Abstract
Binge drinking during adolescence is a risk factor for neuropsychiatric disorders that can develop later in life. Histone acetylation is an important epigenetic mechanism that contributes to neurodevelopment. We investigated the effects of adolescent intermittent ethanol (AIE) exposure, as opposed to normal saline (AIS) exposure, on histone acetylation-mediated regulation of brain-derived neurotrophic factor (BDNF) expression and developmental stages of neurogenesis (proliferating and immature neurons) in the hippocampus in adulthood. AIE exposure increased whole hippocampal histone deacetylase (HDAC) activity and decreased binding protein of cyclic adenosine monophosphate response element binding protein (CBP) and histone H3-K9 acetylation levels in the CA1, CA2, and CA3 regions of the hippocampus. BDNF protein and exon IV mRNA levels in the CA1 and CA3 regions of the hippocampus of AIE exposed adult rats were decreased as compared to AIS exposed adult rats. AIE induced anxiety-like behaviors and deficits in histone H3 acetylation at BDNF exon IV promoter in the hippocampus during adulthood, which were reversed by treatment with the HDAC inhibitor, trichostatin A (TSA). Similarly, neurogenesis was inhibited by AIE in adulthood as demonstrated by the decrease in Ki-67 and doublecortin (DCX)-positive cells in the dentate gyrus, which was normalized by TSA treatment. These results indicate that AIE exposure increases HDACs and decreases CBP levels that may be associated with a decrease in histone H3 acetylation in the hippocampus. These epigenetic changes potentially decrease BDNF expression and inhibit neurogenesis in the hippocampus that may be involved in AIE-induced behavioral abnormalities, including anxiety, in adulthood.
Keywords: Adolescent Alcohol, Anxiety, Brain-derived neurotrophic factor, Hippocampus, Histone H3 acetylation, Neurogenesis
Introduction
Adolescent binge drinking is a major health concern that may interfere with emotional and neurocognitive development resulting in neuropsychological disorders later in life (DeWit et al. 2000; Medina et al. 2007; Keshvan et al. 2014). Clinical as well as preclinical studies have reported the deleterious consequences of adolescent ethanol exposure on hippocampal development and function during adulthood (Nagel et al. 2005; Medina et al. 2007; Ehlers et al. 2013; Vetreno and Crews 2015). The hippocampus is part of the emotional and cognitive brain circuitry that regulates affective states (McNaughton and Gray 2000; Packard 2009). The dentate gyrus (DG) of the hippocampus exhibits regular neurogenic activity throughout adulthood as new neurons develop from neuronal progenitor cells (NPCs) in the subgranular zone (SGZ), a neurogenic region between the granule cell layer (GCL) and the hilus (Zhao et al. 2008; Gu et al. 2013). Neurogenic activity in the hippocampus is altered by environmental experiences or exposure to commonly abused drugs, including alcohol, and altered neurogenesis has also been implicated in psychiatric disorders (Crews et al. 2004, 2006; Ieraci and Herrera 2007; Revest et al. 2009; Chambers 2013; Ehlers et al. 2013; Gu et al. 2013).
The dynamic regulation of histone acetylation by the interaction of histone deacetylases (HDACs) and histone acetyltransferases (HATs), binding protein of cyclic adenosine monophosphate response element binding (CREB) protein (CBP) and p300, represents important epigenetic processes that are involved in brain development and functions throughout life (Kalkhoven 2004; Lilja et al. 2013; Valor et al. 2013; Sheikh 2014; Swaminathan et al. 2014). Histone H3 acetylation of specific lysine residues within given gene promoters regulates transcription and contributes to alcohol-induced changes in the expression of synaptic plasticity associated genes and results in psychopathology, such as anxiety and alcohol use (Pascual et al. 2012; Moonat et al. 2013; Sakharkar et al. 2014a; Krishnan et al. 2014). Using various adult animal models, we have previously shown that alcohol exposure alters the expression of brain-derived neurotrophic factor (BDNF) and neuropeptide Y (NPY), which are regulated by histone H3-K9 acetylation via HDAC and CBP in the amygdala (Pandey et al. 2008; Sakharkar et al. 2012, 2014a; Moonat et al. 2013). Chronic ethanol treatment of adult rats also impacts BDNF expression in the hippocampus (Tapia-Arancibia et al. 2001; Hauser et al. 2011). Binge-like alcohol exposure during adolescence has been shown to decrease BDNF expression and neurogenesis in the hippocampus of adolescent rats (Briones and Woods 2013). BDNF in the hippocampus is also believed to regulate synaptic plasticity and neurogenesis, and these biological processes have been implicated in neuropsychiatric disorders (Duman 2004; Duman and Monteggia 2006; Taliaz et al. 2010; Andero et al. 2014; Schoenfeld and Cameron 2015).
Histone acetylation also regulates adult neurogenesis in preclinical models (Leone et al. 2014; Swaminathan et al. 2014; Yoo et al. 2015). Recently, we observed the ability of HDAC inhibitors, such as trichostatin A (TSA), to reverse adolescent intermittent ethanol (AIE) exposure-induced anxiety-like and alcohol drinking behaviors, as well as reverse the deficits in histone H3-K9&14 acetylation of the BDNF gene in the amygdala during adulthood (Pandey et al. 2015). However, the role of HDAC and CBP-induced histone H3 acetylation in driving long-term effects of AIE on hippocampal BDNF expression and neurogenesis in adulthood remains unexplored. Therefore, we examined the effects of AIE on HDAC activity, CBP expression, histone H3 acetylation and related BDNF expression, and developmental stages of neurogenesis (proliferating and immature neurons) in the adult hippocampus. In addition, we further examined the effects of HDAC inhibitors on anxiety-like behaviors and neurogenesis and histone H3 acetylation of the BDNF gene in the hippocampus following AIE in adulthood.
Materials and methods
Animals and adolescent intermittent ethanol (AIE) exposure
Timed-pregnant Sprague Dawley rats (Harlan Laboratories, Indianapolis, IN, USA) were maintained in a 12:12 hr light/dark cycle with food and water ad libitum. Male pups were weaned at post-natal day (PND) 21 and were group-housed in the same animal facility with ad libitum access to food and water. Adolescent male rats were intraperitoneally (IP) injected with eight doses of ethanol (2.0 g/kg, 20% w/v) or equivalent volume of normal saline during PND 28–41 on an alternating two-day on/two-day off basis (injections on PND 28, 29, 32, 33, 36, 37, 40, 41) as reported previously by our (Pandey et al. 2015) and other laboratories (Pascual et al. 2009; Alaux-Cantin et al. 2013). Adolescent intermittent ethanol (AIE) or saline (AIS) exposed rats were group housed and allowed to grow until adulthood (PND 92) without any intervention. When the animals reached adulthood, they were anesthetized with pentobarbital (50 mg/kg) and either decapitated or perfused with normal saline and 4% paraformaldehyde (Moonat et al. 2011; Sakharkar et al. 2012) for biochemical or histochemical analyses, respectively. All the animal experiments followed the NIH guidelines for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
Trichostatin A treatment of AIE and AIS exposed adult rats
A subset of AIE and AIS exposed adult rats (PND 92) were treated with TSA (AIE+TSA and AIS+TSA groups) or vehicle (AIE+ Vehicle and AIS+ Vehicle groups) for three consecutive days (PND 92, 93, 94) and were subjected to measurement of anxiety-like behavior using the light/dark box (LDB) exploration test. TSA was dissolved in DMSO to a 5.0 mg/ml concentration and further diluted (1:5) in phosphate buffered saline (PBS) to achieve the final concentration of 1.0 mg/ml (Sakharkar et al. 2012; Pandey et al. 2008, 2015). TSA (2.0 mg/kg) or vehicle [DMSO diluted in PBS (1:5)] were injected (IP) daily for three consecutive days (each injection being 24 hrs apart) and rats were subjected to LDB exploration testing 2 hrs after the last TSA or vehicle injections. Immediately after the behavioral measurements, rats were perfused as described above to collect the brains for neurogenesis studies. Another batch of rats was used for the chromatin immunoprecipitation (ChIP) assay (described below).
