Abstract
Objective(s):
Repaglinide (RG) is an antihyperglycemic agent used for the treatment of non-insulin-dependent diabetes mellitus. It has a good safety and efficacy profile in diabetic patients with complications in renal impairment and is an appropriate treatment choice, even for individuals with more severe degrees of renal malfunctions. The aim of the present study was to examine the protective effect of RG on cyclosporine A (CsA)-induced rat renal impairment and to evaluate the antioxidant mechanisms by which RG exerts its protective actions.
Materials and Methods:
Fifty male Sprague-Dawley rats weighing 250–300 g were randomly divided into five groups: administrations of olive oil (control, PO), RG (0.4 mg/kg, PO), CsA (30 mg/kg in olive oil, SC), RG (0.2 or 0.4 mg/kg, PO) plus CsA (30 mg/kg in olive oil SC) every day for 15 days.
Results:
SC administration of CsA (30 mg/kg) to rats produced marked elevations in the levels of renal impairment parameters such as urinary protein, N-acetyl-beta-D-glucosaminidase (NAG), serum creatinine (SCr), and blood urea nitrogen (BUN). It also caused histologic injury to the kidneys. Oral administration of RG (0.2 and 0.4 mg/kg) markedly decreased all the aforementioned changes. In addition, CsA caused increases in the levels of malondialdehyde (MDA) and decreases in superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), glutathione reductase (GSR), glutathione-S-transferase (GST), and glutathione in kidney homogenate, which were reversed significantly by both doses of RG.
Conclusion:
The findings of our study indicate that RG may play an important role in protecting the kidney from oxidative insult.
Keywords: Cyclosporine A-induced renal tubular injury, Glutathione expression rat, Repaglinide antioxidant
Introduction
Repaglinide (RG), a nonsulfonylurea insulin secretagogue, is a prandial glucose regulator used for the treatment of type 2 diabetes (1). In the literature, repaglinide has a good safety and efficacy profile in type 2 diabetic patients with renal impairment complications (2, 3). It has been reported that RG treatment decreases the concentration of lipid hydroperoxide (LPO) and increases the activity of superoxide dismutase (SOD) in kidneys of diabetic nephropathy (4, 5). A study by Gumieniczek suggested that RG possesses antioxidant properties, which are independent of its action on hyperglycaemia (4). The direct antioxidant properties of RG produce better effects at therapeutic doses and can also contribute to its effectiveness in the therapy of type 2 diabetes (4).
The notion that reactive oxygen species (ROS) are involved in progressive renal injury is supported by several lines of evidence: increased generation of oxides occurs in acute and chronic renal damages.
Various antioxidant strategies have beneficial effects in models of acute and chronic renal injury. Therefore, reduction in renal oxidative stress by drugs provides an appealing target for therapies directed towards the retardation of progressive renal damage (6).
Glutathione (GSH) plays an important role in maintaining proper function and in preventing oxidative stress in human cells. GSH is an important antioxidant and can act as a scavenger for hydroxyl radicals, singlet oxygen, and various electrophiles, which makes it a biomarker for preventing damage to important cellular components caused by ROS such as free radicals, peroxides, and lipid peroxides (7).
Cyclosporine A (CsA) is widely used as an immune suppressant for organ transplantation and auto-immune disorders, but its use is limited by its nephrotoxicity (8). Its acute toxicity is tubular necrosis damage, its chronic nephrotoxicity is renal interstitial fibrosis and tubular atrophy (9). Recent studies have shown that oxidative stress plays an important role in mediating CsA-induced nephron-toxicity (10-12).
Although RG has antioxidant effects in the therapy of type 2 diabetes, it is not clearly known whether it has an effect on the expression of GSH and the activity of antioxidant enzymes in non-diabetic kidneys injury. Therefore, we have established a model of CsA-induced renal tubular damage, and study was conducted to elucidate the antioxidant effects of RG in protecting the renal toxicity caused by CsA in rats.
Materials and Methods
All experiments carried out in the current study, were approved by the Animal Ethics Committee of Wuhan University (Protocol #:11.10.2012-2013/7-10). Animals were obtained from the Experimental Research Centre, Wuhan, China, and were cared in accordance with Institutional Guidelines for the Laboratory Animals Care and Use. Fifty male Sprague-Dawley rats age 8-week-old and weighing 250–300 g were housed in standard cages at room temperature (20-24) and regular light cycle (12 light/12 dark). These rats were allowed free access to food and water.
