Abstract
Purpose
We investigated antioxidant effects of CoQ10 supplementation on the prevention of OS-induced ovarian damage and to evaluate the protective effect of such supplementation against OS-related DNA damage.
Methods
Twenty-four adult female Sprague–Dawley rats were randomly divided into three groups (8 rats per group): group 1 (control): saline, ip, and orally; group 2 (cisplatin group): cisplatin, 4.5 mg/kg ip, two times with an interval of 7 days; and group 3 (cisplatin + CoQ10 group): cisplatin, 4.5 mg/kg ip, two times with an interval of 7 days, and 24 h before cisplatin, 150 mg/kg/day orally in 1 mL of saline daily for 14 days. Serum concentrations of anti-Mullerian hormone (AMH), number of AMH-positive follicles, the assessment of the intensity of 8'OHdG immunoreactivity, the primordial, antral and atretic follicle counts in the ovary were assessed.
Result(s)
The mean serum AMH concentrations were 1.3 ± 0.19, 0.16 ± 0.03, and 0.27 ± 0.20 ng/mL in groups 1, 2, and 3, respectively (p < 0.01). Serum AMH levels were significantly higher in group 1 compared to groups 2 and 3 (p < 0.01 and p = 0.01, respectively). There was a statistically significant difference in AMH-positive follicle count between the groups (p < 0.01). Group 1 showed higher numbers of AMH-positive granulosa cells compared to group 2 (p = 0.01). A significant difference was found in the primordial, the atretic, and antral follicle counts between the three groups (p < 0.01, p < 0.01, and p < 0.01, respectively). The atretic follicle count was significantly lower in the cisplatin plus CoQ10 group compared to the cisplatin group (p < 0.01). The antral follicle counts were significantly higher in the cisplatin plus CoQ10 group compared with the cisplatin group (p < 0.01). There was a statistically significant difference in the intensity of staining of the follicles that were positive for anti-8'OHdG between the groups (p = 0.02). Group 1 showed a significant lower intensity of staining of the follicles positive for anti-8'OHdG compared with group 2 (p = 0.03).
Conclusion(s)
CoQ10 supplementation may protect ovarian reserve by counteracting both mitochondrial ovarian ageing and physiological programmed ovarian ageing although the certain effect of OS in female infertility is not clearly known.
Keywords: AMH, Ovarian reserve, Oxidative damage, 8'OHdG, Coenzyme Q10
Introduction
Reactive oxygen species (ROS) consisting of superoxide anion, hydroxyl radical (OH), and hydrogen peroxide are naturally generated by normal oxygen metabolism during some physiological conditions. They are generally regulated by enzymatic and nonenzymatic antioxidant cell systems. Oxidative stress (OS) appears as a failure to convert ROS into an inactive state via the action of cellular antioxidant defences. OS is also known as the imbalance between the production of intracellular ROS and the capacity of antioxidant cell defences. ROS potentially create oxidative damage to basic tissue components due to being unstable and highly reactive when they interact with biomolecules such as proteins, lipids, and DNA, causing the distribution of their functions. ROS-induced DNA damage may potentially cause replication error, bases modification, induction of some signal pathways, genomic instability, mutation, and cell death [1]. ROS are unstable and highly reactive, and they can attack both pyrimidine and purine bases within DNA. OH is considered to be the predominant ROS that results in DNA damage. 8'OHdG resulting from the oxidation of guanosine is the most important modified base that has been identified for oxidative damage to DNA [2, 3]. Therefore, 8'OHdG is the best current available biomarker for determination of ROS-induced DNA damage [4].
Approximately, 90 % of ROS is generated by cell mitochondria [5]. Mitochondrial dysfunction (MD) is characterised by a higher level of ROS accumulation. Therefore, MD plays an important role in OS and OS-induced ageing of cells, which is similar to age-related changes of cells. All tissues as well as ovaries can affect the adverse effects of MD. Moreover, MD based on the free radical theory of ovarian ageing may be one of the main reasons for the onset of premature ageing. MD is strongly associated with poor reproductive performance due to high and long-time exposure to OS, which causes diminishing ovarian reserves, granulosa cell apoptosis, follicular atresia, chromosomal abnormalities, dysfunctional energy production, and poor oocyte quality [6, 7]. Antioxidants may be one of the keys to preventing reproductive performance against OS-induced ageing by improving mitochondrial function [8].
