Abstract
Objective
The goal of this retrospective study was to investigate the effects of perioperative use of dexmedetomidine (Dex) on outcomes for older patients undergoing cardiac surgery.
Design
Retrospective investigation.
Setting
Patients from a single tertiary medical center.
Participants
A total of 505 patients (equal or greater than 65 years old) who underwent coronary artery bypass graft (CABG) or valve surgery, CABG and/or valve surgery plus other procedures were divided into 2 groups: 283 received intravenous Dex infusion (Dex group) and 222 did not(Non-Dex group).
Interventions
Perioperative Dex intravenous infusion (0.24 to 0.6 μg·kg−1·h−1) initiated after cardiopulmonary bypass and continued for < 24 hours postoperatively in the ICU.
Measurements and Main Results
Data were risk adjusted, propensity score weighted, and multivariate logistic regression was used. The primary outcome was mortality. Secondary outcomes included postoperative stroke, coma, myocardial infarction, heart block, cardiac arrest, delirium, renal failure and sepsis. Perioperative Dex infusion significantly decreased in-hospital mortality (0.90% vs. 2.83%; adjusted odds ratio (OR), 0.099; 95% confidence interval (CI), 0.030-0.324; P=0.004) and operative mortality (1.35% vs. 3.18%; adjusted OR, 0.251; 95% CI, 0.077-0.813; P=0.021). Perioperative Dex treatment also reduced the risk of stroke (0.90% vs. 1.77%; adjusted OR, 0.15; 95% CI, 0.038-0.590; P=0.007) and delirium (7.21% vs. 10.95%; adjusted OR, 0.35; 95% CI, 0.212-0.578; P<0.0001).
Conclusions
Results from this study suggested perioperative use dexmedetomidine was associated with a decrease in in-hospital and operative mortality in elderly patients following cardiac surgery. It also reduced incidence of postoperative stroke and delirium in elderly patients.
Introduction
Cardiovascular disease is the most common cause of death in the elderly population. With the advancement of technology, cardiac surgery is feasible in elderly patients with acceptable risks. Currently, more than 67% of patients presenting for cardiac surgery are older than 65 years and with increased comorbidities [1]. Cardiovascular, pulmonary, and renal diseases are more common and contribute to greater perioperative complications and this may influence perioperative outcomes on patients with advanced age [2]. Elderly high-risk patients usually require prolonged ICU stay and are at an increased risk for mortality and morbidity [3-5]. It is well known that surgical stress and cardiopulmonary bypass (CPB) can increase plasma levels of norepinephrine and epinephrine. Dexmedetomidine (Dex) is a highly selective α2-adrenergic agonist that strongly modulates the activity of sympathetic nervous system by binding to the α2-receptors present in both the central and peripheral nervous systems and inhibiting the release of norepinephrine thus modulating sympathetic activity [6, 7]. Because of this, Dex has been used extensively in intensive care unit as a sedative and during surgery as an adjuvant anesthetic to attenuate perioperative hemodynamic abnormalities. Multiple studies have demonstrated that Dex has a protective effect on the heart, brain and kidney [8-11]. It was associated with decreased mortality, time to extubation, and hospital length of stay (LOS) in cardiac surgical patients [12-15]. We have previously published a study describing the effects of Dex on outcomes of patients undergoing cardiac surgery [12]. In that study, Dex reduced in-hospital, 30-day and 1-year mortality and decreased incidence of postoperative complications and delirium. Part of this data has also been used in the study published in JCVA which found that Dex infusion during CABG surgery was more likely to achieve improved in-hospital, 30- day, and 1-year survival rates, and a significantly lower incidence of delirium [15]. We now reported the results of another study using the same database. The aim of this sub-analysis was to investigate the effects of perioperative use of Dex on outcomes for older patients undergoing cardiac surgery.
Methods
Study design
After obtaining the local Institutional Review Board approval, all patients (equal or greater than 65 years old) who underwent cardiac surgery with CPB at the UC Davis Medical Center from January 1, 2006, to December 31, 2011 were entered into the study. This study was a single-center, retrospective cohort study involving 561 consecutive patients. Patients in this study met the following criteria: CABG or valve surgery, CABG or valve surgery combined with other procedures (maze operation). Patients excluded from this study were those undergoing emergency surgery, off-pump or robotic surgery, surgery demanding deep hypothermic circulatory arrest, or surgery involving the thoracic aorta (Figure 1). A total of 505 patients met the inclusion criteria and were divided into 2 groups: those who used Dex (Dex group; n=283, 56.04%) and those who did not use Dex (Non-Dex group; n=222, 43.96%) in surgery (Figure 1).
Figure 1.
