Abstract
Objective(s):
MicroRNA-21 (miR21) is aberrantly elevated in rheumatoid arthritis (RA) patients, the significance of this microRNA in RA pathogenesis and treatment, however, has not been investigated. In this study, by using RA-derived fibroblast-like synoviocyte (FLS) cells as a model, we investigated the effect and corresponding mechanism of miR21 inhibition on FLSs invasion.
Materials and Methods:
miR21 expression in synovial tissue and FLSs in RA patients and non-RA controls were determined by stem-loop RT-PCR. The effect of miR21 on FLSs viability and invasiveness were evaluated using miR21 inhibition. Cell viability was evaluated by MTT assay and the expression of genes at mRNA and protein levels was determined by RT-PCR and Western blot, respectively.
Results:
Our results showed that miR21 expression was highly increased in synovial tissue and FLSs in RA patients. Also, we reported that miR21 inhibitor treatment could significantly suppress the invasiveness of FLSs without affecting cell viability. The decreased FLSs invasion by miR21 inhibition was associated with down-regulated expression of matrix metalloproteinase (MMP)-1, MMP3, and MMP13. Further analysis revealed that miR21 inhibition could suppress the expression of TGFβ1 and Smad4, but promote that of Smad7. Moreover, suppression of FLS invasion and MMPs expression by miR21 treatment could be counteracted by additional TGFβ1 treatment.
Conclusion:
Our results indicated that miR21 inhibition can down-regulate the expression of MMP1, MMP3, and MMP13 and consequently suppress the invasiveness of FLS, which is achieved through TGFβ1/Smad4/7 signaling pathway. The findings of this study could offer a novel approach for RA treatment.
Keywords: Fibroblast-like synoviocyte, Invasion, MicroRNA-21, Rheumatoid arthritis, Smads, Transforming growth factor-beta
Introduction
Rheumatoid arthritis (RA), a systematic auto-immune disorder with progressive articular damage, could result in destruction of bone and cartilage and even life-long disability (1-3). Fibroblast-like syno-viocytes (FLS) are one of the most important cell types in synovial joint tissue and the increased invasiveness of these cells in RA patients is closely associated with RA pathogenesis and progression (4, 5). Matrix metalloproteinases (MMPs) are enzymes that can degrade all components of the extracellular matrix. A previous study has shown that FLSs from RA patients could aberrantly produce high levels of a variety of MMPs including MMP1, MMP3, and MMP13; the elevated MMPs in return, promote FLSs invasiveness which consequently leads to RA progression (6, 7). After years of research, more detailed mechanism underlying RA pathogenesis and progression has been revealed, however, effective treatment strategies for RA still remain to be further developed.
MicroRNAs (miRs) are short non-coding RNAs that can modulate gene expression at posttranscrip-tional level. In recent years, aberrant expression of many miRs have been identified to be associated with various types of diseases including cancer, cardiovascular diseases, and RA, while the regulation of these miRs may offer approaches to treat such diseases (8-10). In RA, unusual expression profiles of more than 40 miRs including miR21 have been detected. However, little is known about their role in RA pathogenesis and progression as well as their potential as therapeutic targets.
MiR21, although its role in RA has not been understood, has been shown to regulate tumor cell invasion in some cancers like esophagus and colon cancers through TGF-β/Smads signaling pathway (11, 12). In this study, we investigated the effect and underlying mechanisms of miR21 inhibition on the invasiveness of RA FLSs. The outcome of the study could provide new approach to RA treatment by targeting miR21.
Materials and Methods
Samples
All human samples used in this study were collected from The First People’s Hospital of Hefei from August, 2013 to March, 2015. A total number of 15 RA cases (female, 9 cases) were recruited according to the ACR/EULAR 2010 criteria for RA (13). Of the 15 RA patients, 10 were in stage III while the other 5 were in stage IV. The recruited patients did not receive drug treatment before the surgery. In parallel, 15 cases of sex- and age-matched non-RA controls were recruited from trauma patients who needed amputation. All recruited subjects were free of cardiovascular, infectious, and other inflammatory diseases. Tissue samples of synovial membrane were collected from all subjects and either used for FLSs isolation or frozen at -80 °C. Informed written consents were obtained from all patients. The protocols involving human specimens were in accordance with the Declaration of Helsinki and were reviewed by the institutional ethical review board (14).
