Abstract
Purpose
To investigate hCG-β level on postovulatory day (POD) 12 and its fold increase as predictors for pregnancy outcome after in vitro fertilization (IVF) cycles.
Methods
A retrospective cohort study was performed in total 1408 fresh and 598 frozen cycles between November 2008 and October 2011, which resulted in biochemical pregnancy, early pregnancy loss, or live birth of singleton pregnancy. The serum hCG-β levels of POD 12 and 14 were compared among biochemical pregnancy, early pregnancy loss, and live birth groups. The cutoff values of POD 12 and 14 hCG-β levels and the degree of hCG-β increase from POD 12 to 14 were determined for each pregnancy outcome.
Results
POD 12 and 14 hCG-β levels stratified based on pregnancy outcomes were significantly different among the biochemical pregnancy, early pregnancy loss, and live birth in both fresh and frozen cycles. Serum hCG-β levels of POD 12 and 14 and the fold increase of hCG-β levels from POD 12 to 14 significantly predict pregnancy outcomes after fresh and frozen cycles. Among these, the cutoff value of POD 14 hCG-β had the highest sensitivity and positive predictive value (PPV). In fresh cycles, the cutoff values of POD 12 and 14 serum hCG-β levels for clinical pregnancies were 30.2 mIU/mL (sensitivity 81.3 %, specificity 79.6 %, and PPV 92.3 %) and 70.5 mIU/mL (sensitivity 88.4 %, specificity 85.2 %, and PPV 94.7 %). In pregnancies with POD 12 serum hCG-β levels ≥30.2 mIU/mL, the cutoff level of increase of hCG-β for clinical pregnancy was 2.56 (sensitivity 73.6 %, specificity 72.4 %, and PPV 97.8 %). Sequential application of cutoff values such as POD 12 hCG-β and fold increase of hCG-β improved predictability of pregnancy outcome as compared with that of POD 12 hCG-β alone. The cutoff values of POD 12 and 14 serum hCG-β levels for live birth were 40.5 mIU/mL (sensitivity 75.2 %, specificity 72.6 %, PPV 78.9 %) and 104.5 mIU/mL (sensitivity 80.3 %, specificity 74.1 %, PPV 80.8 %). In the frozen cycles, the cutoff values of POD 12 and 14 serum hCG-β level for clinical pregnancy were 31.5 IU/L (sensitivity 80.4 %, specificity 71.1 % and PPV 90 %) and 43.5 mIU/mL (sensitivity 72.6 %, specificity 71.7 %, PPV 77.2 %). In pregnancies with POD 12 serum hCG-β level ≥31.5 mIU/mL, the cutoff value for fold increase of hCG-β was 2.38 for clinical pregnancy (sensitivity 81.6 %, specificity 71.4 % and PPV 87.9 %). The cutoff values of POD 12 and 14 for live birth were 43.5 mIU/mL (sensitivity 72.6 %, specificity 71.7 %, PPV 77.2 %) and 101.6 mIU/mL (sensitivity 79.6 %, specificity 71.1 %, PPV 78.4 %). Sequential application of cutoff values for POD 12 hCG-β level and fold increase of hCG-β significantly increased PPV for live birth but not clinical pregnancy in frozen cycles.
Conclusions
Early prediction of pregnancy outcome by using POD 12 and 14 cutoff levels and sequential application of cutoff value of fold increase could provide appropriate reference to health care providers to initiate earlier management of high-risk pregnancies and precise follow-up of abnormal pregnancies.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-016-0744-y) contains supplementary material, which is available to authorized users.
Keywords: IVF, hCG-beta, Pregnancy outcome, Live birth, Biochemical pregnancy
Introduction
Assisted reproductive technology (ART) has been remarkably advanced since its inception in 1978. However, in the USA, the mean implantation rate after each embryo transfer (ET) of women younger than 35 years old still remained 36.8 % in 2010 [1]. Low implantation rate after ART cycles has called for an early reliable biomarker to predict successful pregnancy. Early markers with an abnormal level or rise pattern will allow a clinician to follow patients more closely and expedite diagnosis of abnormal pregnancies [2]. Additionally, early accurate predictor of pregnancy outcome could be helpful in counseling and planning with a patient for a possible consecutive schedule of ART. Hence, the ability to predict pregnancy outcome at the earliest time of pregnancy confirmation would benefit both clinicians and patients [2].
HCG is a glycoprotein hormone which consists with α- and β-subunits [3]. The hCG is expressed by both cyto- and syncytiotrophoblast of the human blastocyst [4]; hence, the level represents the size of trophoblastic mass [5, 6]. hCG-β also can be detected in the culture medium of embryo, and hCG-β mRNA transcripts can be identified in human embryos from two cell stage [7–9]. It has been shown that there is a relationship between hCG level in embryo culture media and embryonic competence [10, 11]. During early pregnancy, hCG can be detected in the maternal serum as early as 6 to 8 days after fertilization [12, 13]. hCG levels are dynamically increased and doubled every 48 h in most normal pregnancies, and this pattern is similar in both in vivo or in vitro (IVF) conceptions [14, 15].
Several studies have reported various cutoff values of initial serum hCG-β levels, drawn a specific number of days after ET to be useful in distinguishing viable with non-viable pregnancies in the early postimplantation period after ART with reasonable sensitivity and specificity [12, 16–25]. Additionally, serial serum hCG-β levels and the application of established logarithmic curves were reported to offer more reliable results for assessing pregnancy progression [2, 21, 25]. However, a significant portion of women under the cutoff value may end up having a normal pregnancy, and contrarily, a hCG-β value above the cutoff may falsely reassure a clinician or a patient when an abnormal pregnancy exists [2]. Therefore, search for the better biological marker or cutoff value is in demand.
In the present study, we defined the three major pregnancy outcomes to set up cutoff values for each pregnancy outcome, such as biochemical pregnancy (pregnancy loss before clinical detection by ultrasound scan [26]), early pregnancy loss (<20 weeks gestation) after a confirmation of a gestational sac by ultrasound, and a live birth. The aim of this study is to establish cutoff values of serum hCG-β and the fold change of hCG-β from postovulatory day (POD) 12 to 14, which can predict pregnancy outcomes in pregnancies achieved with fresh and frozen cycles.
Materials and methods
Study population
The study was designed as a retrospective study. The medical records of patients who underwent fresh IVF-ET (fresh) or frozen-thawed ET (frozen) cycles and got pregnant (positive pregnancy test on POD 12) from November 2008 to October 2011 at the IVF Clinic of Cheil General Hospital, Department of Obstetrics and Gynecology, Dankook University, Seoul, Korea were enrolled and reviewed. A total of 2006 cycles including 1408 fresh cycles and 598 frozen cycles were reviewed, and cycles resulted in either biochemical pregnancy or clinical pregnancy of singleton pregnancy were included in the study. The cycles resulted in heterotopic pregnancies (both extrauterine and intrauterine pregnancy occur simultaneously, n = 3), or pregnancy losses after >20 weeks gestation (n = 35) were excluded. Pregnancies established with embryos with preimplantation genetic diagnosis (PGD) (n = 101) were also excluded, since PGD has been reported to affect hCG-β level [27]. Pregnancies with incomplete or missing POD 12 or 14 data (n = 664) or missing follow-up during pregnancy (n = 66) were excluded. Finally, a total of 784 fresh cycles in 738 patients and 353 frozen cycles in 327 patients were included. This study was approved by the local Institutional Review Board.
