SUMMARY
In 2006, a new virus, xenotropic murine leukemia virus-related virus (XMRV), was discovered in a cohort of U.S. men with prostate cancer. Soon after this initial finding, XMRV was also detected in samples from patients with chronic fatigue syndrome (CFS). The blood community, which is highly sensitive to the threat of emerging infectious diseases since the HIV/AIDS crisis, recommended indefinite deferral of all blood donors with a history of CFS. As XMRV research progressed, conflicting results emerged regarding the importance of this virus in the pathophysiology of prostate cancer and/or CFS. Molecular biologists traced the development of XMRV to a recombination event in a laboratory mouse that likely occurred circa 1993. The virus was propagated via cell lines derived from a tumor present in this mouse and spread through contamination of laboratory samples. Well-controlled experiments showed that detection of XMRV was due to contaminated samples and was not a marker of or a causal factor in prostate cancer or CFS. This paper traces the development of XMRV in the prostate and CFS scientific communities and explores the effect it had on the blood community.
INTRODUCTION
In 2006, the scientific community was introduced to a novel gammaretrovirus that was detected in a cohort of prostate tumors in an article entitled “Identification of a novel gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant” (1). The virus was related to xenotropic murine leukemia viruses (MLVs) but was distinct. Because of its relationship to these viruses, it was given the name xenotropic MLV-related virus (XMRV). The reports of this new virus prompted numerous investigations into the mechanisms of susceptibility, the described association with prostate cancer, and potential associations with other diseases.
RNase L
RNase L plays a significant role in the innate immune reaction to viral infections, and with interferon, it contributes to the regulation of apoptosis and cell proliferation through the 2′-5′-oligoadenylate (2-5A) pathway (2). The pathway can be activated by the presence of double-stranded RNA, a component that is commonly produced in viral infections, and leads to 2-5A being produced (3). The binding of 2-5A with RNase L results in viral and cellular RNAs being degraded and contributing to viral immunity, and those with defects in the RNase L system have an increased risk of viral infections (4). The signaling from 2-5A can lead to apoptosis of cells as a reaction to viral and nonviral triggers, and defects in RNase L may have a role in cancer biology (3). One such defect includes the R462Q variant, which has an arginine-to-glutamine substitution. A report implied a connection in up to 13% of prostatic cancers, and thus, it was a possible candidate for the hereditary prostate cancer gene (HPC-1) (2, 5). Investigations into the functionality of the different variants of the RNase L gene showed that the activity is decreased in lymphocytes from heterozygous individuals compared to those that are homozygous for the wild type (2). The R462Q variant has one-third of the enzymatic activity of the wild type, and a significant association with prostate cancer risk has been described (5). When comparing men who are heterozygous for the mutated allele to those who do not carry a mutation, the risk of developing prostate cancer increases by 50%. For men who are homozygous, the risk more than doubles (5).
Xiang et al. produced a biostable analogue of 2-5A to better understand the effects of different RNase L gene variants on the signaling pathway (6). They then compared the levels of RNase L activity for different naturally occurring mutations and polymorphisms in RNase L. For many of the variants, the levels of RNase L activity were similar to those of the wild-type enzyme; however, while the R462Q variant bound 2-5A at levels similar to those of the wild-type enzyme, it had a three-fold decrease in RNase activity and was deficient in causing apoptosis (6).
ORIGINS IN PROSTATE CANCER
Because of the described impairment in apoptosis, this mechanism has been suspected to play a role in malignancy as a tumor suppressor gene (6). Associations between RNase L mutations and prostate cancer susceptibility have been reported, and additional investigations have continued (2, 5, 7, 8). Urisman et al. set out to investigate the described relationship between prostate cancer and germ line mutations in RNase L (1). Since defects in RNase L may leave patients more susceptible to viral infections, they hoped to identify a virus that could be implicated in the disease.
Urisman used oligonucleotides representing all known viral families to screen a DNA microarray of prostate tumors. With this approach, sequences from a gammaretrovirus were identified in 7 of 11 homozygous R462Q patients and in 1 of 8 homozygous and heterozygous wild-type patients. They widened their search to include a total of 86 tumors and added specific reverse transcriptase PCR to the analysis. The gammaretrovirus was detected in 8 (40%) of the 20 homozygous R462Q samples compared to just a single case from a group of 66 heterozygous and wild-type cases (1.5%). The virus showed some homology with xenotropic MLVs but was distinct, and it was named XMRV.
Laboratory contamination was a potential confounder that concerned Urisman et al.; possible opportunities included the culturing of cell lines and passage through nude mice, but it was ruled out as a potential explanation for their results. The evidence they considered included the following. The detection of XMRV occurred in primary human tissues, no murine sequences were detected by PCR, the presence of the virus was predominately limited to R462Q human tumor samples, polymorphisms in the XMRV clones seen in different samples was consistent with individual acquisition of the virus, and evidence of the virus could be detected by immunohistochemistry and fluorescence in situ hybridization (FISH) in the prostate cancer samples. When considering all of the information available at the time, it was felt that the argument against laboratory contamination with cloned DNA or viral material was strong (1).
The finding of Urisman et al. represented a potential breakthrough in the etiology of some prostate cancers. Many laboratories around the world attempted to reproduce their finding, but there were mixed results. Most notably, labs in the United States detected XMRV in their prostate cancer cohorts with described associations with high-grade tumors (9), but laboratories in Germany, Mexico, Japan, and the Netherlands found, at most, minimal evidence of XMRV DNA in their patient samples (10–13). The international experience with the virus did not come close to corroborating the reports from the United States; was this potentially due to geographic differences in the virus, or were there other contributing factors?
CFS
The introduction of the novel virus to the scientific community prompted further investigation of its presence in other diseases (14–16). Extrapolating from the logic used with prostate cancer, chronic fatigue syndrome (CFS) was investigated because of its similar association with defects in the RNase L pathway and other immune deficiencies (17). In 2009, Lombardi et al. published a report of XMRV DNA detection in 68 (67%) of 101 patients with CFS but in only 8 (3.7%) of 218 of healthy controls (18). They reported the ability to detect the virus in unstimulated peripheral blood mononuclear cells (18–20) and demonstrated the virus's ability to infect human lymphocytes in vitro (18, 21). Electron microscopy demonstrated actual viral particles budding from infected cells in culture.
