Abstract
Objective(s):
The main characteristic of mesenchymal stem cells (MSCs) is their ability to produce other cell types. Electromagnetic field (EMF) stimulates differentiation of MSCs into other cells. In this study, we investigated whether EMF can effect on the differentiation of MSCs into dopaminergic (DA) neurons.
Materials and Methods:
An EMF with a frequency of 50 Hz and two intensities of 40 and 400 µT 1hr/day was generated around the cells for a week. Afterwards, these cells were injected into the left ventricle of Parkinsonian rats. The rats survived for 2 weeks, and then sampling was performed.
Results:
The injected cells differentiated into DA neurons and sporadically settled in the substantia nigra pars compacta (SNpc). Transplanted rats exhibited significant partial correction apomorphine-induced rotational behavior compared to Parkinsonian rats (5.0±0.1 vs 7.57±0.08). Results demonstrated that endogenous serum and brain derived neurotrophic factor (BDNF) were altered in all experimental groups. The greatest increase was in group of 400 µT EMF in comparison with Parkinsonian rats (398±15 vs. 312±11.79 pg ⁄ mg). Current study have shown that 6-Hydroxydopamine can cause severe loss of dopaminergic neurons (68±6.58), but injected MSCs that exposed to 40 and 400 µT EMF increased dopaminergic neurons in SNpc (108±2.33 & 126±3.89) (P<0.001).
Conclusion:
Electromagnetic fields with particular frequencies stimulate MSCs. So, these cells had anti-Parkinsonian properties in our studies.
Keywords: Dopaminergic neuron, Electromagnetic field, Mesenchymal stem cells, Parkinson’s disease
Introduction
Parkinson’s disease (PD) is a prevalent neurodegenerative disorder in old people. It is caused by sever loss of dopaminergic neurons (DA) in different part of central nervous system. The greatest reduction of DA is in the substantia nigra pars compacta (SNpc) of mid brain (1). Although the particular cause of neuronal death or loss in PD is currently unknown, research has shown that both apoptotic (2, 3) and non apoptotic cell death may occur (4). 6-Hydroxydopamine (6-OHDA) is the most frequently used toxins to induce Parkinson’s disease-like models. Injection of this toxin into SNpc results in selective lesion to DA via uncoupling mitochondrial oxidative phosphorylation resulting in energy deprivation (4-6). The advantage of this model is the ease of evaluation of motor deficiency by using tests such as apomorphine-induced rotation test (7) and spontaneous motor test. Injection of 6-OHDA into the nigrostriatal pathway causes the loss of motor stability (8).
Many biological activities can be modulated by electromagnetic fields (EMF) (9, 10). In particular, biological effects of EMF exposure is thought to be through changes in ion channels (especially for H+, K+ and Ca2+) (11), which leads to gene up regulation, cell fate, cell differentiation and cell behavior during normal tissue turnover and regenerative repair (12). It was also reported that the low frequency of EMF causes differentiation of BMSC to pituitary cells (13). Moreover, EMF exposure can modulate the stem cells differentiation, so it is useful for tissue engineering or regenerative medicine. Previous studies have revealed that electrical stimulation on mouse stem cells (ESCs) promotes differentiation of these cells (14). However, effects of electrical stimulation on these cells were little known. Other studies have shown that the exposure of cells to 1 mT EMF does not have significant effects on structure and cytoskeleton of the cells and proteins. The cytoskeleton is the main structure of the cells that is responsible for cell shape. According to findings, it is thought that 40 and 400 µT provide appropriate EMF type to cause least damage to the cells and also stimulate differentiation and proliferation of mesenchymal stem cells (MSCs) into neuron. Therefore, this study was performed to investigate the effects of low intensity EMF on the proliferation and differentiation of MSCs into dopaminergic neurons, to evaluate the survival and activity of these cells in rat model of Parkinson’s disease.
Materials and Methods
Animals
Forty eight adult male albino- Wistar rats (200-250 g) were obtained from the animal center of Semnan University of Medical Sciences, Semnan, Iran. The rats were kept in standard environment, in a temperature (22-24 °C), humidity (40–60%), and light period (12 hr). The rats had free access to food and water. All procedure and maintenance were carried out in accordance with Health Guide for Care and Use of Laboratory Animal that permit by Ethical Committee of Semnan Medical University, Semnan, Iran. (Ethical Committee number 92/299518). Rat were randomly divided into six groups (n=8). Each group was housed in a separate cages, the first group received saline as a control group. The second group received saline with ascorbic acid (vehicle of 6-OHDA as a sham). The third group received 4 µg, 6-OHDA in left substantia nigra SNpc as PD model. The forth group received MSCs that exposed in 40 µT low EMF for a week. The fifth group received MSCs that exposed to 400 µT EMF for a week. The sixth group received MSCs without exposure to EMF.
Hydroxydopamine lesion
To obtain unilateral lesion of nigral system, in all rats, 6-OHDA was injected into the left SN. Ketamine hydrochloride/xylazine hydrochloride (100 mg/kg – 20 mg/kg) (sigma-Aldrich) was used and placed into a stereotaxic device (stoelting, USA) to anesthetize the rats. The skin of skull was exposed by a 2 cm incision, and a single hole was drilled over each side of the skull. For injection in SNpc, the following coordinates were used (47): AP= - 4.8 mm posterior to bregma, M L= -1.6 mm lateral to the midline, DV = 8.2 mm vertical from the dura, and finally 4 µl of 6-OHDA (2 µg/µl) was dissolved in vehicle 2 mg/ml anti ascorbic acid (Sigma St. Louis, USA) in saline and was then injected to the SNpc. 6-OHDA was injected to the left side at the rate of 1 µl/min.