Animals were randomly divided into five groups of ten animals each. Control group: the rats were subjected to 1 ml olive oil administration by gavage (IG) every day for 15 days. RG (Sigma, St. Louis, MO, USA) group: the rats were subjected to RG in olive oil (0.4 mg/kg, IG) every day for 15 days. CsA (Novartis Pharma GmbH, Mannheim, Germany) group: the rats were given subcutaneous (SC) CsA injection, 30 ml/kg in olive oil daily for 15 days. RG + CsA group: the rats were subjected to RG (0.2 mg/kg, IG) plus CsA injection, SC, 30 ml/kg daily for 15 days. RG + CsA group: the rats were subjected to RG (0.4 mg/kg, IG) plus CsA injection, SC, 30 ml/kg of body weight daily for 15 days. To prevent RG-induced rat hypoglycemia reaction, 5% glucose administration by IG after RG was given PO daily during the experimental period.
The rats were maintained individually in the metabolic cages for 2 days before beginning the experiments to allow urine collections for the measurement of urinary protein and urinary N-acetyl-beta-D-glucosaminidase (NAG) levels. At the end of the experiments, animals in all groups were anesthetized 4 hr after the last administration with 45 mg/kg sodium pentobarbital and sacrificed. Blood samples were collected and centrifuged at 200 g for 5 min at +4°C for subsequent measurement of serum creatinine (SCr) and blood urea nitrogen (BUN). Kidneys were removed rapidly, sectioned and fixed with 4% paraformaldehyde for histological and immunohistochemical analysis. The renal cortex was separated, kept at −80 °C and subsequently homo-genized in cold Tris-HCl buffer (0.05 mol/l Tris-HCl, 1.15% KCl, pH7.4), using a Polytron tissue homogeniser. The homogenates were centrifuged at 18, 000 × g (+4 °C) for 30 min. The resulting supernatant was used for biochemical analysis.
Urinary protein was measured using the sulfosalicylic acid colorimetric method (13). Urinary NAG activity was determined by colorimetric method utilizing 3-cresolsulphaphytaleinyl-N-acetyl-b-Dglu-cosaminide as a substrate (14). Contents of SCr and BUN were measured using an autoanalyzer (Beckman Instruments, Fullerton, CA, USA). The Level of malondialdehyde (MDA) was determined according to the method based on the reaction with thiobarbituric acid (15). SOD activity was measured in cytosolic fraction following the inhibition of pyrogallol autooxidation (16). The level of GSH was assayed colorimetrically as protein-free sulfhydryl content using 5,5-dithiobis-2-nitrobenzoic acid (17). Glutathione-S-transferase (GST) activity was assayed spectrophotometrically using 1-chloro-2, 4-dinitrobene as a substrate in the presence of GSH (18). Activities of glutathione peroxidase (GSH-Px) and glutathione reductase (GSR) were assayed by the enzymatic method (19, 20). Total protein content was determined by Lowry method using bovine serum albumin as a standard (21).
At the end of the experiment the kidneys were fixed in 10% formalin solution and then embedded in paraffin. Sections at 4-μm thickness were cut by a microtome, stained with hematoxylin and eosin (H&E) and examined with a light microscope to evaluate renal tubule pathological changes.
Kidneys were fixed in 4% formaldehyde and embedded in paraffin. Five micrometer thick sections were prepared from different animal groups and immunohistochemical analysis was performed. Sections were pre-treated in 10 mm sodium citrate buffer (pH 6.0) in a microwave for 20 min. According to the streptavidin peroxidase (SP) method (22), Sections were incubated at room temperature with GSH monoclonal antibody (1:50) and then probed with secondary antibody. Slides were counterstained with hematoxylin, and were visualized through light microscope and the extent of cell immunopositivity was assessed. Positive cells were cytoplasm stained yellow brown.
