Abstract
Background
The phosphodiesterase-5 inhibitor sildenafil has been shown to attenuate delayed cerebral ischemia (DCI) and improve neurologic function in experimental subarachnoid hemorrhage (SAH). We recently demonstrated that it could improve cerebral vasospasm (CVS) in humans after SAH. However, successful therapies for DCI must also restore cerebral blood flow (CBF) and/or autoregulatory capacity. In this study, we tested the effects of sildenafil on CBF in SAH patients at-risk for DCI.
Methods
Six subjects with angiographically confirmed CVS received 30-mg of intravenous sildenafil (mean 9 ± 2 days after aneurysmal SAH). Each underwent 15O-PET imaging to measure global and regional CBF at baseline and post-sildenafil.
Results
Mean arterial pressure declined by 10 mm Hg on average post-sildenafil (8 %, p = 0.01), while ICP was unchanged. There was no change in global CBF (mean 34.5 ± 7 ml/100g/min at baseline vs. 33.9 ± 8.0 ml/100g/min post-sildenafil, p = 0.84). The proportion of brain regions with low CBF (<25 ml/100g/min) was also unchanged after sildenafil infusion.
Conclusions
Infusion of sildenafil does not lead to a change in global or regional perfusion despite a significant reduction in cerebral perfusion pressure. While this could reflect the ineffectiveness of sildenafil-induced proximal vasodilatation to alter brain perfusion, it also suggests that cerebral autoregulatory function was preserved in this group. Future studies should assess whether sildenafil can restore or enhance autoregulation after SAH.
Keywords: Vasospasm, Subarachnoid hemorrhage, Cerebral blood flow, Sildenafil, Phosphodiesterase
Introduction
Delayed cerebral ischemia (DCI) following aneurysmal subarachnoid hemorrhage (SAH) is a multifactorial process linked to reductions in cerebral blood flow (CBF). DCI has been related to the development of cerebral vasospasm (CVS), cerebrovascular autoregulatory dysfunction, and cortical spreading ischemia [1]. Numerous studies in SAH have focused on CVS prevention and treatment, with the goal of improving CBF and preventing cerebral infarction. While several interventions have shown success in decreasing CVS, few have proven beneficial in improving clinical outcomes [2]. From this, it has become evident that therapies must not only target proximal CVS, but other mechanisms involved in DCI. Assessing the ability of promising interventions to improve or maintain CBF may provide a better mechanistic surrogate marker of downstream efficacy for DCI.
Phosphodiesterase-V (PDE-V), the regulatory enzyme of the endothelial nitric oxide synthase → nitric oxide → cyclic guanosine monophosphate pathway, has been identified as a potential target, as its dysfunction has been linked to DCI [3, 4]. Sildenafil, a PDE-V inhibitor, has been shown in preclinical SAH models to not only attenuate CVS but also reduce neuronal cell death, and improve neurological outcomes [5–7]. We recently published evidence that sildenafil can ameliorate angiographic CVS after SAH in humans [8]. In this exploratory proof-of-principle study, we tested the acute effects of sildenafil on CBF in SAH patients at-risk for DCI.
Methods
We enrolled patients with aneurysmal SAH and CVS, diagnosed on digital-subtraction angiography (DSA). On the day following DSA (when subjects received sildenafil as previously described) [8], patients underwent PET imaging. Two PET measurements of CBF (using injection of , as described in detail elsewhere [9]) were made to obtain stable baseline followed by infusion of 30-mg of sildenafil over 30-min. Physiologic parameters including mean arterial pressure (MAP) and intracranial pressure (ICP) were monitored throughout. Vasopressor doses (for subjects with DCI) were not titrated between PET studies. PET measurement of CBF was repeated 15-min after infusion to allow physiologic status to stabilize as much as possible (given the acute drop in MAP expected based on experience from our angiographic study).