Cell culture
BMSCs were isolated from tibias and femurs of rats under deep anesthesia. The bone marrow was flashed onto the Hank’s buffered salt solution (HBSS). For removal of debris, solution was filtered through a cell strainer (100 μm) and then all cells centrifuged and incubated in a 5% CO2 at 37 °C in Dulbecco’s modified Eagle Medium (DMEM, Invitrogen), supplemented with fetal bovine serum (FBS, 10%), amphotericin B (2.5 μg/ml), strepto-mycin (50 μg/ml). After 3 days, suspended cells were removed with the medium and adherent cells were used as the BMSCs (34). Subsequently, incubation was continued and medium was changed at three days intervals. MSCs were allowed to grow. After third passage, when the density of cultured BMSCs was approximately 5×104 cells/cm2, other cells such as fat and fibroblast were removed and only the MSCs were able to multiply and survive (34). To identify of MSCs used differentiation method to bone and fat cells.
After identification, BMSCs with density of 5×105 cells in flask, treated with EMF with the frequency of 40 or 400 µT 1hr/day for a week.
DiI labeling
Understanding cell morphology is a key part to recognize neuronal cells. Several special staining techniques such as immunofluorescence staining of DiI (1, 1′-dioctadecyl-3, 3, 3′, 3′-tetramethylin-docarbocyanine perchlorate) have been developed to help the morphological recognition of neurons. The stain agent is a carbocyanine membrane dye that increases the fluorescence upon the insertion of its lipophilic hydrocarbon chains into the lipid membrane of cells. The high photo stability and continual fluorescence of the dye serves as an effective dye for recognition of neuronal structure. Before injection, MSCs were labeled with the fluorescent dye CM-DiI (Molecular Probes, Invitro-gen, USA). The MSCs were incubated with 5 µg/106 cells DiI for 2 hr at 37 °C in a 95% air per 5% CO2. Then 2×105 cells separated and injected in the left ventricle.
Exposure system and field characteristics
The exposure system was produced by a horizontal Helmholtz coil (300 turns, distance of 6 cm, and internal diameter of 16 cm) embedded in an open Plexiglas rectangular frame and placed in a CO2 incubator (5% CO2, 37 °C). The 50 Hz electrical current was provided by a signal generator and regulated with a 35 W acoustic amplifier. The EMF at the center of exposure system was measured by a Gauss meter (MG-701, MAGNA Japan), and 40 (the same as earth magnetic field) or 400 µT flux density were chosen for exposing the cell cultures, 1 hr/day for a week. The condition of sham group was quite similar to other groups, except that the EMF was turned off (14).
Immunohistochemical and histological study
Transcardial perfusion was performed at first with 300 ml normal saline followed by 300 ml 4% paraformaldehyde (PFA) in 0.1mmol/phosphate buffered saline (PBS). Animals were deeply sedated (pentobarbital, 50 mg/kg, IP) and perfused Transcardial with 4% paraformaldehyde. After removing the brain, stems were fixed with 4% par formaldehyde; the tissue was then cut (5-7 µm thick). Deparafinized, next step was antigen retrieval with tris/EDTA pH 9.0 buffer and 3% hydrogen peroxide, blocked/permeabilized was done with 10% normal goat serum in PBS containing 0.5% Triton –X100, stained with an anti-tyrosine hydroxylase (TH) polyclonal antibody (1-200 dilution, with 10% normal goat serum) (ab75875 UK), followed by a biotin – conjugated goat anti-mouse IgG (1-300 dilution), (abcam, UK)
After washing 3 times, all sections were incubated with an avidin and biotinylated horseradish peroxidase (HRP) complex then followed by diaminoazoben-zidine (DAB) (abcam, UK). Dopaminergic cells were found and quantified using Olympus microscope.
Behavioral testing
Motor asymmetry following unilateral lesion of the nigro striatal pathway or DA neurons in SNpc was assessed by apomorphine-induced rotational behavior. All behavioral tests were performed by a technician blinded to the project. All groups were tested for rotational behavior 6 days after the first surgery and after treatment. Rats received subcutaneously 0.5 mg/kg apomorphine hydrochloride (sigma- Geramany) dissolved in 0.9% NaCl. Twenty minutes after injection, contralateral turns to the lesion were counted over a period of 60 min. Data were expressed as a contralateral turns/min.
Morphometric studies
Ten coronal sections (a section after each 5 sections) from rostral to caudal of the SNpc in each animal were analyzed. The picture of each section was taken by Olympus AX70 microscope and digital camera DP11 with magnification of 40×. An area of 10,000 μm2 was measured randomly in the region of SNpc in five separate microscopic fields. To count the number of neuronal cells in Nissl staining (interneuron and neuronal cells) and IHC (TH positive cells), the pictures were transferred to the computer using OLYSIA autobiorepot (Olympus optical, Japan) software. A grid was superimposed on the picture and the cells with normal nucleus were counted.
