Abstract
Purpose
Using a rabbit model, we assessed the influence of sperm DNA longevity on female reproductive outcomes.
Methods
Semen was collected from 40 bucks, incubated at 38 °C for 24 h, and the rate of sperm DNA fragmentation (rSDF) was determined using the sperm chromatin dispersion assay. Males were allocated into high rSDF (>0.5 units of increase per hour) or low rSDF (<0.5 units of increase per hour) groups. High and low rSDF semen samples were sequentially artificially inseminated into the same doe to reduce female factor variability, and pregnancy outcomes were recorded.
Results
While there was no difference in SDFs between rSDF groups immediately after collection (T0), differences were significant after 2 h of incubation; SDFs determined at collection and rSDF behaved as independent characters (Pearson correlation = 0.099; P = 0.542). Following artificial insemination, the rate of stillborn pups was significantly higher in does inseminated by males with a high rSDF (14/21) compared to those with low rSDF (15/6); (contingency χ2 5.19; p = 0.022). The risk of stillborn when low rSDF rabbits were used for insemination was 0.16, but increased to 0.36 when high rSDF animals were used (odds ratio = 2.85; 95 % confidence interval = 1.4–2.7).
Conclusion(s)
Dynamic assessment of SDF coupled with natural multiple ovulation, high fecundity of the rabbit and control over female factor influence, provided a useful experimental model to demonstrate the adverse effect of reduced sperm DNA longevity on reproductive outcome.
Keywords: Oryctolagus cuniculus, Sperm DNA fragmentation, Still born, Sperm DNA longevity, Dynamic assay, Animal model
Introduction
Sperm DNA fragmentation (SDF) as a predictive index of human reproductive outcome is highly controversial [1]. SDF is usually assessed as a static or single value determined following ejaculation and then correlated with embryonic development or pregnancy [2–4]. We propose that this controversy is an unintended consequence of several issues. The first issue may be the fact that a proportion of the damaged sperm DNA may not necessarily be apparent immediately after collection but remains “cryptic.” For example, human sperm exposed to a range of damaging agents will only show an increase in DNA fragmentation once incubated under in vitro conditions [5]. The second issue is possibly associated with the lack of standardized technical processing conditions of sperm that could give a false indication of the true SDF value [6].
It is reasonable to assume that the most resistant gamete to iatrogenic damage should also be the one with a better chance of obtaining a positive reproductive outcome. Hence, SDF measured immediately after ejaculation may not be as informative of DNA integrity as to when the sperm is “challenged” over time by means of incubation. We suggest that it is possible to partially mimic the conditions in the female reproductive tract by conducting a dynamic assessment of DNA damage, overtime, and representing it as a rate of DNA fragmentation (rSDF); the loss of DNA integrity during incubation is not only time, temperature and species dependent, it is also associated with the sperm protamine composition of the individual or species in question [7–9].
Humans are a monotocous species, so females typically produce and release only one oocyte and the pregnancy either fails or succeeds. Although ART procedures allow the recovery of several oocytes, the implanted embryos are also usually limited to one or two, limiting the ability to effectively evaluate the influence of sperm DNA stability on reproductive outcome. Moreover, oocyte quality may vary substantially between females making it a somewhat unavoidable but confounding influence when investigating the effect of sperm DNA quality on reproductive outcome. Polytocous species on the other hand, produce and release multiple oocytes such that the probability of detecting embryonic failure is increased. The rabbit is well known for its high fecundity, so it may be possible to obtain a higher resolution as regards the quality of the pregnancy in this polytocous species.
The probability of detecting SDF-associated embryonic failure can be further increased in the reproductive model, if female factors are reduced by using biologically similar oocytes and if it is possible to select semen samples that are known to exhibit a high rate of dynamic DNA damage and compare this directly to males with low or moderate rates of sperm DNA damage. Consequently, to confidently evaluate the influence of sperm DNA stability on reproductive outcome, a rabbit model may prove useful. The current study extends our initial validation experiments of rabbit sperm DNA fragmentation dynamics [10], categorising the sperm DNA longevity into a high and low rate of fragmentation and then exploring the fertility of the sample via artificial insemination of the same females on reproductive outcome. Our underlying hypothesis was that samples from bucks presenting a high rSDF are likely to produce a lower number of live kittens than those from individuals where in vitro DNA longevity is high.