Laboratory
The serum hCG-β levels on POD 12 and 14 were collected from medical record review. In frozen cycles, the compatible days based on embryonic age of frozen-thawed embryos were used: In a case of 3 day ET of frozen cycle, the day of ET was calculated as POD 3. The serum hCG-β was rechecked on POD 14, and the fold change of hCG-β was calculated by dividing POD 14 hCG-β level with that of POD 12. The serum hCG-β levels were measured by electrochemiluminescence immunoassay (HCG + β, Roche Diagnostics, Indianapolis, IN). The sensitivity of the assay was 0.6 mIU/mL.
Treatment protocols and clinical procedures
For ovarian hyperstimulation, patients were treated with a gonadotropin-releasing hormone (GnRH) agonist or antagonist using the short or long protocol. ET was performed 3 or 5 days after oocyte retrieval (cleavage or blastocyst stage embryos). In frozen cycles, the endometrial priming was started on cycle day 2 of the conception cycle. After at least 12 days of estradiol (E2) replacement, if the endometrium was ≥7 mm, the frozen-thawed ET was scheduled. The number of cleavage stage embryos or blastocysts to transfer was determined according to individual clinical conditions, including patient age, embryo quality, and the opportunity for cryopreservation, as previously reported [28].
Pregnancy outcomes
If the serum hCG-β was ≥5 mIU/mL, it was considered as a pregnancy. Biochemical pregnancy was defined as a pregnancy that fails to progress to the point of ultrasound confirmation of gestational sac in spite of a positive pregnancy test. It is characterized by a positive pregnancy test which is not maintained, manifested with a low peak and rapid fall of hCG-β levels [29, 30]. Clinical pregnancy was defined as the presence of a gestational sac using ultrasonography between 5 and 6 weeks of gestation. Early pregnancy loss was defined as a pregnancy loss before 20 weeks of gestation after a confirmation of a gestational sac by ultrasound.
Out of 784 fresh cycles, 196 cycles resulted in biochemical pregnancies, 136 cycles in early pregnancy losses, and 452 cycles in live births. Out of 353 frozen cycles, 83 cycles resulted in biochemical pregnancies, 69 cycles in early pregnancy losses, and 201 cycles in live births.
Statistical analysis
Data were analyzed using SPSS version 21.0 (SPSS Inc., Chicago, IL, USA). The serum hCG-β levels (POD 12 and 14) and a fold change of serum hCG-β between POD 12 and 14 were compared among biochemical pregnancy, early pregnancy loss, and live birth groups. Student’s t test or Mann-Whitney test was used for two group comparison. One-way ANOVA followed by Bonferroni’s multiple comparison test or Kruskal-Wallis test was applied to determine significance for multiple comparisons as indicated. Receiver operating characteristic (ROC) curve analysis was applied to determine sensitivity and specificity of the cutoff values. The percentage for the area under curve (AUC) and 95 % confidence intervals were reported for each ROC curve analysis. A chi-square analysis was performed to evaluate differences in frequencies between groups. Negative and positive predictive values (NPV and PPV) were calculated based on the prevalence of clinical pregnancy or live birth. The effect of predictors on pregnancy outcome was expressed through adjusted odds ratio (OR) and 95 % confidence interval (CI) which was calculated using regression. For P values, <0.05 were considered to be significant.
Results
Study population
The age of total study group was 34.7 ± 3.8 years old (mean ± SD). The age of the women in the live birth group (34.1 ± 3.5) was significantly younger than those of biochemical pregnancy group (35.2 ± 3.9) (P < 0.001) and early pregnancy loss group (36.2 ± 4.1) (P < 0.001). IVF-cycle-related parameters including BMI, number of previous IVF, number of transferred embryos, basal FSH, E2 level on hCG injection day, and indication of IVF cycles are listed in Table 1.
Table 1.
Patient characteristics, cycle parameters and serum hCG-β levels (mean ± SD), and the fold change of hCG-β levels between postovulatory day (POD) 12 and 14 of pregnancies achieved after fresh IVF-ET and frozen-thawed ET cycles
| Fresh cycles (784 cycles in 738 patients) |
Frozen-thawed cycles (353 cycles in 327 patients) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Biochemical pregnancy | Early pregnancy loss | Live Birth | P valuea | Biochemical pregnancy | Early pregnancy loss | Live Birth | P valuea | |
| No. of cycles | 196 | 136 | 452 | 83 | 69 | 201 | ||
| Age (years) | 35.4 ± 3.9 | 36.7 ± 4.3 | 34.4 ± 3.5 | <0.001 | 34.8 ± 3.9 | 35.3 ± 3.6 | 33.3 ± 3.5 | <0.001 |
| Age of husband (years) | 37.3 ± 4.4 | 38.2 ± 4.4 | 36.9 ± 4.3 | 0.014 | 37.8 ± 4.3 | 37.1 ± 4.3 | 35.9 ± 4.5 | 0.004 |
| BMI (kg/m2) | 20.8 ± 3.3 | 21.3 ± 2.6 | 21.0 ± 2.8 | 0.371 | 21.5 ± 3.9 | 21.2 ± 3.7 | 20.8 ± 2.6 | 0.195 |
| No. of IVF | 2.2 ± 1.6 | 2.2 ± 1.9 | 2.2 ± 1.7 | 0.849 | 3.8 ± 2.2 | 3.3 ± 1.8 | 3.0 ± 1.4 | 0.002 |
