Abstract
Purpose
In the present study, we reported two cases of TS with mosaic ring X chromosome showing common clinical characteristics of TS like growth retardation and ovarian dysfunction. The purpose of the present study was to cytogenetically characterize both cases.
Methods
Whole blood culture and G-banding were performed for karyotyping the cases following standard protocol. Origin of the ring chromosome and degree of mosaicism were further determined by fluorescence in situ hybridization (FISH). Breakpoints and loss of genetic material in formation of different ring X chromosomes r (X) in cases were determined with the help of cytogenetic microarray.
Results
Cases 1 and 2 with ring chromosome were cytogenetically characterized as 45, X [114]/46Xr (X) (p22.11q21.32) [116] and 45, X [170]/46, Xr (X) (p22.2q21.33) [92], respectively. Sizes of these ring X chromosomes were found to be ~75 and ~95 Mb in cases 1 and 2, respectively, using visual estimation as part of cytogenetic observation. In both cases, we observed breakpoints on Xq chromosome were within relatively narrow region between Xq21.33 and Xq22.1 compared to regions in previously reported cases associated with ovarian dysgenesis.
Conclusions
Our observation agrees with the fact that despite of large heterogeneity, severity of the cases with intact X-inactive specific transcript (XIST) is dependent on degree of mosaicism and extent of Xq deletion having crucial genes involved directly or indirectly in various physiological involving ovarian cyclicity.
Keywords: Turner syndrome, Ovarian dysfunction, Ring X chromosome, Fluorescence in situ hybridization (FISH), Cytogenetic microarray
Introduction
Turner syndrome (TS) is a common chromosomal disorder in females with prevalence of 1 in 2500 live births [39]. TS is cytogenetically characterized as 45, X. In 6–15 % of the cases, individuals with TS also have another cell line with 46 chromosomes due to presence of an extra ring chromosome X [11, 40]. Ring chromosomes may be formed by either terminal breaks of two arms of the chromosome and their rejoining leading to loss of genetic material [38]. They may also be formed by telomere-telomere fusion with no deletion, resulting in formation of a complete ring [10]—the so-called Mc Clintock mechanism [3]. Other complex mechanisms resulting in its formation includes terminal deletion and a contiguous inverted duplication due to an inv-dup-del rearrangement [15, 37].
Phenotypes associated with ring X chromosome are common features of TS which include short stature, peripheral edema, characteristic facial features, low posterior hairline, ovarian dysgenesis, endocrine disorders and autoimmune conditions. Besides these, individuals with r (X) can also present with mental disorders, learning difficulties, autistic spectrum disorders, craniofacial abnormalities, cardiovascular problems, skeletal system problems and dermatological problems in severe conditions [7, 18, 19, 30, 45]. Clinical manifestation in these cases are dependent on origin, size, replication timing of the ring chromosome, genes affected by copy number variations, level of mosaicism and status of X inactivation [17].
In the present study, we reported molecular cytogenetic characterization of two cases of ring X chromosome with different degree of mosaicism showing common clinical characteristics of TS. Breakpoints on Xq were restricted within a narrow region between Xq21.33 to Xq22.1 compared to other such reported cases.
Material and methods
Cases referred from the Department of Gynaecology, Sir Sunderlal Hospital, Banaras Hindu University, Varanasi, India, were registered at our centre, Centre for Genetic Disorders, Banaras Hindu University, Varanasi. Parents had given their written consent for the detailed study. Clinical history was recorded. Five millilitres peripheral blood was drawn in a sterilized syringe under complete aseptic conditions. Serum was separated from 2 ml of peripheral blood by centrifugation at 3000 rpm for 5 min and stored at −80 °C till hormonal assay was performed. Rest of the blood was transferred in heparinized vial. Whole blood culture was set with 0.5 ml peripheral blood for cytogenetic experiment and rest of the peripheral blood was used for extraction of genomic DNA following modified salting out method [25] for cytogenetic microarray experiment.
Case presentation
The first case was a 16-year-old girl who was presented with complaint of primary amenorrhoea and poorly developed secondary sexual characters. She was diagnosed with bilateral retinoschisis, hypoplastic uterus and small ovaries. The second case was a 21-year-old married woman who had oligoamenorrhoea and primary subfertility. She also had short stature and a ultrasonographic scan showed a hypoplastic uterus. The clinical features of both the cases are summarized in Table 1.
Table 1.