Light/dark box exploration test
Anxiety-like behaviors were measured using the LDB exploration test, as previously described (Pandey et al. 2008, 2015; Sakharkar et al. 2014a, b). In brief, each rat was acclimated to the procedure room for 5 min and then placed in the dark compartment of the LDB apparatus. The activity of the rat in the light and dark compartment of the apparatus was monitored by a computer program during the 5 min test session in terms of time and ambulation in each compartment equipped with infrared beams. Ambulation of each rat was measured by number of times the infrared light beam was crossed. The results of anxiety behaviors and general activity of rats were calculated in terms of mean (±SEM) percent time spent in each compartment and mean (±SEM) total ambulation, respectively.
Doublecortin and Ki-67 immunohistochemistry and image analysis
Immunohistochemical analysis was performed for the measurement of doublecortin (DCX) and Ki-67 positive cells in the hippocampal regions of TSA or vehicle-treated AIS and AIE adult rats as reported earlier (Crews et al. 2006; Ehlers et al. 2013; Broadwater et al. 2014; Vetreno and Crews 2015). Free-floating sections were washed in phosphate-buffered saline (PBS; 0.1 M), incubated in 0.3% hydrogen peroxide, and blocked with normal goat or rabbit serum (MP Biomedicals, Solon, OH). Sections were incubated in goat anti-DCX [1:200 dilution (Santa Cruz Biotechnology, Dallas, TX)] or rabbit anti-Ki-67 antibodies (Abcam, Cambridge, MA) for 24 hrs at 4°C. Sections were then washed with PBS, incubated for 1 hr in biotinylated anti-goat or anti-rabbit secondary antibodies (Vector Laboratories, Burlingame, CA), and incubated for 1 hr in avidin-biotin complex solution (Vectastain ABC Kit, Vector Laboratories). The chromogen, nickel-enhanced diaminobenzidine (Sigma-Aldrich, St. Louis, MO), was used to visualize immunoreactivity. Tissue was mounted onto slides, dehydrated, and cover slipped. Negative control for non-specific binding was conducted on separate sections using above procedures without primary antibody.
BioQuant Nova Advanced Image Analysis (R and M Biometric, Nashville, TN) was used for image capture and quantification. Representative images were captured using an Olympus BX50 microscope and Sony DXC-390 video camera linked to a computer. The dentate gyrus (DG) (Bregma −2.12 mm to −3.80 mm) in each hemisphere was circumscribed in each section for quantification of DCX and Ki-67 immunoreactivity. Since DCX is densely distributed throughout the granule cell layer (GCL) of the hippocampal DG, pixel density was used to assess DCX immunoreactivity in cells and processes as reported earlier by us (Vetreno and Crews 2015). The outlined region of interest (mm2) was determined, and staining density was calculated by dividing the overall pixel count by the overall area. Ki-67 is heterogeneously distributed throughout the subgranular zone (SGZ) of the hippocampal DG. Therefore, a modified stereological profile cell counting method was used to assess Ki-67 immunoreactive cells (Crews et al. 2004). The cell counts were calculated by dividing the overall cell count by the overall area. The regions of interest volumes across treatment groups were assessed and were not found to differ significantly between groups. The microscope, camera, and software were background corrected and normalized to preset light levels in order to ensure the fidelity of data acquisition.
Gold Immunolabeling and counting of immunogold particles
The gold-immunolabeling procedure was employed for the measurement of acetylated histone H3-K9 (H3-K9Ac), CBP, and BDNF protein levels in the hippocampus of the rat brain as described previously (Sakharkar et al. 2014a; Moonat et al. 2013; Pandey et al. 2008, 2015). Free floating, 20 µm thick coronal sections at the level of the hippocampus were immunostained using antibodies against BDNF (Santa Cruz Biotechnology, Santa Cruz, CA), CBP (Santa Cruz Biotechnology, Santa Cruz, CA), and acetylated histone H3-K9 (Millipore, Billerica, MA). After washing with PBS, the sections were incubated in gold particle-labeled goat anti-rabbit secondary antibodies (Nanoprobes, Yaphank, NY) and were counter-stained using the silver enhancement kit (Ted Pella, Redding, CA). Immunogold particles were counted using an image analysis program on a computer-assisted light microscope after adjusting the threshold to zero in a non-immunostained area in the hippocampal regions. The numbers of immunogold particles/100 µm2 area from each of three brain sections of each rat (total 9 object fields for each brain area) were measured and the values were averaged for each rat. Results are presented as mean (±SEMs) of the number of immunogold particles per 100 µm2 area of CA1, CA2, CA3, and DG.
In situ reverse transcription (RT)-PCR
In situ RT-PCR was performed in 40 µm thick coronal brain sections, as described previously (Moonat et al. 2011, 2013; Pandey et al. 2015), for the mRNA measurements of BDNF exon I and IV using the primers (BDNF exon I: Forward-5’-AGGACAGCAAAGCCACAATGTTCC-3’ and Reverse-5’-TGGACGTTTGCTTCTTTCATGGGC-3’; and BDNF exon IV: Forward-5’-TCTCACTGAAGGCGTGCGAGTATT-3’ and Reverse-5’-TGGTGGCCGATATGTACTCCTGTT-3’) and digoxigenin (DIG)-11-dUTP (Roche Diagnostics, Indianapolis, IN) instead of dTTP. Following PCR, sections were processed for immunostaining with alkaline phosphatase-conjugated anti-DIG antibody (Roche Diagnostics), and followed by staining with nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indolylphosphate (NBT/BCIP; Roche Diagnostics). The optical density (OD) of the NBT/BCIP-positive cell bodies from three object fields within hippocampal brain regions from three different coronal sections was measured. All values from three sections were averaged for each rat. The mRNA levels are presented as mean (±SEM) OD/100 pixels of CA1, CA2, CA3, and DG hippocampal area.
Hippocampal HDAC activity in AIE- and AIS-exposed adult rats
The whole hippocampal HDAC activity of AIS and AIE exposed adult rats was measured as previously described (Pandey et al. 2008; 2015; Sakharkar et al. 2012; 2014b). Cytosolic and nuclear fractions were prepared from the hippocampal tissues using a CelLytic™ NuCLEAR™ extraction Kit (Sigma, St. Louis, MO). HDAC activity assay was performed according to the manufacturer’s instructions using the colorimetric HDAC activity assay kit (BioVision Research, Mountain View, CA). The OD of the colorimetric reaction was analyzed at 405 nm using an ELISA plate reader (Spectra MR; DynexTechnologies, Chantilly, VA) and was calculated as OD/mg of protein. The results were presented as % of control.
Chromatin immunoprecipitation (ChIP) using histone H3 acetylated antibody
ChIP assay was performed to examine the level of histone H3-K9&14 acetylation (H3-K9&14Ac) of BDNF exon I and IV in the whole hippocampus of vehicle or TSA-treated AIS and AIE exposed adult rats (Moonat et al. 2013; Sakharkar et al. 2014a; Pandey et al. 2015). Hippocampal tissues were homogenized in PBS, fixed in formaldehyde for 15 min at 37°C and the fixation reaction was stopped by glycine. The tissue homogenate was re-suspended in SDS-lysis buffer for 30 min on a rotating shaker at 4°C and sonicated to shear the chromatin to yield 200–500 base-pair long DNA fragments. Equal volumes of chromatin from each sample were used for ChIP using the antibodies against H3K9&14Ac (Millipore) after removing a portion of the sample for inputs. For immunoprecipitation, chromatin was incubated overnight at 4°C along with H3-K9&14Ac antibodies and protein agarose beads (Santa Cruz Biotechnology). Following immunoprecipitation, chromatin was serially washed with low salt, high salt, lithium chloride, and TE buffers and was eluted using sodium bicarbonate elution buffer. Immunoprecipitated and input chromatin were reverse cross-linked by heating at 67oC for 2 hrs and proteinase K digestion at 37oC for 2 hrs followed by DNA cleaning using the phenol-chloroform method. The qRT-PCR was performed using the SYBR Green PCR Master Mix (Thermo Scientific, Pittsburgh, PA) using primers for promoter regions of BDNF exons I and IV (BDNF exon I: forward 5’-GCGCCCAAAGCCCACCTTCT-3’, reverse 5’-GCGTCGGCTCCGTGCTTCTT-3’ and BDNF exon IV: forward 5’-GTTCGCTAGGACTGGAAGTGG-3’, reverse 5’-CCTCTGCCTCGAAATAGACAC-3’). Fold changes in histone H3-K9&14Ac levels at BDNF exons I and IV promoters were calculated after normalizing them to input using the 2-ΔΔCT method (Schmittgen and Livak 2008; Moonat et al. 2013; Pandey et al. 2015). Results are represented as mean fold changes (±SEM) with respect to the control group (AIS exposed adult + Vehicle group).