Materials and methods
Animals, semen collection and artificial insemination
This study used HYLA commercial breed rabbits (Oryctolagus cuniculus) selected from a population resident in a commercial Spanish rabbit-breeding centre. The semen samples were collected as part of normal commercial operations of TARLAP REPRODUCCIÓ (Vila-Rodona, Tarragona, Spain), so no specific animal ethics approval was required. Hence, rabbit sperm DNA fragmentation and fertility data reported in this study were obtained retrospectively as a result of the standard quality control procedures within TARLAP REPRODUCCIÓ commercial operations and not from a purposely designed experiment. Methods to obtain semen from rabbits were performed in accordance with ethical standards and operational protocols of the commercial rabbit artificial insemination company TARLAP REPRODUCCIÓ (Vila-Rodona, Tarragona, Spain). Initially, semen samples were obtained using an artificial vagina (Amantea; IMV, Technologies, France) from 68 sexually mature males during the peak of the breeding season in spring. All sperm samples included in this study were selected after evaluating for a minimum level of sperm quality [11, 12] for ejaculate volume (>0.7 mL), sperm concentration (>250 × 106 sperm mL−1) and sperm motility (total motility >60 % and progressive motility >50 %); ejaculates not up to this minimum standard were not considered for insemination.
Experiment design
From the 68 bucks initially selected as proven breeders, 40 sexually mature males with a satisfactory seminogram were selected for further study according to their respective rSDF. To determine the sperm DNA dynamics of these ejaculates, freshly collected semen samples (E1) were stored at 38 °C for a period of 24 h in RPMI Media 1640 (Life Technologies; Carlsbad, California, US) at a sperm concentration of approximately 10 × 106 sperm mL−1. SDF was assessed using the sperm chromatin dispersion test following incubation for 0, 2, 7 and 24 h. Semen samples from these 68 bucks were then examined for the incidence of sperm DNA fragmentation, and two groups of animals showing high (n = 20) or low rSDF (n = 20) were arbitrarily created. Those males with a high rate of SDF (H-rSDF) showed an increase of >0.5 units per hour during a period of sperm incubation of 24 h at 38 °C, while those with a low rate of SDF (L-rSDF) displayed an increase of <0.5 units per hour under the same conditions. Immediately prior to artificial insemination, semen was once again collected (E2) from the designated L-rSDF (2 days after E1) or H-rSDF (approximately 2 months after E1) bucks and the ejaculates were re-evaluated for consistency of SDF over a 24-h incubation period at 38 °C. In the first phase of the artificial insemination experiment, L-rSDF males were analyzed for sperm DNA longevity and used for insemination purposes using 20 proven fertile females. Once these parturient females had given birth and passed through puerperium, the second phase of the experiment was conducted in which the same females were inseminated with semen from H-rSDF males; this aspect of the experimental design helped to reduce the influence of female factor. Following artificial insemination, the reproductive outcomes of does inseminated with either L-rSDF or H-rSDF in two separate AI trials were assessed in terms of their pregnancy rate and the number of live and stillborn kittens delivered. Pregnancy diagnosis was performed by means of abdominal palpation 12 to 15 days following artificial insemination.
The Halomax assay
The degree of DNA damage in each sample was quantified using a commercial version of the sperm chromatin dispersion test (Halomax, Halotech SL Madrid, Spain) validated for rabbit spermatozoa by Gosálvez et al. [10]. Given that the sperm concentration was relatively high (150 to 500 × 106 mL−1), all semen samples were adjusted to a concentration of 10 × 106 mL−1 using RPMI Media 1640 (Life Technologies; Carlsbad, California, US). A volume of 25 μL of diluted sample was added to a vial containing low melting point agarose at 38 °C and gently mixed. The agarose-sperm mixture (10 μL) was then prepared on pre-treated slides provided in the kit and covered with a glass coverslip. After gently pressing on the coverslip, each slide was placed in a refrigerator (4 °C) for 5 min to produce a microgel; once formed, the coverslip was removed from the microgel and the slide placed horizontally in 10 mL of the lysing solution provided in the kit for 5 min to achieve the controlled protein depletion. The treated microgel containing partially deproteinized sperm and was then subsequently washed in dH2O for 5 min and dehydrated in a series of ethanol baths (70, 90 and 100 %). Once dehydrated, sperm were stained with the DNA binding fluorochrome DAPI (D9542 Sigma-Aldrich; St. Louis, MO, USA) using a stock prepared at 10 % and diluted 1:10 with an anti-fading solution at the time of analysis under the microscope. Discrimination between fragmented and non-fragmented sperm DNA is very obvious after visualization of two or three different microscope fields (Fig. 1b, c). Not only was it possible to clearly characterize damaged DNA by the diameter of the halo (image analysis is not necessary), but there were also marked differences in the texture of the dispersed chromatin forming the halo. While halos of spermatozoa with damaged DNA were “spotty” and disorganized, spermatozoa without DNA fragmentation only possessed a small halo that was compact and homogenous (Fig. 1b, c).