All values were expressed as mean±standard deviations (n= 10). The results were analyzed by Student’s t-test and one-way analysis of variance (ANOVA). The effect was considered significant when the P-value was < 0.05. All statistical analyses were performed using SPSS software 17.0 (Released Aug. 23, 2008), Chicago, USA.
Results
A single dose of CsA caused a significant increase in urinary protein and urinary NAG levels 15 days after treatment. Compared to rats treated with CsA alone, co-administration with RG (0.2, 0.4 mg/kg) significantly alleviated urinary protein and urinary NAG levels (Table 1). In addition, rats treated with CsA alone showed significant elevation in both BUN and SCr levels in comparison with the control group (P<0.01). The administration of RG (0.2 and 0.4 mg/kg) dose-dependently inhibited the elevation of BUN and SCr levels compared to CsA alone group (P<0.05, P<0.01) (Table 2).
Table 1.
Effect of repaglinide on cyclosporine A-induced changes in urinary protein and urinary N-acetyl-beta-D-glucosaminidase contents
| Group | Urinary protein mg/24 hr urine | Urinary NAG U/g creatinine |
|---|---|---|
| Control | 1.58 ± 0.64 | 24.22 ± 4.62 |
| RG (0.4 mg/kg) | 1.68 ± 0.54 | 25.42 ± 2.86 |
| CsA (30mg/kg) | 13.54 ± 2.86** | 56.76 ± 5.48** |
| RG (0.2 mg/kg) + CsA | 9.34 ± 2.47# | 38.18 ± 8.28# |
| RG (0.4 mg/kg) + CsA | 2.880 ± 1.48## | 28.86 ± 6.28## |
Data given are the mean±standard deviations (n= 10);
Significantly different from control group (P<0.01);
Significantly different from CsA group (P<0.05);
Significantly different from CsA group (P<0.01)
NAG: N-acetyl-beta-D-glucosaminidase; RG: Repaglinide; CsA: cyclosporine A
Table 2.
Effect of repaglinide on cyclosporine A-induced changes in blood urea nitrogen and serum creatinine contents
| Group | BUN (nmol/l) | SCr (µmol/l) |
|---|---|---|
| Control | 10.26±0.72 | 34.68±3.74 |
| RG (0.4 mg/kg) | 10.86±0.44 | 36.84±5.43 |
| CsA (30mg/kg) | 19.80±2.80** | 70.68±4.82** |
| RG (0.2 mg/kg) + CsA | 15.72±0.45# | 48.62±8.52# |
| RG (0.4 mg/kg) + CsA | 11.08±0.96## | 40.76±5.28## |
Data given are the mean ± standard deviations (n = 10);
Significantly different from control group (P<0.01);
Significantly different from CsA group (P<0.05);
Significantly different from CsA group (P<0.01)
BUN: blood urea nitrogen; SCr: serum creatinine; RG: Repaglinide; CsA: cyclosporine A
Histological analysis revealed that kidney tissues in control rats showed normal tubules structure (Figure 1a). The rats treated with RG (0.4 mg/kg) alone had no effect on kidneys histology (Figure 1b). In comparison, CsA treatment caused evident morphological alterations, which included tubular swelling and protein casts (Figure 1c). RG (0.2 mg/kg) reduced swelling and protein casts in tubules (Figure 1d), while RG (0.4 mg/kg) significantly ameliorated tubular injury induced by CsA, and the tubules showed only a few and slight protein casts (Figure 1e).
Figure 1.
