Abstract
Objective(s):
Due to the prevalence and pervasiveness of stress in modern life and exposure to both chronic and acute stresses, it is not clear whether prior exposure to chronic stress can influence the impairing effects of acute stress on memory retrieval. This issue was tested in this study.
Materials and Methods:
Adult male Wistar rats were randomly assigned to the following groups: control, acute, chronic, and chronic + acute stress groups. The rats were trained with six trials per day for 6 consecutive days in the water maze. Following training, the rats were either kept in control conditions or exposed to chronic stress in a restrainer 6 hr/day for 21 days. On day 22, a probe test was done to measure memory retention. Time spent in target and opposite areas, platform location latency, and proximity were used as indices of memory retention. To induce acute stress, 30 min before the probe test, animals received a mild footshock.
Results:
Stressed animals spent significantly less time in the target quadrant and more time in the opposite quadrant than control animals. Moreover, the stressed animals showed significantly increased platform location latency and proximity as compared with control animals. No significant differences were found in these measures among stress exposure groups. Finally, both chronic and acute stress significantly increased corticosterone levels.
Conclusion:
Our results indicate that both chronic and acute stress impair memory retrieval similarly. Additionally, the impairing effects of chronic stress on memory retrieval were not influenced by acute stress.
Keywords: Acute stress, Chronic stress, Morris water maze, rat, Spatial memory retrieval
Introduction
Stress is a biologically important and ubiquitous circumstance that can influence brain functions. Because of the importance of both the beneficial and deleterious effects of acute and chronic stresses on cognitive functions, they have been the subject of numerous studies during the past 8 decades. The response to stress involves the activation of the hypothalamic-pituitary-adrenocortical (HPA) axis and its final product glucocorticoids.
The hippocampus, which has the highest density of glucocorticoid receptors in the brain, is involved in the regulation of the HPA and the behavioral respon-ses to stress.
This sea-horse-shaped structure is a part of a medial temporal lobe system necessary for the formation of stable declarative memory in humans (1-3) and spatial memory in rodents (4, 5). Focusing on the effects of stress on the hippocampus, a large body of work has uncovered effects of chronic and short-term stress on learning and memory (6-8).
These effects have been accompanied by morpho-logical changes: specifically, reduced dendritic arbors after chronic stress (9-11) and reduced spine density after acute stress (12-14) have been described.
Numerous studies have shown that stressful experiences and/or corticosterone can dramatically impair subsequent cognitive processes (15), such as the acquisition (16, 17) or the retrieval of informa-tion (18, 20). Previous findings indicated that acute administration of glucocorticoids impairs memory retrieval (20-22) Systemic injections of stress-level doses of corticosterone administered to rats shortly before retention testing impair retrieval in tasks that rely on spatial or contextual information, including water-maze and inhibitory avoidance (20).
Furthermore, stress-level glucocorticoid adminis-tration to human subjects shortly before retention testing impairs hippocampus-dependent recall of previously learned verbal material (23, 24)
On the other hand, chronic stress is known to increase levels of adrenal glucocorticoids resulting in deleterious cognitive functioning (25, 26). Chronic restraint stress causes alterations in biochemistry, pharmacology, and morphology within the hippo-campus, especially in CA1 and CA3 (27-29), and cognitive alterations have been systematically reported after repeated exposures to stress (29-34)
We are exposed to various forms of stress daily, a common occurrence in the lives of most individuals, which have both positive and negative effects on brain function. In its acute form, stress may be a necessary adaptive mechanism for survival and with only transient changes in the brain. Although, prolonged stress causes overactivation and dysre-gulation of the HPA axis thus induces detrimental changes in the brain structure and function. Therefore, chronic stress is often considered a negative modulator of the cognitive functions including the learning and memory processes. Exposure to long-lasting stress reduces health and increases vulnerability to mental disorders (35).
The mechanisms of impairment of cognition and synaptic plasticity following stress are largely unknown. However, based on studies in adult and older animals and humans, a “glucocorticoid cascade” hypothesis is suggested: there is a relationship between cumulative exposures to high glucocorticoid levels and hippocampal atrophy (36). In an attempt to clarify the mechanism by which glucocorticoid levels correlate with hippocampal atrophy, a “neurotoxicity hypothesis” was introduced. This hypothesis suggests that prolonged exposure to glucocorticoids diminishes the ability of neurons to resist insults, thus increasing the rate at which they are damaged (37).
Several studies have shown that chronic and acute stress produce adverse effects on learning and memory (20, 38-40). Since two type of stress influence plasma glucocorticoid levels that are involved in learning and memory impairments, we, therefore, hypothesized that the combination of acute and chronic stresses could exert a greater deleterious effect on learning and memory than either factor alone. In the current study, this hypothesis was tested on hippocampus-dependent learning and memory using the Morris water maze tasks.
Materials and Methods
Animals
The experimental protocol was approved by the Research and Ethics Committee of Damghan University. Male Wistar rats (weighing 200±20 g) were purchased from Pasteur Institute of Iran. Animals were kept under standard laboratory conditions with a 12-hr light/dark cycle with ad libitum food and water throughout the experiments.
