Summary
VIP is a peptide hormone capable of activating the cAMP/PKA pathway and modifying gonadal steroidogenic capacity. Less is known about the molecular mechanisms of VIP-mediated steroidogenesis and its role in regulating the steroidogenic acute regulatory protein (STAR). We examined the impact of VIP on STAR expression and function in immortalized (KK1) and primary mouse granulosa cells, where VIP strongly upregulated STAR expression and steroidogenesis. Inhibitors of the PKA and PKC pathways suggested that both are activated by VIP. VIP did not efficiently phosphorylate STAR (P-STAR); however, VIP together with cAMP-analogs that activate Type II PKA increased P-STAR and further increased steroidogenesis. Our results suggest that VIP-induced STAR expression and function in granulosa cells result from the preferential activation of Type I PKA. Furthermore, the PKA and PKC pathways appear to converge at regulating VIP-mediated Star transcription and translation.
Keywords: VIP, steroidogenesis, STAR, granulosa cells
Introduction
Vasoactive intestinal peptide (VIP) is a 28-amino acid molecule located in multiple tissues, and capable of exerting a variety of biological effects, including well documented neuroendocrine actions (Abe et al., 1985; Lopez et al., 1989). One of them is the function of VIP as a physiological PRL-releasing factor of pituitary origin (Abe et al., 1985). In the female genital tract the localization of VIP immunoreactive nerves have been described in association with the smooth muscles of blood vessels and the uterus, as well as with the ovarian stroma (Larsson et al., 1977; Alm et al., 1980; Huslhof et al., 1994). In bovine granulosa cells, immunohistochemical signals for VIP increased in the growing and preovulatory follicles and were coincident with the LH-peak suggesting a role for VIP in the final stages of follicle development and ovulation (Hulshof et al., 1994). This is in agreement with the findings of Schmidt et al., 1990, who reported on the increased number of in-vitro ovulations with VIP-perfused ovaries from PMSG primed rats.
Within the ovary, VIP is involved in the regulation of steroidogenic activity, stimulating estradiol and progesterone production in cultured granulosa cells (Davoren and Hsueh, 1985; Ahmed et al., 1986; Parra et al., 2007). At the same time VIP decreases activity of 20αHSD in these cells, and thus maintains the biological activity of progesterone by decreasing the rate of its metabolism to biologically inactive 20α-OH-progesterone (Davoren and Hsueh, 1985).
In the periphery, the denervation of ovaries during the early luteal phase of the estrus cycle leads to changes in their morphology and impairs steroidogenic activity in pigs (Jana et al., 2005). Similarly, inhibition of ovarian secretory function and delayed pubertal onset were observed in rats after denervation (Ojeda et al., 1983; Lara et al., 1990; Forneris et al., 2002). The alterations in gonadal endocrine function are attributed to the loss of the peptidergic supply of neuronal fibers (Jana et al., 2005; Ojeda et al., 1983; Lara et al., 1990; Forneris et al., 2002).
The biological action of VIP is mediated through two G-coupled receptors designated as VIPR1 and -2 (respectively, VIPAC-1 and VIPAC-2) (Vaudry et al., 2000). Both receptors are expressed in the ovaries of different species (Hulshof et al., 1994; Vaccaris et al., 2006; Bao et al., 2000; Cecconi et al., 2004). Acting through its receptors, VIP dose-dependently increases intracellular cAMP content (Robberecht et al., 1979) and subsequently leads to protein kinase A (PKA) activation, which in turn induces steroidogenesis in granulosa cells. Nevertheless, the molecular mechanisms by which VIP induces steroidogenesis, and specifically VIP’s role in regulating STAR expression, remain unclear. STAR mediates the rate-limiting step in steroidogenesis, the transfer of cholesterol from the outer to the inner mitochondrial membrane, and it is the hormonal regulation of STAR expression and activity that allows tissues to accurately control their steroid production. The cAMP/PKA pathway is the major route in the trophic hormone-stimulated regulation of STAR expression and function, and both known subtypes of PKA (type I and type II) are present in steroidogenic tissues. In mouse Leydig tumor cells, type I PKA is more responsible for Star gene expression, while type II PKA impacts the post-translational regulation of STAR by more readily phosphorylating newly synthesized STAR protein (Dyson et al., 2009). Moreover, VIP may affect steroid production through mechanisms downstream of STAR. Recently, a reduction of STAR and 3β-hydroxysteroid dehydrogenase (3βHSD; enzyme converting pregnenolone to progesterone) expression accompanied by decreased serum levels of FSH was demonstrated in young VIP knockout mice (Lacombe et al., 2007).
These findings, together with the previously reported decrease in gonadal secretory function after neurectomy, strongly suggest an important role for the peptidergic supply from the peripheral neuronal system, and in particular for VIP, in regulating STAR-mediated steroidogenesis in the gonad. In order to address this hypothesis in the present study we used VIP to examine the molecular mechanisms involved in the VIP-mediated regulation of STAR expression in immortalized (KK1) and primary mouse granulosa cells.
Material and Methods
Chemicals and Reagents
N6,2-dibutyryladenosine-3,5-cyclic monophosphate (dbcAMP), phorbol 12-myristate 13-acetate (PMA), H89, pregnant mare serum gonadotropin (PMSG; Gonadotropin), Triton X-100 (TX-100), protease inhibitor cocktail, high glucose (4.5g/l) Dulbecco’s modified Eagle’s medium (DMEM) and F12 medium and recombinant VIP were purchased from Sigma-Aldrich (St. Loius, MO).
PKC-specific inhibitor GF-10923X (GFX) was obtained from AG Scientific (San Diego, CA, USA). 8-Piperidinoadenosine-3’, 5’-cyclic monophosphate (PIP-cAMP) and N6-mono-tert-butylcarbamoyl-adenosine-3’, 5’-cyclic monophosphate (MBC-cAMP) were purchased from BioLog (Bremen, Germany). Tissue culture-grade antibiotics, TRIzol, PCR primers, trypsin EDTA and PBS were purchased from Invitrogen (Carlsbad, CA). The 6-carboxyfluorescein (6-FAM) and 6-carboxytetramethyl-rhodamine (TAMRA) labelled probes (TaqMan Probes) were from Eurogentec, North America (San Diego, CA). Fetal bovine serum (FBS) was from Life Technologies Inc. (Gaithersburg, MD). TaqMan Reverse Transcription Reagents® were from Applied Biosystems (Foster City, CA). Fast Start Universal Probe Master (ROX)®, DNase I recombinant, RNase-free and FuGENE HD transfection reagent were purchased from Roche Diagnostics (Indianapolis, IN). The PaphDetect® in Vivo Signal Transduction Pathway trans-Reporting System was from Stratagene (La Jolla, CA). The dual-luciferase reporter assay system was from Promega Corp., Madison, WI.
Primary granulosa cells
Primary granulosa cells were obtained from mature, female CD-1 strain mice (Charles River, Wilmington, MA; donated by Dr. GA Cornwall from the Department of Cell Biology and Biochemistry, Texas Tech University Health Sciences Center, Lubbock). Animals were housed under a constant 12L:12D photoperiod and were allowed free access to food and water. Experiments were carried out in accordance with the principles and procedures described in the NIH Guidelines for Care and Use of Experimental Animals. Mice were killed by cervical dislocation 48h after an ip injection of 8 IU PMSG. A previously described protocol for primary granulosa cell isolation (Salustri et al., 1985) was used. Briefly, the ovaries were removed, trimmed of the surrounding tissue and placed in a HEPES-buffered culture DMEM/F12 (1:1) medium containing heat inactivated 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. The largest follicles were punctured under a dissecting microscope control and cumuli oophori and granulosa cells were released in the medium. Ovarian remnants and cumuli oophori were removed and cells were washed twice in the medium with centrifugation steps in between (600 × g, 10 min). After final centrifugation cells were resuspended in culture medium. Cell number and viability were assessed by trypan blue (0.5%) staining and hemocytometer counting. Cells were seeded in 6-well plates at a density of 2×106 cells per well and used for experiments 24 hrs later.
Cell culture and Radioimmunoassay
Immortalized KK1 mouse granulosa cells (kindly provided by Dr. Ilpo Huhtaniemi, Hammersmith Campus, Imperial College London, London UK) and primary granulosa cells were cultured in a humified incubator at 37°C and 5% CO2. KK1 cells were maintained in HEPES-buffered DMEM/F12 (1:1) medium containing 10% heat inactivated FBS, 100 U/ml penicillin and 100 µg/ml streptomycin. 24hrs prior to stimulation experiments cells were seeded in 6-well plates. For stimulation experiments, cells grown to 70–80% confluency were washed with PBS in order to remove the serum-containing media, which was then replaced by serum-free media containing the agonists. For all experiments cells were stimulated over a 6 hr time period. This time point was chosen based on preliminary experiments in which the highest levels of STAR, P-STAR and progesterone output were observed (not shown). For experiments in which the time course of VIP-mediated PKA activity and CREB and cJUN phosphorylation are presented, a 4 h time-span is shown since PKA activity started to decrease after 30 min, reaching basal levels after 120 min. This was accompanied by a decrease in P-cJUN; CREB remained phosphorylated over the entire time period studied. Finally the medium was collected and stored at −20°C for subsequent radiommunoassay (RIA). The concentration of progesterone was assayed as previously described (Resko et al., 1974) and expressed as pg of progesterone per µg of protein.