Comparative clinical features of the two cases
| Clinical detail | Case 1 | Case 2 |
|---|---|---|
| Age (in years) | 16 | 21 |
| Height (in cm) | 144 | 147.2 |
| Weight (in kg) | 30 | 60 |
| BMI | 14.5 | 27.7 |
| Growth retardation | − | + |
| Menstrual status | Primary amenorrhoea | Oligoamenorrhoea |
| Mental retardation | − | − |
| Lymphedema | − | − |
| Web neck | − | − |
| Low set ears | + | − |
| Cubitus vulgus | − | − |
| Short fourth metacarpals | − | − |
| Cardiovascular abnormality | − | − |
| Autoimmune disorder | − | − |
| High arched palate | − | − |
| TSH (μIU/ml) | 1.91 | 3.41 |
| FSH (mIU/ml) | 114.34 | 38.36 |
| LH (mIU/ml) | 39.28 | 15.06 |
| Development of secondary sexual characters | Breast development—Tanner stage I, axillary hairs and pubic hairs absent and shield chest | Breast development—Tanner stage IV, axillary hairs—Tanner stage III and Pubic hairs—Tanner stage IV |
| Renal malformation | − | − |
| Ultrasonographic report | Hypoplastic uterus and small ovaries and bilateral retinoschisis | Hypoplastic uterus |
| Neurocognitive phenotype | − | − |
Cytogenetic study
Whole blood culture was performed in RPMI-1640 pH 7.2 (Sigma-Aldrich, Inc., St. Louis, MI, USA) culture media supplemented with 10 % foetal bovine serum (Himedia, India) and stimulated by phytohaemagglutinin-M (Sigma-Aldrich, Inc., St. Louis, MI, USA). Chromosome preparation and karyotyping was performed following the methods described elsewhere [12].
Fluorescence in situ hybridization (FISH)
Fluorescence in situ hybridization (FISH) was performed using Spectrum Green labelled probe for pericentromeric region of chromosome X [CEP X (DXZ1)] (Abbott Vysis) as per the manufacturer’s instructions. Twenty-five metaphases and 200 interphases were captured under fluorescent microscope with the help of Isis—Metasystems software (Carl Zeiss Microscopy Gmbh, Göttingen, Germany).
Hormonal assay
Follicular-stimulating hormone (FSH) and luteinizing hormone (LH) were measured by chemiluminescence method (Immulite-1000 Analyser, SIEMENS Diagnostic Products, USA) as per the manufacturer’s instructions.
Cytogenetic microarray
Cytogenetic microarray experiment was performed with patient genomic DNA using cyto-HD array (Affymetrix, Inc. Santa Clara, CA, USA) as per manufacturers’ instruction. Data were analysed with Chromosome Analysis Suite (ChAS) software (Affymetrix, Inc. Santa Clara, CA, USA).
Results
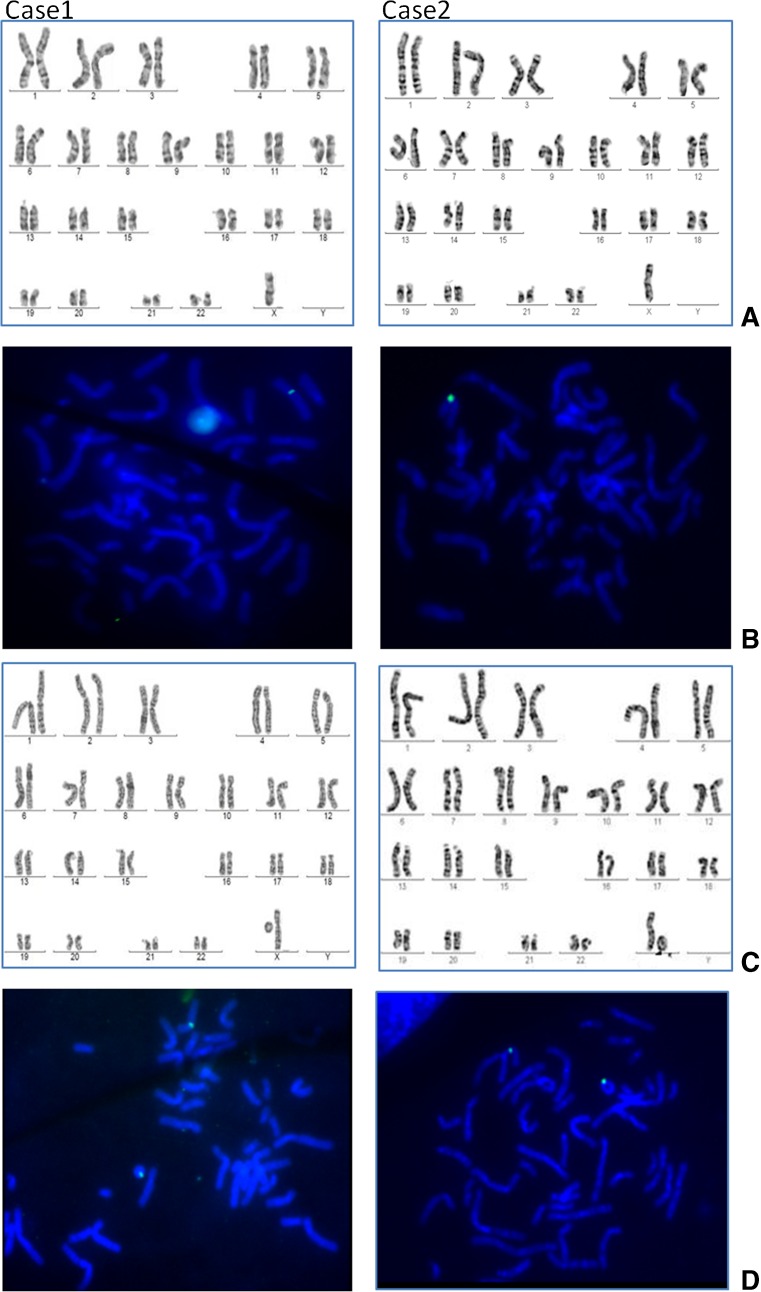
Both the cases presented common clinical characteristics of TS like growth retardation and ovarian dysgenesis. Karyotyping revealed mosaicism for ring X chromosome with two types of cell lines 45, X and 46, Xr (X) in both the cases (Fig. 1). The percentage of the cell line with 46, Xr (X) in case 1 and case 2 was 50 and 35 %, respectively (Table 2). Origin of ring chromosome was determined by FISH using probe for peri centromeric region of chromosome X (Fig. 1). Degree of mosaicism was further confirmed by scoring FISH signals on metaphases and interphases (Fig. 1).
