SYNOPSIS
There are three biologic groups of liposarcoma: well- and dedifferentiated liposarcoma (WD/DDLS), myxoid/round cell liposarcoma (M/RCLS) and pleomorphic liposarcoma. WD/DDLS is characterized by amplification of 12q13-15 (including the oncogenes MDM2 and CDK4), M/RCLS by FUS-DDIT3 translocations, and pleomorphic liposarcoma by loss of p53 and Rb. In all three groups, complete surgical resection is central in treatment aimed at cure and is based on grade. Radiation can reduce risk of local recurrence in high-grade lesions or minimize surgical morbidity in the highly radiosensitive M/RCLS group. The biologic groups differ greatly in their chemosensitivity, so adjuvant chemotherapy is selectively utilized in chemosensitive histologies with metastatic potential (i.e. round cell and pleomorphic liposarcomas) but not in the relatively resistant subtype DDLS. An improved understanding of the genetic aberrations that lead to liposarcoma initiation is also allowing for the rapid development of targeted therapies for liposarcoma. Among such therapies are CDK4 inhibitors in WD/DDLS and trabectedin, which prevents FUS-DDIT3 binding to DNA, in M/RCLS.
Keywords: Sarcoma, liposarcoma, myxoid liposarcoma, pleomorphic liposarcoma, CDK4, MDM2, FUS-CHOP, trabectedin
INTRODUCTION
Liposarcoma (LPS) is one of the most common histologies of soft tissue sarcoma (STS), representing 50% of retroperitoneal and 25% of extremity STS.1 There are three separate biologic groups of LPS encompassing five histologic subtypes. Each group is characterized by specific genetic alterations presumed to drive tumor initiation (Table 1). Well- and dedifferentiated liposarcomas (WD/DDLS) represent over 60% of all LPS and are almost universally associated with amplification of chromosome segment 12q13–15, which carries the oncogenes MDM2, CDK4, and HMGA2.2–6 Over 95% of myxoid and round cell liposarcomas carry a translocation of FUS and DDIT3 (CHOP) genes.7–9 Pleomorphic liposarcoma has a complex karyotype often causing loss of tumor suppressors p53 and Rb.5 This subtype, which is associated with a poor prognosis, is the rarest subtype of LPS, comprising approximately 5% of cases (Table 2).6
Table 1.
Genomic alterations in liposarcoma
| Histologic subtypes | Genomic alterations |
Affected oncogenes | Clinical correlation |
|---|---|---|---|
| Well- and dedifferentiated | 12q13-15 amplification | MDM2 and CDK4 | N/A |
| 3p14-21 loss | Unknown | Dedifferentiation | |
| 11q23-24 loss | Unknown | Dedifferentiation, genomic instability |
|
| 19q13 loss | Unknown | Dedifferentiation, poor prognosis |
|
| Myxoid/round cell | FUS-DDIT3 translocation | Unknown | N/A |
| Pleomorphic | Rb/p53 loss | Rb and p53 | N/A |
Table 2.
Clinical characteristics of liposarcoma histologic subtypes
| Subtype | Local recurrence rate |
Distal recurrence rate |
Chemosensitivity | Radiosensitivity |
|---|---|---|---|---|
| Well-differentiated | Low | Low | Low | Moderate |
| Dedifferentiated | Moderate | Low | Low | Moderate |
| Myxoid | Low | Low | High | High |
| Round cell | Moderate | High | High | High |
| Pleomorphic | Moderate | High | High | Moderate |
Surgery remains the mainstay of treatment for LPS, but the three subgroups have highly variable response to systemic therapies, affecting recommendations regarding adjuvant therapy. The different genomic underpinnings that define the groups mean that research has identified variable means of targeting these diseases using novel therapies. Here we examine the data supporting current treatment strategies, including multimodality paradigms that integrate radiation and chemotherapy. We will also examine ongoing genomic and molecular studies elucidating novel methods for treating the diseases and results of clinical trials aimed at translating these findings into clinical practice.
WELL-AND DEDIFFERENTIATED LIPOSARCOMA
As noted, WDLS and DDLS are the most common histologic variants of LPS. DDLS represents progression of WDLS from an indolent, sometimes locally aggressive lesion to more rapidly growing disease with metastatic potential.6,10,11 Five-year disease-specific survival in patients with DDLS is 44%, compared to 93% in patients diagnosed with pure WDLS.6 Genomic alterations are more complex in DDLS than in WDLS. In addition to amplification of 12q13–15, copy number alterations affecting segments of chromosome 11, 19, and 3, among others, are common in retroperitoneal DDLS and may affect genomic stability as well as prognosis.12
Management of primary well- and dedifferentiated liposarcoma in the extremity
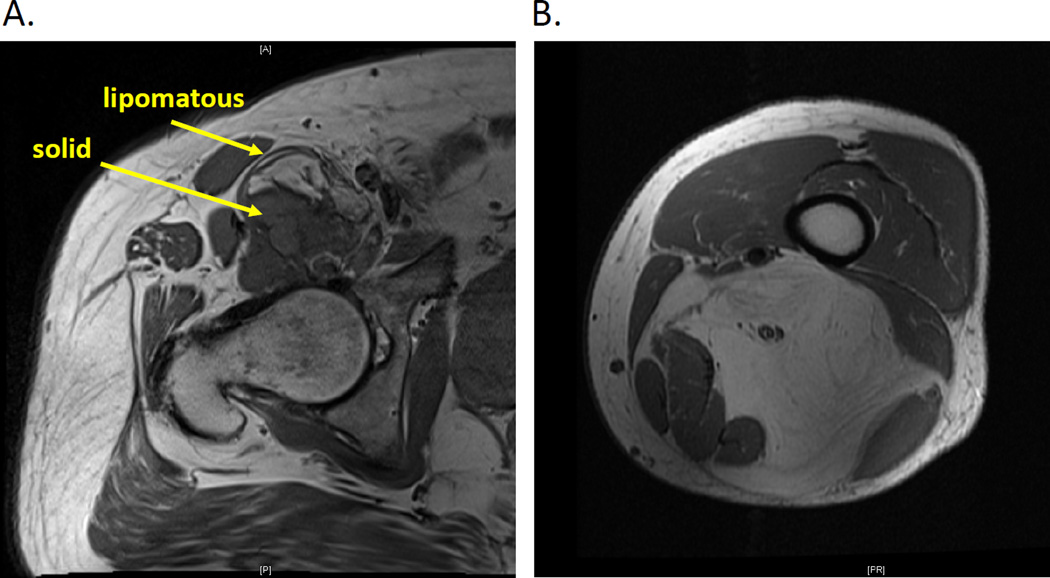
Treatment strategies for WD/DDLS diagnosed in the extremity parallel those of STS in general;1 however, diagnosis can often be made radiographically and not based on core biopsy. DDLS appears as an enhancing nodule in association with a lipomatous tumor (WDLS); the adipogenic component appears similar to fat on CT or MRI (Figure 1A). High-grade DDLS is managed primarily with surgical resection. The tumor is removed with margins of 1 cm of normal tissue or a major fascial barrier circumferentially. Encasement of a major neurovascular structure may require resection with arterial reconstruction. When the tumor abuts the neurovascular bundle or bone, then the neurovascular sheath, perineurium or periosteum can be resected as closest margin. Adjuvant radiation is employed to reduce risk of local recurrence in the case of high-grade DDLS of the extremity that is greater than 5 cm in diameter or after R1 resection that cannot be improved without causing major morbidity. Radiation planned in the neoadjuvant setting is an indication for pre-operative biopsy as opposed to clinical diagnosis. Because DDLS is relatively chemoresistant, systemic therapies are rarely employed for localized DDLS.13,14
Figure 1.