Luciferase Assays
The −151/−1 bp fragment of the Star-promoter cloned upstream of the Firefly luciferase reporter gene in the pGL2 basic vector (Promega Corp., Madison, WI) was used for the reporter assay as previously reported (Manna et al., 2002). 24 hrs before transfection KK1 cells were seeded in 6-well plates and then transfected using FuGENE HD transfection reagent as described in Kowalewski et al., 2009. 1µg of Star-promoter plasmid DNA was used per well. pRL-SV40 plasmid expressing Renilla luciferase driven by CMV immediate-early enhancer and promoter was used to normalize for the transfection efficiency. The activity of the −151/−1 bp Star-promoter construct in response to the VIP and/or dbcAMP treatment was determined after 6 hrs of incubation. The dual-luciferase reporter system was used according to the manufacturer’s instructions (Promega). Luminescence was measured using TD-20/20 luminometer (Turner Designers, Sunnyvale, CA). Promoter activity in the samples is reported in relative light units (RLU) and expressed as the ratio of Firefly luciferase luminescence to that of Renilla luciferase.
Protein preparation and Western blot analyses
Following treatments, whole cell lysates were prepared with NET-2 lysis buffer (50 mM Tris-HCl [pH 7.4], 300 mM NaCl, 0.05% NP-40; Ishigaki et al., 2001) containing 10 µl/ml protease inhibitor cocktail (Sigma Aldrich). Cells were homogenized by sonication with three 10s pulses using a Tekmar TK300 sonic disruptor (Tekmar, Cincinnati, OH). After centrifugation at 10,000×g for 10 min at 4°C the supernatants were used for SDS-PAGE as previously described (Manna et al., 2002).
Primary antibodies were obtained from the following sources: rabbit antiserum generated against STAR protein, was a gift from Dr. W.L. Miller (Department of Pediatrics, University of California, San Francisco, CA; Bose et al., 1999); antiserum against the phosphorylated STAR (phospho-STAR) was a gift from Dr. Steven King (Scott Department of Urology, Baylor College of Medicine, Houston, TX); CREB (48H2) rabbit mAb, phospho-CREB (Ser133) mouse mAb and phospho-c-JUN (Ser63) II rabbit antibody were from Cell Signaling Technology Inc. (Danvers, MA); rabbit polyclonal c-JUN (H-79; sc-1694) and GAPDH (sc 47724) mouse monoclonal antibodies were purchased from Santa Cruz Biotechnology, (Santa Cruz, CA); mouse anti VIPR1 mAB and mouse anti VIPR2 mAb were from Millipore Corporation, USA; Rabbit anti-CYP11A1 (P450scc) antibody was from Chemicon (Temecula, CA); rabbit anti 3βHSD antiserum was a kind gift from Dr. Richard Parker (University of Alabama at Birmingham). Secondary HRP donkey anti-rabbit IgG and SuperSignal West chemiluminescent substrates were obtained from Pierce Biotechnology (Rockford, IL). Anti-mouse IgG conjugated to HRP was obtained from Promega Corp., Madison, WI.
RNA extraction and semi-quantitative Real Time PCR
Semi-quantitative Real Time (TaqMan) PCR was carried out in an automated fluorometer ABI PRISM® 7000 Sequence Detection System (Applied Biosystems, Foster City, CA) according to a previously described protocol (Kowalewski et al., 2006). Briefly, total RNA was isolated using TRIzol ® -Reagent and treated with DNase for the elimination of genomic DNA-contamination. Reverse transcription reagents for TaqMan, obtained from Applied Biosystems, were used with100 ng of DNase-treated total RNA for each sample. Samples were run in duplicates using Fast Start Universal Probe Master (ROX)® from Roche Diagnostics. Amplification conditions were as follows: initial denaturation at 95°C for 10 min followed by 40 cycles: 95°C for 15 s, 60°C for 60 s. Specificity of selected PCR products was confirmed by sequencing (Experimental Sciences Building, Texas Tech University, Lubbock, TX).
Relative quantification was performed using the comparative CT method (ΔΔ CT method), according to the instructions of the manufacturer of the ABI PRISM™ 7000 Sequence Detector and as described in Kowalewski et al., 2006. Results of the Real Time PCR are expressed as the fold-change in gene expression over the non-treated controls. Sequences for primers and TaqMan probes used for detection of Star and GAPDH cDNAs were as follows:
Star (forward): 5´-CCG GGT GGA TGG GTC AA-3´
Star (reverse): 5´-CAC CTC TCC CTG CTG GAT GTA-3´
Star (TaqMan Probe): 5´-CGA CGT CGG AGC TCT CTG CTT GG-3´
Gapdh (forward): 5´- GCA GTG GCA AAG TGG AGA TTG-3´
Gapdh (reverse): 5´-GTG AGT GGA GTC ATA CTG GAA CAT G -3´
Gapdh (TaqMan Probe): 5´-TCA ACG ACC CCT TCA TTG ACC TC -3´
PathDetect® c-JUN Trans-Reporting Systems
KK1 cells were transfected with cJUN signal transduction pathway trans-reporting systems (Strategene, La Jolla, CA) according to the manufacturer’s protocol and as described in Kowalewski et al., 2009. 36 hrs after transfection cells were stimulated for 6 h with VIP and/or cAMP and the luciferase activity in the cell lysates was determined using a TD 20/20 luminometer (Turner Designs).
PKA activity
The levels of PKA activity after treatment with VIP in KK1 cells were determined with the SignaTECT PKA assay system (Promega) as described in Dyson et al., 2008.
Data analysis and Statistics