Experimental groups and stress paradigm
The animals were randomly divided into 4 groups: control, acute, chronic, and chronic + acute stress groups. To induce acute stress, animals received three footshocks (0.8 mA for 1 sec with a 5-sec inter-shock interval) 30 min before the probe test. For chronic stress, rats were daily restrained for 6 hr/day (from 9: 00 to 15: 00) for a total of 21 days in well-ventilated plexiglass tubes without access to food and water. During restraint stress, control animals were handled. In the chronic + acute stress group, rats were restrained daily for 6 hr/day for a total of 21 days in plexiglass tubes and received three footshocks 30 min before the probe test. It is important to note that this really examines the effects of heterotypic stress and that the responses to heterotypic stress may not exhibit habituation as is often observed during exposure to homotypic stress.
Immediately after the chronic stress, 13 of animals in chronically stressed groups and the control group were decapitated, and trunk blood was collected for corticosterone assay (see below). The rest of the animals were subjected to retention test. Probe test was performed on 22 days for all groups. Timeline of experiments is shown in Figure 1.
Figure 1.
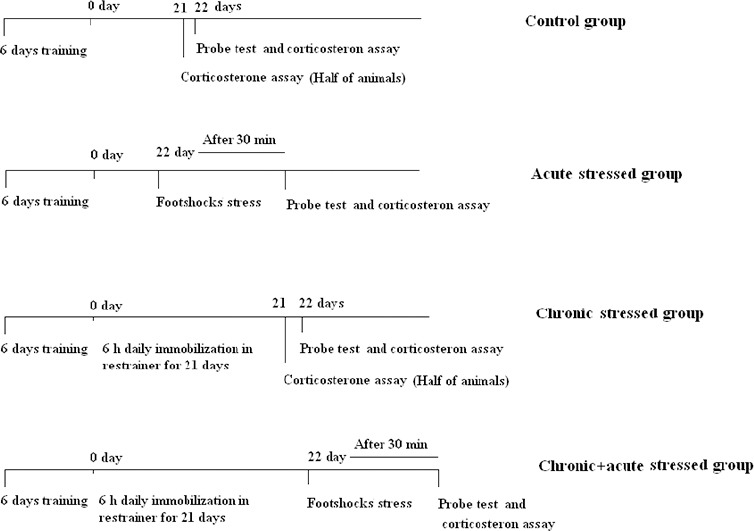
Timelines of experiments
Morris water maze (MWM) task
The MWM used in our study was a black circular pool (140 cm diameter, 45 cm high) filled with water (30 cm depth) at 24±2 °C. The pool was divided into four quadrants of equal size. An invisible escape platform (10 cm diameter) was placed in the middle of one of the quadrants (1.5 cm below the water surface) equidistant from the side wall and middle of the pool. The behavior of the animal (latency, distance and swim speed) was monitored by a video camera, mounted in the ceiling above the center of the pool, and a computerized tracking system (Ethovision; Noldus IT, The Netherlands). Four different starting positions were equally spaced around the perimeter of the pool. The training session consisted of six trials per day for 6 consecutive days, which were started from one of the four start positions, used in a random sequence identically for every rat. A trial began by placing the rat into the water facing the wall of the pool at one of the starting points. When a rat failed to escape within 60 sec, it was guided to the platform by the experimenter. Once the rat reached the platform, it was allowed to remain for 30 sec and then placed in a holding cage for an inter-trial interval of 30 sec. After the last trial, each animal was towel dried and returned to its home cage.
Retention of the spatial training was assessed 22 days after the last training session with a 60 sec free-swim probe trial using a new starting position. The parameters measured in the probe trial were time spent in the quadrant containing the platform during training (target quadrant), time spent in the quadrant opposite to the training quadrant (opposite quadrant), initial latency to cross the platform location, number of crossing platform location, proximity (the average distance from the center of the platform during the probe test), swimming speed, and total swim distance.
Corticosterone assay
To measure corticosterone, the rats were decapitated at immediately after the probe test in four experimental groups and at the end of 21 days restraint stress (see timeline of experiments in Figure 1), their trunk blood was collected in tubes with EDTA, centrifuged (3500 × g, 15 min), and the plasma was stored at -70 °C until used for the corticosterone assay. Corticosterone levels were determined by an ELISA assay (Cayman Chemical, Item Number 500655).
Statistical analysis
Data is expressed as the mean ± standard error of the mean (SEM). Behavioral data were analyzed by one-way and two-way (ANOVA), followed by Tukey’s test for post hoc comparison of the means. For the analysis of the corticosterone levels, a one-way ANOVA with LSD post hoc test was conducted. Statistical differences were considered significant when P<0.05. All analyses were performed using the Statistical Package for the Social Science (SPSS) software in a PC-compatible computer.
Results
Spatial learning in the MWM
Distance traveled, escape latency and swimming speed data of the animals during the 6 days of training in water maze are illustrated in Figure 2 A, two-way ANOVA (group × training days) was used to analyze the escape latencies during training. All animals were able to improve their performance as shown by the reduction of escape latencies (Figure 2A). Statistical analysis showed significant differences in escape latency between different training days (F5,80=58.8, P=0.000, n=84), no significant differences among groups (F3,80=0.364, P= 0.779, n=84), and no interaction between days and groups (F15,80=1.12, P= 0.338, n=84).