CFS is a widespread illness characterized by profound fatigue, cognitive dysfunction, sleep abnormalities, autonomic manifestations, pain, and other symptoms exacerbated by exertion of any sort. Between 836,000 and 2.5 million individuals in the United States are estimated to be affected (22). An accurate diagnosis is wrought with difficulty, as many of these symptoms are commonly encountered and can overlap those of other syndromes. The underlying pathophysiology has not been delineated, and the diagnostic criteria are contested, so the prospect of an etiology in the form of XMRV was met with great excitement.
Many researchers attempted to duplicate the findings described by Lombardi et al.; however, none was able to demonstrate the presence of XMRV in CFS patients (23–28). International groups in the United Kingdom (26), Canada (29), China (30), the Netherlands (27), and Germany (21) examined their own populations and found no evidence of infection. Multiple groups within the United States scrupulously tried to duplicate the protocols of Lombardi et al., applying the same methodology and patient selection criteria and even retesting a subset of those previously identified as being XMRV positive (24, 31). These continued to be negative.
One U.S. group, led by Lo et al., screened 41 retrospective DNA samples from 37 individuals who met the criteria for a diagnosis of CFS. While they did not detect XMRV sequences, in 32 (86.5%) of 37 patients, they did find MLV-related sequences and compared that to healthy volunteer blood donors, in only 3 out of 44 of whom they detected such sequences (16). Even though the MLV-related virus sequences were not closely related to XMRV, this finding was frequently cited as a confirmation of the presence of XMRV in CFS patients.
BLOOD SUPPLY
Despite the inconsistent results obtained when screening donor samples, the blood community was alarmed by the specter of a virus that might cause disease and may be transmissible via transfusion. A panel of experts convened by the FDA met to discuss whether XMRV was a threat to the safety of the blood supply. The history of the AIDS epidemic and the blood community's historically slow response to the possibility of transfusion-transmitted HIV have helped to create a highly conservative approach to all potential blood-borne pathogens. The panel voted nine to four in favor of an indefinite deferral of all individuals presenting to donate blood with a medical history and/or diagnosis of CFS. The American Red Cross was quick to adopt this policy, and other international blood suppliers followed suit. The Chronic Fatigue and Immune Dysfunction Syndrome Association of America issued a statement to “…individuals with a past or present diagnosis of CFS to refrain from giving blood and donating organs to protect the safety of the blood and transplant organs supply for all recipients.”
CONTAMINATION
Any new retrovirus with proposed chronic disease or tumor association is met with extreme scrutiny by the virology community, as many of these associations do not pan out (32). This skepticism may be justified, as many prior cases of “human retroviruses” were found to be little more than laboratory artifacts (32–34). With the increasing number of XMRV-negative studies, suspicions of contamination continued to rise.
Multiple independent articles in a 2010 issue of Retrovirology addressed the important concern of contamination as the explanation of the findings (33, 35–39). While setting out to investigate XMRV in a group of CFS patients, Sato et al. discovered that the negative control was positive at the expected product size for a region of XMRV. They subsequently investigated multiple commercial kits and found a contaminant of endogenous MLV sequences that was amplified with primer sets designed to detect XMRV (38). Hué et al. demonstrated that mouse DNA was capable of contaminating patient samples with an assortment of endogenous MLV proviruses that are identified with PCR primers designed and reported to be specific for XMRV (35). Their observations suggested that XMRV is not an exogenous virus that is transmitted from person to person, and they proposed that lab contamination could explain the disease-associated XMRV sequences and that XMRV might not be a pathogen affecting humans (35). The groups voiced concerns that future work required more care and rigorous PCR protocols to detect and minimize contamination (37, 38). As time progressed, sources of potential contamination continued to be described, including laboratory reagents, as well as cell lines (37, 40–42).
TRACING THE ORIGIN OF XMRV
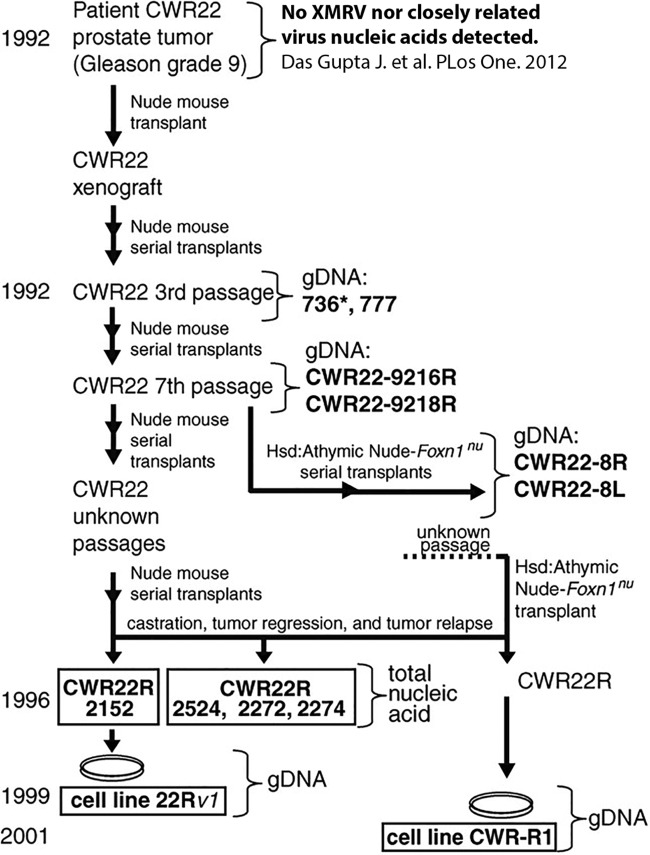
As the evidence of contamination strengthened, a group of virologists tried to trace the origin of XMRV, postulating that a better understanding of the retrovirus might help to explain the discrepant results (43). These researchers returned to the original cell lines cited in the literature that first uncovered the association between XMRV and prostate carcinoma patients (1). They analyzed cell lines CWR22Rv1 and CWR-R1, derived from human prostate cancer, and found XMRV sequences nearly identical to the viral sequences seen in human subjects (Fig. 1). They traced these cell lines back in time by analyzing earlier passages that had been stored in laboratory freezers. This work led them to CWR22, the progenitor human prostate tumor xenograft, isolated originally from a prostate cancer patient and combined with a nude mouse. The CWR22 xenograft was made in 1992 and serially passaged in mice. The original xenograft was not available for analysis at this time; however, genomic DNA from the third and seventh passages was isolated and sequenced. Through careful sleuthing, the researchers eventually uncovered multiple other passages of CWR22 from various laboratories in the United States (Fig. 1) in addition to the related cell lines 22Rv1 and CWR-R1.