All experiments were independently repeated at least 3 times. Shown are the representative Western blots for each experiment. The experiments utilizing Luciferase Reporter Assays and Real Time PCR were performed at least three times in duplicate. Statistical data are expressed as the fold change relative to the non-treated controls. A parametric one-way analysis of variance (ANOVA) followed by Dunnett’s Multiple Comparison Test was applied with the statistical software program, GraphPad 3.06 (GraphPad Software, Inc, San Diego, CA, USA). Numerical data were presented as the mean ± standard deviation.
Results
Role of VIP on STAR, 3β-hydroxysteroid dehydrogenase, P-450scc expression and steroid production in granulosa cells
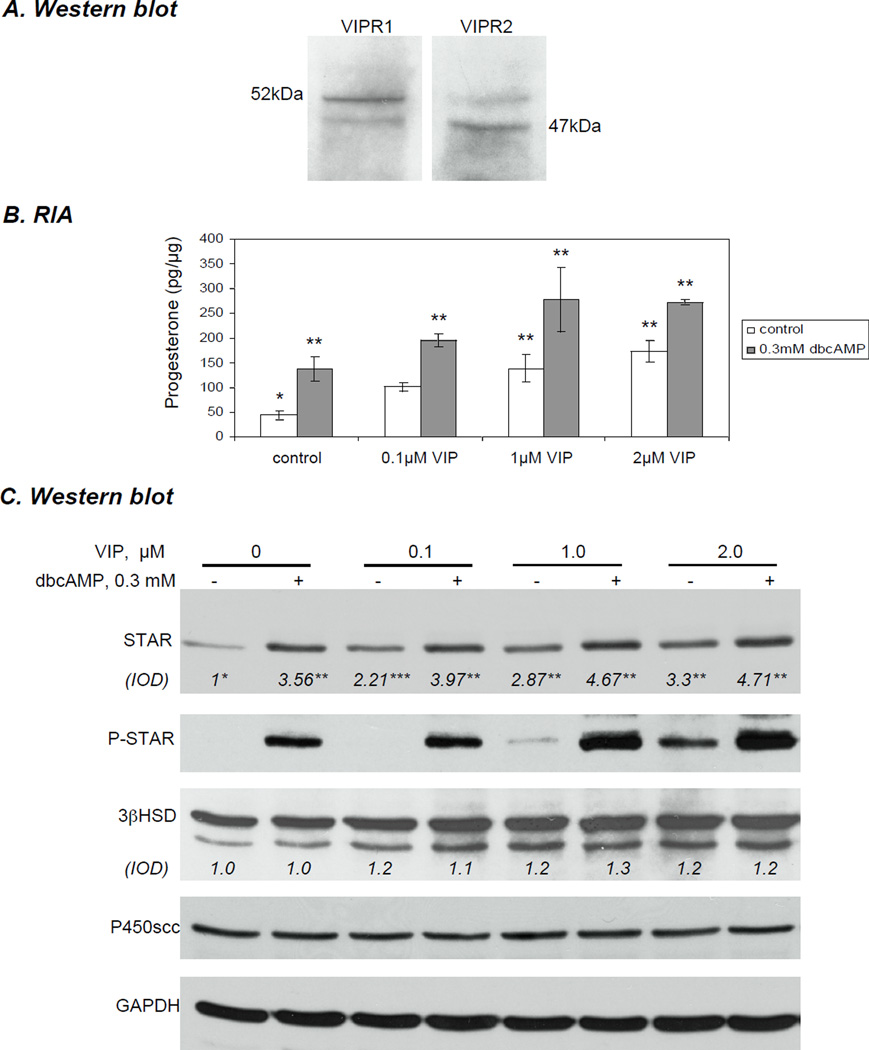
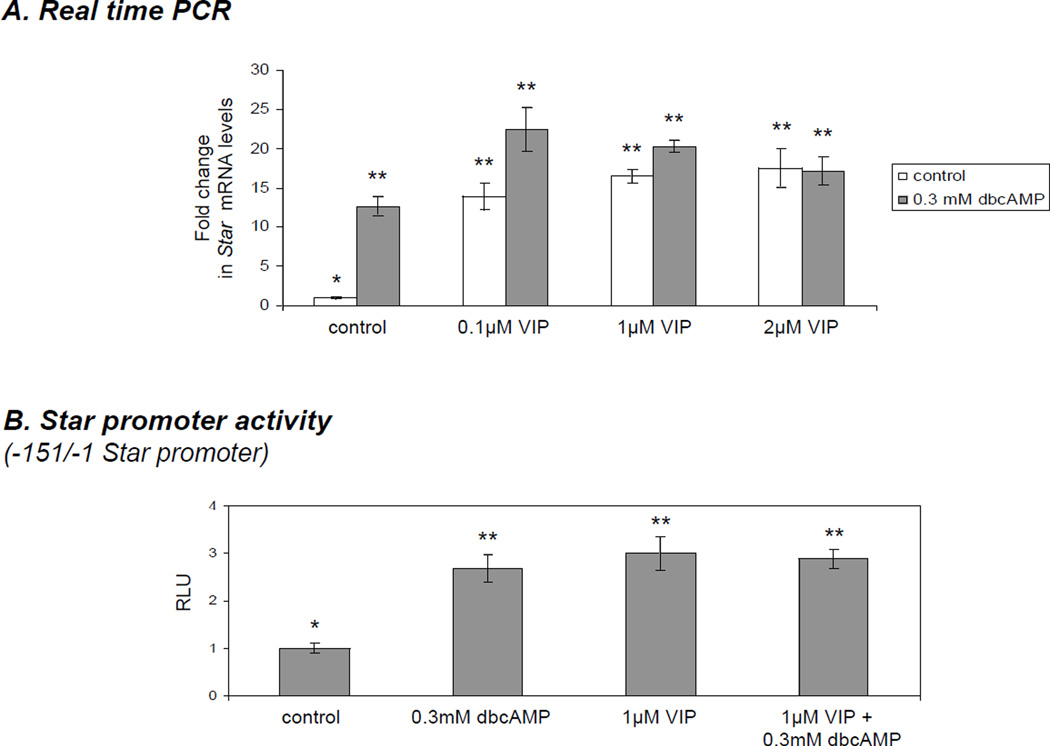
Using western blot analysis, the expression of both VIP receptors (VIPR1 and VIPR2) was detected in the KK1 cells indicating that these cells can potentially respond to VIP stimulation (Fig. 1A). In untreated KK1 cells STAR could be detected, but very little progesterone was synthesized over 6h. When treated with 0.1 µM - 2µM VIP for 6 h, KK1 cells demonstrated a dose-dependent increase in STAR protein and progesterone synthesis (Fig 1B,C). Total STAR increased by approximately 2.2-fold (p<0.05) when treated with 0.1 µm VIP and was further elevated up to 3-fold (p<0.01) with 2.0 µm VIP. Progesterone concentration in the media was elevated by 2.3-, 3.2- and 4-fold with 0.1 µM, 1 µM and 2µM VIP, respectively (p<0.01) and was lower than expected given the very significant increase in total STAR protein expression. Stimulation of KK1 cells with VIP in the presence of 0.3 mM dbcAMP further increased total STAR expression and steroid output, which was enhanced by 60% in the co-treatment experiments (Fig. 1B,C) when compared to the groups treated with VIP alone. Total STAR increased up to 4.71-fold (p<0.01) and steroid production was elevated by 6.43-fold (p<0.01) over non-treated controls (Fig. 1B,C). No P-STAR was detected when 0.1 µM VIP was used to stimulate the cells. STAR protein was only weakly phosphorylated (P-STAR) (and hence activated) when cells were treated with 1.0 µM VIP, and somewhat stronger phosphorylation was observed with 2.0 µM VIP. Addition of dbcAMP to VIP-treated cells synergistically phosphorylated and thus activated STAR (Fig. 1C). To determine whether VIP affected Star gene expression, experiments measuring Star mRNA by semi-quantitative Real Time PCR and experiments examining Star promoter activity were performed. Treatment with 0.1 µM VIP significantly elevated Star mRNA by 13.93-fold (p<0.01) over untreated cells, and maximal values corresponding to 16.52- and 17.52-fold induction were reached with 1.0- and 2.0 µM VIP, respectively (Fig. 2A). Treatment with 0.3 dbcAMP also increased Star expression to similar levels, but quantitatively similar results were obtained when VIP was used individually or in co-treatment with cAMP (Fig. 2C). Similarly, when the proximal −151/−1 bp Star reporter construct was transfected into KK1 cells, promoter activity displayed an approximately 3-fold (p<0.01) increase in response to VIP-treatment when compared with nontreated controls (Fig. 2B). This was comparable to effects observed after stimulation with 0.3 mM dbcAMP individually or in co-treatment (Fig. 2B). As seen with P450scc, the expression of 3βHSD was not significantly affected by VIP treatment either individually or in cotreatment with dbcAMP (Fig. 1C).
Figure 1.
Expression of VIPR1 and VIPR2 and concentration-dependent increase in STAR expression and steroidogenic output in immortalized KK1 mouse granulosa cells treated with vasoactive intestinal peptide (VIP) against the background PKA activity. Cells were cultured in serum-free DMEM/F12 (1:1) medium with increasing concentrations of VIP with or without 0.3 mM dbcAMP for 6 h. A) Expression of VIPR1 and VIPR2 in KK1 control cells. B) Progesterone production in the collected media was determined by radioimmunoassay C) Cells were collected and homogenized, 20 µg of the lysate was used in western blot analysis of STAR (30 kDa), phospho (P)-STAR (30 kDa), 3β-hydroxysteroid dehydrogenase (3βHSD) (42 kDa), P450scc (45 kDa) and GAPDH (37 kDa). Protein expression was normalised against GAPDH; the average integrated optical density (IOD) for STAR and 3βHSD is shown as fold changes relative to the untreated control.
Figure 2.
Concentration-dependent increase in Star mRNA expression in immortalized KK1 mouse granulosa cells treated with vasoactive intestinal peptide (VIP) against the background PKA activity. A) Star mRNA expression as determined by Real Time (TaqMan) PCR normalised against the GAPDH. One-way ANOVA with p < 0.0001 (A,B,C) followed by Dunnett’s multiple comparison test was applied; all samples were compared against the untreated control. (**) indicates p < 0.01 and (***) inicates p < 0.05. B) VIP increases Star promoter activity in immortalized KK1 mouse granulosa cells. Cells were transfected with −151/−1 bp fragment of Star promoter subcloned to pGL2 vector containing Firefly luciferase as a reporter. Transfection efficiency was normalized by cotransfecting pRL-SV40 vector constitutively expressing Renilla luciferase. Cells were treated for 6 h. One-way ANOVA with p < 0.0025 and Dunnett’s multiple comparison test were applied; all samples were compared against the untreated control. Bars with (**) differ at p < 0.01.
