Summary
The wild-type CFTR channel undergoes constitutive internalization and recycling at the plasma membrane. This process is initiated by the recognition of the Tyr- and di-Leu-based endocytic motifs of CFTR by the AP-2 adaptor complex, leading to the formation of clathrin-coated vesicles and the channel delivery to sorting/recycling endosomes. Accumulating evidence suggests that conformationally defective mutant CFTRs (e.g. rescued ΔF508 and glycosylation-deficient channel) are unstable at the plasma membrane and undergo augmented ubiquitination in post-Golgi compartments. Ubiquitination conceivably accounts for the metabolic instability at cell surface by provoking accelerated internalization, as well as rerouting the channel from recycling towards lysosomal degradation. We developed an in vivo fluorescence ratio imaging assay (FRIA) that in concert with genetic manipulation can be utilized to establish the post-endocytic fate and sorting determinants of mutant CFTRs.
Keywords: CF mutations, conformational defect, endosomal sorting, recycling, lysosomal targeting, ubiquitin binding protein, plasma membrane, ESCRT, vesicular pH, siRNA
1. Introduction
1.1. Endocytic membrane trafficking; an overview
Endocytosis is critical in preserving homeostasis at the cellular, organ and organism level by regulating cell surface receptor density, cell polarity and motility, providing nutrient uptake, as well as antigen presentation just to mention a few examples (1). Endocytosis entails the cellular uptake of macromolecules (e.g. hormones, nutrients and viruses) from the extracellular space as well as polypeptides and lipids from the plasma membrane (1). Trafficking pathways of incoming cargo molecules, regardless of their specific entry route, seem to converge at early endosomes (1, 2). Subsequent maturation of early endosomes coincides with progressive acidification by V-ATPases and formation of the tubular endosomal network (TEN), one of the critical structures to accomplish endocytic cargo sorting (1, 3).
The biochemical and functional subcompartmentalization of the TEN in concert with adaptor and coat proteins assembly at the cytoplasmic surface, defined by multiple interactions between sorting signal(s) and oligomeric adaptors with binding surfaces for coat proteins, sorting signals and phospholipid membrane facilitate the concentration and packaging of transmembrane cargo molecules (4). Three major destinations have been delineated from the TEN (1, 5). Membrane proteins can be recycled back to the plasma membrane either by bulk flow or signal-dependent recycling pathway (4, 6, 7). Cargo molecules could also be routed to late endosome/lysosome (e.g. Lamp1 and Lamp2) and lysosome-like organelle, relying on a variety of lysosomal sorting signals (e.g. Tyr-, di-Leu- or ubiquitin-based motifs) (8, 9). The third destination is represented by the trans-Golgi network (TGN) (e.g. TGN38 and CI-MPR), (10, 11) and mediated by retrograde transport, utilizing acidic cluster, Phe, Trp- or Pro-rich motifs (4, 12).
1.2. Endocytic sorting of CFTR
It has been recognized that constitutive, clathrin-dependent internalization of wt CFTR relies on the recognition of tyrosine- and di-leucine-based endocytic motifs by the heterotetrameric clathrin adaptor, the AP-2 complex (13, 14). This association is a prerequisite for CFTR concentration in invaginated plasma membrane patches and recruitment of the clathrin coat (clathrin coated pits, CCP) that culminates in the fission of clathrin coated vesicles (CCV) in a dynamin-mediated process. Interfering with AP-2 binding to CFTR, as well as with dynamin or Rab5 function prevented CFTR internalization (14, 15).
Considering the channel internalization (~5%/min) and limited translational rate (16-18), targeting of endocytosed CFTR back to the cell surface by the endosomal sorting machinery is a prerequisite to preserve its slow metabolic turnover in post-Golgi compartments (t1/2~14-18 h) and avoid premature degradation (2, 17, 19). Since CFTR undergoes numerous internalization and exocytic cycles, even modest inhibition of recycling could lead to intracellular retention and concomitant destabilization of the cell surface pool, as exemplified by the cellular phenotype of conformationally destabilized mutant CFTRs (2). In accord with these considerations, wt CFTR is recycled with high efficiency via Rab-11-positive recycling endosomes to the cell surface, utilizing an Rme1-dependent vesicular transport mechanism (15, 19). In contrast, partially unfolded mutant CFTRs are subjected to ubiquitination at a presently unidentified location in post-Golgi compartments and recognized by ubiquitin-dependent endosomal sorting machinery (ESCRT0-III, ref. (2)). While physical association with ΔF508 CFTR has been detected, the functional consequence of ESCRT interaction with CFTR remains poorly defined. Here we describe a method to determine not only the post-endocytic fate, but also the functional relevance of endocytic adaptors to interorganellar transport based on vesicular pH (pHv) measurements of internalized CFTR containing transport vesicles.