FIG 1.
Characterization of CWR22 xenografts and XMRV-related sequences. The genesis of the 22Rv1 and CWR-R1 cell lines is shown. Bold letters indicate samples from which genomic DNA (gDNA) or nucleic acid was available for analysis. XMRV-positive samples are boxed. (Reproduced from reference 43 with the permission of the American Association for the Advancement of Science, with additional information from reference 44.)
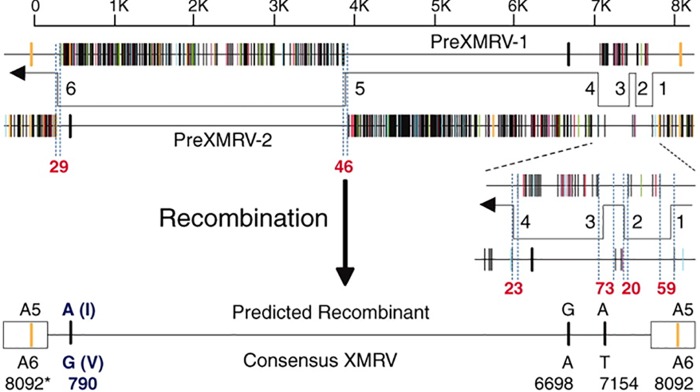
PCR amplification and sequencing of the early passages of the xenografts revealed incomplete XMRV sequences and the presence of a novel XMRV-related provirus the researchers labeled pre-XMRV-1. A survey of additional laboratory and wild-type mice with XMRV-specific primers revealed a second novel XMRV-related provirus, pre-XMRV-2. Full sequencing of these two proviruses showed that the nearly homologous regions in XMRV were shared and nonoverlapping (Fig. 2). This led to the hypothesis that a recombination event between pre-XMRV-1 and -2 resulted in the formation of XMRV. An alignment of the pre-XMRV-1 and pre-XMRV-2 sequences showed near identity (99.92%) with the XMRV sequence, with only a 3-bp difference (Fig. 2) (43). The authors determined that “XMRV was generated as a result of a unique recombination event between two endogenous MLVs that took place around 1993 to 1996 in a nude mouse carrying the CWR22 PC xenograft.” This recombination event involved six template-switching events between the two proviruses, resulting in XMRV. They calculated that the probability of this virus arising by random chance was 1.3 × 10−12; therefore, “… any XMRV isolates with the same or nearly the same sequences identified elsewhere originated from this event” (43). Subsequently, the paraffin blocks from the original prostate cancer tumor were recovered and analyzed by PCR and FISH. Within the primary tissue sources from patient CWR22, there was no evidence of XMRV and no closely related viral components were identified. This helped solidify the interpretation that XMRV was the result of a recombination event, as it was absent from the original prostate cancer tissue (44).
FIG 2.
Predicted recombinant formed by pre-XMRV-1 and pre-XMRV-2 is nearly identical to XMRV. Alignment of plots of pre-XMRV-1 and pre-XMRV-2 reveals the reciprocal and largely nonoverlapping regions of near identity to XMRV. The predicted recombinant and the four nucleotide differences from the consensus XMRV sequence are shown, including the six template-switching events. (Reproduced from reference 43 with the permission of the American Association for the Advancement of Science.)
INVESTIGATION: IS XMRV A CONTAMINANT?
This finding prompted a multilaboratory study to confirm or refute the claim that XMRV was a contaminant in U.S. labs and likely not a causative factor in CFS. While prostate cancer was also linked to XMRV, the transfusion community initiated this study to see whether blood donors with CFS were unnecessarily deferred. Since prostate cancer is already a cause for deferral, the link between XMRV and prostate cancer was not considered.
The researchers assembled blood samples from 15 subjects documented as XMRV positive (14/15 with CFS) and a matching cohort of 15 healthy, XMRV-negative subjects. Nine laboratories, blind to the sample identities, completed tests to detect XMRV/MLV replication, nucleic acids, and antibodies. Extensive precautions were taken to minimize the introduction of potential contaminants and also to keep participating personnel blind to the sample identities. Independent phlebotomists were used to draw samples, which were shipped to a central lab and processed into coded samples. Also, a separate lab prepared aliquots of 22Rv1, the human cell line infected with XMRV. The supernatant from these stocks was used to create positive-control samples.
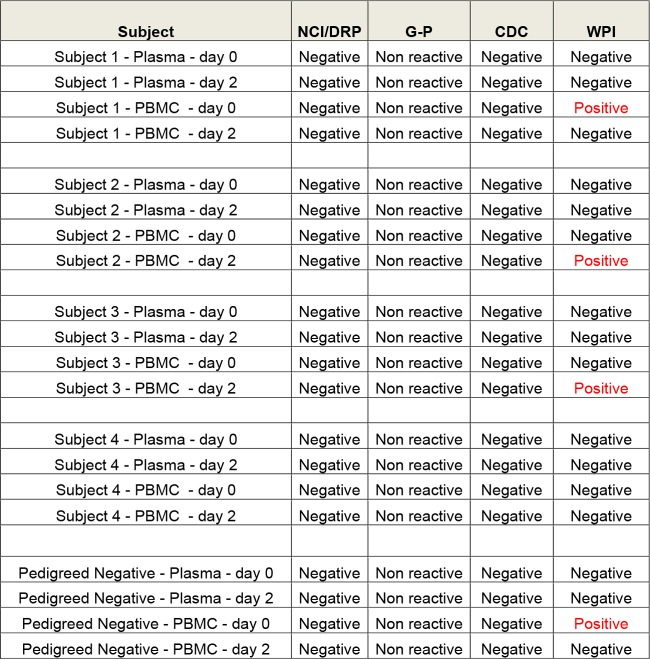
The participating laboratories were largely those that had either reported XMRV present in patient samples or participated in the original investigation of contamination. Initial assays assessed the sensitivity of detection in whole blood and plasma (Fig. 3). The results from the Whittemore Peterson Institute lab indicated technical problems, as the limit of detection skipped several dilutions, jumping from 80 viral RNA copies/ml to 0.128 copy/ml (Fig. 3) (45). The labs also performed a total of 11 nucleic acid tests (NATs) and five serologic and three culture assays. The FDA/Lo laboratory, which had originally reported MLV sequences in a retrospective cohort (16), failed to detect XMRV/MLV sequences in the replicates. Only the Lombardi laboratory, which had made the original finding of XMRV in CFS patient samples (18), reported a positive NAT result for a few of the duplicates from clinical samples (Fig. 4). Further testing in this lab showed that this result was not reproducible in replicate samples. In addition, this lab reported similar reactivity in CFS and negative-control samples. These results indicated that the finding of XMRV/MLV in blood samples was not reproducible and that screening of blood donors was not necessary (46).