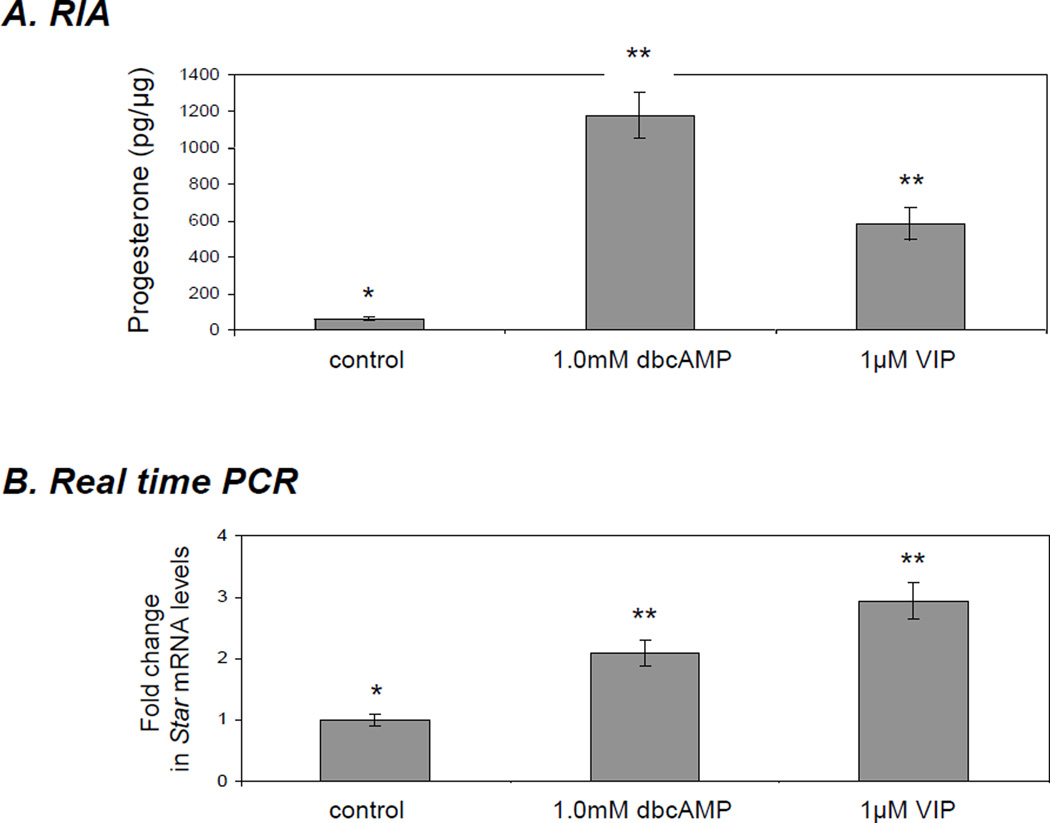
To determine the physiological relevance of the VIP-mediated effects on STAR expression and steroidogenesis, granulosa cells isolated from PMSG-stimulated mice were analyzed using RIA and Real Time PCR (Fig. 3A,B). Treatment with 1µM VIP for 6 h resulted in a 2.8-fold (p<0.01) increase in Star mRNA expression over untreated cells, and this increase closely resembled the induction seen after stimulation with 1mM dbcAMP (2.1-fold increase; p<0.01) (Fig. 3B). Accumulation of progesterone in the media was elevated by 8.9- (p<0.01) and 18.6-fold (p<0.01) by VIP and dbcAMP, respectively, when compared with controls, further confirming the involvement of VIP in the STAR-mediated steroidogenic response.
Figure 3.
Vasoactive intestinal peptide (VIP) increases Star gene expression and steroidogenesis in primary mouse granulosa cells. Mature female CD-1 strain mice were stimulated with 8 IE pregnant mare serum gonadotropin (PMSG). After isolation cells were cultivated for 24h in culture medium and subsequently stimulated for 6 h in serum free media containing either 1mM dbcAMP or 1µM VIP. A) Progesterone production in the collected media was determined by radioimmunoassay B) Star mRNA expression as determined by Real Time (TaqMan) PCR normalised against the GAPDH. One-way ANOVA with p < 0.0001 (A,B) followed by Dunnett’s multiple comparison test was applied; all samples were compared against the untreated control. (**) indicates p < 0.01.
These findings demonstrate that VIP is capable of strongly activating Star gene expression at levels similar to those observed with cAMP analog treatment. Notably, the VIP-mediated increase in Star mRNA did not correlate with a matched increase in steroid production as we observed in the group treated with dbcAMP.
Effects of VIP on specific PKA activity
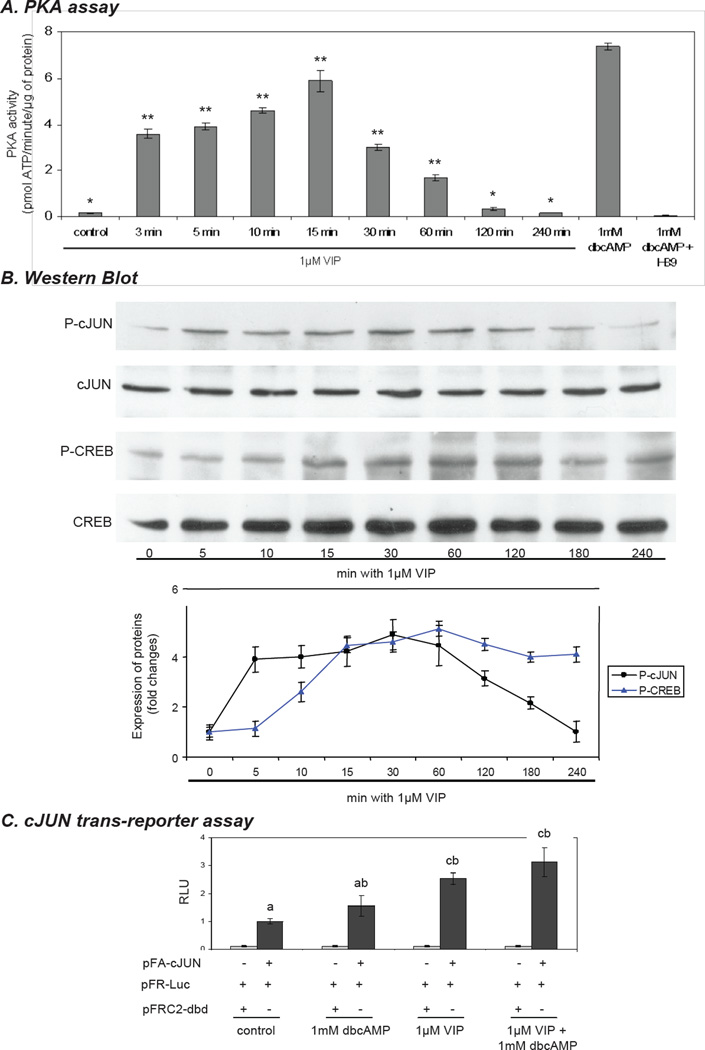
The levels of PKA activity following VIP stimulation were evaluated. We examined the effect of 1 µM VIP on PKA activity in KK1 cells at different time points over a period of 4 h (Fig. 4A). PKA activity at the initial time point was very low; it rose steeply within the first 3 minutes, reaching a maximum after 15 minutes. After 30 minutes the PKA activity started to decrease reaching very low or basal values by 120 and 240 minutes. Cells treated with 1.0 mM dbcAMP were used as a positive control, and consistently yielded high PKA activity, even after 4–6 h of incubation. As a negative control for the assay, PKA activity was quenched with the PKA inhibitor H89.
Figure 4.
Time course of vasoactive intestinal peptide (VIP)– induced PKA, CREB and cJUN activation in immortalized KK1 mouse granulosa cells. The KK1 cells were incubated in the presence of 1µM VIP for the times indicated. A) The effect of 1µM VIP on PKA activity in cultured KK1 cells over a period of 4 h. One-way ANOVA with p < 0.0001 and Dunnett’s multiple comparison test were applied; all samples were compared against the control. (**) indicates p < 0.01. B) Representative immunoblots using antibodies against total and phospho (P)- CREB (43 kDa) and (P)- cJUN (48 kDa). The lower panels show the densitometric values (integrated optical density) normalized against the respective total-CREB and -cJUN. One-way ANOVA with p < 0.0001 (CREB; cJUN) followed by Dunnett’s multiple comparison test was applied; all samples were compared against the control indicating: 0’ min. vs 5’-120’ min p<0.01 and 0’ min vs. 180’ min p < 0.05 for P-cJUN and 0’ min vs. 10-240’ min p< 0.01 for P-CREB. C) KK1 cells were transfected with PathDetect cJUN trans-reporting system; 36h after transfection cells were treated with 1 µM VIP and/or 1mM dbcAMP for additional 6 h and luciferase activity in the cell lysate was determined. Lower case letters are used to designate groups that differ significantly (p < 0.01)
VIP-mediated effects on CREB and cJUN expression
Since VIP effectively induced Star gene and protein expression, it seemed plausible that transcription factors known to regulate Star transcription might be regulated by VIP. Using Western blot analyses, we examined the expression and phosphorylation status of two prominent and well-studied Star promoter activators that are sensitive to PKA, namely, cJUN and CREB. KK1 cells were stimulated with 1µM VIP and changes in the phosphorylation status of both transcription factors were examined at different time points over a period of 4 h (Fig. 4B). Both factors were readily detected in unstimulated cells, with no changes in total protein expression observed during the time course of the experiment. The relative abundance of phospho-CREB and phospho-cJUN were strongly induced following treatment with 1µM VIP when compared to total CREB and cJUN. The phosphorylation of cJUN (4-fold; p<0.01) occurred after 5 minutes, reached a maximum (4.9-fold; p<0.01) after 30 minutes of VIP stimulation, and started to decline afterwards, dropping to basal levels by 240 minutes (p>0.05) (Fig. 4B). The phosphorylation of CREB was significantly increased (2.6-fold; p<0.01) by 10 minutes, reaching a maximum (up to 5-fold) within 15 to 60 minutes and remaining high for the duration of the time course (Fig. 4B). Having seen the rapid phosphorylation of cJUN in response to VIP treatment, we assessed the functional activity of cJUN in response to these treatments. KK1 cells were transfected with a pathway-specific cJUN trans-reporting system. Cells were stimulated with 1mM dbcAMP and 1µM VIP either individually or in co-treatment for 6 h. A 1.55- (p>0.05) and 2.53-fold (p<0.01) increase in luciferase activity, relative to untreated controls, were observed in response to dbcAMP and VIP, respectively. A further, up to 3.12-fold increase (p<0.01), was detected in the groups treated with a combination of VIP and dbcAMP (Fig. 4C). These results reflect how VIP-mediated activation of STAR expression and steroidogenesis could be coordinated by the targeted activation of the Star promoter and its transcriptional machinery.