1.3. Determination of CFTR localization in endo-lysosomal compartment by vesicular pH measurements
Since the pHv of sorting endosome, recycling endosome, late endosome/MVB, lysosome and the trans-Golgi network (TGN) have been relatively well defined (1), the pHv of CFTR containing transport vesicles could be taken as an indicator of the channel localization. The pHv is determined by single cell fluorescence ratiometric image analysis (FRIA). The FRIA technique was originally described for lysosomal pH determination following the labeling of lysosomal compartment with the fluid-phase marker dextran, conjugated to the pH-sensitive fluorescein-isothiocyanate (FITC) (20).
Here we summarize the FRIA developed in order to determine the destination and sorting kinetics of CFTRs after synchronized internalization of the channel in complex with FITC-labeled Ab (Method 3.1). This approach is recommended to define the destination and interorganellar transport kinetics of the CFTR. If the limited plasma membrane density of the cargo precludes the sufficient labeling of endocytic compartments by synchronized internalization, continuous Ab capture is used to increase the sensitivity of the assay, as described in Method 3.2. The authors used these methods to uncover endosomal sorting pathways of multiple cargo molecules, including CFTR variants (21, 22). Determination of the fluorescence intensity ratio as a function of vesicular pH provides the in situ calibration for FRIA experiments as described in Method 3.3. The application of FRIA to determine the functional relevance of ubiquitin-binding ESCRT adaptors is described in Method 3.4. Considering that cell specific variations may occur in the steady-state pHv of organelles, pHv measurements of recycling endosomes and lysosomes are included in the Methods 3.5. Finally, a brief description of immunolocalization experiments is included to validate the results obtained by FRIA (Method 3.6.).
2. Materials
2.1-2.2. Monitoring endocytic trafficking of CFTR by fluorescence ratiometric image analysis (FRIA) of vesicular pH
2.1.1. Cell culture medium
Use the appropriate bicarbonate containing medium (e.g. DMEM, Dulbecco's Modified Eagle's Medium for HeLa cells or DMEM/F12 for BHK cells) supplemented with 10% FBS (Invitrogen) for culturing the cells in a CO2 incubator. Sodium bicarbonate- and phenol-red-free medium, containing 15 mM HEPES and 5% bovine serum (BS) is used for incubating the cells on ice. Medium should be stored at 4°C.
PBS++: Phosphate buffered saline supplemented with 1 mM CaCl2, 0.1 mM MgCl2, 0.5% BSA, bovine serum albumin. Store at 4°C.
2.1.2. CFTR Labeling
Anti-HA antibody (1:500 dilution equivalent to 10 μg/ml, MMS-101R, Covance)
FITC-conjugated goat anti-mouse secondary Fab (Jackson ImmunoResearch Laboratories)
2.1.3. Instrumentation
Tissue culture incubator at 37°C with 5% CO2
Inverted microscope equipped for fluorescence ratio imaging (Note 1.)
6-well culture plates (Falcon #353046)
Glass Coverslip 25 mm with standard #1 or #1.5 thickness
Perfusion chamber (MSC-TD, Warner Instruments Inc.)
2.3. Multi-point in situ pH calibration
10 mg/ml Nigericin (Sigma, #N-7143) in ethanol. Store at −20°C
10 mg/ml Monensin (Sigma, # M-5273) in ethanol. Store at −20°C.
K+-rich buffer for in situ pH calibration: 10 mM NaCl, 135 mM KCl, 10 mM glucose, 1 mM CaCl2, 0.1 mM MgCl2, 20 mM MES for pH ≤ 5.5, 20 mM HEPES for pH > 5.5. Adjust pH with KOH and store at 4°C.
NaKH solution: 140 mM NaCl, 5 mM KCl, 10 mM HEPES, 10 mM glucose, 1 mM CaCl2, 0.1 mM MgCl2. Adjust the pH to 7.3 and store at 4°C.
2.4. FRIA to assess the role ESCRT component in the endocytotic sorting of CFTR
HeLa cell lines stably expressing the reverse tetracyclin transactivator and the Hrs specific shRNAmir plasmid (pTRIPZ, OpenBiosystems) (see section 3.4.1.)