Fig. 1.
Panel with representative karyotype and FISH of two cases showing presence of two cell lines and ring X chromosome. Row A shows karyotype of both the cases with 45, X cell line. Row B represents FISH on 45, X cell line hybridized with spectrum green labelled probe for pericentromeric region of chromosome X. Row C shows karyotype of another cell line with ring X chromosome r (X) present in both cases. Row D represents FISH on 46, Xr (X) cell line hybridized with the same probe showing two signals, one on chromosome X and another on r (X) chromosome
Table 2.
Comparative cytogenetic description of two cases
| Case ID | Cell types | Mosaicism for 46, Xr (X) | Xp deletion | No. of genes on deleted Xp | Xq deletion | No. of genes on deleted Xq |
|---|---|---|---|---|---|---|
| 1 | 45, X/46, Xr (X) | 50 % | Xpter-Xp22.12 (21 Mb) | 221 | Xq21.33-Xq28 (62 Mb) | 897 |
| 2 | 45, X/46, Xr (X) | 35 % | Xpter-Xp22.31 (5.22 Mb) | 49 | Xq22.1-Xq28 (53 Mb) | 736 |
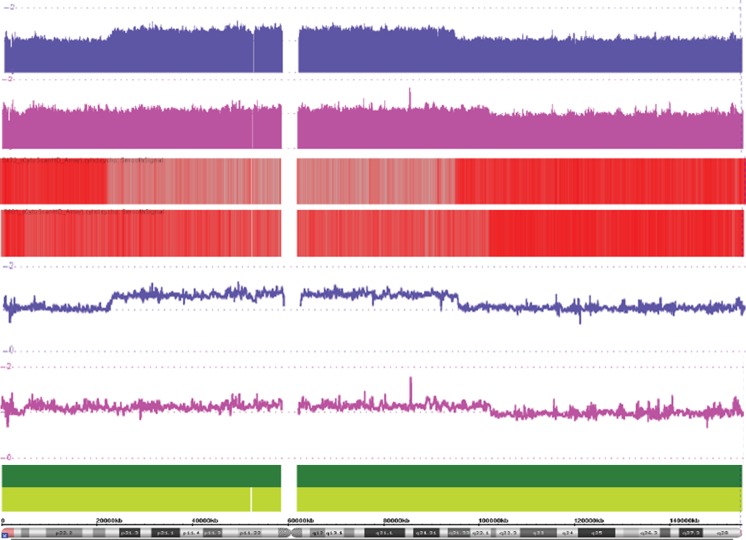
Hormonal assays from serum revealed level of FSH and LH hormones was 114.34 and 39.28 mIU/ml, respectively, in case 1. In case 2, the level of FSH and LH measured was 38.36 and 15.06 mIU/ml, respectively (Table 1). Higher level of both FSH and LH, biomarker of gonadal dysgenesis, was observed in case 1, whereas in case 2 FSH was higher and LH was within normal range (2.5–18 mIU/ml for FSH and 1.9–17.7 mIU/ml for LH). Cytogenetic microarray analysis revealed exact break points on both arms of X chromosomes in each case (Fig. 2). The size of the r (X) chromosome in case 1 was observed as 75 Mb with 21 Mb deletion of Xp (Xpter-Xp22.12) harbouring 221 genes and 62 Mb deletion of Xq (Xq21.33-Xq28) harbouring 897 genes (Table 2). In case 2, the size of r (X) was 100 Mb with 5.22 Mb deletion of Xp (Xpter-Xp22.31) harbouring 49 genes and 53 Mb Xq deletion (Xq22.1-Xq28) having 736 genes (Table 2). Degree of mosaicism was also evident from cytogenetic microarray experiment on comparing deviation of probe intensity signals from the baseline in both the cases (Fig. 2). Combining karyotype, FISH and cytogenetic microarray results, cases 1 and 2 were cytogenetically characterized as 45, X [114]/46Xr (X) (p22.11q21.32) [116] and 45, X [170]/46, Xr (X) (p22.2q21.33) [92], respectively.