Role of PKA and PKC in VIP-mediated STAR expression and steroidogenesis
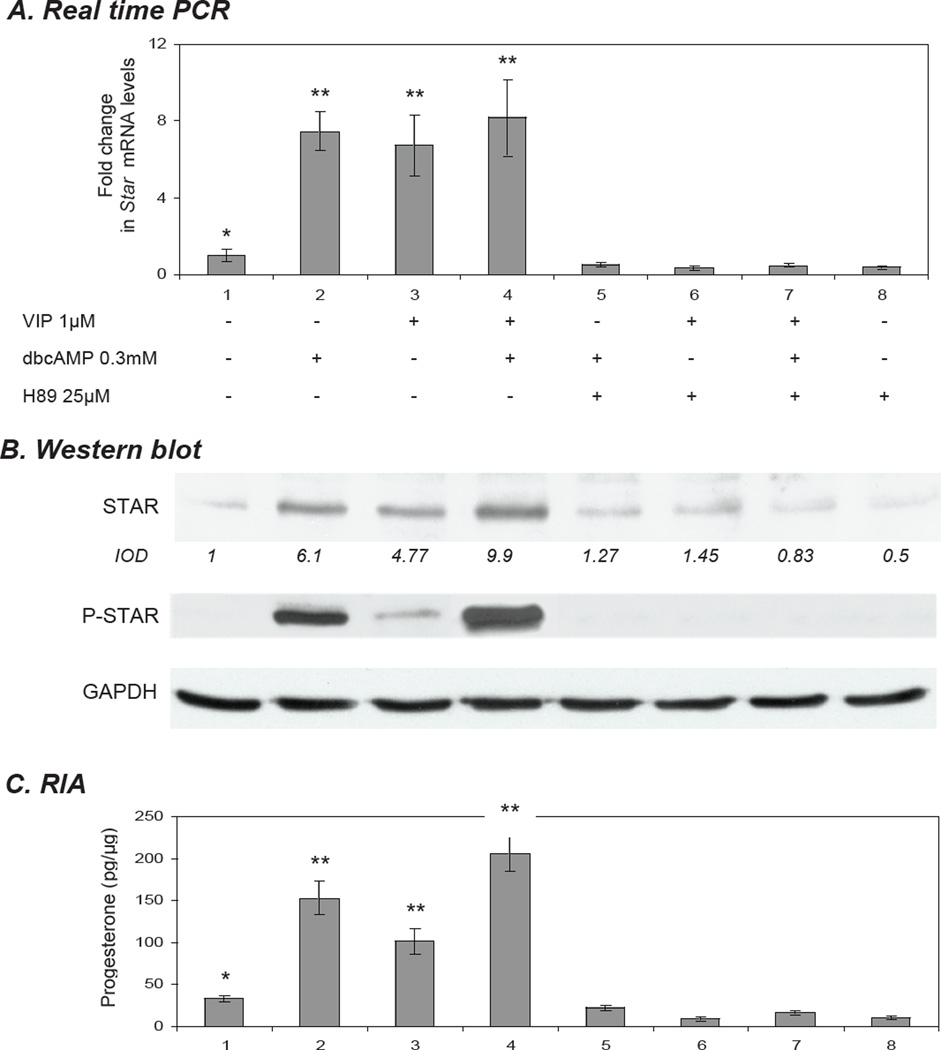
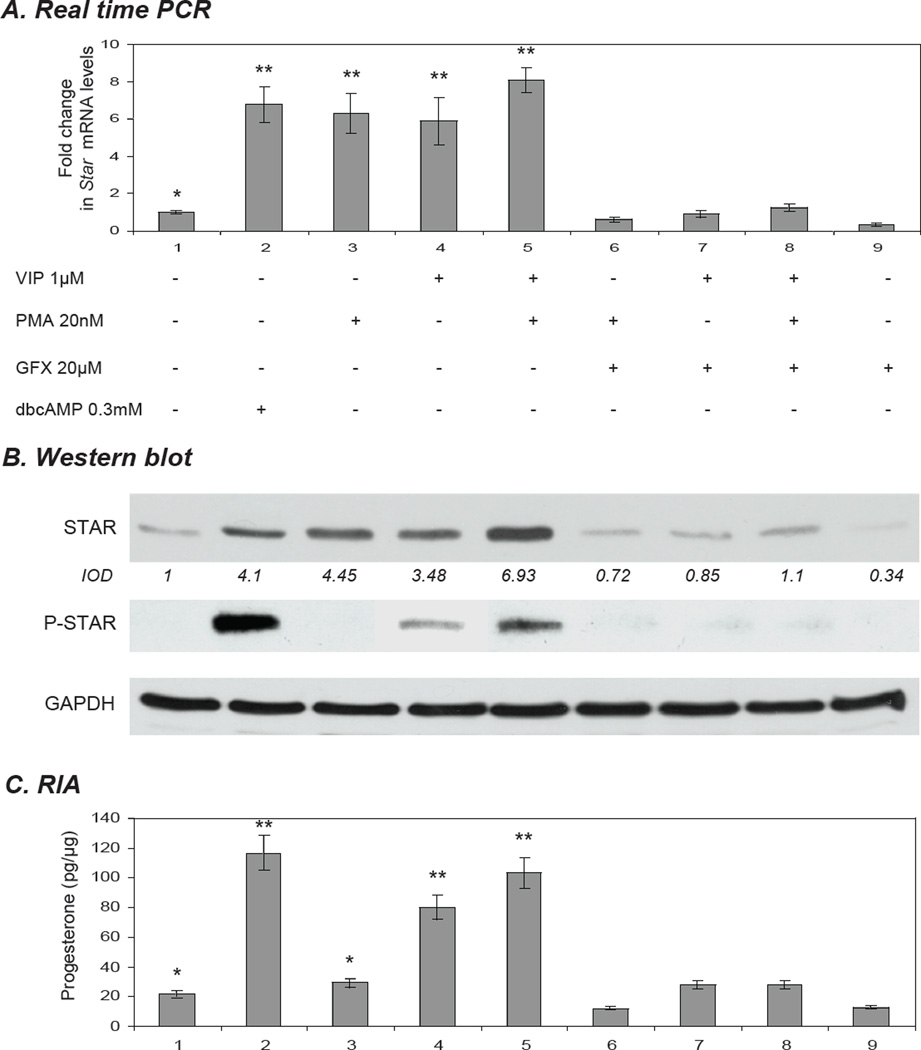
To more specifically evaluate the signalling events in VIP-mediated steroidogenesis, we wished to assess the independent roles of the PKA and PKC pathways as they function downstream of VIP, and to know how these pathways then affect STAR expression. To examine PKA, KK1 cells were treated with 0.3 mM dbcAMP and 1µM of VIP alone and in co-treatment. Cells pre-treated for 30 min with the specific inhibitor of PKA, H89 (20µM) markedly diminished (p<0.01) all responses (Fig. 5A,B,C). In order to elucidate the role of the PKC pathway in VIP-mediated steroidogenesis, StAR expression in response to the PKC activator, phorbol 12-myristate 13-acetate (PMA) was determined and compared with VIP. Stimulation of KK1 cells with 20 nM PMA resulted in a significant (p<0.01) increase in Star-mRNA (6.3-fold; p<0.01) and protein (4.45-fold; p<0.01) expression (Fig. 6A,B). No P-STAR and low steroid levels were observed in response to PMA (Fig. 76,C), corroborating the results of Jo et al., 2005, which were observed in mouse Leydig cells. The addition of 1 µM VIP to PMA–treated cells enhanced total STAR, P-STAR and steroidogenesis when compared to cells treated with VIP alone (Fig. 6B,C). While VIP paired with PMA did result in increased P-STAR relative to the control and either compound alone, their combined use generated less P-STAR than 0.3 mM dbcAMP. Quantitatively similar levels of Star mRNA were observed in response to VIP and PMA (Fig. 6A). The inhibition of the PKC pathway by the specific inhibitor GF-109203X (GFX; 20 µM) markedly reduced the response to both PMA and VIP (Fig. 6). These findings demonstrate that signalling from not only PKA, but also PKC can transduce signals in response to VIP, and that these pathways converge to regulate STAR expression and function, and hence the steroidogenic response in these cells.
Figure 5.
Role of PKA in vasoactive intestinal peptide (VIP) – mediated STAR expression and steroidogenesis in immortalized KK1 mouse granulosa cells. KK1 cells were treated for 6 h with 1.0 µM VIP with or without 0.3 mM dbcAMP. PKA activity was blocked with H89 (25 µM). Untreated cells were used as negative control. A) Star mRNA expression as determined by Real Time (TaqMan) PCR normalised against the GAPDH. B) STAR (30kDa), P-STAR (30 kDa) and GAPDH (37 kDa) levels were examined by Western blot analysis. The average integrated optical density (IOD) for STAR, normalized with GAPDH, is shown as fold changes relative to the untreated control. C) Progesterone production in the collected media was determined by radioimmunoassay. One-way ANOVA with p < 0.0001 (A,C) followed by Dunnett’s multiple comparison test was applied; all samples were compared against the untreated control. Bars with (**) differ at p < 0.01.