Figure 2.
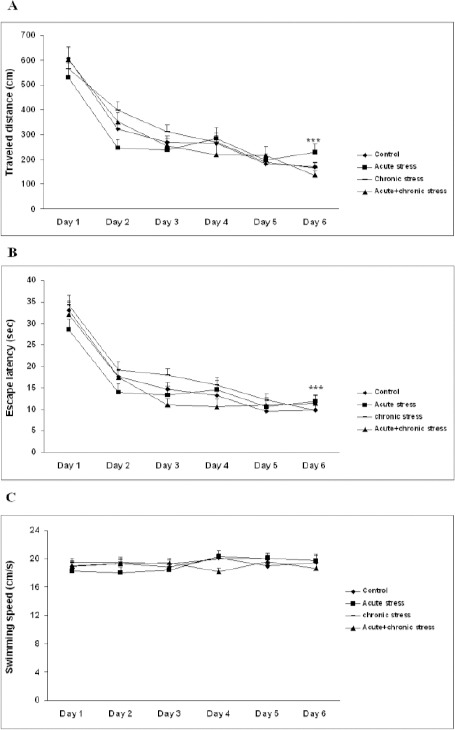
Traveled distance, average escape latency, and swimming speed during 6-day training in MWM
Average escape latency (A), traveled distance (B), and swimming speed (C) across all training days. As shown the mean latency and traveled distance to find the platform declined progressively in all animals. Analysis showed distance traveled and escape latency on the 6th day of training were statistically different (P< 0.001) from the first day of training. Data is expressed as mean±SEM of 84 animals.
***P< 0.001 versus the first day of training in each group
Data related to the distance traveled to reach the platform followed similar to the latency pattern. All groups traveled shorter distances to reach the platform as training progressed (Figure 2B). Two-way ANOVA analysis showed significant differences in swimming distance between different training days (F5,80=54.07, P=0.000, n=84), no significant differences among groups (F3,80=0.777, P=0.513, n=84), and no interaction between days and groups (F15,80=1.27, P=0.221, n=84). The analysis of swimming speed also showed no significant differences as training days progressed (F5,80=0.449, P=0.813, n=84) among groups (F3,80=1.067, P= 0.373, n=84) and no interaction between days and groups (F15,80= 1.442, P= 0.129, n=84) (Figure 2C).
Effects of stress on memory retrieval
The aim of this study was to examine the effect of chronic+acute stress on memory retrieval in Morris water maze task. Exerted stress followed learning in MWM. The probe test was performed 22 days after last acquisition trials. In probe trials, time spent in target quadrant was used to evaluate long-term memory. A two-way analysis of variance (ANOVA), with zones as repeated measure, showed significant effects of group (F3,50=3.02, P= 0.043, n=54) and zone (F1,50=10.73, P=0.007, n=54), and a significant interaction between group and zone (F3,50=3.91, P=0.017, n=54). Between-group comparisons indicated that the acute stress group (P<0.05), the chronic stress group (P<0.01) and the chronic + acute stress group (P<0.01) spent significantly more time in the opposite quadrant and less time in the target quadrant as compared to the control group (Figure 3A). Also, within group comparisons revealed that the control group spent significantly more time in the target quadrant than in the opposite quadrant (P<0.05), and all stress groups spent significantly more time in the opposite quadrant than in the target quadrant (all, P< 0.001).
Figure 3.
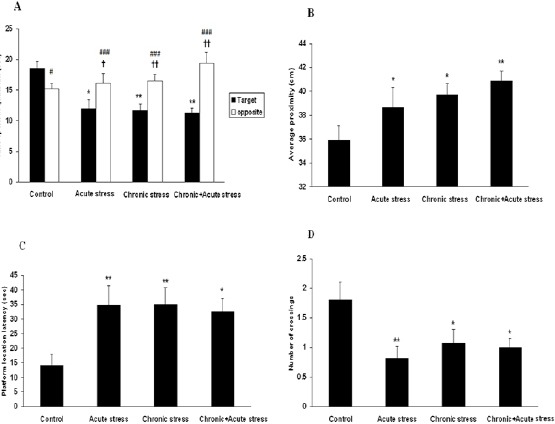
Effects of stress on retention performance in a water maze task
(A) All stress groups spent less time in the target quadrant and spent more time in the opposite quadrant compared to the control group (B) The Average proximity (C) The platform location latency (D) The number of crossings in the area of the original location of the platform. Stress impaired memory retention, as evidenced by the fact that the stressed rats had significantly larger proximity values, more platform location latency, and lower number of crossings compared to the control group. Data are expressed as mean±SEM
*P<0.05, **P<0.01, ***P<0.001 and † P<0.05, ††P<0.01 compared with controls
### P< 0.001 within group comparisons
Figure 3B represents the average proximity to the platform. One-way ANOVA showed a significant difference among the four groups (F3,54=3.427, P= 0.023, n=58). The control group had a smaller average proximity than the acute (P<0.05), chronic (P<0.05), and chronic+acute stress groups (P<0.01).
