Abstract
Alcoholic liver disease (ALD) is a leading cause of liver related mortality worldwide. In contrast to recent advances in therapeutic strategies for patients with viral hepatitis, there is a significant lack of novel therapeutic options for patients with ALD. In particular, there is an urgent need to focus our efforts on effective therapeutic interventions for alcoholic hepatitis (AH), the most severe form of ALD. AH is characterized by an abrupt development of jaundice and complications related to liver insufficiency and portal hypertension in patients with heavy alcohol intake. The mortality of patients with AH is very high (20–50% at 3 months). Available therapies are not effective in many patients and targeted approaches are imminently needed. The development of such therapies requires translational studies in human samples and suitable animal models that reproduce clinical and histological features of AH. In recent years, new animal models that simulate some of the features of human AH have been developed, and translational studies using human samples have identified potential pathogenic factors and histological parameters that predict survival. This review article summarizes the unmet needs for translational studies on the pathogenesis of AH, pre-clinical translational tools, and emerging drug targets to benefit the AH patient.
Keywords: alcoholic liver disease, translational research, ethanol, liver failure, inflammation
The global morbidity and mortality of alcoholic liver disease (ALD) are associated with alcohol abuse over prolonged periods. Recent epidemiologic studies show that liver cirrhosis is the 12th leading cause of death and approximately 50% of the total fatalities are alcohol-related (NIAAA Surveillance report #100). ALD encompasses different stages as a consequence of susceptibility factors, duration and intensity of alcohol consumption. Some of the histological stages can co-exist and include steatosis, alcoholic steatohepatitis (ASH) and progressive fibrosis to cirrhosis and superimposed hepatocellular carcinoma (HCC). Moreover, patients with underlying ALD and heavy alcohol intake can develop a form of acute-on-chronic liver injury called alcoholic hepatitis (AH). Severe AH has very high short-term mortality (20–50% at 3 months) and represents one of the deadliest diseases in clinical hepatology.1 Despite its economical and health burden, ALD has received very limited attention from health policy makers, pharmaceutical companies, and private funding agencies. While research has made significant progress, the pathogenesis of ALD remains incompletely understood and there is little or no impact on development of new therapies. Limitations in patient characterization due to poor diagnostic and prognostic measures, inadequate models of disease and insufficient pre-clinical/translational approaches, have stunted the progress in the field of ALD. One of the most urgent needs in this field is to identify new targeted therapies for patients with AH. This need is further confirmed by the recent STOPAH trial that showed limited efficacy of prednisolone.2 In this article, we review the molecular pathogenesis of AH and highlight translational studies that might lead to targeted approaches for the treatment of AH. Discussion of entry criteria and end-points for clinical trials is beyond the scope of this review.
Alcoholic hepatitis: clinical aspects and unmet needs
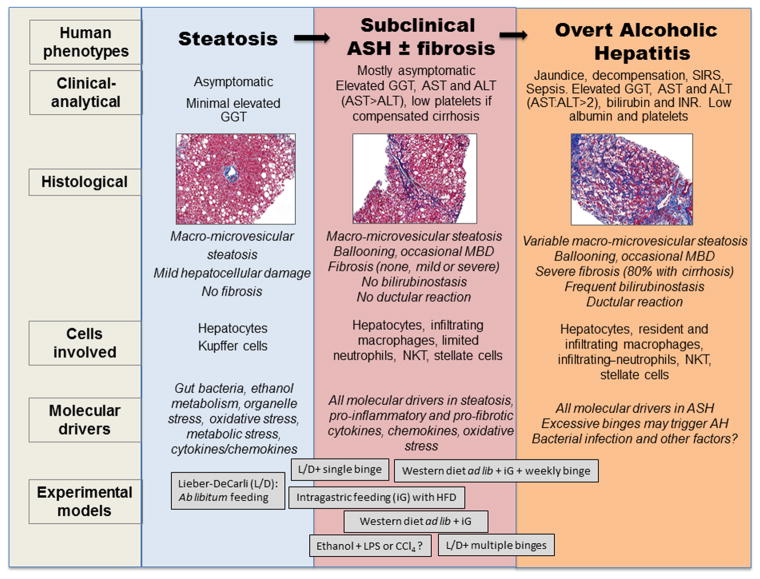
Until it reaches advanced stages, ALD is mostly asymptomatic. Patients with prolonged alcohol misuse develop steatosis and signs of hepatocellular damage (ballooning and Mallory-Denk bodies) and inflammatory infiltrate (typically neutrophils), which define ASH. ASH is a histological concept that can be present both in subclinical patients (defined as subclinical ASH) and in patients with significant clinical syndrome (defined as AH). Patients with subclinical ASH can be asymptomatic for prolonged periods of time (fully compensated and preserved hepatic function).1,3 This subclinical stage of disease is poorly characterized in humans and there is an unmet need to delineate its natural history and prognostic factors as well as to develop non-invasive markers. Eventually, a proportion of patients with subclinical ASH may develop cirrhosis (8–20%), which confers a high risk of complications. Some patients with ASH can also present a form of acute-on-chronic liver failure called AH, which often occurs after recent excessive alcohol binge drinking.1 AH is characterized by an abrupt rise in serum bilirubin levels, jaundice, coagulopathy, and liver-related complications. Traditionally, it was thought that AH could occur in patients with mild underlying ALD. However, more recent studies using tru-cut needles to obtain liver biopsy reveal that the vast majority of patients with AH have underlying cirrhosis.4 In some patients, the episode of AH may occur as the first manifestation in patients who have experienced silent ALD, while in others it is an exacerbation of pre-existing alcoholic cirrhosis. Therefore, AH should be distinguished from subclinical ASH in fully compensated patients (Figure 1).
Figure 1. Histology, pathogenesis, and experimental models for various stages of ALD, including steatosis, subclinical ASH ± fibrosis, and AH.