MRI T1 images showing cross-sectional evaluation of (A) dedifferentiated liposarcoma in the hip and (B) well-differentiated liposarcoma in the thigh. Arrows show lipomatous components representing a well-differentiated component of the dedifferentiated tumor and the nodular high-grade component.
WDLS in the extremity is also termed atypical lipomatous tumors (ALT). As in the case of DDLS, diagnosis of WDLS can be suggested by pre-operative imaging. WDLS appears as a lipomatous mass, with similar signal intensity as normal fat (Figure 1B). The differential diagnosis for lipomatous mass in the extremity includes WDLS and lipoma. Final diagnosis is accurately determined only after pathologic evaluation of the surgically resected specimen, as core biopsy results are susceptible to sampling error, but WDLS may be suspected in deep lesions. WDLS tends to be larger than lipoma (>10 cm), diagnosed in older patients (≥55 years), and identified in the context of recurrence.15 MRI may show enhancing septae in WDLS.16 Outcomes for patients with WDLS of the extremity are good, with five- and ten-year local recurrence–free survival rates of 100% and 78%, respectively; the tumors have no metastatic potential unless they have a dedifferentiated component.17 The surgical approach can, therefore be more conservative than that for DDLS. For example, WDLS that encases a neurovascular bundle may be bivalved to preserve the structures, and margins of less than 1 cm can be planned to optimize function and cosmesis. Similarly, radiation is generally deferred after resection of extremity WDLS, because even in the context of local recurrence, disease-related deaths are exceedingly rare.17
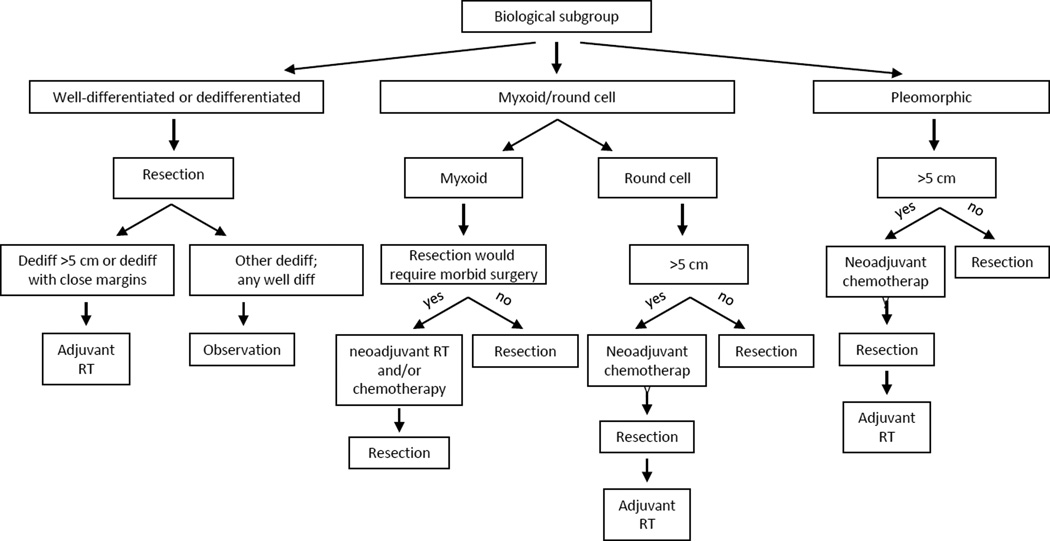
An algorithm for the treatment of localized WD/DDLS of the extremity (and other liposarcomas) is shown in Figure 2.
Figure 2.
Flow-diagram delineating basic treatment algorithm used for management of patients with localized liposarcoma of the extremity.
Management of primary well- and dedifferentiated liposarcoma of the retroperitoneum
WD/DDLS is the most common type of STS in the retroperitoneum. Like extremity WD/DDLS, WD/DDLS of the retroperitoneum can often be diagnosed based on imaging studies. Highly suggestive of WD/DDLS is cross-sectional imaging demonstrating a lipomatous lesion with pushing borders and enhancing septae, with or without solid nodules. Surgery can be planned without biopsy when imaging is reviewed by an experienced diagnostician. The primary goal of surgery for retroperitoneal WD/DDLS should be complete gross resection of all disease. For retroperitoneal sarcoma, R2 resection is associated with significantly poorer outcomes than R0 or R1 resection, and, in most series, patients with gross residual disease have outcomes as poor as patients who did not undergo any surgery.18 Whether R0 resections have better outcomes than R1 resections is unclear, as results have varied among the retrospective analyses of retroperitoneal sarcomas.19,20 Therefore, there is no clear consensus regarding the importance of obtaining an R0 versus R1 resection.