Figure 6.
Role of PKC in vasoactive intestinal peptide (VIP) – mediated STAR expression and steroidogenesis in immortalized KK1 mouse granulosa cells. KK1 cells were treated as indicated for 6h. GFX (20µM) was used to specifically block the PKC activity. Untreated cells and cells treated with 0.3 mM dbcAMP were used as controls. A) Star mRNA expression as determined by Real Time (TaqMan) PCR normalized against the GAPDH. B) STAR (30kDa), P-STAR (30 kDa) and GAPDH (37 kDa) levels were examined by Western blot analysis. The average integrated optical density (IOD) for STAR, normalized with GAPDH, is shown as fold changes relative to the untreated control. C) Progesterone production was determined by radioimmunoassay. One-way ANOVA with p < 0.0001 (A,C) followed by Dunnett’s multiple comparison test was applied; all samples were compared against the untreated control. Bars with (**) differ at p < 0.01.
The induction of STAR phosphorylation in response to vasoactive intestinal peptide (VIP); differential activation of PKA
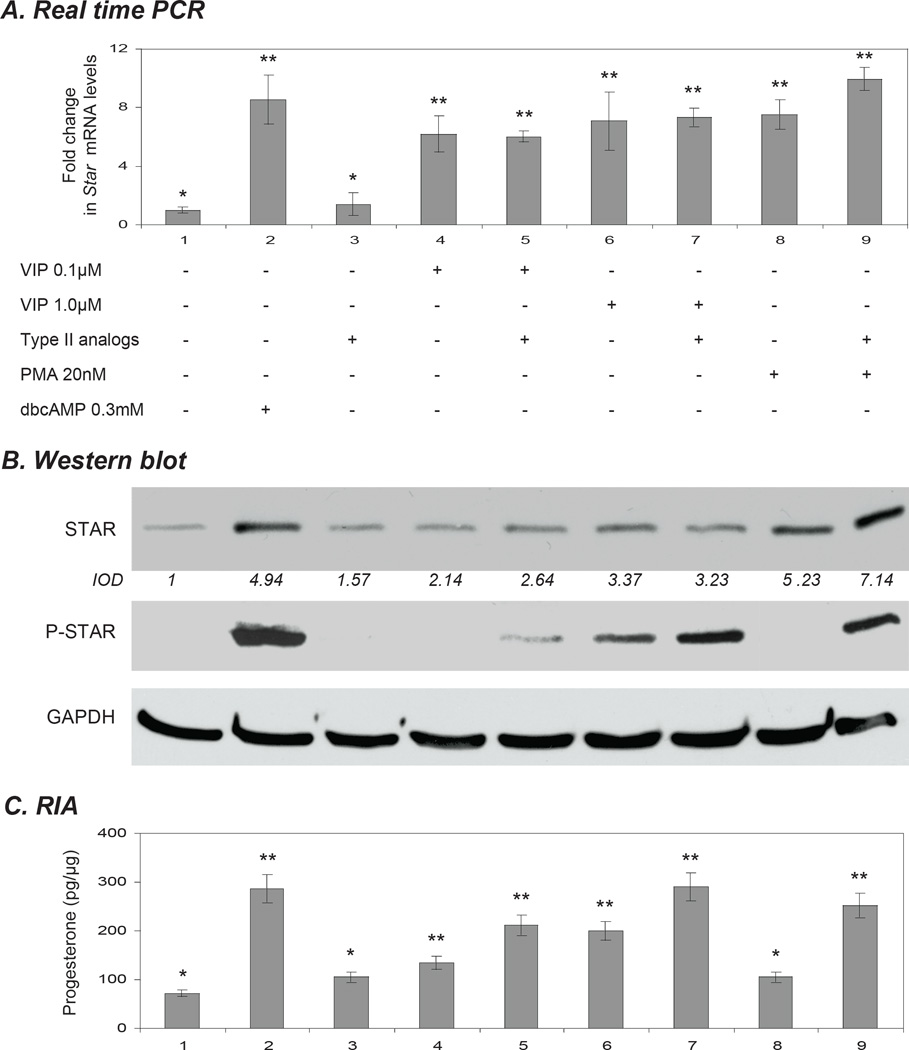
The activation of PKC has been shown to result in the effective transcription and translation of STAR. However, a further activation of the PKA pathway is required to effectively phosphorylate STAR and induce steroid production (Jo, et al., 2005). On the basis of this phenomenon, using specific analogs of cAMP to preferentially activate either type I or type II PKA, we were recently able to show that type I PKA is more involved in Star gene expression, while the activation of type II PKA is essential for efficient phosphorylation of STAR and consequently production of steroids (Dyson et al., 2009). Having observed the VIP-mediated effects predominantly in conjunction with Star gene activation and total STAR expression, we activated type II PKA (MBC-cAMP in combination with PIP-cAMP; Dyson et al., 2009) either alone or in conjunction with 0.1 µM – 1.0 µM VIP to demonstrate the impact of these treatments on STAR expression. The impact of the PKA analogs on the Star promoter was nominal when using 1 µM MBC-cAMP and 100 µM PIP-cAMP, concentrations previously demonstrated to preferentially activate type II PKA (Dyson et al., 2009). Neither STAR expression nor an increase in steroid production were observed in response to this treatment (Fig. 7B,C). As a positive control KK1 cells were treated with 0.3 mM dbcAMP (Fig. 7). To demonstrate the specific activation of type II PKA, 20µM PMA was used either alone or in conjunction with type II analogs. As expected type II PKA activators resulted in the phosphorylation of PMA-induced STAR (Fig. 7B). As before, little or no STAR phosphorylation was observed when KK1 cells were treated with up to 1.0 µM VIP. However, the addition of type II analogs resulted in phosphorylation of STAR to levels comparable with those observed in the group treated with PMA and type II activators and stimulated as much progesterone as in the cells treated with 0.3 mM cAMP (Fig. 7B,C). In this context the cAMP signalling from VIP appears to most directly affect type I PKA activity.
Figure 7.
The induction of STAR phosphorylation in response to vasoactive intestinal peptide (VIP). KK1 cells were treated for 6 h with either 0.1 µM or 1.0 µM VIP with or without type II PKA analogs (25 µm MBC-cAMP together with 100µM PIP-cAMP) and PMA (20 nM). Untreated cells and cells treated with 0.3 mM dbcAMP were used as controls. A) Star mRNA expression as determined by Real Time (TaqMan) PCR normalised against the GAPDH. B) STAR (30kDa), P-STAR (30 kDa) and GAPDH (37 kDa) levels were examined by Western blot analysis. The average integrated optical density (IOD) for STAR, normalized with GAPDH, is shown as fold changes relative to the untreated control. C) Progesterone production was determined by radioimmunoassay. One-way ANOVA with p < 0.0001 (A,C) followed by Dunnett’s multiple comparison test was applied; all samples were compared against the untreated control. Bars with (**) differ at p < 0.01.
Discussion
The role of neuropeptides, and in particular VIP, in regulating gonadal steroidogenic activity has been widely discussed (Larsson et al., 1977; Alm et al., 1980; Davoren and Hsueh, 1985; El-Gehani et al., 1998a; Hulshof et al., 1994; Jana et al., 2005). All previous observations point towards an important role of peptidergic supply at the level of the peripheral neuronal system in regulating reproductive function. However, VIP is also capable of acting directly on the steroidogenic tissues of the gonads, where it effectively stimulates steroid production (Davoren and Hsueh, 1985; Ahmed et al., 1986; El-Gehani et al., 1998b; Parra et al., 2007). Even though there are some indications that VIP could be involved in STAR-mediated steroidogenesis (Lacombe et al., 2007), the role of VIP in this context has not been closely studied, much less fully elucidated. The present study extends our knowledge of the molecular regulatory mechanisms in which VIP acts to increase the steroidogenic response by driving STAR expression and function in immortalized mouse KK1 and primary mouse granulosa cells.
In agreement with previous studies, we observed VIP to consistently induce steroidogenesis in granulosa cells in a concentration-dependent manner (Fig. 1B; Fig. 3A). This is in accordance with results reported by Davoren and Hsueh, 1985, where VIP dose-dependently stimulated progesterone and estrogen production in rat granulosa cells. Similarly, Parra et al., 1985 observed increased steroid release from cultured ovarian tissue in response to stimulation with VIP. In addition, our current data demonstrate that VIP is capable of potently increasing the expression of STAR and steroid production both in KK1 and freshly isolated granulosa cells. Notably, VIP did not seem to regulate the enzymatic availability of P450scc and 3βHSD, since the expression of both enzymes were not significantly affected in response to VIP (Fig. 1C).
