Abstract
Preclinical evidence indicates that inactivation of subthalamic nucleus (STN) may be effective for treating cocaine addiction, and therapies that target STN, e.g. deep brain stimulation, are available indicating that this may have clinical promise. Here, we assessed the therapeutic potential of STN inactivation using a translationally relevant economic approach that quantitatively describes drug-taking behavior, and tested these results with drug-seeking tasks. Economic demand for cocaine was assessed in rats (n = 11) using a within-session threshold procedure in which cocaine price (responses/mg cocaine) was sequentially increased throughout the session. Cocaine demand was assessed in this manner immediately after bilateral microinfusions into STN of either vehicle (artificial cerebrospinal fluid) or the GABAA receptor agonist muscimol. A separate group of animals (n = 8) was tested for changes in cocaine seeking either during extinction or in response to cocaine–associated cues. Muscimol-induced inhibition of STN significantly attenuated cocaine consumption at high prices, drug seeking during extinction, and cued reinstatement of cocaine seeking. In contrast, STN inhibition did not reduce cocaine consumption at low prices or locomotor activity. Thus, STN inactivation reduced economic demand for cocaine and multiple measures of drug seeking during extinction. In view of the association between economic demand and addiction severity in both rat and human, these results indicate that STN inactivation has substantial clinical potential for treatment of cocaine addiction.
Keywords: Addiction, behavioral economics, biomarker, cocaine, demand, subthalamic nucleus
Introduction
Drug dependence is a major public health problem, and effective therapies for many drugs of abuse have remained elusive. Indeed, there are no current therapies that are approved by the US Food and Drug Administration for treatment of cocaine addiction. One promising target for treatment of cocaine addiction is the subthalamic nucleus (STN). Deep brain stimulation (DBS) or lesions in the subthalamic nucleus (STN) in rats have been reported to reduce motivation for cocaine without reducing motivation for non-drug rewards such as sucrose (Baunez et al. 2005; Bezzina et al. 2009; Rouaud et al. 2010; Pratt et al. 2012). Moreover, the neurosurgical approach to targeting the STN is well defined in humans, as it is regularly used to stereotactically implant deep brain stimulators for the treatment of Parkinson’s disease (Okun 2012). Here, we assess the potential of STN inactivation as a treatment for cocaine addiction using behavioral analyses with high translational potential.
An animal model with high predictive validity is required to test STN inactivation as an addiction therapy, and economic methods might provide such a model. They offer a structured quantitative approach to behavioral analysis that is mathematically identical in animals and humans (Hursh 1980; Hursh & Silberberg 2008), and accruing evidence indicates that economic-based descriptors of human behavior may be particularly useful as addiction biomarkers (Heinz et al. 2012; Bickel et al. 2014). Notably, economic demand for drugs of abuse correlates with several addiction related parameters in drug-dependent individuals. For example, economic demand for the respective drug of abuse correlates with lifetime years of cocaine, heroin, marijuana or benzodiazepine use (Petry 2001); severity of alcohol dependence (Murphy et al. 2009; Gray & MacKillop 2014) or craving (MacKillop et al. 2010); as well as severity of nicotine dependence (Murphy et al. 2011; Chase et al. 2013) or craving (MacKillop et al. 2012). We recently developed a mathematical approach that enables rapid testing of cocaine demand in rats (Bentzley et al. 2013) and found that this demand predicts addiction-like behavior – including drug seeking in extinction, relapse propensity, and compulsive (punished) drug taking (Bentzley et al. 2014). Further, we found that demand for cocaine predicted efficacy of treatment with oxytocin (Bentzley et al. 2014) and orexin-1 antagonism (Bentzley & Aston-Jones 2015) in this animal model. Similar associations between drug demand and treatment have been reported in humans: alcohol demand predicts treatment outcomes (MacKillop & Murphy 2007); varenicline treatment both reduces nicotine demand and increases time to relapse in the same patients (McClure et al. 2013); and naltrexone reduces alcohol demand (Bujarski et al. 2012) and increases the time to the first heavy drinking episode (Anton et al. 2006).
We employed an exponential demand equation to analyze results with this model (Hursh & Silberberg 2008), which separately yielded measures of Q0 (drug demand at null price, i.e., the level of consumption that would occur if drug were free), and α (motivation for drug measured by demand elasticity, i.e., the sensitivity of drug demand to drug price). Importantly, α scales inversely with motivation for drug such that animals with a high α display a rapid decrease in drug consumption with rising price, indicating lower motivation for drug. α is unique in that it is a measure of motivation independent of drug consumption at null price (Q0), a result of normalization with respect to both the consumption (ordinate) and price (abscissa) axes (Hursh & Silberberg 2008; Bentzley et al. 2013; 2014). Although we found that Q0 contributes to predictions of compulsive (punished) cocaine taking, only α was found to predict a broad spectrum of addiction-like behaviors (Bentzley et al. 2014).
Here, we evaluated the potential of STN inactivation for treatment of cocaine addiction by assessing changes in cocaine demand (Q0 and α) that occur during STN inactivation with bilateral microinfusion of the GABAA agonist muscimol, which inhibits both metabolic (Féger & Robledo 1991) and electrophysiological (Rouzaire-dubois et al. 1980; Baufreton et al. 2001) activity of STN neurons. We found that inactivation of STN, but not nearby structures, increased demand elasticity (α), indicating that demand became more sensitive to increases in cost; i.e. animals did not value cocaine as highly. There are strong preclinical (Galuska et al. 2011; Bentzley et al. 2014; Bentzley & Aston-Jones 2015) and clinical (McClure et al. 2013) associations between drug demand and relapse to drug seeking. Therefore, we also tested the effect of STN inactivation on cocaine seeking in early extinction and during cue-induced reinstatement of cocaine seeking. We found that STN inactivation reduced drug seeking during extinction as well as during reinstatement of drug seeking by cocaine-associated cues. Taken together, our results indicate that STN inactivation holds promise as a clinical treatment of cocaine addiction.
Materials and Methods
Animals
Male Sprague-Dawley rats (n = 41) with an initial weight of approximately 250–275g (Charles River, Raleigh, NC, USA) were single-housed under a reversed 12-h light/dark cycle (lights on 6 p.m.) and had ad libitum access to food and water. Animals were housed in a temperature and humidity-controlled animal facility at the Medical University of South Carolina (MUSC; AAALAC-accredited). All experiments were approved by the Institutional Animal Care and Use Committee at MUSC and conducted according to specifications of the NIH as outlined in the Guide for the Care and Use of Laboratory Animals.
Drugs
Cocaine HCl powder was provided by the National Institute on Drug Abuse (Research Triangle Park, NC, USA) and was dissolved in 0.9% sterile saline. Muscimol (Tocris, Bristol, UK) was dissolved in sterile artificial cerebrospinal fluid (aCSF) at a concentration of 0.2 mM for all experiments except locomotor activity. Locomotor tests were performed using 0.4mM muscimol, before self-administration testing. However, this dose suppressed self-administration almost completely, precluding analysis of cocaine demand with this dose. Hence, 0.2mM was utilized for all self-administration testing reported herein.