This controversy regarding the role of R0 resection underlies ongoing debate on 'extended compartmental resection' for patients with primary retroperitoneal disease, particularly because retroperitoneal WD/DDLS often recurs locally, and death is often caused by unresectable local recurrence that compresses visceral organs as opposed to distant metastases.11 Retrospective series compared patients treated with excision of primary tumor including surrounding fat and fascial planes (e.g., kidney capsule) where feasible with patients treated by ‘extended compartmental resection,’ i.e. removal of the tumor with surrounding organs where those organs would provide an additional 1-cm margin (e.g., kidney, colon, pancreas and spleen). Decreased rates of local recurrence were reported for the extended compartmental resection group, and, with longer follow-up, results suggested improvements in overall survival.20–22 However, many major sarcoma centers have not adopted this practice, for reasons delineated in depth in multiple reviews.23–25 Briefly, extended compartmental resection is criticized for high rates of post-operative morbidity and for the limited ability to optimize margins because most retroperitoneal lesions lie close to central vessels and unresectable organs (e.g., liver, duodenum). Prior reports have also associated multi-visceral organ resection with poorer outcomes for liposarcoma, suggesting that the need for aggressive surgery to control disease may, in fact, be a function of poor tumor biology.11 More recent data suggest that rates of local recurrence (in the absence synchronous distant metastases) are highest in grade II (FNCLCC) DDLS, so extended resections may be most appropriate for this subset.26
Most STS specialists agree on the need for complete gross resection of retroperitoneal WD/DDLS, and as in the extremity, adjuvant systemic therapy is rarely considered. Given poor rates of response, systemic therapy is generally reserved for unresectable disease, in some instances preoperatively for marginally resectable disease, or in the context of metastatic disease. The role for radiation is debatable; radiation is considered at many institutions because of high rates of local recurrence. When given, it is generally as neoadjuvant to prevent injury to surrounding normal tissues. Adjuvant radiation causes, for example, small bowel enteritis in up to 60% of patients.27 The efficacy of neoadjuvant radiation has been partially defined by two prospective series with a combined 72 patients, though only 40% of the patients had liposarcoma and 25% had recurrent disease. Among the 54 radiation-treated patients who were able to undergo complete gross resection, 5-year local recurrence–free survival was 60% as compared to non-irradiated historic controls, with 5-year local recurrence-free survivals of 30–60%.28 The benefit was not clear-cut, however, and the role of neoadjuvant radiation in primary retroperitoneal sarcoma is currently being examined in a phase III, randomized EORTC trial.29
Management of recurrent disease
Local recurrence rates in the extremity are low, and follow-up can be performed with serial exams or MRI yearly for WDLS (every 6 months for DDLS). Local recurrences of extremity disease are managed in much the same way as primary disease. If reoperation would carry significant morbidity, WDLS recurrence can be observed with serial imaging, given its lack of metastatic potential. When surgery is required, all attempt is made to preserve the limb. Patients with DDLS should undergo chest imaging every 6–12 months, as distant recurrence of extremity DDLS is generally noted in the lung. Metastasectomy can be performed if technically favorable and if the patient has good prognostic indicators, such as a solitary site and long disease-free interval, with the caveat that these recommendations are generally based on studies covering a range of sarcoma histologies. For patients who had retroperitoneal WD/DDLS, distant recurrence is less often a problem though up to 22% of patients may develop metastatic disease in the lungs, but local recurrence is common. CT of the abdomen and pelvis is completed every 3–6 months in the first two years following surgery then every six months for 2 years. 30 Chest imaging is indicated for patients treated for DDLS.
Because of the relative resistance of WD/DDLS to systemic therapy, surgical re-resection has been the standard management for recurrent disease. However, resection of recurrent disease is associated with increased rates of complications, so careful patient selection is essential in determining who may benefit from alternative treatments. Recurrent disease is rarely curable, and surgery should be seen as a temporizing measure and reserved for instances in which significant disease control is anticipated. Recurrence after re-resection is least frequent in patients who had a long disease-free interval and whose recurrence is small and not multifocal, so these characteristics identify ideal surgical candidates. In a multivariate analysis of 393 patients with primary or recurrent disease,31 those with multifocal lesions had significantly poorer outcomes regardless of resectability. Patients with more than seven lesions had 5-year overall survival of only 7%. This outcome was similar to that in patients undergoing incomplete gross resection.31 Both disease-free interval and size of recurrence were examined in a study of 61 patients with retroperitoneal liposarcoma undergoing resection of a first local recurrence. Patients with tumors that grew faster than 0.9 cm per month since the prior complete gross resection had median disease-specific survival of only 13 months.32 Therefore, patients with rapid recurrence and multifocal disease are generally considered for systemic therapies, and operative intervention is reserved for palliation of symptoms.
