Abstract
Defining immunological mechanisms underlying NK cell biology is crucial for the treatment and prevention of immune deficiency and malignancy. The limited availability of human biological specimens presents a challenge to the study of human immunobiology. The use of high throughput, multi-parametric assays will not only aid in the definition and diagnosis of complex human immune disorders affecting NK cell function but also advance NK cell biology through population-based assessment of molecular signaling. In an effort to garner the most information from limited numbers of human cells, we designed a quantitative method to study NK cell function using imaging flow cytometry (IFC), which combines multiparametric flow cytometry and fluorescence microscopy. Specifically, we developed IFC as a tool to measure polarization and secretion of lytic granules at the immunological synapse formed between an NK cell and a susceptible target. We have further validated our approach through quantitative comparison with high-resolution confocal microscopy. We show that IFC can be used as a quantitative, high throughput measure of NK cell biological function possessing greater dimensionality than standard flow cytometry.
Keywords: Imaging flow cytometry, Natural killer cell, Degranulation, Microscopy, Flow cytometry, Immunological synapse
1. Introduction
1.1. NK cell cytotoxicity
Natural killer (NK) cells are lymphocytes critical for human immunity that participate in the survey and killing of virally infected, transformed or stressed cells within the body. Their function is crucial for human host defense, as illustrated by severe, fatal viral infection associated with improper NK cell activity [1,2]. NK cells kill susceptible target cells through the secretion of specialized secretory lysosomes, termed lytic granules, following the formation of a lytic immunological synapse (IS) [3–5]. This process can be broken down into a specific sequence of events with serial checkpoints to ensure specific killing and prevent collateral damage to surrounding healthy tissue [6]. Activation signals begin a series of engagements of surface receptors and ligands on an NK cell, leading to dynamic rearrangement of F-actin at the developing synapse. Concurrently, the lytic granules within an NK cell converge toward the microtubule-organizing center (MTOC) [7]. The NK cell then polarizes the MTOC and granules toward the IS. Exocytosis of lytic granules at the immunological synapse causes specific and directed target cell death. By mediating adhesion and focusing secretion, the NK cell IS promotes target cell killing while limiting damage to surrounding tissues.
1.2. Detection of NK cell exocytosis
Upon degranulation, an individual lytic granule undergoes rapid de-acidification as the lumenal membrane of the granule is exposed at the NK cell plasma membrane. These properties of lytic granule biology have been exploited to quantitatively measure exocytosis. In particular, the use of membrane-permeable dyes that selectively label acidified organelles, such as we have used here, is an important tool for the study of the kinetics of granule movement in living cells. Lumenal membrane proteins such as CD107a (Lysosomal Associated Membrane Protein-1; LAMP1) can be detected by antibody staining on the cell surface for significant times following degranulation, allowing for quantitative measurement and spatiotemporal localization [8,9]. Importantly, the quantitative evaluation by flow cytometry of CD107a reaching the cell surface has emerged as a major experimental paradigm in studies of cytotoxic cell biology [10].
1.3. Advantage of IFC for the study of NK cell polarization and granule externalization
Previous studies of NK cell degranulation have utilized traditional methods of immunological analysis including confocal microscopy and flow cytometry. Despite its excellent spatial resolution, the use of confocal microscopy in analyzing NK cell degranulation is a time-consuming, sample-costly method. As a result, analysis of NK cell degranulation via confocal microscopy is difficult in cases of limited tissue amounts or numbers of peripheral blood cells. Conversely, flow cytometry provides a high throughput, multi-parametric analysis of the physical and chemical properties of events within a sample and has been validated for the quantitative measurement of effector cell degranulation as measured by the expression of CD107a on the cell surface [8,9]. However, the use of flow cytometry forgoes the ability to extract information regarding subcellular localization of proteins of interest. Most notably, flow cytometry is incapable of discerning where the CD107a degranulation event is located relative to an inciting stimulus.
Here, we describe the use of imaging flow cytometry (IFC) to quantitatively measure the formation of NK-target cell conjugates and the location and frequency of NK cell lytic granule polarization and degranulation. IFC is a powerful new technology that combines the statistical power and fluorescence sensitivity of standard flow cytometry with the spatial resolution and quantitative morphology of confocal microscopy [11]. This method enables clinically relevant, sample-sparing assays particularly for the study of the human immunity as it relates to health and disease. Through the use of IFC, we have optimized high-throughput, rapid analysis of NK cell function, a technique that will enable both cell biological investigation and potential clinical application.