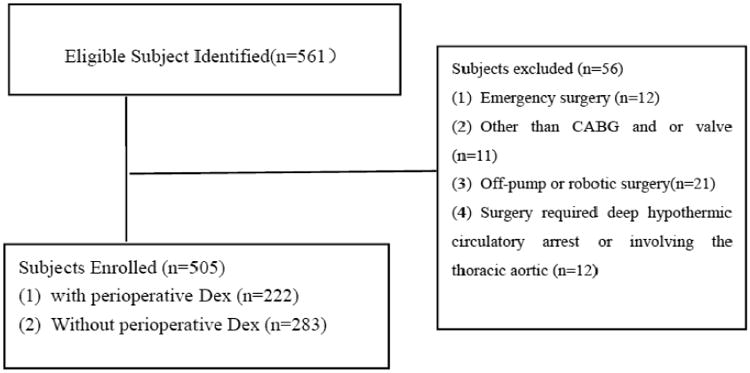
Study population recruitment summary. Dex: dexmedetomidine; CABG: coronary artery bypass graft
Data collection
The patient data were collected and organized following the template of the STS National Adult Cardiac Surgery Database and the hospital medical records that included demographics, patient past medical and surgical histories, preoperative risk factors, preoperative medications, intraoperative data, postoperative stroke, coma, myocardial infarction, heart block, cardiac arrest, acute renal failure, dialysis required, and in-hospital, operative, and 1-year all-cause mortality. Data were collected independently as part of the STS database on each patient during the course of the hospitalization. After routine monitoring, general anesthesia was induced with midazolam, lidocaine, propofol or etomidate, fentanyl, and rocuronium, maintained with sevoflurane. Ventilation with 50% oxygen in air was controlled to an end-tidal CO2 of 35 to 45 mmHg by adjustment of tidal volume and respiratory rate. Arterial catheter, pulmonary artery catheter, and transesophageal echocardiography were used as hemodynamic and cardiac function monitoring after anesthesia induction. Perioperative Dex administration was defined as an intravenous infusion (0.24 to 0.6 μg·kg−1·h−1) initiated after cardiopulmonary bypass and continued for < 24 hours postoperatively in the ICU. The infusion rate of Dex was adjusted according the patients' hemodynamic changes in response to stimulation.
Major and secondary outcomes
Major outcomes of this study were in-hospital, operative, and 1-year mortality. Secondary outcomes included postoperative permanent or transient stroke, coma, myocardial infarction, heart block, and cardiac arrest, renal failure or new dialysis requirement, delirium, postoperative length of mechanical ventilation, length of ICU stay, length of hospital stay, and 30-day readmission. On the basis of the STS criteria, the following definitions were used. Permanent stroke was defined as a postoperative stroke (any confirmed neurological deficit of abrupt onset caused by a disturbance in blood supply to the brain) that did not resolve within 24 hours. Transient ischemic attack was defined as a loss of neurological function that was abrupt in onset but with complete return of function within 24 hours. The definition of coma was a new postoperative coma that persisted for at least 24 hours secondary to anoxic/ischemic or metabolic encephalopathy, thromboembolic event, or cerebral bleed. Cardiac arrest included one of the following: a. ventricular fibrillation, b. rapid ventricular tachycardia with hemodynamic instability, c. asystole, d. implantable cardioverter defibrillator shocks. Heart block was defined as new on-set block requiring the implantation of a permanent pacemaker of any type before discharge. STS definition of postoperative renal failure was used to determine postoperative acute kidney injury (AKI). This definition included the highest Cr level recorded in the post-operative course that is ≥ 3-fold baseline Cr or Cr ≥ 4 with an acute increase of ≥ 0.5mg/dl or new requirement for post-operative dialysis. Finally, sepsis was defined as a systemic inflammatory response syndrome when at least two of the following criteria were present: hypo- or hyperthermia (>38.5 or < 36.0°C), tachycardia or bradycardia, tachypnea, leukocytosis or leukopenia, or thrombocytopenia. Delirium was defined as illusions, confusion, and cerebral excitement in hospital stay and having a comparatively short course. Any complication included all postoperative events occurring during the hospitalization, including the entire postoperative period up to discharge, even if > 30 days.
Statistical Methods
Continuous variables were assessed as mean ± SD and compared by use of the t test; categorical variables were reported as percentages and compared by use of chi-square test (2 tailed). Univariate and multivariate logistic regressions were performed to evaluate the associations of demographic, therapeutic, and clinical outcome variables. To decrease selection bias in patients who received Dex administration, we computed the propensity score, that is, the conditional probability of each patient receiving Dex, with a multivariable logistic regression model that included patient demographic and clinical risk factors (Table 1).
