Summary
African tick bite fever is an emerging infectious disease among travellers caused by the pathogen Rickettsia africae. Most travel-associated cases have been reported from countries in southern Africa. So far it has rarely been reported among travellers to eastern Africa and our patient is one of the first described cases imported from Tanzania. A woman presented with fever, chills, headache, myalgia and a rickettsial eschar on her ankle after returning from Tanzania. The diagnosis of African tick bite fever is often based on clinical grounds due to a lack of reliable diagnostic tests at commencement of symptoms. In this patient direct molecular detection of R. africae was performed by PCR from a sample obtained non-invasively with a swab from the rickettsial eschar. A positive PCR result was achieved although the patient had already started antibiotic treatment with doxycycline. In conclusion, this non-invasive method enables early diagnosis of African tick bite fever by direct molecular detection of R. africae and might improve the management of undifferentiated fever in travellers from Africa.
Keywords: African tick bite fever, Tanzania, Rickettsia africae, Rickettsiosis, Eschar
Introduction
African tick bite fever (ATBF) is an emerging infectious disease among travellers. In febrile travellers returning from sub-Saharan Africa rickettsial infections were the second most common etiology after malaria [1]. Rickettsia africae was the most frequently detected pathogen in tick-bite related diseases from Africa [2]. R. africae was first identified as a new human pathogen in 1992 [3] and since then has been isolated from ticks of different Amblyomma species from many regions across Africa [4, 5]. Most travel-associated cases of ATBF have been reported from South Africa and adjoining countries especially among game hunters [6]. So far ATBF has rarely been reported among travellers to East Africa and our patient is one of the first described cases imported from Tanzania. This fact could be explained by various vectors of R. africae, which might result in different risks of transmission. In eastern African countries R. africae is commonly found in ticks of the species Amblyomma variegatum [5], whereas in South Africa Amblyomma hebraeum is known to transmit the pathogen. Furthermore, different variants of R. africae detected in eastern Africa might be less pathogenic for humans [5, 6].
We describe a case of ATBF with direct molecular detection of R. africae by PCR from a sample obtained non-invasively from the rickettsial eschar. Considering that cases of ATBF imported from Tanzania are rarely reported, we aimed to sequence the detected R. africae to determine the exact genotype of this pathogen.
Case report
A 30-year-old woman presented with fever, chills, headache and myalgia at the outpatient ward for tropical medicine at the University Hospital of Vienna. A skin lesion on the right ankle and a swollen inguinal lymph node were noticed on physical examination. The patient had recently returned from working on a developmental aid project in the southwest of Tanzania. She had spent 2 weeks living in a small rural village and hiking through the adjoining national parks. On a trip through the Kipengere Mpanga Game Reserve she had noticed a tick bite on the right ankle and 1 week later a reddish skin lesion developed at the site of the bite. The next day the patient experienced high fever (39 °C), chills, headache, generalized myalgia and pain in the right lower limb. On the second day of fever the patient visited our outpatient ward. The laboratory analysis showed a moderate leukopenia (3.5 × 103/µl) with an otherwise unremarkable blood count and without elevation of inflammation parameters. A rapid diagnostic test and a thick blood film for malaria were negative. The skin lesion on the right ankle was typical for a rickettsial eschar (Fig. 1) and the diagnosis of ATBF was established on clinical grounds. The patient received 200 mg doxycycline daily for 9 days and the fever resolved after 2 days of treatment. Other symptoms, such as headache, muscle pain and inflammation at the site of the tick bite improved after 1 week of administering doxycycline.
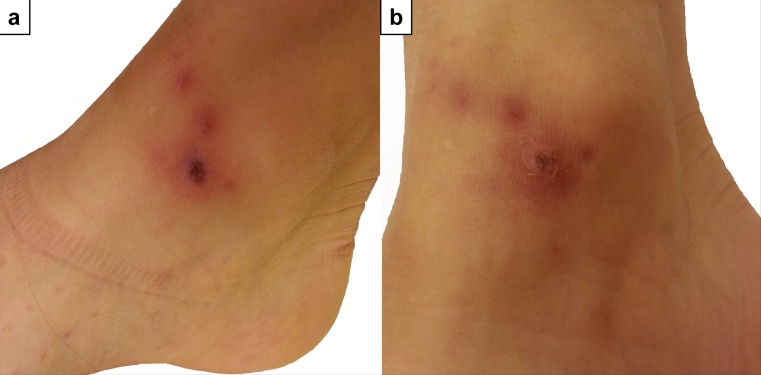
Fig. 1.