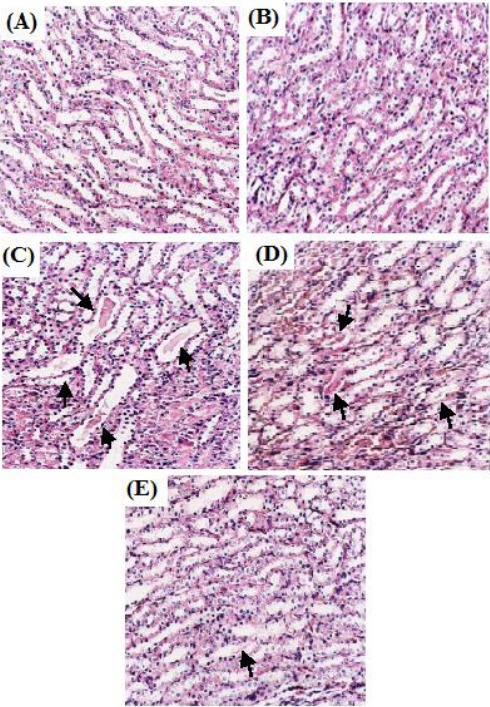
Light microscopy of kidneys tissues from rats (HE stained kidney sections, 400×)
(A) Control group: normal renal tubules. (B) RG (0.4 mg/kg) group: normal renal tubules. (C) CsA group: tubules show extensive and marked swelling and protein casts (arrow). (D) RG (0.2 mg/kg) + CsA group: tubules show swelling and protein casts obvious decrease (arrow). (E) RG (0.4 mg/kg) + CsA group: tubules show only a few and slight protein casts (arrow)
Biochemical analysis showed that GSH levels and SOD, GST, GSH-Px, and GSR activities were lower while MDA levels in kidney tissues were higher in the CsA group alone than those in control rats (P<0.01). Compared with control group, GSH content was increased in rats treated with RG (0.4 mg/kg) alone (P<0.05). RG (0.2, 0.4 mg/kg) significantly decreased MDA levels while increased GSH content and SOD, GST, GSH-Px, and GSR activities when compared to the group treated with CsA alone (P<0.05, P<0.01). RG showed dose-dependent changes in GSH, MDA, SOD, GST, GSH-Px, and GSR levels compared to CsA-treated group (Table 3).
Table 3.
Effect of repaglinide on cyclosporine A-induced changes in rat kidneys malondialdehyde, glutathione levels and superoxide dismutase, glutathione-S-transferase, glutathione peroxidase and glutathione reductase activities
| Group | MDA | GSH | SOD | GST | GSH-Px | GSR |
|---|---|---|---|---|---|---|
| nmol/mg tissue | umol/g tissue | nmol/mg pro | mmol/min/mg pro | mmol/min/mg pro | mmol/min/mg pro | |
| Control | 3.45±0.62 | 19.53±1.07 | 42.15±1.23 | 45.80±4.37 | 84.28±9.93 | 80.17±59.14 |
| RG (0.4 mg/kg) | 3.56±0.87 | 28.86±1.86* | 43.24±1.12 | 46.57±5.8 | 86.35±7.42 | 82.36±40.26 |
| CsA (30 mg/kg) | 7.25±0.30** | 11.20±1.78** | 19.08±4.06** | 19.96±1.07** | 28.84±9.53** | 38.34±21.63** |
| RG (0.2 mg/kg)+ CsA | 5.70±0.68# | 14.65±2.49# | 40.32±6.08## | 20.04±6.89# | 40.79±8.53# | 53.40±25.72# |
| RG (0.4 mg/kg) + CsA | 3.08±0.42## | 23.35±1.44## | 45.81±4.50## | 47.78±3.08## | 87.85±5.40## | 86.46±22.62## |
Data given are the mean ± standard deviations (n=10);
Significantly different from control group (P<0.05);
Significantly different from control group (P<0.01);
Significantly different from CsA group (P<0.05);
Significantly different from CsA group (P<0.01)
RG: Repaglinide; CsA: cyclosporine A; MDA: malondialdehyde; SOD: superoxide dismutase, GSH-Px: glutathione peroxidase, GSR: glutathione reductase, GST:glutathione-S-transferase
Immunohistochemical analysis demonstrated that abundant GSH expression was observed in control tubules (Figure 2a). Expression of GSH was much stronger in rats treated with RG 0.4 mg/kg alone as compared to control group (Figure 2b). In contrast, expression of GSH was much weaker in rats treated with CsA compared to control group (Figure 2c). GSH expression of renal tubules was increased in the rats treated with RG (0.2 mg/kg) plus CsA as compared to CsA group alone (Figure 2d), while RG (0.4 mg/kg) in GSH expression was restored to near normal levels as compared to CsA group alone (Figure 2e).
Figure 2.