Measurement of the brain and serum BDNF concentration
At the end of experiments, the brain and serum level of brain-derived neurotrophic factors (BDNF) were measured with the reagents provided in the BDNF Immunoassay System. The procedure was conducted based on the manufacturer’s protocol (R and D Systems, Minneapolis, MN, USA). The heart blood was collected in free tubes with anticoagulant and incubated at room temperature for 30 min. Next, samples were centrifuged for 15 min at 1500 ×g. The supernatants were collected and stored at -70°C. Then rats decapitated, and brains were quickly out. The extraction of BDNF from the brain sample was performed on ice (48). Briefly, the sample was suspended in 5 volume of lysis buffer containing 20 mM Tris-HCl, 137 mM NaCl, 1% NP40, 0.5 mM PMSF, 10% glycerol, 0.5 mM sodium and protein inhibitor (Calbiochem, USA). The suspension was homogenize-ed on ice for 30 min using a sonicator at power level 1. The brain tissue homogen were then centrifuged at 16000 ×g for 20 min at 4°C. The supernatant was stored at -70°C for subsequent analysis.
Statistical analysis
All data were reported as mean± standard error of the mean (SEM) Enzyme activities was expressed as optical density (OD) value. Statistical analysis was performed by computer using Statistical Package for the Social Sciences version 16 (SPSS16.0). One-way ANOVA and Tukey post hoc multiple comparison tests were used to analyze each tissue. Statistical significance was present at P<0.05.
Results
Drug-induced rotational behavior assessment
As shown in Figure 1, contralateral turns induced by apomorphine hydrochloride were increased in the 6-OHDA group or PD group in the first test (2 weeks after surgery) compared to treatment and control groups (P<0.01). The frequency and duration of the rotation in 6-OHDA group were 8.67± 0.1 circles/min. In the second test, 4 weeks after surgery or 2 weeks after the end of the experiments, the number of contralateral turns observed in the 6-OHDA group was more than all other groups (7.57±0.08, P<0.01). When each group was analyzed considering, in the treatment groups (40 & 400 μT) showed a significant reduction in total contralateral turns (6.50±0.29, 5.0±0.1) but in BMSC treatment group was (7.33±0.52).
Figure 1.
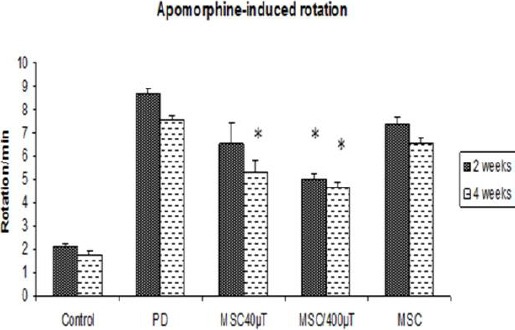
Apomorphine-induced rotations of rats over time reveal a significantly decreased number of rotations in stem cells treatment rats 4 weeks after surgery. All values are mean± SEM.* compared with the PD group (P≤ 0.01)
On the other hand, the number of contralateral turns decreased in all treatment groups, but the decreased value in exposed cell was very noticeable. There were no significant differences between the second and first tests of the control, sham and 6-OHDA group.
Immunohistochemistry
Tyrosine hydroxylase staining was performed for assessment of the dopaminergic neurons. Injection of 6-OHDA into the SNpc significantly reduced the number of TH simmunostaining cells (68±6.58, P<0.001) as illustrated in Figure 2 B,C,D, 3. These cell reduction was observed in all tested groups, but the cell number in the exposed cells treatment groups was higher than other groups (108±2.33 and 126±3.89). Figure 2A shows the boundaries of the SNpc, which were used to measure the cell number in the all tested groups in Nissl and IHC staining. Statistical and comparative light micro-scopic analyses demonstrated that the number of TH and Nissl stained cell in the 6-OHDA group had a prominent decrease compared to the control and treatment groups (68±6.58, P<0.001). The cell number in the control and sham groups were similar (216±5.59 and 212±4.96, respectively), but in the unexposed cell treated groups the number of Nissl-stained cell was 107 ± 5.77 (Figure 3). So, it can be deduced that BMSCs could protect or immigrate into substantia nigra and differentiated into dopaminergic neurons in degenerative disease in rat.
Figure 2.
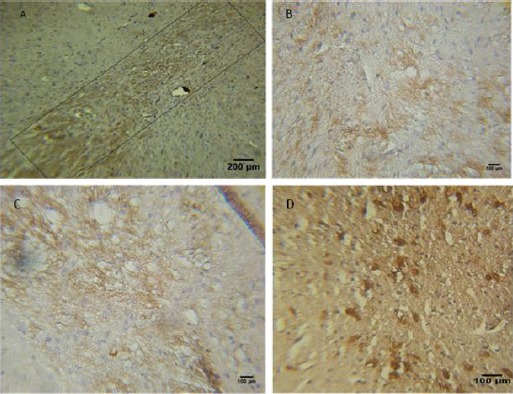
Preservation of dopaminergic systems of 6-OHDA-lesioned rats by mesenchymal stem cells (MSC) transplantation. A-D: TH-positive fibers in the striatum and neurons in the substantia nigra pars compacta (SNpc) of rats. DAPI staining (blue) was used to identify nuclei. (A) The quadrate shows the boundaries of the SNpc, which were used to measure cell number. (B) Received MSCs transplantation and (C-D) received MSCs that exposed to EMF. Scale bar: 200 μm in (A), 100 μm in (B-C-D)
Figure 3.