Fig. 2.
Cytogenetic microarray result representing log 2 ratio for deletion and breakpoints on Xp and Xq in cases 1 and 2 (blue and purple colour probe signals, respectively). Different representation of deletion and breakpoints in both the cases (upper smooth signal, middle heat map view and lower linear view)
Discussion
We presented two cases of TS with varying degree of mosaicism for two types of cells with 45, X and 45Xr (X) (Fig. 1). The cases presented different clinical features associated with TS. Biochemical estimation of the two cases revealed higher level of gonadotropin hormones, LH (in case 1) and FSH (in case 1 and 2), compared to normal range indicating gonadal dysgenesis in both the cases (Table 1). In previous studies, absence of menarche or amenorrhoea has been reported to be associated with higher level of LH and FSH [6].
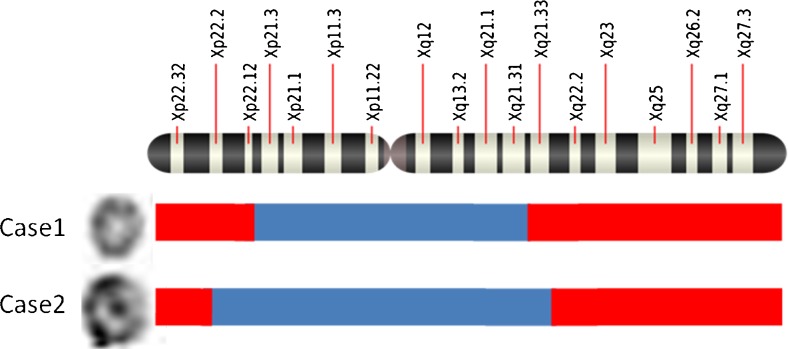
In comparison to case 2, case 1 had more severe symptoms—this could be explained by the amount of Xq deletion [8]. The observed deletion in case 1 (Xpter-Xp22.12 [21 Mb] and Xq21.33-Xq28 [62 Mb]) was larger compared to case 2 (Xpter-Xp22.31 [5.22 Mb] and Xq22.1-Xq28 [53 Mb]) (Fig. 3 and Table 2). It is also possible that the difference in severity between the two cases may possibly be due to difference in degree of mosaicism for two cell lines 45, X and 46, Xr (X) (50:50 in case 1 compared to 65:35 in case 2). But it is difficult to ascertain the impact of mosaicism on clinical phenotype as level of mosaicism has been reported to go down with age due to unstable nature of r (X) chromosomes [2].
Fig. 3.
Sketch diagram showing breakpoints and deleted Xp and Xq arms in the two cases. Ring chromosomes present in the cases are shown on the extreme left, red-coloured bar represents deleted terminal ends of X chromosome and blue-coloured bar represents intact portion with the ring chromosome
Further, we compared Xp deletions and clinical manifestation observed in our cases with previously reported cases where there is no evidence for ovarian dysgenesis with an Xp terminal deletion [4, 13, 26, 28, 47, 49]. Fertility has been reported to be maintained even when more than two third of the Xp is deleted [16]. However, critical region responsible for menarche is present proximal to Xp11.2 [27] and a case having interstitial deletion between Xp11.2 and Xp11.4 with secondary amenorrhoea [48] has been reported. A case similar to ours (Xpter-Xp22.12 and Xpter-Xp22.31, respectively) had been reported in [4, 49] (Xp22.33-Xp22.12) without any sign of ovarian dysgenesis. Therefore, we considered ovarian dysgenesis in our cases might be due to Xq deletion rather than Xp deletion.
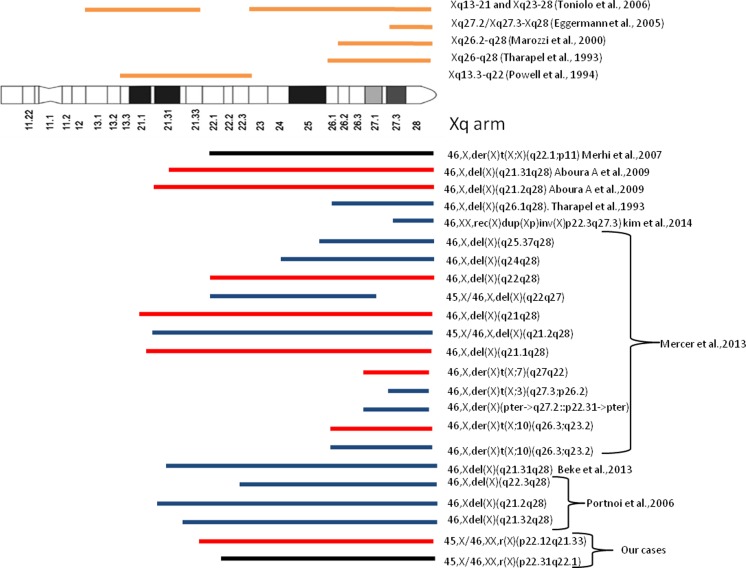
It has been shown that the severity in cases with small ring X chromosome is because of failure of small ring chromosome to be inactivated due to deletion of X-inactive specific transcript (XIST) at Xq13 [22, 43]. In our cases, XIST was intact with the ring chromosome in both the cases negating the role of XIST inactivation in clinical phenotype of patients (Fig. 3). Previous reports have suggested different critical region on Xq arm to be associated with premature ovarian failure (POF) which involve Xq13.3-q22 [29] and Xq26-q28 [41]. Subsequent studies also suggested Xq26.2-q28 [21], Xq27.2/Xq27.3-Xq28 [8] and Xq13-21 and Xq23-28 [44] are the regions associated with POF (Fig. 4). Besides these critical region, breakpoints in Xq13-Xq27 due to balanced X, autosome translocation has been reported in cases of POF [32, 33, 35].