FIG 3.
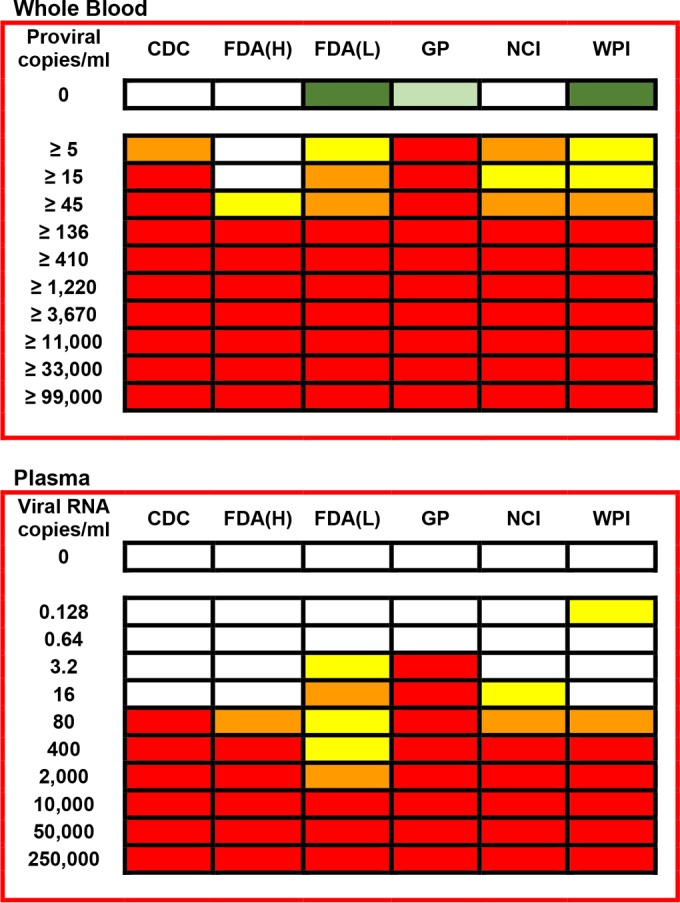
Sensitivity testing for XMRV in laboratories. Analytic sensitivity of spiked XMRV detection in multiple labs is shown. An investigation of XMRV involved carefully controlled testing of sensitivity for different XMRV assays. Serial dilutions of XMRV-infected cells were assayed in triplicate. Labs used a variety of techniques for detection, including nested PCR, quantitative PCR, and transcription-mediated amplification. Abbreviations: GP, GenProbe; FDA(H), lab of Hewlett; FDA(Lo), lab of S. C. Lo; WPI, Whittemore Peterson Institute; NCI, National Cancer Institute. Colors: red, 3/3 positive; orange, 2/3 positive; yellow, 1/3 positive; white, 0/3 positive. Replicates of six negative samples were performed, and white represents 0/6 while green represents 1/6 replicates being positive. In the cases of FDA(Lo) and WPI, subsequent sequencing demonstrated in each case that the amplification product in the single false-positive result for a negative-control sample was of human genomic origin. In the case of GP, a repeat by a separate operator yielded 0/6 controls as positive. (Reproduced from reference 44 with permission of John Wiley and Sons.)
FIG 4.
Results of blind panel testing. Blood was collected from four patients with CFS found to be XMRV positive in the 2009 Whittemore Peterson Institute (WPI) study published in Science, as well as XMRV/MRV-negative controls. Specimen tubes were then divided into three groups, with one set immediately processed into peripheral blood mononuclear cells (PBMC), whole blood, and plasma, while the other two sets were refrigerated and then similarly processed after 2 and 4 days. These samples were coded, blinded, and distributed to three laboratories—the CDC, the National Cancer Institute (NCI), and the WPI, as well as to GenProbe. False-positive results were reported by the Whittemore Peterson Institute (Lombardi) laboratory. DRP, HIV Dynamics and Replication Program. (Based on data from reference 45.)
This conclusion was bolstered by the findings of Dodd et al., who had set out to determine the viral prevalence in blood donors and subsequently the risk of transmission by transfusion (47). They investigated a large cohort of U.S. blood donors, as well as linked donor-recipient samples. In >17,200 blood donors or recipients, no XMRV antibodies could be detected (0%). Additionally, 1,763 specimens tested for XMRV RNA were all nonreactive. International groups found an absence of XMRV sequences in their blood donor populations (48–50). In Brazil, they investigated multiply transfused patients with beta-thalassemia major or sickle cell disease and identified no XMRV/MLV sequences in this group (51). On the basis of their own data and the negative findings previously outlined, Dodd et al. came to the conclusion that XMRV poses no current hazard to people receiving blood and that no additional action related to XMRV and/or MLV and the safety of blood was necessary.
THE END
The original group that found XMRV in prostate cancer samples published a paper showing that further analysis of prostate cancer samples showed no evidence of XMRV sequences (52). The major publications on the topic began to be retracted; one of the longest holdouts ended in 2014 when Schlaberg et al. retracted their article describing the presence of XMRV in prostate cancer cells (53). The original article reporting XMRV in CFS patient samples was at first partially retracted and then fully retracted shortly thereafter (54, 55). The subsequent article by Lo et al., reporting MLV-like sequences in a CFS cohort, was fully retracted (56). Table 1 contains a timeline of events related to XMRV (Table 1). The American Red Cross and other international blood centers withdrew the deferral of donors with CFS or CFS-like symptoms. The CFS Society, however, continues to warn people with CFS not to donate blood. This warning is general, and no mention of XMRV is made. The AABB released an updated fact sheet in 2012 to reflect the work that had been performed related to XMRV, showing that there was no valid evidence that the virus infects humans or is associated with human disease. They note that no FDA guidance or AABB standards exist for XMRV deferrals and they are not necessary since XMRV does not infect humans. Acceptance or deferral of patients with a history of CFS will be at the discretion of the collection facility on the basis of clinical judgment of the health of the donor (57).