Intravenous catheter surgery
Animals were implanted with intravenous jugular catheters after acclimation to the housing facilities as previously described (Smith et al. 2009). Animals were anesthetized prior to surgery using a ketamine/xylazine mixture (56.6/8.7 mg/kg i.p.). Meloxicam was administered as an analgesic (1 mg/kg, s.c.). After obtaining a deep plane of anesthesia (lack of corneal reflex), the free end of the catheter was inserted into the right external jugular vein. The tubing was run subcutaneously and exited through the skin via a biopsy hole placed 2 cm caudal to the mid-scapular region. Beginning 3 days after surgery, catheters were flushed daily with 0.1 ml of heparin (100 mg/mL) and 0.1 ml of cefazolin (100 U/mL). Animals were allowed to recover for at least 1 week before cocaine self-administration training.
Stereotactic surgery
Immediately after catheter surgery, animals were placed in a stereotactic frame (Kopf, Tujunga, CA, USA) and implanted with bilateral stainless steel guide cannulae (22 gauge, 11 mm, Plastics One, Roanoke, VA, USA) 2 mm dorsal to STN (coordinates relative to bregma skull surface in mm: −3.8 posterior, ±2.4 medial–lateral, −6.6 ventral) (Paxinos & Watson 1998). Cannulae were secured to the skull using jeweler’s screws and dental acrylic; stylets were placed into the guide cannulae to prevent occlusion.
STN microinfusions
Just prior to testing, bilateral infusion cannulae (28 gauge, Plastics One, Roanoke, VA, USA) were inserted to a depth of 2 mm below the tip of the guide cannulae, directly into STN. To acclimate rats to the infusion procedure, injection cannulae were bilaterally inserted into the guide cannulae for 1 min (no infusions) after removal from the self-administration chamber the day prior to the first microinfusion test. On test days, muscimol (0.2 mM) or vehicle (aCSF) was microinfused (0.3 μL/side) immediately before being placed into the self-administration chamber. The order of muscimol and vehicle microinfusions was counterbalanced in a within-subjects design, and occurred via polyethylene tubing connected to gastight 10-μL Hamilton syringes (Hamilton, Reno, NV, USA) set in an infusion pump (Model 975, Harvard Apparatus, Holliston, MA, USA) and delivered over a 2.5 min period; injectors were left in place for an additional minute.
Dorsal control microinfusions
Control microinfusions of muscimol were performed along the cannula tract dorsal to STN to confirm that any changes in demand that occurred during injection into STN were not due to diffusion of muscimol along the path of least resistance to fluid flow, i.e., dorsally. This within-subject spatial control was performed using injectors that projected 0.2 mm below the tip of the guide cannulae. Dorsal injections were always performed prior to STN injections, because insertion and removal of STN injectors may produce a low-resistance fluid channel, allowing muscimol delivered from dorsally placed injectors to infiltrate STN.
Acquisition of cocaine self-administration
Animals were trained to self-administer cocaine 1 week following surgery as previously described (Bentzley et al. 2013; 2014). Self-administration sessions were carried out in operant conditioning chambers housed in sound-attenuating cubicles and controlled by a MED-PC IV program (Med-Associates, St Albans, VT, USA). Before beginning the threshold procedure, rats learned to lever-press for 0.19 mg infusions of intravenous cocaine on a fixed-ratio-1 (FR-1) schedule in 2-hr daily sessions. Infusions were delivered over 3.6 sec in 62 μL of saline via a motorized pump with a 20-sec timeout after each infusion during which lever presses were recorded but did not result in further infusions. Cocaine infusions were paired with discrete tone and light cues (78 dB, 2900 Hz; white stimulus light above the active lever). The red house light on the wall opposite the levers was turned off during cocaine infusions and 20-sec timeouts. Presses on an inactive lever had no programmed consequences but were recorded. Rats remained on the acquisition procedure until they achieved at least 20 infusions per session for 5 consecutive days and the variation in number of infusions was within 15% of the mean across the last 3 days. Rats were then switched to either the threshold procedure for training and testing of economic demand, the fixed low-price procedure to test time-dependent effects of treatment, or extinction training to later test drug seeking.
Threshold procedure
The day after completion of FR-1 training, rats were trained and then tested on the within-session threshold procedure (Oleson & Roberts 2012) as described previously (Bentzley et al. 2013; 2014). In this paradigm, the unit price of cocaine (responses/mg cocaine) is increased in successive 10-min intervals by reducing the volume of cocaine infused with a lever press, as apposed to conventional behavioral-economic experiments in which the dose is held constant and the response requirement is increased (Hursh & Silberberg 2008). During a 110-min session, rats received access to decreasing doses of cocaine in successive 10-min intervals on a quarter logarithmic scale (383.5, 215.6, 121.3, 68.2, 38.3, 21.6, 12.1, 6.8, 3.8, 2.2, 1.2 μg per infusion), by decreasing pump infusion duration. The pump rate infusion times (8175, 4597, 2585, 1454, 818, 460, 259, 145, 82, 46, 26 ms) were based on an averaged measurement of the flow rates (1031.9 ± 2.2 μL/min) of 16 individual PMH100 pumps (Med-Associates, St Albans, VT, USA) with a 10 ml syringe (BD, Franklin Lakes, NJ, USA). Importantly, cocaine infusion rate has not been shown to affect the reinforcing efficacy of cocaine in rats (Crombag et al. 2008); thus, the differences in the value of infusions delivered can be assumed to be entirely due to differences in infusion volume. Similarly to the FR-1 paradigm, during an infusion while the pump was on the house-light was turned off and the light and tone cues were presented. However, during the threshold-procedure sessions, there was no timeout, allowing rats to press for the next infusion as soon as the current infusion was finished. The only exception was that while the pump was on presses on the active lever were recorded but did not elicit a second infusion.
Demand curve analyses
Demand curves were fit to data to calculate values for Q0 and α as described previously (Bentzley et al. 2013). Demand curves were constructed individually for each animal and resultant demand parameters (Q0 and α) were averaged across animals for each condition. Briefly, demand curves were constructed as follows: each animal’s brain cocaine concentration was calculated to determine relative stability during a session. Demand data points that occurred during unstable brain cocaine concentrations, i.e. with a change in brain cocaine concentration per change in time (slope) greater or less than a standard deviation from 0, were truncated before demand curves were fit by standard techniques. This always resulted in elimination of the first data point, during which the subject ‘loaded’ on cocaine (Oleson et al. 2011), and typically resulted in elimination of data points that occurred after 2 points following the maximum price at which the subject maintained its preferred level of drug consumption (Pmax), when brain cocaine concentration had dropped enough to provoke increased responding (Bentzley et al. 2013). Demand curves were fit to each subject’s truncated data set using standard regression techniques. In this approach, the values α and Q0 in the exponential demand equation (Hursh & Silberberg 2008) were manipulated to minimize the residual sum of squares, i.e. the square of the difference between the experimentally measured demand and the demand predicted by the exponential demand equation was found for each price and then summed across all prices. α quantifies demand elasticity, a measure of motivation for drug, and Q0 estimates consumption at null effort (Hursh & Silberberg 2008). The parameter k represents the range of the consumption data in Loge units and was held constant at a value of 7.368 (3.2 in Log10 units) across all animals (Hursh & Silberberg 2008). This value of k was chosen based on the maximum observed range of consumption across all experiments in our laboratory.