Histology and pathogenesis of steatosis, subclinical ASH with or without fibrosis, and AH are briefly summarized with more details in the text. Several animal models currently used in the field could represent the different stages of ALD. Chronic ab lib feeding of the Lieber-DeCarli (L/D) ethanol diet is a model to study the early stage of ALD such as steatosis.56 Several models have been used to investigate subclinical ASH with the strongest liver damage in western diet ab lib + iG alcohol +weekly binge,25 followed by western diet ab lib + iG alcohol, 25 iG alcohol with HFD,57 L/D+ multiple binges,23 and L/D+ single binge.23, 49 The western diet ab lib + iG +weekly binge model recapitulates some of histologic and clinical features of human AH 25 and the L/D+ multiple binge model also partially reproduces these features but to a lesser degree.23 Models using second hits such as LPS or CCl4 exhibit coagulative necrosis, failing to recapitulate clinical AH features.
Importantly, patients with ALD can present an episode of jaundice and liver decompensation for reasons other than superimposed AH. These conditions include severe sepsis, biliary obstruction, diffuse HCC, drug-induced liver injury and ischemic hepatitis (i.e, due to massive bleeding or cocaine use). Therefore, not all episodes of jaundice in patients with underlying ALD should be attributable to AH. In patients with other potential causes of jaundice, with severe forms of ALD, or involved in clinical trials, a transjugular liver biopsy is recommended to confirm the existence of AH.5, 6 Future studies including clarification of the nomenclature to define subclinical ASH as well as moderate and severe AH are needed to further understand the heterogeneity and stages of human ALD.
The incidence of AH is not well known and it is likely that many cases are undiagnosed. Population based studies estimate approximately 4.5 hospitalizations for AH per 100,000 persons each year, with a slight male predominance in Western countries.7 AH patients, typically present with symptoms, such as rapidly progressive jaundice, which can be accompanied by fever, abdominal pain, anorexia, and weight loss. There are several clinical scoring systems to assess the severity of AH: Maddrey’s discriminant function-DF-, MELD, ABIC and Glasgow.4, 8, 9 Among them, Maddrey’s DF is the most widely used. Short-term mortality in patients with severe AH is due to sepsis, liver failure and multi-organ dysfunction.10 Moreover, a recent multi-centric study developed a histological scoring system capable of predicting short-term survival in patients with AH.4 The resulting Alcoholic Hepatitis Histological Score (AHHS) comprises 4 parameters that are independently associated with patients’ survival: fibrosis stage, neutrophil infiltration, type of bilirubinostasis, and presence of megamitochondria. By combining these parameters in a semiquantitative manner, patients can be stratified into low, intermediate, or high risk for death within 90 days.4
The management of patients with AH has not evolved substantially over the last decades. In severe AH (e.g. poor mental status or hypotension), patients may need admission to an intensive care unit. Prevention of alcohol withdrawal symptoms and Wernicke’s encephalopathy are recommended. Daily protein intake of 1.5 g/kg body weight is also advised. Because patients with AH are predisposed to severe infections, which may threaten survival, early diagnosis and empiric antibiotic treatment are advised. On discharge, patients should follow alcohol counseling to reach sustained abstinence, a major determinant of long-term survival.11 In addition to these general measures, pharmacological therapy with prednisolone for 4 weeks has been shown to improve short-term survival in patients with severe AH. The efficacy of pentoxifylline is still questionable.1, 2, 12 A recent study has shown some beneficial effects of N-acetylcysteine, a potent but cell-impermeant antioxidant.13 Unfortunately, many patients do not respond to prednisolone, thus novel targeted therapies are urgently needed. Recently, liver transplantation, in highly selected AH patients, is shown to improve survival significantly,14 but a number of clinical and ethical considerations, particularly recidivism, limit eligibility. Furthermore, the relative shortage of donor organs adds to the ethical challenges before consideration of patients for liver transplant.
There are several important unmet needs in diagnosis, management and therapy of AH.15 First, patients often present in the emergency room with very advanced disease, severe sepsis and poor physical status, having high mortality within few days. Informative campaigns in primary care centers, addiction centers and in the general population about the clinical relevance of jaundice in patients with alcohol abuse should be emphasized. Second, the specific mechanisms, outcome, and responses to therapy of the most common secondary complications (encephalopathy, infections, renal failure, etc) are largely unknown. There are only few studies characterizing these complications in this particular population and additional studies to understand their underlying mechanisms are warranted.16, 17 Third, the extra-hepatic consequences of AH that lead to multi-organ dysfunction and death are not well defined. The existence of systemic inflammatory response syndrome (SIRS) is a major predictor of acute organ failure in these patients, suggesting that the extra-hepatic consequences of AH play a major role on disease severity.10, 17 Finally, a meaningful molecular classification of these patients could guide the use of targeted therapies. Not all patients are seen at the same stage of the disease. It is likely that different types and patterns of hepatic inflammation play defining roles for subclinical ASH, moderate and severe AH; whereas impaired liver regeneration could be the major event in patients with severe AH. The development of a molecular classification for these patients is urgently needed and will certainly help in developing tailored therapies for these different spectra of the deadly disease.
Pathogenesis and molecular targets of AH
The cellular and molecular mechanisms of chronic ALD have been extensively studied in animal models over the last forty years. A wide variety of inflammatory mediators and their downstream signaling pathways, as well as several types of inflammatory cells have been identified to contribute to the pathogenesis of steatosis, hepatocellular damage, inflammation, and fibrosis in chronic ALD, which are summarized in several recent reviews.3, 18–21 In the current article, we mainly discuss the potential mechanisms initiated by persistent alcohol drinking causing liver failure as well as multi-organ failure in AH patients (Figure 2). In addition, potential molecular targets of AH are also discussed.
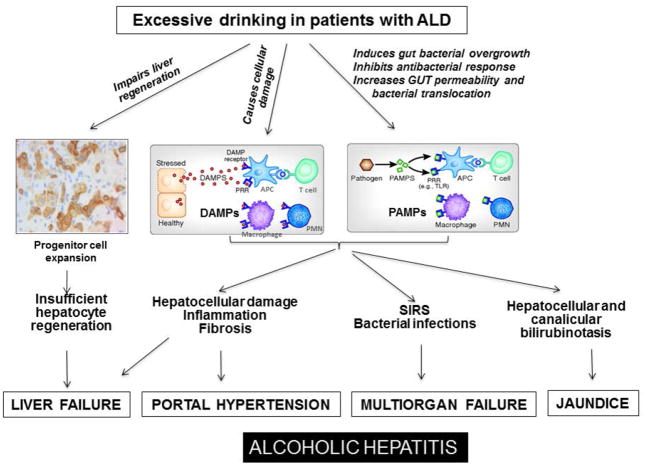
Figure 2. Pathogenesis and molecular mechanisms that trigger liver failure, SIRS, and multi-organ failure in AH.