Fibroblast-like synoviocytes isolation and culture
FLSs were isolated from synovial membrane tissues as previously described with modifications (15). In brief, synovial membrane tissues were first washed with PBS and adipose tissue was removed. Subsequently, tissues were cut into small pieces and digested with Collagenase/Dispase (2 mg/ml, Roche) and DNase I (0.1 mg/ml, Roche) in Dulbecco’s modified eagle medium (DMEM[lz2]) for 1 hr, at 37 °C. After digestion, tissue pieces were forced through cell strainer (BD Biosciences) to obtain single cell suspension. At last, FLSs were obtained by centrifugation and cultured in DMEM supplemented with 10% Fetal Bovine Serum (FBS[lz3]) (GIBICO, Thermo Scientific), 100 U/ml penicillin, and 100 U/ml streptomycin (Sigma-Aldrich).
MiR21 quantification
Quantification of miR21 was performed with real-time qPCR as previously described with modifications (16). In brief, total RNA containing small size RNA was extracted using High Pure miRNA Isolation Kit (Roche) and then miR21 expression was determined using Hairpin-it miRNA real-time PCR Quantitation Kit (GenePharma). U6 snRNA was used as an internal control and the miR21 level was calculated using the 2−ΔCT method.
MiR21 inhibitor transfection
MiR21 specific inhibitor and corresponding negative control (NC) were purchased from Thermo Scientific. Transfection of miR21 inhibitor and NC into FLS was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions.
Cell viability assay
Cell viability was assessed by MTT assay as previously described (17). FLSs were first transfected with miR21 inhibitor or (NC). After 48 hr, cell culture was removed and cells were treated with MTT solution (Sigma-Aldrich) for 4 hr followed by the addition of DMSO (Sigma-Aldrich) for 10 min. Then, the optical density (OD) value was read at 570 nm.
Cell invasion assay
The invasiveness of FLSs was assessed using a Matrigel coated Transwell system (18). FLSs were first transfected with miR21 inhibitor or NC for 48 hr, and then were transferred into Matrigel-coated (1 mg/ml, BD Biosciences) Transwell inserts with 8 mm pore size. The plate was then incubated at 37 °C for 18 hr. Post-incubation, Matrigel inserts were fixed with paraformaldehyde and stained with crystal violet. Cells migrated to the lower surface of the inserts were counted under the microscope. Five random fields were counted for each insert.
RT-PCR
Total RNA was extracted from FLSs and reverse-transcribed into cDNA using Trizol reagent (Invitrogen) and M-MLV reverse transcriptase (Promega), respectively, following the manufac-turer’s instructions. Then target genes were amplified using specific primer pairs with cDNA as template. Relative expression was calculated by grey-scale scanning of gel separated PCR products using GAPDH as an internal control. The primer pairs used in the study are listed in Table 1.
Table 1.
Primers for real-time PCR
| Gene | Primers (5’-3’) |
|---|---|
| TGF-β1 | For: CTGCTACCGCTGCTGCTGTGGCTACTG |
| Rev: CGGTCGCGGGTGCTGTTGT | |
| Smad4 | For: TGCTGTGCAAAGTGTTCAGGTG |
| Rev: CCATCGGGTATCTGGAGTAAGGA | |
| Smad7 | For: TAGCCTTGTCAGATAAGGAAGGA |
| Rev: CACCCACACACCATCCACG | |
| GADPH | For: AGCCACATCGCTCAGACA |
| Rev: TGGACTCCACGACGTACT |
For: Forward primer; Rev: Reverse primer
Western blot
FLSs with or without TGFβ1 treatment were first transfected with miR21 inhibitor or NC for 48 hr, and then were harvested and lysed using radioimmuno-precipitation (RIPA) solution. Whole cell lysates were subsequently separated by SDS-PAGE and transferred onto a PVDF membrane (Millipore). Membrane was then blocked with 5% non-fat milk and incubated with specific primary antibodies and corresponding secondary antibodies for 2 and 1 hr at room temperature, respectively. After incubation, membrane was extensively washed and immune-reactive bands were visualized using ECL substrate (Biotime). The relative expression of the target genes was calculated by gray-scale scanning using Quantity One software (Bio-Rad).