Experimental design for behavioral-economic testing
After acquisition of cocaine self-administration, animals (n = 11) were run daily on the threshold procedure for a minimum of 6 sessions and until they displayed stable motivation for cocaine, i.e. until the last three sessions produced an α that was within a range of ±25% of the mean of those days. All animals then received dorsal muscimol control injections using shortened injectors immediately before testing on the threshold procedure for a single day (control test day). Economic demand was then re-assessed (without microinfusions) by running the animals for a minimum of 3 threshold-procedure sessions (3 days) and until the last 3 sessions produced an α that was within a range of ±25% of the mean of those days. This was done to account for possible changes in economic demand due to prior test microinfusions. On a subsequent (test) session, animals received either muscimol or aCSF microinfusions in a within-subjects design with the order of the treatments counterbalanced. After the first STN microinfusion test session, economic demand was reassessed (without microinfusions) for a minimum of 3 days and until the last 3 sessions produced an α that was within a range of ±25% of the mean of those days. In total, animals received 3 microinfusions: 1 dorsal muscimol control, 1 aCSF in STN, and 1 muscimol in STN.
Fixed low-price procedure and experimental design
A separate group of animals (n = 14) was tested on the fixed low-price procedure after acquisition of cocaine self-administration. The low-price procedure was identical to the threshold procedure in every way except that the cocaine price remained fixed at a single value (4.64 responses/mg) throughout the session by holding the infused dose of cocaine constant at 215.6 μg, the second-highest dose (second-lowest price) of the threshold procedure. The second-highest dose was chosen to increase the sensitivity of the test. The number of infusions per bin at the highest dose is very low on average (0–3 infusions per bin). Using the second-highest dose ensured that all animals would respond in all bins and ensured that small changes in consumption, if present, would be detectable. The experimental design was also identical to threshold procedure testing, except stable responding was defined as the total number of infusions per session being within 15% of the mean across the last 3 days. In total, animals received 3 microinfusions: 1 dorsal muscimol control, 1 aCSF in STN, and 1 muscimol in STN. The 7 animals from this group with histologically confirmed bilateral placement of injection cannulae in STN were included in the analysis.
Extinction and reinstatement procedures and experimental design
A separate group of animals (n = 8) was tested on extinction and reinstatement procedures. After acquiring cocaine self-administration, animals were treated with either muscimol or aCSF in a counterbalanced order in a within-subjects design immediately before being tested in an extinction session. Extinction sessions were identical to self-administration sessions except that responses on the active lever had no consequence. Responses on active and inactive levers were recorded and the house light remained on during the entire 120-min session. In between the 2 extinction test sessions, animals were placed back on the FR-1 acquisition procedure for 3 consecutive days.
After the second extinction test with microinfusions of either muscimol or aCSF, animals were run on daily extinction sessions for a minimum of 7 days and until the last 2 consecutive days had ≤ 25 responses on the previously active lever. The level of extinguished responding was defined as the mean active responding across these 2 days. After animals reached extinction criteria, reinstatement of cocaine seeking by discrete cues was tested in a within-subjects design with the order of muscimol and aCSF microinfusions counterbalanced. During cue-induced reinstatement testing, the same discrete light and tone cues paired with cocaine infusions during acquisition were presented once at the start of the session and in response to active lever presses. Cues were 3.6s in duration and could only be elicited once every 20s; however, all active responses were recorded. In between reinstatement tests animals were run on the extinction procedure for a minimum of 2 days and until 2 consecutive days with ≤ 25 responses on the previously active lever. In total, animals received 4 microinfusions: 1 aCSF in STN and 1 muscimol in STN during the first day of extinction, and 1 aCSF in STN and 1 muscimol in STN during cued reinstatement.
Locomotor testing
A separate group of animals (n = 8) was tested for spontaneous locomotion using locomotor analysis chambers and software as previously described (Smith et al. 2009). Animals were tested in clear acrylic chambers (approximately 40 × 40 × 30 cm) equipped with Digiscan monitors (AccuScan Instruments, Inc., Columbus, OH) containing a 16 × 16 photobeam array for the x / y axes (horizontal activity) and 16 photobeams for the z axis (vertical activity). Photobeam breaks were detected by a Digiscan analyzer and recorded by DigiPro software (Version 1.4). Animals were pretreated with bilateral 0.3 μL microinfusions into STN of either muscimol (0.4 mM) or aCSF vehicle immediately prior to a 120-min test session, and data were collected in 5-min bins. Animals were not pre-exposed to the chambers, and were tested twice in a counter-balanced fashion, with tests 72-hrs apart. The 7 animals from this group with histologically confirmed bilateral placement of injection cannulae in STN were included in the analysis.
Histology
Following completion of behavioral testing animals were anesthetized with ketamine/xylazine (56.6/8.7 mg/kg) and received bilateral microinfusions of pontamine sky blue (0.3 μL) to mark the locations of the injectors. Animals were then decapitated, their brains flash-frozen in methyl-butane at −80C, and then stored at −20C. Brains were then sectioned (40 μm) with a cryostat (Leica CM 3050, Buffalo Grove, IL, USA); every third section was mounted on a slide and Nissl-stained with cresyl violet. Sections were visualized with a Leica DMRXA microscope (5X objective) and photographed with a CCD camera (Princeton Instruments, Trenton, NJ, USA). The distance between the tip of the injector tract and the center of the STN was then measured along the anterior-posterior, medial-lateral, and dorsal ventral axes using ImageJ (National Institutes of Health, Bethesda, MD, USA). The total distance was then calculated as the bilateral sum of the injector offset from STN center; in turn, the offset distance was calculated as the square root of the sum of the squares of the measured distances along the 3 axes measured with ImageJ.
Statistics
All statistical analyses were performed using SPSS Statistics (Version 19). Logarithmic transformations produced Gaussian distributions of α and Q0 (Shapiro-Wilk, p > 0.05), and transformed variables were used for all analyses. Single-factor repeated-measures analysis of variance (ANOVA) with Tukey’s multiple comparison correction was used for all experiments with a single within-subjects variable; this approach was used to determine changes in α, Q0, and reinstatement to cocaine seeking as a consequence of microinfusion treatment. A Pearson correlation was used to relate treatment effect size with microinjector distance from STN. Linear mixed models were used for experiments with multiple within-subjects variables. A linear mixed model was used to test treatment effects during the fixed low-price procedure in which 10-min bin and treatment type were set as the within-subjects factors under compound symmetry covariance. A separate linear mixed model was used to test treatment effects during locomotor testing in which day of test, 5-min bin, and treatment type were set as the within-subjects factors under compound symmetry covariance. Bonferroni corrections were used for all multiple comparisons in linear mixed models.