Systemic therapies for well- and dedifferentiated liposarcoma
Doxorubicin-based chemotherapy (either as a single agent or with ifosfamide) has been a standard treatment for recurrent STS for many years. In a retrospective review of 208 STS patients, the observed response rate to doxorubicin- based chemotherapy was 12%;33 liposomal doxorubicin is also a reasonable option with comparable activity in STS.34 Unfortunately, few patients with liposarcoma were included in clinical trials so histology-specific rates of response are unclear though it is considered as a first line treatment for most patients. Ifosfamide also has some single-agent activity in WD/DDLS.35 Combination therapy with doxorubicin and ifosfamide in STS in general has been shown to improve response rates and progression-free survival compared to doxorubicin alone, but not to improve survival. Thus single agent therapy in the setting of recurrent disease is likely best for most patients unless the disease burden requires an urgent response to palliate symptoms. Dacarbazine shows some activity in second or third line regimens, and the combination of gemcitabine and docetaxel is also widely used in advanced STS, although there are relatively little data on response rates in WD/DDLS.36 In a subgroup analysis of a randomized phase II trial of gemcitabine vs. gemcitabine and docetaxel showed 75% of patients with WD/DDLS had some stabilization of disease though this was only durable (≥24 weeks) in 2 of 12 patients.37
While a range of systemic options are available for patients with advanced WD/DDLS, they appear to have limited efficacy, at least compared to myxoid and pleomorphic liposarcomas. Therefore, significant effort has been made to identify targeted therapies for the disease by understanding the genetic events that drive tumorigenesis. As noted above, WD/DDLS almost universally has amplification of 12q13–15. A high-throughput shRNA screen of genes amplified on this chromosome identified over 20 potential drivers of liposarcomagenesis. One of the most commonly amplified among these was CDK4, a cell cycle regulator that promotes G1 to S phase transition by phosphorylating RB and inducing transcription of E2F targets.5 Palbociclib, an inhibitor of CDK4, was recently FDA-approved for treatment in breast cancer, and an initial phase II trial of palbociclib in WD/DDLS has been reported.38,39 In this report, examining 29 patients with unresectable WD/DDLS and disease progressing through prior treatments, progression-free survival at 12 weeks was 66% and there was one partial response; median progression-free survival was 4.7 months. While these results do not approximate those for targeted therapy in other subtypes of STS (e.g., imatinib in metastatic GIST), a small subset of patients had prolonged progression-free survival of over a year.39 Ongoing research is aimed at discovering predictive markers for response to palbociclib.40
Chromosome 12q13–15 also contains the MDM2 gene. MDM2 promotes degradation of p53 to prevent apoptosis and/or cell cycle arrest, and may have p53-independent effects on alternative tumor suppressors such as p21 and regulators of the epithelial-to-mesenchymal transition.41–45 MDM2 inhibitors that prevent its binding to p53 (e.g. nutlin) have yielded very promising results in vitro and in mouse xenografts.46 In patient trials, however, MDM2 inhibitors have been associated with significant adverse events (up to 40% of patients), which have precluded their chronic administration.47 Recent data do suggest, however, that in vitro abrogation of MDM2 may exert cytostatic effects on the cell (senescence, an irreversible form of growth arrest) without altering cellular levels of p53.40 Specific targeting of the p53-independent activities of MDM2 may have therapeutic potential and minimize toxicity, a hypothesis which warrants further study.
Some additional 12q13–15 amplicon genes, such as YEATS4, have been implicated in WD/DDLS, but research examining their oncogenic functions is limited, minimizing their impact on clinical therapeutics. Similarly, studies of dedifferentiation of WDLS have identified potential oncogenes and tumor suppressors that drive tumor progression, such as 11q23–25, 3p14–21, 3q29, and 19q13.12 While integrated copy number and gene expression data has implicated the loss of tumor suppressors such as CEBP-α in dedifferentiation, mechanisms for inducing re-expression of such proteins have been elusive. One possible mechanism is targeting the epigenetic modification that contributes to downregulation of these tumor suppressors. For example, methylation of the CEBP-α gene was identified in 10 of 42 DDLS samples (24%). Treatment of DDLS cell lines with demethylating agents induced cellular apoptosis in increased expression of CEBP-α.48 Future studies are likely to examine these findings in greater depth, as well as the findings of aberrant expression of receptor tyrosine kinases (e.g., MET and AXL) and microRNAs (e.g., mir-193b) in subsets of WD/DDLS.48,49
MYXOID LIPOSARCOMA AND ROUND CELL LIPOSARCOMA
Myxoid liposarcoma (MLS) is identified primarily in the extremity and, unlike WD/DDLS, only rarely in the retroperitoneum. MLS uniformly has a translocation involving FUS-DDIT3 or EWSR1-DDIT3 fusion. Like most other translocation-associated soft tissue sarcomas, MLS occurs in young patients (typically ages 35–55). Its histology spans a spectrum, with more small blue round cells or primitive round cells in the high-grade form (round cell liposarcoma; RCLS). Some debate exists regarding the percentage of round cells associated with poor prognosis, though our institution uses a 5% cut-point to define the high-grade round cell liposarcoma as opposed to the low-grade myxoid liposarcoma histology and generally considers diagnosis of the high-grade form a poor prognostic indicator.6
Multimodality management of primary disease
Unlike in WD/DDLS, core biopsy is required to confirm diagnosis of M/RCLS and provide some insight into tumor grade. The surgical approach to myxoid and round cell liposarcoma of the extremity is, however, almost identical to that of WD/DDLS, with function-sparing procedures the standard of care.
The local recurrence risk of a low-grade MLS is exceedingly low. Based on a nomogram for extremity sarcomas, even a tumor over 5 cm in diameter with a microscopically positive margin has a risk of local recurrence of only ~15% 3 years post-operatively.50 If margins are widely negative, this rate falls to less than 10%. This is concordant with a report that only 8% of patients with MLS experienced local recurrence at median follow-up of over 5 years.51 Given this fact, our practice has generally been to defer adjuvant radiation in patients with completely resected MLS, reserving radiation for patients with a significant round cell component. M/RCLS is most often in the thigh, and given the high morbidity associated with preoperative radiation in this region, we would tend to prescribe radiation in the adjuvant setting unless a tumor were particularly close to the joint. Both MLS and RCLS are, however, more sensitive to radiation therapy than are other histologic subtypes; mean tumor volume of MLS on average is reduced by approximately 50% after neoadjuvant radiation.52 Therefore, in rare cases where surgical resection is expected to be morbid, neoadjuvant radiation can be considered, even for a low-grade lesion, to minimize the size of the surgical bed.
