky M, Grefhorst A, Anderson NN, Racie TS, Bramlage B, Akinc A, et al. Therapeutic RNAi targeting PCSK9 acutely lowers plasma cholesterol in rodents and LDL cholesterol in nonhuman primates. Proc Natl Acad Sci USA. 2008;105(33):11915–11920. doi: 10.1073/pnas.0805434105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duff CJ, Scott MJ, Kirby IT, Hutchinson SE, Martin SL, Hooper NM. Antibody-mediated disruption of the interaction between PCSK9 and the low-density lipoprotein receptor. Biochem J. 2009;419(3):577–584. doi: 10.1042/BJ20082407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan JC, Piper DE, Cao Q, Liu D, King C, Wang W, et al. A proprotein convertase subtilisin/kexin type 9 neutralizing antibody reduces serum cholesterol in mice and nonhuman primates. Proc Natl Acad Sci USA. 2009;106(24):9820–9825. doi: 10.1073/pnas.0903849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khera AV, Everett BM, Caulfield MP, Hantash FM, Wohlgemuth J, Ridker PM, et al. Lipoprotein(a) concentrations, rosuvastatin therapy, and residual vascular risk: an analysis from the JUPITER trial (justification for the use of statins in prevention: an intervention trial evaluating rosuvastatin) Circulation. 2014;129(6):635–642. doi: 10.1161/CIRCULATIONAHA.113.004406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rader DJ, Cain W, Ikewaki K, Talley G, Zech LA, Usher D, et al. The inverse association of plasma lipoprotein(a) concentrations with apolipoprotein(a) isoform size is not due to differences in Lp(a) catabolism but to differences in production rate. J Clin Invest. 1994;93(6):2758–2763. doi: 10.1172/JCI117292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double-blind, placebo-controlled, phase 2 study. Lancet. 2012;380(9858):1995–2006. doi: 10.1016/S0140-6736(12)61771-1. [DOI] [PubMed] [Google Scholar]
- 49.Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, et al. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2531–2540. doi: 10.1016/j.jacc.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 50.Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380(9858):2007–2017. doi: 10.1016/S0140-6736(12)61770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380(9858):2007–2017. doi: 10.1016/S0140-6736(12)61770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, et al. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA. 2014;311(18):1870–1882. doi: 10.1001/jama.2014.4030. [DOI] [PubMed] [Google Scholar]
- 53.Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, et al. A 52-week placebo-controlled trial of evolocumab in hyperlipidemia. The New England journal of medicine. 2014;370(19):1809–1819. doi: 10.1056/NEJMoa1316222. [DOI] [PubMed] [Google Scholar]
- 54.Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, et al. Low-density lipoprotein cholesterol-lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the reduction of LDL-C with PCSK9 inhibition in heterozygous familial hypercholesterolemia disorder (RUTHERFORD) randomized trial. Circulation. 2012;126(20):2408–2417. doi: 10.1161/CIRCULATIONAHA.112.144055. [DOI] [PubMed] [Google Scholar]
- 55.Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):331–340. doi: 10.1016/S0140-6736(14)61399-4. [DOI] [PubMed] [Google Scholar]
- 56.Hobbs HH, Brown MS, Goldstein JL. Molecular genetics of the LDL receptor gene in familial hypercholesterolemia. Hum Mutat. 1992;1(6):445–466. doi: 10.1002/humu.1380010602. [DOI] [PubMed] [Google Scholar]
- 57.Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9965):341–350. doi: 10.1016/S0140-6736(14)61374-X. [DOI] [PubMed] [Google Scholar]
- 58.Alfirevic A, Neely D, Armitage J, Chinoy H, Cooper RG, Laaksonen R, et al. Phenotype standardization for statin-induced myotoxicity. Clin Pharmacol Ther. 2014;96(4):470–476. doi: 10.1038/clpt.2014.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther. 2005;19(6):403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 60.Zhang H, Plutzky J, Skentzos S, Morrison F, Mar P, Shubina M, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158(7):526–534. doi: 10.7326/0003-4819-158-7-201304020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, et al. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012;308(23):2497–2506. doi: 10.1001/jama.2012.25790. [DOI] [PubMed] [Google Scholar]
- 62.Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63(23):2541–2548. doi: 10.1016/j.jacc.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 63.Cho L, Rocco M, Colquhoun D, Sullivan D, Rosenson RS, Dent R, et al. Design and rationale of the GAUSS-2 study trial: a double-blind, ezetimibe-controlled phase 3 study of the efficacy and tolerability of evolocumab (AMG 145) in subjects with hypercholesterolemia who are intolerant of statin therapy. Clin Cardiol. 2014;37(3):131–139. doi: 10.1002/clc.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, et al. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012;380(9836):29–36. doi: 10.1016/S0140-6736(12)60771-5. [DOI] [PubMed] [Google Scholar]