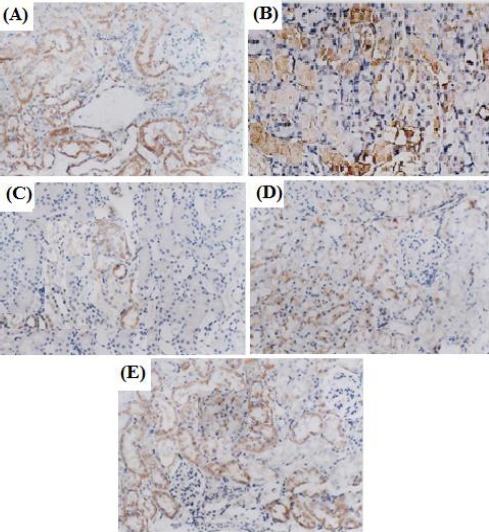
Effect of repaglinide and cyclosporine A treatment on the expression of tubules GSH (SP, 400×). Cytoplasm was brown-yellow stained in GSH expression. (A) Control group: expression of GSH was abundant. (B) RG (0.4 mg/kg) group: expression of GSH was much stronger. (C) CsA group: expression of GSH was weaker. (D) RG (0.2 mg/kg) + CsA group: expression of GSH was increased. (E) RG (0.4 mg/kg) + CsA group: expression of GSH was abundant
Discussion
The end product of lipid peroxidation is MDA. ROS-induced lipid peroxidation and form MDA (23, 24). ROS function as an inducer of cell death and survival or proliferative factor. All of these roles are critical to ischemia-induced renal functional impairment and progressive fibrotic changes in the kidney (25).
Oxidative stress and the antioxidant system may play important roles in the pathophysiology in renal disorders. Activity of SOD is susceptible to oxidative changes. SOD, the cytoprotective antioxidant enzyme, could convert superoxide to hydrogen peroxide to prevent oxidation (26), while MDA was used as an indicator of cell oxidative damage (27). Lipid peroxidation represents the most frequent injury resulting from the activation of ROS (28).
GSH provides the first line of defense for the body by scavenging ROS. The decreased level of GSH in the renal damage might be due to GSH utilization in the exclusion of peroxides (29). GSH-dependent enzymes offer a second line of defense because they detoxify noxious byproducts generated by ROS and also help to avert the dissemination of free radicals (30). The primary role of GSR is to maintain high levels of reduced GSH within the cell, resulting in low levels of ROS (7). GSH-Px detoxifies peroxides by reacting with GSH and converting it to oxidized glutathione (GSSG), which is reduced to GSH by GSR (31). GST is a phase II metabolic isozyme best known for its ability to catalyze the conjugation of the reduced form of GSH to xenobiotic substrates for the purpose of detoxification. The main function of GST is to detoxify xenobiotics by catalyzing the nucleophilic attack by GSH on electrophilic carbon and sulfur, thereby preventing their interaction with crucial cellular proteins and nucleic acids (32). GSR, GSH-Px, and GST are basic antioxidant enzymes in the GSH system. Therefore, the profile of these enzymes and their alteration are strongly linked to renal diseases.
Tubular cell injury in renal diseases involves complex etiological and pathophysiological processes. Growing evidence has shown that ROS mediated damage plays a key role in this pathogenesis process affecting renal tubular cells (33-35). The mechanisms of CsA-induced renal tubular injury have not been fully understood. Several investigators have shown that ROS or free radicals are closely related to the renal tubular injury induced by CsA (10-12). Previous reports have shown that RG has a good safety and efficacy profile in type 2 diabetic patients complicated by renal impairment (2, 3). It has been reported that the direct antioxidative properties of RG produce measurable effects at therapeutic doses and can contribute to its effectiveness in the therapy of type 2 diabetes (4, 5). However, it is not clear whether or not RG plays a protective role in CsA-induced renal tubular injury. In this study, RG is shown to ameliorate the renal tubular injury effect of CsA in rats. The changes induced by CsA in urinary protein, urinary NAG, BUN, SCr levels and kidney histopathological damages were significantly ameliorated in the presence of RG towards the normal values. Simultaneously, we observed that the increase in MDA and decreases in SOD, GST, GSH-Px, GR activities, and down-regulation of GSH expression in the kidney tissues were reversed by RG. Therefore, the protective effects of RG against CsA renal toxicity may be due to its free radical scavenging and the reversal of GSH depletion and increase in SOD, GST, GSH-Px, GR antioxidant activity, and up-regulation of GSH expression as well as the reduction of lipid peroxidation of renal tubular cells.