Results
Inactivation of STN increases α without altering Q0
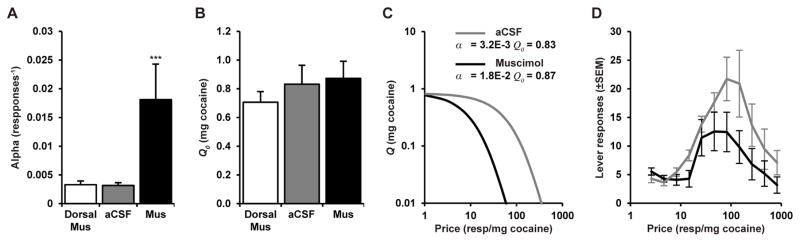
Animals (n = 11) were trained to self-administer cocaine, and baseline cocaine demand (α and Q0) was assessed for each animal. All animals then received bilateral control microinfusions of muscimol dorsally to STN immediately prior to testing on the within-session threshold procedure. On subsequent sessions, animals were pretreated with microinfusions of either muscimol or aCSF (in a counterbalanced manner) into STN immediately prior to testing on the within-session threshold procedure. Demand curves were constructed individually for each animal and resultant demand parameters (Q0 and α) were averaged across animals for each condition, and these averaged values of (Q0 and α; Fig. 1A and 1B) were used to construct average demand curves (Fig. 1C). Microinfusion of muscimol into STN significantly increased demand elasticity (α; decreased motivation) compared to injections of aCSF vehicle or dorsal infusions of muscimol (Fig. 1A, 1C, and 1D) (Repeated measures ANOVA, F2,20 = 15.33, p < 1E-4). In contrast, muscimol microinfusion into STN did not alter cocaine consumption at low effort (Q0; Fig. 1B – 1D); repeated measures ANOVA, F2,20 = 0.54, p = 0.59).
Figure 1.
Inactivation of STN with muscimol increases demand elasticity (α) but not low price cocaine consumption (Q0). Demand curves were constructed individually for each animal and resultant demand parameters (Q0 and α) were averaged across animals (n = 11) for each condition. (A) Demand elasticity (α) was significantly increased following inactivation of STN with muscimol (mus) microinfusion compared to microinfusion of aCSF vehicle or microinfusion of muscimol just dorsal to STN (n = 11, repeated ANOVA, F2,20 = 15.33, ***p < 0.001). (B) Low-price cocaine consumption (Q0) was not altered by muscimol microinfusion into STN (n = 11, repeated ANOVA, F2,20 = 1.68, p = 0.59), indicating STN inactivation does not alter the hedonic properties of cocaine. (C) Demand curves constructed from the mean values of α and Q0 illustrate that reductions in cocaine consumption with STN inactivation are most pronounced at high prices. (D) Average responses at each price show the reduction in responding that occurs at higher, but not lower, prices with muscimol pretreatment. Animals received a mean total of 126.0 (±16.2) and 79.6 (±15.4) infusions with aCSF and muscimol pretreatment respectively. Importantly, statistical testing of average responses is not reported here, as it ignores the effects of unstable brain cocaine concentration that confound within-session measures of demand and preclude this type of analysis (Bentzley et al. 2013). Error bars are +/− the standard error of the mean (SEM).
Effect of STN inactivation is correlated with injector distance from STN
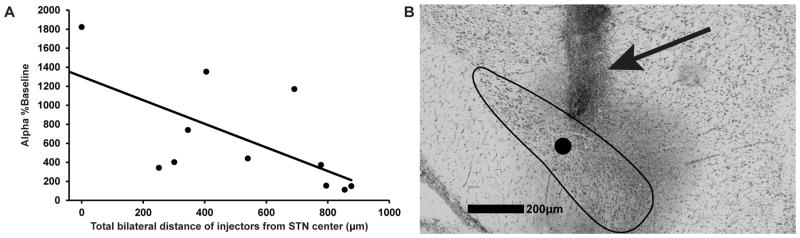
We tested the effect of distance from STN on behavioral results obtained for microinfusions. For this, the proportional increase in α over each animal’s baseline value was plotted against total bilateral distance of microinjectors from STN center (Fig. S1 in Supporting Information). A significant negative correlation between these parameters was found (Pearson, r = −0.64, p < 0.05), with the largest increase in α occurring with injectors nearest to STN center (Fig. 2). This confirms that increased α with muscimol microinfusions was due to actions in STN.
Figure 2.
Effect magnitude of muscimol microinfusion correlates with microinjector distance from STN. After termination of behavioral testing, pontamine sky blue dye was microinjected to mark the location of microinjectors, and total bilateral microinjector distance from STN center was measured histologically in the animals (n = 11) analyzed in Fig. 1. (A) Injectors most proximal to STN produced the largest increase in demand elasticity (α) in proportion to the animal’s baseline α (Pearson, r = −0.64, p < 0.05), indicating that changes in α with muscimol microinfusion were due to inactivation of STN and not nearby structures. (B) Representative Nissl-stained coronal section (cresyl violet) after 4 microinfusions in an animal, consisting of a control microinfusion (dorsal to STN), an aCSF microinfusion in STN, a muscimol microinfusion in STN, and a pontamine microinfusion in STN. The STN boundary is outlined, the circle indicates the center of STN, and the arrow denotes the cannula tract.
STN inactivation does not reduce cocaine consumption when price remains low throughout the session
Time in session and price are directly related in the within-session threshold procedure; hence, it is possible that the effect of STN inactivation to increase α without altering Q0 could reflect a time-dependent (rather than price-dependent) reduction in cocaine consumption, i.e., decreased consumption at later time points during the session. To test this possible confound, a separate group of animals (n = 7) trained to self-administer cocaine and implanted with bilateral guide cannula above STN were tested on a procedure identical to the within-session threshold procedure, except that the price remained low throughout the session, equal to the second-lowest price in the threshold procedure. We hypothesized that if effects of STN inactivation were dependent on price, and not time, then reductions in cocaine consumption would not be observed with STN inactivation. Indeed, we found no change in consumption with microinfusion of muscimol compared to aCSF vehicle or dorsal infusions of muscimol in 7 animals with histologically confirmed bilateral injector placement in STN (Fig. 3; and Fig. S2 in Supporting Information). Linear mixed-model analysis revealed no significant treatment effect (F2,192 = 0.06, p = 0.95) or treatment × bin interaction (F20,192 = 0.55, p = 0.94).
Figure 3.
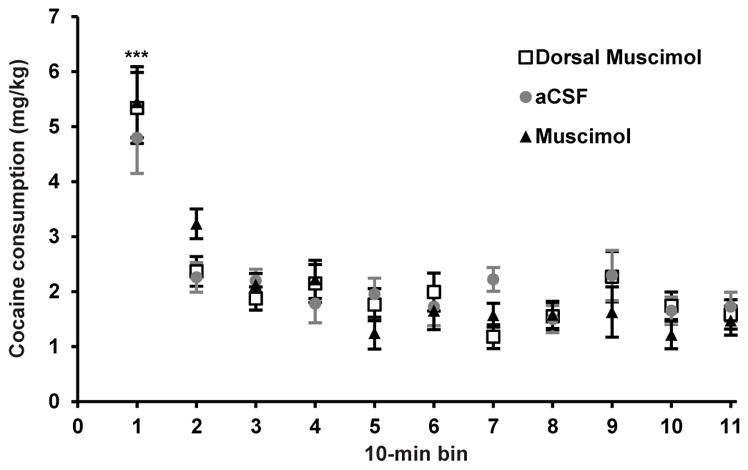
Inactivation of STN with muscimol does not decrease consumption when price remains low throughout the session. Animals (n = 7) with histologically confirmed bilateral injectors in STN were pretreated with microinfusions of either muscimol, aCSF vehicle, or microinfusion of muscimol dorsal to STN. Animals were then tested with a version of the within-session threshold procedure in which price was held low and constant (215 μg per infusion) throughout the 110-min session. Results showed that cocaine consumption was constant throughout the session regardless of treatment, and mixed-model analysis indicated no significant treatment effect (F2,192 = 0.06, p = 0.95) or treatment × bin interaction (F20,192 = 0.55, p = 0.94). As is typically observed, animals consumed significantly more cocaine in the first bin (Bonferroni, ***p < 0.001); this was similar in subjects with vehicle or muscimol microinfusion into STN or dorsally to STN.