Excessive drinking in alcoholics with underlying ALD induces hepatocellular damage, induces systemic inflammation, and impairs liver regeneration, resulting in liver failure and other complications in AH.
Most patients with underlying ALD develop AH after excessive and/or binge alcohol intake, suggesting that excessive binge drinking may contribute to development of AH. This notion is also supported by several recent studies from the chronic-plus-binge ethanol feeding model showing that acute ethanol binge markedly exacerbates liver injury and hepatic neutrophil infiltration in chronically ethanol-fed mice 22, 23 or in high-fat diet fed mice.24 Moreover, recent efforts to recapitulate histologic and clinical features of AH in mice have resulted in the development of diffuse hepatic neutrophil infiltration, fibrosis, ductular reaction, splenomegaly, hypoalbuminemia, and hyperbilirubinemia.25 This model is based on intragastric feeding which assures heavy alcohol intake with sustained blood alcohol levels, the most important requirement for reproduction of AH patient behavior, and combination of common AH patient life-style factors such as Western diet and weekly binge drinking.25 Interestingly, without weekly binge, this hybrid feeding model (intra-gastric and ad libitum feeding) produces chronic ASH with macrophage inflammation and liver fibrosis. When weekly binge is added, inflammation shifts to neutrophil infiltration, and histologic and clinical features of clinical AH ensue despite the same overall alcohol intake,25 further highlighting the critical role of binge drinking in inducing AH phenotype. At the present, how binge drinking initiates AH is not clear. It is likely that excessive binge drinking induces massive hepatocellular damage in patients with underlying chronic ALD who are prone to liver injury, and these damaged hepatocytes subsequently release a variety of damage-associated molecular patterns (DAMPs) to promote systemic inflammatory response syndrome (SIRS).20, 26
In addition to DAMPs, pathogen associated molecular patterns (PAMPs) likely also play an important role in inducing SIRS in AH patients. Chronic alcohol consumption is known to suppress antibacterial immune responses and increase bacterial infection, resulting in elevation of PAMPs in the liver and circulation.27 In addition, chronic alcohol intake induces gut dysbiosis and increases gut permeability, resulting in translocation of PAMPs such as lipopolysaccharide (LPS) into the portal circulation.28, 29 These elevated PAMPs then activate Kupffer cells and other inflammatory cells to produce a variety of pro-inflammatory cytokines such as TNFα, MCP-1 and IL-1β, which contribute to the pathogenesis of ALD.20 Superimposing binge drinking aggravates gut permeability and bacterial and PAMP translocation, contributing to manifestation of SIRS 30 and multiple organ failure in alcoholics with underlying chronic ALD, resulting in AH (Figure 2).
So far, a large number of inflammatory mediators have been identified from the studies of animal models and human AH biopsies and some of them are listed in Table 1. Many of these inflammatory mediators are elevated as a consequence of PAMPs, bacterial translocation and severe hepatocellular damage in AH, and may further promote liver injury or deteriorate liver regeneration. Among these inflammatory mediators, TNFα has received the greatest attention in the early 1990s and shown to play a critical role in promoting liver injury in a rodent model of mild ALD.31 Consequently, TNFα-blocking agents were tested in patients with AH, albeit, with disappointing results due to the development of severe bacterial infections.32 This serves as an important lesson that TNFα has pleiotropic effects and that inflammation stimulated by TNFα or its downstream mediators may have protective anti-microbial and pro-regenerative roles that should be preserved. Later, a variety of chemokines including IL-8, CXCL-5, Gro-γ and CXCL-6, IL-1, osteopontin, MCP1/CCL2 were found to be up-regulated, contributing to macrophage activation and neutrophil recruitment in AH, and in some cases correlating with AH patient survival.33–35 Another report identified molecular chaperone Hsp90, increased in AH, as a potential therapeutic target to modulate inflammatory responses.36 Activation of canonical inflammasome pathway, required for proteolytic cleavage of pro-IL-1β and pro-IL-18, is also implicated in experimental ALD,34, 37 and inhibition of IL-1 is currently under investigation for the treatment of AH patients.
Table 1.
Molecular targets for AH: Anti-inflammatory and Hepatoprotective
| Targets | Potential mechanisms | Beneficial effects | Detrimental effects, or obscure functions in AH |
|---|---|---|---|
| Anti-Inflammatory targets | |||
| Steroids | Broad immunosuppression. | Inhibits systemic inflammation; Improves short-term survival rates in AH patents. | Increases infection; Inhibits liver regeneration;44 Exacerbates neutrophilia. |
| Modulation of gut microbiome | ALD is associated with intestinal bacterial overgrowth and dysbiosis.28, 58 | Probiotics improve liver functions in patients with ALD.59 | Clinical trial using probiotics for the treatment of AH is under consideration. |
| Gut leakage-LPS-TNF-α | Chronic alcohol drinking increases gut leakage and elevates LPS and TNF-α. | Inhibition of gut leakage, LPS, and TNF-α improves liver functions in animal models of ALD. | Anti-TNF-α therapy did not improve survival rate in AH patients.32 Anti-LPS trial and Zinc nutritional supplement trial for the treatment of AH are under consideration. |
| IL-1 inhibitor | IL-1 promotes liver injury and inflammation in animal models. 34 | Inhibition of IL-1 improves liver functions in animal models of ALD. 34 | Anti-IL-1 trial for the treatment of AH is under consideration. |
| Chemokines and their receptors. | Human AH is associated with upregulation of a variety of chemokines and their receptors, which promote inflammation. 35, 53 | Inhibition of various chemokines and their receptors ameliorates liver injury in animal models of ALD. | Targeting multiple chemokines or their receptors may be required for AH therapy because of chemokine receptor redundancy. |