Statistical analysis
All data were expressed as mean ± SD and statistical analysis was performed with SPSS 17.0 (SPSS, Inc.). Student’s t test was adopted for comparisons between two groups and One-way ANOVA with SNK post hoc was used for multiple comparisons. A P value less than 0.05 was considered statistically significant.
Results
MiR21 is elevated in synovial tissue and fibroblast-like synoviocytes in RA patients
We first tested the miR21 expression in synovial tissue and FLSs in RA patients. Synovial tissue samples and FLSs were isolated from both non-RA controls and RA patients, and miR21 expression was quantified. As shown in Figure 1, miR21 level was significantly increased in both synovial tissue and FLSs in RA patients, indicating that aberrant miR21 expression is probably associated with RA.
Figure 1.
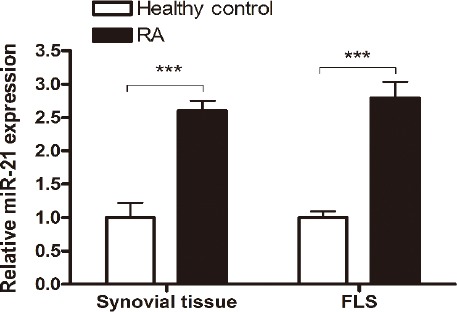
MiR-21 expression is elevated in synovial tissue and FLS cells in RA patients. Synovial tissue samples and FLS cells were isolated from non-RA controls and RA patients, and miR-21 level was determined by stem-loop RT-PCR. All samples were performed in triplicates for each condition. Data shown are mean ± SD of three independent experiments. ***, P<0.001
MiR21 inhibitor decreases the invasiveness of fibroblast-like synoviocytes
Since miR21 was detected to be likely associated with RA, we further investigated whether inhibition of miR21 could offer an approach to treat RA. The invasiveness of FLSs plays an important role in RA pathogenesis, therefore we tested the inhibition of miR21 using miR21 inhibitors. FLSs were first treated with miR21 inhibitor or negative control (NC), and then cell viability and invasiveness were determined. The results showed that miR21 inhibitor, but not miR21 NC, treatment could significantly decrease the invasiveness of FLSs while not showing apparent impact on cell viability (Figure 2 A-C). After miR21 inhibitor treatment, the invasion ability of FLSs decreased to 47%, comparing to that of NC treatment (Figure 2 B-C). These results together indicated that miR21 inhibitor treatment could suppress the invasion of FLSs, but not affect the viability of the cells.
Figure 2.
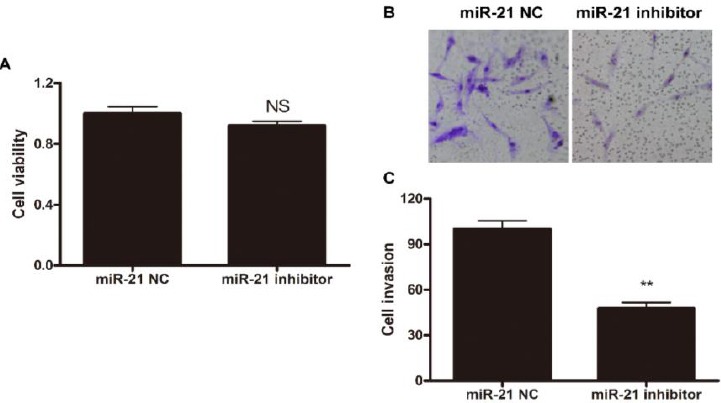
MiR-21 inhibitor treatment decreases the invasiveness of FLS cells. FLS cells were first treated with miR-21 NC or inhibitor, and then (A) cell viability was determined by MTT assay and (B and C) cell invasion was measured by Matrigel-coated Transwell system. (A) Data shown are mean±SD of three independent experiments. (B) Representative results are shown. (C) Data shown are mean±SD of three independent experiments. NS, statistically not significant; **, P< 0.01
MiR21 inhibitor down-regulates the expression of MMP1, MMP3, and MMP13
MMPs are enzymes that can degrade all the components of the extracellular matrix and conse-quently enhance cell migration and invasion. In RA, MMP1, MMP3, and MMP13 have been found to be at high levels and partially responsible for the increased invasiveness of FLSs in RA patients (6, 7). In this study, we then determined whether the suppression of FLSs invasion via miR21 inhibition was achieved by down-regulation of MMPs expression. Western blot analysis of MMP1, MMP3, and MMP13 showed that the expression of all three MMPs was decreased in FLSs after miR21 inhibitor treatment (Figure 3 A-B). Notably, the down-regulation efficiency of miR21 inhibitor on the three MMPs was quite different. MMP1 was the one showed the sharpest decrease (18% of NC group), followed by MMP13 (30% of NC group), while the least decreased expression was seen in MMP3 (36% of NC group).