Statistical analysis
The significance of differences between two groups was analyzed using the Student’s t-test, whereas analysis involving more than two groups was performed using the two-way analysis of variance (ANOVA). Post hoc analysis for all ANOVA comparisons was performed using the Tukey's test and p<0.05 was considered to be significant.
Results
AIE exposure causes anxiety-like behaviors in adulthood: Reversal by TSA
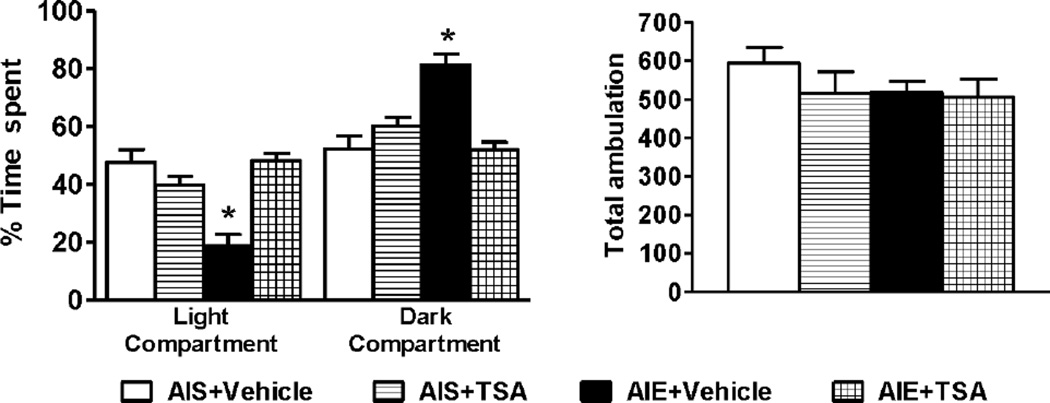
We first repeated our previous behavioral findings on anxiety measures (Pandey et al. 2015) using the LDB exploration test 53 days after the last AIE at PND 94. AIE+ Vehicle group of rats spent significantly (p<0.001) more time in the dark compartment and less time in the light compartment as compared to AIS+ Vehicle group of rats (Fig. 1), which is consistent with increased anxiety-like behaviors. TSA treatment attenuated the AIE-induced preference for the dark compartment. This is consistent with reduced anxiety-like behaviors, as suggested by the significant (p<0.001) increase in time spent in the light compartment in TSA-treated AIE adult rats, when compared to vehicle-treated AIE adult rats (Fig. 1). TSA treatment did not affect the anxiety measures of the AIS adult rats. In addition, no significant differences were observed in total ambulation among the various groups suggesting that neither AIE exposure nor TSA treatment affected general activity of rats in adulthood (Fig. 1). There were no significant differences in body weight (g) of rats (n=10, mean± SEM) among the groups (AIS+ Vehicle, 361± 5.4; AIE+ Vehicle, 351±5.8; AIS+ TSA, 362± 4.8; AIE+ TSA, 347± 8.2). These results replicate our previous findings (Pandey et al. 2015) that long lasting anxiety-like behaviors following AIE exposure in adulthood are reversible by TSA treatment.
Figure 1.
Effects of trichostatin A (TSA) on anxiety-like behaviors of adolescent intermittent ethanol (AIE) - and normal saline (AIS)-exposed adult rats, as measured by the light dark box (LDB) exploration test. The general activity measured as total ambulation was not different among the treatment groups. Values are represented as the mean (±SEM) of 10 rats per group. *Significantly different from respective control groups [p<0.001, two-way ANOVA (AIE effect, F1, 36= 8.7, p<0.01; TSA effect, F1, 36= 9.4, p<0.01; AIE × TSA interaction, F1, 36= 28.5, p<0.001) followed by Tukey’s test].
AIE exposure inhibits adult hippocampal neurogenesis: Reversal by TSA
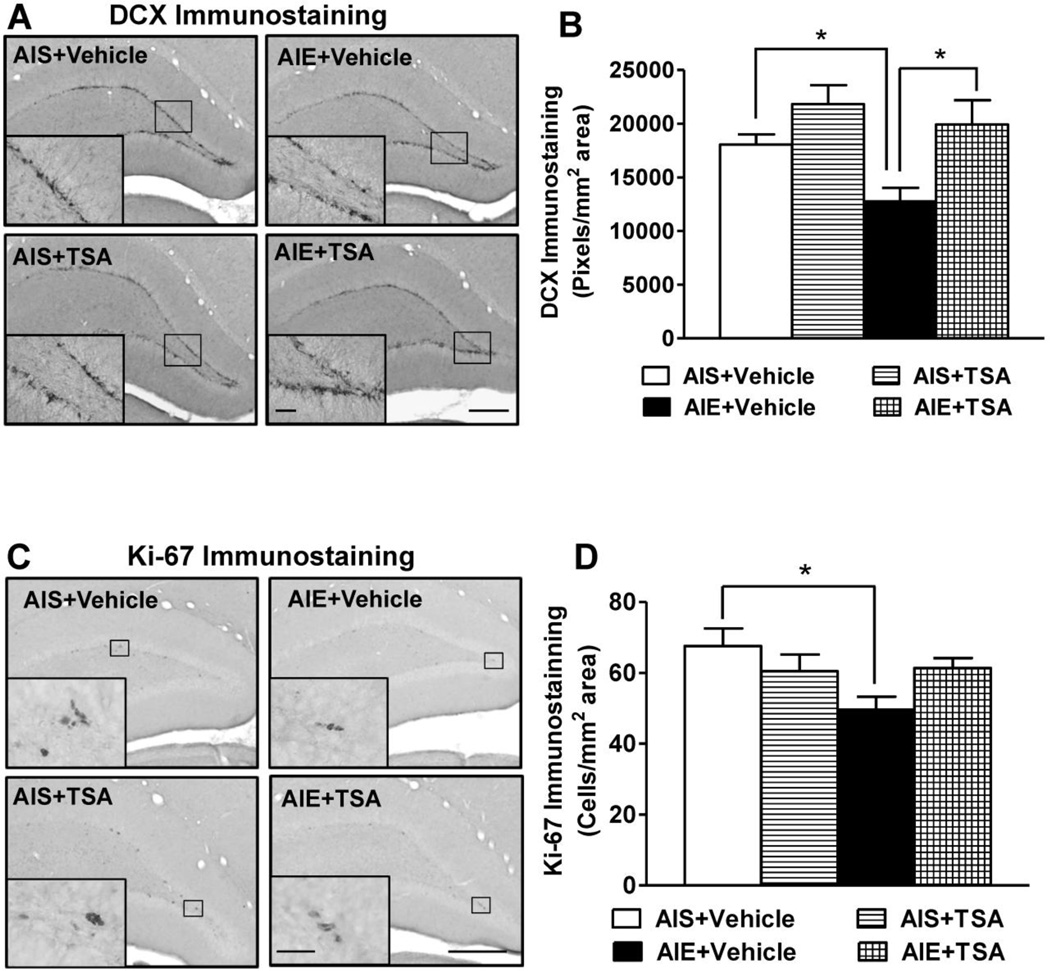
We extended the above behavioral findings to better understand the molecular basis of AIE-induced changes in hippocampal neurogenesis. A number of studies have found a persistent decrease in hippocampal neurogenesis during adulthood following AIE exposure (Ehlers et al. 2013; Broadwater et al. 2014; Vetreno and Crews 2015). However, the mechanism underlying this reduction remains to be elucidated. In the present study, we administered the HDAC inhibitor, TSA, in adulthood and assessed neurogenesis by measuring DCX immunostaining, a neuroprogenitor microtubule-associated protein expressed in immature neurons (Brown et al. 2003), and Ki-67 immunostaining, an endogenous nuclear protein expressed by dividing cells (Scholzen and Gerdes 2000). We observed that DCX immunostaining was significantly reduced (p<0.05) in AIE exposed adult rats compared to AIS exposed adult rats, which is consistent with AIE causing a long lasting decrease of neurogenesis (Fig. 2A, B). Similarly, Ki-67 immunopositive cells were significantly decreased (p<0.01) in the sub granular zone of the DG of AIE exposed adult rats (Fig. 2C, D). These reductions are consistent with AIE causing a persistent reduction in NPC proliferation that contributes to the reduced neurogenesis in adulthood. Although we did not assess NPC death in this study due to tissue limits, several previous studies have found AIE reduced neurogenesis is also associated with increased markers of NPC cell death that likely also contribute to the decrease in neurogenesis (Crews et al. 2006; Ehlers et al. 2013; Broadwater et al. 2014; Vetreno and Crews 2015). DCX immunostaining was significantly increased (p<0.01) in AIE+TSA treated rats relative to the AIE+ Vehicle group (Fig. 2A, B). TSA treatment in adulthood of AIE (AIE+TSA group) did not show significant differences in Ki-67 immunopositive cells as compared with AIS exposed adult rats treated with vehicle (AIS+ Vehicle) (Fig. 2C,D). These findings are consistent with AIE producing long lasting changes in hippocampal neurogenesis that are normalized in adulthood by TSA treatment. This data suggests that reduction in neurogenesis markers (deficits in proliferating and immature neurons) produced by AIE is likely related to deficits in histone acetylation in the hippocampus.