Fig. 1.
Sperm chromatin dispersion (SCD) assay applied to rabbit spermatozoa: a untreated sperm; b sperm cells evaluated immediately (T0h) following SCD assay and c sperm cells evaluated at 24 h after incubation at 38 °C. Sperm with non-fragmented DNA show small homogenous halos of compact chromatin as a consequence of limited spreading of DNA loops, whereas those with fragmented DNA show larger halos composed of diffused and “spotty” DNA fragments
Statistical analysis
Statistical data was analysed using AnalystSoft, StatPlus: mac-statistical analysis program for Mac OS Version 2009 (http://www.analystsoft.com/en/) and SPSS 7.1 statistics package (IBM, NY, USA). SDF data for repeated ejaculates of the same individual bucks were correlated using Pearson’s R correlation. The means of SDF of each group (L- H-rSDF) were compared using non parametric statistics. Contingency χ2, odds ratios were calculated using the SPSS 7.1 statistics package (IBM, NY, USA). The alpha value for all statistical analysis was set at 0.05.
Results
General parameters for SDF
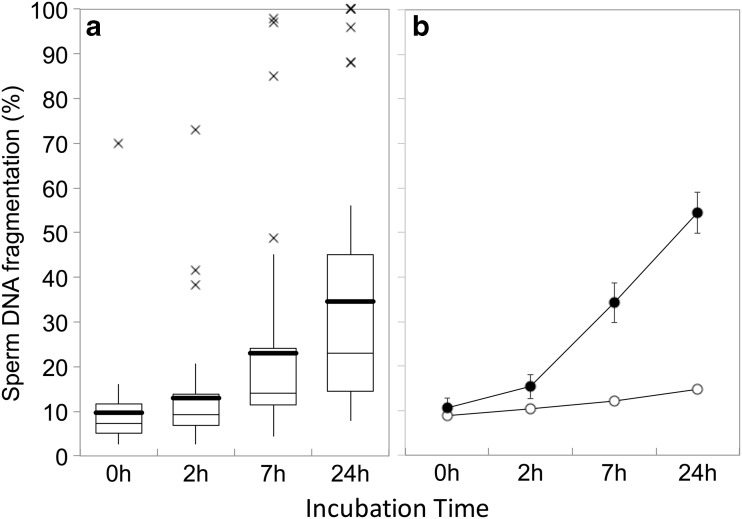
Rabbit sperm exposed to the SCD test and which are representative of a low and high level of SDF are shown in Fig. 1. Descriptive statistics of SDF obtained over a 24-h period of incubation (38 °C) of all 40 bucks with a satisfactory seminogram are shown in Fig. 2a. Figure 2b summarises the mean (±SEM) SDF for each group over the 24-h incubation period. Mean L-rSDF and H-rSDF was 0.25 ± 0.01 and 1.83 ± 0.18 SDF h−1, respectively. These means were statistically different (P < 0.001) since these groups were arbitrarily created by pre-allocating a cut-off value for rSDF of 0.5 units of increase in the rSDF per hour. Despite a clear difference in rSDF, L-rSDF and H-rSDF buck semen could not be separated statistically based on their respective mean initial SDF values (T0h: L-rSDF: 8.8 %; SD = 4.14; H-rSDF: 14.38 %; SD = 14.38; Mann-Whitney U test 178; P = 0.565). Immediately prior to insemination, semen from each group of bucks was again collected and evaluated for SDF dynamics over another 24-h incubation period in order to ensure a degree of consistency in the incidence of SDF in both ejaculates. Table 1 reports the rSDF of each low and high rSDF male determined from the first ejaculate (E1) and the subsequent ejaculate used for the artificial insemination procedure (E2; data showed in Fig. 1). While there was no significant correlation in the rSDF between E1 and E2 ejaculates of either L-rSDF (R = 0.20; P = 0.397) or H-rSDF (R = -0.183; P = 0.440) males, there was no significant difference in the mean rSDF values of E1 and E2 ejaculates in either L-rSDF (P = 0.486) or H-rSDF (P = 0.671) males. Hence, although there was variation in the rSDF between ejaculates, all males originally allocated to L-rSDF or H-rSDF remained within their respective groups immediately prior to AI; mean values of E2 for L-rSDF and H-rSDF were also significantly different (P = 0.003). SDF at T0 and rSDF behaved as independent characters since the correlation between both variables was low (Pearson correlation = 0.099; P = 0.542).