Table 1. Demographic and Clinical Characteristics.
| Characteristics | Dex (N=222) | Non-Dex (N=283) | P value |
|---|---|---|---|
| Age, year | 73.6 (6.1) | 73.5 (6.2) | 0.798 |
| Female sex, n (%) | 71(32.0) | 87(30.7) | 0.766 |
| BMI, mean (SD) | 28.3 (5.7) | 29.7 (7.3) | 0.020 |
| Non-white race n (%) | 57(25.7) | 64(22.6) | 0.424 |
| Past medical history n (%) | |||
| Smoking | 24(10.8) | 43(15.2) | 0.150 |
| Chronic lung disease | 36(16.2) | 50(17.7) | 0.667 |
| Cerebrovascular disease | 48(21.6) | 84(29.7) | 0.041 |
| Peripheral vascular disease | 48(21.6) | 43(15.2) | 0.062 |
| Family History of CAD | 41(18.5) | 69(24.4) | 0.110 |
| Diabetes mellitus | 70(31.5) | 90(31.8) | 0.948 |
| Hypertension | 176(79.3) | 224(79.2) | 0.972 |
| Dyslipidemia | 147(66.2) | 155(54.8) | 0.009 |
| History of Renal Failure | 10 (4.5) | 2 (0.7) | 0.005 |
| Preoperative myocardial infarction | 67 (30.2) | 102 (36.0) | 0.166 |
| CHF | 85 (38.3) | 19 (6.7) | <0.0001 |
| EF % | 52.8 (12.6) | 51.0 (13.2) | 0.119 |
| Preoperative last creatinine level | 1.18 (0.66) | 1.11 (0.53) | 0.159 |
| Preoperative medication n (%) | |||
| ACEI | 106 (47.8) | 154 (54.4) | 0.137 |
| β-blockers | 147 (66.2) | 183 (64.7) | 0.716 |
| ADP Inhibitor | 16 (7.2) | 24 (8.5) | 0.599 |
| Nitrates | 7 (3.2) | 9 (3.2) | 0.986 |
| Anticoagulants | 43 (19.4) | 75 (26.5) | 0.060 |
| Coumadin | 20(9.0) | 30(10.6) | 0.552 |
| Inotropes | 1(0.5) | 2(0.7) | 0.710 |
| Steroids | 5(2.3) | 12(4.2) | 0.219 |
| Aspirin | 179(80.6) | 225(79.5) | 0.754 |
| Lipid lowering | 148(66.7) | 160(56.5) | 0.021 |
| Glycoprotein IIb/IIIa inhibitor | 7(3.2) | 16(5.7) | 0.181 |
| Propensity score | 0.611(0.256) | 0.305(0.197) | <0.0001 |
Dex: dexmedetomidine; BMI: body mass index; ACEI: angiotensin-converting enzyme inhibitors; CAD: coronary artery disease; CHF: congestive heart failure; and EF: ejection fraction. Values are n (%) for categorical variables and mean±SD for continuous variables.
The multivariate model analysis was used to assess any complication included Dex use, age, gender, status of procedure, smoking, chronic lung disease, cerebrovascular disease, peripheral vascular disease, family history of CAD, dyslipidemia, hypertension, MI, congestive heart failure, surgical type, perfusion time. The multivariate model assessing delirium included Dex use, age, gender, body mass index, cerebrovascular disease, family history of CAD, dyslipidemia, ejection fraction, and surgical type. The multivariate model used to assess stroke included Dex use and surgical types. The multivariate model used to assess in-hospital mortality included Dex use and surgical types. The multivariate model used to assess operative mortality included Dex use and status of procedure (urgent vs. elective). The multivariate model used to assess 1-year mortality included Dex use, age, status of procedure, chronic lung disease, diabetes, myocardial infarction, renal failure, last creatinine level, dyslipidemia, IABP and perfusion time.
To achieve model parsimony and stability, the backward selection procedure was applied with a dropout criterion of P > 0.1. The candidate risk factors were selected on the basis of the literature reviews, clinical possibility, and variables collected in the database. The candidate independent variables included age, sex, race, status of procedure, body mass index, creatinine level, smoking, chronic lung disease, cerebrovascular disease (CVD), peripheral vascular disease (PVD), family history of coronary artery disease (CAD), diabetes mellitus, hypertension, hypercholesterolemia, dyslipidemia, renal failure, myocardial infarction, congestive heart failure (CHF), intra-aortic balloon pump (IABP), β-blockers, nitrates, angiotensin-converting enzyme inhibitors (ACEI), ADP inhibitors, glycoprotein IIb/IIIa inhibitors, Coumadin, inotropes, aspirin, lipid-lowering drugs, surgery type, ejection fraction, perfusion time, cross-clamp time, and year of surgery. The parsimonious multivariable propensity model for Dex infusion included status of produces, preoperative CVD, preoperative PVD, preoperative dyslipidemia, preoperative CHF, surgery type, and year of surgery (Figure 2). Then we created a propensity-weighted logistic regression model for 1-year mortality, The C statistic was reported as a measure of predictive power. We compared the propensity weighted and risk-adjusted 1-year mortality between the cohort with Dex use and with no Dex use. The results are reported as percentages and odds ratios (OR) with corresponding 95% confidence intervals (CI). A parsimonious Cox proportional hazards model was created to estimate the effect of Dex for 1-year survival. All reported P values were 2 tailed, and values of P< 0.05 were considered to be statistically significant. All statistical analyses were performed with SAS version 9.3 for Windows (SAS Institute, Cary, NC).