PET images were processed to obtain global and regional CBF (gCBF and rCBF) using standard algorithms, including co-registration and alignment to reference brain atlas [10]. Baseline gCBF was calculated as the average of the two pre-sildenafil measurements and this was compared to post-sildenafil CBF. Regions-of-interest were placed in 36 predetermined locations covering the vascular territories (3 in each ACA and PCA, 6 in each MCA, 4 in vascular borderzones and 1 in each cerebellar hemisphere). rCBF was then calculated within each region. Hypoperfusion was defined as rCBF < 25 ml/100g/min. The number of regions with hypoperfusion was then compared between baseline and post-sildenafil. We also compared the proportion of brain volume with hypoperfusion by segmenting the PET images (excluding sulci, ventricles and regions of infarction or hemorrhage) into pixel-wise measurements and combining all pixels into CBF bins of width 5 ml/100g/min. The proportion of brain pixels with hypoperfusion was calculated at both time points and compared. All comparisons were made using the Wilcoxon signed-ranks test due to the non-normal nature of the small dataset. We also used the variance in our data to estimate the power afforded at alpha of 0.05 to detect a 10 % change in CBF.
Results
Six patients were studied on average 9 ± 2 days after SAH. Clinical characteristics are shown in Table 1. All were confirmed to have CVS prior to PET studies; two had clinical DCI and were being treated with pressors for induced hypertension. MAP declined from 134 ± 17 at baseline to 125 ± 14 post-sildenafil (mean change 8 %, p = 0.01). ICP, temperature, and PaCO2 were unchanged.
