Abstract
Background
One of the most important side effects of contrast pharmaceutical agents, which are used very common in routine radiology practice, is contrast induced nephropathy. Even ischemia, oxidative stress and osmolality related cytotoxic effects are considered, the molecular mechanisms underlying this pathology have not been identified completely yet.
Objectives
The aim of the current study was to reveal the role of oxidative stress and antioxidant enzymatic defence mechanisms in the aetiopathogenesis of contrast-induced nephropathy. We also studied possible alleviating effects of N-acetylcysteine (NAC), a potent antioxidant, to obtain extra information regarding the molecular mechanisms underlying this pathology.
Materials and Methods
This is an clinical-experimental study, This study was conducted of Istanbul/Turkey between September 15, 2012 and April 15, 2013. Three groups of male rats were randomly set up as a control group (C), a 100 mg/kg intraperitoneal NAC + 7 mL/kg contrast agent group (N + CIN) and a 7 mL/kg intraperitoneal contrast agent group (CIN). They were placed in individual metabolic cages 48 hours after agent administration to obtain 24-hour urine samples. Renal function tests (albumin, urea, creatinine, total protein) were conducted, oxidative stress parameters (Cu, Zn superoxide dismutase activity - Cu, Zn-SOD; advanced oxidation protein products - AOPP; protein carbonyls - PCO; total thiol groups - T-SH; and lipid hydroperoxides -LHP) were measured and tissues were analysed histopathologically.
Results
Compared with the control group, groups CIN and N + CIN had significantly higher urea and LHP levels (P < 0.05 and P < 0.001, respectively) and significantly lower Cu, Zn-SOD activity and creatinine clearance (P < 0.05). There was no statistically significant difference between the groups in PCO or AOPP levels despite differences in descriptive statistics.
Conclusions
Contrast-agent-induced nephropathic changes are more closely related to the magnitude of lipid peroxidation than protein oxidation.
Keywords: Contrast Media, Lipid Peroxidation, Nephropathy, Oxidative Stress, Oxidation Protein
1. Background
In daily contemporary clinical practice, some patients commonly require medications that are well known to be nephrotoxic. Among these pharmaceuticals, contrast agents may lead to significant morbidities, such as acute kidney injury, in some patients; in others, they may cause no specific complications at all. This kidney injury can occur in a broad spectrum from asymptomatic status to dialysis-needing renal impairment. The pharmacological constituents, osmolality, dosage, concentration, route and rate of application of the contrast agent all may contribute to adverse reactions (1). Acute kidney injury (AKI), which affects 13-18% of all patients admitted to hospitals, is a potentially reversible problem. Improvements in its recognition and early interventions could have a major impact on patient outcome (2). The incidence of contrast induced nephropathy (CIN) has been calculated to be > 2% in the general population (3). CIN is the third most common cause of hospital-acquired AKI (4). The aetiopathogenesis of CIN has not yet been fully understood or clearly explained by a single mechanism. In terms of CIN, high-risk patients include those individuals with co-morbid conditions, such as impaired renal function, diabetes mellitus, or congestive heart failure. Other risk factors include advanced age, female gender, dehydration and excessive use of contrast agents (1).
There are many studies confirming the protective effects of N-acetylcysteine (NAC) against CIN. It has been shown that NAC causes a significant decrease in urinary levels of 15-isoprostane F2t, a specific marker of oxidative stress (5). N-acetylcysteine is a frequently used medication that is recommended for preventing an acidic environment and the formation of free radicals in the renal tubules (6). N-acetylcysteine also increases the production of nitric oxide, which has vasodilatory properties, and the concentration of glutathione, which acts as a free radical scavenger (3). Hence, NAC is commonly used as a prophylactic for CIN, and it is even used as a treatment for it. However, recent studies have yielded conflicting and contradictory results about the prophylactic use of NAC against CIN (7). Currently, the major treatment modality that many authors agree upon for the prophylaxis of CIN is hydration (8).
Reactive oxygen species (ROS) are atoms, molecules and particles that are unstable and reactive because of having unpaired valence electrons. ROS exist as natural mediators of metabolism to maintain cellular homeostasis. They are integral components of multiple cellular pathways, functioning as anti-microbial effector molecules and as signalling molecules that regulate processes such as nuclear factor-kB (NF-kB) transcriptional activity and signal transduction (9). There is increasing evidence that ROS may act as signalling intermediates in a variety of cellular responses, such as gene transcription, activation of signalling kinases, antigen-IgE-induced mast cell degranulation and leukotriene release (10). However, ROS production may increase significantly in response to environmental stressors, resulting in extensive cellular damage (11). To reach a reasonably stable molecular state, endogenous and/or exogenous ROS react with proteins, nucleic acids, and polyunsaturated fatty acids (PUFA) in phospholipids and thus cause oxidative damage. Oxidative stress occurs when the balance between ROS production and antioxidant defence capacity is disrupted in favour of a pro-oxidant state, which may result in cellular death (12). Emerging biomarkers that may be used to assess oxidative stress have always been an alluring subject for all health care professionals. State of the art, easily applied, rapid and correct tools for measuring such stress with validated methods are beneficial and crucial to investigate its role in the pathophysiology of diseases and responses to treatment modalities, as well as to obtain survey observations and valuable information for disease prognosis. Free radical molecules have very short half-lives (on the order of a few seconds), and their in vivo measurement encounters many difficulties due to their nature. However, oxy radical derivatives (such as hydrogen peroxide, lipid hydroperoxides, peroxynitrite, and hypochlorite ion) are stable and have longer half-lives, and they may be measured and monitored repeatedly (12). Starting with this point of view, the concept of this study was to investigate the role and magnitude of oxidative stress during the development of kidney injury and possible effects of NAC administration as an anti-oxidative agent for the treatment of this pathological condition by using an experimental vertebrate animal method. The markers of oxidative stress that were analysed for this study are summarized in Table 1.