2. Materials and methods
2.1. Cell culture and isolation of ex vivo NK cells
Human NK92 cells [12] were cultured and maintained in supplemented Myelocult culture media (StemCell Technologies) containing 10% penicillin/streptomycin (Life Technologies) and 100 U/mL IL-2 (Roche). The human K562 cell line [13] was maintained in supplemented RPMI 1640 media. Ex vivo NK cells were negatively selected from peripheral blood with RosetteSep NK cell selection kit (StemCell Technologies) and confirmed by conventional flow cytometry to be >90% CD56+ CD3−.
2.2. Preparation of conjugates for IFC
Effector cells and target cells were harvested, counted, washed once in serum free media and resuspended in phosphate buffered saline (PBS, Life Technologies). Effector and target cells were pre-incubated separately Lysotracker Red DND-99 (Life Technologies) or Cell Mask Deep Red (Life Technologies), respectively, for 30 min at 37 °C. Following incubation, cells were washed with supplemented media. Live effector and target cells were mixed and incubated for 60 min at 37 °C at a ratio of 1:5 (effector:target) in media in the absence of detergent or permeabilization agent but in the presence of anti-human CD107a antibody directly conjugated to AlexaFluor 488 (clone H4A3; eBioscience). Conjugates were gently resuspended and 2–3 × 105 events per condition were acquired on the ImageStreamX Mk II imaging cytometer (EMD Millipore).
2.3. Confocal microscopy
Acquisition and analysis of fixed cell conjugates by confocal microscopy was performed as previously described [14]. Briefly, primary human NK cells were incubated with K562 targets, then fixed, permeabilized and stained for lytic granules using anti-perforin antibody directly conjugated to AlexaFluor 647 (clone δG9; Life Technologies) and F-actin with phalloidin AlexaFluor 568 (Life Technologies). Images were acquired on a Leica SP8 laser scanning confocal microscope with tunable white light laser and hybrid detectors. Analysis was performed with Volocity 6.3 (PerkinElmer) [15].
2.4. Data collection by IFC
All IFC samples prepared as outlined in Section 2.2 were acquired using an ImageStreamX Mk II at 40× magnification. Events with areas smaller than 100 pixels (25 nm) were excluded from acquisition. Spectral compensation was applied prior to analysis based on single stained controls prepared as outlined in Section 2.2.
2.5. Analysis of IFC data
Acquired events were analyzed using Image Data Exploration And Analysis Software (IDEAS, EMD Millipore). We first distinguished NK-target conjugates and then analyzed the specific quantity and location of each outlined protein of interest. Detailed analysis methods are described in Section 3.
2.6. Statistical tests
Unpaired Student's T-test was used to compare IFC and confocal microscopy. Data was graphed using Prism 6.0 (GraphPad Software).
3. Results
3.1. Analysis workflow
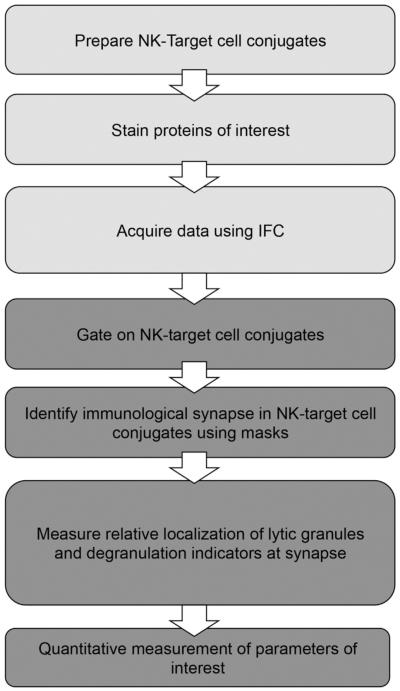
In the acquisition component of the assay (outlined in Fig. 1), NK-target cell conjugates were prepared and acquired as outlined in Section 2. Briefly, the NK92 cell line was conjugated to susceptible target cells (K562) which had been pre-labeled with vital dye. NK cells were labeled with Lysotracker Red to detect lytic granules in live cells. The assay was performed in the presence of anti-CD107a antibody which, as the cells were not permeabilized, specifically detected exocytosed LAMP-1 and thus acted as an indicator of granule externalization[8–10]. For the analysis component, a stringent sequential gating strategy was applied to find NK-target cell conjugates (Fig. 2, Section 3.2). The IS was further identified using Boolean logic to define the region of interest (ROI) within each cell in this population. This ROI was labeled using specific masks and features within the IDEAS interface (Fig. 3, Section 3.3). Measurements of the quantity of specific fluorescently tagged proteins of interest were made by IDEAS software.