Coenzyme Q10 (CoQ10), a fat-soluble component of nearly all cell membranes, is located in the inner mitochondrial membrane. CoQ10 is an essential component for transporting electrons in the mitochondrial respiratory chain to produce cellular energy. Moreover, the reduced form of CoQ10, known as ubiquinol, acts as an antioxidant in cellular metabolism via inhibition of lipid peroxidation, protein, and DNA oxidation [9, 10]. It was demonstrated that an adaptive response to OS is increased antioxidant defence against ROS products. The amount of CoQ10 in tissues is often decreased in many pathological conditions associated with OS, such as diabetes and Alzheimer’s and prion diseases. Although CoQ10 is synthesised by virtually all normal tissues, the tissue levels of CoQ10 gradually decrease with ageing [11]. Deficiencies in CoQ10 synthesis are associated with certain clinical disorders related to high energy-consuming tissues, including skeletal muscles, endocrine glands, and the nervous system [12]. Studies have demonstrated that CoQ10 supplementation causes a significant increase of CoQ10 levels in some tissues, such as muscle, sperm, and plasma, as well as a remarkable increase in the adrenal glands and ovaries [13–16]. Therefore, the supplementation of CoQ10 protects cells against ROS-induced damage due to its antioxidant properties, which strengthen endogenous cellular antioxidant systems.
Cisplatin is a platinum-derived chemotherapeutic agent that is commonly used in the treatment of some cancers. Cisplatin primarily causes DNA damage through DNA cross-linking and then cell death through the activation of signal transduction pathways triggering off the initiation of apoptosis [17]. Although primary action of cisplatin appears to be induction of DNA damage, it can also result in apoptosis of cell via the activation of an intrinsic mitochondrial pathway leading to a release of cytochrome c into the cytosol and the activation of endoplasmic reticulum stress [18]. It can result in toxic effects on gonads [19]. The negative effects of cisplatin on ovaries depend on several factors, including the dosage, the patient’s age at the time of treatment, ovarian cell types, and the specific agent administered. Different ovarian cell types may have differential vulnerabilities to chemotherapeutic agent [20]. Oocytes and somatic cells of growing follicles in ovary would be highly susceptible to the damaging effects of cisplatin. Although the mechanism of cisplatin toxicity on ovaries has not been clearly explained, in addition to DNA damage, it may be due to increased ROS production and decreased antioxidant capacity, which is similar to the demonstrated toxic effect of cisplatin on the kidneys and internal ear [19, 21, 22]. In our previous study, we demonstrated that cisplatin may cause oxidative stress-related ovarian damage, and this effect can be handled by an antioxidant [23]. Therefore, in the present study, cisplatin was used for OS occurring in ovarian tissue.
In this study, we focused on identifying antioxidant effects of CoQ10 supplementation on the prevention of OS-induced ovarian damage and evaluating the protective effect of such supplementation against OS-related DNA damage.
Materials and methods
Animals
Twenty-four adult female Sprague–Dawley rats (weight 200–250 g; age 65–75 days), which were used for this study, were provided by the Yeditepe University Animal Reproduction Center and housed in the Animal Laboratory of Yeditepe University (Istanbul, Turkey). The rats were caged under standard housing conditions with a 12 h light/dark cycle and ad libitum access to food and water. The study protocol was approved by the Institutional Animal Care and Use Committee of Yeditepe University. All procedures were performed in accordance with the National Academy of Science’s Guide for Care and Use of Laboratory Animals (1996).
Experimental design
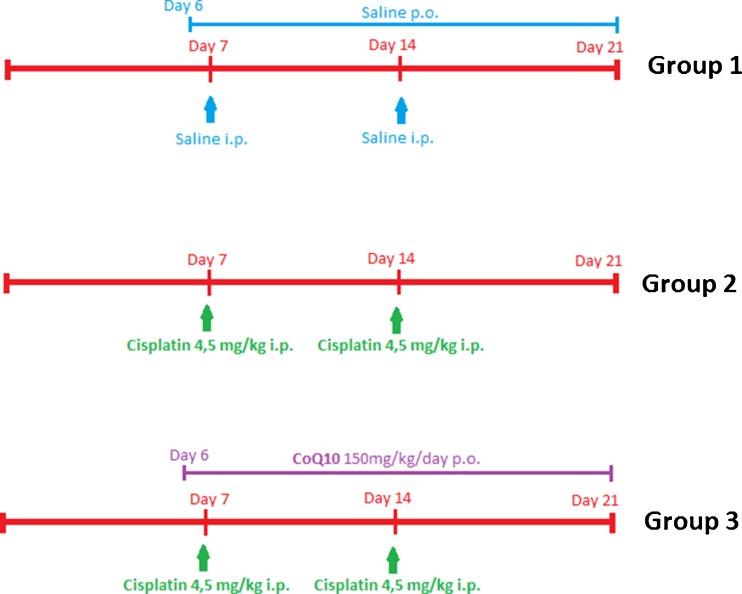
All rats were randomly divided into three groups (8 rats per group): group 1 (control): saline, ip, and orally; group 2 (cisplatin group): cisplatin, 4.5 mg/kg ip, two times with an interval of 7 days; and group 3 (cisplatin + CoQ10 group): cisplatin, 4.5 mg/kg ip, two times with an interval of 7 days, and CoQ10, 150 mg/kg/day orally in 1 mL of saline daily for 14 days via an orogastric tube, starting 24 h before the first cisplatin administration (Fig. 1).