Both MLS and RCLS have metastatic potential. For MLS, metastasis is estimated to occur in ~10% of patients, while in RCLS risk of developing distant disease leads to disease-specific survival of only ~60% of patients 10 years following complete resection.6 The minimal risk associated with MLS means that adjuvant chemotherapy is not generally considered. In general, randomized trials have not shown benefit to adjuvant chemotherapy. Some have argued, however, that because most of these trials are performed on a cohort with mixed histologies, the treatment may still benefit patients with select histologies, and retrospective data support its use in patients with high-risk M/RCLS. In a retrospective cohort study including 61 patients undergoing resection for M/RCLS, treatment with adjuvant or neoadjuvant ifosfamide-based chemotherapy was associated with a 22% improvement in disease specific survival.13 Up to 44% of patients with M/RCLS have objective responses to doxorubicin, suggesting that it is a uniquely chemosensitive subtype of soft tissue sarcoma.53 Our own practice has been to consider neoadjuvant doxorubicin and ifosfamide in high-risk lesions (RCLS >5 cm in largest diameter).
Management of recurrent disease
As noted previously, M/RCLS has metastatic potential regardless of grade, unlike most STSs where low-grade lesions generally show little metastatic potential. The location of metastases also differs significantly from that of other STS. While most STSs metastasize primarily to the lung, and NCCN guidelines suggest that regular imaging of the chest is sufficient to follow for evidence of distant disease, M/RCLS more commonly metastasizes to soft tissue sites such as the retroperitoneal and axillary fat pads. M/RCLS can also metastasize to spine, so complete staging of a high-risk patient requires CT of the chest, abdomen and pelvis as well as MRI of the total spine.54
The relative chemo- and radiosensitivity of M/RCLS means that, in many patients, metastases can be managed for years. Surgical resection of oligometastases is an option, and doxorubicin and ifosfamide yield at least partial responses in over 40% of patients. Radiation yields 100% pathologic treatment response in a subset of radiated lesions, making palliative RT an important means of controlling disease as well.52 An improved understanding of the biology of M/RCLS may also lead to novel therapeutics for advanced disease. Mutations in PI3 kinase and consequent Akt activation have been identified in almost 20% of M/RCLS, so PI3 kinase inhibitors have potential for select patients.5
More recently, results of clinical trials for trabectedin have been published and the FDA has approved its use for patients with metastatic liposarcoma. The drug binds to the minor groove of DNA and causes the FUS-DDIT3 chimera to be displaced from promoters.55 This promotes adipogenic differentiation in vitro. Trabectedin has been available in Europe since 2007 and was FDA-approved in the US in 2015 based on a randomized phase 3 trial. Patients with liposarcoma (all subtypes) and leiomyosarcoma were eligible and the trial showed an improvement in progression-free survival compared to treatment with dacarbazine.56 Of note, the most substantial improvement in PFS was seen in patients with M/RCLS (median PFS 5.6 months with trabectedin vs 1.5 months with dacarbazine). Indeed, a retrospective review of 51 patients with M/RCLS treated in Europe demonstrated a remarkable response rate of 51%.57 Thus, although trabectedin is now available as a treatment for all patients with liposarcoma, it is uniquely useful for the myxoid/round cell subtype.
The newest drug for liposarcoma is eribulin, a novel microtubule inhibitor, which was approved by the FDA in 2016 based on a large phase 3 study comparing eribulin to dacarbazine.58 Treatment with eribulin was associated with improved overall and progression-free survival. Outcomes for the different liposarcoma subtypes have not been reported. Thus, eribulin is a reasonable option for patients with all liposarcoma subtypes, although trabectedin is preferred for M/RCLS. Another new drug for STS, pazopanib, has been tested in multiple subtypes of STS and ultimately was approved by the FDA in 2012. However the phase 3 study excluded patients with liposarcoma because of low progression-free rates observed in the earlier phase 2 study.59 Thus, pazopanib is approved for STS but specifically not for liposarcoma, and its use in liposarcoma patients would be considered “off-label” and supported by little data.
PLEOMORPHIC LIPOSARCOMA
Pleomorphic liposarcoma is uniformly high grade and is the rarest subtype of liposarcoma, representing only ~5% of cases. Surgical management and application of radiation parallel the management of other high-grade tumors as noted above; all but the smallest tumors resected with wide margins are treated with adjuvant radiation. Outcomes for patients with pleomorphic liposarcoma are poor. Over 50% of patients will develop metastatic disease, and overall survival is relatively poor.6 However, like RCLS, pleomorphic liposarcoma responds to the doxorubicin and ifosfamide combination, and in retrospective studies neoadjuvant/adjuvant therapy has been associated with improved disease-specific survival.13 Given this fact, our practice has been to treat eligible patients and tumors >5cm in diameter with neoadjuvant chemotherapy before surgical resection.
Metastases generally occur in the lung, and serial pulmonary imaging is used to follow patients after complete resection. Doxorubicin and ifosfamide are used in the metastatic setting as in the adjuvant setting, though the tumors may also respond to gemcitabine-based therapies. As noted above, trabectedin and eribulin are also options for advanced disease. Significant work remains to be done to develop novel therapies for this disease. To date, most studies have failed to identify targetable aberrations and noted only consistent losses in p53 and Rb pathway proteins.5 These genomic alterations are notoriously difficult to exploit for therapeutic benefit.
CONCLUSIONS/SUMMARY
Liposarcoma is one of the most common types of STS, though it includes three heterogeneous groups, WD/DDLS, M/RCLS, and pleomorphic liposarcoma. Careful analyses have determined optimal surgical and adjuvant approaches to these diseases and delineated both commonalities between groups and variations in treatment response and genomic drivers. Surgery remains the gold standard to cure localized disease, but increased understanding of how each subtype of tumor responds to systemic and radiation therapies has improved our ability to manage these diseases (Figure 2). Similarly, an improved understanding of the genomic underpinnings of each disease is allowing us to rapidly identify potential novel therapies. These therapies may have the ability to further alter treatment paradigms over the coming years.
Key points.
Common genomic events define three biological groups of liposarcoma – amplification of 12q13-15 in well and dedifferentiated liposarcomas, FUS-DDIT3 translocation in myxoid/round cell liposarcomas, and complex genomic changes in pleomorphic liposarcoma.
Surgery is the gold standard for cure of liposarcoma, but grade, histology, and tumor site (retroperitoneal versus extremity) determines prognosis and pattern of recurrence.