VIP acts through its two receptors, both of which are expressed in granulosa cells in vivo (Barberi et al., 2007) and which we also observed here in KK1 cells (Fig. 1A). As members of the secretin family of receptors, VIPR1 and VIPR2 are conventionally thought to activate adenylate cyclase, and the activation of the VIPR leads to a dose-dependent increase in the intracellular cAMP content (Robberecht et al., 1979) that is potentially capable of increasing the PKA activity. Based on this, it is presumable that VIP would stimulate STAR expression via the PKA pathway. In our study, VIP proved to be a very potent activator of PKA. Maximal catalytic activity of the enzyme was reached within 15 minutes (Fig. 4A). During this time CREB and cJUN, two prominent factors in the Star transcriptional machinery were strongly phosphorylated (Fig. 4B), reflecting the targeted activation of Star gene expression in response to the peptidergic stimulation. This observation may mirror the pituitary, where previously published observations showed that VIP could strongly induce the phosphorylation of CREB (Fernandez et al., 2005). This data pairs well with the results from our real time PCR and promoter activity data indicating that incubation of KK1 and primary granulosa cells with VIP strongly induced Star promoter activity, resulting in mRNA levels comparable to those observed in response to dbcAMP. This suggests that cAMP signalling from VIP strongly enhances STAR expression at the transcriptional level.
The activation of the PKA pathway is unquestionably the major signalling route in STAR-mediated steroidogenesis. In this regard, the ability of VIP to activate the PKA pathway was a requirement for increases in STAR and steroidogenesis, since H89 diminished all VIP effects (Fig. 5). While it was clear that cAMP transduces signals from the activated VIPR in these cells, we noticed that STAR protein expression and steroidogenesis from VIP treatment lagged behind the transcription of Star. Notably, the lower doses of VIP consistently produced elevated Star promoter activity and Star mRNA levels that matched our dbcAMP controls, but STAR protein expression, phosphorylation and steroid production were typically lower even as VIP concentrations increased. This closely resembled the phenomenon described by Jo et al., 2005 regarding how PKC could regulate Star expression. Given that PKC and other signalling pathways can also be activated by VIP, we investigated the potential role of the PKC pathway in VIP mediated steroidogenesis. Blocking of PKC activity resulted in a significant reduction of the VIP response (Fig. 6), suggesting that PKC may also serve downstream of VIP. Additionally, the increased STAR expression and enhanced steroidogenic output in response to the combined treatment with VIP and PMA demonstrate an important role for PKC activity in mediating VIP effects. Thus, it appears that the cooperation between different signalling pathways is involved in the regulatory events in the VIP-mediated effects on STAR function and gonadal steroid production.
Given that PKA activity was dramatically increased in these cells in response to VIP alone, and that Star was being actively synthesized, it was perplexing to observe reduced levels of steroidogenesis. Specifically, increased levels of P-STAR and progesterone were observed when VIP was used in concert with dbcAMP. Thus VIP by itself, although capable of increasing STAR protein, did not permit effective phosphorylation of STAR, and resulted in lower steroidogenic output when compared to dbcAMP stimulation (Fig. 1B). This suggests that VIP requires further cAMP-mediated activation of PKA to produce a full response. Recently, we were able to illustrate how the different isoforms of PKA act in regulating STAR function (Dyson et al., 2009) with Type I PKA being more responsible for Star gene expression and Type II PKA appearing to be much more efficient at phosphorylating STAR.
In view of these earlier reports, an interesting finding in our current study is the observation that the effects of VIP on STAR expression in granulosa cells appear to result from the preferential activation of type I PKA. We further observed that VIP-induced Star transcription and translation was augmented by the activation of Type II PKA, which also resulted in the efficient phosphorylation and thus, activation of STAR and consequently enhanced steroid production (Fig. 7). Similarly, in the experiments where PMA was used alone to activate PKC and induce Star expression, VIP was demonstrated to be a very potent activator of Star transcription. However, even when PMA and VIP were used together, P452 STAR levels remained much lower than that observed using dbcAMP, suggesting that some aspect of PKA signalling was still limited. Since the activation of type II PKA appeared sufficient to allow for a full steroidogenic response to VIP treatment, it is likely that VIPR cAMP signals are predominantly transduced through Type I PKA. Observations, such as the rapid and significant phosphorylation of CREB and cJUN in response to VIP indicate that our hypothesis is even more probable, since we have previously observed the phosphorylation of those two factors by type I PKA in MA10 mouse Leydig tumor cells (Dyson et al., 2009). Clearly, further studies will be required to distinguish the specific roles of Type I and Type II PKA in VIP action.
The steroidogenic effects of VIP, though weaker than those seen with cAMP, could play a supportive role in trophic hormone regulated (e.g. FSH, LH) ovarian endocrine function. This becomes more plausible if the increased VIP immunoreactivity that has been seen in the bovine ovary immediately prior and during the LH-peak (Huslhof et al., 1994) is taken into account. Similarly the VIP concentration in the rat ovary is cycle stage dependent with the highest levels seen during dioestrus (Parra et al., 2007).
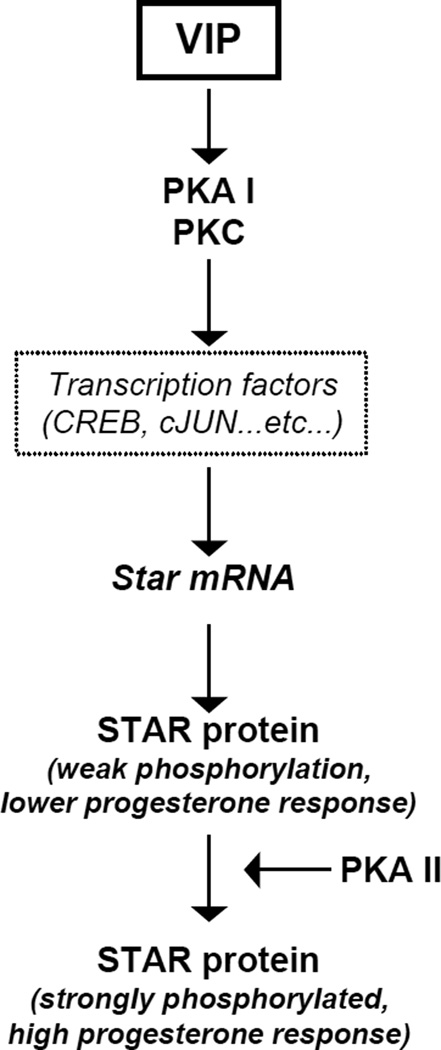
In conclusion and as summarized in Fig. 8, our findings demonstrate that the capacity for VIP to induce steroidogenesis is mediated by PKA and PKC through STAR, and that these pathways appear to converge to regulate the levels of Star transcription and translation. Importantly, VIP induced STAR expression and function in granulosa cells most likely results from the preferential activation of Type I PKA in KK1 cells. Hence, VIP appears to be a neuropeptide that is significantly involved in the regulation of steroidogenesis in granulosa cells.
Figure 8.
The role of Vasoactive Intestinal Peptide (VIP) in regulating STAR. VIP-mediated induction of PKC and type I PKA can induce StAR expression but results only in weak P-STAR and hence lower steroidogenic response. However, together with selective type II PKA activators, VIP increases P-STAR and enhances progesterone output.