TABLE 1.
Timeline of events related to XMRV
| Yr | Event |
|---|---|
| 1992 | Prostate cancer xenograft made from CWR22 sample |
| 1999 | New prostate carcinoma cell line 22Rv1 used in labs |
| 2006 | XMRV identified in prostate cancer samples |
| 2008–2011 | Multiple labs test prostate cancer samples for XMRV |
| 2009 | XMRV identified in CFS patient samples |
| 2010 | MLV-related sequences identified in CFS patient samples |
| 2010 | Multiple labs test CFS samples for XMRV |
| 2010 | Food and Drug Administration panel of experts recommends indefinite deferral of blood donors with CFS |
| 2010–2011 | Red Cross in multiple countries defers donors with history of or symptoms consistent with CFS |
| 2011 | Investigation of XMRV and possible contamination |
| 2011 | Article published tracing origin of XMRV to laboratory recombination event |
| 2012 | Key XMRV and MLV papers retracted |
What Is Next?
The gammaretrovirus XMRV was “created” in a lab, stumbled upon and amplified in the search for a cause of prostate cancer, and elevated into a cause célèbre for CFS. Warnings that XMRV had been detected in the normal population and may cause CFS led to a strong response in the blood community, with permanent deferral of all donors with a diagnosis or symptoms of CFS. This response was appropriate, given the importance of blood safety; however, the XMRV crisis highlights the risk represented by any new pathogen. Currently in the United States, there is concern over the rise in West Nile, dengue, Chikungunya, Ebola, Zika, and other viruses. Each new disease requires a massive effort to understand the clinical presentation, characterize the means of transmission, measure infectivity, and assess the morbidity and mortality that might occur. Additional research is then required to develop screening tests, as well as therapeutic interventions.
Screening tests take time to implement, are expensive, and are only useful for a given pathogen. Nonspecific pathogen reduction/inactivation technologies for blood components have recently become available in the United States. Solvent detergent (SD) treatment of plasma reduces the risk of transmission of most clinically relevant infectious diseases, although SD treatment is not effective against nonenveloped viruses such as hepatitis A virus (58). Pathogen inactivation (PI) is another technology that uses agents such as amotosalen or riboflavin to disrupt nucleic acids (59). The FDA has approved one PI method for treating platelets and plasma, and additional products for red blood cells and whole blood are under way (60). The currently available PI method can inactivate bacteria, fungi, protozoa, and enveloped and some nonenveloped viruses (61). The advent and universal application of PI in the United States will go far to ensure blood safety when new diseases emerge; however, no method is perfect. As a result, continued vigilance and well-managed responses are needed to minimize the loss of blood donors while maintaining the integrity of the blood supply.
ACKNOWLEDGMENTS
C.S.C. has received honoraria and research funding from Fenwal, a Fresenius-Kabi company; has received research funding from Octapharma; and has received research funding and consulting fees from Ortho Diagnostics. A.D.J. has received research funding from Octapharma.
Biographies

Andrew D. Johnson is currently an assistant professor at the University of Minnesota in the Division of Transfusion Medicine in the Department of Laboratory Medicine and Pathology. He recently joined the faculty after completing an anatomic and clinical pathology residency and blood banking/transfusion medicine fellowship at the University of Minnesota. He serves as the medical director of the Apheresis Blood Donor Center, and his additional responsibilities include coverage of the special coagulation laboratory and cellular therapy laboratory, as well as teaching medical students, residents, and fellows. He has had a specific affinity for transfusion medicine for over 7 years, and his interest in XMRV originated with its impact on the blood supply and deferral of potential donors.

Claudia S. Cohn is the medical director of the blood bank laboratory and associate medical director of the HLA laboratory at the University of Minnesota. She earned her Ph.D. in immunology and infectious diseases from the Bloomberg School of Public Health at Johns Hopkins, working on the molecular biology and biochemistry of the protozoan parasite Leishmania donovani. She went on to earn her medical degree from the Louisiana State University School of Medicine in New Orleans, LA. After medical school, she finished a residency program in pathology at New York Presbyterian Hospital, Weill-Cornell School of Medicine, and a fellowship in Transfusion Medicine at the University of California, San Francisco. In her current role at University of Minnesota, she practices clinical pathology, oversees the blood bank, and teaches medical students.