| Heat shock protein (Hsp90) | Hsp90 functions as an important chaperone of LPS signaling and is required for the production of proinflammatory cytokines. | Hsp90 inhibitor attenuates liver inflammatory and injury induced by LPS and alcohol in animal models. 36 | Many Hsp90 inhibitors are currently undergoing clinical evaluation in various types of diseases. Hsp90 inhibitors have not been tested in AH. |
| Complements | Activation of complements plays a role in promoting alcoholic liver injury in mice. | Inhibition of complement activation ameliorates chronic alcoholic liver injury in mice. 60 | The role of complement activation in AH has not been tested. |
| Hepatoprotective Reagents | |||
| IL-22 | Promotes hepatocyte survival and proliferation; Inhibits bacterial infection. | Ameliorates steatosis, liver injury, bacterial infection, kidney injury, and promotes liver repair.45 | A clinical trial of IL-22 for AH is under consideration.45 |
| Caspase inhibitors | AH is associated with hepatocyte apoptosis. | Prevents hepatocyte apoptosis. | A clinical trial using caspase inhibitors for the treatment of AH was stopped (Dr. Vijay Shah, personal communication). |
| HGF | Promotes hepatocyte proliferation and survival. | Ameliorates steatosis, liver injury and promotes liver repair. | Promotes liver cancer cell proliferation. |
| IL-6 | Promotes hepatocyte survival and proliferation. | Ameliorates steatosis, liver injury, and promotes liver repair. | Promotes inflammation and liver cancer cell proliferation; Clinical application is halted due to many side effects. |
| Anti-oxidants | Oxidative stress plays an important role in the pathogenesis of ALD. | Treatment with anti-oxidants shows beneficial effects in animal models of chronic ALD and in patients with NASH. | Treatment with anti-oxidants did not improve survival rate in patients with severe AH. 48 |
Although the importance of liver resident macrophages (Kupffer cells) in chronic ALD is well documented, recent studies suggest that infiltrating macrophages derived from monocytes contribute to the pathogenesis of ASH.38 The migration of monocyte-derived macrophages is dependent on Notch-1 and plays an important role in promoting M1 macrophage inflammation in experimental sub-clinical ASH.39 However, the precise biologic or pathologic significance of resident vs. infiltrating macrophages during progression to AH or whether M1 macrophage activation is required for AH is still unclear. Neutrophil infiltration is a hallmark of sub-clinical ASH and is believed to induce hepatocellular damage and inflammation in AH. However, a recent study reported that infiltration of neutrophils is associated with better prognosis in AH, suggesting that neutrophils may also play beneficial roles in promoting liver repair and controlling bacterial infection in these patients.4 To this end, an ideal therapeutic agent may need to suppress the release of cytotoxic mediators by neutrophils while preserving their efficient bacterial killing properties.40
In addition to inflammation, poor hepatic regenerative response is probably another important mechanism contributing to the liver failure in some AH patients. A detailed analysis of liver explants from AH patients that underwent liver transplantation revealed that patients who failed to respond to medical therapy had reduced hepatic expression of liver regeneration-related cytokines and the lack of proliferative hepatocytes.41 This observation was further confirmed by another study, which showed that presence of proliferating hepatocytes in AH is associated with a better prognosis.42 In addition, a massive expansion of liver progenitor cells (LPCs) called “ductular reaction” is often observed in AH patients, but these LPCs fail to differentiate into mature hepatocytes and correlate positively with severity of liver disease and short-term mortality in these patients.43 At present, the mechanisms leading to inefficient liver regeneration in AH are unknown and deserve further investigation. In addition, the treatment of AH patients with steroids may further block liver regeneration in these patients because steroids suppress inflammation and subsequently inflammation-mediated liver regeneration.44
In summary, in overt AH patients, a large number of inflammatory mediators are activated most likely contributing to SIRS, which together with impaired liver regeneration causes liver failure and multiple organ failure (Figure 2). These inflammatory mediators cooperatively promote liver inflammation and injury in AH. Thus it may be difficult to demonstrate clinical efficacy for the treatment of AH by targeting a single inflammatory mediator. Because many inflammatory mediators including TNFα also play an important roles in promoting liver regeneration, anti-inflammatory modalities for AH patients must preserve effective liver regeneration, and anti-inflammatory drugs may be combined with hepatoprotective agents. Indeed, a combination therapy with anti-inflammatory drugs (such as steroids) plus hepatoprotective drugs (such as interleukin-22) has been proposed and is currently under consideration for AH.45 In addition to inflammation and poor hepatic regenerative response, other challenges associated with AH patients include bacterial infections, hepatic encephalopathy, renal failure or hepatorenal syndrome, portal hypertension, ascites, and bleeding tendencies, and malnutrition. Those patients likely require combination therapies such as immunosuppressive drugs plus hepato- and renal-protective drugs and anti-bacterial drugs.45 In AH, macrophages and T cells are often defective in their anti-bacterial functions,27 which in turn increase the risk of bacterial infection in these patients. Identifying new approaches to correct these defects and restore normal immune responses, rather than to eliminate or suppress, appears a most logical and important future direction.