Table 1.
Clinical characteristics of study participants
| Variable | Frequency, n (%) |
|---|---|
| Age | 57.3 ± 17 years |
| Gender, female | 3 (50 %) |
| Race, African-American | 3 (50 %) |
| WFNS on admission: grade I–III | 3 (50 %) |
| Modified Fisher Score: 3–4 | 2 (33 %) |
| Clip/Coil Ruptured Aneurysm | 2/4 |
| Hydrocephalus | 6 (100 %) |
| Angiographic Vasospasm, moderate-severe | 5 (83 %) |
| Delayed cerebral ischemia | 2 (33 %) |
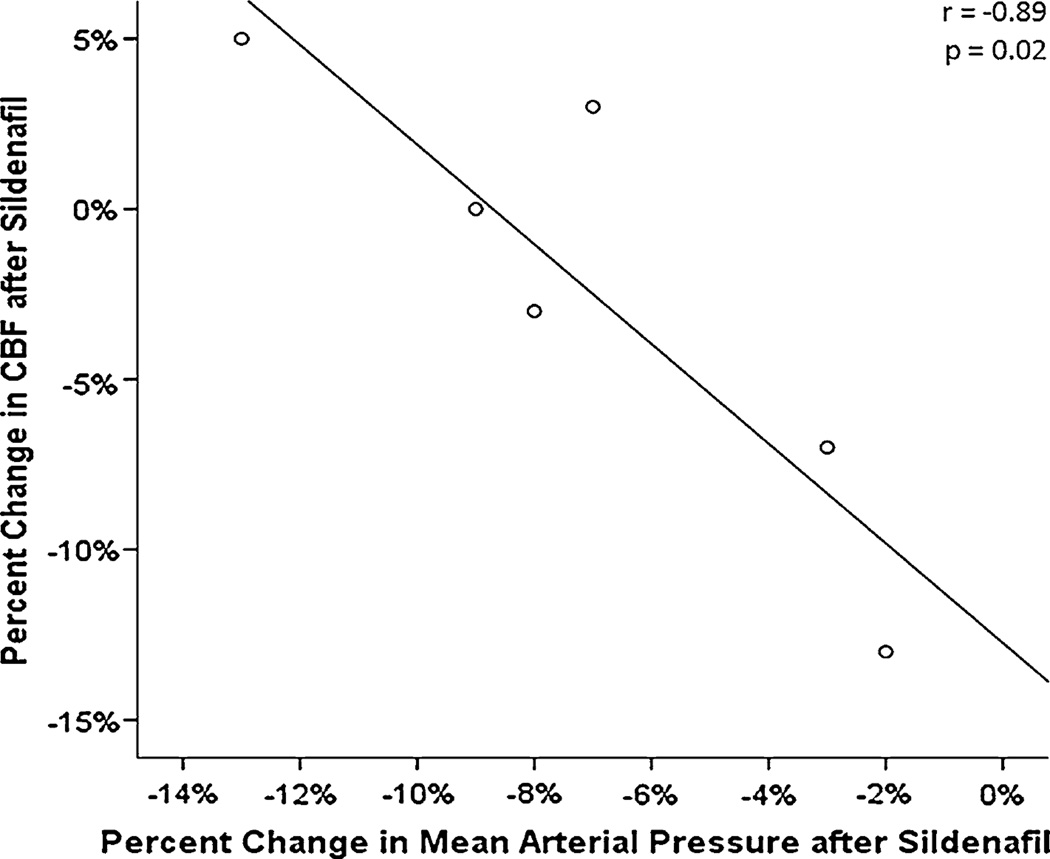
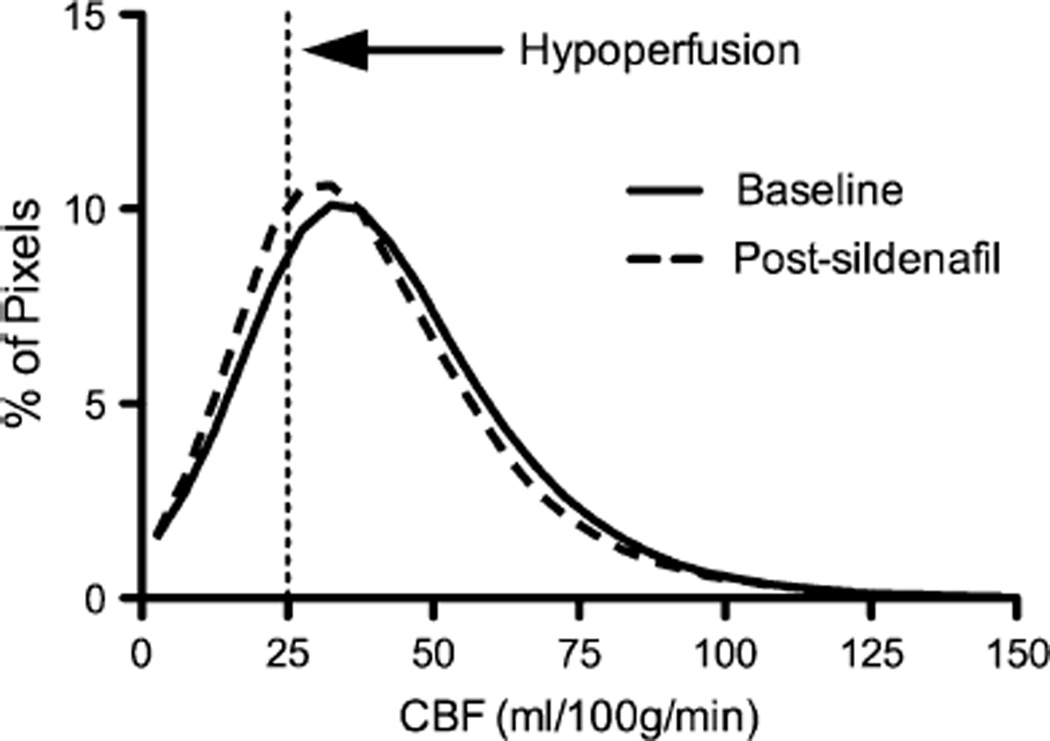
Mean gCBF was 34.5 ± 7 ml/100g/min at baseline and did not change post-sildenafil (CBF 33.9 ± 8.0 ml/100g/min, p = 0.84) (Fig. 1). Using patient data, we determined that this study was sensitive to detect or rule-out a 10 % change in CBF with a power of 91 % (at alpha of 0.05). We also noted an unexpected negative correlation between change in MAP and change in CBF with sildenafil (r = −0.89, p = 0.02) as shown in Fig. 2. That is, those subjects with a greater fall in blood pressure actually had the most stable or even improved CBF while those with lower CBF had stable not lower MAP after infusion. The number of brain regions with low rCBF was a median of 3.5 (out of 36 per patient), both before and after sildenafil. The proportion of brain volume with hypoperfusion was 22 ± 9 % at baseline and 26 ± 14 % post-sildenafil; mean difference of −4 ± 5 %, (p = 0.44) (Fig. 3).