One-way ANOVA on platform location latency data indicated a significant difference among the four groups (F3,54=3.111, P=0.034, n=58). Acute stress (P<0.01), chronic stress (P< 0.01), and chronic+acute stress groups (P<0.05) showed increased escape latency as compared with controls (Figure 3C). One-way ANOVA on quadrant entries also displayed a significant difference between groups (F3,54=3.281, P=0.027, n=58). As represented in Figure 3D, the control group had more crossings as compared to acute (P<0.01), chronic (P<0.05) and chronic+acute stressed groups (P<0.05). These findings show that stress could impair memory retrieval.
To control the differences in the MWM per-formance, we recorded swimming speed of animals (Figure 4A). One-way ANOVA showed no significant differences of swimming speed among the four groups (F3,54=1.90, P= 0.14, n=58). Also, there were no significant differences in total distance traveled in the four groups (F3,54=1.97, P= 0.13, n=58, Figure 4B).
Figure 4.
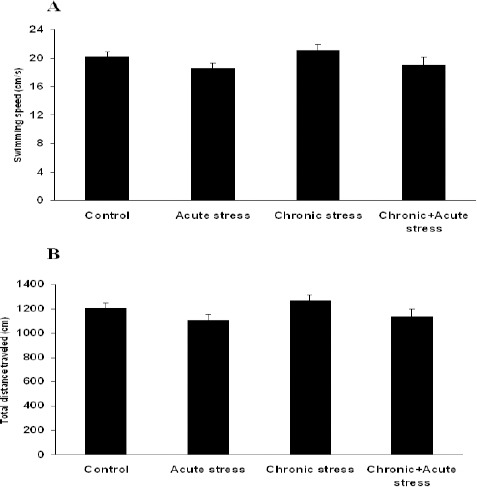
Effect of stress on total distance traveled and swimming speed in a water maze task during the retention test
Total traveled distance (A), and swimming speed (B) in the probe trial. The figures show there were no significant differences in both total traveled distance and swimming speed in the four groups. Data is expressed as mean ± SEM of 15-16 animals in each group
Corticosterone assay
There was a significant difference in cortico-sterone levels between control and chronically stressed groups after 21st days’ stress (8.37±3.3 ng/ml in control versus 103.9±8.9 ng/ml in the chronically stressed group, P<0.01). Also, one-way ANOVA analysis indicated that there were significant differences in corticosterone levels between groups (Figure 5, F3,36=10.67, P=0.000, n=40). As was expected, post hoc Tukey analysis showed acute, chronic, and chronic+acute stress groups had significantly elevated levels of corticosterone in comparison to controls at the post-probe time, respectively (P<0.01, P<0.001, P<0.001, Figure 5). There were no significant differences in cortico-sterone levels among acute, chronic, and chronic+acute stress groups.
Figure 5.
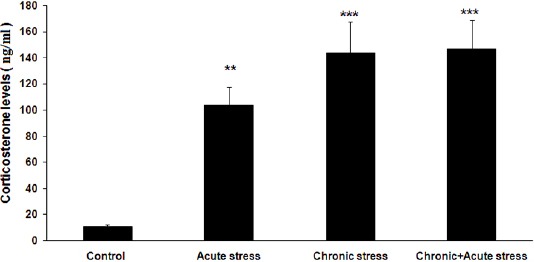
Mean plasma corticosterone levels for stressed groups, immediately following immobilization stress and immediately after probe test
(A): Mean±SEM corticosterone levels of 10 animals immediately following the immobilization stress on the last day of the stress treatment. (B): Mean±SEM of corticosterone levels in stressed groups immediately following the probe test
**P< 0.01, ***P<0.001 versus controls
Discussion
Extensive evidence from animal and human studies indicate that stress and glucocorticoids influence cognitive function (41-43). Previous studies have shown stress levels of glucocorticoids impair memory retrieval in animals as well as humans (39, 44-47). In this study, we found that both chronic and acute stress impair memory retrieval. Moreover, the impairing effect of chronic stress was not affected by acute stress.
During learning, all animals were able to improve their performance as shown by the reduction of escape latencies and traveled distance. Statistical analysis showed no significant difference in swi-mming speed, distance traveled, and escape latency among the four groups during training days.
Thus, memory performance impairment in stress exposure groups was due to disruption of memory retrieval.
Findings indicated chronic stress impaired memory retention as the stressed animals spent significantly less time in target quadrant, had longer platform location latencies, and larger average of proximity than their non-stressed control group. Also, corticosterone levels significantly increased in chronically stressed animals as measured immediately after the period of 21st days’ stress and after the probe test.
McLaughlin et al (2007) reported the use of chronic restraint stress, with wire mesh, for 6 hr/day for 21 days as a reliable and efficient method to produce psychological stress and to cause CA3 dendritic retraction and spatial memory deficits in male Sprague–Dawley rats (48). In this study, we used the same method for chronic stress and found that corticosterone levels significantly increased and spatial memory retrieval reduced in the stressed rats as compared to the control groups. The results of previous studies are in agreement with our finding that chronic stress has an impairing effect on learning and memory (40, 49, 50).