Figure 2.
Representative low and high magnification (inset derived from marked location on low-magnification images) photomicrographs and quantification of doublecortin (DCX) immunostaining (A,B) in granule cell layer (GCL) and Ki-67 immunostaining(C,D) in subgranular zone (SGZ) in the hippocampus of trichostatin A (TSA) or vehicle treated adolescent intermittent ethanol (AIE)- and normal saline (AIS)-exposed adult rats. Scale bar, DCX immunostaining low-magnification image, 500 µm and high magnification image, 10 µm; Ki-67 immunostaining low-magnification image, 100 µm and high magnification image, 10 µm. Values are represented as the mean (±SEM) of the pixels/mm2 area for DCX immunostaining (n=10 rats in each group) and the mean (±SEM) of the cells/mm2 area for Ki-67 immunostaining (n=10 rats in each group except in AIE+TSA group where n=9). *Significantly different from respective control groups [p<0.05–0.01, two-way ANOVA (AIE effect, F1, 36= 4.8, p<0.05; TSA effect, F1, 36= 11.1, p<0.01; AIE x TSA interaction, F1, 36= 1.1, p=0.306) followed by Tukey’s test for DCX immunostaining and p<0.01, two-way ANOVA (AIE effect, F1, 35= 4.1, p<0.05; TSA effect, F1, 35= 0.304, p=0.59; AIE x TSA interaction, F 1, 35= 5.0, p<0.05) followed by Tukey’s test for Ki-67 immunostaining].
AIE exposure decreases BDNF expression in the hippocampus during adulthood
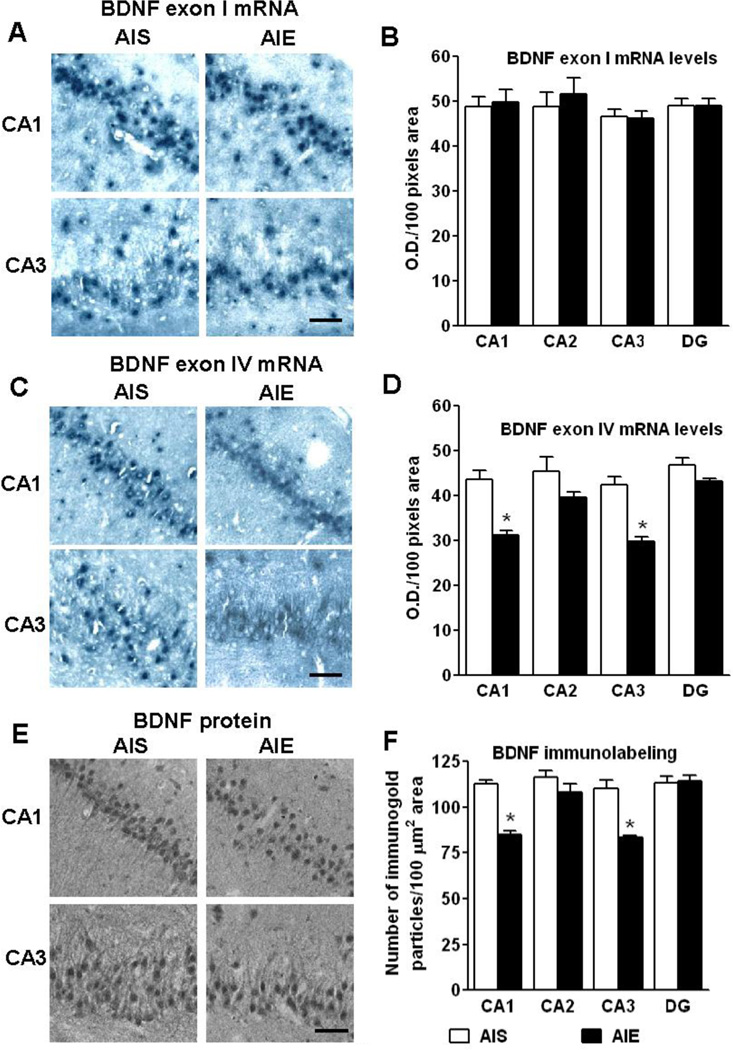
Previous studies have found that BDNF facilitates hippocampal neurogenesis and chronic ethanol treatment reduces BDNF expression during adolescence (Briones and Woods 2013). To determine if AIE exposure resulted in long-lasting changes in the hippocampal BDNF expression, we measured mRNA encoded by BDNF exons I and IV and BDNF protein levels in different hippocampal regions (e.g., CA1, CA2, CA3, and DG) of AIS- and AIE-exposed adult (PND 92) rats. Whereas BDNF exon I mRNA levels were not altered in any regions of the hippocampus by AIE exposure (Fig. 3A, B), BDNF exon IV mRNA levels were significantly (p<0.001) decreased in CA1 and CA3 regions of the hippocampus of AIE adult rats, as compared to AIS adult rats (Fig. 3C, D). In line with this, we also observed a significant (p<0.001) decrease in BDNF protein levels in the CA1 and CA3 regions of AIE exposed rats, as compared to AIS exposed rats (Fig. 3E, F). This data suggests that AIE exposure leads to a decrease in BDNF protein and BDNF exon IV but not exon I mRNA levels in the CA1 and CA3 regions of the hippocampus in adulthood (Fig. 3).
Figure 3.
Representative low-magnification photomicrographs (Scale bar = 50 µm) and quantification of brain-derived neurotrophic factor (BDNF) exon I (A, B) and IV mRNA (C, D) by in situ RT-PCR and BDNF protein (E, F) by gold immunolabeling in the hippocampal structures [CA1, CA2, CA3 and dentate gyrus (DG)] of adolescent intermittent ethanol (AIE)- and normal saline (AIS)-exposed adult rats. Values are represented as the mean ±SEM optical density (O.D.)/100 pixels area for mRNA (BDNF exon I, n=6 rats in each group; BDNF exon IV, n=5 rats in each group) and the number of immunogold particles/100 µm2 area for gold immunolabeling (n=5 rats in each group). *Significantly different from AIS exposed adult group (p<0.001, Student’s t test).