Translational approaches in AH: Human specimens and experimental models
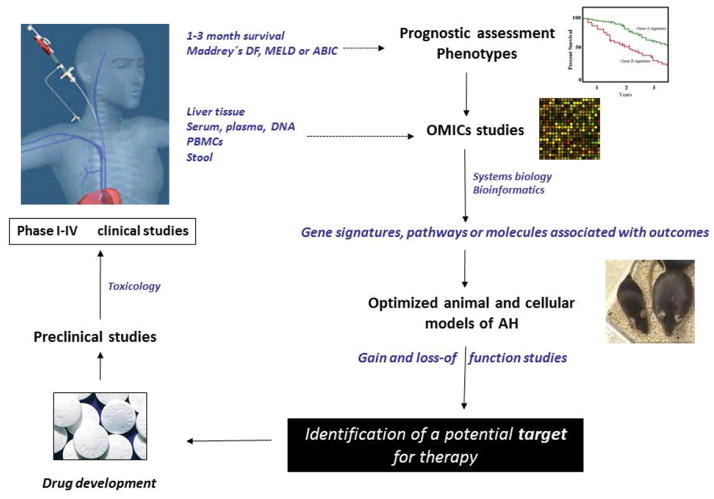
The traditional approach to develop new targets for therapy in the field of AH consists of identifying molecular drivers in pre-clinical omics studies, in vitro and in vivo, that are ultimately tested in randomized clinical trials in patients (Figure 3). Early human studies identified molecular drivers such as TNFα and macrophage-derived ROS in AH patients.46, 47 Later studies in patients with AH using TNFα blocking agents and antioxidant cocktails were carried out with minimal positive outcomes.32, 48 Thus, a more rational approach would be to perform integrative “omics” studies in human samples from patients with AH and use pathway/network computational analysis to identify cellular and molecular mediators that correlate with disease outcome (i.e. short-term mortality or scoring systems that predict survival like Maddrey’s discriminant function-DF-, ABIC score, etc). Such studies could enable identification of disease-specific pathways, correlating expression or activation of molecular targets to clinical outcomes. Ideally, the functional role of such potential disease mediators should then be tested in preclinical models of overt “AH”. As discussed below in this article, while recent animal models of sub-clinical ASH offer some promise, they do not show all the features of overt AH (i.e. jaundice and cirrhosis). Developing a reliable model that closely reproduces the histological and clinical features of AH will advance this field. However, ALD is a disease of man and not animals, and the inherent anatomical and biological differences among the species likely dictate this gap, and development of a “true” AH model may be too idealistic. For this reason, subsequent studies testing plausible molecular targets in preclinical models should utilize them based on clear pathologic criteria. Since patients with AH are severely ill and have profound hepatic and renal dysfunctions, careful pharmacokinetic and pharmacodynamics studies as well as good laboratory practice (GLP) toxicology studies are mandatory. Moreover, as detailed later, phase I studies in healthy controls and ideally in patients with advanced liver disease are required before phase II trials can be carried out.
Figure 3. Clinical and Translational Methods in AH.
Description of the step-wise process to test plausible drug targets is briefly described here. The method involves omics analysis to animal models, preclinical drug development culminating in testing drugs systematically in phase I-IV clinical trials in AH patients.
Human studies in patients with AH can be performed systematically using different types of biospecimens followed by correlation of function or expression of a molecule/pathway with the clinical outcome or phenotype (Figure 3). The first step consists of obtaining prospectively anthropometric, clinical, analytical and histological data. When a liver biopsy is not possible due to hemodynamic instability or unavailability in the research center, a precise clinical diagnosis should be established. In patients with confounding factors such as sepsis at admission, massive bleeding or hypotension, uncertain alcohol assessment or recent intake of hepatotoxic drugs, a liver biopsy is required to establish a proper diagnosis of AH. The second step is to define the phenotype of the patient; 1 and 3-month mortality rates are the most widely used parameters to define disease severity. Alternatively, existing scoring systems (i.e. Maddrey’s DF, ABIC or MELD) can be used to establish patient’s prognosis. Then, genetic signatures or the expression of genes or proteins found in human biospecimens can be correlated with clinical or histological features or patient outcome to identify potential molecular drivers and/or pathways. The type of biospecimens that can be used for translational studies includes liver tissue (typically obtained through a transjugular approach), suprahepatic and peripheral blood (total blood, serum or plasma) and peripheral blood mononuclear cells (PBMCs) and neutrophils. These biospecimens can be used for genomic, proteomic or metabolomic studies. Due to increasing evidence that gut-derived bacterial products play a significant role in pathophysiology, collecting stools can be useful for translational studies in patients with AH. Overall, initiation of translational research should involve hypothesis-generating omics studies, in which disease-associated pathways or signatures linked to clinical outcome are identified. Key molecules of disease pathways should be further tested in pre-clinical in vivo and in vitro models to identify druggable molecular drivers. To overcome complexity of studies assessing different biological levels (i.e. DNA, RNA, proteins, metabolites), an integrative analysis by systems biology experts could further aid in understanding interdisciplinary complex interactions within biological systems of AH.
Over the last four decades, chronic ethanol feeding studies in rodents using either ad libitum or intragastric feeding models have significantly enhanced our understanding of the pathogenesis of ALD (Table 2). However, these models may produce some features of chronic ALD but not acute-on-chronic liver injury observed in AH patients. Recently, a new mouse model of chronic-plus-binge ethanol feeding was developed.23, 49 The feeding protocol in this model is similar to the drinking patterns of many AH patients: a history of chronic drinking superimposed by episodic excessive alcohol consumption. Chronic-plus-binge ethanol feeding synergistically induced steatosis, liver injury, and hepatic neutrophil infiltration in mice,23, 49 which may be useful for the study of neutrophils and early stages of alcoholic liver injury in AH patients. Using this chronic-plus-binge alcohol model, researchers have begun to identify novel mechanisms that participate in the pathogenesis of alcoholic liver injury, thereby revealing novel therapeutic targets.22, 23, 36, 50, 51 However, hepatocellular damage and inflammation caused by chronic-plus-binge ethanol feeding are moderate and transient. 23, 49 Further, many AH patients are on Western diet and binge drink weekly on top of heavy daily alcohol intake, and it is important to study how alcohol drinking and obesity synergistically induce liver injury. Indeed, a single acute binge of ethanol induces acute steatohepatitis in high-fat diet-fed mice, resulting in steatohepatitis with neutrophil infiltration and liver fibrosis.24 However, these mouse models do not exhibit sustained high blood alcohol levels, which exemplify human alcoholics. To circumvent these weaknesses, a hybrid feeding model was developed by allowing ad lib feeding of solid Western diet at 40% of their caloric intake while assuring high alcohol intake and blood concentration by intragastric infusion of ethanol liquid diet at 60% calories.25 This model reproduces chronic ASH characterized by ballooned cell degeneration, macrophage activation and infiltration, and progression of liver fibrosis. Further, addition of weekly binge to the model produces diffuse neutrophil infiltration and ductular reaction similar to AH in patients, as well as clinical features of AH such as splenomegaly, hypoalbuminemia, and hyperbilirubinemia. However, this model has not achieved decompensation such as jaundice and ascites seen in severe AH although mild abdominal effusion and hyperbilirubinemia are noted.25 Thus, our challenge now is to manipulate this model to tip the pathologic progression toward decompensated AH. In summary, the chronic-plus-binge model of ethanol feeding and the hybrid feeding model represent moderate and advanced ASH, respectively (Figure 1). These models are beginning to highlight potential differences in the significance of inflammation and inflammatory mediators in subclinical ASH and to reinforce the notion that the pathogenesis of ALD is complex and multifactorial, and stage-dependent (Figure 1). Although we are far from achieving a “true” model which reproduces overt AH features, these subclinical ASH models still prove useful for comparative “omic” studies with AH patient samples, identification of potential molecular drivers that initiate the pathogenesis of ASH, and screening for therapeutic drugs targeting these molecules.