2.5. Measuring the pH of recycling endosomes and lysosomes
FITC-Transferrin (Tf) (Molecular Probes, Inc.)
Holotransferrin (#T-0665, Sigma)
Albumin Chicken (ovalbumin) (#A-2153, Sigma)
FITC-Dextran (M.W.: 10 kDa anionic, #D1822 Molecular Probes, Inc.)
Dextran (M.W.: 68.8 kDa, #D4876 Sigma).
2.6. Immunocolocalization of internalized CFTR with organellar markers
Paraformaldehyde 4 %: Add 1g PFA to 20 ml PBS and heat to 65°C to dissolve. Add 750 [.proportional]l μM sucrose and make up the volume to 25 ml with PBS. Filter and store the solution in the dark at −20°C up to two weeks.
TRITC-conjugated goat anti-mouse Fab (1:500 dilution, Jackson ImmunoResearch Laboratories, West Grove, PA, USA)
FITC-Tf (Molecular Probes, Inc.), FITC-Dextran (M.W.: 10 kDa anionic, Molecular Probes, Inc.)
Mounting medium (Vectashield H-1200, Vector Laboratories, Burlingame, CA)
Laser confocal fluorescence microscope (e.g. LSM 510 Carl Zeiss)
3. Methods
To monitor the post-endocytic trafficking, CFTR variants with the 3HA-tag in the 4th extracellular loop, were expressed heterologously (e.g. BHK, HeLa and CFBE (2, 22, 23)) Labeling of CFTR-3HA with primary anti-HA IgG and FITC-conjugated secondary Fab by Ab capture in vivo enabled to determine the luminal pH of endocytic vesicles harboring the CFTR channel (2, 22, 23).
3.1. Monitoring synchronized endocytic trafficking of CFTR by fluorescence ratiometric image analysis (FRIA) of vesicular pH
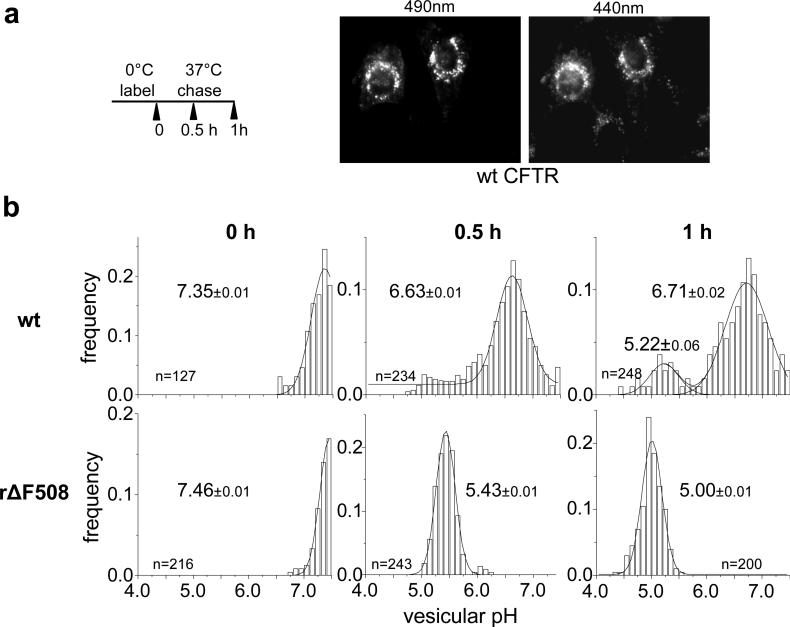
This method illustrates the distinct post-endocytic sorting of the wt and ΔF508 CFTR following synchronized internalization. First, the anti-HA primary and FITC-conjugated secondary Fab were bound to CFTR-3HA expressing BHK cells on ice. Then internalization was initiated by raising the temperature to 37°C for 0-60 min, followed by FRIA using an inverted epifluorescence microscope. Cells were illuminated alternatively with 495 nm and 440 nm fluorescent light and the emitted fluorescence was detected at 535 nm with a cooled CCD camera (Fig.1). Since FITC fluorescence at 495 nm excitation, but not at 440 nm, is sensitive to pH, normalization ensures that the fluorescence light intensity ratio (495nm/440nm) is largely pH-dependent and independent of the dye concentration (24).
Figure 1.