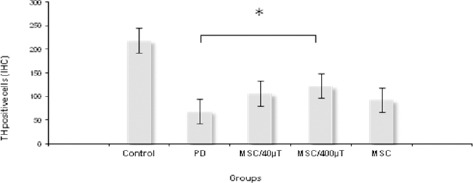
The mean number of TH-positive neurons in the substantia nigra pars compacta (SNpc) of all groups. The number of TH-positive neurons in the SNPc was significantly higher in group that received MSCs exposed to 400 µT, compared with the Parkinson’s rats. (*P ≤ 0.01)
DiI labeling
DiI staining was performed for assessment of location of injected cells. In histological sections, labeled cells have been placed in different area in the mid brain, but mojarity of the cells were in the damaged area (Figure 4). Since fluorescent marker DiI was used as neuronal markers, so it can show the distribution of neurons in this region.
Figure 4.
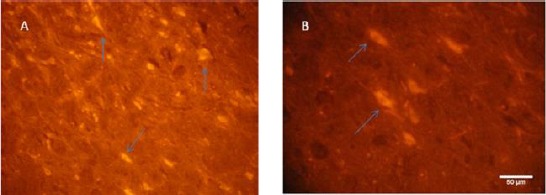
Light photomicrograph of rat substantia nigra. Mesenchymal stem cells (MSCs) were detected in mid brain by fluorescence microscope. (A, B) Arrows show that MSCs were labeled with CM-DiI and appeared clear under fluorescent microscopy. The labeled cells were mainly detected in substantia nigra after injection
BDNF assay
Neurotrophic factors, such as the BDNF, have been considered to play a principal role in protection of neurons. In this study, we have demonstrated that endogenous BDNF are altered in PD, and the stem cells can change the BDNF level in the serum and brain. We found that the level of brain BDNF was higher and significant in exposed cells (400 µT) group in comparison with the 6-OHDA group (398±15 vs 312±11.79 pg⁄mg protein, respectively) (P= 0.001). In this group, the level of BDNF was higher than control group (398±15 vs 342±8.79 pg⁄mg protein). There were not significant differences between the other treatment groups. The results were slightly different in the serum. Results have shown that after injury, the level of serum BDNF were increased in all treatment groups compared to control group. There were not also significant differences between the levels of serum BDNF in all treatment groups (Figure 5).
Figure 5.
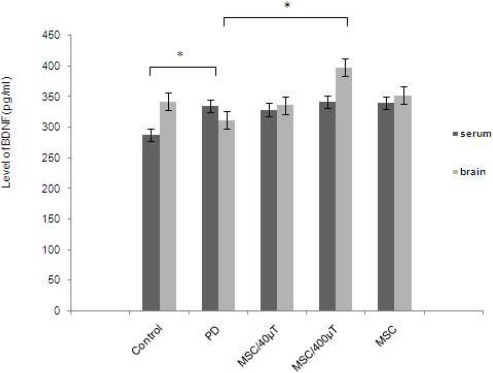
Brain derived neurotrophic factor (BDNF) levels in serum and brain of rats. In serum, the ratio was significantly elevated by 6-OHDA treatment in comparison with the control group. At the same time, it was induced in the stem cell treatment. All values are mean±SEM. *compared with the Parkinson’s disease (PD) group (P ≤ 0.01)
Discussion
The most important specification of MSCs is the ability to self-renew and generation of other cells including different kinds of neurons, astrocytes, and oligodendrocytes. In addition to these cells, here we explained the multi-potential stem cells, which were placed in the EMF and differentiated into dopaminergic neurons and also deployed in damaged area in the brain. These activated cells could increase some important factors that supported neurons. MSCs have clinical potential. These cells have been used for the treatment of different neurodegenerative diseases such as Parkinson’s disease, multiple sclerosis, peripheral nervous lesion, and traumatic spinal cord injuries (15, 16). Researches are now focused on neurogenesis in cerebral degenerative diseases. Different types of SCs such as mesenchymal and embryonic stem cells may be a suitable source for clinical applications. If MSCs could be proliferated rapidly in high quantities over a short period of time, and could be induced to differentiate into specific neurons, it would be a super excellence. In this paper, we focused on attempting to activate MSCs in suspended culture medium, and differentiate in vivo to develop a new method, which allows MSCs to be expanded and activated rapidly in a short time and be capable of differentiating into dopaminergic neurons successfully. In this study, we observed that cells that isolated from the rat bone marrow may be proliferated in vitro, and after injection can be transferred to mid brain. Dopaminergic neurons can be found in different areas of brain and brain stem such as the substantia nigra of midbrain, hypothalamus, some part of retina, and sheet of olfactory bulbs. The most dominant groups of DA neurons stationed in the ventral tegmental area and substantia nigra of the midbrain; both of these areas participate in the formation of extra pyramidal motor system that controls postural reflexes and are responsible for initiation of movement (2). It is estimated that striatal environment and cells might be responsible for producing neurotrophic factors that lead to major differentiation of progenitor cells into TH-positive neurons. Therefore, we injected MSCs into left ventricle, and then cells suspend in the cerebro spinal fluid (CSF) and migrate to damaged area. We observed that the labeled cells that were injected in the left ventricle, reside in midbrain. Some of these cells were in substantia nigra and the others were spread sporadically in the mid brain. Results have shown that MSCs are able to pass through blood brain barrier and be stationed in the affected areas. But, how these cells are capable of interacting with other cells or differentiate into dopaminergic neurons and produce dopamine are not correctly known.