Table 2.
Commonly used animal models for ALD
| Models (References) | Characteristics | Mechanisms | Represent features of human ALD | Deficiencies |
|---|---|---|---|---|
| Acute binge ethanol feeding model. 61 |
|
|
|
|
| Chronic Ad libitum ethanol feeding.56 |
|
|
|
|
| Intragastric chronic ethanol feeding. 57 |
|
|
|
|
| Chronic-plus-binge feeding model.23, 49 |
|
|
|
|
| HFD-plus-binge ethanol model.24 |
|
|
|
|
| Hybrid model with Western diet ad lib plus intragastric ethanol and binge. 25 |
|
|
|
|
| “Second hit” or “Multiple hits” model.52 |
|
|
|
|
The “second or multiple hits” used experimentally include nutritional modifications, pharmacologic agents (e.g., Concanavalin A or CCl4, hormones, cytochrome P450 inducers, toll-like receptor ligands), genetic manipulation, and viral infections.52 When introducing these “second/multiple” hit(s), it becomes important to assess whether the hits are clinically relevant and whether liver pathology produced mimics clinical ALD. For example, alcohol feeding is used to prime and sensitize the liver for the second hit such as single injection of LPS or CCl4. In these models, coagulative necrosis and accompanying inflammation occur, but this is a primary consequence of the second hit, deviating from balloon cell degeneration seen in ALD. Recently, Affo et al. 53 developed a model of acute-on-chronic liver injury by chronically administering mice with CCl4 followed by injecting LPS, and this model has some features of liver inflammation observed in AH, but no alcohol was involved. Investigators using the “second hit” model must be cautious when interpreting the results since it may be difficult to determine whether the observed mechanisms are a consequence of the ethanol feeding or the “second hit.”
Challenges and future perspectives
AH was first described in 1961 as an acute disease that often ensues due to episodic excessive drinking in chronic alcoholics and is characterized by jaundice with severe clinic syndromes such as anorexia, nausea, upper abdominal pain, hepatomegaly and fever.54 Today, it is generally accepted that AH is a form of acute-on-chronic liver failure in patients with underlying ALD. Since the mechanisms underlying AH pathogenesis remain largely unknown, and current therapeutic options for this severe disease are largely ineffective, there is an urgent need for systematic translational research to identify therapeutic targets. Many investigators have collaborated and started to use integrative approaches to investigate the pathogenesis of AH and explore the novel therapeutic targets for AH by analyzing human AH biopsy samples and animal models. For example, a recent collaborative study have compared transcriptome data from a clinically relevant chronic-plus-binge model and biopsy-proven AH and identified similar alterations in expression of many hepatic genes in this animal model and human AH samples.23 Among these genes, fat-specific protein 27 (Fsp27)/CIDEC, which was highly upregulated in this animal model and in AH samples, plays an important role in promoting ASH in mice and possibly also in humans.23 In addition, the NIAAA released a major initiative to support four large multi-institutional consortia for conducting translational research on the pathogenesis of AH, identification of new therapeutic targets and performing clinical trials of plausible drugs for AH therapy. These efforts will likely enhance our understanding of AH pathogenesis and help identify novel therapies for AH. Despite these recent efforts on AH, many challenges remain for the research and therapy of this severe disease.15 First, the current models do not fully reproduce all features of severe human AH, and many of these models represent subclinical ASH. The requisite to continue to develop the appropriate in vivo models that closely mimic human AH are warranted. Second, chronic ethanol feeding models in rodents that have been used for the last forty years have revealed therapeutic targets, and these targets may be relevant to early stages of ALD but not acute-on-chronic liver injury in AH. It is essential to re-examine these targets in chronic-plus-binge ethanol feeding models or severe “multi-hit” hybrid models before testing in AH patients. Third, so far, many therapeutic targets have been identified from the studies of human AH samples and animal models. Here, it is important to recognize that targets identified in mild vs. severe ASH models may have different functional significance, and targeting such “presumed-culprits” may yield completely different or even opposite outcomes as recently noted for osteopontin.25, 55 Carefully designed clinical trials to test these targets in AH patients are urgently needed. Finally, AH is a major severe form of liver disease worldwide, with no effective drugs for this disease. Therefore, there is an urgent need to train young hepatologists and basic/discovery scientists, and foster global collaborations in both clinical and basic research related to ALD, particularly AH.
Acknowledgments
Financial Support: Pranoti Mandrekar is supported by RO1 #2AA017986-01, Dept of Defense-PRMRP #W81XWH-11-1-0420, PRMRP#W81XWH-13-1-0498; Ramon Bataller is supported by 1U01AA021908-01 and 1U01AA020821; H. Tsukamoto is supported by NIAAA grants 5P50AA011999, 1U01AA018663, and 5R24AA012885 and the Department of Veterans Affairs Merit Review award 5I01BX001991; and Bin Gao is supported by the NIAAA Intramural Program.
The authors apologize to the colleagues whose work was not mentioned or cited in this paper because of space constraints.