Table 1. Oxidative Stress Parameters Measured and Their Brief Descriptions.
| Evaluated Parameter | Brief Explanation |
|---|---|
| Lipid hydroperoxides | Prominent non-radical intermediates of lipid peroxidation (13) |
| Protein carbonyl groups | Early biomarkers of oxidative stress to protein, which have been observed in Alzheimer's disease, rheumatoid arthritis, diabetes, sepsis, chronic renal failure, and respiratory distress syndrome (14) |
| Cu, Zn superoxide dismutase activity | One of the key enzymatic antioxidants of the defence system, by which the free radicals that are produced during metabolic reactions are removed (15) |
| Advanced oxidation protein products | A potential diagnostic and/or prognostic indicator for diseases marking oxidative stress presence (16) |
| Total thiols | Reducing agents that are not susceptible to oxidative modification or regulation and also have important roles in the stability and solubility of proteins (17) |
| Urea | Antioxidant effect on tissues through inhibiting oxidation of divalent iron, which is protective against lipid peroxidation (18) |
| Albumin | One of the most potent extracellular antioxidant defences in blood plasma (19) |
| Creatinine | A significant antioxidant scavenger (20) |
2. Objectives
The aim of the current study was to reveal the role of oxidative stress and antioxidant enzymatic defence mechanisms in the aetiopathogenesis of contrast-induced nephropathy.
3. Materials and Methods
3.1. Location and Time of the Study
This study was conducted of Istanbul/Turkey between September 15, 2012 and April 15, 2013. All the equipments (pipettes, precision scale analyzer, dispensers etc.) were calibrated periodically and checked during the study. All the variables were chosen based on oxidative stress, routine clinical chemistry and their relation to the disease.
3.2. Experimental Animals and Treatment
In this clinical-experimental study, 23 male Wistar rats were randomly divided (simple randomization) into tree groups. One of these groups was randomly selected as the control. In this study, determing the sample size was calculated using statistical power analysis. The effect size of this study was 0.05, the value of alpha was 0.05 and power is 1-beta was calculated 0.80 and the sample size was found minimum 23 rats. Male Wistar albino rats (n: 23, weight 350 ± 50 g, 20 weeks old) were included in the study. They were obtained from Istanbul Bezm-i Alem Vakif University faculty of medicine experimental animal laboratory. The rats were housed in standard cages in an air-conditioned room at 22°C under controlled light conditions. The experimental animals were randomly divided into 3 groups: group C (control group, n = 7), group N + CIN (NAC + contrast agent group, n = 8) and group CIN (contrast agent group, n = 8). Group C underwent intraperitoneal application of only saline. Group N + CIN was administered 100 mg/kg NAC in 15 minutes. through intraperitoneal infusion followed by 7 mL/kg meglumine diatrizoate in 45 minutes. through the same way. Group CIN received 7 mL/kg meglumine diatrizoate in 45 minutes. through intraperitoneal infusion. Twenty-four hours after the infusions, the rats were placed in metabolic cages in order to collect 24-hour urine samples individually. Forty-eight hours after the onset of the experiment, the rats were anaesthetised with ketamine/xylazine (44 mg/kg and 33 mg/kg) and sacrificed. Blood samples were drawn into tubes containing a gel separator and then centrifuged at 1500 rpm for 15 minutes. Serum samples were stored at -80°C until the analytical experiments were performed.
3.3. Ethical Considerations
The experiments were performed in accordance with the internationally accepted standard ethical guidelines for laboratory animal use and care as described in the European community guidelines. The study protocols were approved by the institutional animal ethics committee of Istanbul Bezm-i Alem Vakif University of Medical Sciences in Istanbul-Turkey (reference code: 2012/259). All chemicals and reagents were supplied by Merck (Darmstadt, Germany) or Sigma-Aldrich (St Louis, MO, USA).
3.4. Preparation of Supernatant Fractions of Kidney Tissues
Samples of kidney tissue were first washed in cooled 0.9 % NaCl solution and then placed on an ice-cold plate. The samples were immediately frozen in liquid N2 until homogenization. The tissue samples (200 mg) were homogenized manually in 2 mL of homogenizing buffer (100 mM KH2PO4 - K2HPO4, pH 7.4). Finally, the homogenate was centrifuged at 5000 rpm for 10 minutes. and the supernatant fraction was collected for analyses of various parameters. All the homogenates were stored at -80°C till the analytical experiments were performed.
3.5. Measurement of Protein Carbonyls
Protein carbonyl groups (PCO) were measured spectrophotometrically by using the Reznick and Packer method (21). The principle of this method depends on the reaction of PCO groups with 2, 4-dinitrophenylhydrazine (DNPH) to yield chromophoric dinitrophenylhydrazones. DNPH was dissolved in HCl, and after the DNPH reaction, proteins were precipitated with an equal volume of 20 % (w/v) trichloroacetic acid and washed three times with 4 mL of an equal ethanol/ethyl acetate mixture (1: 1). Washing was accomplished by mechanical disruption of pellets using a small spatula and repelleting by centrifugation at 6000 g for 5 minutes. Finally, the protein precipitates were dissolved in 6 M-guanidine-HCl solution, and the absorbance values were measured at 360 nm using the molar extinction coefficient of DNPH, ε = 22,000 M−1 cm−1.