Fig. 1.
Workflow of IFC assays describing two main steps: acquisition (light gray) and assessment (dark gray). IFC assays, acquired as outlined in Section 2, were analyzed to assess the quantification and localization of granules relative to the IS. Assessment included a three step process: identification of appropriate NK-target populations, identification of the IS within specific population and localization and quantitative measurement of parameters of interest.
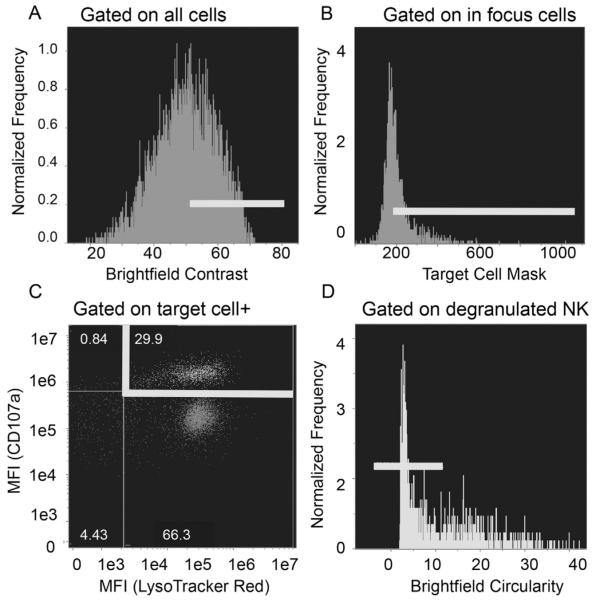
Fig. 2.
Representative gating strategy for identifying NK92 and target cell conjugates. Cells were stained and data collected as described in Section 2. Conjugates were identified using IDEAS software. A) Events in focus were identified as those with high contrast values and labeled as “In Focus”. B) Events with a target cell stain within the “In Focus” group were labeled as “Target+”. C) Lysotracker Red+ and CD107a+ events within the “Target+” population were labeled as “Degranulated NK cell”. D) Events with low circulatory scores were identified as NK-target conjugates and their microscopy images were subsequently quantitatively analyzed.
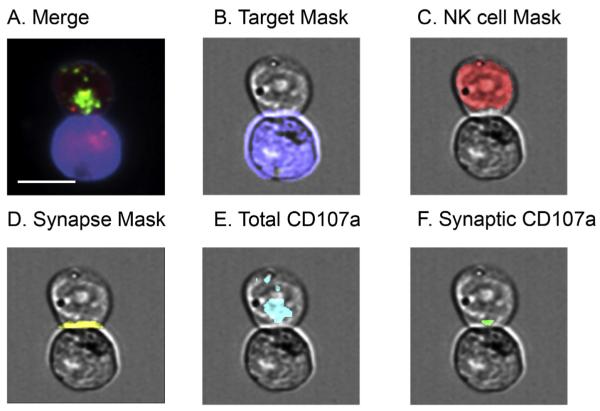
Fig. 3.