It is widely accepted that EMF can influence several biological functions, modulate intracellular reactive oxygen species (ROS) levels and the cell cycle progression (17-19). Exposing cells to 50 Hz EMF lead to increase in cell proliferation rate (20). Stimulating the cells with 0.1 μT EMF activates the protein kinase C. This activation caused an increase in cell proliferation. An increase in [Ca2+] in cells upon EMF exposure was reported by numerous researchers (21, 22), and it is known that this function is able to modulate proteasome activity (23).
MSCs are multipotential cells and have high capacity for replication. Cells have a potential to differentiate into other lineages of mesenchymal tissues (24). The exposure of MSCs to 600 μT causes the MSCs differentiate into other cells such as adipocytes. Therefore, EMF exposure may also lead to overexpression of lipoprotein lipase and peroxisome (25). However, it is reported that exposure of MSCs to EMF increase cells differentiation (26).
Sinusoidal EMF of 800 μT with frequency of 50 Hz is able to differentiate stem cells. Real-time quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis shows a significantly increase of GATAAGA2-4 and Nkx-2.5 mRNA expression (27). However, exposing of embryonic stem cells with the above described EMF caused differentiation into cardiac cells (27). GATA-4 and Nkx-2.5 mRNA are essential for encoding zinc finger containing transcription factor and homeodomain, and both of these are essential for cardiogenesis in different species (28), especially in human (29, 30). The EMF has been previously tested on P19 embryonic carcinoma cells (P19 cells) (31). EMF with intensity of 1 mT and frequency of 50 Hz leads to differentiation of P19 cells; however, the result was not very significant. By exposing P19 cells into sever EMF with the intensity of 10 mT, it differentiates into neuronal cells (31). Exposure of bone morrow stem cells to EMF with intensity of 1.1 mT leads to differentiation to osteogenic cells (32). Differentiation of BMSC into osteogenic cells is due to increase of intracellular Ca2+ after EMF stimulation. According to these results, it has been deduced that the elevation of Ca2+ in intracellular is one of the important factors for activation of biochemical mechanism that is responsible for the induction of terminal differentiation (32).
The above findings revealed that EMF can cause proliferation and differentiation of stem cells into other cells. And this may open a new prospective in the use of EMF for differentiation of stem cells into a specific cells without the aid of gene transfer technologies.
In conclusion, in accordance with the results of other studies, we suggest that the range of EMF, which is effective on differentiation of MSCs, is between 10 μT and 400 μT. Also the differentiation will not occur if the intensity is less than 10 μT. The results of this research have shown that the intensity of 400 μT have more effective on the settle of the cells on injured area and recovery of the disease. According to the data, the number of DA neurons in SNpc in group of 400 µT was more than other groups. Also, these cells that were exposed to EMF are much more active and can differentiate into DA neurons in vivo.
Function and survival of striatal neurons dependent on BDNF, which is chiefly provided by anterograde transport from corticostriatal afferents (33). The present study reports that the elevation of brain and serum BDNF following 6-OHDA treatment support dopaminergic neurons of SNpc. One of important mechanisms that MSCs might protect DA neurons in striate is through the production or up-regulation of neurotrophines such as BDNF. High concentrations of BDNF have not only been found in the central nervous system, but also in other non-neuronal cells, particularly in platelets. A positive correlation between serum and cortical BDNF concentrations has been observed in rats (34, 35) and humans (36). In this study, the level of BDNF was increased in brain in all cell therapy groups, but the level of brain BDNF was only significant in group of 400 μT. It was concluded that there was a constitutive up-regulation of brain BDNF concentrations that might compensate for defective intracellular protein signaling in the SNpc of brain stem. Study of brain tissue from patients with Parkinson’s disease after death has shown that degeneration in striatal neurons are closely related with reduction in BDNF expression. Moreover, in the brain tissue of Parkinson’s, BDNF of striatum is more reduced as compared to age or sex-matched controls (37).
Now, there is no effective treatment for PD patients (38). Neuroprotective growth factors have also been used for PD (36). Among the growth factors, BDNF is the best candidate to modulate the onset and severity of movement and cognitive functions in PD mouse models (35). In animal models, a striatal stab wound in 6- to 8-week old mice increases BDNF level around the injury site and activates microglia and macrophages (39). Furthermore, the production of BDNF occurs in dopaminergic fibers and dopamine-transporter positive neuritis (40). Thus, these studies suggest that increased BDNF expression following administration of activated MSCs protects dopaminergic neurons from death, and supports the residence of new cells in damage area and production of BDNF.