REFERENCES
- 1.Urisman A, Molinaro RJ, Fischer N, Plummer SJ, Casey G, Klein EA, Malathi K, Magi-Galluzzi C, Tubbs RR, Ganem D, Silverman RH, DeRisi JL. 2006. Identification of a novel gammaretrovirus in prostate tumors of patients homozygous for R462Q RNASEL variant. PLoS Pathog 2:e25. doi: 10.1371/journal.ppat.0020025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Carpten J, Nupponen N, Isaacs S, Sood R, Robbins C, Xu J, Faruque M, Moses T, Ewing C, Gillanders E, Hu P, Bujnovszky P, Makalowska I, Baffoe-Bonnie A, Faith D, Smith J, Stephan D, Wiley K, Brownstein M, Gildea D, Kelly B, Jenkins R, Hostetter G, Matikainen M, Schleutker J, Klinger K, Connors T, Xiang Y, Wang Z, De Marzo A, Papadopoulos N, Kallioniemi OP, Burk R, Meyers D, Gronberg H, Meltzer P, Silverman R, Bailey-Wilson J, Walsh P, Isaacs W, Trent J. 2002. Germline mutations in the ribonuclease L gene in families showing linkage with HPC1. Nat Genet 30:181–184. doi: 10.1038/ng823. [DOI] [PubMed] [Google Scholar]
- 3.Liu W, Liang SL, Liu H, Silverman R, Zhou A. 2007. Tumour suppressor function of RNase L in a mouse model. Eur J Cancer 43:202–209. doi: 10.1016/j.ejca.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 4.Silverman RH. 2007. A scientific journey through the 2-5A/RNase L system. Cytokine Growth Factor Rev 18:381–388. doi: 10.1016/j.cytogfr.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey G, Neville PJ, Plummer SJ, Xiang Y, Krumroy LM, Klein EA, Catalona WJ, Nupponen N, Carpten JD, Trent JM, Silverman RH, Witte JS. 2002. RNASEL Arg462Gln variant is implicated in up to 13% of prostate cancer cases. Nat Genet 32:581–583. doi: 10.1038/ng1021. [DOI] [PubMed] [Google Scholar]
- 6.Xiang Y, Wang Z, Murakami J, Plummer S, Klein EA, Carpten JD, Trent JM, Isaacs WB, Casey G, Silverman RH. 2003. Effects of RNase L mutations associated with prostate cancer on apoptosis induced by 2′,5′-oligoadenylates. Cancer Res 63:6795–6801. [PubMed] [Google Scholar]
- 7.Rennert H, Bercovich D, Hubert A, Abeliovich D, Rozovsky U, Bar-Shira A, Soloviov S, Schreiber L, Matzkin H, Rennert G, Kadouri L, Peretz T, Yaron Y, Orr-Urtreger A. 2002. A novel founder mutation in the RNASEL gene, 471delAAAG, is associated with prostate cancer in Ashkenazi Jews. Am J Hum Genet 71:981–984. doi: 10.1086/342775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rökman A, Ikonen T, Seppala EH, Nupponen N, Autio V, Mononen N, Bailey-Wilson J, Trent J, Carpten J, Matikainen MP, Koivisto PA, Tammela TL, Kallioniemi OP, Schleutker J. 2002. Germline alterations of the RNASEL gene, a candidate HPC1 gene at 1q25, in patients and families with prostate cancer. Am J Hum Genet 70:1299–1304. doi: 10.1086/340450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlaberg R, Choe DJ, Brown KR, Thaker HM, Singh IR. 2009. XMRV is present in malignant prostatic epithelium and is associated with prostate cancer, especially high-grade tumors. Proc Natl Acad Sci U S A 106:16351–16356. doi: 10.1073/pnas.0906922106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10.Hohn O, Krause H, Barbarotto P, Niederstadt L, Beimforde N, Denner J, Miller K, Kurth R, Bannert N. 2009. Lack of evidence for xenotropic murine leukemia virus-related virus (XMRV) in German prostate cancer patients. Retrovirology 6:92. doi: 10.1186/1742-4690-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez-Fierro ML, Leach RJ, Gomez-Guerra LS, Garza-Guajardo R, Johnson-Pais T, Beuten J, Morales-Rodriguez IB, Hernandez-Ordonez MA, Calderon-Cardenas G, Ortiz-Lopez R, Rivas-Estilla AM, Ancer-Rodriguez J, Rojas-Martinez A. 2010. Identification of viral infections in the prostate and evaluation of their association with cancer. BMC Cancer 10:326. doi: 10.1186/1471-2407-10-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuta RA, Miyazawa T, Sugiyama T, Kuratsune H, Ikeda Y, Sato E, Misawa N, Nakatomi Y, Sakuma R, Yasui K, Yamaguti K, Hirayama F. 2011. No association of xenotropic murine leukemia virus-related virus with prostate cancer or chronic fatigue syndrome in Japan. Retrovirology 8:20. doi: 10.1186/1742-4690-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verhaegh GW, de Jong AS, Smit FP, Jannink SA, Melchers WJ, Schalken JA. 2011. Prevalence of human xenotropic murine leukemia virus-related gammaretrovirus (XMRV) in Dutch prostate cancer patients. Prostate 71:415–420. doi: 10.1002/pros.21255. [DOI] [PubMed] [Google Scholar]
- 14.Danielson BP, Ayala GE, Kimata JT. 2010. Detection of xenotropic murine leukemia virus-related virus in normal and tumor tissue of patients from the southern United States with prostate cancer is dependent on specific polymerase chain reaction conditions. J Infect Dis 202:1470–1477. doi: 10.1086/656146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arnold RS, Makarova NV, Osunkoya AO, Suppiah S, Scott TA, Johnson NA, Bhosle SM, Liotta D, Hunter E, Marshall FF, Ly H, Molinaro RJ, Blackwell JL, Petros JA. 2010. XMRV infection in patients with prostate cancer: novel serologic assay and correlation with PCR and FISH. Urology 75:755–761. doi: 10.1016/j.urology.2010.01.038. [DOI] [PubMed] [Google Scholar]
- 16.Lo SC, Pripuzova N, Li B, Komaroff AL, Hung GC, Wang R, Alter HJ. 2010. Detection of MLV-related virus gene sequences in blood of patients with chronic fatigue syndrome and healthy blood donors. Proc Natl Acad Sci U S A 107:15874–15879. doi: 10.1073/pnas.1006901107. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Suhadolnik RJ, Peterson DL, O'Brien K, Cheney PR, Herst CV, Reichenbach NL, Kon N, Horvath SE, Iacono KT, Adelson ME, De Meirleir K, De Becker P, Charubala R, Pfleiderer W. 1997. Biochemical evidence for a novel low molecular weight 2-5A-dependent RNase L in chronic fatigue syndrome. J Interferon Cytokine Res 17:377–385. doi: 10.1089/jir.1997.17.377. [DOI] [PubMed] [Google Scholar]
- 18.Lombardi VC, Ruscetti FW, Das Gupta J, Pfost MA, Hagen KS, Peterson DL, Ruscetti SK, Bagni RK, Petrow-Sadowski C, Gold B, Dean M, Silverman RH, Mikovits JA. 2009. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science 326:585–589. doi: 10.1126/science.1179052. [DOI] [PubMed] [Google Scholar]