Representative masks for analysis of synaptic granules using IFC. Cells were stained as outlined in Section 2 and acquired using IFC. Conjugates were identified using multi-parametric IDEAS algorithm as shown in Fig. 2 and confirmed visually. Masks were developed using IDEAS software to establish regions of interest within each conjugate. A) Example of conjugate identified by IDEAS. Green, CD107a; red, Lysotracker Red; blue, target cell mask. B) Target cell mask (blue): created by using “Morphology” mask that captured all pixels that had fluorescence in target cell. C) NK cell mask (red): created by subtracting target cell mask from total conjugate mask (not pictured). D) Synapse mask (yellow): created by identifying furthest pixels between conjugate mask (not pictured) and target cell mask. E) Total CD107a (cyan): created by using “Intensity” mask to limit region to fluorescent proteins of interest. F) Synaptic CD107a (green): created by identifying pixels common in both the “synapse mask” and “total CD107a” mask. Scale bar = 10 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
An analysis algorithm designed in IDEAS was used to gate events based on area, intensity, and aspect ratio (length:width). Once established, this algorithm was used to analyze NK cell degranulation within all NK-target conjugates. The data analysis steps that were used to accurately identify conjugates are described in more detail below.
3.2. Design and validation of an algorithm for the detection of conjugates by IFC
To identify events that were biologically relevant, a strict definition for an NK-target cell conjugate was applied to all images acquired by IFC. Specifically, an NK-target conjugate is composed of two cells (one NK and one target cell) joined by an identifiable IS. Cells were identified by fluorescence of expected markers in the expected cell regions (i.e. the NK cell contains CD107a and Lysotracker Red while the target cell contains target cell vital dye). Events composed of two cells positive for NK cell markers were denoted as “NK–NK cell conjugates”; likewise, events in which neither cell expressed these quantities were denoted as “target–target cell conjugates”. These events were rejected from further analysis.
To identify true NK-target cell conjugates using flow cytometric gating (Fig. 2), we first identified in-focus cells using the brightfield contrast parameter (Fig. 2A). Next, we gated on events that were positive for target cell dye (Fig. 2B). These selected cells were then further gated on those positive for both Lysotracker Red and CD107a in order to identify NK-target cell conjugates in which NK cells had undergone degranulation (Fig. 2C). Since many cells were identified that were CD107a negative but Lysotracker Red positive, the degranulation event detected by CD107a is not simply a marking of the granule compartment. Finally, events were selected based on low circularity in order to enrich for 1:1 conjugates that have a high aspect ratio (length:width) and corresponding low circularity (Fig. 2D).
3.3. Advanced analysis of identified conjugates using IFC
In order to perform advanced analysis on these isolated conjugate images, we identified ROI through custom masks (Fig. 3). In IDEAS, a mask is defined as a malleable set of pixels containing the ROI. To create a mask that specifically identifies the NK cell lytic synapse, the following steps were employed in sequential order on the NK-target cell conjugates identified by our flow cytometric gating (Fig. 3A). First, we created a “conjugate mask” that included all positive pixels in the brightfield image. From this, we created a “target cell mask” by using the predefined IDEAS morphology mask to identify any region that was positive for the target cell dye (Fig. 3B). Similarly, we created an NK cell mask by excluding the target cell from the conjugate mask (Fig. 3C). Next, we created the “contact point mask” using the predefined IDEAS Interface mask to identify the pixels where the NK cell mask and target cell mask came into contact. The contact point mask was then dilated to form the “synapse mask” (Fig. 3D). The target cell was excluded from the synapse mask in order to identify only the region of IS in NK cells. Lastly, intensity masks were used to identify total protein of interest within the NK cell (Fig. 3E). From this, pixels overlapping between total protein and the synapse mask were identified as the synaptic ROI of each protein of interest (Fig. 3F). The intensity and area of total and synaptic lytic granules respectively were then calculated from this ROI.
In order to validate the multi-parametric IDEAS gating strategy (Fig. 2), microscopy images generated as IFC data were qualitatively assessed to ensure that they fit all conditions of an NK-target cell conjugate stated above (Section 3.2). Events that did not fall under these categories were removed from the pool. Continual feedback from the microscopy images was used to evaluate and refine the flow cytometric algorithm. We estimate that the false discovery rate of NK-target cell conjugates was <15% and these were removed by visual identification following flow cytometric gating.
3.4. Measurement of synaptic v. non-synaptic granule localization and degranulation determined by IFC
Following the development of masks for each ROI (Section 3.3), specifically synaptic and total NK cell regions (Fig. 3), we made quantitative measurements of the mean fluorescent intensity of synaptic versus total protein. The identification of the target cell and NK cell masks individually allows us to consider only signal contributed from the NK cell, thus eliminating unintentional measurement of any non-specific Lysotracker Red dye on the target cell. Through the use of our high throughput algorithm, we were able to measure greater than 100 conjugates per experiment and distinguish synaptic from non-synaptic localization (Fig. 4A).