3.6. Measurement of Advanced Oxidation Protein Products
Advanced oxidation protein products (AOPPs) were analysed by using a method validated and optimized by Hanasand et al. (22). Forty μL supernatant fractions of homogenate and serum were first diluted using 160 μL of 0.2 M citric acid. Then, 10 μL of potassium iodide was added to each well of the microplate. The absorbance of each well was measured at 340 nm against a solvent blank (190 μL of 0.20 mol/L citric acid and 10 μL of potassium iodide). Sample absorbance was expressed as µmol/L (µmol/L chloramine-T equivalents) using a chloramine-T standard curve.
3.7. Measurement of Lipid Hydroperoxides
The range of lipid hydro peroxide (LHP and LOOHs) levels was detected spectrophotometrically by a method that uses oxidation of ferrous ions with xylenol orange (23). LOOHs oxidize ferrous to ferric ions selectively in diluted acid, and the amount of resultant ferric ions is determined by using a ferric-sensitive dye, which indicates the concentration of LOOHs. Fifty μL aliquots of the samples were transferred into microcentrifuge reaction vials. Ferrous xylenol orange reagent (950 μL) was added into each sample vial at the same time with vortex mixing. After incubation at room temperature for 30 minutes, all of the samples were centrifuged at 3000 g at 20°C for 10 minutes., and the supernatant was transferred into microplate wells. Finally, the absorbance was read at 560 nm against a reagent blank.
3.8. Measurement of Cu, Zn Superoxide Dismutase Activity
Cu, Zn superoxide dismutase (SOD) (EC 1.15.1.1) activity levels were measured using the method described by Sun et al. (24), which depends on the fact that when superoxide radicals, which are produced by the xanthine-xanthine oxidase system, are not removed by the enzyme Cu, Zn SOD, nitro blue tetrazolium (NBT), which is present in the reaction medium, is reduced, and the colour change of which is measured spectrophotometrically. Assay mixture aliquots of 972 μL (containing 0.3 mmol/L xanthine, 0.6 mmol/L Na2EDTA, 150 μmol/L NBT, 400 mmol/L Na2CO3, and 1 g/L albumin) and 13 μL XO (167 U/L) were added into 25 μL plasma sample tubes separately. At the end of a 20 minutes. incubation period, 250 μL of 0.8 mmol/L CuCl2 was added to each well in order to terminate the reaction. Finally, absorbance values were read at 560 nm against a reagent blank.
3.9. Measurement of Thiol Groups
Total thiol group (T-SH) values were analysed using the method described by Sedlak and Lindsay (25). The principle of the method is the production of 1 mole of 2-nitro-5-thiobenzoic acid for each mole of thiol groups as a result of the reduction of 5, 5’-dithiobis 2-nitrobenzoic acid (DTNB) (26). Sample aliquots of 125 μL were mixed with 375 μL of 0.2 M tris buffer pH 8.2, 25 μL of 0.01 M DTNB and then with 1975 μL absolute methanol. A reagent blank (without sample) and a sample blank (without DTNB) were prepared in a similar manner. After a 15 minutes. incubation period, the mixtures were centrifuged at 3000 g at room temperature for 15 minutes. The absorbance values of the supernatant fractions were read at 412 nm with a spectrophotometer against a blank.
3.10. Measurement of Serum Total Protein, Albumin, Urea, Creatinine, and Creatinine Clearance
Routine biochemical analyses were run immediately using an automated multi-item analyser (TMS-1024, Tokyo Boeki Medical System Ltd., Japan measuring protein with Biuret method, albumin with bromocresol gren method, urea with urease method, and creatinine with Jaffe method).
3.11. Histopathological Examination
Renal tissue samples were fixed in 10% paraformaldehyde in phosphate buffered saline for histological examination. They were postfixed in the same fixative overnight for preparing paraffin and frozen sections (30% sucrose in 0.1 M phosphate buffer saline for 2 days after post-fixation). Serial coronal paraffin sections (4 - 5 µm thick) were cut for haematoxylin and eosin staining.
3.12. Statistical Analysis
For the statistical analysis of the findings obtained during the study, NCSS (Number cruncher statistical system) 2007 and PASS (power analysis and sample size) 2008 Statistical Software (Utah, USA) were used. Besides descriptive statistical (mean, standard deviation, minimum, maximum) methods, The one-way ANOVA test (one-way variance analysis) was used to compare three or more groups with normal distribution, chi-squared test to identify the potential difference between two groups or more and the Turkey HSD test to determine the group responsible of the difference. Kruskal-Wallis test and Mann-Whitney U-test were used to compare three or more groups with abnormal distribution. Significance was considered to be at a level of P < 0.05.
4. Results
The average weight of rats in the control group was 332.71 ± 20.62 g, whereas it was 317.25 ± 13.74 g in the N + CIN group and 349.5 ± 32.68 g in the CIN group. There was no significant difference between the groups (P = 0.057). The average weight of kidney tissue in the control group was 2.2 ± 0.25 g, whereas it was 2.5 ± 0.41 g in the N + CIN group and 2.4 ± 0.27 g in the CIN group. There was no significant difference between these groups either (P = 0.233).
Routine serum renal functional test results are given in Table 2. There was no significant difference between the three groups in total protein or albumin levels (P = 0.083 and P = 0.094). The difference in serum urea levels between the three groups was statistically significant (P = 0.008). Serum urea levels were significantly higher in the N + CIN and CIN groups than in group C (P = 0.02). There was no significant difference between the three groups in terms of serum creatinine levels (P = 0.058). In addition, there was a significant difference in total urine output between the groups (P = 0.027). The rats in group N + CIN excreted urine higher volumes than the rats in group CIN (P = 0.015). Creatinine clearance values also differed significantly between the groups (P = 0.048) (Table 2).
