Abstract
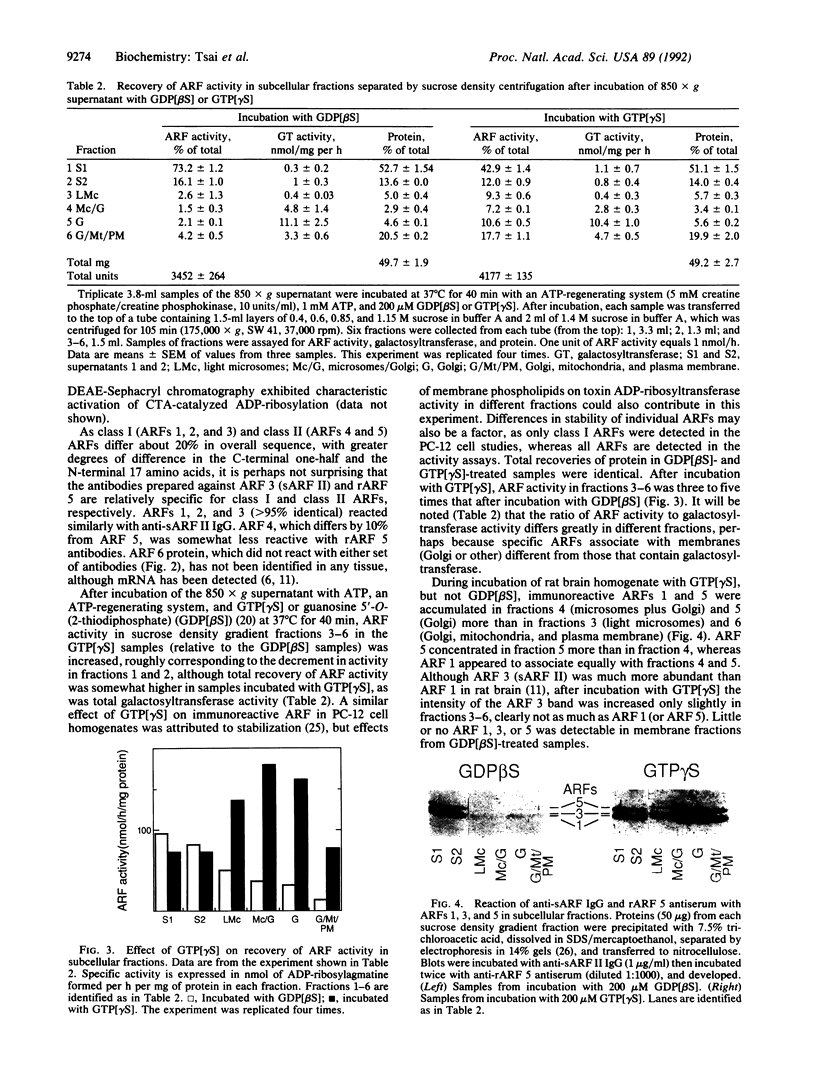
Six mammalian ADP-ribosylation factors (ARFs) identified by cDNA cloning were expressed as recombinant proteins (rARFs) that stimulated cholera toxin ADP-ribosyltransferase activity. Microsequencing of soluble ARFs I and II (sARFs I and II), purified from bovine brain, established that they are ARFs 1 and 3, respectively. Rabbit antibodies (IgG) against sARF II reacted similarly with ARFs 1, 2, and 3 (class I) on Western blots. ARFs 1 and 3 were distinguished by their electrophoretic mobilities. Antiserum against rARF 5 cross-reacted partially with rARF 4 but not detectably with rARF 6 and minimally with class I ARFs. Guanosine 5'-O-(3-thiotriphosphate) (GTP[gamma S]) increased recovery of ARF activity and immunoreactivity in organelle fractions separated by density gradient centrifugation, after incubation of rat brain homogenate with ATP and a regenerating system. ARF 1 accumulated in microsomes plus Golgi and Golgi fractions, whereas ARF 5 seemed to localize more specifically in Golgi; the smaller increment in ARF 3 was distributed more evenly among fractions. On incubation of Golgi with a crude ARF fraction, GTP[gamma S], and an ATP-regenerating system, association of ARF activity with Golgi increased with increasing ATP concentration paralleled by increases in immunoreactive ARFs 1 and 5 and, to a lesser degree, ARF 3. Golgi incubated with GTP[gamma S] and purified ARF 1 or 3 bound more ARF 1 than ARF 3. Based on immunoreactivity and assay of ARF activity, individual ARFs 1, 3, and 5 appeared to behave independently and selectively in their GTP-dependent association with Golgi in vitro.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bobak D. A., Nightingale M. S., Murtagh J. J., Price S. R., Moss J., Vaughan M. Molecular cloning, characterization, and expression of human ADP-ribosylation factors: two guanine nucleotide-dependent activators of cholera toxin. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6101–6105. doi: 10.1073/pnas.86.16.6101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariappa R., Kilberg M. S. Hormone-induced system A amino acid transport activity in rat liver plasma membrane and Golgi vesicles. Evidence for a differential sensitivity to inactivation by N-ethylmaleimide during carrier maturation. J Biol Chem. 1990 Jan 25;265(3):1470–1475. [PubMed] [Google Scholar]
- Chavrier P., Gorvel J. P., Stelzer E., Simons K., Gruenberg J., Zerial M. Hypervariable C-terminal domain of rab proteins acts as a targeting signal. Nature. 1991 Oct 24;353(6346):769–772. doi: 10.1038/353769a0. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Kahn R. A., Lippincott-Schwartz J., Klausner R. D. Binding of ARF and beta-COP to Golgi membranes: possible regulation by a trimeric G protein. Science. 1991 Nov 22;254(5035):1197–1199. doi: 10.1126/science.1957170. [DOI] [PubMed] [Google Scholar]