Figure 2.
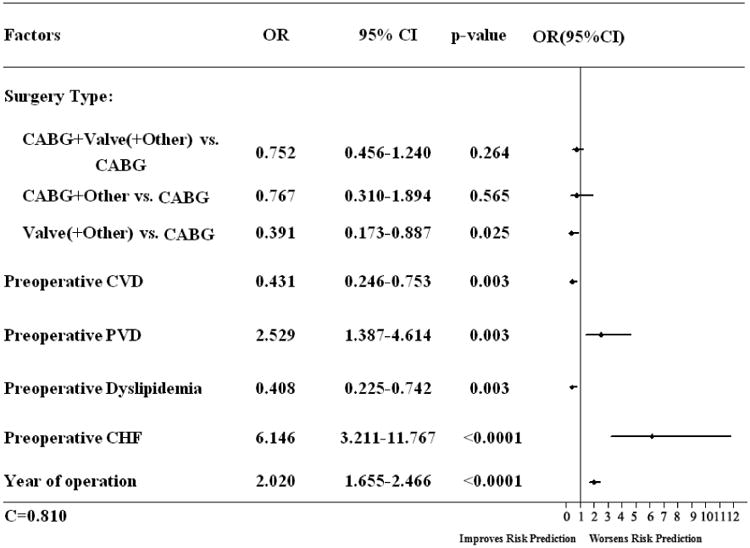
Represented the Parsimonious multivariable propensity model for dexmedetomidine use. CABG: coronary artery bypass graft; CVD: Cerebrovascular Disease; CAD: coronary artery disease; CHF: congestive heart failure; CI: confidence interval; EF: ejection fraction; and OR: odds ratio.
Results
Baseline and Intraoperative Parameters
The demographic and clinical data were presented in Table 1. There were no significant differences between the two groups with respect to age, sex, race, medical history (smoking, chronic lung disease, peripheral vascular disease, family history of CAD, diabetes mellitus, hypertension, myocardial infarction, ejection fraction or last creatinine level) and preoperative medical therapy (ACEI, β-blockers, ADP inhibitor, nitrates, Coumadin, inotropes, glycoprotein IIb/IIIa inhibitor or aspirin). However, the patients in the Dex group presented with a greater incidence of renal failure (4.5% vs. 0.7%, P=0.005), congestive heart failure (38.3% vs. 6.7%; P<0.0001), dyslipidemia (66.2% vs. 54.8%; P=0.009), and the use of lipid-lowering medications (66.7% vs. 56.5%; P<0.0001). The patients in the Dex group presented with a lower body mass index (28.3% vs. 29.7%; P=0.02), less urgent surgery (45.5% vs. 58%, P=0.005), lower incidence of cerebrovascular disease (21.6% vs. 29.7%, P=0.041).
Procedural Characteristics
In terms of surgery types, CABG only (45.5% vs. 53.4%, P< 0.0001) was less and valve/valve + other (21.6% vs. 6.7%, P < 0.0001) was more in the Dex group. Cardiopulmonary bypass time (181.7 ± 61.2 vs. 203.7 ± 79.4 minutes; P=0.001) and aortic cross-clamp time (129.2 ± 50.8 vs. 141.9 ± 58.9 minutes; P=0.009) were significantly shorter and the incidence of perioperative intra-aortic balloon pump use (5% vs. 13.1%; P=0.002) was significantly lower in the Dex group (Table 2).
Table 2. Procedural Characteristics.
| Characteristics | Dex (n=222) | Non-Dex (n=283) | P value |
|---|---|---|---|
| Perfusion time, min | 181.7 (61.2) | 203.7 (79.4) | 0.001 |
| Cross clamp time, min | 129.2 (50.8) | 141.9 (58.9) | 0.009 |
| IABP used, n (%) | 11(5.0) | 37(13.1) | 0.002 |
| Surgery type, n (%) | |||
| CABG only | 101(45.5) | 151(53.4) | <0.0001 |
| CABG+valve+(other) | 61(27.5) | 93(32.9) | 0.192 |
| CABG+other | 12(5.4) | 20(7.1) | 0.447 |
| Valve+(valve+other) | 48(21.6) | 19(6.7) | <0.0001 |
Dex: dexmedetomidine; CABG: coronary artery bypass graft; IABP: intra-aortic balloon pump. Values are n (%) for categorical variables and mean±SD for continuous variables.