Monitoring the wt and rΔF508 CFTR-3HA postendocytic sorting by by vesicular pH (pHv) measurement using the FRIA. a) Anti-HA antibody and FITC-conjugated secondary Fab was bound to CFTR expressing BHK cells for 1 h at 0°C. Then the temperature was raised to 37°C for 0, 0.5 or 1 h and the pHv was measured by FRIA. Representative fluorescent micrographs of wt CFTR containing vesicles at 490 nm (pH-sensitive) and 450 nm (pH-insensitive) excitation wavelengths expressed in BHK cells after 30 min chase. b) The lysosomal delivery kinetics of rΔF508 CFTR. Data are expressed as frequency of pHv and means (± SEM) pHv of the major endosomal population. The number of vesicles analyzed in a single experiment is indicated.
3.1.1. Cell preparation
Seed BHK cells stably expressing CFTR-3HA (2, 23) on sterile glass cover slips at least 24 h before the experiment. Use 6-well tissue culture plates and 2 ml DMEM/F12 supplemented with 5% v/v FBS. Prepare non-transfected cells to verify that the non-specific fluorescent signal is negligible. By the time of the FRIA, cells should be at 60-70% confluence.
3.1.2. Labeling with the pH-sensitive probe
Rinse the cells, twice with 2 ml ice-cold medium (bicarbonate-free DMEM/F12) gently to avoid cell loss.
Bind the primary anti-HA antibody (1:400-800 dilution) in 0.8 ml bicarbonate-free DMEM/F12 containing 5% v/v bovine serum (BS) for 1 h on ice (Note 2.).
Rinse the cells with 2 ml ice-cold bicarbonate-free DMEM/F12 supplemented and 5% (v/v) BS three times.
Bind FITC-conjugated goat anti-mouse Fab (1:500 dilution) in 0.8 ml bicarbonate-free DMEM/F12 supplemented 5% v/v BS on ice.
Rinse the cells with 2 ml ice-cold DMEM/F12 no bicarbonate three times.
3.1.3. Internalization
CFTR endocytosis is induced by the addition of 2 ml pre-warmed DMEM/F12 supplemented with bicarbonate and 5% v/v FBS and by transferring the plate into the tissue culture incubator (37°C) for the desired time.
Terminate the internalization by rinsing the cells with 2 ml ice-cold PBS++ solution and placing the coverslip in 35 mm tissue culture dish on ice in 2 ml NaKH solution.
3.1.4. Acquisition of fluorescent ratio images
Mount the coverslip in the perfusion chamber and add 0.8 ml of NaKH solution (25°C). Place the chamber in the thermostated coverslip holder pre-warmed to 25°C. The microscope objective temperature should be maintained at 25°C with a dedicated heater.
Acquire image pairs at 495 and 440 nm excitation wavelengths by selecting at least 10-15 areas on the coverslip. This step should be performed in a timely manner to avoid significant cargo processing (Note 3).
3.1.5. Single-point in situ pH calibration
After completing image acquisition, single-point calibration is performed to verify that the microscope optical characteristics correspond to that observed during the multi-point calibration.
Aspirate the NaKH solution and add 0.8 ml of freshly prepared K+- rich medium containing 10 μM nigericin and 10 μM monensin (pH=6.5, 25°C).
To ensure that vesicular H+ concentration is equilibrated with the extracellular medium pH, replace the K+-rich medium (0.8 ml) supplemented with nigericin and monensin once more (Note 4).
Record the fluorescence ratio. If the fluorescence ratio is different from that observed by the multi-point calibration technique, the system has to be recalibrated as described in Method 3.3.
3.1.6. Vesicular pH analysis
Setup the MetaFluor program to obtain the average fluorescence intensity values of selected regions of interest (ROI) at 495 nm and 440 nm excitation wavelength in an Excel table. The average fluorescence intensity represents the integrated fluorescence intensity divided by the area of the ROI (Note 1.)
-
Select all of the ROIs corresponding to labeled vesicles in the first cell (Note 5.).
In the same cells select three cytoplasmic ROIs that are free of labeled vesicles. These ROIs with comparable size to that of selected in the previous step will serve to calculate the background fluorescence.
-
Acquire the mean fluorescence intensity value of ROIs for all cells repeating steps 1 and 2.
The Excel file contains the mean fluorescence intensity value for each ROI at 495 nm and 440 nm excitation wavelengths and the background signal for each cell. Calculate the mean background fluorescence intensity at 495 nm and 440 nm excitation wavelengths for each cell. Subtract the mean background fluorescence values from the mean fluorescence intensity of each ROI at 495 nm and 440 nm excitation wavelengths to obtain the specific fluorescent signal.