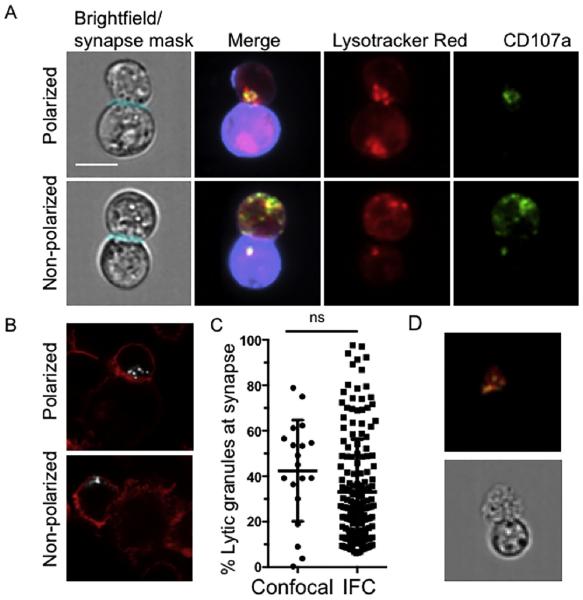
Fig. 4.
Analysis of conjugates from IFC analysis. Cells were stained as outlined in Section 2 and acquired using IFC. Conjugates were identified using the multi-parametric IDEAS algorithm shown above and confirmed visually with CD107a (degranulated granules, green), Lysotracker Red (lytic granules, red) and Cell Mask Deep Red (target cell stain, purple). Masks were developed using IDEAS software to establish regions of interest within each conjugate. A) Representative examples of polarized (top) and non-polarized (bottom) lytic granules and degranulation B) Representative images acquired by confocal microscopy of NK cells conjugated to K562 targets and fixed, permeabilized and stained for F-actin (phalloidin, red) and perforin as a marker of lytic granules (white). Examples of polarized (top) and non-polarized (bottom) lytic granule localization are shown. C) Percentage of the total NK cell lytic granules localized to the immunological synapse was calculated for IFC and confocal microscopy. p = 0.09 by Student's T-test. Scale bar = 10 μm. D) Representative example of an ex vivo human NK cell conjugated to a K562 target cell. Conjugates were identified using multi-parametric IDEAS algorithm and confirmed visually with CD107a (degranulated granules, green), LysoTracker Red (lytic granules, red). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. Validation of quantitative IFC measurements by conventional confocal microscopy
In order to validate the algorithms we developed for IFC using a “gold standard” approach for molecular localization, we performed comparison of our data of NK cell granule polarization with that obtained by fixed cell confocal microscopy (Fig. 4B) and image analysis. Specifically, we calculated the relative localization of synaptic granules for both IFC and confocal microscopy. This was expressed as the percentage of lytic granules relative to the total granule population that were found at the synapse (Fig. 4C). Values obtained using the two techniques were found to be non-significant (p = 0.09 by unpaired Student's T-test), suggesting our approach and algorithm utilizing IFC is sensitive and specific enough, when compared to high-resolution methods, to detect relative subcellular localization of lytic granules. This validates the use of IFC for future efforts to quantify granule polarization and provides a quantitative comparison between the two methods. Values obtained by IFC are comparable to current analysis methods and therefore are likely to be biologically relevant in a quantitative high-throughput format. Finally, we negatively selected human NK cells from peripheral blood and performed the same assay as was done with NK cell lines. As expected, ex vivo NK cells conjugated to susceptible K562 targets showed lytic granule polarization and granule externalization as seen in NK cell lines (Fig. 4D).
4. Discussion
A frequent barrier to studying immunity in the context of human diseases is the limited availability of biological specimens for study, particularly in pediatric patients. The development of assays that take advantage of limited numbers of cells will allow investigations for immune phenotype and function within these patient populations. Here, we have developed an objective and systematic approach to identify and analyze effector-target conjugates through a novel application of quantitative IFC technology.