It has been reported that CsA can markedly increase the plasma concentrations of RG. Their concomitant use may enhance the blood glucose-lowering effect of RG, but this positive effect is associated with an increased risk of hypoglycemia. Therefore, care is warranted when using RG in patients receiving CsA immunosuppression (36).
Conclusion
Our present study demonstrated that the protective effect of GR on renal injury in CsA-induced renal tubular toxicity was associated with its antioxidant activity. Thus, GR might be a beneficial agent for the prevention and treatment of renal injury.
Conflict of interest
The authors state no conflict of interest.
Acknowledgment
The authors would like to thank Hubei Provincial Department of Educatinn (Grant number 2015460), Wuhan, China for providing financial supports. The results described in this paper were part of student thesis.
References
- 1.Polonsky KS, Given BD, Hirsch LJ, Tillil H, Shapiro ET, Beebe C, et al. Abnormal patterns of insulin secretion in non-insulindependent diabetes. N Engl J Med. 1988;318:1231–1239. doi: 10.1056/NEJM198805123181903. [DOI] [PubMed] [Google Scholar]
- 2.Hasslacher C. Safety and efficacy of repaglinide in type 2 diabetic patients with and without impaired renal function. Diabetes Care. 2003;26:886–891. doi: 10.2337/diacare.26.3.886. [DOI] [PubMed] [Google Scholar]
- 3.Yale JF. Oral antihyperglycemic agents and renal disease: new agents, new concepts. J Am Soc Nephrol. 2005;16:7–10. doi: 10.1681/asn.2004110974. [DOI] [PubMed] [Google Scholar]
- 4.Gumieniczek A. Oxidative stress in kidney and liver of alloxan-induced diabetic rabbits: effect of repaglinide. Acta Diabetol. 2005;42:75–81. doi: 10.1007/s00592-005-0182-2. [DOI] [PubMed] [Google Scholar]
- 5.Tankova T, Koev D, Dakovska L, Kirilov G. The effect of repaglinide on insulin secretion and oxidative stress in type2 diabetic patients. Diabetes Res Clin Pract. 2003;59:43–49. doi: 10.1016/s0168-8227(02)00179-1. [DOI] [PubMed] [Google Scholar]
- 6.Haugen E, Nath KA. The involvement of oxidative stress in the progression of renal injury. Blood Purif. 1999;17:58–65. doi: 10.1159/000014377. [DOI] [PubMed] [Google Scholar]
- 7.Deponte M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim Biophys Acta. 2013;1830:3217–3266. doi: 10.1016/j.bbagen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 8.Ponticelli C. Cyclosporine: from renal transplan-tation to autoimmune diseases. Ann NY Acad Sci. 2005;1051:551–558. doi: 10.1196/annals.1361.099. [DOI] [PubMed] [Google Scholar]
- 9.Busauschina A, Schnuelle P, Van der Woude FJ. Cyclosporine nephrotoxicity. Transplant Proc. 2004;36:229S–233S. doi: 10.1016/j.transproceed.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 10.O’Connell S, Tuite N, Slattery C, Ryan MP, McMorrow T. Cyclosporine A induced oxidative stress in human renal mesangial cells: a role for ERK 1/2 MAPK signaling. Toxicol Sci. 2012;1261:101–113. doi: 10.1093/toxsci/kfr330. [DOI] [PubMed] [Google Scholar]
- 11.Ateşşahin A, Çeribaı OA, Yılmaz S. Lycopene, a carotenoid, attenuates cyclosporine-induced renal dysfunction and oxidative stress in rats. Basic Clin Pharmacol Toxicol. 2007;100:372–376. doi: 10.1111/j.1742-7843.2007.00060.x. [DOI] [PubMed] [Google Scholar]
- 12.Hagar HH, Eman EE, Maha A. Taurine attenuates hypertension and renal dysfunction induced by cyclosporine A in rats. Clin Exp Pharmacol Physiol. 2006;33:189–196. doi: 10.1111/j.1440-1681.2006.04345.x. [DOI] [PubMed] [Google Scholar]
- 13.Salant DJ, Cybulsky AV. Experimental glomerulo-nephritis. Methods Enzymol. 1988;162:421–461. doi: 10.1016/0076-6879(88)62096-9. [DOI] [PubMed] [Google Scholar]
- 14.Price RJ. Urinary N-acetyl-β-D- glucosaminidase (NAG) as an indicator of renal disease. Curr Probl Clin Biochem. 1979;9:150–163. [PubMed] [Google Scholar]