Table 2. Evaluation of Routine Clinical Chemistry Parametersa.
| Group C (n = 7) | Group N + CIN (n = 8) | Group CIN (n = 8) | |
|---|---|---|---|
| Serum total protein, g/dL | 5.81 ± 0.42 | 6.36 ± 0.39 | 6.13 ± 0.46 |
| Serum albumin, g/dL | 3.47 ± 0.13 | 3.3 ± 0.15 | 3.34 ± 0.19 |
| Serum urea, mg/dL | 40.83 ± 5.34b | 55.67 ± 6.31b | 48.17 ± 6.14b |
| Serum creatinine, mg/dL | 0.66 ± 0.07 | 0.65 ± 0.08 | 0. 66 ± 0.05 |
| Total urine output, mL/24 h | 9.57 ± 1.62c | 12.38 ± 3.29c | 7.63 ± 2.83c |
| Creatinine clearance, mL/min | 1.44 ± 0.32c | 1.11 ± 0.11c | 1.00 ± 0.32c |
aValues are expressed as mean ± SD.
bKruskal Wallis test, P < 0.01.
cKruskal Wallis test, P < 0.05.
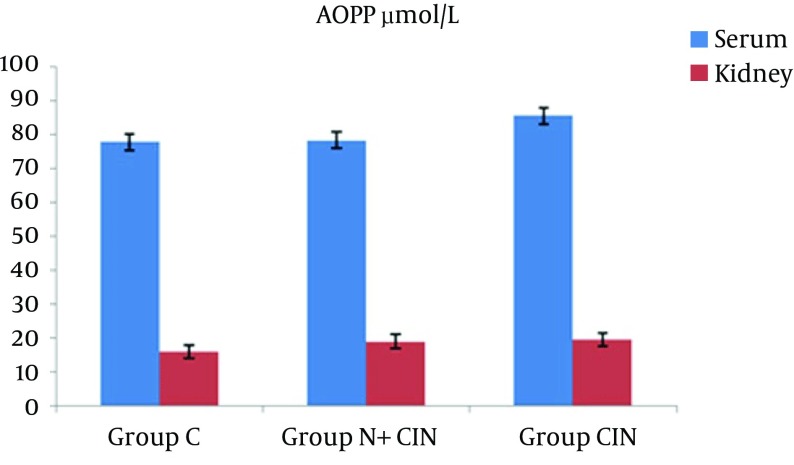
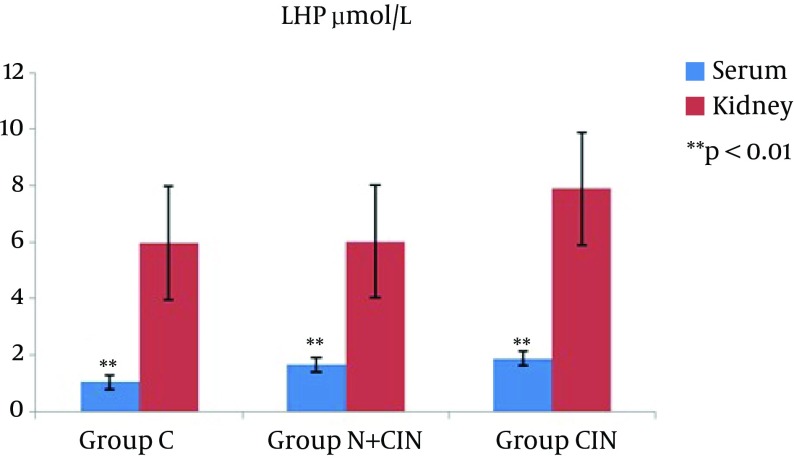
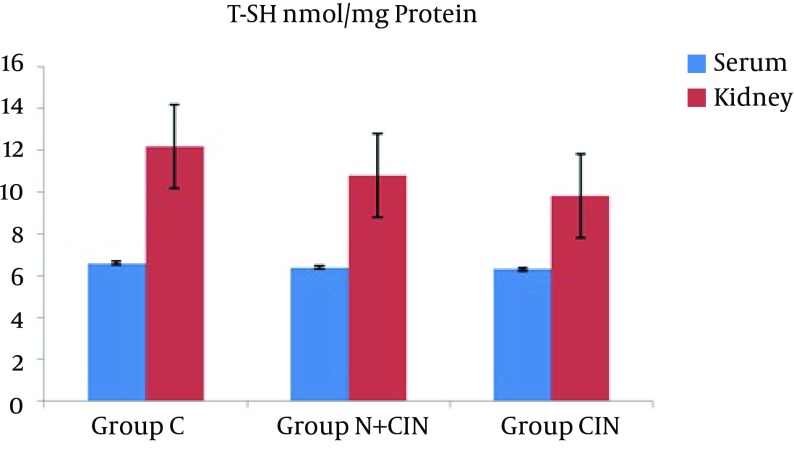
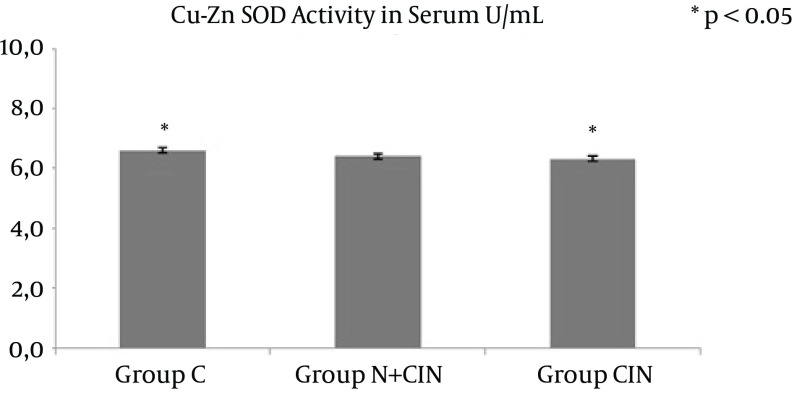
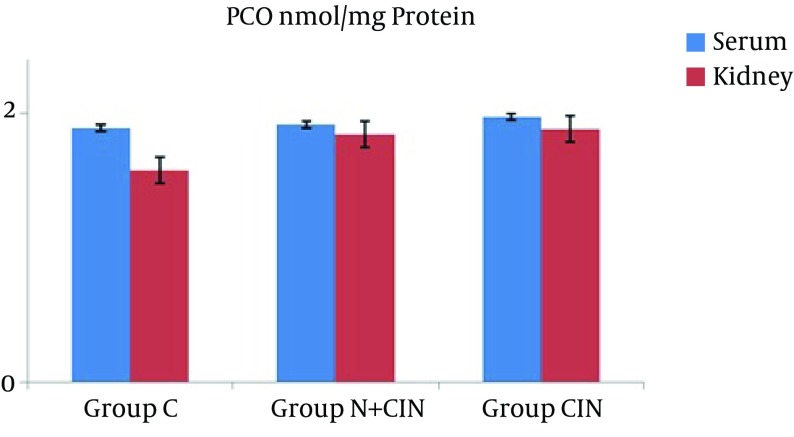
AOPP levels in serum and kidney homogenates did not differ significantly from each other (P = 0.994 and P = 0.661) (Figure 1). LHP levels in the kidney did not differ significantly between groups (P > 0.236). However, there was a significant difference between the groups in terms of systemic LHP levels (P = 0.008). Groups N + CIN and CIN each had significantly higher systemic LHP levels when compared to group C (P = 0.004, P = 0.009) (Figure 2). Neither systemic nor tissue levels of T-SH showed any statistically significant difference among the three groups (P = 0.971 and P = 0.597) (Figure 