Abstract
Bronchoalveolar lavage fluid (BALF) in patients with idiopathic pulmonary fibrosis (IPF) is typically characterized by a neutrophil inflammatory pattern and to a lesser extent (<25%) a mild eosinophil alveolitis. We here present two patients with a definite usual interstitial pneumonia (UIP) pattern on high-resolution computed tomography of the thorax (HRCT) which demonstrated unusually high eosinophil counts in the BALF (40% and 51%). Based on HRCT, lack of response to steroids and the disease course they were both diagnosed as IPF after a multidisciplinary team discussion. This report discusses the diagnostic and etiological considerations of a coexisting UIP pattern and an eosinophil alveolitis. We conclude that these cases illustrate that high level BALF eosinophilia (40–50%) may occur among patients with IPF.
Keywords: Bronchoalveolar lavage, Eosinophilia, Idiopathic pulmonary fibrosis
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is a rare, progressive fibrotic lung disease of unknown cause with a dismal outcome. IPF is limited to the lungs and associated with the radiologic and/or histopathologic pattern of usual interstitial pneumonia (UIP) [1]. Bronchoalveolar lavage fluid (BALF) in patients with idiopathic pulmonary fibrosis (IPF) is typically characterized by a neutrophil inflammatory pattern and to a lesser extent (<25%) a mild eosinophil alveolitis [2], [3].
Only 50% of patients with IPF have a definite UIP pattern on high-resolution computed tomography of the thorax (HRCT). A surgical lung biopsy is often considered too risky in many patients [4], [5], [6]. According to the recent guidelines on IPF [3], bronchoalvelar lavage (BAL) should not be performed in the diagnostic evaluation in the majority of patients, but may be appropriate in a minority. However, this is a weak recommendation with low-quality evidence focusing solely on patients with a definite UIP pattern. Patients with a possible UIP pattern on HRCT represent a grey zone group and BAL may be diagnostically helpful in this setting. Many centers including our center do BAL in patients with both a possible and a definite UIP pattern on HRCT as part of the standard diagnostic work up.
A previous study reported of six patients with a definite radiologic and histopathologic pattern of UIP and with coexistent eosinophilic pneumonia in surgical lung biopsies [7].
We here present two patients with a high level BALF eosinophilia (40–50%) and a definite UIP pattern on HRCT. We further discuss combination of an eosinophil alveolitis and a UIP pattern. No previous studies have reported this high eosinophil count in patients with IPF.
1.1. Case 1
A 63-year-old man was referred due to progressive dyspnea when walking on stairs (Medical Research Council Dyspnea Scale (MRC) grade 2) during two months and a cough with clear sputum. He was a smoker (40 pack years) but previously healthy. He had no environmental or occupational exposures, no history of a recent infection, no history of drugs with pulmonary toxicity, no autoimmune disorder or family history.
1.2. Case 2
A 64-year-old man, with randomly discovered radiological signs of interstitial lung disease prior to methotrexate treatment for severe psoriasis, was referred for assessment. Because of the findings, methotrexate was not initiated. He had had dyspnea when walking on stairs (MRC grade 2) for some years and a slight cough with clear sputum. He had stopped smoking 15 years ago but had cumulated 30 pack years. He had no history of recent infection, no history of drugs with pulmonary toxicity, no autoimmune disorder or family history.