STN inactivation attenuates cocaine seeking in extinction
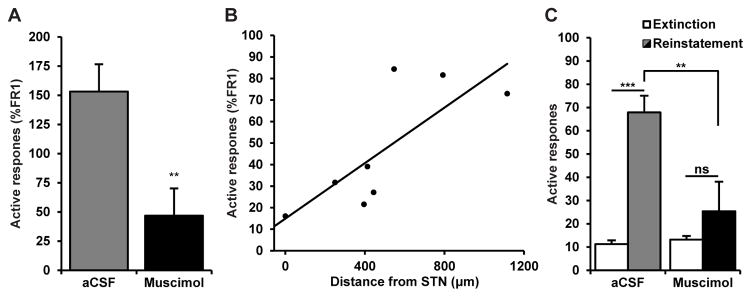
We previously showed that α predicts inter-individual variability in cocaine seeking during extinction (Bentzley et al. 2014). Therefore, in view of the marked increase in demand elasticity (α) with STN inactivation we hypothesized that STN inactivation may also reduce cocaine seeking during extinction. Twenty-four hrs after their final cocaine self-administration session, a separate group of 8 rats received bilateral muscimol microinfusions into STN before being tested in an initial extinction session in which responses on the previously active lever no longer resulted in cocaine infusions or cocaine-associated light and tone cues. Unreinforced responses during this extinction session were normalized to active responding during the two previous self-administration sessions to account for large inter-individual differences in drug seeking. Muscimol microinfusion into STN significantly reduced responding on the previously active lever compared to microinfusion with aCSF vehicle (Fig. 4A; paired t-test, t7 = 4.31, p < 0.01), indicating a reduction in cocaine seeking during early extinction with STN inactivation. This difference in responding was also present when non-normalized response rates were compared with mean±SEM response rates for aCSF and muscimol 73.3±11.4 and 29.9±6.0 respectively (paired t-test, t7 = 3.75, p < 0.01). Further, similarly to the association between microinjector distance from STN and proportional increase in α, there was a significant association between injector distance (Fig. S3 in Supporting Information) and proportional change in responding during extinction (Fig. 4B; Pearson, r = 0.77, p < 0.05), thereby indicating that reductions in unreinforced drug seeking were due to muscimol action in STN.
Figure 4.
Inactivation of STN attenuates cocaine seeking. (A) Twenty-four hrs following the last cocaine self-administration session, bilateral STN muscimol microinfusion pretreatment significantly reduced unreinforced responding on the first day of extinction compared to pretreatment with aCSF vehicle (n = 8, paired t-test, t7 = 4.31, p < 0.01). (B) This suppression of unreinforced responding with muscimol microinfusion into STN was significantly correlated with microinjector distance from STN center (n = 8, Pearson, r = 0.77, p < 0.05), indicating inactivation of STN, not a nearby structure, was driving reduced drug seeking in early extinction. (C) After extinction of cocaine self-administration, responding was significantly reinstated with cocaine-associated light and tone cues (n = 8, extinction vs. aCSF reinstatement, Tukey, q = 6.78, ***p < 0.001). Muscimol pretreatment into STN blocked cued reinstatement of cocaine seeking (n = 8, extinction vs. muscimol reinstatement, Tukey, q = 1.46, p = 0.74, n.s. = not significant), and cue-reinstated responding was significantly lower with muscimol pretreatment compared to aCSF pretreatment (n = 8, Tukey, q = 5.09, **p < 0.01).
STN inactivation attenuates cue-induced reinstatement of cocaine seeking
Next, animals (n = 8) were run on daily extinction sessions until lever responding decreased to less than 25 active presses per session for two consecutive days. Reinstatement of cocaine seeking was then tested with response-contingent, cocaine-associated light and tone cues (Fig. 4C). Microinfusions of muscimol or aCSF (counterbalanced) were made into STN immediately prior to these reinstatement tests. Repeated measures ANOVA revealed significant differences in responding during cue-induced reinstatement (F3,21 = 9.98, p < 0.001), and Tukey post-hoc testing revealed that active lever responding was significantly reinstated by cocaine-associated cues after vehicle pretreatment (q = 6.78, p < 0.001) but not after muscimol pretreatment (q = 1.46, p = 0.74). Furthermore, active responding after muscimol microinfusion into STN was significantly less than after vehicle pretreatment (q = 5.09, p < 0.01). The effect of muscimol on reducing cue-induced reinstatement [(ResponsesaCSF − ResponsesMus)/ResponsesaCSF] was similar across animals and unrelated to injector distance from STN (Pearson, r = 0.60, p = 0.11).
STN inactivation does not alter locomotor activity
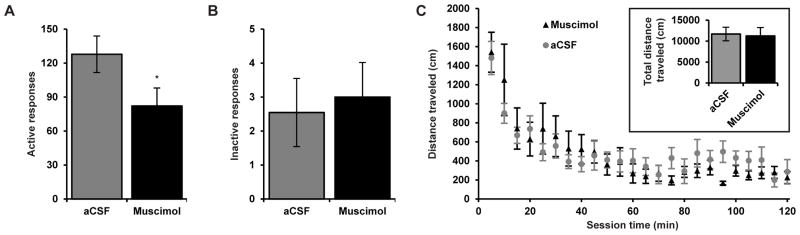
Animals must respond at increasing rates to defend initial, low-price cocaine consumption (Q0); thus, interventions that constrain motor output may also limit cocaine seeking or demand at high prices. Hence, we tested for changes in responding on the inactive lever in the economic cohort of animals (n = 11) to see if muscimol pretreatment resulted in a generalized reduction in motor activity. Although muscimol pretreatment significantly reduced active lever responding during threshold procedure testing (as expected due to decreased responding at high prices; Fig. 5A; paired t-test, t10 = 2.85, p < 0.05), pretreatment did not reduce inactive lever responding (Fig. 5B; paired t-test, t10 = 0.39, p = 0.71). Furthermore, we tested the effect of STN inactivation on activity in a locomotor testing chamber by microinfusing a high dose of muscimol (0.4 mM) bilaterally into STN in a separate group of rats (n = 7) that had self-administered cocaine on the within-session threshold procedure. Pretreatment with muscimol did not alter the distance traveled in the locomotor chamber compared to microinfusion of aCSF vehicle in the 7 animals with histologically confirmed bilateral injector placement in STN (Fig. 5C; and Fig. S4 in Supplement 1). Although this dose of muscimol had no effect on locomotor activity, it caused a near complete suppression in active lever responding in the same animals during threshold procedure testing (data not shown). Linear mixed-model analysis revealed no significant treatment effect (F1,281 = 1.36, p = 0.24), treatment × day interaction (F1,5 = 0.25, p = 0.64), or treatment × bin interaction (F23,281 = 0.82, p = 0.71).