Figure 3.
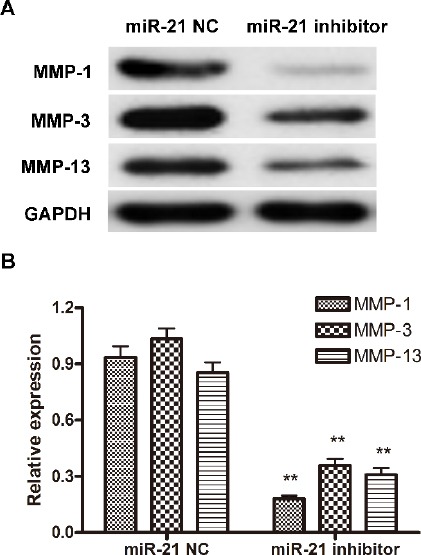
MiR-21 inhibitor treatment down-regulate the expression of MMP-1, MMP-3 and MMP-13. FLS cells were first treated with miR-21 NC or inhibitor, and then cells were harvested and the expression of MMP-1, MMP-3 and MMP-13 was determined by Western blot. (A) One representative result out of three is shown. (B) Gray-scale of the immune-bands were measured by Quantity One software and the relative expression of MMP-1, MMP-3 and MMP-13 to the internal control GAPDH was calculated. Data shown are mean±SD of three independent experiments. **, P<0.01
MiR21 inhibitor decreases fibroblast-like synoviocytes invasion through TGFβ1/Smads signaling pathway
In esophagus and colon cancers, miR21 enhances tumor cell invasion through TGFβ/Smads signaling pathway (11, 12). Here we also investigated the involvement of TGFβ/Smads signaling pathway in miR21-associated FLSs invasion in RA, and the effect of miR21 inhibition suppression of this signaling pathway. Within TGFβ/Smads signaling pathway, Smad4 is the common-mediator participating in the pathway, while Smad7 is the inhibitory regulator which blocks the activation of this pathway (19, 20).
First, we determined the expression profiles of TGFβ1, Smad4, and Smad7 on both mRNA and protein levels in miR21 inhibitor or NC treated FLSs. As shown in Figure 4, miR21 inhibitor treatment changed the expression pattern of the three proteins; TGFβ1 and Smad4 exhibitted decreased expression, while Smad7 showed increased expression at mRNA level (Figure 4A and C). Similar results were observed regarding their expression at protein level (Figure 4B and D).
Figure 4.