Postoperative Complications and Mortality
Univariate analysis demonstrated that 11 out of the total 505 patients (2.18%) died in hospital, 12 patients (2.37%) died during the hospitalization, 39 patients (7.72%) died within 1 year. In-hospital, operative, and 1-year mortality was 0.9%, 1.35%, and 7.21% respectively in Dex group and was 2.83%, 3.18%, and 8.13% in Non-Dex group. Total length of ICU stay (142.5 ± 191.5 vs. 106.2 ± 132.8 hours, P=0.017) was is longer in the Dex group. No differences were seen in the incidence of postoperative complications: MI, coma, heart block, cardiac arrest, delirium, renal failure, sepsis, ventilation time, or length of hospital stay, readmission within 30-day, in-hospital, operative, and 1-year mortality in two groups (Figure 3).
Figure 3.
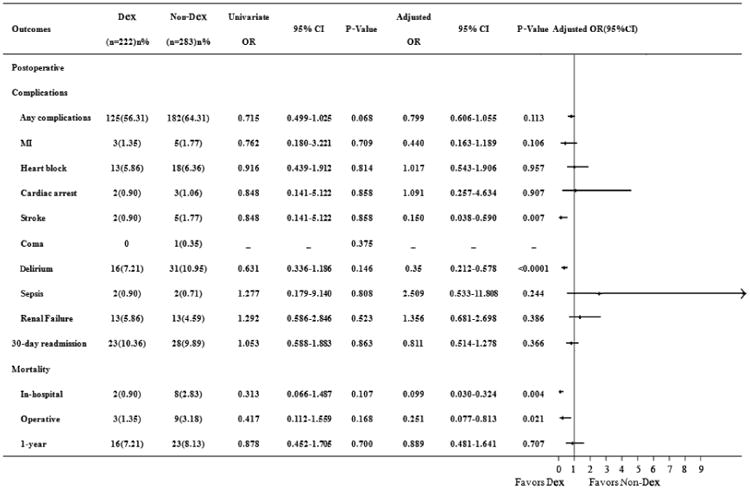
Showed the effects of dexmedetomidine on postoperative complications and mortality in patients undergoing cardiac surgery. Values are numbers (percent) for categorical variables. CI: confidence interval; Dex: dexmedetomidine; MI: myocardial infarction; and OR: odds ratio.
Results of the multivariate analysis are summarized in Figure 3. The observed reduction in-hospital (adjusted OR, 0.099; 95% CI, 0.030-0.324; P=0.004), operative (adjusted OR, 0.251; 95% CI, 0.077-0.813; P=0.021) mortality in patients receiving Dex persisted after propensity adjustment. Postoperative delirium (adjusted OR, 0.350; 95% CI, 0.212-0.578; P < 0.0001) and stroke (adjusted OR, 0.15; 95% CI, 0.038-0.590; P=0.007) were also significant decreased in the Dex group. There were no statistical differences in the incidence of any complication (adjusted OR, 0.799; 95% CI, 0.606-1.055; P=0.113), or cardiac arrest (adjusted OR, 1.213; 95% CI, 0.280-5.261; P=0.796) or postoperative renal failure (adjusted OR, 1.356; 95% CI, 0.681-2.698; P=0.386) or sepsis (adjusted OR, 2.509; 95% CI, 0.533-11.808; P=0.244) between groups after adjustment (Figure 3). Postoperative ventilation times(33.9 vs. 53.1 hours, P=0.046)were shorter and total ICU times (153.4 vs. 103.0 hours, P<0.0001) were longer after adjustment in the Dex group.
One-Year Mortality
After risk adjustment, a Cox proportional hazard model analysis revealed that older age, urgent surgery, preoperative last creatinine level, perfusion time significantly increased the 1-year mortality, Dex was no longer a contributing factor for 1-year survival rate (Figure 4).
Figure 4.
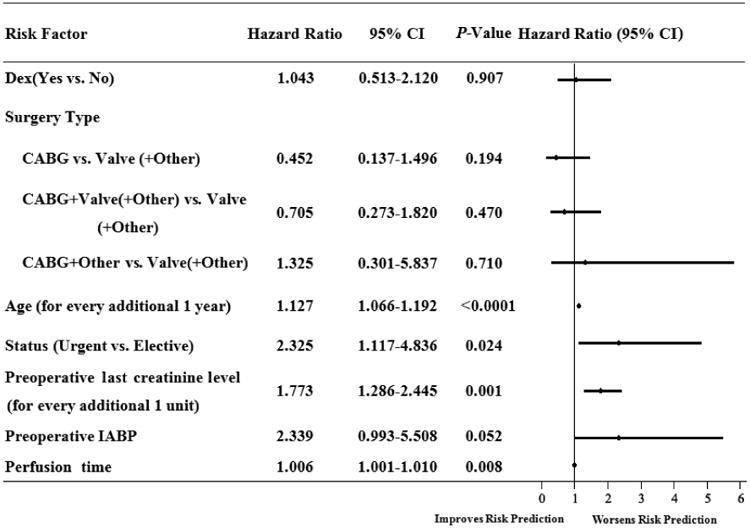
Demonstrated the Cox proportional hazard model for 1-year mortality after cardiac surgery in elderly patients. CABG: coronary artery bypass graft; IABP: Intra-aortic balloon pump; and CI: confidence interval.