Fig. 2.
SDF dynamics of 40 bucks incubated at 38 °C for 24 h showing the increasing rate of SDF. a Whisker box diagram showing the increasing values for all samples. Thick horizontal line denotes the mean; box denotes the 1st and 3rd quartile; thin horizontal line in box denotes the median; cross denotes outliers. b Mean (±SEM) changes in SDF for each pre-established group showing L- and H-rSDF over 24 h incubation based on an arbitrary value for r-SDF of 0.5 h−1. Open circles are L-SDF; closed circles are H-SDF
Table 1.
Comparison of L-rSDF and H-rSDF in the initial allocating ejaculate (E1) and the ejaculate collected for use with artificial insemination (E2)
| Low rSDF | rSDF E1 | rSDF E2 | High rSDF | rSDF E1 | rSDF E2 |
|---|---|---|---|---|---|
| L01 | 0.03 | 0.40 | H01 | 0.61 | 1.01 |
| L02 | 0.07 | 0.03 | H02 | 0.67 | 0.63 |
| L03 | 0.08 | 0.26 | H03 | 0.75 | 1.54 |
| L04 | 0.15 | 0.07 | H04 | 0.79 | 2.13 |
| L05 | 0.17 | 0.08 | H05 | 0.85 | 1.34 |
| L06 | 0.17 | 0.32 | H06 | 0.92 | 2.67 |
| L07 | 0.21 | 0.10 | H07 | 1.08 | 1.89 |
| L08 | 0.25 | 0.23 | H08 | 1.08 | 2.94 |
| L09 | 0.25 | 0.22 | H09 | 1.36 | 1.33 |
| L10 | 0.28 | 0.21 | H10 | 1.43 | 12.25 |
| L11 | 0.28 | 0.12 | H11 | 1.47 | 1.03 |
| L12 | 0.29 | 0.14 | H12 | 1.56 | 3.10 |
| L13 | 0.29 | 0.46 | H13 | 1.78 | 1.69 |
| L14 | 0.30 | 0.39 | H14 | 1.88 | 2.01 |
| L15 | 0.32 | 0.19 | H15 | 2.08 | 1.10 |
| L16 | 0.35 | 0.21 | H16 | 3.16 | 2.33 |
| L17 | 0.35 | 0.21 | H17 | 3.54 | 0.68 |
| L18 | 0.36 | 0.10 | H18 | 3.78 | 0.89 |
| L19 | 0.38 | 0.43 | H19 | 3.85 | 0.85 |
| L20 | 0.43 | 0.35 | H20 | 3.93 | 0.84 |
| Mean ± SEM | 0.25 ± 0.02 | 0.23 ± 0.03 | Mean ± SEM | 1.83 ± 0.26 | 2.11 ± 0.56 |
SDF and reproductive outcome
The reproductive outcomes of does following AI with L-rSDF and H-rSDF semen are reported in Table 2. No statistical difference was found in the rate of pregnancy of does inseminated with L-rSDF (15/20) or H-rSDF (14/20) (contingency χ20.13; P = 0.723). However, there was a significantly lower number of individuals born alive produced in females inseminated with H-rSDF (Table 2; contingency χ214.26; P = 0.0002). There was also a significantly higher number of stillborn young produced in females inseminated with H-rSDF (14/21) compared to L-rSDF (15/6; contingency χ25.19; P = 0.022). The risk of stillborn when L-rSDF rabbits were used for insemination was 0.16; this risk increased to 0.36 when H-rSDF animals were used (odds ratio = 2.85; 95 % confidence interval = 1.4–2.7). One of the females that was inseminated with an H-rSDF male died because of multiple abortion without fetal reabsorption.
Table 2.