| No. of embryos transferred | 2.8 ± 0.9 | 2.9 ± 1.0 | 2.8 ± 0.9 | 0.654 | 3.2 ± 0.9 | 3.2 ± 0.9 | 3.0 ± 0.8 | 0.097 |
| Basal FSH on cycle day 2 | 9.8 ± 5.5 | 9.4 ± 5.2 | 9.4 ± 4.0 | 0.671 | – | – | – | – |
| E2 level on hCG injection day | 1744.5 ± 1683.2 | 1653.9 ± 1282.4 | 1827.6 ± 1658.6 | 0.524 | – | – | – | – |
| Indication of IVF | P valueb | P valueb | ||||||
| Male factor (%) | 51 (26.0) | 22 (16.2) | 111 (24.6) | 0.139 | 20 (24.1) | 18 (26.1) | 60 (29.9) | 0.580 |
| Tubal factor (%) | 31 (15.8) | 18 (13.2) | 73 (16.2) | 0.709 | 27 (32.5) | 16 (23.2) | 45 (22.4) | 0.186 |
| Ovulatory factor (%) | 13 (6.6) | 17 (12.5) | 40 (8.8) | 0.182 | 5 (6.0) | 5 (7.2) | 11 (5.5) | 0.865 |
| Endometriosis (%) | 34 (17.3) | 19 (14.0) | 65 (14.4) | 0.399 | 2 (2.4) | 4 (5.8) | 20 (10.0) | 0.074 |
| Advanced age (%) | 8 (4.1) | 14 (10.3) | 16 (3.5) | 0.005 | 1 (1.2) | 2 (2.9) | 4 (2.0) | 0.757 |
| Uterine (%) | 4 (2.0) | 3 (2.2) | 6 (1.3) | 0.694 | 5 (6.0) | 2 (2.9) | 4 (2.0) | 0.204 |
| Unexplained (%) | 55 (28.1) | 43 (31.6) | 141 (31.2) | 0.693 | 23 (27.7) | 22 (31.9) | 58 (28.9) | 0.843 |
| Serum β-hCG level | P valuea | P valuea | ||||||
| POD 12 hCG-β (mIU/mL) | 20.0 ± 17.2c,d | 47.9 ± 30.3e | 65.2 ± 32.4 | <.001 | 29.3 ± 29.1c,d | 51.5 ± 39.4e | 73.0 ± 42.1 | <.001 |
| POD 14 hCG-β (mIU/mL) | 38.6 ± 42.1c,d | 137.5 ± 87.1e | 197.2 ± 98.1 | <.001 | 60.2 ± 71.0c,d | 148.9 ± 122.1e | 222.6 ± 131.6 | <.001 |
| Increase of hCG-β between POD 12 and 14 (fold) | 2.0 ± 1.3c,d | 3.0 ± 1.0e | 3.1 ± 0.8 | <.001 | 1.9 ± 0.9c,d | 3.0 ± 1.0e | 3.1 ± 0.9 | <.001 |
a P values were calculated among study groups, including biochemical pregnancy loss and live birth groups, using ANOVA or Kruskal-Wallis test
b P values were calculated among three groups using chi-square test
cBiochemical pregnancy vs early pregnancy loss, P < 0.05
dBiochemical pregnancy vs live birth, P < 0.05
eEarly pregnancy loss vs live birth, P < 0.05
POD 12 and 14 serum hCG-β levels
The mean serum hCG-β level on POD 12 in the live birth group was 65.2 ± 32.4 mIU/mL in fresh cycles, which was significantly higher than those of biochemical pregnancy (20.0 ± 17.2 mIU/mL) and early pregnancy loss groups (47.9 ± 30.3 mIU/mL) (P < 0.001). In frozen cycles, the mean serum hCG-β levels on POD 12 in live birth group was 73.0 ± 42.1 mIU/mL, which was significantly higher than those of biochemical pregnancy (29.3 ± 29.1 mIU/mL) and early pregnancy loss groups (51.5 ± 39.4 mIU/mL) (P < 0.001) (Table 1).
The mean serum hCG-β levels checked on POD 14 in the live birth group of fresh and frozen cycles were 197.2 ± 98.1 mIU/mL and 222.6 ± 131.6 mIU/mL each. These were significantly higher than those of biochemical pregnancy (fresh cycle, 38.6 ± 42.1 mIU/mL; frozen cycle, 60.2 ± 71.0 mIU/mL, P < 0.001) and early pregnancy loss groups (137.5 ± 87.1 mIU/mL, 148.9 ± 122.1 mIU/mL, P < 0.001) (Table 1). The mean hCG-β levels on POD 12 and 14 after frozen cycles were significantly higher than those after fresh cycles regardless of pregnancy outcomes (P < 0.004 and P < 0.005, respectively).
Fold change of hCG-β from POD 12 to 14
The increase of hCG-β between POD 12 and 14 was calculated by dividing POD 14 hCG-β level with that of POD 12. In fresh cycles, the increase in biochemical pregnancy, early pregnancy loss, and live birth groups were 2.0 ± 1.3, 3.0 ± 1.0, and 3.1 ± 0.8, respectively (P < 0.001). In frozen cycles, the increase were 1.9 ± 0.9, 3.0 ± 1.0, and 3.1 ± 0.9 in biochemical pregnancy, early pregnancy loss, and live birth groups, respectively (P < 0.001) (Table 1). The increase of hCG-β level was not different between fresh and frozen cycles.
Cutoff values of hCG-β to predict clinical pregnancy and live birth
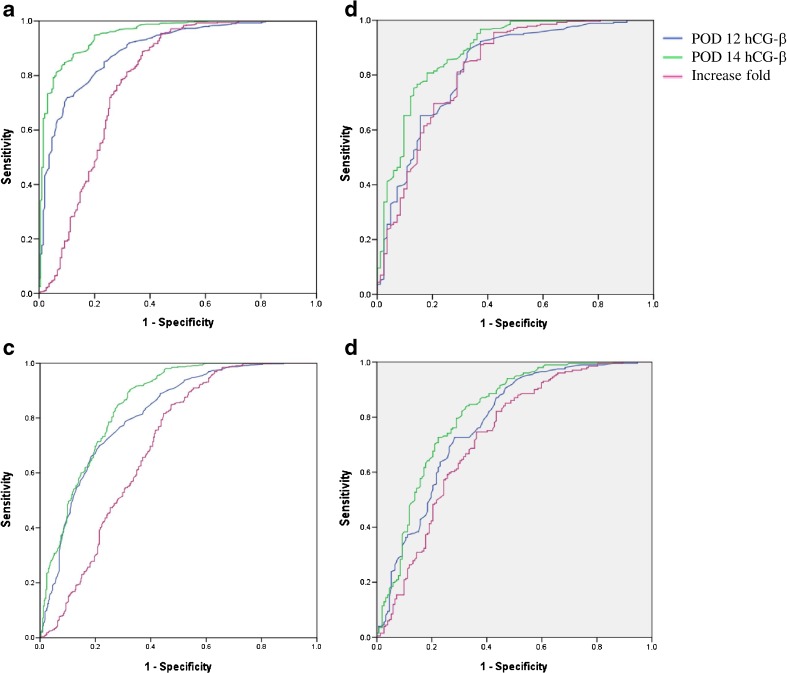
To determine the cutoff value of serum hCG-β for clinical singleton pregnancies from biochemical pregnancies, ROC curves were generated in both fresh (Fig. 1a) and frozen cycles (Fig. 1b). The AUC for POD 12 hCG-β levels (blue line in Fig. 1a) was 0.893 (95 % confidence interval [CI], 0.868–0.919). The AUC for POD 14 was 0.951 (95 % CI, 0.933–0.968). The hCG-β of POD 14 was a better predictor for clinical pregnancy than that of POD 12 in both of fresh and frozen cycles (Table 2). The AUC for the combined POD 14 hCG-β levels and hCG-β fold increase was 0.868 (95 % CI, 0.817–0.919).
Fig. 1.
Receiver-operating characteristic curve of postovulatory day (POD) 12 and POD 14 serum hCG-β levels in order to distinguish between biochemical pregnancies and singleton clinical pregnancies in a fresh IVF-ET cycles and b frozen-thawed ET cycles, and either ended as biochemical pregnancies or early pregnancy loss and live birth in c fresh IVF-ET cycles and d frozen-thawed ET cycles. Blue line reflects POD 12 hCG-β levels; green line reflects POD 14 hCG-β levels; violet line reflects increase fold between POD 12 and POD 14 hCG-β levels
Table 2.