Non-standard abbreviations
- AH
alcoholic hepatitis
- AHHS
Alcoholic Hepatitis Histological Score
- ALD
Alcoholic liver disease
- ASH
alcoholic steatohepatitis
- CCl4
carbon tetrachloride
- DAMP
danger-associated molecular pattern
- DF
discriminant function
- HCC
hepatocellular carcinoma
- HFD
high-fat diet
- HSC
hepatic stellate cell
- LPS
lipopolysaccharide
- NIAAA
National Institute on Alcohol Abuse and Alcoholism
- PAMP
pathogen associated molecular pattern
- ROS
reactive oxygen species
- SIRS
systemic inflammatory response syndrome
References
- 1.Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–69. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
- 2.Thursz MR, Richardson P, Allison M, Austin A, Bowers M, Day CP, et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619–28. doi: 10.1056/NEJMoa1412278. [DOI] [PubMed] [Google Scholar]
- 3.Bataller R, Gao B. Liver fibrosis in alcoholic liver disease. Semin Liver Dis. 2015;35:146–56. doi: 10.1055/s-0035-1550054. [DOI] [PubMed] [Google Scholar]
- 4.Altamirano J, Miquel R, Katoonizadeh A, Abraldes JG, Duarte-Rojo A, Louvet A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014;146:1231–9. e1–6. doi: 10.1053/j.gastro.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Shea RS, Dasarathy S, McCullough AJ Practice Guideline Committee of the American Association for the Study of Liver D, Practice Parameters Committee of the American College of G. Alcoholic liver disease. Hepatology. 2010;51:307–28. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of L. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. 2012;57:399–420. doi: 10.1016/j.jhep.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Sandahl TD, Jepsen P, Thomsen KL, Vilstrup H. Incidence and mortality of alcoholic hepatitis in Denmark 1999–2008: a nationwide population based cohort study. J Hepatol. 2011;54:760–4. doi: 10.1016/j.jhep.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Papastergiou V, Tsochatzis EA, Pieri G, Thalassinos E, Dhar A, Bruno S, et al. Nine scoring models for short-term mortality in alcoholic hepatitis: cross-validation in a biopsy-proven cohort. Aliment Pharmacol Ther. 2014;39:721–32. doi: 10.1111/apt.12654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louvet A, Labreuche J, Artru F, Boursier J, Kim DJ, O’Grady J, et al. Combining Data From Liver Disease Scoring Systems Better Predicts Outcomes of Patients With Alcoholic Hepatitis. Gastroenterology. 2015 doi: 10.1053/j.gastro.2015.04.044. [DOI] [PubMed] [Google Scholar]
- 10.Michelena J, Altamirano J, Abraldes JG, Affo S, Morales-Ibanez O, Sancho-Bru P, et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology. 2015;62:762–72. doi: 10.1002/hep.27779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Potts JR, Goubet S, Heneghan MA, Verma S. Determinants of long-term outcome in severe alcoholic hepatitis. Aliment Pharmacol Ther. 2013;38:584–95. doi: 10.1111/apt.12427. [DOI] [PubMed] [Google Scholar]
- 12.Singh S, Murad MH, Chandar AK, Bongiorno CM, Singal AK, Atkinson SR, et al. Comparative Effectiveness of Pharmacological Interventions for Severe Alcoholic Hepatitis: A Systematic Review and Network Meta-analysis. Gastroenterology. 2015;149:958–70. e12. doi: 10.1053/j.gastro.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen-Khac E, Thevenot T, Piquet MA, Benferhat S, Goria O, Chatelain D, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781–9. doi: 10.1056/NEJMoa1101214. [DOI] [PubMed] [Google Scholar]
- 14.Mathurin P, Moreno C, Samuel D, Dumortier J, Salleron J, Durand F, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. 2011;365:1790–800. doi: 10.1056/NEJMoa1105703. [DOI] [PubMed] [Google Scholar]
- 15.Sanyal AJ, Gao B, Szabo G. Gaps in Knowledge and Research Priorities for Alcoholic Hepatitis. Gastroenterology. 2015;149:4–9. doi: 10.1053/j.gastro.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louvet A, Wartel F, Castel H, Dharancy S, Hollebecque A, Canva-Delcambre V, et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology. 2009;137:541–8. doi: 10.1053/j.gastro.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 17.Altamirano J, Fagundes C, Dominguez M, Garcia E, Michelena J, Cardenas A, et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2012;10:65–71. e3. doi: 10.1016/j.cgh.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–85. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. 2015;148:30–6. doi: 10.1053/j.gastro.2014.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mandrekar P, Szabo G. Signalling pathways in alcohol-induced liver inflammation. J Hepatol. 2009;50:1258–66. doi: 10.1016/j.jhep.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32:343–68. doi: 10.1146/annurev-nutr-072610-145138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bertola A, Park O, Gao B. Chronic plus binge ethanol feeding synergistically induces neutrophil infiltration and liver injury in mice: a critical role for E-selectin. Hepatology. 2013;58:1814–23. doi: 10.1002/hep.26419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu MJ, Cai Y, Wang H, Altamirano J, Chang B, Bertola A, et al. Fat-Specific Protein 27/CIDEC Promotes Development of Alcoholic Steatohepatitis in Mice and Humans. Gastroenterology. 2015;149:1030–1041. e6. doi: 10.1053/j.gastro.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang B, Xu MJ, Zhou Z, Cai Y, Li M, Wang W, et al. Short- or long-term high-fat diet feeding plus acute ethanol binge synergistically induce acute liver injury in mice: An important role for CXCL1. Hepatology. 2015;62:1070–85. doi: 10.1002/hep.27921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazaro R, Wu R, Lee S, Zhu NL, Chen CL, French SW, et al. Osteopontin deficiency does not prevent but promotes alcoholic neutrophilic hepatitis in mice. Hepatology. 2015;61:129–40. doi: 10.1002/hep.27383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–72. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 27.Markwick LJ, Riva A, Ryan JM, Cooksley H, Palma E, Tranah TH, et al. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology. 2015;148:590–602. e10. doi: 10.1053/j.gastro.2014.11.041. [DOI] [PubMed] [Google Scholar]
- 28.Chen P, Torralba M, Tan J, Embree M, Zengler K, Starkel P, et al. Supplementation of Saturated Long-Chain Fatty Acids Maintains Intestinal Eubiosis and Reduces Ethanol-induced Liver Injury in Mice. Gastroenterology. 2015;148:203–214. e16. doi: 10.1053/j.gastro.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen P, Starkel P, Turner JR, Ho SB, Schnabl B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology. 2015;61:883–94. doi: 10.1002/hep.27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One. 2014;9:e96864. doi: 10.1371/journal.pone.0096864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, et al. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942–52. doi: 10.1016/s0016-5085(99)70354-9. [DOI] [PubMed] [Google Scholar]