Fig. 1.
The global CBF at baseline and post-sildenafil for each patient as well as the average for all patients
Fig. 2.
Relationship between change in mean arterial pressure after sildenafil and change in global CBF
Fig. 3.
Distribution of brain volume across CBF. There was no difference between proportion of brain volume being hypoperfused between baseline and post-sildenafil measurements
Discussion
Sildenafil has shown promise as a treatment for DCI in SAH-animal models, and more recently, we demonstrated sildenafil’s ability to acutely improve CVS in SAH patients [7, 8]. In this study, we analyzed the effects of intravenous sildenafil on CBF and found that despite inducing a significant acute reduction in MAP, global and regional CBF remained unchanged. In fact, those with a greater fall in MAP still had stable CBF. One interpretation for this neutral finding is that the degree of sildenafil-induced vasodilatation at the level of intracranial arteries (as seen in our prior angiographic study) does not translate into improved downstream perfusion, reflecting a discordance between ameliorating proximal CVS and reversing tissue hypoperfusion [11]. This could also represent the lack of effect of sildenafil on smaller parenchymal vessels that play an important role in regulating tissue perfusion or the need for a higher-dose of sildenafil to induce greater arteriolar vasodilatation.
However, a second plausible complementary hypothesis is that sildenafil may concurrently be improving dysfunctional cerebral autoregulation after SAH. Such dysfunction has been reported after SAH, especially in association with CVS [12], and may make the brain more susceptible to reductions in CBF and developing ischemia when CPP is reduced, as has been demonstrated with hypotension after nimodipine administration [13]. The stability of CBF seen in those with larger fall in MAP may indicate a strong vasodilatory response to lower CPP in these subjects (i.e., cerebrovascular autoregulation). Sildenafil has been shown to improve cerebrovascular reactivity in neurologically healthy patients [14, 15]. While this study provides only speculative evidence that a similar mechanism may be responsible for the stable CBF we observed after sildenafil, it points to the importance of further evaluating the role of autoregulation (and not solely vasodilator capacity) in studies evaluating therapies for CVS and DCI.
In summary, the findings of this preliminary study are encouraging from a safety and mechanistic perspective. We have demonstrated that sildenafil does not result in CBF being adversely affected in the setting of reduced MAP and CPP. We also suggest that sildenafil’s potential effectiveness for DCI may be driven not primarily by augmentation of blood flow but by enhancement of cerebrovascular reactivity. This study provides a basis for the design of future trials assessing sildenafil’s impact on autoregulatory dysfunction following SAH.
Acknowledgments
Funding American Heart Association (SDG3440008 to RD) and Washington University Institute of Clinical and Translational Sciences (UL1 TR000448 to GZ).