Calculate the mean fluorescence intensity ratio for each ROI by dividing the background subtracted mean fluorescence intensity at 495 nm and 440 nm. Based on the multi-point calibration curve (see Method. 3.3.), the fluorescence ratio values of ROIs are converted to pHv values.
Frequency distributions of pHv values are plotted by the Origin 7.0 software using single or multi-component Gaussian distribution (Fig.1b). The mean pHv is calculated for the individual peak. Partitioning of cargo molecule into various organelles can be estimated based on the fractional distribution of vesicles in the individual peak relative to the total number of vesicles.
3.2. Monitoring endocytic trafficking of CFTR by continuous antibody capture and FRIA
If the steady-state expression of the mutant CFTR (e.g. low temperature rescued ΔF508 CFTR) at the cell surface is insufficient for synchronised labeling and endocytosis (Method 3.1.), association of Ab with a larger cohort of channels could be achieved by continuous labeling in the presence of extracellular primary anti-HA and FITC-labeled secondary Fab at 37°C. This 0.5-1 h labeling usually provides sufficient signal followed by 0-2 h chase at 37°C. Although the temporal resolution of this assay is reduced as compared to that of described in Method 3.1., the lysosomal targeting of ΔF508 and the glycosylation-deficeint CFTR and the highly efficient recycling of the wt form was readily demonstrated (2, 22, 23). The assay could be used in both stably and transiently transfected cells. The detaild methodology, including cell preparation, imaging and data analysis are similar to that described in Method 3.1. except the labeling step of CFTR.
3.2.1. Labeling CFTR with pH sensitive probe
Incubate the cells on individual coverslip with anti-HA antibody and FITC-conjugated goat anti-mouse Fab for 30-60 min in 1 ml cell medium supplemented with FBS at 37°C in tissue culture incubator. The usual antibody dilution is decreased due to the loss of respective antibody affinity (1/100-500) when they are complexed simultaneously.
Thoroughly wash away the extracellular antibodies with 2 ml PBS++ at room temperature (RT) and resume the incubation of cells in 2 ml cell medium + FBS for 0-200 min at 37°C in the tissue culture incubator.
3.3. Multi-point in situ pH calibration
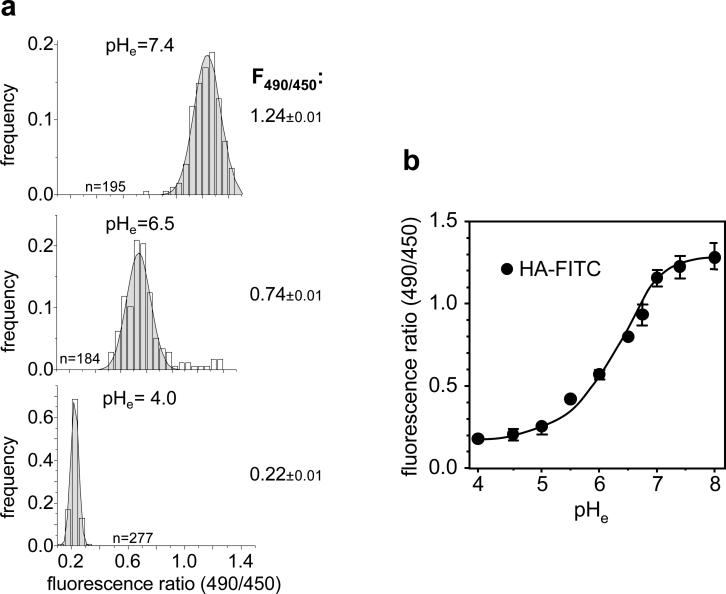
To calibrate the fluorescence ratio values as a function of pHv, FRIA was performed after clamping the luminal pH of intracellular organelles loaded with primary Ab and FITC-conjugated secondary Fab. To optimize signal to noise ratio, calibration was usually, but not exclusively, performed on cells expressing cargo molecule that was retained intracellularly (e.g. ΔF508 CFTR). If the cargo is recycled with high efficiency, plasma membrane bound Abs should be removed by acid washes (Note 6.). Following the loading of intracellular vesicles with FITC-conjugated Fab, the luminal pH of intracellular compartments was clamped to the extracellular pH (pHe) in the presence of monensin and nigericin, ionophores that mediate H+/Na+ and H+/K+ exchange (25, 26). Multi-point calibration curve was obtained by conducting FRIA at various pHe in the range of 7.4-4.5. The theoretical basis of this method has been previously described (24). For each pHe value ~200-300 vesicles are determined and the mean pHv is calculated, based on single-peak Gaussian distribution of pHv (Fig.2a-b). Considering that the pH-sensitivity of FITC is reduced at the pH < 5.4 (pKa ~ 6.4), the methodology at pHv < 5.4 can be validated using a mixture of Oregon-Green-dextran (70%) and FITC-dextran (30%) (Note 7.)