This study provides a stepwise methodology to acquire, identify and analyze effector-target conjugates using IFC. Specifically, we have designed and validated a modular algorithm for (1) the identification of NK-target conjugates, (2) the identification of the lytic immune synapse within these conjugates and (3) the localization of lytic granules, both before and after degranulation, at the synapse. While a representative gating strategy was shown here, analysis of additional immunological parameters are added easily.
Previously published uses of IFC include measuring nuclear translocation of transcription factors, Ca++ mobilization and cell death [16–19]. The use of cell conjugates and the spatiotemporal localization of immune synapse components is a powerful use for this technology. In addition, modifications to our currently described protocol, such as measurement of F-actin accumulation, will yield further valuable information, as has been recently shown in T cells [20]. The ability to monitor conjugation frequency, polarization and target cell death with minimal sample volume has important implications for both clinical and cell biological questions. In addition, our algorithm can be used to quantitate the kinetics of granule movement and degranulation at multiple time points in large numbers of conjugates, an undertaking which is challenging by conventional confocal microscopy. While both confocal microscopy and IFC have similar sample preparation times, the rapid acquisition of samples by IFC and the standardized, high-throughput analysis provide considerable time savings for IFC relative to confocal. Unlike confocal, this method makes it feasible to prepare samples, acquire data and analyze in a single session.
The use of high throughput analyses allowed us to distinguish synaptic from non-synaptic granule localization and exocytosis rapidly. In addition, it allowed us to selectively analyze the population of NK cells that had undergone degranulation. The variation in polarization that we observed by IFC was also borne out by confocal microscopy analysis, albeit using a much smaller sample size. While it is difficult to compare groups of such varying sample sizes, the difference in means was not statistically different between groups.
IFC provides an advantage by simultaneously providing quantitative and qualitative data about multiple parameters. In this study, we provide quantitative analyses of lytic synapse formation and granule polarization of NK cells in conjugates with susceptible target cells. Further, we show detection of lytic granule exocytosis, as we did not treat the cells with detergents, or drugs and were able to detect CD107a within a relatively short incubation time. Since there were many Lysotracker Red positive NK cells that did not demonstrate CD107a, a specificity to the granule externalization is strongly suggested in IFC. While we do not measure target cell death per se, the use of IFC in combination with a gold standard assay of cytotoxic function, such as Cr51 release, would provide a quantitative measure of the rate of target cell killing. In addition, we have validated results obtained from IFC with more traditional methods of acquisition, proving that IFC is a practical, potentially clinically relevant tool for immunological study. This would potentially include relatively higher throughput screens of different drugs or molecular tools interfering with the expression of various genes.
With regard to clinical applicability, we would envision this approach being particularly relevant in the context of evaluating patients suspected of having hemophagocytic lymphohistiocytosis (HLH) [21]. Here the current clinical evaluation requires a combination of NK cell cytotoxicity and flow-cytometry based degranulation evaluation, particularly because some types of HLH have reduced killing and normal degranulation. It is plausible that being able to quantify and localize degranulation in an IFC assay that could also detect early stages of cell death would provide further useful information still and may allow for further biological dissection of the different HLH sub-types. Thus, future work includes the application of IFC to patient samples with known functional cytotoxicity defects. Most importantly, by combining novel technology with the rigorous application of quantitative algorithms, we can minimize research sample volume for maximal biological information and answer important questions about NK cell biology. Perhaps most importantly, we can add a relevant tool to the clinical and experimental immunologist's cell biological toolbox.
Acknowledgements
The authors wish to thank Christine Probst from Amnis/Millipore for technical support and Dr. Audrea Burns, Dr. George Makedonas and Dr. Pinaki Banerjee for their helpful discussion. DV is a participant in the Rice Undergraduate Scholars Program and the Texas Children's Hospital Developing Investigative Scholars Program. This work was supported by National Institute of Allergy and Infectious Diseases R01AI067946 and R01AI120989 to J.S.O.
Abbreviations
- (NK)
natural killer
- (IFC)
imaging flow cytometry
- (ROI)
region of interest
- (IS)
immunological synapse
- (MTOC)
microtubule organizing center
- (HLH)
hemophagocytic lymphohistiocytosis
Footnotes
Conflict of interest
None of the authors has any potential financial conflict of interest related to this manuscript.