- Fleischer B. Isolation and characterization of Golgi apparatus and membranes from rat liver. Methods Enzymol. 1974;31:180–191. doi: 10.1016/0076-6879(74)31020-8. [DOI] [PubMed] [Google Scholar]
- Gordon J. I., Duronio R. J., Rudnick D. A., Adams S. P., Gokel G. W. Protein N-myristoylation. J Biol Chem. 1991 May 15;266(14):8647–8650. [PubMed] [Google Scholar]
- Haun R. S., Moss J. Ligation-independent cloning of glutathione S-transferase fusion genes for expression in Escherichia coli. Gene. 1992 Mar 1;112(1):37–43. doi: 10.1016/0378-1119(92)90300-e. [DOI] [PubMed] [Google Scholar]
- Kahn R. A. Fluoride is not an activator of the smaller (20-25 kDa) GTP-binding proteins. J Biol Chem. 1991 Aug 25;266(24):15595–15597. [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. Purification of a protein cofactor required for ADP-ribosylation of the stimulatory regulatory component of adenylate cyclase by cholera toxin. J Biol Chem. 1984 May 25;259(10):6228–6234. [PubMed] [Google Scholar]
- Kahn R. A., Gilman A. G. The protein cofactor necessary for ADP-ribosylation of Gs by cholera toxin is itself a GTP binding protein. J Biol Chem. 1986 Jun 15;261(17):7906–7911. [PubMed] [Google Scholar]
- Kahn R. A., Goddard C., Newkirk M. Chemical and immunological characterization of the 21-kDa ADP-ribosylation factor of adenylate cyclase. J Biol Chem. 1988 Jun 15;263(17):8282–8287. [PubMed] [Google Scholar]
- Kahn R. A., Randazzo P., Serafini T., Weiss O., Rulka C., Clark J., Amherdt M., Roller P., Orci L., Rothman J. E. The amino terminus of ADP-ribosylation factor (ARF) is a critical determinant of ARF activities and is a potent and specific inhibitor of protein transport. J Biol Chem. 1992 Jun 25;267(18):13039–13046. [PubMed] [Google Scholar]
- Kinsella B. T., Maltese W. A. rab GTP-binding proteins implicated in vesicular transport are isoprenylated in vitro at cysteines within a novel carboxyl-terminal motif. J Biol Chem. 1991 May 5;266(13):8540–8544. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. M., Haun R. S., Tsai S. C., Moss J., Vaughan M. Characterization of the human gene encoding ADP-ribosylation factor 1, a guanine nucleotide-binding activator of cholera toxin. J Biol Chem. 1992 May 5;267(13):9028–9034. [PubMed] [Google Scholar]
- Monaco L., Murtagh J. J., Newman K. B., Tsai S. C., Moss J., Vaughan M. Selective amplification of an mRNA and related pseudogene for a human ADP-ribosylation factor, a guanine nucleotide-dependent protein activator of cholera toxin. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2206–2210. doi: 10.1073/pnas.87.6.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtagh J. J., Jr, Mowatt M. R., Lee C. M., Lee F. J., Mishima K., Nash T. E., Moss J., Vaughan M. Guanine nucleotide-binding proteins in the intestinal parasite Giardia lamblia. Isolation of a gene encoding an approximately 20-kDa ADP-ribosylation factor. J Biol Chem. 1992 May 15;267(14):9654–9662. [PubMed] [Google Scholar]