Reproductive outcomes of same does following artificial insemination with L-rSDF and H-rSDF semen
| ID | L-rSDF | Preg | AB | SB | ID | H-rSDF | Preg | AB | SB |
|---|---|---|---|---|---|---|---|---|---|
| L01 | 0.40 | Yes | 1 | 0 | H01 | 1.01 | Yes | 6 | 1 |
| L02 | 0.03 | Yes | 5 | 0 | H02 | 0.63 | No | 0 | 0 |
| L03 | 0.26 | Yes | 7 | 0 | H03 | 1.54 | Yes | 8 | 3 |
| L04 | 0.07 | Yes | 4 | 1 | H04 | 2.13 | Yes | 8 | 2 |
| L05 | 0.08 | Yes | 7 | 2 | H05 | 1.34 | No | 0 | 0 |
| L06 | 0.32 | No | 0 | 0 | H06 | 2.67 | No | 0 | 0 |
| L07 | 0.10 | Yes | 4 | 0 | H07 | 1.89 | Yes | 3 | 0 |
| L08 | 0.23 | No | 0 | 0 | H08 | 2.94 | Yes | 5 | 0 |
| L09 | 0.22 | Yes | 9 | 0 | H09 | 1.33 | No | 0 | 0 |
| L10 | 0.21 | Yes | 6 | 0 | H10 | 12.25 | Yes | 2 | 4 |
| L11 | 0.12 | Yes | 10 | 0 | H11 | 1.03 | No | 0 | 0 |
| L12 | 0.14 | No | 0 | 0 | H12 | 3.10 | Yes | 3 | 0 |
| L13 | 0.46 | Yes | 12 | 0 | H13 | 1.69 | Yes | 5 | 0 |
| L14 | 0.39 | No | 0 | 0 | H14 | 2.01 | Yes | 6 | 1 |
| L15 | 0.19 | No | 0 | 0 | H15 | 1.10 | No | 0 | 0 |
| L16 | 0.21 | Yes | 8 | 0 | H16 | 2.33 | Yes | 9 | 0 |
| L17 | 0.21 | Yes | 11 | 0 | H17 | 0.68 | Yes | 7 | 1 |
| L18 | 0.10 | Yes | 8 | 3 | H18 | 0.89 | Yes | 2 | 2 |
| L19 | 0.43 | Yes | 6 | 0 | H19 | 0.85 | Yes | 0 | 7 |
| L20 | 0.35 | Yes | 7 | 0 | H20 | 0.84 | Yes | 3 | 0 |
| Mean ± SEM | 0.23 ± 0.03 | N = 15 | N = 105 | N = 6 | 2.11 ± 0.56 | N = 14 | N = 67 | N = 21 |
L-rSDF low rate of SDF, H-rSDF high rate of SDF, Preg successful pregnancy, AB number of alive-born kittens, SB number of stillborn kittens
Discussion
The primary outcome of this study was that artificial insemination of rabbits with semen from bucks exhibiting low sperm DNA longevity resulted in a lower number of live young born and a higher number of still born kittens. We suggest that this result was discernible because of three important aspects of our experimental design: (1) the characterisation of insemination donors into groups with high and low levels of sperm DNA longevity based on a dynamic assessment of SDF; (2) the polytocous highly fecund nature of the doe rabbit, which allowed us to define the efficiency of pregnancy at a much greater resolution than in humans and (3) the fact that the same does were sequentially inseminated with semen that displayed a low and high rSDF in order to reduce oocyte variability and the influence of female factor.
The majority of human studies that have attempted to correlate SDF and pregnancy rate only use a single static value of SDF, typically obtained at some undefined time period after ejaculation [13]. In clinical practice, the evaluation of SDF can be either conducted at the time of ejaculation, immediately after sperm liquefaction or when the sperm has been selected for insemination purposes. As we now have clear evidence from a range of species that DNA integrity declines following in vitro incubation [8, 9, 14–16], a single SDF assessment determined at random time points makes direct comparison of these data, without some form of standardization, virtually meaningless. Consequently, we advocate determination of an rSDF over a defined time period to obtain a more informed measure of the quality and longevity of the sperm chromatin and so as to allow comparison between laboratories. The results of the current study appear to support this concept, as there was no difference in the SDF values of either L-rSDF or H-rSDF when the sample was assessed immediately after ejaculation (T0h), but there were clear differences detected at 2 h and massive differences after 24 h of incubation.
Although there are clear experimental and ethical limitations when using human gametes, FIV-ICSI procedures allow the assessment of the influence of SDF at initial embryonic development. There are different investigations that point to the fact that either embryonic failure or abortion is significantly correlated with the presence of abnormal levels of sperm DNA damage [3, 17, 18]. Nevertheless, given the low fecundity of human reproduction, investigations into the possible influence of SDF in the later stages of pregnancy (e.g. still birth) are more problematic. The rabbit model allows this assessment, with clear evidence presented in this study, that shows the proportion of stillborn kittens was significantly higher following insemination with H-rSDF semen. The presence of more than one oocyte per mating increases the probability of spermatozoa with damaged DNA being able to fertilize. The high number of oocytes ovulated per individual in the doe rabbit provides the researcher with a greater opportunity to detect the effect of sperm DNA fragmentation on embryonic development since the male and the female contributions to successful pregnancy are being assessed within the same physiological environment.