AIE exposure increases HDAC activity and decreases CBP levels in the hippocampus during adulthood
In previous studies, HDACs have been linked to regulation of BDNF expression in the amygdala (Moonat et al. 2013; Pandey et al. 2015). To determine if AIE also altered HDAC activity in the hippocampus, we measured the HDAC activity in nuclear and cytosolic fractions isolated from the whole hippocampus of AIS and AIE adult rats (Fig. 4A). AIE significantly increased nuclear HDAC activity (p<0.01) without affecting cytosolic HDAC activity in the hippocampus as compared to AIS rats in adulthood. AIE exposure significantly (p<0.001) decreased CBP protein levels in the CA1, CA2, and CA3 regions but not in the DG (Fig. 4B, C). These data reveal that AIE treatment leads to an increase in nuclear HDAC activity as well as a decrease in CBP expression in the adult hippocampus.
Figure 4.
Histone deacetylase (HDAC) activity in the cytosolic and nuclear fractions of the whole hippocampus obtained from adolescent intermittent ethanol (AIE) - and normal saline (AIS)-exposed adult rats (A). Values are represented as % of control (Mean ±SEM) of 6 rats per group. *Significantly different from AIS adult group (p<0.01, Student’s t test). Representative low-magnification photomicrographs (Scale bar = 50 um) of CREB binding protein (CBP) (B) and H3-K9 acetylated (D) gold immunolabeling as well as quantification of CBP (C) and acetylated histone H3-K9 (E) proteins in the hippocampal structures [CA1, CA2, CA3 and dentate gyrus (DG)] of AIS and AIE exposed adult rats. Values are represented as the mean of the number of immunogold particles/100 µm2 area (±SEM) and derived from 6 rats per group. *Significantly different from AIS exposed adult rats (p<0.001, Student’s t test).CREB, cyclic adenosine monophosphate response element binding protein.
AIE exposure decreases histone H3 acetylation in the hippocampus: Reversal by TSA
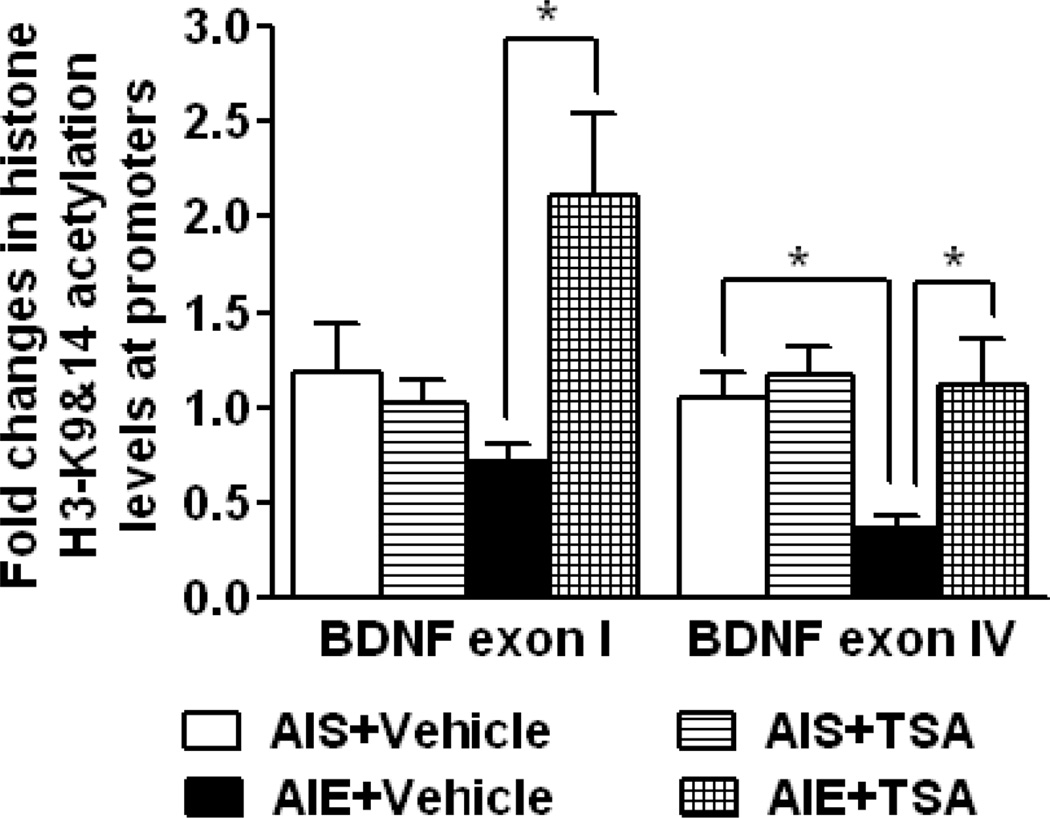
In order to determine if AIE alters hippocampal histone acetylation, we used gold immunolabeling to examine the global acetylated H3-K9 protein levels in CA1, CA2, CA3, and DG regions of the hippocampus of AIS and AIE exposed adult rats (Fig. 4D, E). AIE exposure significantly (p<0.001) decreased the H3-K9Ac protein levels in the CA1, CA2, and CA3 regions, but not in the DG (Fig. 4D, E). We further examined gene-specific histone acetylation (H3-K9&14) in the hippocampus of AIS- and AIE-exposed adult rats using ChIP assay (Fig. 5). The levels of H3-K9&14Ac were not significantly altered at the BDNF exon I promoter in the hippocampus of AIE adult rats, as compared to AIS adult rats. However, AIE caused a marked reduction in H3-K9&14Ac levels (p<0.01) in the BDNF exon IV promoter in the hippocampus (Fig. 5). It should be noted (as described above) that, AIE exposure did not alter BDNF exon I mRNA levels in the hippocampus (Fig. 3A, B), but it did decrease BDNF exon IV mRNA levels in the CA1 and CA3 regions of the hippocampus (Fig. 3C, D) with concomitant down-regulation of BDNF protein levels in the CA1 and CA3 regions (Fig. 3E, F). These findings indicate that AIE causes long lasting decreases in hippocampal BDNF likely due to reduced histone H3 acetylation at the BDNF IV exon promoter.
Figure 5.
Effect of trichostatin A (TSA) treatment on acetylated histone H3-K9&14 levels in the promoters of brain-derived neurotrophic factor (BDNF) exon I and IV in the whole hippocampus of adolescent intermittent ethanol (AIE) - and normal saline (AIS)-exposed adult rats as measured by the chromatin immunoprecipitation (ChIP) assay. Values are represented as the mean fold changes (±SEM) of 6 rats per group except in AIS+TSA group where 7 rats were used. *Significantly different from respective control groups [p<0.001, two-way ANOVA (AIE effect, F1,21= 1.5, p=0.24; TSA effect, F1,21= 6.1, p<0.05; AIE x TSA interaction, F1,21= 9.7, p<0.01) followed by Tukey’s test for BDNF exon I and p<0.01, two-way ANOVA (AIE effect, F1,21= 5.2, p<0.05; TSA effect, F1,21= 7.2, p<0.05; AIE x TSA interaction, F1,21= 3.9, p=0.06) followed by Tukey’s test for BDNF exon IV].
We also examined the effect of TSA on histone H3 acetylation in the promoter regions of BDNF exons I and IV in the whole hippocampus of AIS and AIE exposed adult rats (Fig. 5). TSA treatment increased H3-K9&14Ac levels at BDNF exon I (p<0.001) and exon IV (p<0.01) promoters in the hippocampus of AIE adult rats (AIE+TSA group) as compared to vehicle treated AIE adult rats (AIE+ Vehicle group). However, TSA treatment did not alter the histone acetylation status in these promoters in the hippocampus of AIS adult rats (Fig. 5). These results suggest that AIE decreases histone H3-K9&14 acetylation of BDNF exon IV that is normalized by TSA treatment in adulthood.