- 65.Farnier M, Kastelein JJP, Roth E, Taskinen MR, Ginsberg HN, Colhoun HM, et al. Relationship between alirocumab, PCSK9 and LDL-C levels: results from the odyssey mono phase 3 trial of alirocumab 75 mg every 2 weeks. Atherosclerosis. 2014;235(2):e34–e35. [Google Scholar]
- 66.Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U, et al. (2015) Efficacy and safety of the PCSK9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J [DOI] [PubMed]
- 67.Colhoun HM, Robinson JG, Farnier M, Cariou B, Blom D, Kereiakes DJ, et al. Efficacy and safety of alirocumab, a fully human PCSK9 monoclonal antibody, in high cardiovascular risk patients with poorly controlled hypercholesterolemia on maximally tolerated doses of statins: rationale and design of the ODYSSEY COMBO I and II trials. BMC Cardiovasc Disord. 2014;14(1):121. doi: 10.1186/1471-2261-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwartz GG, Bessac L, Berdan LG, Bhatt DL, Bittner V, Diaz R, et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY outcomes trial. Am Heart J. 2014;168(5):682–689. doi: 10.1016/j.ahj.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 69.Bays H, Gaudet D, Weiss R, Ruiz JL, Watts GF, Gouni-Berthold I, et al. Alirocumab as add-on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metabol. 2015;100(8):3140–3148. doi: 10.1210/jc.2015-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kastelein JJP, Robinson JG, Farnier M, Krempf M, Langslet G, Lorenzato C, et al. Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia not adequately controlled with current lipid-lowering therapy: design and rationale of the ODYSSEY FH studies. Cardiovasc Drugs Ther. 2014;28(3):281–289. doi: 10.1007/s10557-014-6523-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roth EM, Diller P. Alirocumab for hyperlipidemia: physiology of PCSK9 inhibition, pharmacodynamics and Phase I and II clinical trial results of a PCSK9 monoclonal antibody. Future Cardiol. 2014;10(2):183–199. doi: 10.2217/fca.13.107. [DOI] [PubMed] [Google Scholar]
- 72.Ginsberg HN, Rader D, Raal FJ, Guyton J, Lorenzato C, Pordy R, et al. ODYSSEY high FH: efficacy and safety of alirocumab in patients with severe heterozygous familial hypercholesterolemia. Circulation. 2014;130(23):2119. doi: 10.1007/s10557-016-6685-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 74.Kastelein JJP, Ginsberg HN, Langslet G, Hovingh GK, Ceska R, Dufour R, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36(43):2996–3003. doi: 10.1093/eurheartj/ehv370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cicero AF, Tartagni E, Ertek S. Safety and tolerability of injectable lipid-lowering drugs: a review of available clinical data. Exp Opin Drug Saf. 2014;13(8):1023–1030. doi: 10.1517/14740338.2014.932348. [DOI] [PubMed] [Google Scholar]
- 76.Roth EM, Taskinen MR, Ginsberg HN, Kastelein JJ, Colhoun HM, Robinson JG, et al. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double-blind, randomized Phase 3 trial. Int J Cardiol. 2014;176(1):55–61. doi: 10.1016/j.ijcard.2014.06.049. [DOI] [PubMed] [Google Scholar]
- 77.Roth EM, McKenney JM, Hanotin C, Asset G, Stein EA. Atorvastatin with or without an antibody to PCSK9 in primary hypercholesterolemia. N Engl J Med. 2012;367(20):1891–1900. doi: 10.1056/NEJMoa1201832. [DOI] [PubMed] [Google Scholar]
- 78.McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59(25):2344–2353. doi: 10.1016/j.jacc.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 79.Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372(16):1500–1509. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 80.Prospective Studies C Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55 000 vascular deaths. Lancet. 2007;370(9602):1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 81.Zhao Z, Tuakli-Wosornu Y, Lagace TA, Kinch L, Grishin NV, Horton JD, et al. Molecular characterization of loss-of-function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79(3):514–523. doi: 10.1086/507488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.The Institute for Clinical and Economic Review (ICER) PCSK9 inhibitor therapies for high cholesterol: effectiveness, value, and value-based price benchmarks (draft) Boston: The Institute for Clinical and Economic Review (ICER); 2015. [Google Scholar]
- 83.Schulman KA, Balu S, Reed SD. Specialty pharmaceuticals for hyperlipidemia—impact on insurance premiums. N Engl J Med. 2015;373(17):1591–1593. doi: 10.1056/NEJMp1509863. [DOI] [PubMed] [Google Scholar]
- 84.Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305(24):2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 85.Hirayama A, Honarpour N, Yoshida M, Yamashita S, Huang F, Wasserman SM, et al. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin-treated Japanese patients at high cardiovascular risk–primary results from the phase 2 YUKAWA study. Circ J. 2014;78(5):1073–1082. doi: 10.1253/circj.cj-14-0130. [DOI] [PubMed] [Google Scholar]