Stem cells have the ability to regulate immune responses (41, 42) and differentiate into special cells with the aim of replacing injured cells (43). These cells produce trophic factors for protection and repair of cells by inhibition of apoptotic pathways (44). Therefore, MSCs might express and produce growth factors such as, epidermal growth factor (EGF), glial cell-derived neurotrophic factor (GDNF), BNDF, and stromal-derived factor (SDF-1α) (45). The neuroprotective effect of BDNF on cultured neurons is through the Pl3kinase/Akt pathway by inhibition of neuronal death (46). The most important discovery of this research is that in treatment groups, the number of injected cells was equal, but in cells exposed to 400 μT, the level of brain and serum BDNF was higher than other cell treatment groups. The cells exposed to 400 μT are more active and are able to convert to dopaminergic neurons. Therefore, it can be concluded that EMF with intensity 400 μT and frequency 50 HZ will stimulate and differentiate MSCs to neuronal cells.
Conclusion
MSCs that were exposed to EMF with 400 μT increase brain BDNF and subsequently lead to increased tyrosine hydroxylase neurons in SNpc after 6-OHDA. We propose that these activated MSCs have good effects on Parkinson’s dieses. However, future studies are required to reveal further detailed mechanisms of action.
Acknowledgment
We would like to thank the Research Center of Nervous System Stem Cells of Semnan University of Medical Sciences, Semnan, Iran, for cooperation and providing facilities to this work. This work was supported by Grant no. 506.
Conflict of interest
The authors declare no conflict of interest regarding the publication of this paper.
References
- 1.Chen CC, Shih YY, Chang C. Dopaminergic imaging of nonmotor manifestations in a rat model of Parkinson’s disease by fMRI. Neurobiol Dis. 2013;49:99–106. doi: 10.1016/j.nbd.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Danielyan L, Beer-Hammer S, Stolzing A, Schäfer R, Siegel G, Fabian C, et al. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transplant. 2014;1:123–139. doi: 10.3727/096368914X684970. [DOI] [PubMed] [Google Scholar]
- 3.Lin YC, Hsieh AR, Hsiao CL, Wu SJ, Wang HM, Lian IeB, et al. Identifying rare and common disease asso-ciated variants in genomic data using Parkinson’s disease as a model. J Biomed Sci. 2014;21:88. doi: 10.1186/s12929-014-0088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capitelli CS, Lopes CS, Alves AC, Barbiero J, Oliveira LF, da Silva VJ, et al. Opposite effects of bone marrow-derived cells transplantation in MPTP-rat model of Parkinson’sdisease: a comparison study of mononuclear and mesenchymal stem cells. Int J Med Sci. 2014;11:1049–1064. doi: 10.7150/ijms.8182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glavaski-Joksimovic A, Bohn MC. Mesenchymal stem cells and neurodegeneration in Parkinson’s disease. Exp Neurol. 2013;247:25–38. doi: 10.1016/j.expneurol.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Yang J, Li H, Wang X, Zhu L, Fan M, et al. Hypoxia promotes dopaminergic differentiation of mesenchymal stem cells and shows benefits for transplantation in a rat model of Parkinson’s disease. PLoS One. 2013;8:54296. doi: 10.1371/journal.pone.0054296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan MS, Tabrez S, Priyadarshini M, Priyamvada S, Khan MM. Targeting Parkinson’s-tyrosine hydroxyl-lase and oxidative stress as points of interventions. CNS Neurol Disord Drug Targets. 2012;11:369–380. doi: 10.2174/187152712800792848. [DOI] [PubMed] [Google Scholar]
- 8.Shukla A, Mohapatra TM, Parmar D, Seth K. Neuroprotective potentials of neurotrophin rich olfactory ensheathing cell’s conditioned media against 6OHDA-induced oxidative damage. Free Radic Res. 2014;48:560–571. doi: 10.3109/10715762.2014.894636. [DOI] [PubMed] [Google Scholar]
- 9.Golbach LA, Scheer MH, Cuppen JJ, Savelkoul H, Verburg-van Kemenade BM. Low-frequency electromagnetic field exposure enhances extra-cellular trap formation by human neutrophils through the NADPH Pathway. J Innate Immun. 2015;7:459–465. doi: 10.1159/000380764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amaroli A, Chessa MG, Bavestrello G, Bianco B. Effects of an extremely low-frequency electro-magnetic field on stress factors: a study in Dictyostelium discoideum cells. Eur J Protistol. 2013;49:400–405. doi: 10.1016/j.ejop.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Ketabi N, Mobasheri H, Faraji-Dana R. Electromagnetic fields (UHF) increase voltage sensitivity of membrane ion channels; possible indication of cell phone effect on living cells. Electromagn Biol Med. 2015;34:1–313. doi: 10.3109/15368378.2013.844706. [DOI] [PubMed] [Google Scholar]
- 12.Kim HJ, Jung J, Park JH, Kim JH, Ko KN, Kim CW. Extremely low-frequency electromagnetic fields induce neural differentiation in bone marrow derived mesenchymal stem cells. Exp Biol Med (Maywood) 2013;238:923–931. doi: 10.1177/1535370213497173. [DOI] [PubMed] [Google Scholar]