Fig. 4.
Comparative schematic presentation showing portion of Xq material missing in each case and their menstruation pattern (red bar for primary amenorrhoea, black bar for oligoamenorrhoea and blue bar for secondary amenorrhoea) from already published data and in our cases. Above ideogram, bars represent critical regions for ovarian dysgenesis suggested in different studies. Diagram modified from [14]
In our cases, breakpoints on Xq due to ring chromosome formation fall within relatively narrow region Xq21.33 to Xq22.1 coinciding with critical region suggested in [29] (Fig. 4). We observed two cases reported in [23] with 45, X [29]/46, X, del (X) (q22q27) [51] and 45, X [6]/46, X, del (X) (q21.2q28) [24] having short stature and secondary amenorrhoea with almost similar chromosomal anomalies (mosaicism and Xq deletion) as in our cases 1 and 2 (Fig. 4) with difference in severity (primary amenorrhoea in case 1 and oligoamenorrhoea amenorrhoea in case 2). Similar to case 2, Xq22.1 - Xter deletion has been reported in a 12-year-old girl with irregular menses [24] (Fig. 4). As case 1 had a smaller r (X) chromosome compared to case 2, we analysed the additional genes deleted and found that the extra region deleted in case 1 (Xq21.33-Xq22.1) were harbouring genes involved in thyroid hormone signalling, insulin receptor signalling, meiosis regulation, cell cycle regulation and chromatin organization. Alterations in these pathways are reported to be responsible for ovarian dysfunction [1, 5, 9, 31, 36, 46].
Moreover, it has been observed that irrespective of the size of genetic content loss, majority of patient with Xq deletion have oligoamenorrhoea followed by secondary amenorrhoea or premature menopause [42]. On comparing our cases with already reported cases of partial Xq deletion with ovarian dysfunction, it was observed that the same deletion may have different clinical consequences on ovarian function similar to the observation reported in [20]. Therefore, ovarian dysgenesis related to chromosomal abnormalities can be due to disrupted expression of critical genes [34], inappropriate gene expression following incomplete paring of X chromosome at pachytene leading to meiotic arrest [34, 35] or position effect of the breakpoints on the flanking X-linked gene or autosomal linked genes.
In conclusion, we cytogenetically characterized the two cases of ring (X) chromosomes and compared phenotypic heterogeneity of these cases with already reported such cases. Cytogenetic microarray exactly mapped breakpoints within comparatively narrow region between Xq21.33 and Xq22.1 in the two cases. Karyotype-phenotype correlation in the two cases suggested manifestation is dependent on degree of genetic material loss in ring (X) formation as well as mosaicism.
Acknowledgments
We thank the parents for giving consent to publish the data. We record words of gratitude to our teacher Professor Rajiva Raman for critical analysis and comments. We are thankful to anonymous reviewers for their suggestions in improving the manuscript. We are thankful to Centre for Genetic Disorders, BHU, for chromosomal analysis and innovative programme of UGC for hormonal assay experiment and Interdisciplinary School of Life Sciences, BHU, Varanasi, for cytogenetic microarrays. Banaras Hindu University, Varanasi, and Indian Council of Medical Research, New Delhi, are highly acknowledged for providing fellowship. This work forms part of the contribution of the Disease biology thrust area under DBT, New Delhi, sponsored by Interdisciplinary School of Life Sciences, BHU.
Footnotes
Capsule
In the present study, molecular cytogenetic mapping of ring X chromosomes narrowed down the critical region associated with ovarian dysgenesis between Xq21.33 to Xq22.1. The study further suggested that severity of clinical manifestation in the cases is dependent on degree of mosaicism and extent of Xq deletion.
Pooja Chauhan and Sushil Kumar Jaiswal contributed equally to this work.