References
- 1.Macdonald RL. Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol. 2014;10:44–58. doi: 10.1038/nrneurol.2013.246. [DOI] [PubMed] [Google Scholar]
- 2.Etminan N, Vergouwen MDI, Ilodigwe D, MacDonald RL. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis. J Cereb Blood Flow Metab. 2011;31:1443–1451. doi: 10.1038/jcbfm.2011.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pluta RM. Dysfunction of nitric oxide synthases as a cause and therapeutic target in delayed cerebral vasospasm after SAH. Acta Neurochirurgica, Supplementum. 2008:139–147. doi: 10.1007/978-3-211-75718-5_28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vellimana AK, Milner E, Azad TD, et al. Endothelial nitric oxide synthase mediates endogenous protection against subarachnoid hemorrhage-induced cerebral vasospasm. Stroke. 2011;42:776–782. doi: 10.1161/STROKEAHA.110.607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atalay B, Caner H, Cekinmez M, Ozen O, Celasun B, Altinors N. Systemic administration of phosphodiesterase V inhibitor, sildenafil citrate, for attenuation of cerebral vasospasm after experimental subarachnoid hemorrhage. Neurosurgery. 2006;59:1102–1107. doi: 10.1227/01.NEU.0000245605.22817.44. [DOI] [PubMed] [Google Scholar]
- 6.Gokce C, Gulsen S, Yilmaz C, Guven G, Caner H, Altinors N. The effect of the sildenafil citrate on cerebral vasospasm and apoptosis following experimental subarachnoid hemorrhage in rats. J Neurosurg Sci. 2010;54:29–37. [PubMed] [Google Scholar]
- 7.Han BH, Vellimana AK, Zhou ML, Milner E, Zipfel GJ. Phosphodiesterase 5 inhibition attenuates cerebral vasospasm and improves functional recovery after experimental subarachnoid hemorrhage. Neurosurgery. 2012;70:178–186. doi: 10.1227/NEU.0b013e31822ec2b0. discussion 86–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Washington CW, Derdeyn CP, Dhar R, et al. A Phase I proof-of-concept and safety trial of sildenafil to treat cerebral vasospasm following subarachnoid hemorrhage. J Neurosurg. 2015;110:1–10. doi: 10.3171/2015.2.JNS142752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raichle ME, Martin WR, Herscovitch P, Mintun MA, Markham J. Brain blood flow measured with intravenous H2(15)O. II. Implementation and validation. J Nucl Med. 1983;24:790–798. [PubMed] [Google Scholar]
- 10.Dhar R, Zazulia AR, Videen TO, Zipfel GJ, Derdeyn CP, Diringer MN. Red blood cell transfusion increases cerebral oxygen delivery in anemic patients with subarachnoid hemorrhage. Stroke. 2009;40:3039–3044. doi: 10.1161/STROKEAHA.109.556159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhar R, Scalfani MT, Blackburn S, Zazulia AR, Videen T, Diringer M. Relationship between angiographic vasospasm and regional hypoperfusion in aneurysmal subarachnoid hemorrhage. Stroke. 2012;43:1788–1794. doi: 10.1161/STROKEAHA.111.646836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yundt KD, Grubb RL, Diringer MN, Powers WJ. Autoregulatory vasodilation of parenchymal vessels is impaired during cerebral vasospasm. J Cereb Blood Flow Metab. 1998;18:419–424. doi: 10.1097/00004647-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Choi HA, Ko SB, Chen H, et al. Acute effects of nimodipine on cerebral vasculature and brain metabolism in high grade subarachnoid hemorrhage patients. Neurocrit Care. 2012;16:363–367. doi: 10.1007/s12028-012-9670-8. [DOI] [PubMed] [Google Scholar]
- 14.Rosengarten B, Schermuly RT, Voswinckel R, et al. Sildenafil improves dynamic vascular function in the brain: studies in patients with pulmonary hypertension. Cerebrovasc Dis. 2006;21:194–200. doi: 10.1159/000090555. [DOI] [PubMed] [Google Scholar]
- 15.Diomedi M, Sallustio F, Rizzato B, et al. Sildenafil increases cerebrovascular reactivity: a transcranial Doppler study. Neurology. 2005;65:919–921. doi: 10.1212/01.wnl.0000173835.12308.bb. [DOI] [PubMed] [Google Scholar]