The rickettsial eschar on the right ankle of the patient during the first visit before treatment was initiated (a) and at the follow-up 1 week later after treatment with doxycycline (b)
For further diagnostic work-up serological testing for IgG and IgM against R. conorii was performed at initial presentation. Both the immunofluorescence assay (Focus Diagnostics, Cypress, CA) and the Weil Felix agglutination assay (DiaMondial, Sees, France) gave negative results. These serological tests are designed to detect R. conorii but may also give positive results for R. africae due to cross-reactivity. Furthermore, PCR performed on DNA extracted from an EDTA blood sample yielded a negative result for Rickettsia spp. At a follow-up visit 5 days later, the crust of the eschar was removed and two samples from the base of the lesion were taken with a dry swab to perform PCR for Rickettsia spp. At that time treatment with doxycycline had already been administered for 5 days. A serological follow-up was performed on day 17 after the tick bite and seroconversion of IgG was observed in the immunofluorescence assay (titre 1:512). For PCR testing of the eschar samples, DNA was extracted from each sample using the PeqGOLD Tissue DNA Mini Kit (Peqlab, Erlangen, Germany) and carried out according to the manufacturer’s protocol. For the detection of Rickettsia spp. samples were subjected to a real time PCR targeting the gltA gene [7]. Rickettsia DNA could be detected in one of the two swabs. To further identify the species present in the sample, additional PCR targeting the 16S rRNA gene [8], 23S-5S intergenic spacer [9], gltA gene [10] and ompB gene [11] were performed as previously described. Primers and probes used are displayed in Table 1. Amplicons were purified using QIAquick PCR purification kit and Qiagen gel extraction kit (Qiagen, Hilden, Germany) if multiple bands were observed. Purified products were sent to MWG (Eurofins, Ebersberg, Germany) for bidirectional sequencing. Consensus sequences were created and ompB fragments were assembled by using CLC Main Workbench (version 7.6). A BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) yielded a 100 % identity to R. africae strain ESF-5. The sequences obtained in this study were submitted to GenBank (accession numbers: KU721068, KU721069, KU721070, KU721071). A maximum likelihood-based phylogenetic tree (Fig. 2) for the obtained ompB sequence was constructed by using the analysis software package MEGA7 (version 7.0.14).
Table 1.
Primer and probe sequences used to identify Rickettsia africae
| Oligonucleotide name | Target gene | Oligonucleotide sequence (5’ → 3’) | Reference |
|---|---|---|---|
| Real time PCR | |||
| RickF | gltA gene | GGT ATA CCG TCG CAA ATG TTC AC | [7] |
| RickR | gltA gene | GGG TCT TCG TGCATT TCT TTC C | [7] |
| RickTM | gltA gene | TGT GCC ATC CAG CCT ACG GTT CTT Ga | [7] |
| Primers used for sequencing | |||
| Rick-F1 | 16S rRNA gene | GAA CGC TAT CGG TAT GCT TAA CAC A | [8] |
| Rick-R2 | 16S rRNA gene | CAT CAC TCA CTC GGT ATT GCT GGA | [8] |
| RCK/23-5-F | 23S-5S IGS | GAT AGG TCR GRT GTG GAA GCA C | [9] |
| RCK/23-5-R | 23S-5S IGS | TCG GGA YGG GAT CGT GTG TTT C | [9] |
| CS-78 | gltA gene | GCA AGT ATC GGT GAG GAT GTA AT | [10] |
| CS-323 | gltA gene | GCT TCC TTA AAA TTC AAT AAA TCA GGA T | [10] |
| M59 | ompB (I) | CCGCAGGGTTGGTAACTGC | [11] |
| 120-807 R | ompB (I) | CCTTTTAGATTACCGCCTAA | [11] |
| 120-607 F | ompB (II) | AATATCGGTGACGGTCAAGG | [11] |