Figure 2.
In situ calibration of the FRIA. a) Distributions of fluorescence ratios of intracellular vesicles after clamping the intracellular space at the indicated extracellular pH (pHe) in the presence of monensin and nigericine. Anti-HA primary Ab and FITC-conjugated secondary Fab were complexed with CFTR and chased for 30 min before FRIA as described in Method 3.3. b) Calibration curve using CFTRwt-3HA in BHK cells. Internalization of Ab was performed as described in method 3.1. Data were obtained on indicated number of vesicles on 15 fields (means ± SEM).
3.3.1. Cell preparation
See Method 3.1.1.
3.3.2. Preparation of calibration solutions
Prepare 50 ml aliquots of K+-rich medium and adjust the pH to 4.5, 5.0, 5.5, 6.0, 6.3, 6.6, 6.9, 7.2 and 7.4. Add nigericin (10 μM) and monensin (10 μM) from 10 mM stocks before the experiment. The buffer composition of the calibration medium should be changed according to its final pH. Use 20 mM MES and 20 mM HEPES for medium with pH ≤ 5.5 and pH > 5.5, respectively. The medium pH should be adjusted with 1M KOH at 25°C and filter sterilized. Do not store buffers longer than 4 weeks at 4°C and check the medium pH prior to use at 25°C.
3.3.3. Labeling CFTR with pH-sensitive probe
Internalize anti-HA primary IgG and FITC-conjugated secondary Fab in complex with CFTR-3HA as described in Method 3.2. The internalization time could be extended to increase the fluorescence signal-to-noise ratio.
Wash the cells three times with 2 ml PBS++ at RT.
3.3.4. Acquisition of fluorescence ratio images
Place the coverslips in the perfusion chamber and add 0.8 ml K+-rich calibration medium containing 10 μM nigericin and 10 μM monensin at 25°C.
Incubate for 1-2 min and replace the medium to facilitate fast equilibration of the intracellular and extracellular pH (identical volume as step 4). Wait an additional 2-3 min and proceed with image acquisition by selecting 10-20 areas on the coverslip (Note 8.).
3.3.5. In situ calibration of fluorescence ratio values as a function of pHv
Calculation of the intracellular vesicle pH is based on the relationship between fluorescence ratio and pHv values, established by the in situ multi-point calibration technique (Fig.2).
- The fluorescence ratios of intracellular vesicles clamped to pH 4.5-7.4 are fitted by non-linear regression analysis according to a modified version of the Henderson-Hasselbalch equation, as described originally (24):
where Ri is the fluorescence ratio (I495nm/I440nm) after background subtraction, Rmax and Rmin are the asymptotes of minimum and maximum ratio values at highly acidic and alkaline pH, respectively. The apparent dissociation constant of FITC is indicated by pKa and pHi is the intracellular pH. Rearranging the equation for defining R yields:
(27) Based on measured ratio values at different pH, the Rmax and Rmin values were iterated by the Prism 4® (GraphPad software, San Diego, USA) software. The pHv of CFTR containing vesicles was calculated by Excel, using extrapolated constants and the calculated R value.