- 13.Ross CL, Siriwardane M, Almeida-Porada G, Porada CD, Brink P, Christ GJ, et al. The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation. Stem Cell Res. 2015;15:96–108. doi: 10.1016/j.scr.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Safari M, Jadidi M, Baghian A, Hasanzadeh H. Proliferation and differentiation of rat bone marrow stem cells by 400 µT electromagnetic field. Neurosci Lett. 2015;612:1–6. doi: 10.1016/j.neulet.2015.11.044. [DOI] [PubMed] [Google Scholar]
- 15.Datta I, Bhonde R. Can mesenchymal stem cells reduce vulnerability of dopaminergic neurons in the substantia nigra to oxidative insult in individuals at risk to Parkinson’s disease? Cell Biol Int. 2012;36:617–624. doi: 10.1042/CBI20110602. [DOI] [PubMed] [Google Scholar]
- 16.García Santos JM, Blanquer M, Torres del Río S, Iniesta F, Espuch JG, Pérez-Espejo MA, et al. Acute and chronic MRI changes in the spine and spinal cord after surgical stem cell grafting in patients with definite amyotrophic lateral sclerosis: post-infusion injuries are unrelated with clinical impairment. Magn Reson Imaging. 2013;31:1298–1308. doi: 10.1016/j.mri.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Poniedzialek B, Rzymski P, Nawrocka-Bogusz H, Jaroszyk F, Wiktorowicz K. The effect of electromagnetic field on reactive oxygen species production in human neutrophils in vitro. Electromagn Biol Med. 2013;32:333–341. doi: 10.3109/15368378.2012.721845. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Li X, Bai L, Uchida K, Bai W, Wu B, et al. Effects of low frequency electromagnetic field on proliferation of human epidermal stem cells: An in vitro study. Bioelectromagnetics. 2013;34:74–80. doi: 10.1002/bem.21747. [DOI] [PubMed] [Google Scholar]
- 19.Zhong C, Zhang X, Xu Z, He R. Effects of low-intensity electromagnetic fields on the proliferation and differentiation of cultured mouse bone marrow stromal cells. Phys Ther. 2012;92:1208–1219. doi: 10.2522/ptj.20110224. [DOI] [PubMed] [Google Scholar]
- 20.Eleuteri AM, Amici M, Bonfili L, Cecarini V, Cuccioloni M, Grimaldi S, et al. 50 Hz extremely low frequency electromagnetic fields enhance protein carbonyl groups content in cancer cells: effects on proteasomal systems. J Biomed Biotechnol. 2009;2009:834239. doi: 10.1155/2009/834239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pall ML. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J Cell Mol Med. 2013;17:958–965. doi: 10.1111/jcmm.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo FL, Yang N, He C, Li HL, Li C, Chen F, et al. Exposure to extremely low frequency electromagnetic fields alters the calcium dynamics of cultured entorhinal cortex neurons. Environ Res. 2014;135:236–246. doi: 10.1016/j.envres.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Platano D, Mesirca P, Paffi A, Pellegrino M, Liberti M, Apollonio F, et al. Acute exposure to low-level CW and GSM-modulated 900 MHz radiofrequency does not affect Ba 2+ currents through voltage-gated calcium channels in rat cortical neurons. Bioelectromagnetics. 2007;28:599–607. doi: 10.1002/bem.20345. [DOI] [PubMed] [Google Scholar]
- 24.Ge W, Ren C, Duan X, Geng D, Zhang C, Liu X, et al. Differentiation of mesenchymal stem cells into neural stem cells using cerebrospinal fluid. Cell Biochem Biophys. 2015;71:449–455. doi: 10.1007/s12013-014-0222-z. [DOI] [PubMed] [Google Scholar]
- 25.Schäfer R, Kehlbach R, Muller M, Bantleon R, Kluba T, Ayturan M, et al. Labeling of human mesenchymal stromal cells with superparamagnetic iron oxide leads to adecrease in migration capacity and colony formation ability. Cytotherapy. 2009;11:68–78. doi: 10.1080/14653240802666043. [DOI] [PubMed] [Google Scholar]
- 26.Schäfer R, Bantleon R, Kehlbach R, Siegel G, Wiskirchen J, Wolburg H, et al. Functional investigation on human mesenchymal stem cells exposed to magnetic fields and labeled with clinically approved iron nanoparticles. BMC Cell Biol. 2010;11:22. doi: 10.1186/1471-2121-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ventura C, Maioli M, Asara Y, Santoni D, Mesirca P, Remondini D, et al. Turning on stem cell cardiogenesis with extremely low frequency magnetic fields. FASEB J. 2005;19:155–157. doi: 10.1096/fj.04-2695fje. 470. [DOI] [PubMed] [Google Scholar]
- 28.Mascheck L, Sharifpanah F, Tsang SY, Wartenberg M, Sauer H. Stimulation of cardiomyogenesis from mouse embryonic stem cells by nuclear translocation of cardiotrophin-1. Int J Cardiol. 2015;193:23–33. doi: 10.1016/j.ijcard.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Zhang H, Nie L, Xu L, Chen M, Ding Z. Myogenic differentiation and reparative activity of stromal cells derived from pericardial adipose in comparison to subcutaneous origin. Stem Cell Res Ther. 2014;5:92. doi: 10.1186/scrt481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ventura C, Maioli M, Asara Y, Santoni D, Mesirca P, Remondini D, et al. Turning on stem cell cardiogenesis with extremely low frequency magnetic fields. FASEB J. 2004;19:155–157. doi: 10.1096/fj.04-2695fje. [DOI] [PubMed] [Google Scholar]