References
- 1.Andreeva P. Thyroid gland and fertility. Akush Ginekol (Sofiia) 2014;53:18–23. [PubMed] [Google Scholar]
- 2.Atkins L, Sceery RT, Keenan ME. An unstable ring chromosome in a female infant with hypotonia, seizures, and retarded development. J Med Genet. 1966;3:134. doi: 10.1136/jmg.3.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin EL, May LF, Justice AN, Martin CL, Led-better DH. Mechanisms and consequences of small supernumerary marker chromosomes: from Barbara McClintock to modern genetic counseling issues. Am J Hum Genet. 2008;82:398–410. doi: 10.1016/j.ajhg.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cho EH, Kim SY, Kim JK. A case of 9.7 Mb terminal Xp deletion including OA1 locus associated with contiguous gene syndrome. J Korean Med Sci. 2012;27:1273–7. doi: 10.3346/jkms.2012.27.10.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho MK. Thyroid dysfunction and subfertility. Clin Exp Reprod Med. 2015;42:131–135. doi: 10.5653/cerm.2015.42.4.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Moraes-Ruehsen M, Jones GS. Premature ovarian failure. Fertil Steril. 1967;18:440–461. doi: 10.1016/S0015-0282(16)36362-2. [DOI] [PubMed] [Google Scholar]
- 7.Dennis N, Coppin B, Turner C, Skuse D, Jacobs P. A clinical, cytogenetic and molecular study of 47 females with r (X) chromosomes. Ann Hum Genet. 2000;64:277–293. doi: 10.1046/j.1469-1809.2000.6440277.x. [DOI] [PubMed] [Google Scholar]
- 8.Eggermann T, Meschede D, Schuler H, Palm S, Glaser D, Horsthemke B, Eggermann K, Haverkamp F, Zerres K. Premature ovarian failure associated with a small terminal Xq deletion: narrowing the POF1 region down to Xq27.2/Xq27.3-qter. Clin Genet. 2005;67(5):434–437. doi: 10.1111/j.1399-0004.2005.00427.x. [DOI] [PubMed] [Google Scholar]
- 9.Gazdag E, Santenard A, Ziegler-Birling C, Altobelli G, Poch O, Tora L, Torres-Padilla ME. TBP2 is essential for germ cell development by regulating transcription and chromatin condensation in the oocyte. Genes Dev. 2009;23:2210–2223. doi: 10.1101/gad.535209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guilherme RS, Klein E, Hamid AB, Bhatt S, Volleth M, Polityko A, Kulpanovich A, Dufke A, Albrecht B, Morlot S, Brecevic L, Petersen MB, Manolakos E, Kosyakova N, Liehr T. Human ring chromosomes—new insights for their clinical significance. BJMG. 2013;16:13–20. doi: 10.2478/bjmg-2013-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobs P, Dalton P, James R, Mosse K, Power M, Robinson D, Skuse D. Turner syndrome: a cytogenetic and molecular study. Ann Hum Genet. 1997;61:471–483. doi: 10.1017/S0003480097006507. [DOI] [PubMed] [Google Scholar]
- 12.Jaiswal SK, Kumar A, Ali A, Rai AK. Co-occurrence of mosaic supernumerary isochromosome 18p and intermittent 2q13 deletions in a child with multiple congenital anomalies. Gene. 2015;559:94–98. doi: 10.1016/j.gene.2015.01.042. [DOI] [PubMed] [Google Scholar]
- 13.James RS, Coppin B, Dalton P, Dennis NR, Mitchell C, Sharp AJ, Skuse DH, Thomas NS, Jacobs PA. A study of females with deletions of the short arm of the X chromosome. Hum Genet. 1998;102:507–516. doi: 10.1007/s004390050733. [DOI] [PubMed] [Google Scholar]
- 14.Kim MK, Seok HH , Kim YS, Chin MU, Sung SR, Lee WS, et al. Molecular genetic and cytogenetic characterization of a partial Xp duplication and Xq deletion in a patient with premature ovarian failure. Gene. 2014; 534:54–59. [DOI] [PubMed]
- 15.Knijnenburg J, van Haeringen A, Hansson KB, Lankester A, Smit MJ, Belfroid RD, Bakker E, Rosenberg C, Tanke HJ, Szuhai K. Ring chromosome formation as a novel escape mechanism in patients with inverted duplication and terminal deletion. Eur J Hum Genet. 2007;15:548–555. doi: 10.1038/sj.ejhg.5201807. [DOI] [PubMed] [Google Scholar]
- 16.Lachlan KL, Youings S, Costa T, Jacobs PA, Thomas NS. A clinical and molecular study of 26 females with Xp deletions with special emphasis on inherited deletions. Hum Genet. 2006;118:640–651. doi: 10.1007/s00439-005-0081-1. [DOI] [PubMed] [Google Scholar]
- 17.Leppig KA, Disteche CM. Ring X and other structural X chromosome abnormalities: X inactivation and phenotype. Semin Reprod Med. 2001;19:147–57. [DOI] [PubMed]