In our experience, if oocyte quality can be somehow homogenised or standardised, using for example donor oocytes, then it may be more likely to elucidate a stronger relationship between high levels of baseline SDF and reduced pregnancy rate [19]. There are numerous studies that have shown that the oocyte has the capacity to repair sperm DNA damage [20–22]. Even if good quality oocytes are sourced from donors, individual variability in DNA repair of these cells will still potentially influence the expression of adverse effects on reproductive outcome associated with SDF; moreover, the restricted implantation rate in the clinic is also an investigative drawback for this purpose. In the current experiment, we attempted to reduce the influence of female factor by using the same females to sequentially inseminate selected semen samples with high and low rates of SDF. We suggest that this element of our experimental design, in combination with the dynamic assessment of SDF and the polytocous nature of the rabbit, increased the resolution for the detection of a statistically adverse effect of elevated SDF on the quality of the pregnancy that might otherwise be very difficult to demonstrate in humans. As indicated, some studies have suggested that high levels of SDF are associated with a higher incidence of miscarriage but these reports have primarily been based on observational data rather than experimental design.
Consequently, we may need to turn to animal studies to clarify this relationship between SDF and pregnancy outcome. In the fresh water fish (Tinca tinca), the rate of sperm DNA fragmentation is triggered within minutes of sperm activation [15]; zebra fish spermatozoa also experience an equivalent loss of sperm DNA quality after sperm activation. Using the fish model, we have discovered that if a certain level of DNA damage is produced following sperm activation, SDF appears to have no adverse effect on fertilization rates or early embryonic development. However, when embryos reach a developmental stage of approximately 1000 cells, a significant decline in embryo viability was detected [23]; similar observations have also been reported in rainbow trout by Pérez-Cerezales et al. [24]. Again in trout, Devaux et al. [25] found a positive correlation between the level of sperm DNA damage and the incidence of skeletal abnormalities in the progeny; this morphological alteration resulted in an increased mortality rate of up to three times higher than when spermatozoa of low DNA damage was used. While there is less evidence in mammalian species, studies of mice ICSI have shown that sperm with a high level of SDF resulted in a proportion of offspring performing abnormally in behavioural tests and displaying malformations, tumours and premature aging [26].
In our experimental model, it appears that SDF may be specifically contributing to a diminished number of offspring per pregnancy and an increased number of stillborn kittens. It is likely that sperm samples with lower DNA longevity undergo DNA fragmentation more rapidly after insemination and progression within the female reproductive tract, so that a higher proportion of sperm with fragmented DNA would be found in the oviduct; even so, these spermatozoa are still potentially motile and able to fertilize the oocyte. After fertilization, fragmented DNA of the pronucleus may not be totally repairable in amount and/or fidelity; in fact, the main double-strand DNA break repair pathway (the non-homologous end joining (NHEJ)), may be error-prone leading to chromosome aberrations [21]. In addition, massive single-stranded DNA breaks also increase the possibility of unrepair or misrepair. These conditions are likely to disrupt initial developmental stages of the embryo, thus explaining the observed decreased number of offspring in the current study.
Our experimental model focuses on the effect of increased SDF as a consequence of decreased DNA longevity on the final outcome of pregnancy or the embryos which were able to develop to term. Under this scenario, we are exploring presumed DNA damage that did not necessarily prevent embryonic development but rather resulted to stillbirth. This may present as subtle rather than major DNA fragmentation. Despite a high rate of SDF, apparent normal kittens in the current study were born mixed with stillborns, suggesting at least in these neonates, fertilization was by normal sperm or sperm that were apparently repaired. Moreover, this damage could interfere with the epigenetic control of gene expression during embryo or early adult developmental stages. The epigenetic implications of a failure of sperm DNA repair after fertilization is an argument that has been used to explain embryonic loss and congenital abnormality; it is likely that humans are also not exempt from the similar phenomena [27–29]. It is possible that a larger sample size of experimental animals may have also revealed an adverse effect of high r-SDF on pregnancy rate so that future studies should employ the use of ultrasonography to more precisely determine early pregnancy status; both these limiting factors were not possible to control under the constraints of the rabbit breeding centre’s commercial operations. In addition, future experiments conducted in a laboratory setting could also incorporate further controls that better account for any confounding effect of sequential insemination; for example, the order of high and low rSDF inseminations could be balanced and/or if possible, the female inseminated twice with high and low rSDF semen from the same male.
