Abstract
Objective(s):
Scientific interest in potential mobile phone impact on human brain and performance has significantly increased in recent years. The present study was designed to evaluate the effects of mobile phone radiation on seizure threshold in mice.
Materials and methods:
BALB/c male mice were randomly divided into three groups: control, acute, and chronic mobile phone radiation for 30, 60, and 90 min with frequency 900 to 950 MHz and pulse of 217 Hz. The chronic group received 30 days of radiation, while the acute group received only once. The intravenous infusion of pentylenetetrazole (5 mg/ml) was used to induce seizure signs.
Results:
Although acute mobile radiation did not change seizure threshold, chronic radiation decreased the clonic and tonic seizure thresholds significantly.
Conclusion:
Our data suggests that the continued and prolonged contact with the mobile phone radiation might increase the risk of seizure attacks and should be limited.
Keywords: Mice, Mobile phone radiation, Pentylenetetrazole, Seizure
Introduction
The application of mobile phone technology and scientific interest in its potential impact on human brain and performance has significantly increased in the recent years (1). Epilepsy is the most common brain disorder after stroke, and its prevalence is growing due to various reasons (2). Epilepsy is commonly associated with the brain dysfunctions leading to hyperexcitability of neurons. It is assumed that there is an imbalance between inhibitory GABA-mediated and excitatory glutamate-mediated neurotransmission (3). Several studies have demons-
trated the effects of electromagnetic mobile phone waves on central nervous system (4, 5). It was shown that brain changes caused by electromagnetic waves are well reflected in the EEG of rats; also the largest changes are reported in brain activity of the limbic and olfactory cortex and the subcortical parts (6, 7). It was also demonstrated that if the electromagnetic fields (EMFs) are exactly radiated in the contralateral brain cortex, the patient’s cortex nerve stimulation increases (8). A few studies investigated the effects of exposure to EMFs on epileptic disorders in human and experimental subjects, but the results are controversial (6, 9). The present study was designed to examine the effects of acute and chronic mobile phone radiation on clonic and tonic seizure thresholds induced by intravenous infusion of pentylenetetrazole (PTZ) in mice.
Materials and Methods
We used adult male BALB/c mice, weighing 25–30 g, aged 10–12 weeks, and kept at 21°C and 12 hr light–dark cycle with easy access to food and water. All experiments were performed between 9:00–11:00 AM during the light cycle. All procedures were carried out in accordance with institutional guidelines for animal care and use and possible measures were undertaken to minimize the number of animals used and also to minimize animals’ discomfort including immediate euthanasia after acute experiments (10, 11). Groups consisted of at least eight animals and each animal was used only once (12). Mice were randomly divided into seven groups. Group one was the control group with mice unexposed to the mobile phone. In three groups mice were exposed for 30, 60, and 90 min just once and in another three groups mice were exposed for 30, 60, and 90 min per day for 30 days while the cell phone was in answering state. Special plastic cages (45 cm diameter by 11 cm height) were designed; in these cages, the maximum distance from the source of radiation was about 10 cm. Since the GSM (Global System for Mobile Communications) system with frequency 900 to 950 MHz and pulse of 217 Hz is the most common system in Asia and Europe and in most studies, we used it in our project (6).
Seizure induction
To induce the animal model of seizure, intra-venous PTZ infusion (5 mg/ml) was used. PTZ was dissolved in heparinized normal saline (0.9%) and infused at the rate of 0.5 ml/min in all animals (control and experimental groups). The test was conducted on mice with some modification in the previously mentioned method (3). PTZ was dissolved in heparinized sterile saline to prepare a fresh solution with a concentration of 5 mg/ml before IV infusion. The dose and infusion rate of administered PTZ were 5 mg/ml and 0.3 ml/min, respectively. Before testing, each mouse was weighed, placed in a clear acrylic plastic restrainer and its tail was immersed in a warm water bath (40–45°C) for 60 sec to dilate the tail veins. A dental carpule (27 G, Heraeus Kulzer, Germany) connected by a poly-ethylene tube (no. 10) to a 50 ml syringe prefilled with heparinized PTZ solution was used. The needle was inserted into the mid-length of the lateral vein of the tail. After verifying the proper insertion by the appearance of blood in the infusion tube, the needle was secured to the tail using a special tape. The syringe was held in the adjustable motor driven infusion pump (TOP Syringe Pump, Japan). During the infusion period, the animal could move freely in the plexiglass observation box with the aid of attached cannula. Each animal was observed throughout the infusion period and the duration between the start of infusion and onset of seizure stages was recorded in seconds and then converted to threshold convulsant dosage (i.e. mg of drug per kg of body weight). Sudden involuntary muscle jerk followed by rapid writhing movements of the head and neck and/or forelimb clonus were considered as the signs of clonic seizure, and tonic hind limb extension, extreme rigidity with forelimbs, and hindlimbs extended caudally were considered as the signs of tonic seizure. The threshold concentration in mg/kg for the appearance of seizure signs was calculated using the following formula (13):
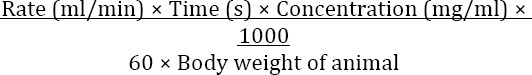
Statistical analysis
Results are expressed as mean values±standard error of the mean (SEM). Statistical analysis of the results was done by one-way ANOVA for seizure thresholds followed by Tukey’s test for multiple comparisons. A P-value less than 0.05 was regarded as statistically significant (14, 15).