Fig. 1.
The time line of the experiment
Drug administration
Cisplatin (Hospira, UK) was diluted in normal saline immediately before use, and two injections were administered with an interval of 7 days [24]. The lethal dose (50) of cisplatin in rats was 7.4 mg/kg [25]. CoQ10 (Gfn-Selco, Germany) was started 24 h before the first administration of cisplatin. A total of 150 mg/kg/day CoQ10 was given orally for 14 days via an orogastric tube [26]. The animals were sacrificed 14 days after initiation of CoQ10 treatment.
Sample collection
All the rats were anesthetised by intramuscular administration of 50 mg/kg ketamine hydrochloric acid (Ketalar; Eczacibasi Warner-Lambert Ilac Sanayi, Levent, Istanbul, Turkey) and 7 mg/kg xylazine hydrochloric acid (Rompun, Bayer Sisli, Istanbul, Turkey). After immobilising rats on a standard surgery board, their reproductive organs were exposed with a ventral midline incision utilising the aseptic technique. The ovaries were removed, and blood samples were collected.
Histological evaluations
The histopathologic examination was performed by a pathologist blinded to the groups. For light microscopy, specimens were fixed in 10 % neutral buffered formalin (NBF, 10 %) for 72 h, dehydrated in increasing concentrations of alcohol series (70, 90, 96, and 100 %), cleared in xylene, and embedded in paraffin. Paraffin sections (sectioned at 5-mm thickness, four sections per sample) were stained with haematoxylin and eosin (H&E) for morphological analysis. Atretic, antral, and primordial follicles were evaluated using the techniques described by Tilly [27].
Immunohistochemistry
Three sections of formalin-fixed ovarian tissues stained with H&E were randomly selected from one ovary for immunohistochemistry examination. The immunohistochemistry procedure was performed as described in our previous study [23]. The anti-Mullerian hormone (AMH)-positive follicles were analysed according to two factors: the intensity (0 to 3 as follows: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining) and the distribution of staining (1 or 2, as follows: 1, ≤50 % of the structure staining and 2, ≥50 % of the structure staining) [28]. The total score was calculated by multiplying the intensity by the distribution. A score of ≥2 was considered positive for AMH. Immunohistochemical staining was scored in a semi-quantitative manner in order to determine differences between the control group and the experimental groups.
8'OHdG Immunohistochemistry
Sections from paraffine blocks were incubated overnight at 37 °C. After deparaffinisation with xylene and rehydration in descendant grades of ethanol, the sections were incubated in 3 % hydrogen peroxide in methanol for 10 min to inhibit endogenous enzyme blockage and then washed with tap and distilled water. Then, the sections were microwaved at 200 W with a citrate buffer (pH 6.1) for 20 min for antigen retrieval. The slices were cooled at room temperature. After they were washed with phosphate buffered saline (PBS), they were incubated in blocking solution for 10 min and then incubated in mouse anti 8'OHdG primary antibody (1:2000, Abcam, Cat: ab62623) at 4 °C overnight. Secondary antibody staining was performed using the Histostain®-Plus 3rd Gen IHC Detection Kit (Cat: 85-9073, Invitrogen, CA, USA) following the manufacturer’s protocol. After washing, sections were incubated with streptavidin–peroxidase (ready-to-use) for 10 min at room temperature, followed by incubation with 3, 3′-diaminobenzidine (DAB) for 5 min. Slides were finally counterstained with Mayer’s haematoxylin and covered with mounting medium. Control samples were processed in the same manner, except that the primary antibody was omitted. 8'OHdG immunohistochemistry was examined under a photomicroscope (Nikon Eclipse i5, Tokyo, Japan). An observer blinded to the experimental groups evaluated the staining intensity semi-quantitatively. The staining intensity of follicles was graded as none (0), less (1), weak (2), dense (3), and intense (4), respectively.
Serum AMH concentrations
Serum AMH levels were measured by USCN Life Science enzyme-linked immunosorbent assays (ELISA). The assay range was 0.31–20 ng/mL, and the minimum detectable dose of this assay was less than 0.078 ng/mL. The technician was unaware of the treatment allocation.
Statistical analysis
Based on power analysis, 4 rats in each group were required to assess statistical significance (power of 0.95 and α = 0.05). Power calculation was based on serum AMH levels [23]. The results were analysed using the Statistical Package for the Social Sciences version 21.0 (SPSS, Chicago, IL). Data were reported as mean ± standard deviation (SD). Kruskal-Wallis tests were conducted to compare the variables amongst the groups. Differences in measured parameters between the three groups were analysed using a Kruskal–Wallis test followed by Mann–Whitney U post hoc test to test the significance of pairwise differences using Bonferroni correction to adjust for multiple comparisons. After Bonferroni correction, p < .016 was considered statistically significant. P < .05 was considered statistically significant.