- 19.Mikovits JA, Lombardi VC, Pfost MA, Hagen KS, Ruscetti FW. 2010. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Virulence 1:386–390. doi: 10.4161/viru.1.5.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mikovits JA, Huang Y, Pfost MA, Lombardi VC, Bertolette DC, Hagen KS, Ruscetti FW. 2010. Distribution of xenotropic murine leukemia virus-related virus (XMRV) infection in chronic fatigue syndrome and prostate cancer. AIDS Rev 12:149–152. [PubMed] [Google Scholar]
- 21.Hohn O, Strohschein K, Brandt AU, Seeher S, Klein S, Kurth R, Paul F, Meisel C, Scheibenbogen C, Bannert N. 2010. No evidence for XMRV in German CFS and MS patients with fatigue despite the ability of the virus to infect human blood cells in vitro. PLoS One 5:e15632. doi: 10.1371/journal.pone.0015632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clayton EW. 2015. Beyond myalgic encephalomyelitis/chronic fatigue syndrome: an IOM report on redefining an illness. JAMA 313:1101–1102. doi: 10.1001/jama.2015.1346. [DOI] [PubMed] [Google Scholar]
- 23.Robinson MJ, Erlwein O, McClure MO. 2011. Xenotropic murine leukaemia virus-related virus (XMRV) does not cause chronic fatigue. Trends Microbiol 19:525–529. doi: 10.1016/j.tim.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Knox K, Carrigan D, Simmons G, Teque F, Zhou Y, Hackett J Jr, Qiu X, Luk KC, Schochetman G, Knox A, Kogelnik AM, Levy JA. 2011. No evidence of murine-like gammaretroviruses in CFS patients previously identified as XMRV-infected. Science 333:94–97. doi: 10.1126/science.1204963. [DOI] [PubMed] [Google Scholar]
- 25.Erlwein O, Kaye S, McClure MO, Weber J, Wills G, Collier D, Wessely S, Cleare A. 2010. Failure to detect the novel retrovirus XMRV in chronic fatigue syndrome. PLoS One 5:e8519. doi: 10.1371/journal.pone.0008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Groom HC, Boucherit VC, Makinson K, Randal E, Baptista S, Hagan S, Gow JW, Mattes FM, Breuer J, Kerr JR, Stoye JP, Bishop KN. 2010. Absence of xenotropic murine leukaemia virus-related virus in UK patients with chronic fatigue syndrome. Retrovirology 7:10. doi: 10.1186/1742-4690-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Kuppeveld FJ, de Jong AS, Lanke KH, Verhaegh GW, Melchers WJ, Swanink CM, Bleijenberg G, Netea MG, Galama JM, van der Meer JW. 2010. Prevalence of xenotropic murine leukaemia virus-related virus in patients with chronic fatigue syndrome in the Netherlands: retrospective analysis of samples from an established cohort. BMJ 340:c1018. doi: 10.1136/bmj.c1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Switzer WM, Jia H, Hohn O, Zheng H, Tang S, Shankar A, Bannert N, Simmons G, Hendry RM, Falkenberg VR, Reeves WC, Heneine W. 2010. Absence of evidence of xenotropic murine leukemia virus-related virus infection in persons with chronic fatigue syndrome and healthy controls in the United States. Retrovirology 7:57. doi: 10.1186/1742-4690-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cool M, Bouchard N, Masse G, Laganiere B, Dumont A, Hanna Z, Phaneuf D, Morisset R, Jolicoeur P. 2011. No detectable XMRV in subjects with chronic fatigue syndrome from Quebec. Virology 420:66–72. doi: 10.1016/j.virol.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Hong P, Li J, Li Y. 2010. Failure to detect xenotropic murine leukaemia virus-related virus in Chinese patients with chronic fatigue syndrome. Virol J 7:224. doi: 10.1186/1743-422X-7-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin CH, Bateman L, Schlaberg R, Bunker AM, Leonard CJ, Hughen RW, Light AR, Light KC, Singh IR. 2011. Absence of XMRV retrovirus and other murine leukemia virus-related viruses in patients with chronic fatigue syndrome. J Virol 85:7195–7202. doi: 10.1128/JVI.00693-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voisset C, Weiss RA, Griffiths DJ. 2008. Human RNA “rumor” viruses: the search for novel human retroviruses in chronic disease. Microbiol Mol Biol Rev 72:157–196. doi: 10.1128/MMBR.00033-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith RA. 2010. Contamination of clinical specimens with MLV-encoding nucleic acids: implications for XMRV and other candidate human retroviruses. Retrovirology 7:112. doi: 10.1186/1742-4690-7-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss RA. 2010. A cautionary tale of virus and disease. BMC Biol 8:124. doi: 10.1186/1741-7007-8-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hué S, Gray ER, Gall A, Katzourakis A, Tan CP, Houldcroft CJ, McLaren S, Pillay D, Futreal A, Garson JA, Pybus OG, Kellam P, Towers GJ. 2010. Disease-associated XMRV sequences are consistent with laboratory contamination. Retrovirology 7:111. doi: 10.1186/1742-4690-7-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oakes B, Tai AK, Cingoz O, Henefield MH, Levine S, Coffin JM, Huber BT. 2010. Contamination of human DNA samples with mouse DNA can lead to false detection of XMRV-like sequences. Retrovirology 7:109. doi: 10.1186/1742-4690-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robinson MJ, Erlwein OW, Kaye S, Weber J, Cingoz O, Patel A, Walker MM, Kim WJ, Uiprasertkul M, Coffin JM, McClure MO. 2010. Mouse DNA contamination in human tissue tested for XMRV. Retrovirology 7:108. doi: 10.1186/1742-4690-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato E, Furuta RA, Miyazawa T. 2010. An endogenous murine leukemia viral genome contaminant in a commercial RT-PCR kit is amplified using standard primers for XMRV. Retrovirology 7:110. doi: 10.1186/1742-4690-7-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoye JP, Silverman RH, Boucher CA, Le Grice SF. 2010. The xenotropic murine leukemia virus-related retrovirus debate continues at first international workshop. Retrovirology 7:113. doi: 10.1186/1742-4690-7-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uphoff CC, Lange S, Denkmann SA, Garritsen HS, Drexler HG. 2015. Prevalence and characterization of murine leukemia virus contamination in human cell lines. PLoS One 10:e0125622. doi: 10.1371/journal.pone.0125622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tuke PW, Tettmar KI, Tamuri A, Stoye JP, Tedder RS. 2011. PCR master mixes harbour murine DNA sequences. Caveat emptor! PLoS One 6:e19953. doi: 