- 18.Leppig KA, Sybert VP, Ross JL, Cunniff C, Trejo T, Raskind WH, Disteche CM. Phenotype and X inactivation in 45, X/46, X, r (X) cases. Am J Med Genet A. 2004;128:276–284. doi: 10.1002/ajmg.a.30002. [DOI] [PubMed] [Google Scholar]
- 19.Lindgren V, Chen CP, Bryke CR, Lichter P, Page DC, Yang-Feng TL. Cytogenetic and molecular characterization of marker chromosomes in patients with mosaic 45, X karyotypes. Hum Genet. 1992;88:393–398. doi: 10.1007/BF00215672. [DOI] [PubMed] [Google Scholar]
- 20.Maraschio P, Tupler R, Barbierato L, Dainotti E, Larizza D, Bernardi F, Hoeller H, Garau A, Tiepolo L. An analysis of Xq deletions. Hum Genet. 1996;97:375–381. doi: 10.1007/BF02185777. [DOI] [PubMed] [Google Scholar]
- 21.Marozzi A, Manfredini A, Tibileth MG, Furlan D, Villa N, Vegetti W, Crosignani PG, Ginelli E, Menever R, Dalpra L. Molecular definition of Xq common-deleted region in patients affected by premature ovarian failure. Hum Genet. 2000;1074:304–311. doi: 10.1007/s004390000364. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo M, Muroya K, Adachi M, Tachibana K, Asakura Y, Nakagomi Y, Hanaki K, Yokoya S, Yoshizawa A, Igarashi Y, Hanew K, Matsuo N, Ogata T. Clinical and molecular studies in 15 females with ring X chromosomes: implications for r (X) formation and mental development. Hum Genet. 2000;107:433–439. doi: 10.1007/s004390000377. [DOI] [PubMed] [Google Scholar]
- 23.Mercer CL, Lachlan K, Karcanias A, Affara N, Huang S, Jacobs PA, Thomas NS. Detailed clinical and molecular study of 20 females with Xq deletions with special reference to menstruation and fertility. Eur J Med Genet. 2013;56:1–6. doi: 10.1016/j.ejmg.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Merhi ZO, Roberts JL, Awonuga AO. A case of 46, X, der (X) t (X;X) (q22.1;p11) Xq22.1> Xqter in a 12 year old girl with premature ovarian failure. Gynecol Obstet Invest. 2007;63:1379. doi: 10.1159/000096436. [DOI] [PubMed] [Google Scholar]
- 25.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucl Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogata T, Matsuo N. Turner syndrome and female sex chromosome aberrations: deduction of the principal factors involved in the development of clinical features. Hum Genet. 1995;95:607–629. doi: 10.1007/BF00209476. [DOI] [PubMed] [Google Scholar]
- 27.Ogata T, Matsuo N, Fukushima Y, Saito M, Nose O, Miharu N, Uehara S, Ishizuka B. FISH analysis for apparently simple terminal deletions of the X chromosome: identification of hidden structural abnormalities. Am J Med Genet. 2001;104:307–311. doi: 10.1002/ajmg.10071. [DOI] [PubMed] [Google Scholar]
- 28.Pfeiffer RA. Observations in a case of an X/Y translocation, t (X; Y) (p22; q11), in a mother and son. Cytogenet Cell Genet. 1980;26:150–157. doi: 10.1159/000131436. [DOI] [PubMed] [Google Scholar]
- 29.Powell CM, Taggart RT, Drumheller TC, Wangsa D, Qian C, Nelson LM, White BJ. Molecular and cytogenetic studies of an X autosome translocation in a patient with premature ovarian failure and review of the literature. Am J Med Genet. 1994;52:19–26. doi: 10.1002/ajmg.1320520105. [DOI] [PubMed] [Google Scholar]
- 30.Prandstraller D, Mazzanti L, Picchio FM, Magnani C, Bergamaschi R, Perri A, Tsingos E, Cacciari E. Turner’s syndrome: cardiologic profile according to the different chromosomal patterns and long-term clinical follow-up of 136 nonpreselected patients. Pediatr Cardiol. 1999;20:108–112. doi: 10.1007/s002469900416. [DOI] [PubMed] [Google Scholar]
- 31.Rice S, Christoforidis N, Gadd C, Nikolaou D, Seyani L, Donaldson A, Margara R, Hardy K, Franks S. Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum Reprod. 2005;20:373–381. doi: 10.1093/humrep/deh609. [DOI] [PubMed] [Google Scholar]
- 32.Rizzolio F, Bione S, Sala C, Goegan M, Gentile M, Gregato G, Rossi E, Pramparo T, Zuffardi O, Toniolo D. Chromosomal rearrangements in Xq and premature ovarian failure: mapping of 25 new cases and review of the literature. Hum Reprod. 2006;21:1477–83. doi: 10.1093/humrep/dei495. [DOI] [PubMed] [Google Scholar]