- Pagano R. E., Sepanski M. A., Martin O. C. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells: interaction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J Cell Biol. 1989 Nov;109(5):2067–2079. doi: 10.1083/jcb.109.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price S. R., Nightingale M., Tsai S. C., Williamson K. C., Adamik R., Chen H. C., Moss J., Vaughan M. Guanine nucleotide-binding proteins that enhance choleragen ADP-ribosyltransferase activity: nucleotide and deduced amino acid sequence of an ADP-ribosylation factor cDNA. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5488–5491. doi: 10.1073/pnas.85.15.5488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T., Orci L., Amherdt M., Brunner M., Kahn R. A., Rothman J. E. ADP-ribosylation factor is a subunit of the coat of Golgi-derived COP-coated vesicles: a novel role for a GTP-binding protein. Cell. 1991 Oct 18;67(2):239–253. doi: 10.1016/0092-8674(91)90176-y. [DOI] [PubMed] [Google Scholar]
- Sewell J. L., Kahn R. A. Sequences of the bovine and yeast ADP-ribosylation factor and comparison to other GTP-binding proteins. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4620–4624. doi: 10.1073/pnas.85.13.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Willingham M. C., Botstein D., Kahn R. A. ADP-ribosylation factor is functionally and physically associated with the Golgi complex. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1238–1242. doi: 10.1073/pnas.87.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. C., Adamik R., Tsuchiya M., Chang P. P., Moss J., Vaughan M. Differential expression during development of ADP-ribosylation factors, 20-kDa guanine nucleotide-binding protein activators of cholera toxin. J Biol Chem. 1991 May 5;266(13):8213–8219. [PubMed] [Google Scholar]
- Tsai S. C., Noda M., Adamik R., Chang P. P., Chen H. C., Moss J., Vaughan M. Stimulation of choleragen enzymatic activities by GTP and two soluble proteins purified from bovine brain. J Biol Chem. 1988 Feb 5;263(4):1768–1772. [PubMed] [Google Scholar]
- Tsai S. C., Noda M., Adamik R., Moss J., Vaughan M. Enhancement of choleragen ADP-ribosyltransferase activities by guanyl nucleotides and a 19-kDa membrane protein. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5139–5142. doi: 10.1073/pnas.84.15.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya M., Price S. R., Tsai S. C., Moss J., Vaughan M. Molecular identification of ADP-ribosylation factor mRNAs and their expression in mammalian cells. J Biol Chem. 1991 Feb 15;266(5):2772–2777. [PubMed] [Google Scholar]
- Walker M. W., Bobak D. A., Tsai S. C., Moss J., Vaughan M. GTP but not GDP analogues promote association of ADP-ribosylation factors, 20-kDa protein activators of cholera toxin, with phospholipids and PC-12 cell membranes. J Biol Chem. 1992 Feb 15;267(5):3230–3235. [PubMed] [Google Scholar]
- Waters M. G., Serafini T., Rothman J. E. 'Coatomer': a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 1991 Jan 17;349(6306):248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]