Results
Acute exposure
Acute exposure (30, 60, and 90 min for one time) to GSM mobile waves did not induce any significant changes in clonic and tonic seizure thresholds (Figures 1 and 2). The mean dose of clonic seizure threshold in the control group was 79.2±2.7 mg/kg and for test groups that received acute exposure for 30, 60, and 90 min were 78.1±2.6 mg/kg, 73.5±3.1 mg/kg, and 70.4±4.2 mg/kg, respectively (Figure 1). The mean dose of tonic seizure threshold in the control group was 143.4±4.7 mg/kg and in the test groups that received acute exposure for 30, 60, and 90 min were 137.2±5.1 mg/kg, 142±5.8 mg/kg, and 142.1±6.4 mg/kg, respectively, which compared with the control group, were not significantly different (Figure 2).
Figure 1.

Effect of acute (once) mobile phone radiation in various exposure times (30, 60, and 90 min) on clonic seizure threshold induced by pentylenetetrazole (PTZ) in mice. The results are expressed as mean± SEM of eight mice
Figure 2.

Effect of acute (once) mobile phone radiation in various exposure times (30, 60, and 90 min) on tonic seizure threshold induced by pentylenetetrazole (PTZ) in mice. The results are expressed as mean± SEM of eight mice
Chronic exposure
Analysis of the groups that were receiving chronic exposure (30, 60, and 90 min per day for 30 days), showed that the tonic and clonic seizure thresholds in all groups were mostly reduced; increasing the exposure time from 30 to 60 led to higher attenuation seizure thresholds, in both clonic and tonic (P<0.001) (Figures 3 and 4) seizures. The mean dose for clonic seizure threshold, in the control group, was 80±2.7 mg/kg. These doses for 30, 60, and 90 min exposures were 68.1±2.5, 55±2.7 and 57±4.7, respectively (Figure 3). The mean dose of tonic seizure threshold in the control group was 141±4.4 mg/kg and in the groups receiving chronic exposure (30, 60, and 90 min was 125±4.5 and 106±5.1 and 1106±5.9 mg/kg, respectively (Figure 4).
Figure 4.

Effect of chronic (30 days) mobile phone radiation in various exposure times (30, 60, and 90 min per day) on tonic seizure threshold induced by pentylenetetrazole (PTZ) in mice. The results are expressed as mean± SEM of eight mice. ** P<0.01, *** P<0.001 versus control and # P<0.05 versus exposure 30 min
Figure 3.

Effect of chronic (30 days) mobile phone radiation in various exposure times (30, 60, and 90 min per day) on clonic seizure threshold induced by pentylenetetrazole (PTZ) in mice. The results are expressed as mean± SEM of eight mice. * P<0.05, *** P<0.001 versus control and # P<0.05 versus exposure 30 min
Discussion
In the present study, for the first time, we show that the chronic exposure to GSM mobile phone waves reduces the seizure threshold induced by intravenous infusion of PTZ in mice. While the physiological effects of EMFs on living organisms are entirely sensible and researchers have warned about their effects (16), more than a third of the world population uses mobile phones daily. GSM with 900-950 MHz and pulse frequency of 217 Hz is the most common mobile phone system in Asia and Europe (4), which is the system used in the present study. Few studies have evaluated the effects of GSM mobile radiation on seizure (7, 8). It was shown that radiation can induce seizures in rats following their facilitation by sub-convulsive doses of picrotoxin (9). It was also reported that mobile phone emissions modulate brain excitability in patient with focal epilepsy (8).