Prediction of clinical pregnancy (either ended as early pregnancy loss or live birth) and live birth by hCG-β levels and the fold increases of hCG-β levels between POD 12 and 14
| Prediction by hCG-β levels | |||||||||
| Pregnancy outcome | hCG-β | Cutoff (mIU/mL) | AUC (95 % CI) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | OR (95 % CI) | P value |
| Fresh cycle | |||||||||
| Clinical pregnancy | POD 12 | 30.2 | 0.893 (0.868–0.919) | 81.3 | 79.6 | 92.3 | 58.6 | 16.947 (11.309–25.396) | <0.001 |
| POD 14 | 70.5 | 0.951 (0.933–0.968) | 88.4 | 85.2 | 94.7 | 71.1 | 44.037 (27.569–70.342) | <0.001 | |
| Live birth | POD 12 | 40.5 | 0.813 (0.782–0.844) | 75.2 | 72.6 | 78.9 | 68.3 | 8.040 (5.826–11.095) | <0.001 |
| POD 14 | 104.5 | 0.850 (0.822–0.879) | 80.3 | 74.1 | 80.8 | 73.4 | 11.667 (8.323–16.353) | <0.001 | |
| Frozen-thawed cycle | |||||||||
| Clinical pregnancy | POD 12 | 31.5 | 0.825 (0.770–0.881) | 80.4 | 71.1 | 90.0 | 52.7 | 10.065 (5.740–17.649) | <0.001 |
| POD 14 | 76.4 | 0.885 (0.840–0.930) | 84.8 | 74.7 | 91.6 | 60.2 | 16.490 (9.085–29.931) | <0.001 | |
| Live birth | POD 12 | 43.5 | 0.776 (0.726–0.826) | 72.6 | 71.7 | 77.2 | 66.5 | 6.729 (4.206–10.764) | <0.001 |
| POD 14 | 101.6 | 0.814 (0.767–0.861) | 79.6 | 71.1 | 78.4 | 72.5 | 9.579 (5.865–15.643) | <0.001 | |
| Prediction by fold change of hCG-β level between POD 12 and 14 | |||||||||
| Pregnancy outcome | Cutoff Of increase (fold) | AUC (95 % CI) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | OR (95 % CI) | P value | |
| Fresh cycle | |||||||||
| Clinical pregnancy | 2.52 | 0.784 (0.738–0.829) | 76.4 | 71.9 | 89.1 | 50.4 | 8.281 (5.748–11.930) | <0.001 | |
| Live birth | 2.78 | 0.697 (0.658–0.737) | 65.7 | 63.0 | 70.6 | 57.3 | 3.224 (2.400–4.332) | <0.001 | |
| Frozen-thawed cycle | |||||||||
| Clinical pregnancy | 2.37 | 0.823 (0.765–0.881) | 81.1 | 71.1 | 89.8 | 53.2 | 9.962 (5.695–17.428) | <0.001 | |
| Live birth | 2.60 | 0.726 (0.670–0.781) | 71.6 | 64.5 | 72.7 | 63.2 | 4.585 (2.917–7.206) | <0.001 | |
POD postovulatory day, AUC area under receiver operating characteristic curve, PPV positive predictive value, NPV negative predictive value, OR odds ratio
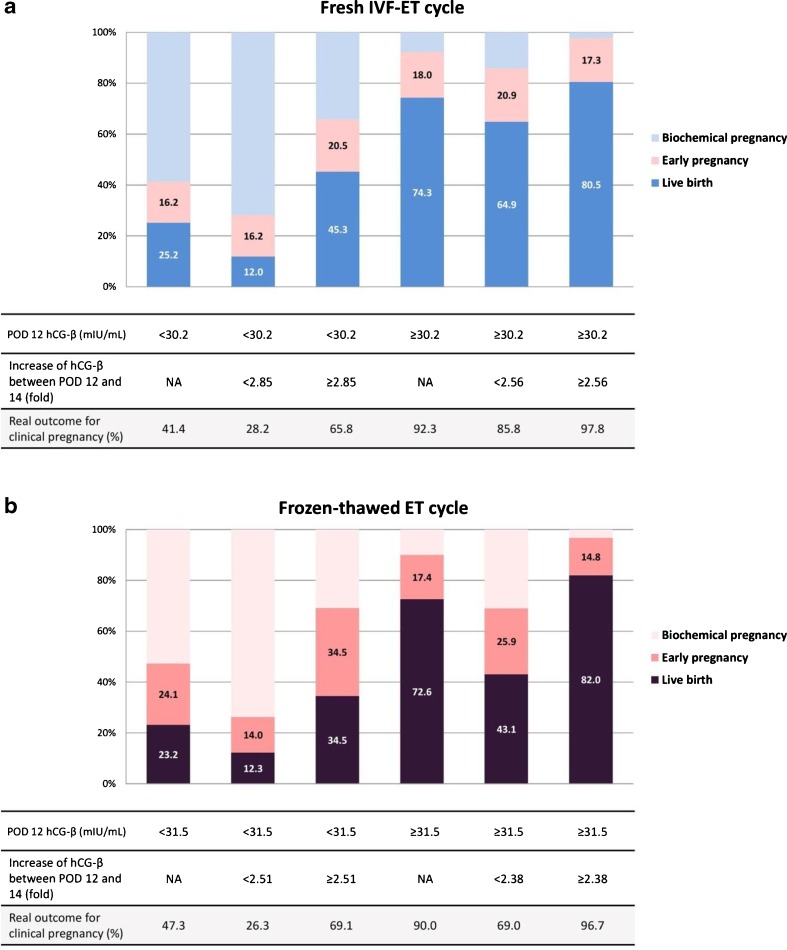
In fresh cycle, the cutoff value of POD 12 hCG-β for predicting clinical pregnancy was 30.2 mIU/mL with 81.3 % sensitivity and 79.6 % specificity. The hCG-β level ≥30.2 mIU/mL predicts a clinical pregnancy with PPV 92.3 % and NPV 58.6 % (OR 16.947, P < 0.001). In pregnancies with POD 12 serum hCG-β level <30.2 mIU/mL, the biochemical pregnancy rate was 58.6 %, which was significantly higher than 7.7 % in pregnancies with POD 12 serum hCG-β levels ≥30.2 IU/L (P < 0.001). Pregnancies with POD 12 hCG-β levels ≥30.2 mIU/mL resulted in 74.3 % live birth rate as compared to 25.2 % in pregnancies with <30.2 mIU/mL. A cutoff value of 70.5 mIU/mL on POD 14 revealed 88.4 % sensitivity and 85.2 % specificity for predicting a clinical pregnancy. hCG-β levels ≥70.5 mIU/mL on POD 14 predicts clinical pregnancies with PPV 94.7 % NPV 71.1 % (OR 44.037, P < 0.001) (Table 2). The cutoff value of POD 12 hCG-β levels to predict live birth was 40.5 mIU/mL with 75.2 % of sensitivity and 72.6 % of specificity. A cutoff value of POD 14 hCG-β level was 104.5 mIU/mL with 80.3 % of sensitivity and 74.1 % of specificity (Fig. 1c, Table 2).