Retroperitoneal liposarcomas are almost always well- and dedifferentiated tumors that recur locally even after complete surgical resection, so that active research focuses on optimizing surgical protocols and defining the role of radiation in multimodality therapy.
Well- and dedifferentiated liposarcoma are relatively chemoresistant; however, myxoid/round cell liposarcomas and pleomorphic liposarcomas respond well to cytotoxic therapies and myxoid/round cell liposarcoma is particularly radiosensitive.
Among targeted therapies, CDK4 inhibitors are effective in well- and dedifferentiated liposarcoma and trabectedin, which prevents FUS-DDIT3 binding to DNA, is effective in myxoid/round cell liposarcoma.
Acknowledgments
This work was supported by the Memorial Sloan Kettering Cancer Center Core Grant (P30 CA008748) and the Kristen Ann Carr Fund. The authors thank Janet Novak for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have nothing to disclose.
REFERENCES
- 1.Crago AM, Brennan MF. Principles in Management of Soft Tissue Sarcoma. Advances in surgery. 2015;49(1):107–122. doi: 10.1016/j.yasu.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mandahl N, Akerman M, Aman P, et al. Duplication of chromosome segment 12q15-24 is associated with atypical lipomatous tumors: a report of the CHAMP collaborative study group. CHromosomes And MorPhology. Int J Cancer. 1996 Sep 4;67(5):632–635. doi: 10.1002/(SICI)1097-0215(19960904)67:5<632::AID-IJC7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Pedeutour F, Forus A, Coindre JM, et al. Structure of the supernumerary ring and giant rod chromosomes in adipose tissue tumors. Genes Chromosomes Cancer. 1999 Jan;24(1):30–41. [PubMed] [Google Scholar]
- 4.Fletcher CD, Akerman M, Dal Cin P, et al. Correlation between clinicopathological features and karyotype in lipomatous tumors. A report of 178 cases from the Chromosomes and Morphology (CHAMP) Collaborative Study Group. Am J Pathol. 1996 Feb;148(2):623–630. [PMC free article] [PubMed] [Google Scholar]
- 5.Barretina J, Taylor BS, Banerji S, et al. Subtype-specific genomic alterations define new targets for soft-tissue sarcoma therapy. Nat Genet. 2010 Aug;42(8):715–721. doi: 10.1038/ng.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalal KM, Kattan MW, Antonescu CR, Brennan MF, Singer S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006 Sep;244(3):381–391. doi: 10.1097/01.sla.0000234795.98607.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knight JC, Renwick PJ, Dal Cin P, Van den Berghe H, Fletcher CD. Translocation t(12;16)(q13;p11) in myxoid liposarcoma and round cell liposarcoma: molecular and cytogenetic analysis. Cancer Res. 1995 Jan 1;55(1):24–27. [PubMed] [Google Scholar]
- 8.Crozat A, Aman P, Mandahl N, Ron D. Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma. Nature. 1993 Jun 17;363(6430):640–644. doi: 10.1038/363640a0. [DOI] [PubMed] [Google Scholar]
- 9.Rabbitts TH, Forster A, Larson R, Nathan P. Fusion of the dominant negative transcription regulator CHOP with a novel gene FUS by translocation t(12;16) in malignant liposarcoma. Nat Genet. 1993 Jun;4(2):175–180. doi: 10.1038/ng0693-175. [DOI] [PubMed] [Google Scholar]
- 10.McCormick D, Mentzel T, Beham A, Fletcher CD. Dedifferentiated liposarcoma. Clinicopathologic analysis of 32 cases suggesting a better prognostic subgroup among pleomorphic sarcomas. Am J Surg Pathol. 1994 Dec;18(12):1213–1223. doi: 10.1097/00000478-199412000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Singer S, Antonescu CR, Riedel E, Brennan MF. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg. 2003 Sep;238(3):358–370. doi: 10.1097/01.sla.0000086542.11899.38. discussion 370–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crago AM, Socci ND, Decarolis P, et al. Copy Number Losses Define Subgroups of Dedifferentiated Liposarcoma with Poor Prognosis and Genomic Instability. Clin Cancer Res. 2012 Feb 23; doi: 10.1158/1078-0432.CCR-11-2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eilber FC, Eilber FR, Eckardt J, et al. The impact of chemotherapy on the survival of patients with high-grade primary extremity liposarcoma. Ann Surg. 2004 Oct;240(4):686–695. doi: 10.1097/01.sla.0000141710.74073.0d. discussion 695–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. The lancet oncology. 2012 Oct;13(10):1045–1054. doi: 10.1016/S1470-2045(12)70346-7. [DOI] [PubMed] [Google Scholar]
- 15.Fisher SB, Baxter KJ, Staley CA, 3rd, et al. The General Surgeon's quandary: atypical lipomatous tumor vs lipoma, who needs a surgical oncologist? Journal of the American College of Surgeons. 2013 Nov;217(5):881–888. doi: 10.1016/j.jamcollsurg.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jelinek JS, Kransdorf MJ, Shmookler BM, Aboulafia AJ, Malawer MM. Liposarcoma of the extremities: MR and CT findings in the histologic subtypes. Radiology. 1993 Feb;186(2):455–459. doi: 10.1148/radiology.186.2.8421750. [DOI] [PubMed] [Google Scholar]
- 17.Kooby DA, Antonescu CR, Brennan MF, Singer S. Atypical lipomatous tumor/well-differentiated liposarcoma of the extremity and trunk wall: importance of histological subtype with treatment recommendations. Ann Surg Oncol. 2004 Jan;11(1):78–84. doi: 10.1007/BF02524350. [DOI] [PubMed] [Google Scholar]