Literature review suggests several possible mechanisms for the effect of mobile phone radiation on seizure (17). It was demonstrated that long-term exposure to electromagnetic waves can change the level of calcium in neurons, which modifies the cellular oxidative status (18). It has been shown that brain excitability is significantly modified by mobile phone emission (19, 20). It was suggested this hyperexcitability might depend on decreased GABAA-mediated inhibition or increased N-methyl-D-aspartate (NMDA)-mediated excitatory activity or both, such a disruption in neurotransmitter balance leading to hyperexcitability may also be associated with the monohemispheric increase of regional cerebral blood flow as actually observed via neurometabolic measurements during GSM mobile phone utilization (21). It has also been shown that the electromagnetic radiation emitted by mobile phones increases the permeability of the blood-brain barrier (BBB) immediately and 14 days after exposure (22). This increased permeability can weaken the protective role of BBB from potentially harmful substances like PTZ circulating in the blood and therefore reduce the PTZ-induced seizure threshold. Recently it was demonstrated that cellular phone radiation may increase the oxidative damage, lipid peroxidation, and total nitric oxide level during pentylenetetrazole-induced epileptic seizure in mice (23).
Further studies are needed to clarify the exact mechanisms involved in the effect of mobile phone radiation on seizure.
Conclusion
Based on our findings, although short-term exposure to mobile phone radiation cannot have a significant effect on seizure threshold in mice, chronic radiation in the long term (30 days) reduced the seizure threshold and the continuous use of mobile phones might increase the risk of seizure attacks and should be limited.
Acknowledgment
This paper on an MD degree thesis and supported financially by Vice Chancellor of Research, Kashan University of Medical Sciences, Kashan, Iran.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Hamblin DL, Wood AW. Effects of mobile phone emissions on human brain activity and sleep variables. Int J Radiat Biol. 2002;78:659–669. doi: 10.1080/09553000210132298. [DOI] [PubMed] [Google Scholar]
- 2.Sander JW. The epidemiology of epilepsy revisited. Curr Opin Neurol. 2003;16:165–170. doi: 10.1097/01.wco.0000063766.15877.8e. [DOI] [PubMed] [Google Scholar]
- 3.Mesdaghinia A, Yazdanpanah H, Seddighi M, Banafshe HR, Heydari A. Effect of short-term lead exposure on PTZ-induced seizure threshold in mice. Toxicol Lett. 2010;199:6–9. doi: 10.1016/j.toxlet.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Kaprana AE, Chimona TS, Papadakis CE, Velegrakis SG, Vardiambasis IO, Adamidis G, et al. Auditory brainstem response changes during exposure to GSM-900 radiation: an experimental study. Audiol Neurootol. 2011;16:270–276. doi: 10.1159/000321337. [DOI] [PubMed] [Google Scholar]
- 5.Rağbetli MC, Aydinlioğlu A, Koyun N, Rağbetli C, Bektas S, Ozdemir S. The effect of mobile phone on the number of Purkinje cells: a stereological study. Int J Radiat Biol. 2010;86:548–554. doi: 10.3109/09553001003734527. [DOI] [PubMed] [Google Scholar]
- 6.López-Martín E, Bregains J, Relova-Quinteiro JL, Cadarso-Suárez C, Jorge-Barreiro FJ, Ares-Pena FJ. The action of pulse-modulated GSM radiation increases regional changes in brain activity and c-Fos expression in cortical and subcortical areas in a rat model of picrotoxin-induced seizure proneness. J Neurosci Res. 2009;87:1484–1499. doi: 10.1002/jnr.21951. [DOI] [PubMed] [Google Scholar]
- 7.Carballo-Quintás M, Martínez-Silva I, Cadarso-Suárez C, Alvarez-Figueiras M, Ares-Pena FJ, López-Martín EA. Study of neurotoxic biomarkers, c-fos and GFAP after acute exposure to GSM radiation at 900 MHz in the picrotoxin model of rat brains. Neurotoxicology. 2011;32:478–494. doi: 10.1016/j.neuro.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Tombini M, Pellegrino G, Pasqualetti P, Assenza G, Benvenga A, Fabrizio E, et al. Mobile phone emissions modulate brain excitability in patients with focal epilepsy. Brain Stimulat. 2013;6:448–454. doi: 10.1016/j.brs.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 9.López-Martín E, Relova-Quinteiro JL, Gallego-Gómez R, Peleteiro-Fernández M, Jorge-Barreiro FJ, Ares-Pena FJ. GSM radiation triggers seizures and increases cerebral c-Fos positivity in rats pretreated with sub convulsive doses of picrotoxin. Neurosci Lett. 2006;398:139–144. doi: 10.1016/j.neulet.2005.12.082. [DOI] [PubMed] [Google Scholar]