Discussion
This study contributes to our understanding of the persistent effects of adolescent alcohol exposure on epigenetic mechanisms and neurogenesis as measured by DCX (immature neurons) and Ki-67 (proliferating NPC) immunostaining in the hippocampus in adulthood. We report here that AIE causes long lasting decreases in BDNF exon IV-specific histone H3 acetylation and BDNF expression as well as decreases in neurogenesis markers in the hippocampus during adulthood. AIE increases nuclear HDAC activity and decreases CBP that, in turn, decreases histone H3-K9Ac levels in the hippocampus during adulthood. The long lasting AIE-induced changes in adulthood appear to be linked to histone H3 acetylation mechanisms, as inhibition of HDAC by TSA normalizes deficits in histone H3 acetylation at the BDNF exon IV promoter, and DCX and Ki-67 immunostaining in the hippocampus. As observed earlier (Pandey et al. 2015), AIE-induced anxiety-like behaviors in adulthood were rescued by TSA treatment. Clinical studies have indicated that alcohol drinking during adolescence is a risk factor for the development of psychiatric disorders including alcohol abuse later in life (Grant and Dawson 1998; DeWit et al. 2000; Donovan 2004). Recent preclinical studies also found that binge-like alcohol exposure during adolescence causes anxiety and depression-like behaviors (Briones and Woods 2013; Pandey et al. 2015), and increased alcohol intake in adulthood (Pascual et al. 2009; Alaux-Cantin et al. 2013; Pandey et al. 2015). To our knowledge, the present study is the first to implicate histone H3 acetylation as a possible epigenetic mechanism by which binge-like ethanol exposure can decrease BDNF expression and neurogenesis in the hippocampus and possibly contributes to risks and/or symptoms of adult psychopathology.
Although the specific role of hippocampal neurogenesis in brain function is not clear, both human and animal studies suggest that formation of new neurons in the adult brain contribute to healthy brain function (Gu et al. 2013; Schoenfeld and Cameron 2015). Previous studies have reported that adolescents are more sensitive to acute ethanol-induced inhibition of neurogenesis than adults (Crews et al. 2006) and that adult binge ethanol treatment reduces neurogenesis during intoxication with ethanol, which recovers during abstinence (Crews and Nixon 2009). We report here that AIE decreases Ki-67 positive cells in the adult DG, which is consistent with decreased NPC proliferation, and reduces immature neurons as measured by DCX immunostaining. Multiple studies have found that AIE exposure reduces neurogenesis markers regardless of animal strain [e.g., Sprague-Dawley (present study) or Wistar rats (Ehlers 2013; Vetreno and Crews 2015)] and ethanol exposure paradigm [e.g., intragastric (Crews et al. 2006; Broadwater et al. 2014), vapor (Ehlers et al. 2013) or voluntary self-administration (Briones and Woods 2013)]. Furthermore, studies directly comparing identical intermittent adolescent and adult treatments find that ethanol inhibition of adolescent neurogenesis persists during abstinence into adulthood, while ethanol inhibition of neurogenesis following adult exposure recovers after an equal period of abstinence (Broadwater et al. 2014). In our previous studies we consistently found decreases in Ki-67 and DCX immunostaining and increased markers of cell death (Broadwater et al. 2014; Crews et al. 2006; Ehlers et al. 2013; Vetreno and Crews 2015) which together support the interpretation that AIE reduces neurogenesis through reduced proliferation and increased death of NPCs during maturation. However, the molecular mechanisms by which AIE attenuate adult neurogenesis are currently less clear. One possible explanation for the long lasting decrease in neurogenesis following AIE is the persistent reduction in BDNF levels in the hippocampus. BDNF contributes to synaptic plasticity as well as NPC survival and differentiation (Duman and Monteggia 2006; Alme et al. 2007). BDNF has been consistently shown to regulate adult hippocampal neurogenesis and to contribute to the efficacy of therapeutic drugs that increase neurogenesis and reverse psychopathology (Duman 2004; Duman and Monteggia 2006; Kozisek et al. 2008). Here, we found that AIE reduced adult neurogenesis markers in the DG and BDNF expression in the hippocampus in CA1 and CA3, but not CA2 and DG. Studies have found that CA3 has reciprocal connections with the DG (Scharfman 2007) and lesions of CA3 lead to long lasting decreases in neurogenesis due to disrupted maturation of NPC to mature granule cells (Liu et al. 2011). Lesioning of CA3 also blocks the fluoxetine-induced increase in neurogenesis despite the fact that fluoxetine increases BDNF in the DG (Liu et al. 2011). It has been shown that binge-like adolescent self-administered ethanol exposure reduced hippocampal neurogenesis and BDNF expression in late adolescence (7 days after ethanol exposure). Furthermore, treatment with a BDNF-like agonist acting at the TrkB receptor restored neurogenesis (Briones and Woods 2013), suggesting the role of BDNF in hippocampal neurogenesis. Although the lack of change in histone H3-K9 acetylation, CBP, and BDNF expression in the DG by AIE in adulthood is one of the limitations of the study, nonetheless these findings suggest the possibility that a reduction in BDNF expression in CA3 due to decreases in global and BDNF gene-specific histone acetylation after AIE may be associated with the inhibition of adult hippocampal neurogenesis.
As discussed above, many studies implicate BDNF in the regulation of neurogenesis and other studies have found that reduced neurogenesis may be related to increased neuroimmune gene expression in response to stress (Iwata et al. 2013) or alcohol exposure (Zou and Crews 2012; Vetreno and Crews 2015). In particular, it has been shown that AIE exposure produced increases in neuroimmune gene expression, including toll-like receptor 4 (TLR4) that persisted into adulthood (Crews and Vetreno 2014; Vetreno and Crews 2015). Previous studies have suggested a reciprocal relationship between cAMP response-element binding (CREB) protein transcriptional activation of BDNF with NF-κB-induced activation of proinflammatory gene expression (Pandey et al. 2005). Microglia cells are associated with NF-κB proinflammatory responses, but they also produce and respond to BDNF (Parkhurst et al. 2013). Interestingly, NF-κB transcription factor activation in glia is linked to proinflammatory gene induction, also shown to reduce neurogenesis (Crews and Vetreno 2014). This is further supported by a recent report that treatment of adult rats (PND70) with lipopolysaccharide, a gram-negative endotoxin agonist to TLR4, produces a reduction in DCX immunostaining in the hippocampus (Vetreno and Crews 2015). Regardless, increased BDNF could overcome AIE-induced neuroimmune suppression of neurogenesis, and our findings reveal that epigenetic suppression of BDNF persists into adulthood following AIE and possibly contributes to reduced DCX immunostaining that is reversible by TSA treatment.