In frozen cycle, a cutoff value of hCG-β 31.5 mIU/mL (POD 12) revealed 80.4 % sensitivity and 71.1 % specificity for predicting clinical pregnancy. The hCG-β ≥31.5 mIU/mL on POD 12 predicted clinical pregnancy with PPV 90.0 % NPV 52.7 % (OR 10.065, P < 0.001). In pregnancies with POD 12 hCG-β levels ≥31.5 mIU/mL resulted in 72.6 % live birth as compared to 23.2 % in pregnancies with POD 12 hCG-β <31.5 mIU/mL. A cutoff value of POD 14 hCG-β ≥76.4 IU/L revealed 84.8 % sensitivity and 74.7 % specificity. POD 14 hCG-β ≥76.4 mIU/mL revealed PPV 91.6 % NPV 60.2 % (Table 2). A cutoff of POD 12 hCG-β level for live birth was 43.5 mIU/mL, which revealed 72.6 % sensitivity and 71.7 % specificity. A cutoff of 101.6 IU/L on POD 14 had 79.6 % of sensitivity and 71.1 % of specificity (Fig. 1d, Table 2).
Serial hCG-β levels and fold change of hCG-β level for clinical pregnancy and live birth
In fresh cycles, 43 cycles resulted in biochemical pregnancy that had decreased hCG-β levels on POD 14 as compared to POD 12. The fold changes of hCG-β from POD 12 to 14 were calculated after excluding these cases. In the cycles with POD 12 hCG-β level <30.2 mIU/mL (cutoff for clinical pregnancy), the cutoff level of increase for clinical pregnancy was 2.85 with 70.0 % of sensitivity and 67.7 % of specificity (PPV 65.8 %, NPV 71.8 %, OR 4.900, P < 0.001). The cutoff of fold changes in cycles with POD 12 hCG-β level ≥30.2 mIU/mL was 2.56 with 73.6 % sensitivity and 72.4 % specificity (PPV 97.8 %, NPV 14.2 %, OR 7.255, P < 0.001). In regards of predicting live birth, a cutoff of fold change birth in the fresh cycles with POD 12 hCG-β level <40.5 mIU/mL was 2.87 with 71.4 % of sensitivity and 61.9 % of specificity (PPV 51.0 %, NPV 79.6 %, OR 4.058, P < 0.001). In pregnancies with POD 12 hCG-β level ≥40.5 mIU/mL, a cutoff for live birth was 2.79 with 61.8 % of sensitivity and 50.6 % of specificity (PPV 82.7 %, NPV 24.9 %, OR 1.579 P = .039) (Table 3).
Table 3.
Cutoffs of the fold changes of hCG-β for clinical pregnancy and live birth according to the initial hCG-β levels
| Pregnancy outcome | Sequential application | AUC (95 % CI) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | OR (95 % CI) | P value | |
|---|---|---|---|---|---|---|---|---|---|
| Initial hCG-β (mIU/mL) | Cutoff of increase (fold) | ||||||||
| Fresh cycles | |||||||||
| Clinical pregnancy | <30.2 | 2.85 | 0.761 (0.699–0.822) | 70.0 | 67.7 | 65.8 | 71.8 | 4.900 (2.813–8.536) | <0.001 |
| ≥30.2 | 2.56 | 0.771 (0.666–0.876) | 73.6 | 72.4 | 97.8 | 14.2 | 7.255 (3.134–16.792) | <0.001 | |
| Live birth | <40.5 | 2.87 | 0.723 (0.667–0.723) | 71.4 | 61.9 | 51.0 | 79.6 | 4.058 (2.465–6.682) | <0.001 |
| ≥40.5 | 2.79 | 0.592 (0.518–0.665) | 61.8 | 50.6 | 82.7 | 24.9 | 1.579 (0.983–2.535) | 0.039 | |
| Frozen-thawed cycles | |||||||||
| Clinical pregnancy | <31.5 | 2.51 | 0.775 (0.684–0.867) | 71.7 | 65.3 | 69.1 | 68.1 | 4.769 (2.062–11.030) | <0.001 |
| ≥31.5 | 2.38 | 0.805 (0.697–0.913) | 81.6 | 71.4 | 87.9 | 45.5 | 6.044 (4.118–8.871) | <0.001 | |
| Live birth | <43.5 | 2.72 | 0.679 (0.593–0.765) | 65.5 | 63.9 | 50.0 | 76.3 | 3.211 (1.607–6.412) | <0.001 |
| ≥43.5 | 2.56 | 0.692 (0.591–0.793) | 77.4 | 61.9 | 87.6 | 44.1 | 5.564 (2.672–11.589) | <0.001 | |
AUC area under receiver operating characteristic curve, PPV positive predictive value, NPV negative predictive value, OR odds ratio
In frozen cycles, increase was calculated with the exclusion of 13 cycles (increase fold ≤1). In the group with POD 12 hCG-β level <31.5 mIU/mL, the cutoff level of fold change for clinical pregnancy was 2.51 with 71.7 % of sensitivity and 65.3 % of specificity (PPV 69.1 %, NPV 68.1 %, OR 4.769, P < 0.001). The cutoff of cycles with POD 12 hCG-β level ≥31.5 mIU/mL was 2.38 with 81.6 % sensitivity and 71.4 % specificity (PPV 87.9 %, NPV 45.5 %, OR 6.044, P < 0.001). The pregnancies with POD 12 hCG-β level <43.5 mIU/mL, the cutoff of fold change for live birth was 2.72 with 65.5 % of sensitivity and 63.9 % of specificity (PPV 50.0 %, NPV 76.3 %, OR 3.211, P < 0.001). The cutoff of cycles with POD 12 hCG-β level ≥43.5 mIU/mL was 2.56 with 77.4 % sensitivity and 61.9 % specificity (PPV 87.6 %, NPV 44.1 %, OR 5.564, P < 0.001) (Table 3). Figure 2 shows the distribution of pregnancy outcomes in groups divided with cutoff values of POD 12 hCG-β alone and sequential values.
Fig. 2.
The distribution of pregnancy outcomes (biochemical pregnancy, clinical pregnancy, or live birth) in groups divided with cutoff values of POD 12 hCG-β alone and with the sequential application of hCG-β fold change. a Fresh IVF-ET cycles. b Frozen-thawed ET cycles
Discussion
Few data exist regarding the predictability of early hCG levels and its fold change on pregnancy outcome of ART cycles based on embryonic age. Our data shows that in both fresh and frozen cycles, the hCG-β levels on POD 12 and 14 were significantly higher in pregnancies resulting in live birth compared to those of non-viable pregnancies such as biochemical or early pregnancy losses, which was consistent with previous studies [31, 32]. The proportions of live birth were similar between fresh and frozen cycles; however, the POD 12 and 14 hCG-β levels of frozen cycles were higher in each pregnancy outcome than those of fresh cycles. Contradictory to our results, higher hCG-β levels in early pregnancies after fresh cycles as compared to frozen cycles have been previously reported [31]. The other report also demonstrated the mean of hCG-β levels after fresh cycle were higher than after frozen cycles; however, the mean hCG-β levels between these two groups were not significantly different when pregnancies were stratified according to pregnancy outcomes [32]. In our data mean hCG-β levels after frozen cycles were significantly higher than those after fresh cycles regardless of pregnancy outcomes. It is noteworthy that mid-trimester hCG-β levels in pregnancies achieved after frozen cycles are higher than those of fresh cycles [33, 34]. The differences in hCG-β levels among these studies could be explained by various factors including day of ET, embryo biopsy, method of fertilization, method of freezing and thawing, media of embryo culture and etiology of infertility, etc. [27, 35–37]. In our study, hCG-β levels were not affected by the embryonic age or the number of embryos transferred (data not shown). Interestingly, even with the different hCG-β levels in pregnancies achieved after fresh and frozen cycles, the fold change of hCG-β after frozen cycles was same as that of fresh cycles (data not shown).