Most studies on the relationship between chronic stress and spatial memory have focused on the hippocampus because of its crucial role in spatial learning and its well-known susceptibility to stress (33). The hippocampus plays a critical role in spatial memory’s ability because damage to the hippo-campus corresponds with spatial memory impairments in both animals (30, 50, 51) and humans (52, 54). Stress resulted in enhanced release of catecholamines and glucocorticoids due to the activation of sympathoadrenal and hypothalamic-pituitary-adrenal axes (55). The impairing effects of chronic stress on learning and memory are mainly mediated via elevated levels of glucocorticoids. Chronic stress may affect hippocampal function through such mechanisms as CA3 neuronal remodeling (56), suppression of synaptic activity (45, 57), and altered neurogenesis (51, 58, 59). These changes in the hippocampus following chronic stress or elevation of glucocorticoids have been related to changes in spatial learning and memory (60).
Retention was impaired when the animals were tested 30 min after the footshock. Also, acute stress increased average proximity and escape location latency compared with the controls. The finding that plasma levels of corticosterone, from blood collected immediately after the probe trial, was elevated in the acutely stressed group compared with the control group, suggests that increased adrenocortical function induced by the stressor may have disrupted memory retrieval.
There is evidence that glucocorticoids impair retention performance when rats or human subjects are tested shortly after training when circulating levels of glucocorticoids are still elevated (44-46) These effects on retention performance suggest that the retention impairment is directly related to increased adrenocortical function.
Glucocorticoids can affect retention performance by selectively influencing memory retrieval proce-sses and these effects appear to depend on GR activation. In one study (20), rats that were given an aversive experience of footshock exposure 30 min before retention testing, failed to remember the platform location as indicated by equal swim times in both target and opposite quadrants. Stress effects on memory retrieval are time-dependent and retention performance was not impaired when rats were tested either 2 min or 4 hr after footshock exposure. This time course on retention impairment correlated with plasma corticosterone levels that peak 30 min after stress exposure and return to baseline within 4 hr. Our result in this study was in agreement with findings of de Quervain et al that indicated foot-shock exposure 30 min before retention testing impaired memory retention (20).
Also, our study indicated chronic plus acute stress impaired memory retention as the stressed animals spent significantly less time in the target quadrant and had longer platform location latencies and average of proximity than their non-stressed control group. Also, corticosterone levels increased signi-ficantly more in these animals than controls at immediately after the probe test.
As mentioned before, animal studies (20), as well as studies on healthy human volunteers (23), have demonstrated that retrieval of learned information is susceptible to glucocorticoid-induced impairment. By administrating cortisone at different times to different groups of healthy volunteers (one hour before word presentation to test consolidation; and one hour before the delayed recall test to test retrieval), it was found that only declarative memory was affected by acute exposure to glucocorticoids; there was no effect on acquisition or consolidation.
In our study, there was no significant difference in platform location latency, average of proximity, time in target quadrant, and number of crossings between acute, chronic, and chronic + acute stress groups. Furthermore, there was no significant difference in corticosterone levels between chronic stress and chronic + acute stress groups.
Wright et al reported novel findings that chronic stress impairs spatial memory through changes in the HPA axis and that attenuating corticosterone levels can restore spatial memory (61). These findings are consistent with the hypothesis presented by Roozendaal et al (2001) and Roozendaal (2002) that elevated corticosterone levels at the time of memory assessment may mediate spatial memory impairment in rats with a compromised hippocampus (62, 63). Our data may indicate that enhanced corticosterone secretion as a result of exposure to behavioral tasks (probe test) may mediate this effect. Corticosterone levels in chronically stressed animals immediately after the probe test was significantly more than chronically stressed group immediately after termination of stress. One limitation of the results in this study that needs to be addressed is that corticosterone levels must be measured in all groups immediately after ending of stress and after the probe test, but we measured it only in the chronically stressed and the control groups.
Chronic stress and the subsequent corticosterone hypersecretion increase adrenal weight (64), which can release additional corticosterone in response to a stressor. There is evidence that chronic stress increased total corticosterone levels in response to the Y-maze procedure and down-regulated hippo-campal GR mRNA expression, which may have functional consequences at the level of receptor capacity. GR reduction or changes in other brain areas may have been responsible for the enhanced corticosterone response to the Y-maze procedure because GR are proposed to mediate corticosterone release during the stress response and diurnal rhythms (65, 67), and alterations in extrahippo-campal brain areas have been shown to be involved in enhanced corticosterone response to novel stressors after chronic stress (68, 69). The reduction in GR mRNA and an enhanced corticosterone response to the Y-maze procedure in chronically stressed rats is consistent with the hypothesis that stress-induced corticosterone elevations on the day of memory assessment impairs spatial memory in chronically stressed rats.
In our study, probably alterations of rats’ hippocampus in both chronic and chronic plus acute stress groups and similar corticosterone levels after the probe test are related to similar memory retrieval impairment in the two groups.