The regulation of BDNF expression via epigenetic mechanisms, such as histone acetylation and DNA methylation under different experimental paradigms including drug addiction, has been implicated in the regulation of synaptic plasticity (Tsankova et al. 2004, 2006; Kumar et al. 2005; Newton and Duman 2006; Lubin et al. 2008; Renthal and Nestler 2008; Roth et al. 2009; Fuchikami et al. 2010). Previous studies have shown the differential regulation of BDNF exons by histone acetylation in the hippocampus (Lubin et al. 2008; Fuchikami et al. 2010). We observed down-regulation of histone H3 acetylation at the BDNF exon IV promoter in the hippocampus of adult rats following AIE, with a concordant decrease in BDNF exon IV expression and BDNF protein levels. Similarly, a single bout of immobilization stress decreases histone H3 acetylation and mRNA expression of both exons I and IV in the rat hippocampus, suggesting that stress impacts BDNF expression via histone H3 acetylation (Fuchikami et al. 2010). Reciprocally, increases in histone H3 acetylation at the BDNF exon IV promoter following fear memory consolidation were paralleled by an increase in BDNF exon IV expression (Lubin et al. 2008). Furthermore, we observed that AIE-induced deficits in histone H3 acetylation at BDNF exon IV were rescued after TSA treatment. Therefore, increased HDAC activity in adulthood following AIE seems to be regulating BDNF exon IV promoter activity via histone H3 acetylation and thereby its expression in the hippocampus. In line with this, innately high HDAC activity and HDAC2 expression and deficits in histone H3 acetylation was observed at the BDNF exon IV and NPY promoter in the amygdala of alcohol preferring (P) rats, as compared to non-preferring (NP) rats leading to lowered expression, which was reversed after specific knockdown of the HDAC2 isoform in the CeA or TSA treatment (Moonat et al. 2013; Sakharkar et al. 2014a). These findings implicate the role of HDAC-mediated histone H3 acetylation in the regulation of BDNF expression in relation to alcoholism and anxiety-like behaviors. As discussed earlier, BDNF is a likely regulator of adult neurogenesis in the hippocampus in response to AIE exposure. We have further observed that TSA is able to normalize AIE-induced deficits in adult hippocampal neurogenesis (proliferating and immature neurons). Several HDAC inhibitors, including TSA have been shown to promote neuronal differentiation from multipotent adult neural progenitor cells via increase in histone H3 acetylation (Hsieh et al. 2004). It may be speculated that the normalization of deficits in histone H3 acetylation of the BDNF gene by TSA treatment may be involved in restoring AIE-induced inhibition of hippocampal neurogenesis. AIE also produced deficits in global and BDNF gene-specific histone H3 acetylation in the amygdala and induced anxiety-like and alcohol drinking behaviors in adulthood (Pandey et al. 2015). Interestingly, the deficits in histone H3 acetylation of BDNF exon I and IV in the amygdala and behavioral phenotypes of AIE were normalized by TSA treatment in adulthood (Pandey et al. 2015). After combining this data with the present data on AIE-induced inhibition of neurogenesis and deficits in histone H3 acetylation and BDNF expression, it is evident that the HDAC-induced histone modifications produced by AIE in emotional and cognitive circuitry may be responsible for AIE-induced adult psychopathology. Furthermore, we found that CBP levels also decreased in the hippocampal structures, which correlated with reductions in histone H3-K9 acetylation, and BDNF expression after AIE in adulthood. CBP has been shown to regulate hippocampal neurogenesis and histone acetylation and modulate long-term memory (Barrett et al. 2011; Chatterjee et al. 2013). It is possible that AIE-induced reductions in CBP and increases in HDAC activity may be responsible for reductions in histone H3 acetylation and BDNF expression thereby inhibiting neurogenesis in adulthood. The direct role of CBP and specific HDAC isoforms, along with histone H3 acetylation, in the regulation of AIE-induced changes in hippocampal neurogenesis and associated anxiety and alcohol intake (Pandey et al. 2015) needs to be investigated in future studies.
In conclusion, ethanol exposure in early life can change histone H3 acetylation at BDNF gene promoters to decrease its function in the hippocampus, likely affecting adult neurogenesis, which might be implicated in anxiety-like behaviors during adulthood. Furthermore, HDAC inhibition is able to reverse the deficits in histone H3 acetylation-regulated molecular events and anxiety phenotypes. In addition, we reported earlier that TSA treatment in adulthood also attenuated AIE-induced alcohol intake and corrected the deficits in histone H3 acetylation of BDNF gene in the amygdala (Pandey et al. 2015). Therefore, the present and our previous studies (Pandey et al. 2015) together pinpoint the role of histone H3 acetylation/deacetylation mechanisms in AIE-induced deficits in BDNF expression and neurogenesis as well as behavioral phenotypes of anxiety and alcohol intake in adulthood.
Acknowledgments
This work was supported by the grants from National Institute on Alcohol Abuse and Alcoholism [Neurobiology of Adolescent Drinking in Adulthood (NADIA, AA-019971 and U24AA-024605), AA-010005, AA-013341, and P50 AA-022538], and by the Department of Veterans Affairs (Merit Review Grant, I01BX000143; Senior Research Career Scientist award) to SCP and AA020023 (NADIA project), AA020024, and AA020022 to FTC. DMK was supported by Raman post-doctoral research fellowship from the University Grant Commission, New Delhi, India.
SCP reports that a US patent application entitled “Histone acetyl transferase activators and histone deacetylase inhibitors in the treatment of alcoholism” (serial number 60/848237 filed on September 29th, 2006) is currently pending. No biomedical financial interests or potential conflicts of interest were reported by other authors.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Alme MN, Wibrand K, Dagestad G, Bramham CR. Chronic fluoxetine treatment induces brain region-specific upregulation of genes associated with BDNF-induced long-term potentiation. Neural Plast. 2007;2007:26496. doi: 10.1155/2007/26496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Choi DC, Ressler KJ. BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Prog Mol Biol Transl Sci. 2014;122:169–192. doi: 10.1016/B978-0-12-420170-5.00006-4. [DOI] [PubMed] [Google Scholar]
- Barrett RM, Malvaez M, Kramar E, Matheos DP, Arrizon A, Cabrera SM, Lynch G, Greene RW, Wood MA. Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharmacology. 2011;36:1545–1556. doi: 10.1038/npp.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briones TL, Woods J. Chronic binge-like alcohol consumption in adolescence causes depression-like symptoms possibly mediated by the effects of BDNF on neurogenesis. Neuroscience. 2013;254:324–334. doi: 10.1016/j.neuroscience.2013.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadwater MA, Liu W, Crews FT, Spear LP. Persistent loss of hippocampal neurogenesis and increased cell death following adolescent, but not adult, chronic ethanol exposure. Dev Neurosci. 2014;36:297–305. doi: 10.1159/000362874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neuro. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Chambers RA. Adult hippocampal neurogenesis in the pathogenesis of addiction and dual diagnosis disorders. Drug Alcohol Depend. 2013;130:1–12. doi: 10.1016/j.drugalcdep.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Mizar P, Cassel R, Neidl R, Selvi BR, Mohankrishna DV, Vedamurthy BM, Schneider A, Bousiges O, Mathis C, Cassel JC, Eswaramoorthy M, Kundu TK, Boutillier AL. A novel activator of CBP/p300 acetyltransferases promotes neurogenesis and extends memory duration in adult mice. J Neuroscience. 2013;33:10698–10712. doi: 10.1523/JNEUROSCI.5772-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33:63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Vetreno RP. Neuroimmune basis of alcoholic brain damage. Int Rev Neurobiol. 2014;118:315–357. doi: 10.1016/B978-0-12-801284-0.00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Donovan JE. Adolescent alcohol initiation: a review of psychosocial risk factors. J Adolesc Health. 2004;35 doi: 10.1016/j.jadohealth.2004.02.003. 529.e7–18. [DOI] [PubMed] [Google Scholar]