- 31.Saito A, Takayama Y, Moriguchi H, Kotani K, Jimbo Y. Developmental effects of low frequency magnetic fields on P19-derived neuronal cells. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:5942–5945. doi: 10.1109/IEMBS.2009.5334755. [DOI] [PubMed] [Google Scholar]
- 32.Zhao W, Ma W, Zhao Z, Fang Z, Wu H. Preliminary research on the proliferation and differentiation of rat bone marrow mesenchymal stem cells with exposure to 50 Hz magnetic fields. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2015;22:510–513. [PubMed] [Google Scholar]
- 33.Zhang HY, Song N, Jiang H, Bi MX, Xie JX. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor inhibit ferrous iron influx via divalent metal transporter 1 and iron regulatory protein 1 regulation in ventral mesencephalic neurons. Biochim Biophys Acta. 2014;1843:2967–2975. doi: 10.1016/j.bbamcr.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Safari M, Sameni HR, Badban L, Bandegi AR, Vafaei AA, Rashidy Pour, et al. Protective effects of water extract of propolis on dopaminergic neurons, brain derived neurotrophic factor, and stress oxidative factors in the rat model of Parkinson’s disease. Int J Pharmacol. 2015;11:300–308. [Google Scholar]
- 35.Ventriglia M, Zanardini R, Bonomini C, Zanetti O, Volpe D, Pasqualetti P, et al. Serum brain-derived neurotrophic factor levels in different neurological diseases. Biomed Res Int. 2013;2013:901082. doi: 10.1155/2013/901082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziebell M, Khalid U, Klein AB, Aznar S, Thomsen G, Jensen P, et al. Striatal dopamine transporter binding correlates with serum BDNF levels in patients with striatal dopaminergic neurodegene-ration. Neurobiol Aging. 2012;33:1–5. doi: 10.1016/j.neurobiolaging.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Białecka M, Kurzawski M, Roszmann A, Robowski P, Sitek EJ, Honczarenko K, et al. BDNF G196A (Val66Met) polymorphism associated with cognitive impairment in Parkinson’sdisease. Neurosci Lett. 2014;561:86–90. doi: 10.1016/j.neulet.2013.12.051. [DOI] [PubMed] [Google Scholar]
- 38.Gopalakrishna A, Alexander SA. Understan-ding Parkinson Disease: A Complex and Multifaceted Illness. J Neurosci Nurs. 2015;47:320–326. doi: 10.1097/JNN.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 39.Rizzo F, Riboldi G, Salani S, Nizzardo M, Simone C, Corti S, et al. Cellular therapy to target neuroinflammation in amyotrophic lateral sclerosis. Cell Mol Life Sci. 2014;71:999–1015. doi: 10.1007/s00018-013-1480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Razgado-Hernandez LF, Espadas-Alvarez AJ, Reyna-Velazquez P, Sierra-Sanchez A, Anaya-Martinez V, Jimenez-Estrada I, et al. The transfection of BDNF to dopamine neurons potentiates the effect of dopamine D3 receptor agonist recovering the striatal innervation, dendritic spines and motor behavior in an aged rat model of Parkinson’s disease. PLoS One. 2015;10:0117391. doi: 10.1371/journal.pone.0117391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou Y, Singh AK, Hoyt RF, Jr, Wang S, Yu Z, Hunt T, et al. Regulatory T cells enhance mesenchymal stem cell survival and proliferation following autologous cotransplantation in ischemic myocar-dium. J Thorac Cardiovasc Surg. 2014;148:1131–1137. doi: 10.1016/j.jtcvs.2014.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: what do we know so far? Biomed Res Int. 2014;2014:216806. doi: 10.1155/2014/216806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng R, Wang LW, Hu ZB, Guo WT, Wei JS, Lin H, et al. Differentiation of human bone marrow mesenchymal stem cells into neuron-like cells in vitro. Spine. 2011;36:997–1005. doi: 10.1097/BRS.0b013e3181eab764. [DOI] [PubMed] [Google Scholar]
- 44.Jager M, Hernigou P, Zilkens C, Herten M, Li X, Fischer J, et al. Cell therapy in bone healing disorders. Orthop Rev. 2010;2:79–87. doi: 10.4081/or.2010.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Croft AP, Przyborski SA. Mesenchymal stem cells expressing neural antigens instruct a neurogenic cell fate on neural stem cells. Exp Neurol. 2009;216:329–341. doi: 10.1016/j.expneurol.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Wilkins A, Kemp K, Ginty M, Hares K, Mallam E, Scolding N. Human bone marrow-derived mesenchymal stem cells secrete brain-derived neurotrophic factor which promotes neuronal survival in vitro. Stem Cell Res. 2009;3:63–70. doi: 10.1016/j.scr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 47.Paxinos G, Franlin k. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 48.Szapacs ME, Mathews TA, Tessarollo L, Ernest Lyons W, Mamounas LA, Andrews AM. Exploring the relationship between serotonin and brainderived neurotrophic factor: analysis of BDNF protein and extraneuronal 5-HT in mice with reduced serotonin transporter or BDNF expression. J Neurosci Methods. 2004;140:81–92. doi: 10.1016/j.jneumeth.2004.03.026. [DOI] [PubMed] [Google Scholar]