Discussion
This is a sub-analysis using the data we used before [12] to investigate the effect of Dex on outcomes for elderly cardiac surgery population. The current study found that perioperative Dex use significantly reduced in-hospital and operative mortality and was associated with improved early survival rate in elderly patients undergoing cardiac surgery. Our results further suggest that perioperative intravenous Dex is associated with a reduced incidence of postoperative stroke and delirium after cardiac surgery.
Perioperative Dex infusion may affect the mortality rates in the elder cardiac surgical patients. Age has been suggested as an independent risk factor for cardiac surgery outcomes [12]. Frilling and colleagues [16] found that in-hospital mortality in geriatric patients undergoing aortic valve replacement was 2.56% in isolated valve replacement and 3.38% in patients who had additional bypass surgery. Our results revealed the in-hospital mortality (2.83%) and operative mortality (3.18%) in Non-Dex group is similar to the previous reports (2%-4.4%) [12, 16-18]. However, in-hospital (0.9%) and operative mortality (1.35%) was significantly lower in patients who received Dex. The 1-year total mortality was 7.72% that was similar to the other published study [19]. Barreto-Filho and colleagues found 30-day and 1-year mortality were 3.5% and 9.9% in isolated aortic valve replacement (AVR) vs. 5.1% and 12.3% in AVR with concomitant CABG, respectively [19]. Our study found that although in-hospital and operative mortality rates were lower in the Dex group, there were no statistical differences in 1- year mortality (7.21% vs. 8.13%, P=0.878) between the two groups. Dex was no longer a contributing factor in the Cox proportional hazard analysis as to one-year survival rate which was different from the other study [12, 15]. So perioperative Dex use may be associated with improved short-term survival rate and doesn't improve 1-year survival rate in older cardiac surgical patients.
Stroke is a devastating complication following cardiac surgery and has been reported that the prevalence of stroke was 1.6-5.25% among patients undergoing different cardiac surgeries [3, 21-23]. Dex, a highly selective α2-adrenoceptor agonist, provides sedation, sympatholysis and analgesia without respiratory depression [20]. In the current study, Dex use decreased the postoperative stroke after risk adjustment. The exact cause of stroke after surgery is poorly understood. A pre-clinical study showed significant neurological improvement in Dex-treated animals following stroke [24]. The neuroprotective effect of Dex was suggested to be mediated by activation of the α2A adrenergic receptor subtype that modulates neurotransmitter release in the central and peripheral sympathetic nervous system [9]. Micro-embolization produced during CPB, O2 supply/demand imbalance and the systemic inflammatory response may be contributing factors [9, 25]. Chi and colleagues suggested Dex could improve micro-regional O2 supply/consumption balance and may have contributed to the decreased size of cortical infarction in the early stage of reperfusion in an animal study [25].
Postoperative delirium is common among older patients undergoing cardiac surgery [26, 27]. The reported incidence of postoperative delirium is reported to range from 30% to 55% in elder patients undergoing cardiac surgery [26, 28-32]. Our study also found that those who received Dex had a significantly lower incidence of delirium after cardiac surgery. At the same time, we observed the prevalence of delirium in our study was lower than most reported results [26, 28-31]. The reason may be that we included only patients with hyperactive delirium in this database, many patients with hypoactive delirium were therefore likely to be undetected. A study had shown that hypoactive delirium was the most common motor subtype in geriatric patients [32]. Pandharipande and colleagues found the hyperactive delirium was no more than 1% in surgical ICU patients, whereas the majority of patients had hypoactive (64%) delirium in the postoperative period [33]. On the other hand, delirium assessment is somewhat subjective and may be susceptible to observational bias. A datum showed that Dex could provide neurocognitive protection in an animal model [34]. In a retrospective cohort study, use of Dex was associated with a decrease in postoperative acute kidney injury [11], particularly in patients with normal preoperative kidney function or mild chronic kidney disease [10]. However, our data failed to show the relationship between the use of Dex and the reduction of AKI in aged population.
In a randomized trial, Dex-treated patients spent less time on the ventilator [35]. A meta-analysis confirmed the result [14]. The mean postoperative time to extubation and length of hospital stay were shorter in the Dex group when compared with the propofol group and Dex-based sedation resulted in achievement of early extubation more frequently than propofol-based sedation [13]. In this study, while durational mechanical ventilation was shorter in the Dex treated patients, the ICU length of stay is longer, it is different from the other study [12, 13, 35], however the length of hospital stay was no difference between two groups. The reason for the discrepancy in the results was unclear. Further studies to confirm this result are needed.