2. Results
2.1. Case 1
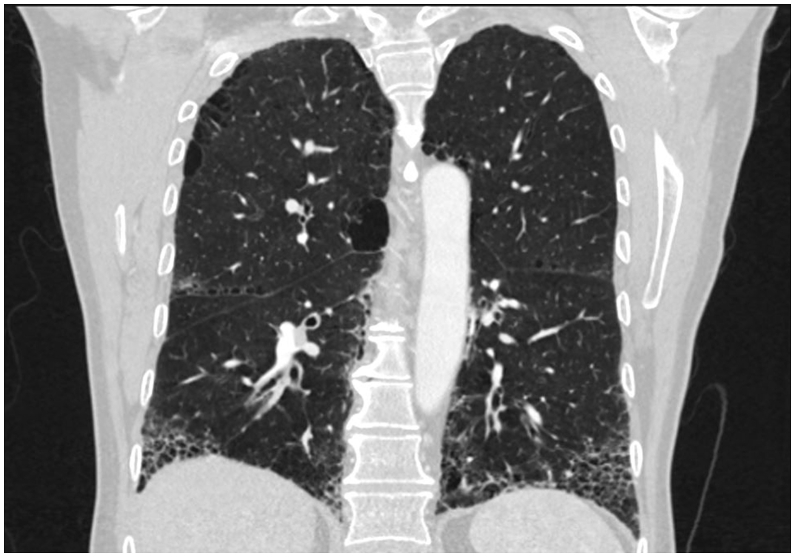
Blood samples, including peripheral blood eosinophils (EOS), antinuclear antibodies (ANA), anti-neutrophil cytoplasmic antibodies (ANCA), and rheumatoid factor (IgM-RF), were normal. The patients had no signs of infection and no recent traveling. The physical examination was normal without clubbing. Six minute walk test distance was 612 m, with desaturation from 99% to 92%. The pulmonary function test (PFT) showed FEV1 2.72 (75%), FVC 4.19 (90%), FEV1/FVC 83%. DLCO 47% and TLC 79%. HRCT (Image 1) showed a definite UIP pattern with basal predominance of honeycombing, reticulation and traction bronchiectasis. BALF cell count showed 40% eosinophils, 51% macrophages and 9% neutrophils. Treatment with high-dose prednisolone was initiated with a gradual taper after 4 months. PFT did not improve on prednisolone treatment. In a multidisciplinary team discussion, the patient was diagnosed with IPF based on age, smoking history, definite UIP pattern on HRCT and non-responsiveness to steroids. Pirfenidone was initiated. Lung function and symptoms has been stable since.
Image 1.
High-resolution computed tomography (HRCT) of the thorax in coronal plane of case 1. It shows honeycombing, traction bronchiectasis and reticulation with basal predominance, consistent with definite usual interstitial pneumonia (UIP) pattern.
2.2. Case 2
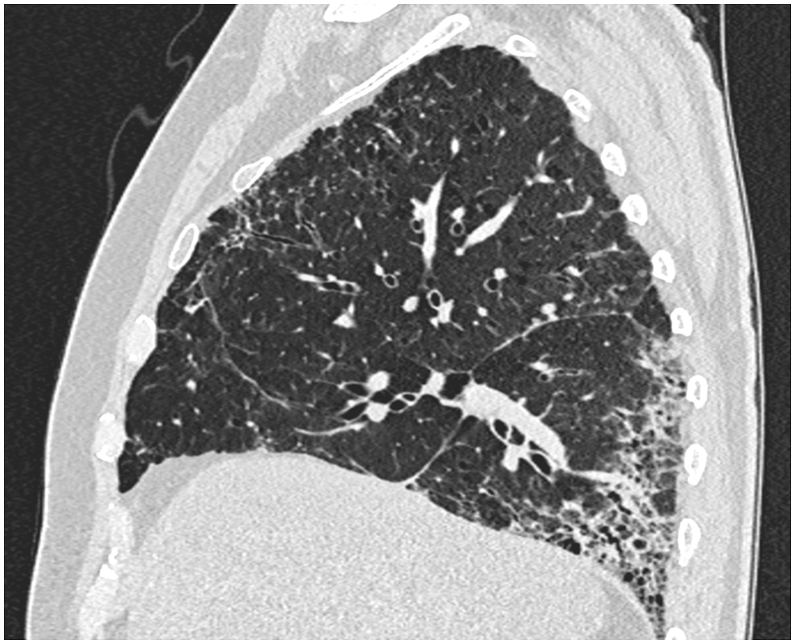
Blood samples, including EOS, ANA, ANCA and IgM-RF, were normal. The patients had no signs of infection and no recent traveling. The physical examination was normal. Six minute walk test distance was 500 m with desaturation from 97% to 91%. PFT showed a FEV1 2.82 L (90%), FVC 3.77 (94%), ratio of 96%, DLCO 49% and TLC 73%. HRCT (Image 2) showed definite UIP pattern with honeycombing, traction bronchiectasis and reticulation with basal predominance. BALF cell count showed 51% eosinophils, 48% macrophages and 2% mast cells. Treatment with high-dose prednisolone was initiated, but PFT did not improve. In a multidisciplinary team discussion, the patient was diagnosed with IPF based on age, smoking history, definite UIP pattern on HRCT and non-responsiveness to steroids. Subsequently, the patient was recommended anti-fibrotic therapy to prevent progression, but rejected it. Lung function and symptoms has been stable for 14 months.
Image 2.
High-resolution computed tomography of the thorax in sagittal plane of case 2. It shows honeycombing, traction bronchiectasis and reticulation with basal predominance, consistent with definite UIP pattern.