3). There was a significant difference between the groups in terms of Cu, Zn-SOD activity (P = 0.044). Levels of serum Cu, Zn-SOD activity were lower in groups N + CIN than in group C (p = 0.026) (Figure 4). Serum and tissue PCO levels were not significantly different between the groups (P = 0.884, P= 0.78) (Figure 5).
Figure 1. Advanced Oxidation Protein Product Levels in Both Serum and Tissue Homogenates.
Figure 2. Lipid Hydroperoxide Levels in Both Serum and Tissue Homogenates.
Figure 3. Total Thiol Levels in Both Serum and Tissue Homogenates.
Figure 4. Cu, Zn Superoxide Dismutase Activity Levels in Serum.
Figure 5. Protein Carbonyl Levels in Both Serum and Tissue Homogenates.
Histopathological examination revealed the effects of contrast agent on renal tissue. While the kidney tissue from group C was physiological in appearance (Figure 6), tubular dilatation, which is the gathering of contrast agent and the presence of necrotic areas around tubules, were noticed in the experimental groups (Figures 7 and 8).
Figure 6. Histopathological Examination of Kidney Tissue From Group C.
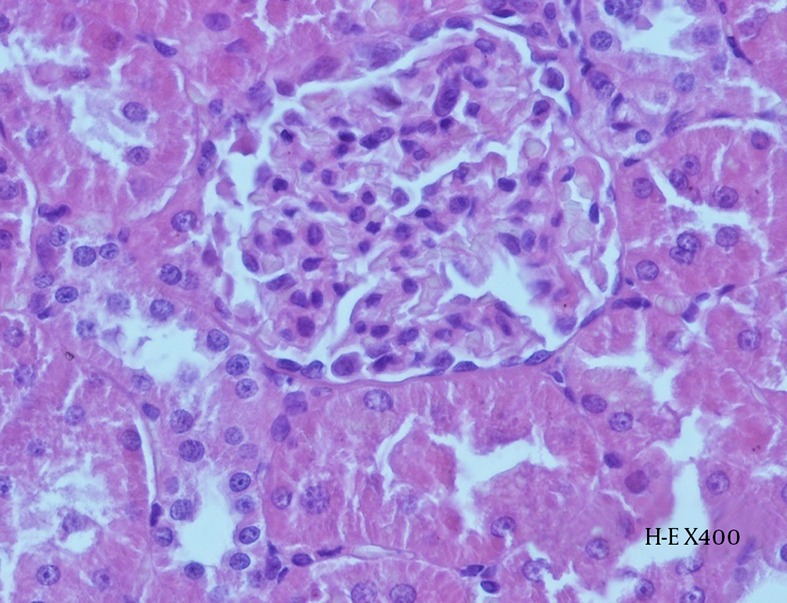
Preserved glomerular and tubular structures can be observed (H-E X 400).
Figure 7. Histopathological Examination of Kidney Tissue From Group N + CIN.
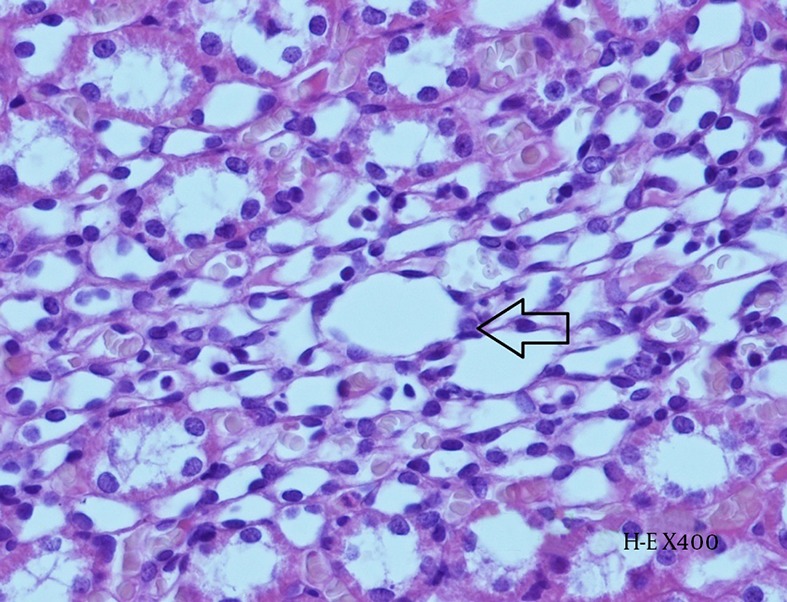
Hydropic degeneration within the tubular epithelium (arrow) and accumulation of contrast agent can be observed in the intertubular space (H-E X 400).