There are several limitations of this study. This was a single-center, observational cohort study. Multivariate regression in combination with propensity score adjustments was applied to this study population to reduce the bias, however, the potential confounding bias associated with a non-randomized study remains. The sample size of this study is relatively small. Further prospective, multicenter, randomized and controlled studies with a larger sample size are required to confirm these effects in this study.
Conclusions
The retrospective analysis demonstrated that elderly cardiac surgical patients who received dexmedetomidine after CPB surgery had better in-hospital and operative survival rates. Perioperative use of dexmedetomidine was also associated with a significant decrease in the incidence of postoperative stroke and delirium. A prospective, randomized, multicenter study focused on the use of dexmedetomidine in elderly cardiac surgery patients is indicated to confirm these findings.
Acknowledgments
Founding: This work was supported by the Department of Anesthesiology and Pain Medicine, Department of Surgery and Department of Internal Medicine of University of California Davis Health System and NIH grant UL1 TR000002 of the University of California Davis Health. This work was also supported by the grant from the National Natural Science Foundation of China NO 81471835 and NO 81471889.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests: The authors have declared that no competing interests exist.
References
- 1.Shehabi Y, Grant P, Wolfenden H, et al. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study) Anesthesiology. 2009;111:1075–1084. doi: 10.1097/ALN.0b013e3181b6a783. [DOI] [PubMed] [Google Scholar]
- 2.Oresanya LB, Lyons WL, Finlayson E. Preoperative assessment of the older patient: a narrative review. JAMA. 2014;311:2110–2120. doi: 10.1001/jama.2014.4573. [DOI] [PubMed] [Google Scholar]
- 3.Fruitman DS, MacDougall CE, Ross DB. Cardiac surgery in octogenarians: can elderly patients benefit? Quality of life after cardiac surgery. Ann Thorac Surg. 1999;68:2129–2135. doi: 10.1016/s0003-4975(99)00818-8. [DOI] [PubMed] [Google Scholar]
- 4.Sündermann S, Dademasch A, Praetorius J, et al. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33–37. doi: 10.1016/j.ejcts.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Lee DH, Buth KJ, Martin BJ, et al. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121(8):973–978. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 6.Gertler R, Brown HC, Mitchell DH, et al. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 2001;14:13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jalonen J, Hynynen M, Kuitunen A, et al. Dexmedetomidine as an anesthetic adjunct in coronary artery bypass grafting. Anesthesiology. 1997;86:331–345. doi: 10.1097/00000542-199702000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Biccard BM, Goga S, de Beurs J. Dexmedetomidine and cardiac protection for non-cardiac surgery: a meta-analysis of randomised controlled trials. Anaesthesia. 2008;63:4–14. doi: 10.1111/j.1365-2044.2007.05306.x. [DOI] [PubMed] [Google Scholar]
- 9.Ma D, Hossain M, Rajakumaraswamy N, et al. Dexmedetomidine produces its neuroprotective effect via the a2A-adrenoceptor subtype. Eur J Pharmacol. 2004;502(1-2):87–97. doi: 10.1016/j.ejphar.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 10.Ji F, Li Z, Young JN, et al. Post-bypass dexmedetomidine use and postoperative acute kidney injury in patients undergoing cardiac surgery with cardiopulmonary bypass. PLoS One. 2013;8:e77446. doi: 10.1371/journal.pone.0077446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balkanay OO, Goksedef D, Omeroglu SN, et al. The dose-related effects of Dexmedetomidine on renal functions and serum neutrophil gelatinase-associated lipocalin values after coronary artery bypass grafting: a randomized, triple-blind, placebo-controlled study. Interact Cardiovasc Thorac Surg. 2015;20:209–214. doi: 10.1093/icvts/ivu367. [DOI] [PubMed] [Google Scholar]
- 12.Ji F, Li Z, Nguyen H, et al. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation. 2013;127:1576–1584. doi: 10.1161/CIRCULATIONAHA.112.000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curtis JA, Hollinger MK, Jain HB. Propofol-based versus dexmedetomidine-based sedation in cardiac surgery patients. J Cardiothorac Vasc Anesth. 2013;27:1289–1294. doi: 10.1053/j.jvca.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Lin YY, He B, Chen J, et al. Can dexmedetomidine be a safe and efficacious sedative agent in post-cardiac surgery patients? a meta-analysis. Crit Care. 2012;16(5):R169. doi: 10.1186/cc11646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji F, Li Z, Young N, et al. Perioperative dexmedetomidine improves mortality in patients undergoing coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2014;28(2):267–273. doi: 10.1053/j.jvca.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frilling B, von Renteln-Kruse W, Riess FC. Evaluation of operative risk in elderly patients undergoing aortic valve replacement: the predictive value of operative risk scores. Cardiology. 2010;116:213–218. doi: 10.1159/000319703. [DOI] [PubMed] [Google Scholar]