It is reasonable to assume that sperm with a low rSDF should be more likely to have a greater chance of successful pregnancy. However, the specific situation seems more complex, as some individuals in the current study with low rSDF still failed to produce a pregnancy, while others presenting with high rSDF were capable of producing live offspring. This level of variability has justifiably been a major contributor to the scepticism of some andrologists as regards the benefit of SDF as a predictor of reproductive outcome, but as we have demonstrated in this study, some of the “noise” of this relationship can be reduced if the experimental design used to investigate the effect of SDF incorporates (1) a dynamic assessment of SDF, (2) a greater proportion and probability of sperm with damaged DNA fertilizing the oocyte (polytocous versus monotocous species) and (3) control over the female contribution to reproductive outcome or the oocyte’s capacity for DNA repair and the capacity to maintain pregnancy.
Acknowledgments
This research was supported by the Spanish Ministry of Economy and Competitiveness, MINECO (BFU-2013-44290-R).
Footnotes
Capsule
Sequential artificial insemination of the same naturally multi-ovulating female makes the rabbit a useful model for investigating the effect of sperm DNA longevity on reproductive outcome. Decreased sperm DNA longevity resulted in a diminished proportion of live offspring per pregnancy and an increased number of stillborn kittens.
References
- 1.Drobnis EZ, Johnson MH. Are we ready to incorporate sperm DNA-fragmentation testing into our male infertility work-up? A plea for more robust studies. Reprod Biomed Online. 2015;30:111–2. [DOI] [PubMed]
- 2.Palermo GD, Neri QV, Cozzubbo T, Rosenwaks Z. Perspectives on the assessment of human sperm chromatin integrity. Fertil Steril. 2014;102:1508–17. doi: 10.1016/j.fertnstert.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Robinson L, Gallos ID, Conner SJ, Rajkhowa M, Miller D, Lewis S, et al. The effect of sperm DNA fragmentation on miscarriage rates: a systematic review and meta-analysis. Hum Reprod. 2012;27:2908–17. doi: 10.1093/humrep/des261. [DOI] [PubMed] [Google Scholar]
- 4.Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med. 2011;57:78–85. doi: 10.3109/19396368.2010.515704. [DOI] [PubMed] [Google Scholar]
- 5.Santiso R, Tamayo M, Gosálvez J, Johnston S, Mariño A, Fernández C, et al. DNA fragmentation dynamics allows the assessment of cryptic sperm damage in human: evaluation of exposure to ionizing radiation, hyperthermia, acidic pH and nitric oxide. Mutat Res. 2012;734:41–9. doi: 10.1016/j.mrfmmm.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Practice Committee of the American Society for Reproductive Medicine The clinical utility of sperm DNA integrity testing: a guideline. Fertil Steril. 2013;99:673–7. doi: 10.1016/j.fertnstert.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 7.Oliva R. Protamines and male infertility. Hum Reprod. 2006;12:417–35. doi: 10.1093/humupd/dml009. [DOI] [PubMed] [Google Scholar]
- 8.Gosálvez J, López-Fernández C, Fernández JL, Gouraud A, Holt WV. Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian sperm from eleven species. Mol Reprod Dev. 2011;78:951–61. [DOI] [PubMed]
- 9.Gosálvez J, Holt WV, Johnston SD. Sperm DNA fragmentation and its role in wildlife conservation. Reproductive sciences in animal conservation. Adv Exp Med Biol. 2014;753:357–84. doi: 10.1007/978-1-4939-0820-2_15. [DOI] [PubMed] [Google Scholar]
- 10.Gosálvez J, López-Fernández C, Arroyo F, Gosálbez A, Gutiérrez-Cortés El, Johnston SD. The assessment of sperm DNA damage in rabbits using the Halomax assay. In: Adamo G, Constanza A, editors. Rabbits: biology, diet and eating habits and disorders. New York: NOVA Science publishers; 2013. p. 87–100.
- 11.Bencheik N. Effect de la fréquence de collecte de la semence sur les caractéristiques du sperme et des spermatozoides récoltés chez la lapin. Ann Zootech. 1995;44:263–79. doi: 10.1051/animres:19950306. [DOI] [Google Scholar]
- 12.Mocé E, Vicente JS, Lavara R. Effect of donor strain and maturation stage of rabbit oocytes on results of penetration test of rabbit semen. World Rabbit Sci. 2002;10:53–62. [Google Scholar]
- 13.Gosálvez J, López-Fernández C, Fernández JL, Esteves SC, Johnston SD. Unpacking the mysteries of sperm DNA fragmentation: ten frequently asked questions. J Reprod Biotech Fertil. 2015;4:1–16.