- 33.Rossetti F, Rizzolio F, Pramparo T, Sala C, Bione S, Bernadi F, Goegan M, Zuffardi O, Toniolo D. A susceptibility gene for premature ovarian failure (POF) maps to proximal Xq28. Eur J Hum Genet. 2004;12:829–834. doi: 10.1038/sj.ejhg.5201186. [DOI] [PubMed] [Google Scholar]
- 34.Sala C, Arrigo G, Torri G, Martinazzi F, Riva P, Larizza L, Philippe C, Jonveaux P, Sloan F, Labella T, Toniolo D. Eleven X chromosome breakpoints associated with premature ovarian failure (POF) map to a 15-Mb Yac contig spanning Xq21. Genomics. 1997;40:123–131. doi: 10.1006/geno.1996.4542. [DOI] [PubMed] [Google Scholar]
- 35.Schlessinger D, Herrera L, Crisponi L, Mumm S, Percesepe A, Pellegrini M, Pilia G, Forabosco A. Genes and translocations involved in POF. Am J Med Genet. 2002;111:328–333. doi: 10.1002/ajmg.10565. [DOI] [PubMed] [Google Scholar]
- 36.Schuh-Huerta SM, Johnson NA, Rosen MP, Sternfeld B, Cedars MI, Reijo Pera RA. Genetic markers of ovarian follicle number and menopause in women of multiple ethnicities. Hum Genet. 2012;131:1709–1724. doi: 10.1007/s00439-012-1184-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seghezzi L, Maraschio P, Bozzola M, Maserati E, Tupler R, Marchi A, Tiepolo L. Ring chromosome 9 with a 9p22.3-p24.3 duplication. Eur J Pediat. 1999;158:791–793. doi: 10.1007/s004310051206. [DOI] [PubMed] [Google Scholar]
- 38.Sigurdardottir S, Goodman BK, Rutberg J, Thomas GH, Jabs EW, Geraghty MT. Clinical, cytogenetic, and fluorescence in situ hybridization findings in two cases of “complete ring” syndrome. Am J Med Genet. 1999;87:384–90. [DOI] [PubMed]
- 39.Stochholm K, Juul S, Juel K, Naeraa RW, Gravholt CH. Prevalence, incidence, diagnostic delay, and mortality in Turner syndrome. J Clin Endocrinol Metab. 2006;91:3897–902. [DOI] [PubMed]
- 40.Sybert VP. The adult with Turners syndrome: May 18-21; Gothenburg, Sweden. Edited by: Albertsson-Wikland K, Ranke MB. Amsterdam, Lausanne, New York, Oxford, Shannon, Tokyo: Elsevier 1995.
- 41.Tharapel AT, Anderson KP, Simpson JL, Martens PR, Wilroy RS, Llerena JC, Schwartz CE. Deletion (X) (q26.1 –N q28) in a proband and her mother: molecular characterization and phenotypic–karyotypic deductions. Am J Hum Genet. 1993;52:463–471. [PMC free article] [PubMed] [Google Scholar]
- 42.Therman E, Laxova R, Susman B. The critical region on the humanXq. Hum Genet. 1990;85:455–461. doi: 10.1007/BF00194216. [DOI] [PubMed] [Google Scholar]
- 43.Tomkins DJ, McDonald HL, Farrell SA, Brown CJ. Lack of expression of XIST from a small ring X chromosome containing the XIST locus in a girl with short stature, facial dysmorphism and developmental delay. Eur J Hum Genet. 2002;10:44–51. doi: 10.1038/sj.ejhg.5200757. [DOI] [PubMed] [Google Scholar]
- 44.Toniolo D. X-linked premature ovarian failure: a complex disease. Curr Opin Genet Dev. 2006;16:293–300. doi: 10.1016/j.gde.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 45.Van Dyke DL, Wiktor A, Palmer CG, Miller DA, Witt M, Babu VR, Worsham MJ, Roberson JR, Weiss L. Ullrich-Turner syndrome with a small ring X chromosome and presence of mental retardation. Am J Med Genet. 1992;43:996–1005. doi: 10.1002/ajmg.1320430617. [DOI] [PubMed] [Google Scholar]
- 46.Wei Y, Reveal B, Reich J, Laursen WJ, Senger S, Akbar T, Iida-Jones T, Cai W, Jarnik M, Lilly MA. TORC1 regulators Iml1/GATOR1 and GATOR2 control meiotic entry and oocyte development in Drosophila. Proc Natl Acad Sci U S A. 2014;111:5670–5677. doi: 10.1073/pnas.1419156112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wyss D, DeLozier CD, Daniell J, Engel E. Structural anomalies of the X chromosome: personal observation and review of non-mosaic cases. Clin Genet. 1982;21:145–159. doi: 10.1111/j.1399-0004.1982.tb00752.x. [DOI] [PubMed] [Google Scholar]
- 48.Zinn AR, Ross JL. Molecular analysis of genes on Xp controlling Turner syndrome and premature ovarian failure (POF) Semin Reprod Med. 2001;19:141–146. doi: 10.1055/s-2001-15394. [DOI] [PubMed] [Google Scholar]
- 49.Zinn AR, Tonk VS, Chen Z, Flejter WL, Gardner HA, Guerra R, Kushner H, Schwartz S, Sybert VP, Van Dyke DL, Ross JL. Evidence for a Turner syndrome locus or loci at Xp11.2–p22.1. Am J Hum Genet. 1998;63:1757–1766. doi: 10.1086/302152. [DOI] [PMC free article] [PubMed] [Google Scholar]