Figure 5.
Inactivation of STN does not alter locomotor activity or inactive lever responding. (A) Although STN inactivation with muscimol significantly suppressed active lever responding (as expected from increased demand elasticity as in Fig. 1; n = 11, paired t-test, *p < 0.05), (B) STN inactivation did not alter inactive lever responding in the same animals (n = 11, paired t-test, p = 0.71). (C) A separate group of animals (n = 7) with histologically confirmed bilateral microinjector placement within STN displayed no differences in mean distance traveled in locomotor chambers with muscimol compared to vehicle pretreatment. Mixed model analysis indicated no effect of treatment (F1,281 = 1.36, p = 0.24), treatment × day interaction (F1,5 = 0.25, p = 0.64), or treatment × bin interaction (F23,281 = 0.82, p = 0.71). (Insert) Mean total distance traveled across all bins.
Discussion
Here, we showed that STN inactivation markedly reduces economic demand for cocaine (Fig. 1), cocaine seeking in early extinction (Fig. 4A), and cue-induced reinstatement of cocaine seeking (Fig. 4C). Taken together, these findings indicate high clinical potential of STN-targeting therapies for treatment of cocaine addiction. Specifically, this clinical potential is denoted by the economic framework of our analysis (Bentzley et al. 2014) and the availability of STN-targeting therapies for treatment of Parkinson’s disease, e.g. DBS (Okun 2012) and glutamate decarboxylase (GAD) gene transfer (Emborg et al. 2007).
The particular economic framework used in these experiments allows us to make predictions of treatment efficacy across species. Namely, we found here that STN inactivation increases demand elasticity (α) within a particular exponential demand model (Hursh & Silberberg 2008). We previously reported that this measure of cocaine demand elasticity (α) predicts a broad spectrum of addiction-like behavior in rat (Bentzley et al. 2014). In addition, others found that demand elasticity corresponds to several measures of alcohol (Murphy et al. 2009; Gray & MacKillop 2014), nicotine (Murphy et al. 2011; Chase et al. 2013) or cocaine (Bruner & Johnson 2014) abuse in humans, indicating that this model of drug demand is an addiction biomarker in both rat and human and confirming the existence of a conserved model of drug demand across reinforcers and species. Hence, we expect that STN inactivation will reduce drug demand in cocaine-dependent individuals as found here in rats.
Because STN inactivation reduced cocaine demand here, we predict that STN inactivation will also reduce the propensity to relapse to cocaine use in humans. In our previous work in animals we found a strong association between cocaine demand elasticity and propensity to reinstate extinguished drug seeking (Bentzley et al. 2014; Bentzley & Aston-Jones 2015), an association also found for methamphetamine (Galuska et al. 2011). In the current set of studies, after finding that STN inactivation markedly increased demand elasticity, we prospectively hypothesized – and then confirmed – that STN inactivation would also attenuate cued reinstatement behavior. This concurrent increase in demand elasticity and reduction in reinstatement behavior was also reported in animal studies for oxytocin treatment (Bentzley et al. 2014) and orexin-1 receptor antagonism (Bentzley & Aston-Jones 2015). Moreover, a recent human study found that varenicline treatment for nicotine dependence concurrently increased demand elasticity for cigarettes and increased time to relapse (McClure et al. 2013). Also, naltrexone reduces alcohol demand (Bujarski et al. 2012) and increases the time to the first heavy drinking episode (Anton et al. 2006) in human participants. Hence, we expect a similar association between increases in demand elasticity and reductions in relapse behavior to hold true for STN-inactivation in cocaine-dependent patients.
A primary reason we elected to test the effect of STN inactivation on cocaine demand was because the neurosurgical approach to targeting the STN is well defined in humans. STN DBS is frequently utilized for treatment of Parkinson’s disease and psychiatric diseases such as obsessive-compulsive disorder (Hamani & Temel 2012). Indeed, several reports have considered (Luigjes et al. 2012; Pierce & Vassoler 2013) and supported (Baunez et al. 2011; Pelloux & Baunez 2013) STN DBS as a viable treatment for cocaine addiction. Support for STN DBS as a treatment for cocaine dependence stems in part from a study of STN lesions (Baunez et al. 2005) and the results here for pharmacological inactivation of STN. Although inactivation of STN does not always produce the same effects as STN DBS (Gradinaru et al. 2009), there are parallels between our findings and past reports that indicate inactivation of STN and STN DBS produce similar reductions in cocaine seeking. For example, our results indicate that transient inactivation of STN with muscimol selectively reduces demand for cocaine when the price required to obtain cocaine is high, but not when the price is low. Although our manipulation of price was through a reduction in cocaine dose on an FR-1 schedule, manipulations of schedule and dose have been shown to be equivalent (Collier et al. 1986; Hursh et al. 1988; Bickel et al. 1990) and threshold procedure-based measures of demand correlate with other measures of effort such as progressive ratio breakpoint (Oleson & Roberts 2009). Thus, our results here indicate that transient inactivation of STN with muscimol selectively reduces demand for cocaine when the effort required to obtain cocaine is high. Similarly, past reports have found the same effort-dependent effects of permanent STN lesions (Baunez et al. 2005), as well as STN DBS (Rouaud et al. 2010). However, due to the differing mechanisms of muscimol microinfusion and STN DBS, these results must be confirmed with deep-brain stimulators in animals before a strong prediction of clinical efficacy of STN DBS can be made.
The anatomical specificity of muscimol microinfusions was confirmed in our current studies by correlating the magnitude of the behavioral effect of muscimol microinfusions to the distance of microinjectors from STN. Although this approach could be limited by non-linear spread of muscimol and non-linear effects of partial STN inactivation, a significant negative association between STN injector distance and effect of muscimol microinfusion was observed for behavioral-economic and early extinction testing. This indicates that the observed reductions in drug demand were likely due to muscimol action in STN. In contrast, an association between reductions in cued-reinstatement behavior and microinjector distance was not observed. This could be because cued-reinstatement behavior is particularly sensitive to even incomplete STN inactivation, and the lack of an association is possibly a floor-effect. That is, most animals demonstrated a complete block in cued-induced reinstatement behavior with muscimol microinfusion, precluding the possibility of detecting an association with reinstatement behavior. It is also possible that observed reductions in cued-reinstatement might be due to inactivation of structures adjacent to STN. Although this seems unlikely given the significant associations between microinjector distance and the effect of muscimol on other measures of drug seeking; additional studies are needed to examine this further.