- 18.Crago AM, Singer S. Clinical and molecular approaches to well differentiated and dedifferentiated liposarcoma. Current opinion in oncology. 2011 Jul;23(4):373–378. doi: 10.1097/CCO.0b013e32834796e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis JJ, Leung D, Woodruff JM, Brennan MF. Retroperitoneal soft-tissue sarcoma: analysis of 500 patients treated and followed at a single institution. Ann Surg. 1998 Sep;228(3):355–365. doi: 10.1097/00000658-199809000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonvalot S, Rivoire M, Castaing M, et al. Primary retroperitoneal sarcomas: a multivariate analysis of surgical factors associated with local control. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009 Jan 1;27(1):31–37. doi: 10.1200/JCO.2008.18.0802. [DOI] [PubMed] [Google Scholar]
- 21.Bonvalot S, Miceli R, Berselli M, et al. Aggressive surgery in retroperitoneal soft tissue sarcoma carried out at high-volume centers is safe and is associated with improved local control. Ann Surg Oncol. 2010 Jun;17(6):1507–1514. doi: 10.1245/s10434-010-1057-5. [DOI] [PubMed] [Google Scholar]
- 22.Gronchi A, Lo Vullo S, Fiore M, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol. 2009 Jan 1;27(1):24–30. doi: 10.1200/JCO.2008.17.8871. [DOI] [PubMed] [Google Scholar]
- 23.Pisters PW. Resection of some -- but not all -- clinically uninvolved adjacent viscera as part of surgery for retroperitoneal soft tissue sarcomas. J Clin Oncol. 2009 Jan 1;27(1):6–8. doi: 10.1200/JCO.2008.18.7138. [DOI] [PubMed] [Google Scholar]
- 24.Gronchi A, Pollock R. Surgery in retroperitoneal soft tissue sarcoma: a call for a consensus between Europe and North America. Ann Surg Oncol. 2011 Aug;18(8):2107–2110. doi: 10.1245/s10434-011-1746-8. [DOI] [PubMed] [Google Scholar]
- 25.Crago AM. Extended Surgical Resection and Histology in Retroperitoneal Sarcoma. Ann Surg Oncol. 2014 Oct 15; doi: 10.1245/s10434-014-4135-2. [DOI] [PubMed] [Google Scholar]
- 26.Gronchi A, Miceli R, Allard MA, et al. Personalizing the approach to retroperitoneal soft tissue sarcoma: histology-specific patterns of failure and postrelapse outcome after primary extended resection. Ann Surg Oncol. 2015 May;22(5):1447–1454. doi: 10.1245/s10434-014-4130-7. [DOI] [PubMed] [Google Scholar]
- 27.Ballo MT, Zagars GK, Pollock RE, et al. Retroperitoneal soft tissue sarcoma: an analysis of radiation and surgical treatment. International journal of radiation oncology, biology, physics. 2007 Jan 1;67(1):158–163. doi: 10.1016/j.ijrobp.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Pawlik TM, Pisters PW, Mikula L, et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann Surg Oncol. 2006 Apr;13(4):508–517. doi: 10.1245/ASO.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 29.EORTC. [Accessed 29 Jan, 2016];A phase III randomized study of preoperative radiotherapy plus surgery versus surgery alone for patients with Retroperitoneal sarcomas (RPS) - STRASS. http://www.eortc.be/clinicaltrials/Details.asp?Protocol=62092&T=
- 30.Tirumani SH, Tirumani H, Jagannathan JP, et al. Metastasis in dedifferentiated liposarcoma: Predictors and outcome in 148 patients. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2015 Jul;41(7):899–904. doi: 10.1016/j.ejso.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Anaya DA, Lahat G, Liu J, et al. Multifocality in retroperitoneal sarcoma: a prognostic factor critical to surgical decision-making. Ann Surg. 2009 Jan;249(1):137–142. doi: 10.1097/SLA.0b013e3181928f2f. [DOI] [PubMed] [Google Scholar]
- 32.Park JO, Qin LX, Prete FP, Antonescu C, Brennan MF, Singer S. Predicting outcome by growth rate of locally recurrent retroperitoneal liposarcoma: the one centimeter per month rule. Ann Surg. 2009 Dec;250(6):977–982. doi: 10.1097/sla.0b013e3181b2468b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Italiano A, Cioffi A, Penel N, et al. Comparison of doxorubicin and weekly paclitaxel efficacy in metastatic angiosarcomas. Cancer. 2012 Jul 1;118(13):3330–3336. doi: 10.1002/cncr.26599. [DOI] [PubMed] [Google Scholar]
- 34.Judson I, Radford JA, Harris M, et al. Randomised phase II trial of pegylated liposomal doxorubicin (DOXIL/CAELYX) versus doxorubicin in the treatment of advanced or metastatic soft tissue sarcoma: a study by the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. May. 2001;37(7):870–877. doi: 10.1016/s0959-8049(01)00050-8. [DOI] [PubMed] [Google Scholar]
- 35.Martin-Liberal J, Alam S, Constantinidou A, et al. Clinical activity and tolerability of a 14-day infusional Ifosfamide schedule in soft-tissue sarcoma. Sarcoma. 2013;2013:868973. doi: 10.1155/2013/868973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia-Del-Muro X, Lopez-Pousa A, Maurel J, et al. Randomized phase II study comparing gemcitabine plus dacarbazine versus dacarbazine alone in patients with previously treated soft tissue sarcoma: a Spanish Group for Research on Sarcomas study. J Clin Oncol. 2011 Jun 20;29(18):2528–2533. doi: 10.1200/JCO.2010.33.6107. [DOI] [PubMed] [Google Scholar]
- 37.Maki RG, Wathen JK, Patel SR, et al. Randomized phase II study of gemcitabine and docetaxel compared with gemcitabine alone in patients with metastatic soft tissue sarcomas: results of sarcoma alliance for research through collaboration study 002 [corrected] J Clin Oncol. 2007 Jul 1;25(19):2755–2763. doi: 10.1200/JCO.2006.10.4117. [DOI] [PubMed] [Google Scholar]
- 38.Beaver JA, Amiri-Kordestani L, Charlab R, et al. FDA Approval: Palbociclib for the Treatment of Postmenopausal Patients with Estrogen Receptor-Positive, HER2-Negative Metastatic Breast Cancer. Clin Cancer Res. 2015 Nov 1;21(21):4760–4766. doi: 10.1158/1078-0432.CCR-15-1185. [DOI] [PubMed] [Google Scholar]