Single and serial serum hCG-β values have been reported to be reliable predictive markers to assess pregnancy progression after ART cycles [2, 12, 13, 21, 25, 30, 38]. In this study, we investigated serial hCG-β levels on POD 12 and 14 to predict pregnancy outcome, which were significantly earlier than previous studies, in which hCG-β levels were tested on POD 15–19 [35, 39]. Furthermore, we investigated the fold change between POD 12 and 14, though others calculated the doubling time of hCG-β levels or interval increase in 7 days [21, 23, 40]. To reflect embryonic age, postoocyte retrieval day was adopted instead of using post ET day. Previous studies used post ET day with unexplained or mixed 2-, 3-, or 5-day-old ET data for the analysis; hence, it has a limitation to reflect exact embryonic ages [2, 23, 24, 31, 41].
For the prediction of clinical pregnancy, our cutoff values were 30.2 mIU/mL on POD 12 and 70.5 mIU/mL on POD 14 in fresh cycles. Poikkeus et al. demonstrated hCG-β 76 mIU/mL for predicting viable pregnancy, which had a sensitivity of 80 % and a specificity of 82 % when drawn 12 days after ET (POD 15) [24]. Similarly, hCG-β cutoff value for ongoing pregnancy on day 15 after oocyte fertilization (POD 15) was reported to be 78 mIU/mL for day 3 ET [35], and hCG-β cutoff on day 14 of ET (POD 17–19) for the prediction of pregnancies beyond 12 weeks was reported to be 347 mIU/ml with 72.2 % sensitivity and 73.6 % specificity [39]. Hence, our data tend to have a compatible POD 12 cutoff value but higher POD 14 cutoff values when considering hCG-β doubling time as 1.4–3.5 days [41, 42].
In the present study, the serial hCG-β levels and fold changes were analyzed as predictors for pregnancy outcome. Both POD 12 and 14 hCG-β cutoff values significantly predicted pregnancy outcome. In pregnancies with POD 12 hCG-β over its cutoff level, sequential application of the cutoff values for fold change significantly increased PPV for both clinical pregnancy and live birth in fresh cycles, higher than those of POD 12 or 14 hCG-β levels. Furthermore, the cycles with low POD 12 hCG-β levels in fresh cycles (<30.2 mIU/mL), PPV was significantly increased through sequential application of fold change. In frozen cycles, the sequential application of fold change increased the PPV for live birth, but not for clinical pregnancy. This may be due to a slower increase of hCG-β during early pregnancy after frozen cycles as compared to fresh cycles. In order to validate our cutoff values, we reviewed medical records of IVF cycles from January 2012 to December 2014 at the IVF Clinic of Cheil General Hospital, Department of Obstetrics and Gynecology, Dankook University, Seoul, Korea. A total of 1663 cycles including 1004 fresh and 659 frozen cycles which sufficed the inclusion criteria of this study were analyzed. A sequential application of cutoff values predicted a clinical pregnancy with PPV 96.5 % and a live birth with PPV 59.7 % in fresh cycles. In frozen cycles, the sequential application predicted a clinical pregnancy with PPV 95.4 % and a live birth PPV 71.3 % (Supplemental Table 1).
In this study, we excluded multiple pregnancies since twin pregnancy showed higher hCG-β levels, and the levels of monochorionic twin pregnancies were significantly lower than those of dichorionic twin pregnancies [23]. However, there are inherent limitations since biochemical pregnancies can be multiple gestation, ectopic, heterotopic, or genetically abnormal pregnancies, and early pregnancy losses may have abnormal karyotypes, which have decreased or increased hCG-β levels. Age was significantly different among biochemical pregnancy, early pregnancy loss, and live birth groups; however, inclusion of age in the AUC of the prediction model did not improve the outcome.
As far as we understand, this is the first report to analyze the sequential application of cutoff values for prediction of pregnancy outcome. Our data demonstrated a sequential application of cutoff values for POD 12 hCG-β, and its fold change significantly improved a predictability of pregnancy outcome as compared to POD 12 hCG-β alone, and even if the initial serum hCG-β level on POD 12 is lower than the cutoff, the clinical pregnancy can be expected when the fold change is greater than its cutoff. Application of this sequential data to clinical practice could aid the early prediction of pregnancy outcome, and appropriate and timely counseling of patients. Particularly, earlier prediction of pregnancy outcome could provide appropriate reference to health care providers to establish a treatment plan for recurrent pregnancy losses or implantation failures [43–45], initiate earlier management of high risk pregnancies, and precise follow-up of abnormal pregnancies. Therefore, early pregnancy biomarkers such as POD 12 hCG-β level and its fold change based on embryonic age could provide a predictive and appropriate reference to both health care providers and patients and assist earlier management and precise follow-up of women with abnormal or high risk pregnancies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 296 kb)
Acknowledgments
Author’s roles
NS collected the data and analyzed it. JKK supervised the data analysis, study design, and writing of the manuscript.
HSK collected the data. KM Y supervised data collection, analysis, and designed the study.
Compliance with ethical standards
Funding
The study was supported by Department of Obstetrics and Gynecology, Cheil General Hospital and Women’s Healthcare Center, Dankook University College of Medicine, Seoul, Korea.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Capsule Early prediction of pregnancy outcome by using POD 12 and 14 hCG-β cutoff levels and sequential application for cutoff value of fold increase could provide appropriate reference to health care providers after in-vitro fertilization (IVF) cycles.