Results
The mean serum AMH concentrations were 1.3 ± 0.19, 0.16 ± 0.03, and 0.27 ± 0.20 ng/mL in groups 1, 2, and 3, respectively (p < 0.01) (Table 1). Serum AMH levels were significantly higher in group 1 compared to groups 2 and 3 (p < 0.01 and p = 0.01, respectively). There was no significant difference between groups 2 and 3 (p = 1).
Table 1.
The evaluation of serum concentrations of AMH, number of AMH-positive follicles, and semi-quantitative assessment of the intensity of 8'OHdG immunoreactivity in ovary
| Variables | Group 1–control (n = 8) | Group 2—cisplatin (n = 8) | Group 3—cisplatin plus Coenzyme Q10 (n = 8) | p value |
|---|---|---|---|---|
| Serum concentrations of AMH (ng/ml) | 1.3 ± 0.19a,b | 0.16 ± 0.03a | 0.27 ± 0.20b | <0,01* |
| Number of AMH-positive follicles | 8.87 ± 2.64c | 2.87 ± 2.1c | 5 ± 2.61 | <0,01* |
| Intensity of 8'OHdG immunoreactivity | 1.3 ± 0.82d | 2.3 ± 0.95d | 2.2 ± 0.89 | 0,02* |
All values are expressed as mean ± SD
*p < 0.05, significant difference, comparison of all groups
a p = <0.01, b p = 0.01 (comparison of serum concentrations of AMH, acisplatin vs control, bcontrol vs cisplatin + CoQ10)
c p = 0.01 (comparison of number of AMH-positive follicles, control vs cisplatin)
d p = 0.03 (comparison of Intensity of 8'OHdG immunoreactivity, control group and cisplatin group)
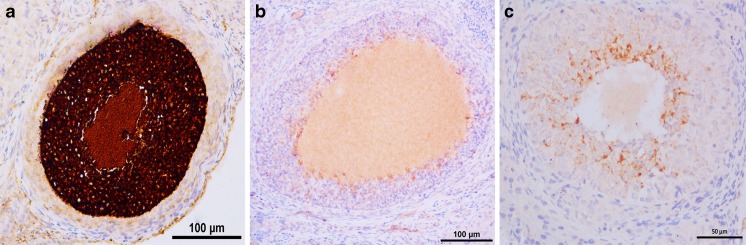
The quantitative analysis for AMH-positive follicles in all groups is shown in Fig. 2. There was a statistically significant difference in AMH-positive follicle count between the groups (p < 0.01) (Table 1). Group 1 showed higher numbers of AMH-positive granulosa cells compared to group 2 (p = 0.01). Although there was no significant difference between group 2 and group 3 in terms of the quantitative analysis of AMH-positive follicle, group 3 had higher numbers of AMH-positive granulosa cells (p = 0.3).
Fig. 2.
The immunohistochemical detection of intensity of staining of the follicles positive for anti-Mullerian hormone. a Group 1 (control, saline), very high, 5 (++++).; b group 2 (cisplatin group, 4.5 mg/kg cisplatin), a few, 2 (+); and c group 3 (cisplatin plus Coenzyme Q10 group, 4.5 mg/kg cisplatin + 150 mg/kg/day Coenzyme Q10), medium, 4 (++). Panel bar = 100 μm
The primordial, antral, and atretic follicle counts of all groups are shown in Table 2. A significant difference was found in the primordial follicle counts between the three groups (p < 0.01). The evaluation of the atretic and antral follicle counts revealed statistically significant differences between the groups (p < 0.01 and p < 0.01, respectively). The cisplatin group has the lowest antral and the highest atretic follicle count. The atretic follicle count was significantly lower in the cisplatin plus CoQ10 group compared to the cisplatin group (p < 0.01). The antral follicle counts were significantly higher in the cisplatin plus CoQ10 group compared with the cisplatin group (p < 0.01).
Table 2.