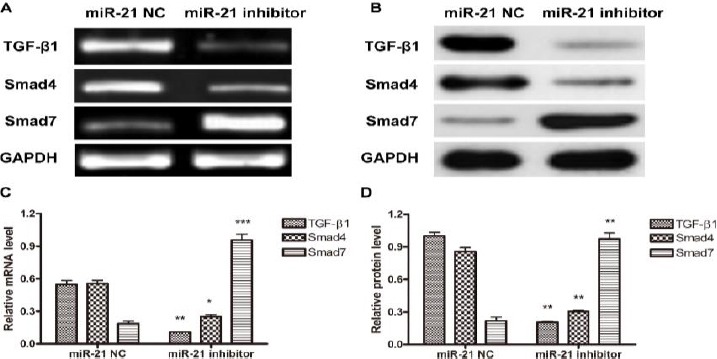
The effect of miR-21 inhibitor treatment on expression of TGF-β/Smads signaling pathway proteins. FLS cell were first treated with miR-21 NC or inhibitor, and then cells were harvested and the expression of TGF-β1, Smad4 and Smad7 on mRNA (A and C) and protein (B and D) levels was detected by RT-PCR and Western blot, respectively. (A and B) One representative result out of three is shown. (C and D) Gray-scale of the immune-bands were measured by Quantity One software and the relative expression of TGF-β1, Smad4 and Smad7 to the internal control GAPDH was calculated. Data shown are mean±SD of three independent experiments. *, P<0.05; **, P<0.01; ***, P<0.001
To further confirm the role of TGFβ1 in miR21 inhibitor suppression of FLSs invasion, miR21 inhibitor treated FLSs were then treated with or without TGFβ1, and the invasiveness and MMPs expression level were determined. The invasion assay showed that the decreased invasiveness of miR21 treated FLSs could be restored by the additional treatment of TGFβ1 (Figure 5A and C). Similarly, TGFβ1 treatment could counteract the impact of miR21 inhibitor on expression of MMP1, MMP3, and MMP13 (Figure 5B and D). These data indicated that TGFβ1/Smads pathway was involved in miR21 associated FLSs invasion, and the inhibition of miR21 could suppress the invasiveness of FLSs through the same pathway.
Figure 5.
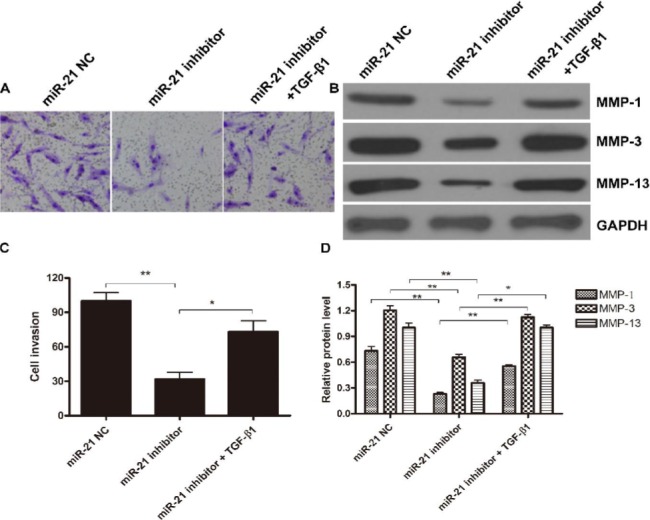
TGF-β1 treatment counteracts the suppression of miR-21 inhibitor on FLS invasion. FLS cells were first treated with miR-21 NC, inhibitor or miR-21 inhibitor + TGF-β1, and then (A and C) cell invasion was detected by Matrigel-coated Transwell system while (B and D) MMP-1, MMP-3 and MMP-13 expression was determined by Western blot. (A and B) One representative result out of three is shown. (C) Data shown are mean±SD of three independent experiments. (D) Gray-scale of the immune-bands were measured by Quantity One software and the relative expression of MMP-1, MMP-3 and MMP-13 to the internal control GAPDH was calculated. Data shown are mean±SD of three independent experiments *, P<0.05; **, P<0.01
Taken together, our results demonstrated that miR21 was aberrantly elevated in RA synovial tissue and FLSs, and inhibition of miR21 could down-regulate the expression of MMPs in FLSs and consequently suppress the invasiveness of FLSs. This suppression of FLSs invasion by inhibition of miR21 was through TGFβ1/Smad4/7 signaling pathway.
Discussion
RA is a systematic autoimmune disorder which affects joints and results in destruction of bone and cartilage and even life-long disability. Up to now, no cure for RA is available. Current treatment strategies for RA aim to reduce joint inflammation and pain, maximize joint function, and prevent joint destruction (21). Therefore, development of effective treatment strategies for RA is essentially needed. In the current study, we tested the efficacy of an inhibitor targeting miR21, an aberrantly expressed miR in RA, in suppressing FLSs invasion. Our results demonstrated that miR21 inhibitor could significantly reduce the invasiveness of FLSs, and this suppression was achieved through TGFβ1/Smad4/7 signaling pathway. The findings of our study indicate a novel approach in RA treatment and warrant further investigation on pre-clinical and clinical trials.