3. Discussion
3.1. Guidelines for diagnosis of IPF: BAL or not?
In the appropriate clinical setting, the presence of a UIP pattern on HRCT is sufficient for the diagnosis of IPF, given the high-quality evidence regarding HRCT specificity for the recognition of histopathologic UIP pattern [4], [8], [9], [10]. Several studies have documented that UIP pattern on HRCT is highly accurate for the presence of UIP pattern on a surgical lung biopsy [3], [8], [11], [12]. A surgical biopsy was not performed in the cases presented, as both had definite UIP pattern on HRCT and no obvious exposures. In retrospect a biopsy would have been academically interesting, but without consequences for diagnosis or treatment. However, it would have been of interest to see whether a biopsy had revealed coexistent eosinophilic pneumonia, as found by Yousem et al. [7], especially as BAL not were performed in his cases.
The clinical utility of BAL cell analysis for the diagnosis and management of patients with ILD has been a subject of debate and controversy. The recommendations concerning the use of BAL in patients with ILD were reversed in 2012, based on an expert group assessment of the relevant literature [13]. Though, when used in conjunction with comprehensive clinical information and HRCT scan, BAL cell patterns and other characteristics frequently provide useful information for the diagnostic evaluation of patients with suspected ILD [4]. Recent retrospective data suggest that 8% of patients with an HRCT UIP pattern may have BAL findings suggestive of an alternative diagnosis. It is unclear whether BAL adds significant diagnostic specificity to a careful exposure history and clinical evaluation [14].
3.2. The importance of eosinophilia
Eosinophils are relatively rare in the normal lung and so they stand out both in tissue and airway lumen samples when present in increased numbers. Eosinophils have been proven to be cytotoxic and pro-inflammatory effector cells in non-infectious disorders where they are prominent, though the extent to which eosinophils cause tissue damage remains controversial [15], [16], [17], [18], [19], [20], [21].
The significance of eosinophil alveolitis in UIP is unclear. A level of eosinophils >3% in BALF of patients with UIP are associated with a poor response to steroids, more severe functional abnormalities, and a worse prognosis [22], [23], [24], [25]. Yousem et al. suggested that eosinophilic pneumonia may be one histologic correlate of these zones of activity [12]. Previous studies of UIP cases report maximum 25% eosinophils in BALF. Modest increases in BAL eosinophils (<25%) have been found in up to 45% of the cases [23], [25], [26], [27]. Histologic correlations with BAL studies fail to show associations of BALF eosinophilia and specific morphologic patterns of injury [25], [28], [29].
3.3. Coexisting eosinophilic pneumonia and UIP in previous study
Yousem et al. report six patients with idiopathic UIP who had patchy areas of eosinophilic pneumonia superimposed on the underlying UIP pattern. Five of the six patients were men and smokers. The average age was 49 years (range: 38–58 years) and neither had a history of peripheral blood eosinophilia. Unfortunately, none of the six patients had BAL performed before biopsy. The study suggested that the interstitial and air space damage associated with air space eosinophils may represent an atypical form of active interstitial injury [7]. Four of the patients had high-dose steroids, two combined with cyclophosphamide and one with methotrexate. One remained alive with evidence of disease and three died of their disease after respectively 4, 13 and 18 months. Two patients had single lung transplantation.
3.4. Differential diagnosis
Eosinophil alveolitis in the setting of a radiologic UIP pattern raises several differential diagnostic possibilities. A markedly increased eosinophil count (≥25%) in BAL is typically a manifestation of idiopathic acute eosinophilic pneumonia (AEP), chronic eosinophilic pneumonia (CEP), Churg Strauss syndrome with active pneumonitis, tropical pulmonary eosinophilia or the hypereosinophilic syndrome (HES). These diseases are characterized by an increased number of eosinophils in peripheral blood, in lung tissue, in sputum, in BALF, or in all of these [6], [26], [30].
Cigarette smoking has been associated with increased BALF eosinophils in the setting of UIP, but masses of air space eosinophils are not seen in smokers' bronchiolitis. There is a strong association between tobacco smoking and AEP. A study from 2014 [31] showed that smoking increases the BAL levels of IL-5 and IL-1RA and eosinophils which is associated with the onset of AEP. Typically, BAL eosinophils are less than 5% in smokers [32], [33]. Smoking is also considered as a possible agent in the pathogenesis of IPF [2], [13].
4. Conclusion
In summary, this report describes two patients with a radiologic definite UIP pattern with coexistent eosinophil alveolitis. Based on HRCT, lack of treatment response and a disease course compatible with IPF, both patients were classified as IPF after a multidisciplinary team discussion. Thus, a high level BALF eosinophilia (40–50%) may occur among patients with IPF.