Figure 8. Histopathological Examination of Kidney Tissue From Group CIN.
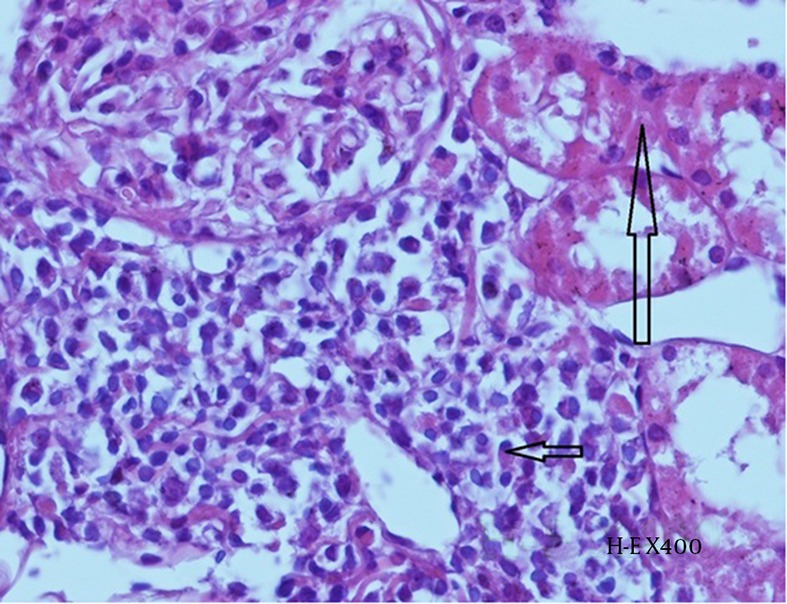
Mild tubular necrosis (long arrow) and interstitial inflammation (plasma cells- short arrow) can be observed (H-E X 400).
5. Discussion
The pathophysiology of CIN has not yet been fully elucidated. Therefore, finding a proper and robust prophylactic and/or treatment is an important clinical issue. In this current study, we aimed to investigate the possible scope and consistency of oxidative damage in experimental kidney injury caused by contrast agents in multiple ways by the use of histopathological examination, routine clinical chemistry and stable oxidative stress markers. Moreover, the effect of NAC, which is a well-known antioxidant, was assessed in the progression, prevention and treatment of CIN. Preliminary histopathological examination and results regarding LHP, Cu Zn SOD activity, urea, creatinine clearance and total urine output levels support the idea that contrast agents damage renal cells in rats through the mechanism of medullar hypoxia and cause acute kidney injury through oxidative stress, such as in many hypotheses regarding the pathophysiology of CIN (27, 28). Although previous researchers have successfully demonstrated that NAC, ascorbic acid, melatonin, caffeic acid phenethyl ester and L-carnitine have renal protective effects in addition to hydration, there is no standardized treatment modality for all age and patient groups. The aforementioned supplements may prevent contrast-induced nephropathy and its consequences by modifying renal haemodynamics and reducing direct oxidative tissue injury (29). The most widely used and well-known agent, NAC, is being recommended by many researchers in preventive clinical approaches where therapy is indicated. Despite this general perspective on NAC, there are some studies that disagree regarding its preventive and curative effects (29).
Unlike all routine clinical chemistry practice, there is no standard predictive and/or prognostic golden biomarker for contrast-induced kidney injury. As part of the biochemical aspect, the statistical significance of elevated serum urea levels, reduced urine excretion, and most importantly decreased creatinine clearance levels in the contrast agent group indicate that CIN was experimentally confirmed. In contrast, the higher total urine excretion in groups C and N + CIN compared to group CIN note the protective effect of NAC, as was previously proven (5). When renal function tests, such as serum urea and creatinine clearance values are considered, our study falls into the category of those suggesting that NAC possesses a partial positive effect. On the other hand, the lack of statistical significance in creatinine levels might be due to the lack of balanced and homogenous hydration of the animals as a result of sustained stress following the experimentation and the possible presence of idiosyncratic contrast agent hepatotoxicity together with nephrotoxicity.
For a comprehensive definition, it is extremely difficult to analyse the degree of oxidation or modification of macromolecules in a time-dependent manner at the present time. Once lipid peroxidation is initiated, a propagating chain of reactions will take place until termination products are produced. Polyunsaturated fatty acids (PUFAs) of cell membranes are major substrates for lipid peroxidation due to their methylene -CH2- groups, which are especially reactive with ROS due to their hydrogen content (30). Such ROS reactions can also lead to protein damage, including DNA repair enzyme and polymerase impairment, as well as the production of aldehyde by-products, such as malondialdehyde and 4-hydroxy-2-nonenal (31). Lipid peroxidation-derived aldehydes can easily diffuse across membranes and can covalently modify any proteins in the cytoplasm and nucleus far from their site of origin (32). On the other hand, antioxidants are substances that delay or prevent the oxidation of molecules even when they are present in low amounts compared to oxidizable substrates. The mechanisms of action of these antioxidants include catalytic removal of ROS (e.g., superoxide dismutase, superoxide reductase, catalase, peroxidase), decreasing ROS formation (e. g., mitochondrial uncoupling proteins, transferrin, haptoglobins, haemopexin, metallotionin), protecting biomolecules against oxidative damage (e.g., chaperones), physical quenching of ROS (e.g., carotenoids), and the replacement of molecules sensitive to oxidative damage with resistant molecules, which are ‘sacrificial agents’ that are oxidized by ROS to preserve more important molecules (e. g., glutathione, α-tocopherol, bilirubin, ascorbate, urate) (33, 34). It can be concluded from this study that these mechanisms preserve proteins more than lipids or that protein oxidation has a relatively minor role in the pathogenesis of nephrotoxicity (Figures 1, 3, and 4). Therefore, the novelty (and to some extent superiority) of this study is that lipid peroxidation is more prominent or persistent than protein oxidation with regard to oxidative damage to macromolecules.