MicroRNAs have been found to be associated with a variety of diseases including cancer, inflammation, cardiovascular disorder, and autoimmune diseases, and treatment targeting microRNAs has been believed to be a promising approach (22-25). MiR21, although its significance in RA has not been previously understood, has been reported to closely correlate to esophagus and colon cancers through TGFβ/Smads signaling pathway (11, 12). Consistent with previous findings in other diseases, our results in this study also revealed that TGFβ/Smads signaling pathway was involved in the regulation of miR21, and the inhibition of miR21 could restored the balance of the pathway and consequently reduce FLSs invasion. Moreover, our study further revealed that miR21 through TGFβ/Smads signaling pathway could result in variated MMPs expression. MMPs are contributing factors to invasiveness of FLSs, and miR21 inhibitor could decrease MMPs expression, consequently rendering in reduced FLSs invasion. Notably, previous study by Wang et al has[GK4] identified 40 aberrantly expressed microRNAs in RA patients, and miR21 was only one out of 40 (26). Therefore, whether other microRNAs could be adopted as RA treatment targets like miR21 warrants further studies.
For disease management, potential treatment candidates should bear the following characteristics besides efficacy: high specificity, non- or low toxicity, and easy-to-use. In the current study, we also tested the cell toxicity of miR21 inhibitor to FLSs. And our results showed that this inhibitor was non-toxic to FLSs with cell viability similar to control group. However, as a proof of concept study, we adopted liposome transfection to deliver miR21 inhibitor into cells, which might be not applicable in clinical use. Therefore, drug delivery method optimization before clinical use is required if treatment using miR21 proves effective in high level evaluations.
Conclusion
Our results demonstrated that miR21 was aberrantly elevated in RA synovial tissue and FLSs, and inhibition of miR21 could down-regulate the expression of MMPs in FLSs, and consequently suppress the invasiveness of FLSs. This suppression of FLSs invasion by inhibition of miR21 was through TGFβ1/Smad4/7 signaling pathway. The findings of this study indicated that inhibition of miR21 might offer a novel approach for RA treatment.
Conflicts of interest
The authors declare that no conflict of interest exists.
Acknowledgement
This work is supported by Natural Science Foundation of Guangdong Province: S2012040007235.
References
- 1.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659–672. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- 2.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 3.Matcham F, Ali S, Hotopf M, Chalder T. Psychological correlates of fatigue in rheumatoid arthritis: a systematic review. Clin Psychol Rev. 2015;39:16–29. doi: 10.1016/j.cpr.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Mirfeizi Z, Noubakht Z, Rezaie AE, Jokar MH, Sarabi ZS. Plasma levels of leptin and visfatin in rheumatoid arthritis patients; is there any relationship with joint damage? Iran J Basic Med Sci. 2014;17:662–666. [PMC free article] [PubMed] [Google Scholar]
- 5.Tolboom TC, van der Helm-Van Mil AH, Nelissen RG, Breedveld FC, Toes RE, Huizinga TW. Invasiveness of fibroblast-like synoviocytes is an individual patient characteristic associated with the rate of joint destruction in patients with rheumatoid arthritis. Arthritis Rheum. 2005;52:1999–2002. doi: 10.1002/art.21118. [DOI] [PubMed] [Google Scholar]