3.4. Using FRIA to assess the role of ESCRT in the endocytotic sorting of CFTR
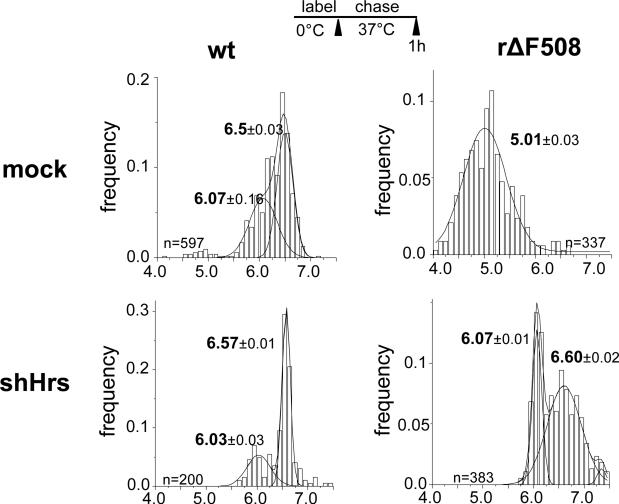
The cell surface density of plasma membrane receptors and transporters is regulated by signal-dependent ubiquitination and coupled downregulation via the endo-lysosomal associated degradation (ELAD) (21, 28). A similar paradigm was proposed for the rapid retreival of rescued (r)ΔF508 and glycosylation-deficient CFTR from the cell surface with the distinction that the destabilized channel downregulation is initiated by partial unfolding (2, 22). Association of the Ub-binding endosomal sorting complex components (e.g. Hrs, STAM and TSG101) with the rΔF508 CFTR has been documented (2). Here FRIA was used to assess whether Hrs binding has any functional role in the lysosomal delivery of the rΔF508 CFTR by monitoring the postendocytic membrane trafficking of the internalized channel in Hrs-depleted cells (Fig.3).
Figure 3.
Hrs knockdown impedes the rΔF508 CFTR delivery into lysosomes. Postendocytic targeting of internalized CFTRs was monitored by vesicular pH (pHv) measurement on HeLa cells transiently expressing the indicated CFTR variant as described in Method 3.5. Anti-HA antibody and FITC-conjugated secondary Fab was bound for 1 h at 0°C to HeLa expressing the shHrs, as well as the wt or rΔF508 CFTR. Cells were chased for 1 h at 37C prior to FRIA. Data were obtained on indicated number of vesicles on 10-15 fields (means ± SEM). The mean pHv of the subpopulations are depicted.
3.4.1. Cell preparation
To achieve Hrs down-regulation, the HeLa cells were stably transfected with pTRIPZ lentivirus expressing microRNA-adapted short hairpin RNA (shRNAMir, Open Biosystem) containing 30miR for Hrs sequence (V2THS_36954). The pTRIPZ is an inducible bicistronic vector containing the tetracycline-inducible promoter driven the shRNA and the Turbo-Red Fluorescent Protein (TurboRFP). The cells were selected in the presence of 5 μg/ml puromycin and the expression of shRNA was verified inducing the cells with 500 ng/ml doxycycline for 24 h and checking the TurboRFP fluorescence marking the inducible shRNA expression. Immunoblotting showed that doxycyclin (500 ng/ml) treatment for 5-days reduced the cellular Hrs expression level by 75% (data not shown). After 3-days of doxycycline-induction HeLa cells were transiently transfected with wt or ΔF508 CFTR-3HA using Fugene (Roche) according the instructions of the manufacturer. On the 4th day the cells were seeded on glass cover slips and incubated for additional 24-36 h at 26°C in the presence of doxycycline to rescue the ΔF508 CFTR processing defect.
3.4.2. CFTR labeling, FRIA, calibration and data analysis
These steps are performed as described in Method 3.1. Fig.3 illustrates that the lysosomal delivery of internalized rΔF508 CFTR-3HA was significanly delayed in Hrs-depldeted, but not in parental cells (Note 9.).
3.5. Measuring the pH of recycling endosomes and lysosomes
To confine the internalized CFTR localization to specific endocytic organelles based on the pHv determination, determination of the luminal pH of early recycling endosmes, lysosomes and TGN is recommended using organelle-specific markers. Here, the labeling of recycling endosomes and lysosomes for FRIA is described (21, 23, 29). Since approximately 80% of the the cellular transferrin receptor (TfR) pool is confined to recycling endosomes in steady-state (1, 23), FITC-Tf was used to monitor the pH of recycling endosomes. Selective labeling of the lysosomal compartment was achieved by the fluid-phase marker, FITC-dextran (30). Following the FITC-dextran uptake, cells were incubated in dextran-free medium to ensure that early and late endosomes were cleared of fluorophores. Colocalization of FITC-dextran loaded lysosomes with Lamp1 can validate the specificity of the dextran labeling (21, 28, 31). Following the labeling of recycling endosomes and lysosomes, FRIA, image acquisition, calibration and data analysis were performed as described in Method 3.1. and 3.3.
3.5.1 Labeling of recycling endosomes with FITC-Tf
Deplete endogenous Tf by incubating cells in 2 ml serum-free, bicarbonate containing DMEM for 45 min at 37°C in the tissue culture incubator.