Acknowledgments
This investigation was supported by NIH grant HD-17481 and with funds from the Robert A. Welch Foundation Grant B1-0028. The skilful technical assistance of Yuping Sun is greatly appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abe H, Engler D, Molitch ME, Bollinger-Gruber J, Reichlin S. Vasoactive intestinal peptide is a physiological mediator of prolactin release in the rat. Endocrinology. 1985;116:1383–1390. doi: 10.1210/endo-116-4-1383. [DOI] [PubMed] [Google Scholar]
- Ahmed CE, Dees WL, Ojeda SR. The immature rat ovary is innervated by vasoactive intestinal peptide (VIP)-containing fibers and responds to VIP with steroid secretion. Endocrinology. 1986;118:1682–1689. doi: 10.1210/endo-118-4-1682. [DOI] [PubMed] [Google Scholar]
- Alm P, Alumets J, Håkanson R, Owman O, Sjöberg NO, Sundler F, Walles B. Origin and distribution of VIP (vasoactive intestinal polypeptide)-nerves in the genito-urinary tract. Cell Tissue Res. 1980;205:337–347. doi: 10.1007/BF00232276. [DOI] [PubMed] [Google Scholar]
- Bajo AM, Juarranz MG, Valenzuela P, Martínez P, Prieto JC, Guijarro LG. Expression of vasoactive intestinal peptide (VIP) receptors in human uterus. Peptides. 2000;21:1383–1388. doi: 10.1016/s0196-9781(00)00282-5. [DOI] [PubMed] [Google Scholar]
- Barberi M, Muciaccia B, Morelli MB, Stefanini M, Cecconi S, Canipari R. Expression localisation and functional activity of pituitary adenylate cyclase-activating polypeptide, vasoactive intestinal polypeptide and their receptors in mouse ovary. Reproduction. 2007;134:281–292. doi: 10.1530/REP-07-0051. [DOI] [PubMed] [Google Scholar]
- Bose HS, Whittal RM, Baldwin MA, Miller WL. The active form of the steroidogenic acute regulatory protein, StAR, appears to be a molten globule. PNAS. 1999;96:7250–7255. doi: 10.1073/pnas.96.13.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecconi S, Rossi G, Barberi M, Scaldaferri L, Canipari R. Effect of pituitary adenylate cyclase-activating polypeptide and vasoactive intestinal polypeptide on mouse preantral follicle development in vitro. Endocrinology. 2004;145:2071–2079. doi: 10.1210/en.2003-1004. [DOI] [PubMed] [Google Scholar]
- Davoren JB, Hsueh AJ. Vasoactive intestinal peptide: a novel stimulator of steroidogenesis by cultured rat granulosa cells. Biol Reprod. 1985;33:37–52. doi: 10.1095/biolreprod33.1.37. [DOI] [PubMed] [Google Scholar]
- Dyson MT, Kowalewski MP, Manna PR, Stocco DM. The differential regulation of steroidogenic acute regulatory protein-mediated steroidogenesis by type I and type II PKA in MA-10 cells. Mol Cell Endocrinol. 2009;300:94–103. doi: 10.1016/j.mce.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gehani F, Tena-Sempere M, Huhtaniemi I. Vasoactive intestinal peptide is an important endocrine regulatory factor of fetal rat testicular steroidogenesis. Endocrinology. 1998a;139:1474–1480. doi: 10.1210/endo.139.4.5861. [DOI] [PubMed] [Google Scholar]
- El-Gehani F, Tena-Sempere M, Huhtaniemi I. Vasoactive intestinal peptide stimulates testosterone production by cultured fetal rat testicular cells. Mol Cell Endocrinol. 1998b;140:175–178. doi: 10.1016/s0303-7207(98)00047-1. [DOI] [PubMed] [Google Scholar]
- Fernández M, Sánchez-Franco F, Palacios N, Sánchez I, Cacicedo L. IGF-I and vasoactive intestinal peptide (VIP) regulate cAMP-response element-binding protein (CREB)-dependent transcription via the mitogen-activated protein kinase (MAPK) pathway in pituitary cells: requirement of Rap1. J Mol Endocrinol. 2005;34:699–712. doi: 10.1677/jme.1.01703. [DOI] [PubMed] [Google Scholar]
- Forneris ML, Aguado LI. Neonatal superior nerve transection disturbs the cyclic activity of the female rats. Journal of Steroids Biochemistry and Molecular Biology. 2002;82:75–82. doi: 10.1016/s0960-0760(02)00149-8. [DOI] [PubMed] [Google Scholar]
- Gozes I, Tsafriri A. Detection of vasoactive intestinal peptide-encoding messenger ribonucleic acid in the rat ovaries. Endocrinology. 1986;119:2606–2610. doi: 10.1210/endo-119-6-2606. [DOI] [PubMed] [Google Scholar]
- Hulshof SC, Dijkstra G, Van der Beek EM, Bevers MM, Figueiredo JR, Beckers JF, Van den Hurk R. Immunocytochemical localization of vasoactive intestinal peptide and neuropeptide Y in the bovine ovary. Biol Reprod. 1994;50:553–560. doi: 10.1095/biolreprod50.3.553. [DOI] [PubMed] [Google Scholar]
- Ishigaki Y, Li X, Serin G, Maquat LE. Evidence for a pioneer round of mRNA translation: mRNAs subject to nonsense-mediated decay in mammalian cells are bound by CBP80 and CBP20. Cell. 2001;106:607–617. doi: 10.1016/s0092-8674(01)00475-5. [DOI] [PubMed] [Google Scholar]
- Jana B, Dzienis A, Pańczyszyn J, Rogozińska A, Wojtkiewicz J, Skobowiat C, Majewski M. Denervation of the porcine ovaries performed during the early luteal phase influenced morphology and function of the gonad. Reprod Biol. 2005;5:69–82. [PubMed] [Google Scholar]
- Jo Y, King SR, Khan SA, Stocco DM. Involvement of protein kinase C and cyclic adenosine 3',5'-monophosphate-dependent kinase in steroidogenic acute regulatory protein expression and steroid biosynthesis in Leydig cells. Biol Reprod. 2005;73:244–255. doi: 10.1095/biolreprod.104.037721. [DOI] [PubMed] [Google Scholar]
- Kowalewski MP, Dyson MT, Manna PR, Stocco DM. Involvement of peroxisome proliferator-activated receptor gamma in gonadal steroidogenesis and steroidogenic acute regulatory protein expression. Reprod Fertil Dev. 2009;21:909–922. doi: 10.1071/RD09027. [DOI] [PubMed] [Google Scholar]
- Kowalewski MP, Schuler G, Taubert A, Engel E, Hoffmann B. Expression of cyclooxygenase 1 and 2 in the canine corpus luteum during diestrus. Theriogenology. 2006;66:1423–1430. doi: 10.1016/j.theriogenology.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Lacombe A, Lelievre V, Roselli CE, Muller JM, Waschek JA, Vilain E. Lack of vasoactive intestinal peptide reduces testosterone levels and reproductive aging in mouse testis. J Endocrinol. 2007;1941:153–160. doi: 10.1677/JOE-07-0102. [DOI] [PubMed] [Google Scholar]
- Lara HE, McDonald JK, Ahmed CE, Ojeda SR. Guanethidine-mediated destruction of ovarian sympathetic nerves disrupts ovarian development and function in rats. Endocrinology. 1990;127:2199–2209. doi: 10.1210/endo-127-5-2199. [DOI] [PubMed] [Google Scholar]
- Larsson LI, Fahrenkrug J, Schaffalitzky de Muckadell OB. Vasoactive intestinal polypeptide occurs in nerves of the female genitourinary tract. Science. 1977;197:1374–1375. doi: 10.1126/science.897673. [DOI] [PubMed] [Google Scholar]
- López FJ, Dominguez JR, Sánchez-Franco F, Negro-Vilar A. Role of dopamine and vasoactive intestinal peptide in the control of pulsatile prolactin secretion Endocrinology. 1989;124:527–535. doi: 10.1210/endo-124-1-527. [DOI] [PubMed] [Google Scholar]
- Manna PR, Dyson MT, Eubank DW, Clark BJ, Lalli E, Sassone-Corsi P, Zeleznik AJ, Stocco DM. Regulation of steroidogenesis and the steroidogenic acute regulatory protein by a member of the cAMP response-element binding protein family. Mol Endocrinol. 2002;16:184–199. doi: 10.1210/mend.16.1.0759. [DOI] [PubMed] [Google Scholar]
- Manna PR, Wang XJ, Stocco DM. Involvement of multiple transcription factors in the regulation of steroidogenic acute regulatory protein gene expression. Steroids. 2003;68:1125–1134. doi: 10.1016/j.steroids.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Ojeda SR, White SS, Aguado LI, Advis JP, Andersen JM. Abdominal vagotomy delays the onset of puberty and inhibits ovarian function in the female rat. Neuroendocrinology. 1983;36:261–267. doi: 10.1159/000123465. [DOI] [PubMed] [Google Scholar]
- Parra C, Fiedler JL, Luna SL, Greiner M, Padmanabhan V, Lara HE. Participation of vasoactive intestinal polypeptide in ovarian steroids production during the rat estrous cycle and in the development of estradiol valerate-induced polycystic ovary. Reproduction. 2007;133:147–154. doi: 10.1530/rep.1.01214. [DOI] [PubMed] [Google Scholar]
- Resko JA, Norman RL, Niswender GD, Spies HG. The relationship between progestins and gonadotropins during the late luteal phase of the menstrual cycle in rhesus monkeys. Endocrinology. 1974;94:128–135. doi: 10.1210/endo-94-1-128. [DOI] [PubMed] [Google Scholar]
- Robberecht P, Deschodt-Lanckman M, Camus JC, de Neef P, Lambert M, Christophe J. VIP activation of rat anterior pituitary adenylate cyclase. FEBS Letters. 1979;103:229–233. doi: 10.1016/0014-5793(79)81333-2. [DOI] [PubMed] [Google Scholar]
- Salustri A, Petrungaro S, Siracusa G. Granulosa cells stimulate in vitro the expansion of isolated mouse cumuli oophori: involvement of prostaglandin E2. Biol Reprod. 1985;33:229–234. doi: 10.1095/biolreprod33.1.229. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Jörgensen J, Kannisto P, Liedberg F, Ottesen B, Owman C. Vasoactive intestinal polypeptide in the PMSG-primed immature rat ovary and its effect on ovulation in the isolated rat ovary perfused in vitro. J Reprod Fertil. 1990;90:465–472. doi: 10.1530/jrf.0.0900465. [DOI] [PubMed] [Google Scholar]
- Vaccari S, Latini S, Barberi M, Teti A, Stefanini M, Canipari R. Characterization and expression of different pituitary adenylate cyclase-activating polypeptide/vasoactive intestinal polypeptide receptors in rat ovarian follicles. J Endocrinol. 2006;191:287–299. doi: 10.1677/joe.1.06470. [DOI] [PubMed] [Google Scholar]
- Vaudry D, Gonzalez BJ, Basille M, Yon L, Fournier A, Vaudry H. Pituitary adenylate cyclase-activating polypeptide and its receptors: from structure to functions. Pharmacol Rev. 2000;52:269–324. Review. [PubMed] [Google Scholar]