- 6.Burrage PS, Mix KS, Brinckerhoff CE. Matrix metalloproteinases: role in arthritis. Front Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 7.Tolboom TC, Pieterman E, van der Laan WH, Toes RE, Huidekoper AL, Nelissen RG, et al. Invasive properties of fibroblast-like synoviocytes: correlation with growth characteristics and expression of MMP-1, MMP-3, and MMP-10. Ann Rheum Dis. 2002;61:975–980. doi: 10.1136/ard.61.11.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen WX, Ren LH, Shi RH. Implication of miRNAs for inflammatory bowel disease treatment: Systematic review. World J Gastrointest Pathophysiol. 2014;5:63–70. doi: 10.4291/wjgp.v5.i2.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chatzikyriakidou A, Voulgari PV, Georgiou I, Drosos AA. miRNAs and related polymorphisms in rheumatoid arthritis susceptibility. Autoimmun Rev. 2012;11:636–641. doi: 10.1016/j.autrev.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Latruffe N, Lancon A, Frazzi R, Aires V, Delmas D, Michaille JJ, et al. Exploring new ways of regulation by resveratrol involving miRNAs, with emphasis on inflammation. Ann N Y Acad Sci. 2015;1348:97–106. doi: 10.1111/nyas.12819. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Pan T, Zhong X, Cheng C. Nicotine upregulates microRNA-21 and promotes TGF-beta-dependent epithelial-mesenchymal transition of esophageal cancer cells. Tumour Biol. 2014;35:7063–7072. doi: 10.1007/s13277-014-1968-z. [DOI] [PubMed] [Google Scholar]
- 12.Cottonham CL, Kaneko S, Xu L. miR-21 and miR-31 converge on TIAM1 to regulate migration and invasion of colon carcinoma cells. J Biol Chem. 2010;285:35293–35302. doi: 10.1074/jbc.M110.160069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alves C, Luime JJ, van Zeben D, Huisman A-M, Weel AEAM, Barendregt PJ, et al. Diagnostic performance of the ACR/EULAR 2010 criteria for rheumatoid arthritis and two diagnostic algorithms in an early arthritis clinic (REACH) Ann Rheum Dis. 2011;70:1645–1647. doi: 10.1136/ard.2010.142299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Association WM. Declaration of Helsinki. Ethical principles for medical research involving human subjects. 2009 [PubMed] [Google Scholar]
- 15.Harada S, Yamamura M, Okamoto H, Morita Y, Kawashima M, Aita T, et al. Production of interleukin-7 and interleukin-15 by fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:1508–1516. doi: 10.1002/1529-0131(199907)42:7<1508::AID-ANR26>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 16.Yan L-X, Huang X-F, Shao Q, Huang MAY, Deng L, Wu Q-L, et al. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 2008;14:2348–2360. doi: 10.1261/rna.1034808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stockert JC, Blázquez-Castro A, Cañete M, Horobin RW, Villanueva Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012;114:785–796. doi: 10.1016/j.acthis.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Shridhar R, Zhang J, Song J, Booth BA, Kevil CG, Sotiropoulou G, et al. Cystatin M suppresses the malignant phenotype of human MDA-MB-435S cells. Oncogene. 2004;23:2206–2215. doi: 10.1038/sj.onc.1207340. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Hata A, Lo RS, Massague J, Pavletich NP. A structural basis for mutational inactivation of the tumour suppressor Smad4. Nature. 1997;388:87–93. doi: 10.1038/40431. [DOI] [PubMed] [Google Scholar]
- 20.Itoh F, Asao H, Sugamura K, Heldin CH, ten Dijke P, Itoh S. Promoting bone morphogenetic protein signaling through negative regulation of inhibitory Smads. EMBO J. 2001;20:4132–4142. doi: 10.1093/emboj/20.15.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 22.Broderick JA, Zamore PD. MicroRNA therapeutics. Gene Ther. 2011;18:1104–1110. doi: 10.1038/gt.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dangwal S, Thum T. microRNA therapeutics in cardiovascular disease models. Annu Rev Pharm Toxicol. 2014;54:185–203. doi: 10.1146/annurev-pharmtox-011613-135957. [DOI] [PubMed] [Google Scholar]
- 24.van Rooij E, Kauppinen S. Development of microRNA therapeutics is coming of age. EMBO Mol Med. 2014;6:851–864. doi: 10.15252/emmm.201100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thum T. MicroRNA therapeutics in cardiovascular medicine. EMBO Mol Med. 2012;4:3–14. doi: 10.1002/emmm.201100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Peng W, Ouyang X, Li W, Dai Y. Circulating microRNAs as candidate biomarkers in patients with systemic lupus erythematosus. Transl Res. 2012;160:198–206. doi: 10.1016/j.trsl.2012.04.002. [DOI] [PubMed] [Google Scholar]