- 10.Hamidi GA, Jafari-Sabet M, Abed A, Mesdaghinia A, Mahlooji M, Banafshe HR. Gabapentin enhances anti-nociceptive effects of morphine on heat, cold, and mechanical hyperalgesia in a rat model of neuropathic pain. Iran J Basic Med Sci. 2014;17:753–759. [PMC free article] [PubMed] [Google Scholar]
- 11.Abed A, Hajhashemi V, Banafshe HR, Minaiyan M, Mesdaghinia A. Venlafaxine attenuates heat hyperalgesia independent of adenosine or opioid system in a rat model of peripheral neuropathy. Iran J Pharm Res. 2015;14:843–850. [PMC free article] [PubMed] [Google Scholar]
- 12.Payandemehr B, Bahremand A, Rahimian R, Ziai P, Amouzegar A, Sharifzadeh M, et al. 5-HT(3) receptor mediates the dose-dependent effects of citalopram on pentylenetetrazole-induced clonic seizure in mice: involvement of nitric oxide. Epilepsy Res. 2012;101:217–227. doi: 10.1016/j.eplepsyres.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Ghasemi M, Shafaroodi H, Nazarbeiki S, Meskar H, Ghasemi A, Bahremand A, et al. Inhibition of NMDA receptor/NO signaling blocked tolerance to the anti-convulsant effect of morphine on pentylenetetrazole-induced seizures in mice. Epilepsy Res. 2010;91:39–48. doi: 10.1016/j.eplepsyres.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Hajhashemi V, Minaiyan M, Banafshe HR, Mesdaghinia A, Abed A. The anti-inflammatory effects of venlafaxine in the rat model of carrageenan-induced paw edema. Iran J Basic Med Sci. 2015;18:654–658. [PMC free article] [PubMed] [Google Scholar]
- 15.Banafshe HR, Ghazi-Khansari M, Ejtemaei Mehr S, Dehpour AR. Cyclosporine attenuates the adenylyl cyclase superactivation induced by chronic cannabinoid treatment. Eur J Pharmacol. 2007;557:20–22. doi: 10.1016/j.ejphar.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Valentini E, Curcio G, Moroni F, Ferrara M, De Gennaro L, Bertini M. Neurophysiological effects of mobile phone electromagnetic fields on humans: a comprehensive review. Bioelectromagnetics. 2007;28:415–432. doi: 10.1002/bem.20323. [DOI] [PubMed] [Google Scholar]
- 17.Hassanzadeh P, Arbabi E, Rostami F. The ameliorative effects of sesamol against seizures, cognitive impairment and oxidative stress in the experimental model of epilepsy. Iran J Basic Med Sci. 2014;17:100–107. [PMC free article] [PubMed] [Google Scholar]
- 18.Hardell L, Sage C. Biological effects from electromagnetic field exposure and public exposure standards. Biomed Pharmacother. 2008;62:104–109. doi: 10.1016/j.biopha.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Ferreri F, Curcio G, Pasqualetti P, De Gennaro L, Fini R, Rossini PM. Mobile phone emissions and human brain excitability. Ann Neurol. 2006;60:188–196. doi: 10.1002/ana.20906. [DOI] [PubMed] [Google Scholar]
- 20.Ziemann U, Hallett M, Cohen LG. Mechanisms of differentiation-induced plasticity in human motor cortex. J Neurosci. 1998;18:7000–7007. doi: 10.1523/JNEUROSCI.18-17-07000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber R, Treyer V, Schuderer J, Berthold T, Buck A, Kuster N, et al. Exposure to pulse modulated radio frequency electromagnetic fields affects regional cerebral blood flow. Eur J Neurosci. 2005;21:1000–1006. doi: 10.1111/j.1460-9568.2005.03929.x. [DOI] [PubMed] [Google Scholar]
- 22.Nittby H, Brun A, Eberhardt J, Malmgren L, Persson BR, Salford LG. Increased blood-brain barrier permeability in mammalian brain 7 days after exposure to the radiation from a GSM-900 mobile phone. Pathophysiology. 2009;16:103–112. doi: 10.1016/j.pathophys.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Esmekaya MA, Tuysuz MZ, Tomruk A, Canseven AG, Yücel E, Aktuna Z, et al. Effects of cell phone radiation on lipid peroxidation, glutathione and nitric oxide levels in mouse brain during epileptic seizure. J Chem Neuroanat. 2016:S0891–0618. doi: 10.1016/j.jchemneu.2016.01.011. 30008-30004. [DOI] [PubMed] [Google Scholar]