Comparision of the primordial, antral, and atretic follicle counts of all groups
| Variables | Group 1—control (n = 8) | Group 2—cisplatin (n = 8) | Group 3—cisplatin plus Coenzyme Q10 (n = 8) | P value |
|---|---|---|---|---|
| Primordial follicle counts | 15.80 ± 7.45 | 8 ± 1.65 | 9.92 ± 4.17 | <0.01* |
| Antral follicle counts | 4.33 ± 1.30 | 2 ± 0.86a | 4.14 ± 1.51a | <0.01* |
| Atretic follicle counts | 1.28 ± 1.03 | 2.08 ± 0.87b | 0.8 ± 0.41b | <0.01* |
All values are expressed as mean ± SD
*p < 0.05, significant difference, comparison of all groups
a p = <0.01 (comparison of antral follicle counts, cisplatin vs cisplatin + CoQ10)
b p = <0.01 (comparison of atretic follicle counts cisplatin vs cisplatin + CoQ10)
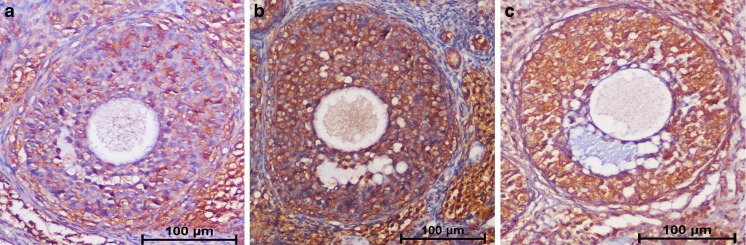
The semi-quantitative analysis of intensity of staining of the follicles positive for anti- 8'OHdG is shown in Fig. 3. There was a statistically significant difference in the intensity of staining of the follicles that were positive for anti-8'OHdG between the groups (p = 0.02) (Table 1). Group 1 showed a lower intensity of staining of the follicles positive for anti-8'OHdG compared with group 2 (p = 0.03). There is no significant difference between groups 1 and 2 in terms of intensity of staining of the follicles positive for anti-8'OHdG (p = 0.06).
Fig. 3.
The immunohistochemical detection of intensity of staining of the follicles positive for anti-8'OHdG. a Group 1 (control, saline), weak, (+); b group 2 (cisplatin group, 4.5 mg/kg cisplatin), dense,(++); and c group 3 (cisplatin plus Coenzyme Q10 group, 4.5 mg/kg cisplatin + 150 mg/kg/day Coenzyme Q10), dense, (++). Panel bar = 100 μm
Discussion
Most stages of human reproduction, including steroidogenesis, oocyte maturation, fertilisation, and embryo development require high energy. Therefore, the number of mitochondria markedly increases to provide adequate energy production to meet the metabolic requirements during the transition of a primordial follicle into a primary follicle. All energy-dependent mechanisms of human fertility are influenced by MD. MD based on OS, known as free radical theory, is the second most commonly accepted theory and is strongly associated with ovarian ageing after physiological programmed ageing, which is the most commonly accepted theory [29]. Because MD results in an increased accumulation of ROS and mtDNA mutations, the increased release of intracellular ROS due to MD, which cannot be counteracted by antioxidant defences similar to those of ageing cells, induces damage to ovarian tissue [6]. The exposure to high levels of ROS may eventually contribute to granulosa cell apoptosis, follicular atresia, chromosomal abnormalities, oocyte ageing, and infertility [7]. Older cells tend to produce more oxidants and less ATP than younger cells from their mitochondria because of a positive correlation between the level of ROS production and chronological age. The potentially adverse effects of OS in most stages of human fertility can be counteracted by administration of several mitochondrial nutrients known as antioxidants, which have been demonstrated to increase energy production in mitochondria and to protect cells from OS. Alpha-lipoic acid (RALA), vitamin C, CoQ10, and resveratrol are the best and most commonly used antioxidants in current studies [8]. Several studies evaluating the supplementation of several mitochondrial nutrients as antioxidants suggest that the abnormalities resulting from MD based on ovarian ageing may be counteracted by the use of antioxidants [7, 8, 30].
In the current study, we focused mainly on the potential protective effect of CoQ10 on OS-related ovarian damage and ovarian reserve. The antral follicle count dramatically increased when using CoQ10 in combination with cisplatin, whereas the atretic follicle count significantly decreased. Serum AMH levels and AMH-positive follicles were found to be higher in the cisplatin plus CoQ10 group than in the cisplatin group, though the difference was not statistically significant. In addition, there was a trend towards decreasing the intensity of 8'OHdG immunoreactivity with CoQ10 in combination with cisplatin. These results suggest that CoQ10 could be effective to protect ovarian reserve and prevent ovarian damage by counteracting OS-related ovarian damage. However, according to our results, the differences between the cisplatin plus CoQ10 group and the cisplatin group, in terms of serum AMH levels and AMH-positive follicles, did not achieve statistically significance. One of the possible reasons for this may be early performed analysis following an acute exposure, whilst the animals were still under the effect of cisplatin. If the animals were operated to remove the ovaries a few weeks after the last administered dose of CoQ10, the results could better reflect the possible beneficial effect of CoQ10 on serum AMH concentration and the number of AMH-positive follicles due to better long-term recovery. Other possible reason may be explained by challenges in dosing and schedule of CoQ10 administration. Improvements in CoQ10 supplementation such as, increasing the dose, longer duration of treatment, and/or earlier initiation of CoQ10 supplementation prior to cisplatin exposure may better demonstrate the possible protective role of CoQ10. In this study, we evaluated the effect of only a single dose and treatment schedule, thus further evaluations are needed to determine optimal dosing and schedule. Lastly, primary action of cisplatin appears to be induction of DNA damage through DNA cross-linking, although cisplatin can also cause the adverse effects on the ovaries via the activation of an intrinsic mitochondrial pathway [18]. Therefore, CoQ10 is effective to likely only protect the ovaries from the ROS-forming component of the cisplatin action. Likewise, our data were obtained using an experimental animal study and do not definitely predict accurate results for human ovaries. Therefore, the dose, schedule, and effect of CoQ10 on human ovaries should be investigated.