References
- [1].Ham H, Billadeau DD. Human immunodeficiency syndromes affecting human natural killer cell cytolytic activity. Front. Immunol. 2014;5:2. doi: 10.3389/fimmu.2014.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Orange JS. Natural killer cell deficiency. J. Allergy Clin. Immunol. 2013;132(3):515–525. doi: 10.1016/j.jaci.2013.07.020. (quiz 526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Grakoui A, et al. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285(5425):221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- [4].Monks CR, et al. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395(6697):82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- [5].Orange JS, et al. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc. Natl. Acad. Sci. U. S. A. 2003;100(24):14151–14156. doi: 10.1073/pnas.1835830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mace EM, et al. Cell biological steps and checkpoints in accessing NK cell cytotoxicity. Immunol. Cell Biol. 2014;92(3):245–255. doi: 10.1038/icb.2013.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mentlik AN, et al. Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment. Mol. Biol. Cell. 2010;21(13):2241–2256. doi: 10.1091/mbc.E09-11-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods. 2004;294(1–2):15–22. doi: 10.1016/j.jim.2004.08.008. [DOI] [PubMed] [Google Scholar]
- [9].Betts MR, Koup RA. Detection of T-cell degranulation: CD107a and b. Methods Cell Biol. 2004;75:497–512. doi: 10.1016/s0091-679x(04)75020-7. [DOI] [PubMed] [Google Scholar]
- [10].Aktas E, et al. Relationship between CD107a expression and cytotoxic activity. Cell. Immunol. 2009;254(2):149–154. doi: 10.1016/j.cellimm.2008.08.007. [DOI] [PubMed] [Google Scholar]
- [11].Basiji DA, et al. Cellular image analysis and imaging by flow cytometry. Clin. Lab. Med. 2007;27(3):653–670. doi: 10.1016/j.cll.2007.05.008. (viii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Gong JH, Maki G, Klingemann HG. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia. 1994;8(4):652–658. [PubMed] [Google Scholar]
- [13].Lozzio CB, Lozzio BB. Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood. 1975;45(3):321–334. [PubMed] [Google Scholar]
- [14].Mizesko MC, et al. Defective actin accumulation impairs human natural killer cell function in patients with dedicator of cytokinesis 8 deficiency. J. Allergy Clin. Immunol. 2013;131(3):840–848. doi: 10.1016/j.jaci.2012.12.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sanborn KB, et al. Analysis of the NK cell immunological synapse. Methods Mol. Biol. 2010;612:127–148. doi: 10.1007/978-1-60761-362-6_9. [DOI] [PubMed] [Google Scholar]
- [16].Cerveira J, et al. An imaging flow cytometry-based approach to measuring the spatiotemporal calcium mobilisation in activated T cells. J. Immunol. Methods. 2015 doi: 10.1016/j.jim.2015.04.030. [DOI] [PubMed] [Google Scholar]
- [17].Kock J, et al. Nuclear factor of activated T cells regulates the expression of interleukin-4 in Th2 cells in an all-or-none fashion. J. Biol. Chem. 2014;289(39):26752–26761. doi: 10.1074/jbc.M114.587865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pietkiewicz S, Schmidt JH, Lavrik IN. Quantification of apoptosis and necroptosis at the single cell level by a combination of imaging flow cytometry with classical Annexin V/propidium iodide staining. J. Immunol. Methods. 2015 doi: 10.1016/j.jim.2015.04.025. [DOI] [PubMed] [Google Scholar]
- [19].Schmidt JH, et al. Quantification of CD95-induced apoptosis and NF-kappaB activation at the single cell level. J. Immunol. Methods. 2015 doi: 10.1016/j.jim.2015.04.026. [DOI] [PubMed] [Google Scholar]
- [20].Wabnitz GH, et al. InFlow microscopy of human leukocytes: a tool for quantitative analysis of actin rearrangements in the immune synapse. J. Immunol. Methods. 2015 doi: 10.1016/j.jim.2015.03.003. [DOI] [PubMed] [Google Scholar]
- [21].Lehmberg K, Ehl S. Diagnostic evaluation of patients with suspected haemophagocytic lymphohistiocytosis. Br. J. Haematol. 2013;160(3):275–287. doi: 10.1111/bjh.12138. [DOI] [PubMed] [Google Scholar]