10.1371/journal.pone.0019953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang DE, Lee MC, Das Gupta J, Klein EA, Silverman RH. 2011. XMRV discovery and prostate cancer-related research. Adv Virol 2011:432837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paprotka T, Delviks-Frankenberry KA, Cingöz O, Martinez A, Kung HJ, Tepper CG, Hu WS, Fivash MJ, Coffin JM, Pathak VK. 2011. Recombinant origin of the retrovirus XMRV. Science 333:97–101. doi: 10.1126/science.1205292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das Gupta J, Luk KC, Tang N, Gaughan C, Klein EA, Kandel ES, Hackett J Jr, Silverman RH. 2012. Absence of XMRV and closely related viruses in primary prostate cancer tissues used to derive the XMRV-infected cell line 22Rv1. PLoS One 7:e36072. doi: 10.1371/journal.pone.0036072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simmons G, Glynn SA, Holmberg JA, Coffin JM, Hewlett IK, Lo SC, Mikovits JA, Switzer WM, Linnen JM, Busch MP, Blood XMRV Scientific Research Working Group . 2011. The Blood Xenotropic Murine Leukemia Virus-Related Virus Scientific Research Working Group: mission, progress, and plans. Transfusion 51:643–653. doi: 10.1111/j.1537-2995.2011.03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simmons G, Glynn SA, Komaroff AL, Mikovits JA, Tobler LH, Hackett J Jr, Tang N, Switzer WM, Heneine W, Hewlett IK, Zhao J, Lo SC, Alter HJ, Linnen JM, Gao K, Coffin JM, Kearney MF, Ruscetti FW, Pfost MA, Bethel J, Kleinman S, Holmberg JA, Busch MP. 2011. Failure to confirm XMRV/MLVs in the blood of patients with chronic fatigue syndrome: a multi-laboratory study. Science 334:814–817. doi: 10.1126/science.1213841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dodd RY, Hackett J Jr, Linnen JM, Dorsey K, Wu Y, Zou S, Qiu X, Swanson P, Schochetman G, Gao K, Carrick JM, Krysztof DE, Stramer SL. 2012. Xenotropic murine leukemia virus-related virus does not pose a risk to blood recipient safety. Transfusion 52:298–306. doi: 10.1111/j.1537-2995.2011.03450.x. [DOI] [PubMed] [Google Scholar]
- 48.Mi Z, Lu Y, Zhang S, An X, Wang X, Chen B, Wang Q, Tong Y. 2012. Absence of xenotropic murine leukemia virus-related virus in blood donors in China. Transfusion 52:326–331. doi: 10.1111/j.1537-2995.2011.03267.x. [DOI] [PubMed] [Google Scholar]
- 49.Tang S, Zhao J, Viswanath R, Nyambi PN, Redd AD, Dastyar A, Spacek LA, Quinn TC, Wang X, Wood O, Gaddam D, Devadas K, Hewlett IK. 2011. Absence of detectable xenotropic murine leukemia virus-related virus in plasma or peripheral blood mononuclear cells of human immunodeficiency virus type 1-infected blood donors or individuals in Africa. Transfusion 51:463–468. doi: 10.1111/j.1537-2995.2010.02932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiu X, Swanson P, Tang N, Leckie GW, Devare SG, Schochetman G, Hackett J Jr. 2012. Seroprevalence of xenotropic murine leukemia virus-related virus in normal and retrovirus-infected blood donors. Transfusion 52:307–316. doi: 10.1111/j.1537-2995.2011.03395.x. [DOI] [PubMed] [Google Scholar]
- 51.Slavov SN, Otaguiri KK, Macedo MD, Rocha-Júnior MC, Silva-Pinto AC, Kashima S, Covas DT. 2014. No evidence of xenotropic murine leukemia virus-related virus infection in Brazilian multiply transfused patients with sickle cell disease and beta-thalassemia major. New Microbiol 37:543–550. [PubMed] [Google Scholar]
- 52.Lee D, Das Gupta J, Gaughan C, Steffen I, Tang N, Luk KC, Qiu X, Urisman A, Fischer N, Molinaro R, Broz M, Schochetman G, Klein EA, Ganem D, Derisi JL, Simmons G, Hackett J Jr, Silverman RH, Chiu CY. 2012. In-depth investigation of archival and prospectively collected samples reveals no evidence for XMRV infection in prostate cancer. PLoS One 7:e44954. doi: 10.1371/journal.pone.0044954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schlaberg R, Choe DJ, Brown KR, Thaker HM, Singh IR. 2014. Retraction for Schlaberg et al., XMRV is present in malignant prostatic epithelium and is associated with prostate cancer, especially high-grade tumors. Proc Natl Acad Sci U S A 111:12270. doi: 10.1073/pnas.1409186111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Silverman RH, Das Gupta J, Lombardi VC, Ruscetti FW, Pfost MA, Hagen KS, Peterson DL, Ruscetti SK, Bagni RK, Petrow-Sadowski C, Gold B, Dean M, Mikovits JA. 2011. Partial retraction. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science 334:176. doi: 10.1126/science.334.6053.176-a. [DOI] [PubMed] [Google Scholar]
- 55.Alberts B. 2011. Retraction. Science 334:1636. doi: 10.1126/science.334.6063.1636-a. [DOI] [PubMed] [Google Scholar]
- 56.Lo SC, Pripuzova N, Li B, Komaroff AL, Hung GC, Wang R, Alter HJ. 2012. Retraction for Lo et al., Detection of MLV-related virus gene sequences in blood of patients with chronic fatigue syndrome and healthy blood donors. Proc Natl Acad Sci U S A 109:346. doi: 10.1073/pnas.1119641109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anonymous. 2012. Xenotropic murine leukemia virus-related virus (XMRV) and other polytropic murine leukemia viruses (pMLV). AABB, Bethesda, MD: http://www.aabb.org/tm/eid/Documents/xmrvfactsheet.pdf#search=xmrv Accessed 26 June 2015. [Google Scholar]
- 58.Canadian Agency for Drugs and Technologies in Health. 2010. Octaplas compared with fresh frozen plasma to reduce the risk of transmitting lipid-enveloped viruses: an economic analysis and budget impact analysis. CADTH Technol Overv 1:e0106. [PMC free article] [PubMed] [Google Scholar]
- 59.Pelletier JP, Transue S, Snyder EL. 2006. Pathogen inactivation techniques. Best Pract Res Clin Haematol 19:205–242. doi: 10.1016/j.beha.2005.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seghatchian J, Putter JS. 2013. Pathogen inactivation of whole blood and red cell components: an overview of concept, design, developments, criteria of acceptability and storage lesion. Transfus Apher Sci 49:357–363. doi: 10.1016/j.transci.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 61.Irsch J, Lin L. 2011. Pathogen inactivation of platelet and plasma blood components for transfusion using the INTERCEPT Blood System. Transfus Med Hemother 38:19–31. doi: 10.1159/000323937. [DOI] [PMC free article] [PubMed] [Google Scholar]