- 17.Ferguson TB, Jr, Coombs LP, Peterson ED Society of Thoracic Surgeons National Adult Cardiac Surgery Database. Preoperative beta-blocker use and mortality and morbidity following CABG surgery in North America. JAMA. 2002;287:2221–227. doi: 10.1001/jama.287.17.2221. [DOI] [PubMed] [Google Scholar]
- 18.Bridgewater B. Almanac 2012-adult cardiac surgery: the national society journals presents elected research that has driven recent advances in clinical cardiology. Heart. 2012;98(19):1412–1417. doi: 10.1136/heartjnl-2011-301539. [DOI] [PubMed] [Google Scholar]
- 19.Barreto-Filho JA, Wang Y, Dodson JA, et al. Trends in aortic valve replacement for elderly patients in the United States, 1999-2011. JAMA. 2013;310(19):2078–2085. doi: 10.1001/jama.2013.282437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoy SM, Keating GM. Dexmedetomidine: a review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs. 2011;71(11):1481–1501. doi: 10.2165/11207190-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Tarakji KG, Sabik JF, 3rd, Bhudia SK, et al. Temporal onset, risk factors, and outcomes associated with stroke after coronary artery bypass grafting. JAMA. 2011;305:381–390. doi: 10.1001/jama.2011.37. [DOI] [PubMed] [Google Scholar]
- 22.Whitlock R, Healey JS, Connolly SJ, et al. Predictors of early and late stroke following cardiac surgery. CMAJ. 2014;186:905–911. doi: 10.1503/cmaj.131214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Almassi GH, Sommers T, Moritz TE, et al. Stroke in cardiac surgical patients: determinants and outcome. Ann Thorac Surg. 1999;68:391–397. doi: 10.1016/s0003-4975(99)00537-8. [DOI] [PubMed] [Google Scholar]
- 24.Sato K, Kimura T, Nishikawa T, et al. Neuroprotective effects of a combination of dexmedetomidine and hypothermia after incomplete cerebral ischemia in rats. Acta Anaesthesiol Scand. 2010;54:377–382. doi: 10.1111/j.1399-6576.2009.02139.x. [DOI] [PubMed] [Google Scholar]
- 25.Chi OZ, Grayson J, Barsoum S, et al. Effects of Dexmedetomidine on Microregional O2 Balance during Reperfusion after Focal Cerebral Ischemia. J Stroke Cerebrovasc Dis. 2015;24:163–170. doi: 10.1016/j.jstrokecerebrovasdis.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Smulter N, Lingehall HC, Gustafson Y, et al. Delirium after cardiac surgery: incidence and risk factors. Interact Cardiovasc Thorac Surg. 2013;17:790–796. doi: 10.1093/icvts/ivt323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koster S, Hensens AG, Schuurmans MJ, et al. Risk factors of delirium after cardiac surgery: a systematic review. Eur J Cardiovasc Nurs. 2011;10:197–204. doi: 10.1016/j.ejcnurse.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Burkhart CS, Dell-Kuster S, Gamberini M, et al. Modifiable and nonmodifiable risk factors for postoperative delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2010;24:555–559. doi: 10.1053/j.jvca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Rudolph JL, Inouye SK, Jones RN, et al. Delirium: an independent predictor of functional decline after cardiac surgery. J Am Geriatr Soc. 2010;58:643–649. doi: 10.1111/j.1532-5415.2010.02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bakker RC, Osse RJ, Tulen JH, et al. Preoperative and operative predictors of delirium after cardiac surgery in elderly patients. Eur J Cardiothorac Surg. 2012;41:544–549. doi: 10.1093/ejcts/ezr031. [DOI] [PubMed] [Google Scholar]
- 31.Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119:229–236. doi: 10.1161/CIRCULATIONAHA.108.795260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robinson TN, Raeburn CD, Tran ZV, et al. Motor subtypes of postoperative delirium in older adults. Arch Surg. 2011;146:295–300. doi: 10.1001/archsurg.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandharipande P, Cotton BA, Shintani A, et al. Motoric subtypes of delirium in mechanically ventilated surgical and trauma intensive care unit patients. Intensive Care Med. 2007;33:1726–31. doi: 10.1007/s00134-007-0687-y. [DOI] [PubMed] [Google Scholar]
- 34.Sanders RD, Xu J, Shu Y, et al. Dexmedetomidine attenuates isoflurane-induced neurocogitive impairment in neonatal rats. Anesthesiology. 2009;110:1077–1085. doi: 10.1097/ALN.0b013e31819daedd. [DOI] [PubMed] [Google Scholar]
- 35.Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs Midazolam for Sedation of Critically Ill Patients. JAMA. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]