| 120-1497 | ompB (II) | CCTATATCGCCGGTAATT | [11] |
| 120-1378 | ompB (III) | TAAACTTGCTGACGGTACAG | [11] |
| 120-2399 | ompB (III) | CTTGTTTGTTTAATGTTACGGT | [11] |
| 120-2113 | ompB (IV) | CGATGCTAACGTAGGTTCTT | [11] |
| 120-2988 | ompB (IV) | CCGGCTATACCGCCTGTAGT | [11] |
| 120-2788 | ompB (V) | AAACAATAATCAAGGTACTGT | [11] |
| 120-3599 | ompB (V) | TACTTCCGGTTACAGCAAAGT | [11] |
| 120-3462 | ompB (VI) | CCACAGGAACTACAACCATT | [11] |
| 120-4346 | ompB (VI) | CGAAGAAGTAACGCTGACTT | [11] |
aReal time PCR probe was labeled with 5’FAM and 3’TAMRA
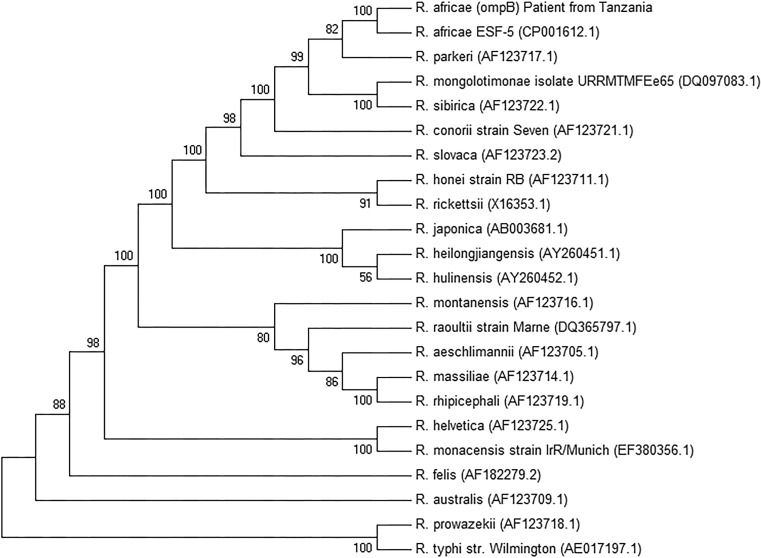
Fig. 2.
Phylogenetic tree based on partial ompB nucleotide sequences of the genus Rickettsia. The tree was constructed by using the maximum likelihood method with the use of the Tamura-3-parameter model and gamma distribution. The bootstrap consensus tree was inferred from 1000 replicates. A total of 23 nucleotide sequences were involved in the analysis. Accession numbers of sequences obtained from GenBank are shown in parenthesis. The final dataset included a total of 4155 positions. Evolutionary analysis was conducted in MEGA (Molecular Evolutionary Genetics Analysis) version 7.0
Discussion
The diagnosis of ATBF is often based on the clinical presentation, especially on the presence of fever and one or multiple eschars. A confirmatory laboratory method is necessary to differentiate ATBF from other febrile diseases. For serological testing the immunofluorescence test is the preferred method but seroconversion usually occurs about 2–3 weeks after the tick bite; therefore, serological testing is often negative at the onset of symptoms as shown in our patient. The use of PCR on skin biopsy samples from the eschar has been proposed as a method with high sensitivity [2]; however, obtaining a skin biopsy may not be practical in certain settings. As an alternative the use of a non-invasive eschar swab has proven to be effective in several case series [12–14]. The eschar crust should be removed and a dry swab circulated on the base of the eschar to yield a sufficient sample. This method also showed high acceptance among physicians and patients [15]. Both the sampling and the transport (optimal temperature 4 °C) are easy to perform for research in field conditions and therefore a good alternative to the more laborious skin biopsy [13]. In the described case one of the two eschar swabs contained sufficient material for a positive PCR result although our patient had already received treatment with doxycycline for 5 days.
In conclusion, this case confirms the usefulness of eschar swabs to detect the agent of ATBF by PCR even after starting antibiotic treatment. Finally, ATBF must be considered in febrile travellers returning from East Africa, although imported infections have rarely been reported so far from this part of Africa.
Acknowledgments
Open access funding provided by Medical University of Vienna.
Compliance with ethical guidelines
Conflict of interest
N. Harrison, H. Burgmann, C. Forstner, M. Ramharter, M. Széll, A.-M. Schötta, G. Stanek and M. Markowicz declare that they have no competing interests.
Ethical standards
All studies on humans described in this article were carried out with the approval of the responsible ethics committee and in accordance with national law and the Helsinki Declaration from 1975 (in its current revised form). The patient signed a written informed consent for the publication of this case report.