References
- 1.Cohen J, Alikani M, Bisignano A. Past performance of assisted reproduction technologies as a model to predict future progress: a proposed addendum to Moore’s law. Reprod Biomed Online. 2012;25(6):585–90. doi: 10.1016/j.rbmo.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Shamonki MI, Frattarelli JL, Bergh PA, Scott RT. Logarithmic curves depicting initial level and rise of serum beta human chorionic gonadotropin and live delivery outcomes with in vitro fertilization: an analysis of 6021 pregnancies. Fertil Steril. 2009;91(5):1760–4. doi: 10.1016/j.fertnstert.2008.02.171. [DOI] [PubMed] [Google Scholar]
- 3.Morgan FJ, Birken S, Canfield RE. The amino acid sequence of human chorionic gonadotropin. The alpha subunit and beta subunit. J Biol Chem. 1975;250(13):5247–58. [PubMed] [Google Scholar]
- 4.Handschuh K, Guibourdenche J, Tsatsaris V, Guesnon M, Laurendeau I, Evain-Brion D, et al. Human chorionic gonadotropin expression in human trophoblasts from early placenta: comparative study between villous and extravillous trophoblastic cells. Placenta. 2007;28(2–3):175–84. doi: 10.1016/j.placenta.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Braunstein GD, Rasor JL, Engvall E, Wade ME. Interrelationships of human chorionic gonadotropin, human placental lactogen, and pregnancy-specific beta 1-glycoprotein throughout normal human gestation. Am J Obstet Gynecol. 1980;138(8):1205–13. doi: 10.1016/s0002-9378(16)32793-4. [DOI] [PubMed] [Google Scholar]
- 6.Dokras A, Sargent IL, Gardner RL, Barlow DH. Human trophectoderm biopsy and secretion of chorionic gonadotrophin. Hum Reprod (Oxford, England) 1991;6(10):1453–9. doi: 10.1093/oxfordjournals.humrep.a137288. [DOI] [PubMed] [Google Scholar]
- 7.Fishel SB, Edwards RG, Evans CJ. Human chorionic gonadotropin secreted by preimplantation embryos cultured in vitro. Science. 1984;223(4638):816–8. doi: 10.1126/science.6546453. [DOI] [PubMed] [Google Scholar]
- 8.Hay DL, Lopata A. Chorionic gonadotropin secretion by human embryos in vitro. J Clin Endocrinol Metab. 1988;67(6):1322–4. doi: 10.1210/jcem-67-6-1322. [DOI] [PubMed] [Google Scholar]
- 9.Adjaye J, Bolton V, Monk M. Developmental expression of specific genes detected in high-quality cDNA libraries from single human preimplantation embryos. Gene. 1999;237(2):373–83. doi: 10.1016/S0378-1119(99)00329-7. [DOI] [PubMed] [Google Scholar]
- 10.Ramu S, Acacio B, Adamowicz M, Parrett S, Jeyendran RS. Human chorionic gonadotropin from day 2 spent embryo culture media and its relationship to embryo development. Fertil Steril. 2011;96(3):615–7. doi: 10.1016/j.fertnstert.2011.06.035. [DOI] [PubMed] [Google Scholar]
- 11.Butler SA, Luttoo J, Freire MO, Abban TK, Borrelli PT, Iles RK. Human chorionic gonadotropin (hCG) in the secretome of cultured embryos: hyperglycosylated hCG and hCG-free beta subunit are potential markers for infertility management and treatment. Reprod Sci. 2013;20(9):1038–45. doi: 10.1177/1933719112472739. [DOI] [PubMed] [Google Scholar]
- 12.Dor J, Rudak E, Rotmench S, Levran D, Blankstein J, Lusky A, et al. The role of early post-implantation beta-HCG levels in the outcome of pregnancies following in-vitro fertilization. Hum Reprod (Oxford, England) 1988;3(5):663–7. doi: 10.1093/oxfordjournals.humrep.a136763. [DOI] [PubMed] [Google Scholar]
- 13.Hay DL, Gronow M, Lopata A, Brown JB. Monitoring early production of chorionic gonadotrophin (HCG) following in vitro fertilization and embryo transfer. Aust N Z J Obstet Gynaecol. 1984;24(3):206–9. doi: 10.1111/j.1479-828X.1984.tb01491.x. [DOI] [PubMed] [Google Scholar]
- 14.Kadar N, Romero R. Observations on the log human chorionic gonadotropin-time relationship in early pregnancy and its practical implications. Am J Obstet Gynecol. 1987;157(1):73–8. doi: 10.1016/S0002-9378(87)80349-6. [DOI] [PubMed] [Google Scholar]
- 15.Lenton EA, Hooper M, King H, Kumar A, Monks N, Verma S, et al. Normal and abnormal implantation in spontaneous in-vivo and in-vitro human pregnancies. J Reprod Fertil. 1991;92(2):555–65. doi: 10.1530/jrf.0.0920555. [DOI] [PubMed] [Google Scholar]
- 16.Liu HC, Kreiner D, Muasher SJ, Jones G, Jones H, Jr, Rosenwaks Z. Beta-human chorionic gonadotropin as a monitor of pregnancy outcome in in vitro fertilization-embryo transfer patients. Fertil Steril. 1988;50(1):89–94. doi: 10.1016/S0015-0282(16)60014-6. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita T, Okamoto S, Thomas A, MacLachlan V, Healy DL. Predicting pregnancy outcome after in vitro fertilization and embryo transfer using estradiol, progesterone, and human chorionic gonadotropin beta-subunit. Fertil Steril. 1989;51(2):304–9. doi: 10.1016/S0015-0282(16)60495-8. [DOI] [PubMed] [Google Scholar]
- 18.Heiner JS, Kerin JF, Schmidt LL, Wu TC. Can a single, early quantitative human chorionic gonadotropin measurement in an in vitro fertilization-gamete intrafallopian transfer program predict pregnancy outcome? Fertil Steril. 1992;58(2):373–7. doi: 10.1016/S0015-0282(16)55232-7. [DOI] [PubMed] [Google Scholar]
- 19.Glatstein IZ, Hornstein MD, Kahana MJ, Jackson KV, Friedman AJ. The predictive value of discriminatory human chorionic gonadotropin levels in the diagnosis of implantation outcome in in vitro fertilization cycles. Fertil Steril. 1995;63(2):350–6. doi: 10.1016/S0015-0282(16)57367-1. [DOI] [PubMed] [Google Scholar]
- 20.Qasim SM, Callan C, Choe JK. The predictive value of an initial serum beta human chorionic gonadotropin level for pregnancy outcome following in vitro fertilization. J Assist Reprod Genet. 1996;13(9):705–8. doi: 10.1007/BF02066422. [DOI] [PubMed] [Google Scholar]
- 21.Chen CD, Ho HN, Wu MY, Chao KH, Chen SU, Yang YS. Paired human chorionic gonadotrophin determinations for the prediction of pregnancy outcome in assisted reproduction. Hum Reprod. 1997;12(11):2538–41. doi: 10.1093/humrep/12.11.2538. [DOI] [PubMed] [Google Scholar]
- 22.Bjercke S, Tanbo T, Dale PO, Morkrid L, Abyholm T. Human chorionic gonadotrophin concentrations in early pregnancy after in-vitro fertilization. Hum Reprod. 1999;14(6):1642–6. doi: 10.1093/humrep/14.6.1642. [DOI] [PubMed] [Google Scholar]
- 23.Urbancsek J, Hauzman E, Fedorcsak P, Halmos A, Devenyi N, Papp Z. Serum human chorionic gonadotropin measurements may predict pregnancy outcome and multiple gestation after in vitro fertilization. Fertil Steril. 2002;78(3):540–2. doi: 10.1016/S0015-0282(02)03278-8. [DOI] [PubMed] [Google Scholar]