Funding source
No funding was secured for this study.
Financial disclosure
The authors have no financial relationships relevant for this article to disclose.
Conflict of interest
The authors have no conflict of interest to disclose.
Acknowledgements
None.
References
- 1.Directors AB of Committie EE American thoracic society idiopathic pulmonary fibrosis: diagnosis and treatment. Am. J. Respir. Crit. Care Med. 2000;161:646–664. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.Travis W.D., Costabel U., Hansell D.M. An official american thoracic society/european respiratory society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am. J. Respir. Crit. Care Med. 2013;188(6):733–748. doi: 10.1164/rccm.201308-1483ST. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raghu G., Collard H.R., Egan J.J. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011;183(6):788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutchinson J.P., Fogarty A.W., McKeever T.M., Hubbard R.B. In-hospital mortality after surgical lung biopsy for interstitial lung disease in the United States. 2000 to 2011. Am. J. Respir. Crit. Care Med. 2016 May 15;193(10):1161–1167. doi: 10.1164/rccm.201508-1632OC. [DOI] [PubMed] [Google Scholar]
- 5.Ravaglia C., Bonifazi M., Wells A.U. Safety and diagnostic yield of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases: a comparative study versus video-assisted thoracoscopic lung biopsy and a systematic review of the literature. Respiration. 2016;91(3):215–227. doi: 10.1159/000444089. [DOI] [PubMed] [Google Scholar]
- 6.Rotolo N., Imperatori A., Dominioni L. Efficacy and safety of surgical lung biopsy for interstitial disease. Experience of 161 consecutive patients from a single institution in Italy. Sarcoidosis Vasc. Diffuse Lung Dis. 2015 Sep 14;32(3):251–258. [PubMed] [Google Scholar]
- 7.Yousem S.A. Eosinophilic pneumonia-like areas in idiopathic usual interstitial pneumonia. [DOI] [PubMed]
- 8.Hunninghake G.W., Bridget Zimmerman M., Schwartz D.A. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2001;164:193–196. doi: 10.1164/ajrccm.164.2.2101090. [DOI] [PubMed] [Google Scholar]
- 9.Flaherty K.R., Thwaite E.L., Kazerooni E.A. Radiological versus histological diagnosis in UIP and NSIP: survival implications. Thorax. 2003;58:143–148. doi: 10.1136/thorax.58.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quadrelli S., Molinari L., Ciallella L. Radiological versus histopathological diagnosis of usual interstitial pneumonia in the clinical practice: does it have any survival difference? Respiration. 2010;79(1):32–37. doi: 10.1159/000225987. [DOI] [PubMed] [Google Scholar]
- 11.Grenier P., Valeyre D., Cluzel P. Chronic diffuse interstitial lung disease: diagnostic value of chest radiography and high-resolution CT. Radiology. 1991;179(1):123–132. doi: 10.1148/radiology.179.1.2006262. [DOI] [PubMed] [Google Scholar]
- 12.Swensen S.J., Aughenbaugh G.L.M.J. Diffuse lung disease: diagnostic accuracy of CT in patients undergoing surgical biopsy of the lung. Radiol. 1997;205(1):229–234. doi: 10.1148/radiology.205.1.9314990. [DOI] [PubMed] [Google Scholar]
- 13.Meyer K.C., Raghu G., Baughman R.P. American thoracic society documents an official american thoracic society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am. J. Respir. Crit. Care Med. 2012;185(9):1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 14.Ohshimo S., Bonella F., Cui A. Significance of bronchoalveolar lavage for the diagnosis of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2009;179:1043–1047. doi: 10.1164/rccm.200808-1313OC. [DOI] [PubMed] [Google Scholar]
- 15.Alberts W.M. Eosinophilic interstitial lung disease. Curr. Opin. Pulm. Med. 2004;10(5):419–424. doi: 10.1097/01.mcp.0000130330.29422.8d. [DOI] [PubMed] [Google Scholar]
- 16.Jeong Y.J., Kim K.-I., Im Seo J. Eosinophilic lung diseases: a clinical, radiologic, and pathologic overview. Radiograpics [Internet] 2007;27:617–637. doi: 10.1148/rg.273065051. Available from: http:// [DOI] [PubMed] [Google Scholar]