Although our finding of reduced economic demand for cocaine with STN inactivation is complementary to past reports (Baunez et al. 2005; Rouaud et al. 2010), our finding initially seems to contrast with two other studies. Uslaner et al. reported increased responding for cocaine on a progressive ratio schedule in STN-lesioned animals (2005). However, this increase in responding may have occurred because animals had not fully acquired cocaine self-administration before testing, as previously hypothesized by others (Lardeux & Baunez 2008). Our current study required that animals stabilize their self-administration behavior over a course of weeks before STN inactivation. This experimental design is important for testing the possible clinical utility of STN inactivation because it required that animals, like presenting patients, have a stable history of drug use before commencement of treatment. Our current study differs from Uslaner et al. in several other ways that could also account for divergent results (2005). We used a transient and immediate form of neural inactivation, as apposed to the permanent lesion method of Uslaner et al. In addition, the location of the STN that was inactivated may have differed between these two studies (2005). Kantak et al. reported no change in cocaine seeking with lidocaine inactivation of STN despite extensively training animals until stable responding was reached (2013). Inconsistencies between these findings and ours could be due to the differences between the threshold procedure used here and the second-order task used by Kantak et al. (2013). The major constraint on intake in the second order task employed was a 5-min fixed interval; whereas, in the economic task we employed cocaine intake was limited only by effort (not time), and the effects of STN inactivation were only present at high effort. Thus, STN inactivation could fail to reduce responding for cocaine in tasks were a fixed interval is the major constraint on delivery of cocaine infusions. Further, the authors in Kantak et al. (Kantak et al. 2013) intended to exclusively target the medial portion of the STN for inactivation. In line with past reports (Baunez et al. 2005), we targeted the entire STN, not a specific subregion; hence, discordance in results may indicate the rat medial STN is not involved in drug seeking or that processing is distributed across STN such that a larger portion needs to be inactivated to induce changes in drug-seeking behavior. However, neither our report nor that by Kantak et al. (2013) used methods that could resolve the precise boundary of brain tissue inactivated.
Impairment of motoric function is a potential confound in all self-administration experiments. Here, we addressed this issue by testing for changes in cocaine consumption throughout the session using the fixed low-price procedure. This was done to determine if STN inactivation preferentially inhibited self-administration behavior later in the session, when the price of cocaine was high and when we observed changes in cocaine consumption in the threshold procedure. We also tested for attenuated travel distance in a novel environment to determine if there were any gross effects on locomotor activity. Neither of these tests, nor inactive lever responding, revealed changes with STN inactivation. Further, several past studies reported increased rates of self-administration with STN inactivation (Baunez et al. 2002; 2005; Uslaner et al. 2005; Lardeux & Baunez 2008). Taken together, it is unlikely that the results reported here are due to changes in motoric activity with STN inactivation.
In summary, we have shown that transient inactivation of STN with muscimol microinfusion significantly increased cocaine demand elasticity (α) but not cocaine intake at low prices (Q0). The economic framework of our analysis indicates high translational potential of these results, and that STN inactivation may be effective for treating cocaine addiction. This extrapolation is bolstered by our finding that STN inactivation attenuated cocaine seeking in early extinction as well as cued reinstatement of extinguished cocaine seeking. Thus, STN inactivation both reduces demand for drug as well as propensity to relapse, indicating substantial potential for therapeutic utility.
Supplementary Material
Acknowledgments
This work was supported by U.S. Public Health Service grant awards R37/R01-DA006214, T32-GM008716, T32-DA007288 and F30-DA035065.
Footnotes
Location work carried out:
Medical University of South Carolina, Department of Neurosciences, Basic Science Building, 404, 173 Ashley Avenue, Charleston, SC 29403
Disclosure/Conflict of Interest
The authors declare no conflict of interest.
Author Contributions
B.S.B designed experiments, collected data, analyzed data, obtained research funding and prepared the manuscript. G.A.-J. designed experiments, analyzed results, obtained research funding and prepared the manuscript. All authors discussed results and commented on the manuscript.
References
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Baufreton J, Garret M, Dovero S, Dufy B, Bioulac B, Taupignon A. Activation of GABA(A) receptors in subthalamic neurons in vitro: properties of native receptors and inhibition mechanisms. Journal of Neurophysiology. 2001;86:75–85. doi: 10.1152/jn.2001.86.1.75. [DOI] [PubMed] [Google Scholar]
- Baunez C, Amalric M, Robbins TW. Enhanced food-related motivation after bilateral lesions of the subthalamic nucleus. Journal of Neuroscience. 2002;22:562–568. doi: 10.1523/JNEUROSCI.22-02-00562.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunez C, Dias C, Cador M, Amalric M. The subthalamic nucleus exerts opposite control on cocaine and “natural” rewards. Nat Neurosci. 2005;8:484–489. doi: 10.1038/nn1429. [DOI] [PubMed] [Google Scholar]
- Baunez C, Yelnik J, Mallet L. Six questions on the subthalamic nucleus: lessons from animal models and from stimulated patients. Neuroscience. 2011;198:193–204. doi: 10.1016/j.neuroscience.2011.09.059. [DOI] [PubMed] [Google Scholar]
- Bentzley BS, Aston-Jones G. Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci. 2015;41:1149–1156. doi: 10.1111/ejn.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Fender KM, Aston-Jones G. The behavioral economics of drug self-administration: a review and new analytical approach for within-session procedures. Psychopharmacology. 2013;226:113–125. doi: 10.1007/s00213-012-2899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, Jhou TC, Aston-Jones G. Economic demand predicts addiction-like behavior and therapeutic efficacy of oxytocin in the rat. Proceedings of the National Academy of Sciences. 2014;111:11822–11827. doi: 10.1073/pnas.1406324111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzina G, Cheung THC, Body S, Deakin JFW, Anderson IM, Bradshaw CM, Szabadi E. Quantitative analysis of the effect of lesions of the subthalamic nucleus on intertemporal choice: further evidence for enhancement of the incentive value of food reinforcers. Behavioural Pharmacology. 2009;20:437–446. doi: 10.1097/FBP.0b013e3283305e4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, DeGrandpre RJ, Higgins ST, Hughes JR. Behavioral economics of drug self-administration. I. Functional equivalence of response requirement and drug dose. Life Sci. 1990;47:1501–1510. doi: 10.1016/0024-3205(90)90178-t. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Johnson MW, Koffarnus MN, MacKillop J, Murphy JG. The behavioral economics of substance use disorders: reinforcement pathologies and their repair. Annu Rev Clin Psychol. 2014;10:641–677. doi: 10.1146/annurev-clinpsy-032813-153724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruner NR, Johnson MW. Demand curves for hypothetical cocaine in cocaine-dependent individuals. Psychopharmacology. 2014;231:889–897. doi: 10.1007/s00213-013-3312-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bujarski S, MacKillop J, Ray LA. Understanding naltrexone mechanism of action and pharmacogenetics in Asian Americans via behavioral economics: a preliminary study. Experimental and Clinical Psychopharmacology. 2012;20:181–190. doi: 10.1037/a0027379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, MacKillop J, Hogarth L. Isolating behavioural economic indices of demand in relation to nicotine dependence. Psychopharmacology. 2013;226:371–380. doi: 10.1007/s00213-012-2911-x. [DOI] [PubMed] [Google Scholar]