T-SH groups act as a redox sensor for the regulation of renal redox status (35). It is well known that NAC is a molecule that possesses thiol (-SH) groups. Therefore, when compared to the control group, the lower decline in T-SH levels in both serum and kidney tissues in group N + CIN than in group CIN may support the significance of timely antioxidant defences and their ability to protect macromolecules. The lack of a significant difference in PCO concentrations in either sera or tissue homogenates, in addition to the similar results regarding AOPP levels, indicate that oxidative stress in CIN is not as effective as anticipated in the redox regulation of plasma and tissue proteins.
5.1. Limitations
The limitation was low sample size. Therefore cannot be generalized this results. More sample size studies recommended for further research.
- The sample size can be varied with old/young, male/female, diseased/healty working groups.
- It can be obtained more sensitivity and high specificity results with Western blot protein analysis.
- Both the contrast agent and the NAC infusion can be applied higher doses or as repeated doses.
- It can be clarified specific relationship of these parameters with contrast nephropathy, in particular lipid peroxidation and later by working basal value of their products.
5.2. Conclusions
If the limitations of this study are to be mentioned, there are plenty of biomarkers and tools for measuring the oxidative stress status of living organisms from biological materials. No single test shows all the modification processes of lipid peroxidation and protein oxidation. We were able to measure only eight oxidative stress markers. The number of animal disease model groups could have been higher or built differently regarding aspects such as animal type, dosage, and pharmaceutical applications. In future studies, we suggest that researchers use a higher dose of contrast agent, a possible period of dehydration, repeated exposure to different contrast agents and a selection of different types of animals or cell culture. We also suggest to add risk factors by application of streptozotocin or alloxan and using aged animals for better comparison to humans. Oxidative damage to DNA and assessment of 8-oxo-2’-deoxyguanosine (8-oxo-dG) should also be included during the conception and design of further studies. Finally, by establishing basal levels and determining better mechanistic relations of lipid peroxidation parameters, further larger studies will lead to more preferable biological markers from a clinical standpoint.
Acknowledgments
We are grateful to the Bezmialem Vakif University in Istanbul-Turkey.
Footnotes
Authors’Contribution:Proposal design: Mehmet Kucuk, Gungor Sitar; material preparation; Mustafa Erinc Sitar, Seval Aydin, Karolin Yanar, carrying out the experiment: Gungor Sitar, Nur Buyukpınarbasili, Ufuk Cakatay, Ozgur Yasar; data analysis: Gungor Sitar, Ozgur Yasar; manuscript preparation: Mehmet Kucuk, Gungor Sitar, Mustafa Erinc Sitar.
References
- 1.Silver SA, Shah PM, Chertow GM, Harel S, Wald R, Harel Z. Risk prediction models for contrast induced nephropathy: systematic review. BMJ. 2015;351:h4395. doi: 10.1136/bmj.h4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larmour KE, Maxwell AP. Early intervention can improve outcomes in acute kidney injury. Practitioner. 2015;259(1783):25–8. 3. [PubMed] [Google Scholar]
- 3.Golshahi J, Nasri H, Gharipour M. Contrast-induced nephropathy; A literature review. J Nephropathol. 2014;3(2):51–6. doi: 10.12860/jnp.2014.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margulis AR, Eisenberg RL. Gastrointestinal radiology from the time of Walter B. Cannon to the 21st century. Radiology. 1991;178(2):297–302. doi: 10.1148/radiology.178.2.1987582. [DOI] [PubMed] [Google Scholar]
- 5.Drager LF, Andrade L, Barros de Toledo JF, Laurindo FR, Machado Cesar LA, Seguro AC. Renal effects of N-acetylcysteine in patients at risk for contrast nephropathy: decrease in oxidant stress-mediated renal tubular injury. Nephrol Dial Transplant. 2004;19(7):1803–7. doi: 10.1093/ndt/gfh261. [DOI] [PubMed] [Google Scholar]
- 6.Heinrich MC, Kuhlmann MK, Grgic A, Heckmann M, Kramann B, Uder M. Cytotoxic effects of ionic high-osmolar, nonionic monomeric, and nonionic iso-osmolar dimeric iodinated contrast media on renal tubular cells in vitro. Radiology. 2005;235(3):843–9. doi: 10.1148/radiol.2353040726. [DOI] [PubMed] [Google Scholar]
- 7.A. C. T. Investigators Acetylcysteine for prevention of renal outcomes in patients undergoing coronary and peripheral vascular angiography: main results from the randomized Acetylcysteine for Contrast-induced nephropathy Trial (ACT). Circulation. 2011;124(11):1250–9. doi: 10.1161/CIRCULATIONAHA.111.038943. [DOI] [PubMed] [Google Scholar]
- 8.Andreucci M, Faga T, Pisani A, Sabbatini M, Russo D, Michael A. Prevention of contrast-induced nephropathy through a knowledge of its pathogenesis and risk factors. ScientificWorldJournal. 2014;2014:823169. doi: 10.1155/2014/823169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dan Dunn J, Alvarez LA, Zhang X, Soldati T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015;6:472–85. doi: 10.1016/j.redox.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ezeamuzie CI, Taslim N. Protein kinase C- and reactive oxygen species-dependent stimulation of intracellular cAMP in human eosinophils. The role of extracellular signal-regulated protein kinases. Med Princ Pract. 2008;17(6):468–74. doi: 10.1159/000151569. [DOI] [PubMed] [Google Scholar]