Gendelman and Roth evaluated the effect of CoQ10 on the improvement of mitochondrial features of bovine oocytes collected during different seasons [31]. Their study demonstrated that the mitochondrial distribution pattern decreased in bovine oocytes maturing in vitro with 50 lM in the fall, and they concluded that CoQ10 incorporation could decrease season-induced deleterious effects on mitochondrial function. Quinzii et al. created a model of CoQ10 deficiency, typically seen in the elderly, with increasing dosages of 4-nitrobenzoate in multiple cell lines, and they evaluated the effect of CoQ10 levels on mitochondrial function, oxidative stress, and cell death [32]. Their findings suggest that CoQ10 deficiency results in an increase in ROS production and oxidative stress.
In an aged mouse model, Burstein et al. compared the effect of CoQ10, resveratrol, and R-ALA for a period of 18 weeks before superovulation on the number of ovulated oocytes and oocyte mitochondrial function [33]. Their study showed that CoQ10 treatment significantly increased the number of ovulated oocytes, improved oocyte mitochondrial function, and reduced ROS levels in elderly mice to levels similar to those of oocytes in young animals. However, their study was only presented as an abstract. Ben-Meir et al evaluated the effect of CoQ10, alpha lipoic acid, and resveratrol supplementation on impaired oocyte mitochondrial function and ovarian reserve. These stimulators of mitochondrial bioenergetics were subcutaneously injected to 9-month-old mice. The results of this study demonstrated that CoQ10 supplementation preserves ovarian reserve and improves mitochondrial performance in oocytes and ovulation rates. In addition, increase in ratio of gametes which are able to support normal development was observed [34]. A controlled randomised trial designed by Bentov et al. evaluated the effect of CoQ10 on post-meiotic oocyte aneuploidy rate in women undergoing IVF, finding that the rate of aneuploidy and clinical pregnancy were, respectively, 46.5 and 33 % in the CoQ10 group and 62.8 and 26.7 % in the control group [35]. According to these results, they concluded that there were no significant differences in outcome between the CoQ10 and control groups. However, due to safety concerns regarding the effects of biopsy on embryo quality and implantation, their study was terminated before reaching a sufficient number to detect a difference in the rate of aneuploidy. In a prospective controlled randomised trial, El Refaeey et al. investigated whether the combination of oral CoQ10 and clomiphene citrate improved the ovulation induction response in women with clomiphene-resistant polycystic ovary syndrome [36]. They found that this combination is an effective and safe option for improving ovulation and clinical pregnancy rates.
In conclusion, to the best of our knowledge, our study is the first to evaluate the protective effect of CoQ10 on ovarian reserve and on oxidative DNA damage in the ovaries. In light of the above-mentioned studies and our study results, we conclude that there is a strong relationship between ovarian ageing and OS, and CoQ10 is effective to likely only protect the ovaries from the ROS-forming component of the cisplatin action. Therefore, CoQ10 supplementation may protect ovarian reserve by improving mitochondrial function, counteracting both mitochondrial ovarian ageing and physiological programmed ovarian ageing although the certain effect of OS in female infertility is not clearly known. We attempt to provide new evidence for the protective effect of CoQ10 on OS-induced ovarian damage, which is one of the most important and widely accepted pathomechanisms underlying cell ageing. This could also lead to biochemical alternatives for the management of women with poor ovarian reserve during IVF treatment. Further experimental studies need to be designed to define the optimum dosage and duration for CoQ10 supplementation to enhance its protective effects. Furthermore, future studies are needed to understand the effect of CoQ10 on ovaries and human reproduction.
Compliance with ethical standards
The study protocol was approved by the Institutional Animal Care and Use Committee of Yeditepe University. All procedures were performed in accordance with the National Academy of Science’s Guide for Care and Use of Laboratory Animals (1996).
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Capsule CoQ10 supplementation may protect ovarian reserve by counteracting both mitochondrial ovarian ageing and physiological programmed ovarian ageing.