- 15.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Misra HP, Fridovich I. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide-dismutase. J Biol Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 17.Beutler E, Durom O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 18.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 19.Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 20.Smith IK, Vierheller TL, Thorne CA. Rassay of glutathione reductase in crude tissue homogenates using 5,5’-dithiobis (2-nitrobenzoic acid) Anal Biochem. 1988;175:408–413. doi: 10.1016/0003-2697(88)90564-7. [DOI] [PubMed] [Google Scholar]
- 21.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 22.Gao H, Zhou YW. Inhibitory effect of picroside II on hepatocyte apoptosis. Acta Pharmacol Sin. 2005;26:729–736. doi: 10.1111/j.1745-7254.2005.00729.x. [DOI] [PubMed] [Google Scholar]
- 23.Marnett LJ. Lipid peroxidation-DNA damage by malondialdehyde. Mutat Res. 1999;424:83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 24.Del RD, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15:316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Jung KJ, Park KM. Reactive oxygen species differently regulate renal tubular epithelial and interstitial cell proliferation after ischemia and reperfusion injury. Am J Physiol Renal Physiol. 2010;298:F1118–1129. doi: 10.1152/ajprenal.00701.2009. [DOI] [PubMed] [Google Scholar]
- 26.Khan RA, Khan MR, Sahreen S. Evaluation of Launaea procumbens use in renal disorders: a rat model. J Ethnopharmacol. 2010;128:452–461. doi: 10.1016/j.jep.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 27.Aikemu A, Yusup A, Umar A, Berké B, Moore N, Upur H. The impact of the Uighur medicine abnormal savda munziq on antitumor and antioxidant activity in a S180 and Ehrlich ascites carcinoma mouse tumor model. Pharmacogn Mag. 2012;8:141–148. doi: 10.4103/0973-1296.96568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids. 2009;157:1–11. doi: 10.1016/j.chemphyslip.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Yadav P, Sarkar S, Bhatnagar D. Action of Capparis deciduas against alloxan-induced oxidative stress and diabetes in rat tissues. Pharmacol Res. 1997;36:221–228. doi: 10.1006/phrs.1997.0222. [DOI] [PubMed] [Google Scholar]
- 30.Gumieniczek A. Effects of repaglinide on oxidative stress in tissues of diabetic rabbits. Diab Res Clin Pract. 2005;68:89–95. doi: 10.1016/j.diabres.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 31.Maritim AC, Sanders RA, Watkins JB. Effects of α-lipoic acid on biomarkers of oxidative stress in streptozotocin-induced diabetic rats. J Nutr Biochem. 2003;14:288–294. doi: 10.1016/s0955-2863(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 32.Raza H. Dual localization of glutathione S-transferase in the cytosol and mitochondria: implications in oxidative stress, toxicity and disease. FEBS J. 2011;278:4243–4251. doi: 10.1111/j.1742-4658.2011.08358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brezniceanu ML, Lau CJ, Godin N, Chénier I, Duclos A, Ethier J, et al. Reactive oxygen species promote caspase-12 expression and tubular apoptosis in diabetic nephropathy. J Am Soc Nephrol. 2010;21:943–994. doi: 10.1681/ASN.2009030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Habib SL. Alterations in tubular epithelial cells in diabetic nephropathy. J Nephrol. 2013;26:865–869. doi: 10.5301/jn.5000287. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int. 1999;56:1627–1637. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- 36.Backman JT, Kajosaari LI, Niemi M, Nevvonen PJ. Cyclosporine A increases plasma concentrations and effects of repaglinide. Am J Transplant. 2006;6:2221–2222. doi: 10.1111/j.1600-6143.2006.01456.x. [DOI] [PubMed] [Google Scholar]