- Duman RS. Role of neurotrophic factors in the etiology and treatment of mood disorders. Neuromolecular Med. 2004;5:11–25. doi: 10.1385/NMM:5:1:011. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Liu W, Wills DN, Crews FT. Periadolescent ethanol vapor exposure persistently reduces measures of hippocampal neurogenesis that are associated with behavioral outcomes in adulthood. Neuroscience. 2013;244:1–15. doi: 10.1016/j.neuroscience.2013.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchikami M, Yamamoto S, Morinobu S, Takei S, Yamawaki S. Epigenetic regulation of BDNF gene in response to stress. Psychiatry Investig. 2010;7:251–256. doi: 10.4306/pi.2010.7.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Janoschka S, Ge S. Neurogenesis and hippocampal plasticity in adult brain. Curr Top Behav Neurosci. 2013;15:31–48. doi: 10.1007/7854_2012_217. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Taylor RE, Tizabi Y. Alcohol induced depressive-like behavior is associated with a reduction in hippocampal BDNF. Pharmacol Biochem Behav. 2011;100:253–258. doi: 10.1016/j.pbb.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH. Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci USA. 2004;101:16659–16664. doi: 10.1073/pnas.0407643101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Single alcohol exposure in early life damages hippocampal stem/progenitor cells and reduces adult neurogenesis. Neurobiol Dis. 2007;26:597–605. doi: 10.1016/j.nbd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105–114. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkhoven E. CBP and p300: HATs for different occasions. Biochem Pharmacol. 2004;68:1145–1155. doi: 10.1016/j.bcp.2004.03.045. [DOI] [PubMed] [Google Scholar]
- Keshvan MS, Giedd J, Lau JY, Lweis DA, Paus T. Changes in the adolescent brain and the pathophysiology of psychotic disorders. Lancet Psychiatry. 2014;1:549–558. doi: 10.1016/S2215-0366(14)00081-9. [DOI] [PubMed] [Google Scholar]
- Kozisek ME, Middlemas D, Bylund DB. Brain-derived neurotrophic factor and its receptor tropomyosin-related kinase B in the mechanism of action of antidepressant therapies. Pharmacol Ther. 2008;117:30–51. doi: 10.1016/j.pharmthera.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Krishnan HR, Sakharkar AJ, Teppen TL, Berkel TD, Pandey SC. The epigenetic landscape of alcoholism. Int Rev Neurobiol. 2014;115:75–116. doi: 10.1016/B978-0-12-801311-3.00003-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Choi KH, Renthal W, Tsankova NM, Theobald DE, Truong HT, Russo SJ, Laplant Q, Sasaki TS, Whistler KN, Neve RL, Self DW, Nestler EJ. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Leone L, Fusco S, Mastrodonato A, Piacentini R, Barbati SA, Zaffina S, Pani G, Podda MV, Grassi C. Epigenetic modulation of adult hippocampal neurogenesis by extremely low-frequency electromagnetic fields. Mol Neurobiol. 2014;49:1472–1486. doi: 10.1007/s12035-014-8650-8. [DOI] [PubMed] [Google Scholar]
- Lilja T, Heldring N, Hermanson O. Like a rolling histone: epigenetic regulation of neural stem cells and brain development by factors controlling histone acetylation and methylation. Biochim Biophys Acta. 2013;1830:2354–2360. doi: 10.1016/j.bbagen.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Liu JX, Pinnock SB, Herbert J. Novel control by the CA3 region of the hippocampus on neurogenesis in the dentate gyrus of the adult rat. PLoS One. 2011;6:e17562. doi: 10.1371/journal.pone.0017562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Roth TL, Sweatt JD. Epigenetic regulation of BDNF gene transcription in the consolidation of fear memory. J Neurosci. 2008;28:10576–10586. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton N, Gray JA. Anxiolytic action on the behavioural system implies types of arousal contribute to anxiety. J Affect Disord. 2000;61:161–176. doi: 10.1016/s0165-0327(00)00344-x. [DOI] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Pandey SC. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2011;16:238–250. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Tang L, Pandey SC. Aberrant histone deacetylase2-mediated histone modifications and synaptic plasticity in the amygdala predisposes to anxiety and alcoholism. Biol Psychiatry. 2013;73:763–773. doi: 10.1016/j.biopsych.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, Duman RS. Chromatin remodeling: a novel mechanism of psychotropic drug action. Mol Pharmacol. 2006;70:440–443. doi: 10.1124/mol.106.027078. [DOI] [PubMed] [Google Scholar]
- Packard M. Anxiety, cognition, and habit: a multiple memory systems perspective. Brain Res. 2009;1293:121–128. doi: 10.1016/j.brainres.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Chartoff EH, Carlezon WA, Jr, Zou J, Zhang H, Kreibich AS, Blendy JA, Crews FT. CREB gene transcription factors: role in molecular mechanisms of alcohol and drug addiction. Alcohol Clin Exp Res. 2005;29:176–184. doi: 10.1097/01.alc.0000153550.31168.1d. [DOI] [PubMed] [Google Scholar]
- Pandey SC, Ugale R, Zhang H, Tang L, Prakash A. Brain chromatin remodeling: a novel mechanism of alcoholism. J Neurosci. 2008;28:3729–3737. doi: 10.1523/JNEUROSCI.5731-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey SC, Sakharkar AJ, Tang L, Zhang H. Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol Dis. 2015;82:607–619. doi: 10.1016/j.nbd.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Do Couto BR, Alfonso-Loeches S, Aguilar MA, Rodriguez-Arias M, Guerri C. Changes in histone acetylation in the prefrontal cortex of ethanol-exposed adolescent rats are associated with ethanol-induced place conditioning. Neuropharmacology. 2012;62:2309–2319. doi: 10.1016/j.neuropharm.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med. 2008;14:341–350. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Lubin FD, Funk AJ, Sweatt JD. Lasting epigenetic influence of early-life adversity on the BDNF gene. Biol Psychiatry. 2009;65:760–769. doi: 10.1016/j.biopsych.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Baxstrom K, Shi G, Moonat S, Pandey SC. Effects of histone deacetylase inhibitors on amygdaloid histone acetylation and neuropeptide Y expression: a role in anxiety-like and alcohol-drinking behaviours. Int J Neuropsychopharmacol. 2014a;17:1207–1220. doi: 10.1017/S1461145714000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Tang L, Zhang H, Chen Y, Grayson DR, Pandey SC. Effects of acute ethanol exposure on anxiety measures and epigenetic modifiers in the extended amygdala of adolescent rats. Int J Neuropsychopharmacol. 2014b;17:2057–2067. doi: 10.1017/S1461145714001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakharkar AJ, Zhang H, Tang L, Shi G, Pandey SC. Histone deacetylases (HDAC)-induced histone modifications in the amygdala: a role in rapid tolerance to the anxiolytic effects of ethanol. Alcohol Clin Exp Res. 2012;36:61–71. doi: 10.1111/j.1530-0277.2011.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. The CA3 "backprojection" to the dentate gyrus. Prog Brain Res. 2007;163:627–637. doi: 10.1016/S0079-6123(07)63034-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, Cameron HA. Adult neurogenesis and mental illness. Neuropsychopharmacology. 2015;40:113–128. doi: 10.1038/npp.2014.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sheikh BN. Crafting the brain - role of histone acetyltransferases in neural development and disease. Cell Tissue Res. 2014;356:553–573. doi: 10.1007/s00441-014-1835-7. [DOI] [PubMed] [Google Scholar]
- Swaminathan A, Kumar M, HalderSinha S, Schneider-Anthony A, Boutillier AL, Kundu TK. Modulation of neurogenesis by targeting epigenetic enzymes using small molecules: an overview. ACS Chem Neurosci. 2014;5:1164–1177. doi: 10.1021/cn500117a. [DOI] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2010;15:80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-Arancibia L, Rage F, Givalois L, Dingeon P, Arancibia S, Beaugé F. Effects of alcohol on brain-derived neurotrophic factor mRNA expression in discrete regions of the rat hippocampus and hypothalamus. J Neurosci Res. 2001;63:200–208. doi: 10.1002/1097-4547(20010115)63:2<200::AID-JNR1012>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Kumar A, Nestler EJ. Histone modifications at gene promoter regions in rat hippocampus after acute and chronic electroconvulsive seizures. J Neurosci. 2004;24:5603–5610. doi: 10.1523/JNEUROSCI.0589-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Valor LM, Viosca J, Lopez-Atalaya JP, Barco A. Lysine acetyltransferases CBP and p300 as therapeutic targets in cognitive and neurodegenerative disorders. Curr Pharm Des. 2013;19:5051–5064. doi: 10.2174/13816128113199990382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetreno RP, Crews FT. Binge ethanol exposure during adolescence leads to a persistent loss of neurogenesis in the dorsal and ventral hippocampus that is associated with impaired adult cognitive functioning. Front Neurosci. 2015;9:35. doi: 10.3389/fnins.2015.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo DY, Kim DW, Kim MJ, Choi JH, Jung HY, Nam SM, Kim JW, Yoon YS, Choi SY, Hwang IK. Sodium butyrate, a histone deacetylase Inhibitor, ameliorates SIRT2-induced memory impairment, reduction of cell proliferation, and neuroblast differentiation in the dentate gyrus. Neurol Res. 2015;37:69–76. doi: 10.1179/1743132814Y.0000000416. [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Zou J, Crews FT. Inflammasome-IL-1β signaling mediates ethanol inhibition of hippocampal neurogenesis. Front Neurosci. 2012;6:77. doi: 10.3389/fnins.2012.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]