- 17.Katz U., Shoenfeld Y. Pulmonary eosinophilia. Allergy Immunol. 2008;34:367–371. doi: 10.1007/s12016-007-8053-y. [DOI] [PubMed] [Google Scholar]
- 18.Roufosse F., Weller P.F. Practical approach to the patient with hypereosinophilia. J. Allergy Clin. Immunol. 2010;126:39–44. doi: 10.1016/j.jaci.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noguchi H., Kephart G.M., Colby T.V. Tissue Eosinophilia and eosinophil degranulation in syndromes associated with fibrosis. Am. J. Pathol. 1992;140(2):521–528. [PMC free article] [PubMed] [Google Scholar]
- 20.Davis W.B, Fells G.A, Sun X, et al. Eosinophil-mediated injury to lung parenchymal cells and interstitial matrix (8):269–278. [DOI] [PMC free article] [PubMed]
- 21.Boomars K.A., Schweizer R.C., Zanen P. Eosinophil chemotactic activity in bronchoalveolar lavage from idiopathic pulmonary fibrosis is dependent on cytokine priming of eosinophils. Eur. Respir. J. 1998;11:1009–1014. doi: 10.1183/09031936.98.11051009. [DOI] [PubMed] [Google Scholar]
- 22.Wells A.U., Cullinan P., Hansell D.M. Fibrosing alveolitis associated with systematic sclerosis has a better prognosis than lone cryptogenic fibrosing alveolitis. Am. J. Respir. Crit. Care Med. 1994;149:1583–1590. doi: 10.1164/ajrccm.149.6.8004317. [DOI] [PubMed] [Google Scholar]
- 23.Wells A.U., Hansell D.M., Rubens M.B. Fibrosing alveolitis in systematic sclerosis. Bronchoalveolar lavage findings in relation to computed tomographic appearance. Am. J. Respir. Crit. Care Med. 1994;150:462–468. doi: 10.1164/ajrccm.150.2.8049830. [DOI] [PubMed] [Google Scholar]
- 24.Noble P.W., Albera C., Bradford W.Z. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011 May;377(9779):1760–1769. doi: 10.1016/S0140-6736(11)60405-4. [DOI] [PubMed] [Google Scholar]
- 25.Boomars K.A., Wagenaar S.S., Mulder P.G.H. Relationship between cells obtained by bronchoalveolar lavage and survival in idiopathic pulmonary fibrosis. Thorax. 1995;50:1087–1092. doi: 10.1136/thx.50.10.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen J., Davis W., Pacht E. Diagnostic significance of increased bronchoalveolar lavage fluid eosinophils. Am. Rev. Respir. Dis. 1990;142(3):642–647. doi: 10.1164/ajrccm/142.3.642. [DOI] [PubMed] [Google Scholar]
- 27.Wells A.U., Hansell D.M., Haslam P.L. Bronchoalveolar lavage cellularity: lone cryptogenic fibrosing alveolitis compared with the fibrosing alveolitis of systemic sclerosis. Am. J. Respir. Crit. Care Med. 1998;157:1474–1482. doi: 10.1164/ajrccm.157.5.9609096. [DOI] [PubMed] [Google Scholar]
- 28.Hyde D.M., King T.E., Jr., McDermott T. Idiopathic pulmonary fibrosis. Quantitative assessment of lung pathology. Comparison of a semiquantitative and a morphometric histopathologic scoring system. Am. Rev. Respir. Dis. 1992;146:1042–1047. doi: 10.1164/ajrccm/146.4.1042. [DOI] [PubMed] [Google Scholar]
- 29.Cherniack R., Colby T., Flint A. Correlation of structure and function in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1995;151:1180–1188. doi: 10.1164/ajrccm/151.4.1180. [DOI] [PubMed] [Google Scholar]
- 30.Akuthota P., Weller P.F. Eosinophilic pneumonias. Clin. Microbiol. Rev. 2012;25:649. doi: 10.1128/CMR.00025-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teng Y., Gao Y. 2014. Tobacco Smoking Associated with the Increases of the Bronchoalveolar Levels of Interleukin-5 and Interleukin-1 Receptor Antagonist in Acute Eosinophilic Pneumonia; pp. 887–893. [PubMed] [Google Scholar]
- 32.Heron M., Grutters J.C., Hijdra D. Bronchoalveolar lavage cell pattern from healthy human lung. Clin. Exp. Immunol. 2011:523–531. doi: 10.1111/j.1365-2249.2011.04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domagala-Kulawik J. BAL in the diagnosis of smoking-related interstitial lung diseases: review of literature and analysis of. Diag. Cytopathol. 2008;36(12):909–915. doi: 10.1002/dc.20944. [DOI] [PubMed] [Google Scholar]