- 39.Dickson MA, Tap WD, Keohan ML, et al. Phase II trial of the CDK4 inhibitor PD0332991 in patients with advanced CDK4-amplified well-differentiated or dedifferentiated liposarcoma. J Clin Oncol. 2013 Jun 1;31(16):2024–2028. doi: 10.1200/JCO.2012.46.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kovatcheva M, Liu DD, Dickson MA, et al. MDM2 turnover and expression of ATRX determine the choice between quiescence and senescence in response to CDK4 inhibition. Oncotarget. 2015 Apr 10;6(10):8226–8243. doi: 10.18632/oncotarget.3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997 May 15;387(6630):296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 42.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997 May 15;387(6630):299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 43.Jin Y, Lee H, Zeng SX, Dai MS, Lu H. MDM2 promotes p21waf1/cip1 proteasomal turnover independently of ubiquitylation. EMBO J. 2003 Dec 1;22(23):6365–6377. doi: 10.1093/emboj/cdg600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Z, Wang H, Li M, Rayburn ER, Agrawal S, Zhang R. Stabilization of E2F1 protein by MDM2 through the E2F1 ubiquitination pathway. Oncogene. 2005 Nov 3;24(48):7238–7247. doi: 10.1038/sj.onc.1208814. [DOI] [PubMed] [Google Scholar]
- 45.Wang SP, Wang WL, Chang YL, et al. p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug. Nature cell biology. 2009 Jun;11(6):694–704. doi: 10.1038/ncb1875. [DOI] [PubMed] [Google Scholar]
- 46.Singer S, Socci ND, Ambrosini G, et al. Gene expression profiling of liposarcoma identifies distinct biological types/subtypes and potential therapeutic targets in well-differentiated and dedifferentiated liposarcoma. Cancer Res. 2007 Jul 15;67(14):6626–6636. doi: 10.1158/0008-5472.CAN-07-0584. [DOI] [PubMed] [Google Scholar]
- 47.Ray-Coquard I, Blay JY, Italiano A, et al. Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: an exploratory proof-of-mechanism study. The lancet oncology. 2012 Nov;13(11):1133–1140. doi: 10.1016/S1470-2045(12)70474-6. [DOI] [PubMed] [Google Scholar]
- 48.Taylor BS, DeCarolis PL, Angeles CV, et al. Frequent alterations and epigenetic silencing of differentiation pathway genes in structurally rearranged liposarcomas. Cancer discovery. 2011 Dec;1(7):587–597. doi: 10.1158/2159-8290.CD-11-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng T, Zhang P, Liu J, et al. An experimental model for the study of well-differentiated and dedifferentiated liposarcoma; deregulation of targetable tyrosine kinase receptors. Lab Invest. 2010 Nov 8; doi: 10.1038/labinvest.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cahlon O, Brennan MF, Jia X, Qin LX, Singer S, Alektiar KM. A postoperative nomogram for local recurrence risk in extremity soft tissue sarcomas after limb-sparing surgery without adjuvant radiation. Ann Surg. 2012 Feb;255(2):343–347. doi: 10.1097/SLA.0b013e3182367aa7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoffman A, Ghadimi MP, Demicco EG, et al. Localized and metastatic myxoid/round cell liposarcoma: clinical and molecular observations. Cancer. 2013 May 15;119(10):1868–1877. doi: 10.1002/cncr.27847. [DOI] [PubMed] [Google Scholar]
- 52.Pitson G, Robinson P, Wilke D, et al. Radiation response: an additional unique signature of myxoid liposarcoma. International journal of radiation oncology, biology, physics. 2004 Oct 1;60(2):522–526. doi: 10.1016/j.ijrobp.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 53.Patel SR, Burgess MA, Plager C, Papadopoulos NE, Linke KA, Benjamin RS. Myxoid liposarcoma. Experience with chemotherapy. Cancer. 1994 Aug 15;74(4):1265–1269. doi: 10.1002/1097-0142(19940815)74:4<1265::aid-cncr2820740414>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 54.Schwab JH, Boland PJ, Antonescu C, Bilsky MH, Healey JH. Spinal metastases from myxoid liposarcoma warrant screening with magnetic resonance imaging. Cancer. 2007 Oct 15;110(8):1815–1822. doi: 10.1002/cncr.22992. [DOI] [PubMed] [Google Scholar]
- 55.Di Giandomenico S, Frapolli R, Bello E, et al. Mode of action of trabectedin in myxoid liposarcomas. Oncogene. 2014 Oct 30;33(44):5201–5210. doi: 10.1038/onc.2013.462. [DOI] [PubMed] [Google Scholar]
- 56.Demetri GD, von Mehren M, Jones RL, et al. Efficacy and Safety of Trabectedin or Dacarbazine for Metastatic Liposarcoma or Leiomyosarcoma After Failure of Conventional Chemotherapy: Results of a Phase III Randomized Multicenter Clinical Trial. J Clin Oncol. 2015 Sep 14; doi: 10.1200/JCO.2015.62.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grosso F, Jones RL, Demetri GD, et al. Efficacy of trabectedin (ecteinascidin-743) in advanced pretreated myxoid liposarcomas: a retrospective study. Lancet Oncol. 2007 Jul;8(7):595–602. doi: 10.1016/S1470-2045(07)70175-4. [DOI] [PubMed] [Google Scholar]
- 58.Schoffski P, et al. Randomized, open-label, multicenter, phase III study of eribulin versus dacarbazine in patients (pts) with leiomyosarcoma (LMS) and adipocytic sarcoma (ADI) J Clin Oncol. 2015. 2015;33(suppl) abstr LBA10502. [Google Scholar]
- 59.Sleijfer S, Ray-Coquard I, Papai Z, et al. Pazopanib, a multikinase angiogenesis inhibitor, in patients with relapsed or refractory advanced soft tissue sarcoma: a phase II study from the European organisation for research and treatment of cancer-soft tissue and bone sarcoma group (EORTC study 62043) J Clin Oncol. 2009 Jul 1;27(19):3126–3132. doi: 10.1200/JCO.2008.21.3223. [DOI] [PubMed] [Google Scholar]