In this study, we expected that prior chronic stress enhances hippocampal vulnerability to acute stress, thereby further increasing spatial memory retrieval impairment induced by acute stress. However, there were no significant differences in memory retrieval impairment of three stress exposure groups, suggesting that memory impair-ment has reached a ceiling effect in the chronic plus acute stress group, and so acute stress could not further increase memory retrieval impairment. Also, one possibility for interpretation of this finding might be that other adaptation systems are activated during chronic stress that can prevent acute stress effects, thus that we did not observe additive effects.
On the other hand, brain regions other than the hippocampus may have been affected by chronic restraint stress paradigm and could have contributed to the memory impairment, since corticosteroid receptors are present ubiquitously in the brain and consequently, every region has the potential to be affected by chronic stress. It will be interesting to see whether chronic restraint stress also induces morphological changes in regions other than the hippocampus. In addition, the interaction of glucocorticoid with several neurotransmitter systems in the brain such as adrenergic (70, 71), dopaminergic (21), and opioidergic (22, 72) systems may influence memory retrieval.
Conclusion
Chronic + acute stress same as acute and chronic stress alone impair retrieval of spatial memory. A similar effect of three types of stressors on memory retrieval suggests that the extent of memory impairment by stress is not influenced by prior stress experience. Future studies may provide a clearer indication of the exact areas of the memory process that are impaired by excess levels of glucocorticoids and the mechanisms by which it happens.
Acknowledgment
This study was supported by Damghan University, Damghan, Iran. The authors thank Dr Taghi lashkarbolouki for his help in the experimental part of the study and Dr Hossein Davari for language editing of the manuscript.
References
- 1.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 3.Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- 4.Morris RG, Garrud P, Rawlins JN, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 5.Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87:873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 7.de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 8.Howland JG, Wang YT. Synaptic plasticity in learning and memory: stress effects in the hippocampus. Prog Brain Res. 2008;169:145–158. doi: 10.1016/S0079-6123(07)00008-8. [DOI] [PubMed] [Google Scholar]
- 9.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- 10.Magarinos AM, McEwen BS, Flugge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16:3534–3540. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alfarez DN, Karst H, Velzing EH, Joels M, Krugers HJ. Opposite effects of glucocorticoid receptor activation on hippocampal CA1 dendritic complexity in chronically stressed and handled animals. Hippocampus. 2008;18:20–28. doi: 10.1002/hipo.20360. [DOI] [PubMed] [Google Scholar]
- 12.Donohue HS, Gabbott PL, Davies HA, Rodriguez JJ, Cordero MI, Sandi C, et al. Chronic restraint stress induces changes in synapse morphology in stratum lacunosum-moleculare CA1 rat hippocampus: a stereological and three-dimensional ultrastructural study. Neuroscience. 2006;140:597–606. doi: 10.1016/j.neuroscience.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 13.Shors TJ, Falduto J, Leuner B. The opposite effects of stress on dendritic spines in male vs. female rats are NMDA receptor-dependent. Eur J Neurosci. 2004;19:145–150. doi: 10.1046/j.1460-9568.2003.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Dube CM, Rice CJ, Baram TZ. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 16.Oitzl MS, de Kloet ER. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav Neurosci. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- 17.Roozendaal B, McGaugh JL. Basolateral amygdala lesions block the memory-enhancing effect of glucocorticoid administration in the dorsal hippocampus of rats. Eur J Neurosci. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 18.Diamond DM, Rose GM. Stress impairs LTP and hippocampal-dependent memory. Ann N Y Acad Sci. 1994;746:411–414. doi: 10.1111/j.1749-6632.1994.tb39271.x. [DOI] [PubMed] [Google Scholar]
- 19.Diamond DM, Fleshner M, Ingersoll N, Rose GM. Psychological stress impairs spatial working memory: relevance to electrophysiological studies of hippocampal function. Behav Neurosci. 1996;110:661–672. doi: 10.1037//0735-7044.110.4.661. [DOI] [PubMed] [Google Scholar]