References
- 1.Wilson DM, Sofinowski TM, McNeill DR. Repair mechanisms for oxidative DNA damage. Front Biosci. 2003;1(8):963–81. doi: 10.2741/1109. [DOI] [PubMed] [Google Scholar]
- 2.Kasai H, Tanooka H, Nishimura S. Formation of 8-hydroxyguanine residues in DNA by X-irradiation. Gan. 1984;75(12):1037–9. [PubMed] [Google Scholar]
- 3.Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2'-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat Res. 1997;387(3):147–63. doi: 10.1016/S1383-5742(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 4.Klaunig JE, Wang Z, Pu X, Zhou S. Oxidative stress and oxidative damage in chemical carcinogenesis. Toxicol Appl Pharmacol. 2011;254(2):86–99. doi: 10.1016/j.taap.2009.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Nickel A, Kohlhaas M, Maack C. Mitochondrial reactive oxygen species production and elimination. J Mol Cell Cardiol. 2014;73:26–33. doi: 10.1016/j.yjmcc.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Meldrum DR. Aging gonads, glands, and gametes: immutable or partially reversible changes? Fertil Steril. 2013;99(1):1–4. doi: 10.1016/j.fertnstert.2012.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Li Q, Geng X, Zheng W, Tang J, Xu B, Shi Q. Current understanding of ovarian aging. Sci China Life Sci. 2012;55(8):659–69. doi: 10.1007/s11427-012-4352-5. [DOI] [PubMed] [Google Scholar]
- 8.Bentov Y, Casper RF. The aging oocyte—can mitochondrial function be improved? Fertil Steril. 2013;99(1):18–22. doi: 10.1016/j.fertnstert.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 9.Santos-Ocana C, Do TQ, Padilla S, Navas P, Clarke CF. Uptake of exogenous coenzyme Q and transport to mitochondria is required for bc1 complex stability in yeast coq mutants. J Biol Chem. 2002;277(13):10973–81. doi: 10.1074/jbc.M112222200. [DOI] [PubMed] [Google Scholar]
- 10.Villalba JM, Navas P. Plasma membrane redox system in the control of stress-induced apoptosis. Antioxid Redox Signal. 2000;2000(2):213–30. doi: 10.1089/ars.2000.2.2-213. [DOI] [PubMed] [Google Scholar]
- 11.Pignatti C, Cocchi M, Weiss H. Coenzyme Q10 levels in rat heart of different age. Biochem Exp Biol. 1980;16(1):39–42. [PubMed] [Google Scholar]
- 12.Quinzii CM, Hirano M, DiMauro S. CoQ10 deficiency diseases in adults. Mitochondrion. 2007;(7Suppl):122–6 [DOI] [PMC free article] [PubMed]
- 13.Balercia G, Mosca F, Mantero F, Boscaro M, Mancini A, Ricciardo-Lamonica G, et al. Coenzyme Q(10) supplementation in infertile men with idiopathic asthenozoospermia: an open, uncontrolled pilot study. Fertil Steril. 2004;81(1):93–8. doi: 10.1016/j.fertnstert.2003.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Bentinger M, Tekle M, Brismar K, Chojnacki T, Swiezewska E, Dallner G. Stimulation of coenzyme Q synthesis. Biofactors. 2008;32(1-4):99–111. doi: 10.1002/biof.5520320112. [DOI] [PubMed] [Google Scholar]
- 15.Cooke M, Iosia M, Buford T, Shelmadine B, Hudson G, Kerksick C, et al. Effects of acute and 14-day coenzyme Q10 supplementation on exercise performance in both trained and untrained individuals. J Int Soc Sports Nutr. 2008;4(5):8. doi: 10.1186/1550-2783-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mizuno K, Tanaka M, Nozaki S, Mizuma H, Ataka S, Tahara T, et al. Antifatigue effects of coenzyme Q10 during physical fatigue. Nutrition. 2008;24(4):293–9. doi: 10.1016/j.nut.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Siddik Z. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–79. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 18.Morgan S, Anderson RA, Gourley C, Wallace WH, Spears N. How do chemotherapeutic agents damage the ovary? Hum Reprod Update. 2012;18(5):525–35. doi: 10.1093/humupd/dms022. [DOI] [PubMed] [Google Scholar]
- 19.Tangir J, Zelterman D, Ma W, Schwartz PE. Reproductive function after conservative surgery and chemotherapy for malignant germ cell tumors of the ovary. Obstet Gynecol. 2003;101(2):251–7. doi: 10.1016/s0029-7844(02)02508-5. [DOI] [PubMed] [Google Scholar]