- 11.Zuo L, Best TM, Roberts WJ, Diaz PT, Wagner PD. Characterization of reactive oxygen species in diaphragm. Acta Physiol (Oxf). 2015;213(3):700–10. doi: 10.1111/apha.12410. [DOI] [PubMed] [Google Scholar]
- 12.Gorlach A, Dimova EY, Petry A, Martinez-Ruiz A, Hernansanz-Agustin P, Rolo AP, et al. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox Biol. 2015;6:372–85. doi: 10.1016/j.redox.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girotti AW. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J Lipid Res. 1998;39(8):1529–42. [PubMed] [Google Scholar]
- 14.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329(1-2):23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 15.Jeeva JS, Sunitha J, Ananthalakshmi R, Rajkumari S, Ramesh M, Krishnan R. Enzymatic antioxidants and its role in oral diseases. J Pharm Bioallied Sci. 2015;7(Suppl 2):S331–3. doi: 10.4103/0975-7406.163438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piwowar A. [The advanced oxidation protein products as potential diagnostic and prognostic factor in diseases of the indicated participation of oxidative stress]. Postepy Hig Med Dosw (Online). 2014;68:446–58. doi: 10.5604/17322693.1101545. [DOI] [PubMed] [Google Scholar]
- 17.Maulik VT, Jennifer SL, Teruna JS. The role of thiols and disulfides in protein chemical and physical stability. Curr Protein Pept Sci. 2009;10:614–25. doi: 10.2174/138920309789630534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Wu L, Aouffen M, Mateescu MA, Nadeau R, Wang R. Novel cardiac protective effects of urea: from shark to rat. Br J Pharmacol. 1999;128(7):1477–84. doi: 10.1038/sj.bjp.0702944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sitar ME, Aydin S, Cakatay U. Human serum albumin and its relation with oxidative stress. Clin Lab. 2013;59(9-10):945–52. [PubMed] [Google Scholar]
- 20.Lawler JM, Barnes WS, Wu G, Song W, Demaree S. Direct antioxidant properties of creatine. Biochem Biophys Res Commun. 2002;290(1):47–52. doi: 10.1006/bbrc.2001.6164. [DOI] [PubMed] [Google Scholar]
- 21.Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Methods Enzymol. 1994;233:357–63. doi: 10.1016/s0076-6879(94)33041-7. [DOI] [PubMed] [Google Scholar]
- 22.Hanasand M, Omdal R, Norheim KB, Goransson LG, Brede C, Jonsson G. Improved detection of advanced oxidation protein products in plasma. Clin Chim Acta. 2012;413(9-10):901–6. doi: 10.1016/j.cca.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 23.Wolff SP. [18] Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994;233:182–9. [Google Scholar]
- 24.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34(3):497–500. [PubMed] [Google Scholar]
- 25.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25(1):192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 26.Hu ML. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994;233:380–5. doi: 10.1016/s0076-6879(94)33044-1. [DOI] [PubMed] [Google Scholar]
- 27.Wang N, Wei RB, Li QP, Yang X, Li P, Huang MJ, et al. Renal Protective Effect of Probucol in Rats with Contrast-Induced Nephropathy and its Underlying Mechanism. Med Sci Monit. 2015;21:2886–92. doi: 10.12659/MSM.895543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunak CS, Ugan RA, Cadirci E, Karakus E, Polat B, Un H, et al. Nephroprotective potential of carnitine against glycerol and contrast-induced kidney injury in rats through modulation of oxidative stress, proinflammatory cytokines, and apoptosis. Br J Radiol. 2016;89(1058):20140724. doi: 10.1259/bjr.20140724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izcovich A, Rada G. Should acetylcysteine be used to prevent contrast induced nephropathy? Medwave. 2015;15(3):e37331. doi: 10.5867/medwave.2015.03.6122. [DOI] [PubMed] [Google Scholar]
- 30.Repetto M, Boveris A, Semprine J. Lipid peroxidation: chemical mechanism, biological implications and analytical determination. INTECH Open Access Publisher; 2012. [Google Scholar]
- 31.Aikaterini TV, Donald CM. Lipid Peroxidation. In: Schwab M, editor. Encyclopedia of Cancer. New York: Springer; 2012. pp. 2054–5. [Google Scholar]
- 32.Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olisekodiaka MJ, Igbeneghu CA, Onuegbu AJ, Oduru R, Lawal AO. Lipid, lipoproteins, total antioxidant status and organ changes in rats administered high doses of cadmium chloride. Med Princ Pract. 2012;21(2):156–9. doi: 10.1159/000333385. [DOI] [PubMed] [Google Scholar]
- 34.Rent M, Halliwell B, Gutteridge J. Antioxidant defences: endogenous and diet derived; in Free radicals in biology and medicine. New York: Oxford University Press; 2007. [Google Scholar]
- 35.De Chiara B, Sedda V, Parolini M, Campolo J, De Maria R, Caruso R, et al. Plasma total cysteine and cardiovascular risk burden: action and interaction. ScientificWorldJournal. 2012;2012:303654. doi: 10.1100/2012/303654. [DOI] [PMC free article] [PubMed] [Google Scholar]