References
- 1.Freedman DO, Weld LH, Kozarsky PE, Fisk T, Robins R, von Sonnenburg F, et al. Spectrum of disease and relation to place of exposure among ill returned travelers. N. Engl. J. Med. 2006;354(2):119–130. doi: 10.1056/NEJMoa051331. [DOI] [PubMed] [Google Scholar]
- 2.Raoult D, Fournier PE, Fenollar F, Jensenius M, Prioe T, de Pina JJ, et al. Rickettsia africae, a tick-borne pathogen in travelers to sub-Saharan Africa. N. Engl. J. Med. 2001;344(20):1504–1510. doi: 10.1056/NEJM200105173442003. [DOI] [PubMed] [Google Scholar]
- 3.Kelly PJ, Beati L, Matthewman LA, Mason PR, Dasch GA, Raoult D. A new pathogenic spotted fever group rickettsia from Africa. J Trop Med Hyg. 1994;97(3):129–137. [PubMed] [Google Scholar]
- 4.Lorusso V, Gruszka KA, Majekodunmi A, Igweh A, Welburn SC, Picozzi K. Rickettsia africae in Amblyomma variegatum ticks, Uganda and Nigeria. Emerging Infect. Dis. 2013;19(10):1705–1707. doi: 10.3201/eid1910.130389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maina AN, Jiang J, Omulo SA, Cutler SJ, Ade F, Ogola E, et al. High prevalence of Rickettsia africae variants in Amblyomma variegatum ticks from domestic mammals in rural western Kenya: implications for human health. Vector Borne Zoonotic Dis. 2014;14(10):693–702. doi: 10.1089/vbz.2014.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensenius M, Fournier PE, Vene S, Hoel T, Hasle G, Henriksen AZ, et al. African tick bite fever in travelers to rural sub-Equatorial Africa. Clin. Infect. Dis. 2003;36(11):1411–1417. doi: 10.1086/375083. [DOI] [PubMed] [Google Scholar]
- 7.Leschnik MW, Khanakah G, Duscher G, Wille-Piazzai W, Horweg C, Joachim A, et al. Species, developmental stage and infection with microbial pathogens of engorged ticks removed from dogs and questing ticks. Med. Vet. Entomol. 2012;26(4):440–446. doi: 10.1111/j.1365-2915.2012.01036.x. [DOI] [PubMed] [Google Scholar]
- 8.Nijhof AM, Bodaan C, Postigo M, Nieuwenhuijs H, Opsteegh M, Franssen L, et al. Ticks and associated pathogens collected from domestic animals in the Netherlands. Vector Borne Zoonotic Dis. 2007;7(4):585–595. doi: 10.1089/vbz.2007.0130. [DOI] [PubMed] [Google Scholar]
- 9.Jado I, Escudero R, Gil H, Jimenez-Alonso MI, Sousa R, Garcia-Perez AL, et al. Molecular method for identification of Rickettsia species in clinical and environmental samples. J. Clin. Microbiol. 2006;44(12):4572–4576. doi: 10.1128/JCM.01227-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Labruna MB, Whitworth T, Horta MC, Bouyer DH, McBride JW, Pinter A, et al. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J. Clin. Microbiol. 2004;42(1):90–98. doi: 10.1128/JCM.42.1.90-98.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roux V, Raoult D. Phylogenetic analysis of members of the genus Rickettsia using the gene encoding the outer-membrane protein rOmpB (ompB) Int J Syst Evol Microbiol. 2000;50(Pt 4):1449–1455. doi: 10.1099/00207713-50-4-1449. [DOI] [PubMed] [Google Scholar]
- 12.Socolovschi C, Renvoise A, Brouqui P, Parola P, Raoult D. The use of eschar swabs for the diagnosis of African tick-bite fever. Ticks Tick Borne Dis. 2012;3(5–6):361–363. doi: 10.1016/j.ttbdis.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Bechah Y, Socolovschi C, Raoult D. Identification of rickettsial infections by using cutaneous swab specimens and PCR. Emerging Infect. Dis. 2011;17(1):83–86. doi: 10.3201/eid1701.100854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang JM, Hudson BJ, Watts MR, Karagiannis T, Fisher NJ, Anderson C, et al. Diagnosis of Queensland tick typhus and African tick bite fever by PCR of lesion swabs. Emerging Infect. Dis. 2009;15(6):963–965. doi: 10.3201/eid1506.080855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mouffok N, Socolovschi C, Benabdellah A, Renvoise A, Parola P, Raoult D. Diagnosis of rickettsioses from eschar swab samples, Algeria. Emerging Infect. Dis. 2011;17(10):1968–1969. doi: 10.3201/eid1710.110332. [DOI] [PMC free article] [PubMed] [Google Scholar]