- Collier GH, Johnson DF, Hill WL, Kaufman LW. The economics of the law of effect. J Exp Anal Behav. 1986;46:113–136. doi: 10.1901/jeab.1986.46-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag HS, Ferrario CR, Robinson TE. The rate of intravenous cocaine or amphetamine delivery does not influence drug-taking and drug-seeking behavior in rats. Pharmacol Biochem Behav. 2008;90:797–804. doi: 10.1016/j.pbb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg ME, Carbon M, Holden JE, During MJ, Ma Y, Tang C, Moirano J, Fitzsimons H, Roitberg BZ, Tuccar E, Roberts A, Kaplitt MG, Eidelberg D. Subthalamic glutamic acid decarboxylase gene therapy: changes in motor function and cortical metabolism. J Cereb Blood Flow Metab. 2007;27:501–509. doi: 10.1038/sj.jcbfm.9600364. [DOI] [PubMed] [Google Scholar]
- Féger J, Robledo P. The Effects of Activation or Inhibition of the Subthalamic Nucleus on the Metabolic and Electrophysiological Activities Within the Pallidal Complex and Substantia Nigra in the Rat. Eur J Neurosci. 1991;3:947–952. doi: 10.1111/j.1460-9568.1991.tb00030.x. [DOI] [PubMed] [Google Scholar]
- Galuska CM, Banna KM, Willse LV, Yahyavi-Firouz-Abadi N, See RE. A comparison of economic demand and conditioned-cued reinstatement of methamphetamine-seeking or food-seeking in rats. Behavioural Pharmacology. 2011;22:312–323. doi: 10.1097/FBP.0b013e3283473be4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradinaru V, Mogri M, Thompson KR, Henderson JM, Deisseroth K. Optical deconstruction of parkinsonian neural circuitry. Science. 2009;324:354–359. doi: 10.1126/science.1167093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JC, MacKillop J. Interrelationships among individual differences in alcohol demand, impulsivity, and alcohol misuse. Psychol Addict Behav. 2014;28:282–287. doi: 10.1037/a0032766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Temel Y. Deep brain stimulation for psychiatric disease: contributions and validity of animal models. Sci Transl Med. 2012;4:142rv8. doi: 10.1126/scitranslmed.3003722. [DOI] [PubMed] [Google Scholar]
- Heinz AJ, Lilje TC, Kassel JD, de Wit H. Quantifying reinforcement value and demand for psychoactive substances in humans. Curr Drug Abuse Rev. 2012;5:257–272. doi: 10.2174/1874473711205040002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR. Economic concepts for the analysis of behavior. J Exp Anal Behav. 1980;34:219–238. doi: 10.1901/jeab.1980.34-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Raslear TG, Shurtleff D, Bauman R, Simmons L. A cost-benefit analysis of demand for food. J Exp Anal Behav. 1988;50:419–440. doi: 10.1901/jeab.1988.50-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Kantak KM, Yager LM, Brisotti MF. Impact of medial orbital cortex and medial subthalamic nucleus inactivation, individually and together, on the maintenance of cocaine self-administration behavior in rats. Behavioural Brain Research. 2013;238:1–9. doi: 10.1016/j.bbr.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardeux S, Baunez C. Alcohol preference influences the subthalamic nucleus control on motivation for alcohol in rats. Neuropsychopharmacology. 2008;33:634–642. doi: 10.1038/sj.npp.1301432. [DOI] [PubMed] [Google Scholar]
- Luigjes J, van den Brink W, Feenstra M, van den Munckhof P, Schuurman PR, Schippers R, Mazaheri A, De Vries TJ, Denys D. Deep brain stimulation in addiction: a review of potential brain targets. Mol Psychiatry. 2012;17:572–583. doi: 10.1038/mp.2011.114. [DOI] [PubMed] [Google Scholar]
- MacKillop J, Brown CL, Stojek MK, Murphy CM, Sweet L, Niaura RS. Behavioral economic analysis of withdrawal- and cue-elicited craving for tobacco: an initial investigation. Nicotine Tob Res. 2012;14:1426–1434. doi: 10.1093/ntr/nts006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKillop J, Murphy JG. A behavioral economic measure of demand for alcohol predicts brief intervention outcomes. Drug Alcohol Depend. 2007;89:227–233. doi: 10.1016/j.drugalcdep.2007.01.002. [DOI] [PubMed] [Google Scholar]
- MacKillop J, O’Hagen S, Lisman SA, Murphy JG, Ray LA, Tidey JW, McGeary JE, Monti PM. Behavioral economic analysis of cue-elicited craving for alcohol. Addiction. 2010;105:1599–1607. doi: 10.1111/j.1360-0443.2010.03004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Vandrey RG, Johnson MW, Stitzer ML. Effects of varenicline on abstinence and smoking reward following a programmed lapse. Nicotine Tob Res. 2013;15:139–148. doi: 10.1093/ntr/nts101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J, Skidmore JR, Pederson AA. Reliability and validity of a demand curve measure of alcohol reinforcement. Experimental and Clinical Psychopharmacology. 2009;17:396–404. doi: 10.1037/a0017684. [DOI] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J, Tidey JW, Brazil LA, Colby SM. Validity of a demand curve measure of nicotine reinforcement with adolescent smokers. Drug Alcohol Depend. 2011;113:207–214. doi: 10.1016/j.drugalcdep.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun MS. Deep-brain stimulation for Parkinson’s disease. N Engl J Med. 2012;367:1529–1538. doi: 10.1056/NEJMct1208070. [DOI] [PubMed] [Google Scholar]
- Oleson EB, Richardson JM, Roberts DCS. A novel IV cocaine self-administration procedure in rats: differential effects of dopamine, serotonin, and GABA drug pre-treatments on cocaine consumption and maximal price paid. Psychopharmacology. 2011;214:567–577. doi: 10.1007/s00213-010-2058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Roberts DCS. Behavioral economic assessment of price and cocaine consumption following self-administration histories that produce escalation of either final ratios or intake. Neuropsychopharmacology. 2009;34:796–804. doi: 10.1038/npp.2008.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleson EB, Roberts DCS. Cocaine self-administration in rats: threshold procedures. Methods Mol Biol. 2012;829:303–319. doi: 10.1007/978-1-61779-458-2_20. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pelloux Y, Baunez C. Deep brain stimulation for addiction: why the subthalamic nucleus should be favored. Curr Opin Neurobiol. 2013;23:713–720. doi: 10.1016/j.conb.2013.02.016. [DOI] [PubMed] [Google Scholar]
- Petry NM. A behavioral economic analysis of polydrug abuse in alcoholics: asymmetrical substitution of alcohol and cocaine. Drug Alcohol Depend. 2001;62:31–39. doi: 10.1016/s0376-8716(00)00157-5. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Vassoler FM. Deep brain stimulation for the treatment of addiction: basic and clinical studies and potential mechanisms of action. Psychopharmacology. 2013;229:487–491. doi: 10.1007/s00213-013-3214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WE, Choi E, Guy EG. An examination of the effects of subthalamic nucleus inhibition or μ-opioid receptor stimulation on food-directed motivation in the non-deprived rat. Behavioural Brain Research. 2012;230:365–373. doi: 10.1016/j.bbr.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaud T, Lardeux S, Panayotis N, Paleressompoulle D, Cador M, Baunez C. Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proceedings of the National Academy of Sciences. 2010;107:1196–1200. doi: 10.1073/pnas.0908189107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzaire-dubois B, Hammond C, Hamon B, Féger J. Pharmacological blockade of the globus palidus-induced inhibitory response of subthalamic cells in the rat. 1980;200:321–329. doi: 10.1016/0006-8993(80)90923-3. [DOI] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Yang P, Robinson TE. Subthalamic nucleus lesions enhance the psychomotor-activating, incentive motivational, and neurobiological effects of cocaine. Journal of Neuroscience. 2005;25:8407–8415. doi: 10.1523/JNEUROSCI.1910-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.