- 20.Yuksel A, Bildik G, Senbabaoglu F, Akin N, Arvas M, Unal F, et al. The magnitude of gonadotoxicity of chemotherapy drugs on ovarian follicles and granulosa cells varies depending upon the category of the drugs and the type of granulosa cells. Hum Reprod. 2015;30(12):2926–35. doi: 10.1093/humrep/dev256. [DOI] [PubMed] [Google Scholar]
- 21.Chirino YI, Pedraza-Chaverri J. Role of oxidative and nitrosative stress in cisplatin-induced nephrotoxicity. Exp Toxicol Pathol. 2009;61(3):223–42. doi: 10.1016/j.etp.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Yucebilgin MS, Terek MC, Ozsaran A, Akercan F, Zekioglu O, Isik E, et al. Effect of chemotherapy on primordial follicular reserve of rat: an animal model of premature ovarian failure and infertility. Aust N Z J Obstet Gynaecol. 2004;44(1):6–9. doi: 10.1111/j.1479-828X.2004.00143.x. [DOI] [PubMed] [Google Scholar]
- 23.Ozcan P, Fıçıcıoğlu C, Yıldırım ÖK, Özkan F, Akkaya H, Aslan İ. Protective effect of resveratrol against oxidative damage to ovarian reserve in female Sprague-Dawley rats. Reprod Biomed Online. 2015;31(3):404–10. doi: 10.1016/j.rbmo.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Yang S, Lv X, Sun H, Weng J, Liang Y, et al. The mechanism of mesna in protection from cisplatin-induced ovarian damage in female rats. J Gynecol Oncol. 2013;24(2):177–85. doi: 10.3802/jgo.2013.24.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dale O, Mortensen B, Thommesen L, Hagen B. Cisplatin toxicity in the rat may be influenced by anaesthetic agents. Acta Anaesthesiol Scand. 2000;44(6):770. doi: 10.1034/j.1399-6576.2000.440619.x. [DOI] [PubMed] [Google Scholar]
- 26.Kwong LK, Kamzalov S, Rebrin I, Bayne AC, Jana CK, Morris P, et al. Effects of coenzyme Q(10) administration on its tissue concentrations, mitochondrial oxidant generation, and oxidative stress in the rat. Free Radic Biol Med. 2002;33(5):627–38. doi: 10.1016/S0891-5849(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 27.Tilly JL. Ovarian follicle counts—not as simple as 1, 2, 3. Reprod Biol Endocrinol. 2003;6(1):11. doi: 10.1186/1477-7827-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeh J, Kim BS, Peresie J. Protection against cisplatin-induced ovarian damage by the antioxidant sodium 2-mercaptoethanesulfonate (mesna) in female rats. Am J Obstet Gynecol. 2008;198(4):463.e1-6. doi: 10.1016/j.ajog.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 29.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 30.Bentov Y, Esfandiari N, Burstein E, Casper RF. The use of mitochondrial nutrients to improve the outcome of infertility treatment in older patients. Fertil Steril. 2010;93(1):272–5. doi: 10.1016/j.fertnstert.2009.07.988. [DOI] [PubMed] [Google Scholar]
- 31.Gendelman M, Roth Z. Incorporation of coenzyme Q10 into bovine oocytes improves mitochondrial features and alleviates the effects of summer thermal stress on developmental competence. Biol Reprod. 2012;87(5):118. doi: 10.1095/biolreprod.112.101881. [DOI] [PubMed] [Google Scholar]
- 32.Quinzii CM, Tadesse S, Naini A, Hirano M. Effects of inhibiting CoQ10 biosynthesis with 4-nitrobenzoate in human fibroblasts. PLoS ONE. 2012;7(2):30606. doi: 10.1371/journal.pone.0030606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burstein E, Perumalsamy A, Bentov Y, Esfandiari N, Jurisicova A, Casper RF. Co-enzyme Q10 supplementation improves ovarian response and mitochondrial function in aged mice. Fertil Steril. 2009;92(3-1):31. doi: 10.1016/j.fertnstert.2009.07.121. [DOI] [Google Scholar]
- 34.Ben-Meir A, Burstein E, Borrego-Alvarez A, Chong J, Wong E, Yavorska T, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14(5):887–95. doi: 10.1111/acel.12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bentov Y, Hannam T, Jurisicova A, Esfandiari N, Casper RF. Coenzyme Q10 Supplementation and Oocyte Aneuploidy in Women Undergoing IVF-ICSI Treatment. Clin Med Insights Reprod Health. 2014;8:31–6. doi: 10.4137/CMRH.S14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Refaeey A, Selem A, Badawy A. Combined coenzyme Q10 and clomiphene citrate for ovulation induction in clomiphene-citrate-resistant polycystic ovary syndrome. Reprod Biomed Online. 2014;29(1):119–24. doi: 10.1016/j.rbmo.2014.03.011. [DOI] [PubMed] [Google Scholar]