- 20.de Quervain DJ, Roozendaal B, McGaugh JL. Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 21.Pakdel R, Rashidy-Pour A. Glucocorticoid-induced impairment of long-term memory retrieval in rats: an interaction with dopamine D2 receptors. Neurobiol Learn Mem. 2006;85:300–306. doi: 10.1016/j.nlm.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Rashidy-Pour A, Sadeghi H, Taherain AA, Vafaei AA, Fathollahi Y. The effects of acute restraint stress and dexamethasone on retrieval of long-term memory in rats: an interaction with opiate system. Behav Brain Res. 2004;154:193–198. doi: 10.1016/j.bbr.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 23.de Quervain DJ, Roozendaal B, Nitsch RM, McGaugh JL, Hock C. Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nature Neurosci. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- 24.Wolf OT, Convit A, McHugh PF, Kandil E, Thorn EL, De Santi S, et al. Cortisol differentially affects memory in young and elderly men. Behav Neurosci. 2001;115:1002–1011. doi: 10.1037//0735-7044.115.5.1002. [DOI] [PubMed] [Google Scholar]
- 25.Lupien SJ, Gaudreau S, Tchiteya BM, Maheu F, Sharma S, Nair NP, et al. Stress-induced declarative memory impairment in healthy elderly subjects: relationship to cortisol reactivity. J Clin Endocrinol Metab. 1997;82:2070–2075. doi: 10.1210/jcem.82.7.4075. [DOI] [PubMed] [Google Scholar]
- 26.Krugers HJ, Douma BR, Andringa G, Bohus B, Korf J, Luiten PG. Exposure to chronic psychosocial stress and corticosterone in the rat: effects on spatial discrimination learning and hippocampal protein kinase Cgamma immunoreactivity. Hippocampus. 1997;7:427–436. doi: 10.1002/(SICI)1098-1063(1997)7:4<427::AID-HIPO8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 27.Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: comparison of stressors. Neuroscience. 1995;69:83–88. doi: 10.1016/0306-4522(95)00256-i. [DOI] [PubMed] [Google Scholar]
- 28.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 29.McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- 30.Conrad CD, Galea LA, Kuroda Y, McEwen BS. Chronic stress impairs rat spatial memory on the Y maze, and this effect is blocked by tianeptine pretreatment. Behav Neurosci. 1996;110:1321–1334. doi: 10.1037//0735-7044.110.6.1321. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs E, Flugge G, Ohl F, Lucassen P, Vollmann-Honsdorf GK, Michaelis T. Psychosocial stress, glucocorticoids, and structural alterations in the tree shrew hippocampus. Physiol Behav. 2001;73:285–291. doi: 10.1016/s0031-9384(01)00497-8. [DOI] [PubMed] [Google Scholar]
- 32.Sandi C, Merino JJ, Cordero MI, Touyarot K, Venero C. Effects of chronic stress on contextual fear conditioning and the hippocampal expression of the neural cell adhesion molecule, its polysialylation, and L1. Neuroscience. 2001;102:329–339. doi: 10.1016/s0306-4522(00)00484-x. [DOI] [PubMed] [Google Scholar]
- 33.Sandi C, Davies HA, Cordero MI, Rodriguez JJ, Popov VI, Stewart MG. Rapid reversal of stress induced loss of synapses in CA3 of rat hippocampus following water maze training. Eur J Neurosci. 2003;17:2447–2456. doi: 10.1046/j.1460-9568.2003.02675.x. [DOI] [PubMed] [Google Scholar]
- 34.Cordero MI, Kruyt ND, Sandi C. Modulation of contextual fear conditioning by chronic stress in rats is related to individual differences in behavioral reactivity to novelty. Brain Res. 2003;970:242–245. doi: 10.1016/s0006-8993(03)02352-7. [DOI] [PubMed] [Google Scholar]
- 35.Alkadhi K. Brain Physiology and Pathophysiology i n Mental Stress. ISRN Physiol. 2013;2013:23. [Google Scholar]
- 36.Sapolsky RM. Glucocorticoids, stress, and their adverse neurological effects: relevance to aging. Exp Gerontol. 1999;34:721–732. doi: 10.1016/s0531-5565(99)00047-9. [DOI] [PubMed] [Google Scholar]
- 37.Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohammadi HS, Goudarzi I, Lashkarbolouki T, Abrari K, Elahdadi Salmani M. Chronic administration of quercetin prevent spatial learning and memory deficits provoked by chronic stress in rats. Behav Brain Res. 2014;270:196–205. doi: 10.1016/j.bbr.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 39.Rashidy-Pour A, Vafaei AA, Taherian AA, Miladi-Gorji H, Sadeghi H, Fathollahi Y, et al. Verapamil enhances acute stress or glucocorticoid-induced deficits in retrieval of long-term memory in rats. Behav Brain Res. 2009;203:76–80. doi: 10.1016/j.bbr.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F, et al. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol. 2011;667:222–229. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Salin PA, Bullier J. Corticocortical connections in the visual system: structure and function. Physiol Rev. 1995;75:107–154. doi: 10.1152/physrev.1995.75.1.107. [DOI] [PubMed] [Google Scholar]
- 42.Nelson JI, Frost BJ. Orientation-selective inhibition from beyond the classic visual receptive field. Brain Res. 1978;139:359–365. doi: 10.1016/0006-8993(78)90937-x. [DOI] [PubMed] [Google Scholar]
- 43.Vanduffel W, Orban GA, Lomber SG, Payne BR. Functional impact of cerebral projection systems. Mol Psychiatry. 1998;3:215–219. doi: 10.1038/sj.mp.4000358. [DOI] [PubMed] [Google Scholar]
- 44.Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH. Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life Sci. 1996;58:1475–1483. doi: 10.1016/0024-3205(96)00118-x. [DOI] [PubMed] [Google Scholar]