- 14.Aurich C. Recent advances in cooled-semen technology. Anim Reprod Sci. 2008;107:268–75. doi: 10.1016/j.anireprosci.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 15.López-Fernández C, Gage MJ, Arroyo F, Gosálbez A, Larrán AM, Fernández JL, et al. Rapid rates of sperm DNA damage after activation in tench (Tinca tinca: Teleostei, Cyprinidae) measured using a sperm chromatin dispersion test. Reproduction. 2009;138:257–66. doi: 10.1530/REP-09-0105. [DOI] [PubMed] [Google Scholar]
- 16.Wdowiak A, Bojar I. Relationship between pregnancy, embryo development, and sperm deoxyribonucleic acid fragmentation dynamics. Saudi J Biol Sci. 2015 (in press). Published online ahead of print 10 August 2015. doi:10.1016/j.sjbs.2015.08.001. [DOI] [PMC free article] [PubMed]
- 17.Zhao J, Zhang Q, Wang Y, Li Y. Whether sperm deoxyribonucleic acid fragmentation has an effect on pregnancy and miscarriage after in vitro fertilization/intracytoplasmic sperm injection: a systematic review and meta-analysis. Fertil Steril. 2014;102:998–1005. doi: 10.1016/j.fertnstert.2014.06.033. [DOI] [PubMed] [Google Scholar]
- 18.Leach M, Aitken RJ, Sacks G. Sperm DNA fragmentation abnormalities in men from couples with a history of recurrent miscarriage. Aust NZ J Obst Gyn. 2015;55:379–83. [DOI] [PubMed]
- 19.Gosálvez J, Caballero P, López-Fernández C, Ortega L, Guijarro JA, Fernández JL, et al. Can DNA fragmentation of neat or swim-up spermatozoa be used to predict pregnancy following ICSI of fertile oocyte donors? Asian J Androl. 2013;15:812–8. doi: 10.1038/aja.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genescà A, Caballín MR, Miró R, Benet J, Germà JR, Egozcue J. Repair of human sperm chromosome aberrations in the hamster egg. Hum Genet. 1992;89:181–6. [DOI] [PubMed]
- 21.Derijck A, van der Heijden G, Giele M, Philippens M, de Boer P. DNA double-strand break repair in parental chromatin of mouse zygotes, the first cell cycle as an origin of de novo mutation. Hum Mol Genet. 2008;17:1922–37. [DOI] [PubMed]
- 22.Marchetti F, Bishop J, Gingerich J, Wyrobek AJ. Meiotic interstrand DNA damage escapes paternal repair and causes chromosomal aberrations in the zygote by maternal misrepair. Sci Rep. 2015;5:7689. [DOI] [PMC free article] [PubMed]
- 23.Gosálvez J, López-Fernández C, Hermoso A, Fernández JL, Kjelland ME. Sperm DNA fragmentation in zebrafish (Danio rerio) and its impact on fertility and embryo viability —Implications for fisheries and aquaculture. Aquaculture. 2014;433:173–82. doi: 10.1016/j.aquaculture.2014.05.036. [DOI] [Google Scholar]
- 24.Pérez-Cerezales S, Martínez-Páramo S, Beirão J, Herráez MP. Fertilization capacity with rainbow trout DNA-damaged sperm and embryo developmental success. Reproduction. 2010;139:989–97. doi: 10.1530/REP-10-0037. [DOI] [PubMed] [Google Scholar]
- 25.Devaux A, Fiat L, Gillet C, Bony S. Reproduction impairment following paternal genotoxin exposure in brown trout (Salmo trutta) and arctic charr (Salvelinus alpinus) Aquat Toxicol. 2011;101:405–11. doi: 10.1016/j.aquatox.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 26.Fernández-Gonzalez R, Moreira PN, Pérez-Crespo M, Sánchez-Martín M, Ramırez MA, Pericuesta E, et al. Long-term effects of mouse intracytoplasmic sperm injection with DNA fragmented sperm on health and behavior of adult offspring. Biol Reprod. 2008;78:761–72. [DOI] [PubMed]
- 27.De Rycke M, Liebaers I, Van Steirteghem A. Epigenetic risks related to assisted reproductive technologies. Risk analysis and epigenetic inheritance. Hum Reprod. 2002;17:2487–94. doi: 10.1093/humrep/17.10.2487. [DOI] [PubMed] [Google Scholar]
- 28.Kelly TL, Trasler JM. Reproductive epigenetics. Clin Genet. 2004;65:247–60. doi: 10.1111/j.0009-9163.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- 29.Urrego R, Rodriguez-Osorio N, Niemann H. Epigenetic disorders and altered gene expression after use of assisted reproductive technologies in domestic cattle. Epigenetics. 2014;9:803–15. doi: 10.4161/epi.28711. [DOI] [PMC free article] [PubMed] [Google Scholar]