- 32.Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, et al. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953–60. doi: 10.1053/j.gastro.2008.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mandrekar P, Ambade A, Lim A, Szabo G, Catalano D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology. 2011;54:2185–97. doi: 10.1002/hep.24599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–89. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dominguez M, Miquel R, Colmenero J, Moreno M, Garcia-Pagan JC, Bosch J, et al. Hepatic expression of CXC chemokines predicts portal hypertension and survival in patients with alcoholic hepatitis. Gastroenterology. 2009;136:1639–50. doi: 10.1053/j.gastro.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 36.Ambade A, Catalano D, Lim A, Kopoyan A, Shaffer SA, Mandrekar P. Inhibition of heat shock protein 90 alleviates steatosis and macrophage activation in murine alcoholic liver injury. J Hepatol. 2014;61:903–11. doi: 10.1016/j.jhep.2014.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DeSantis DA, Ko CW, Liu Y, Liu X, Hise AG, Nunez G, et al. Alcohol-induced liver injury is modulated by Nlrp3 and Nlrc4 inflammasomes in mice. Mediators Inflamm. 2013;2013:751374. doi: 10.1155/2013/751374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang M, You Q, Lor K, Chen F, Gao B, Ju C. Chronic alcohol ingestion modulates hepatic macrophage populations and functions in mice. J Leukoc Biol. 2014;96:657–65. doi: 10.1189/jlb.6A0114-004RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, Chi F, Guo T, Punj V, Lee WN, French SW, et al. NOTCH reprograms mitochondrial metabolism for proinflammatory macrophage activation. J Clin Invest. 2015;125:1579–90. doi: 10.1172/JCI76468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruchaud-Sparagano MH, Mills R, Scott J, Simpson AJ. MPLA inhibits release of cytotoxic mediators from human neutrophils while preserving efficient bacterial killing. Immunol Cell Biol. 2014;92:799–809. doi: 10.1038/icb.2014.55. [DOI] [PubMed] [Google Scholar]
- 41.Dubuquoy L, Louvet A, Lassailly G, Truant S, Boleslawski E, Artru F, et al. Progenitor cell expansion and impaired hepatocyte regeneration in explanted livers from alcoholic hepatitis. Gut. 2015;64:1949–60. doi: 10.1136/gutjnl-2014-308410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lanthier N, Rubbia-Brandt L, Lin-Marq N, Clement S, Frossard JL, Goossens N, et al. Hepatic cell proliferation plays a pivotal role in the prognosis of alcoholic hepatitis. J Hepatol. 2015;63:609–21. doi: 10.1016/j.jhep.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Sancho-Bru P, Altamirano J, Rodrigo-Torres D, Coll M, Millan C, Jose Lozano J, et al. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology. 2012;55:1931–41. doi: 10.1002/hep.25614. [DOI] [PubMed] [Google Scholar]
- 44.Kwon HJ, Won YS, Park O, Feng D, Gao B. Opposing effects of prednisolone treatment on T/NKT cell- and hepatotoxin-mediated hepatitis in mice. Hepatology. 2014;59:1094–106. doi: 10.1002/hep.26748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gao B, Shah VH. Combination therapy: New hope for alcoholic hepatitis? Clin Res Hepatol Gastroenterol. 2015;39(Suppl 1):S7–S11. doi: 10.1016/j.clinre.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diluzio NR. Prevention of the Acute Ethanol-Induced Fatty Liver by the Simultaneous Administration of Antioxidants. Life Sci. 1964;3:113–8. doi: 10.1016/0024-3205(64)90189-4. [DOI] [PubMed] [Google Scholar]
- 47.Bird GL, Sheron N, Goka AK, Alexander GJ, Williams RS. Increased plasma tumor necrosis factor in severe alcoholic hepatitis. Ann Intern Med. 1990;112:917–20. doi: 10.7326/0003-4819-112-12-917. [DOI] [PubMed] [Google Scholar]
- 48.Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, O’Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis--a randomised clinical trial. J Hepatol. 2006;44:784–90. doi: 10.1016/j.jhep.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 49.Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–37. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Williams JA, Ni HM, Ding Y, Ding WX. Parkin regulates mitophagy and mitochondrial function to protect against alcohol-induced liver injury and steatosis in mice. Am J Physiol Gastrointest Liver Physiol. 2015;309:G324–40. doi: 10.1152/ajpgi.00108.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ni HM, Bhakta A, Wang S, Li Z, Manley S, Huang H, et al. Role of hypoxia inducing factor-1beta in alcohol-induced autophagy, steatosis and liver injury in mice. PLoS One. 2014;9:e115849. doi: 10.1371/journal.pone.0115849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsukamoto H, Machida K, Dynnyk A, Mkrtchyan H. “Second hit” models of alcoholic liver disease. Semin Liver Dis. 2009;29:178–87. doi: 10.1055/s-0029-1214373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Affo S, Morales-Ibanez O, Rodrigo-Torres D, Altamirano J, Blaya D, Dapito DH, et al. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut. 2014;63:1782–92. doi: 10.1136/gutjnl-2013-306098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beckett AG, Livingstone AV, Hill KR. Acute alcoholic hepatitis. Br Med J. 1961;2:1113–9. doi: 10.1136/bmj.2.5260.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morales-Ibanez O, Dominguez M, Ki SH, Marcos M, Chaves JF, Nguyen-Khac E, et al. Human and experimental evidence supporting a role for osteopontin in alcoholic hepatitis. Hepatology. 2013;58:1742–56. doi: 10.1002/hep.26521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lieber CS, DeCarli LM, Sorrell MF. Experimental methods of ethanol administration. Hepatology. 1989;10:501–10. doi: 10.1002/hep.1840100417. [DOI] [PubMed] [Google Scholar]
- 57.Ueno A, Lazaro R, Wang PY, Higashiyama R, Machida K, Tsukamoto H. Mouse intragastric infusion (iG) model. Nat Protoc. 2012;7:771–81. doi: 10.1038/nprot.2012.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan AW, Fouts DE, Brandl J, Starkel P, Torralba M, Schott E, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675–82. doi: 10.1016/j.alcohol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen JI, Roychowdhury S, McMullen MR, Stavitsky AB, Nagy LE. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology. 2010;139:664–74. 674 e1. doi: 10.1053/j.gastro.2010.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shukla SD, Pruett SB, Szabo G, Arteel GE. Binge ethanol and liver: new molecular developments. Alcohol Clin Exp Res. 2013;37:550–7. doi: 10.1111/acer.12011. [DOI] [PMC free article] [PubMed] [Google Scholar]