- 45.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 46.Diamond DM, Park CR, Heman KL, Rose GM. Exposing rats to a predator impairs spatial working memory in the radial arm water maze. Hippocampus. 1999;9:542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 47.de Quervain DJ-F, Benno R, Nitsch, Roger M, McGaugh, James L, Hock, Christoph Acute cortisone administration impairs retrieval of long-term declarative memory in humans. Nat Neurosci. 2000;3:313. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- 48.McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song L, Che W, Min-Wei W, Murakami Y, Matsumoto K. Impairment of the spatial learning and memory induced by learned helplessness and chronic mild stress. Pharmacol Biochem Behav. 2006;83:186–193. doi: 10.1016/j.pbb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Kleen JK, Sitomer MT, Killeen PR, Conrad CD. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav Neurosci. 2006;120:842–851. doi: 10.1037/0735-7044.120.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- 52.Astur RS, Taylor LB, Mamelak AN, Philpott L, Sutherland RJ. Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav Brain Res. 2002;132:77–84. doi: 10.1016/s0166-4328(01)00399-0. [DOI] [PubMed] [Google Scholar]
- 53.Kessels RP, de Haan EH, Kappelle LJ, Postma A. Varieties of human spatial memory: a meta-analysis on the effects of hippocampal lesions. Brain Res Brain Res Rev. 2001;35:295–303. doi: 10.1016/s0165-0173(01)00058-3. [DOI] [PubMed] [Google Scholar]
- 54.King JA, Burgess N, Hartley T, Vargha-Khadem F, O’Keefe J. Human hippocampus and viewpoint dependence in spatial memory. Hippocampus. 2002;12:811–820. doi: 10.1002/hipo.10070. [DOI] [PubMed] [Google Scholar]
- 55.Joels M, Karst H, Krugers HJ, Lucassen PJ. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol. 2007;28:72–96. doi: 10.1016/j.yfrne.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav Cogn Neurosci Rev. 2006;5:41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stewart MG, Davies HA, Sandi C, Kraev IV, Rogachevsky VV, Peddie CJ, et al. Stress suppresses and learning induces plasticity in CA3 of rat hippocampus: a three-dimensional ultrastructural study of thorny excrescences and their postsynaptic densities. Neuroscience. 2005;131:43–54. doi: 10.1016/j.neuroscience.2004.10.031. [DOI] [PubMed] [Google Scholar]
- 58.Shors TJ. Significant life events and the shape of memories to come: a hypothesis. Neurobiol Learn Mem. 2006;85:103–115. doi: 10.1016/j.nlm.2005.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- 60.McEwen BS. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann N Y Acad Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- 61.Wright RL, Lightner EN, Harman JS, Meijer OC, Conrad CD. Attenuating corticosterone levels on the day of memory assessment prevents chronic stress-induced impairments in spatial memory. Eur J Neurosci. 2006;24:595–605. doi: 10.1111/j.1460-9568.2006.04948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roozendaal B. Stress and memory: opposing effects of glucocorticoids on memory consolidation and memory retrieval. Neurobiol Learn Mem. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- 63.Roozendaal B, Phillips RG, Power AE, Brooke SM, Sapolsky RM, McGaugh JL. Memory retrieval impairment induced by hippocampal CA3 lesions is blocked by adrenocortical suppression. Nat Neurosci. 2001;4:1169–1171. doi: 10.1038/nn766. [DOI] [PubMed] [Google Scholar]
- 64.Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 65.Canny BJ. Hippocampal glucocorticoid receptors and the regulation of ACTH secretion. Mol Cell Endocrinol. 1990;71:C35–38. doi: 10.1016/0303-7207(90)90067-i. [DOI] [PubMed] [Google Scholar]
- 66.de Kloet ER, Oitzl MS, Joels M. Functional implications of brain corticosteroid receptor diversity. Cell Mol Neurobiol. 1993;13:433–455. doi: 10.1007/BF00711582. [DOI] [PubMed] [Google Scholar]
- 67.Bradbury MJ, Akana SF, Dallman MF. Roles of type I and II corticosteroid receptors in regulation of basal activity in the hypothalamo-pituitary-adrenal axis during the diurnal trough and the peak: evidence for a nonadditive effect of combined receptor occupation. Endocrinology. 1994;134:1286–1296. doi: 10.1210/endo.134.3.8119168. [DOI] [PubMed] [Google Scholar]
- 68.Bhatnagar S, Dallman M. Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- 69.Dallman MF. Stress update Adaptation of the hypothalamic-pituitary-adrenal axis to chronic stress. Trends Endocrinol Metab. 1993;4:62–69. doi: 10.1016/s1043-2760(05)80017-7. [DOI] [PubMed] [Google Scholar]
- 70.Roozendaal B, de Quervain DJ, Schelling G, McGaugh JL. A systemically administered beta-adrenoceptor antagonist blocks corticosterone-induced impairment of contextual memory retrieval in rats. Neurobiol Learn Mem. 2004;81:150–154. doi: 10.1016/j.nlm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 71.Roozendaal B, Hahn EL, Nathan SV, de Quervain DJ, McGaugh JL. Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala. J Neurosci. 2004;24:8161–8169. doi: 10.1523/JNEUROSCI.2574-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sajadi AA, Samaei SA, Rashidy-Pour A. Blocking effects of intra-hippocampal naltrexone micro-injections on glucocorticoid-induced impairment of spatial memory retrieval in rats. Neuropharmacology. 2007;52:347–354. doi: 10.1016/j.neuropharm.2006.08.021. [DOI] [PubMed] [Google Scholar]