- 24.Poikkeus P, Hiilesmaa V, Tiitinen A. Serum HCG 12 days after embryo transfer in predicting pregnancy outcome. Hum Reprod. 2002;17(7):1901–5. doi: 10.1093/humrep/17.7.1901. [DOI] [PubMed] [Google Scholar]
- 25.Alahakoon TI, Crittenden J, Illingworth P. Value of single and paired serum human chorionic gonadotropin measurements in predicting outcome of in vitro fertilisation pregnancy. Aust N Z J Obstet Gynaecol. 2004;44(1):57–61. doi: 10.1111/j.1479-828X.2004.00172.x. [DOI] [PubMed] [Google Scholar]
- 26.Winter E, Wang J, Davies MJ, Norman R. Early pregnancy loss following assisted reproductive technology treatment. Hum Reprod. 2002;17(12):3220–3. doi: 10.1093/humrep/17.12.3220. [DOI] [PubMed] [Google Scholar]
- 27.Cho YJ, Kim JY, Song IO, Lee HS, Lim CK, Koong MK, et al. Does blastomere biopsy in preimplantation genetic diagnosis affect early serum beta-hCG levels? Clin Exp Reprod Med. 2011;38(1):31–6. doi: 10.5653/cerm.2011.38.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Practice Committee of the American Society for Reproductive Medicine and the Practice Committee of the Society for Assisted Reproductive Technology Guidelines on number of embryos transferred. Fertil Steril. 2009;92(5):1518–9. doi: 10.1016/j.fertnstert.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 29.Annan JJ, Gudi A, Bhide P, Shah A, Homburg R. Biochemical pregnancy during assisted conception: a little bit pregnant. J Clin Med Res. 2013;5(4):269–74. doi: 10.4021/jocmr1008w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Confino E, Demir RH, Friberg J, Gleicher N. The predictive value of hCG beta subunit levels in pregnancies achieved by in vitro fertilization and embryo transfer: an international collaborative study. Fertil Steril. 1986;45(4):526–31. doi: 10.1016/S0015-0282(16)49282-4. [DOI] [PubMed] [Google Scholar]
- 31.Lawler CC, Budrys NM, Rodgers AK, Holden A, Brzyski RG, Schenken RS. Serum beta human chorionic gonadotropin levels can inform outcome counseling after in vitro fertilization. Fertil Steril. 2011;96(2):505–7. doi: 10.1016/j.fertnstert.2011.05.071. [DOI] [PubMed] [Google Scholar]
- 32.Reljič M, Knez J, Vlaisavljević V. Human chorionic gonadotropin levels are equally predictive for pregnancy outcome after fresh and vitrified-warmed blastocyst transfer. J Assist Reprod Genet. 2013;30(11):1459–63. doi: 10.1007/s10815-013-0099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raty R, Virtanen A, Koskinen P, Anttila L, Forsstrom J, Laitinen P, et al. Serum free beta-HCG and alpha-fetoprotein levels in IVF, ICSI and frozen embryo transfer pregnancies in maternal mid-trimester serum screening for Down’s syndrome. Hum Reprod. 2002;17(2):481–4. doi: 10.1093/humrep/17.2.481. [DOI] [PubMed] [Google Scholar]
- 34.Hui PW, Tang MH, Lam YH, Ng EH, Yeung WS, Ho PC. Maternal serum hCG and alpha-fetoprotein levels in pregnancies conceived after IVF or ICSI with fresh and frozen-thawed embryos. Hum Reprod. 2003;18(3):572–5. doi: 10.1093/humrep/deg153. [DOI] [PubMed] [Google Scholar]
- 35.Kathiresan AS, Cruz-Almeida Y, Barrionuevo MJ, Maxson WS, Hoffman DI, Weitzman VN, et al. Prognostic value of beta-human chorionic gonadotropin is dependent on day of embryo transfer during in vitro fertilization. Fertil Steril. 2011;96(6):1362–6. doi: 10.1016/j.fertnstert.2011.09.042. [DOI] [PubMed] [Google Scholar]
- 36.Orasanu B, Jackson KV, Hornstein MD, Racowsky C. Effects of culture medium on HCG concentrations and their value in predicting successful IVF outcome. Reprod Biomed Online. 2006;12(5):590–8. doi: 10.1016/S1472-6483(10)61185-6. [DOI] [PubMed] [Google Scholar]
- 37.Gold RS, Azem F, Yovel I, Wagman I, Amit A, Lessing JB. Does ICSI affect early serum beta-HCG in pregnancies achieved after IVF? Hum Reprod (Oxford, England) 2000;15(6):1221–4. doi: 10.1093/humrep/15.6.1221. [DOI] [PubMed] [Google Scholar]
- 38.al-Sebai MA, Diver M, Hipkin LJ. The role of a single free beta-human chorionic gonadotrophin measurement in the diagnosis of early pregnancy failure and the prognosis of fetal viability. Hum Reprod (Oxford, England) 1996;11(4):881–8. doi: 10.1093/oxfordjournals.humrep.a019271. [DOI] [PubMed] [Google Scholar]
- 39.Singh N, Goyal M, Malhotra N, Tiwari A, Badiger S. Predictive value of early serum beta-human chorionic gonadotrophin for the successful outcome in women undergoing in vitro fertilization. J Hum Reprod Sci. 2013;6(4):245–7. doi: 10.4103/0974-1208.126312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugantha SE, Webster S, Sundar E, Lenton EA. Predictive value of plasma human chorionic gonadotrophin following assisted conception treatment. Hum Reprod. 2000;15(2):469–73. doi: 10.1093/humrep/15.2.469. [DOI] [PubMed] [Google Scholar]
- 41.Stone BA, Vargyas JM, Ringler GE, March CM, Marrs RP. The rate at which serum total β-subunit human chorionic gonadotropin increases after embryo transfer is a predictor of the viability of pregnancy and an identifier of determinants of pregnancy. Fertil Steril. 2006;86(6):1626–33. doi: 10.1016/j.fertnstert.2006.04.048. [DOI] [PubMed] [Google Scholar]
- 42.Batzer FR, Weiner S, Corson SL, Schlaff S, Otis C. Landmarks during the first forty-two days of gestation demonstrated by the beta-subunit of human chorionic gonadotropin and ultrasound. Am J Obstet Gynecol. 1983;146(8):973–9. doi: 10.1016/0002-9378(83)90977-8. [DOI] [PubMed] [Google Scholar]
- 43.Badawy AM, Khiary M, Sherif LS, Hassan M, Ragab A, Abdelall I. Low-molecular weight heparin in patients with recurrent early miscarriages of unknown aetiology. J Obstet Gynaecol. 2008;28(3):280–4. doi: 10.1080/01443610802042688. [DOI] [PubMed] [Google Scholar]
- 44.Fawzy M, Shokeir T, El-Tatongy M, Warda O, El-Refaiey AA, Mosbah A. Treatment options and pregnancy outcome in women with idiopathic recurrent miscarriage: a randomized placebo-controlled study. Arch Gynecol Obstet. 2008;278(1):33–8. doi: 10.1007/s00404-007-0527-x. [DOI] [PubMed] [Google Scholar]
- 45.Han AR, Ahn H, Vu P, Park JC, Gilman-Sachs A, Beaman K, et al. Obstetrical outcome of anti-inflammatory and anticoagulation therapy in women with recurrent pregnancy loss or unexplained infertility. Am J Reprod Immunol. 2012;68(5):418–27. doi: 10.1111/j.1600-0897.2012.01178.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 296 kb)