Load the cells in 1 ml serum-free DMEM containing 5-15 μg/ml FITC-Tf and bicarbonate for 1 h at 37°C in the tissue culture incubator.
Transfer the cells on ice and rinse the cells 4 times with 1 mg/ml ovalbumin and 0.1 mg/ml holotransferrin containing 2 ml bicarbonate-free DMEM with 5% v/v BS.
3.5.2. Lysosomes labeling with FITC-dextran
Incubate cells with 50 μg/ml FITC-dextran overnight in 2 ml DMEM in the tissue culture incubator at 37°C. Filter-sterilize the dextran solution (0.22 μm disposable sterile filter unit) to avoid contamination of cells.
Rinse the cells three times with 2 ml PBS++.
Incubate the cells in 2 ml DMEM containing 10% FBS and 100 μg/ml dextran for 3 h in the tissue culture incubator at 37°C.
3.5.2. CFTR labeling, FRIA, calibration and data analysis
These steps can be performed as described in the Method 3.1.
3.6. Immunocolocalization of internalized CFTR with organellar markers
Subcellular distribution of internalized CFTR, determined by FRIA, should be validated by colocalization studies using organellar markers. To this end internalized CFTR is labeled with the Ab capture assay as described for FRIA experiment (Method 3.2.). Although recycling endosomes and lysosomes could be labeled with fluorophore-conjugated Tf and dextran, respectively (21, 23), these organelles could also be visualized by organelle specific antibodies e.g. TfR or rab11 (recycling endosomes), Lamp1, Lamp2 and CD63 (MVB/lysosomes) and TGN46 or giantin (TGN) that are available commercially.
3.6.1. Labeling internalized CFTR by Ab capture
Internalize the anti-HA primary and secondary Abs complexed with cargo molecule of interest as described in Method 3.2. The use of secondary TRITC- or Cy3-conjugated Fab is recommended to minimize photobleaching. If mouse primary Ab is only available for organelle identification, CFTR immunostaining should be performed using goat anti-HA Ab (#A190-107P/F, Bethyl, Montgomery, TX, USA).
The loading protocol for recycling endosomes and lysosomes with TRITC-Tf and TRITC-dextran have been described (21, 23, 29). It is possible to label two different cargoes simultaneously. For example during the 45 min TRITC-Tf (5 μg/ml) loading period, CFTR labeling was carried out with anti-HA Ab.
Incubate the cells in 2 ml Ab free medium for 30 min or as performed in the FRIA experiment (Note 10).
Wash the cells twice with 2 ml ice cold PBS++.
Fix the cells in 1 ml PBS++ containing 4% PFA for 10 minutes at RT.
Wash the cells three times (5 min each) with 2 ml PBS++ solution, once with 0.1 M glycine containing PBS++ to quench the PFA.
Mount the cells in mounting medium.
Collect single optical sections with laser confocal fluorescence microscope (21, 23).
The extent of colocalization can be analyzed quantitatively by using an appropriate software program (e.g. Volocity®, PerkinElmer or Imaris®, Bitplane).
Footnotes
The FRIA measurement could be performed on a fluorescence microscope (e.g. Zeiss Axiovert 100 TV) equipped an excitation filter wheel and high sensitivity cooled CCD camera. Our microscope had the following configuration; Planachromat objective (63X NA 1.4, Carl Zeiss MicroImaging, Inc.), excitation filters, D495/10 and D440/20; emission filter, D535/25 (these filters are included in the filter set for ratiometric analysis of BCECF by Chroma. Inc.) incorporated into the Lambda 10-2 (Sutter Instrument) filter wheel, X-Cite 120 fluorescence illumination system (EXFO) allowing the adjustment of the Xe-lamp intensity and Hamamatsu ORCA-ER CCD camera. The temperature was maintained by a thermostated coverslip holder (Bipolar temperature controller, TC-202, Med Systems Corp., Greenvale NY) and objective heater (TempControl mini, Carl Zeiss MicroImaging, Inc.). Data acquisition and analysis was performed by MetaFluor® (MDS Analytical Technologies) Imaging System and OriginPro7® (OriginLab® Corporation) software run on a PC computer.
Optimize the antibody concentration to maximize the fluorescence signal and minimize the background fluorescence. This could be achieved by using serial dilutions of the primary and secondary Abs on transfected and non-transfected cells in pilot studies in concert with quantitative fluorescence video image analysis.
It is advised that the first coverslip is used to adjust the exposure time, the camera gain and the fluorescence light intensity and/or the lamp intensity to avoid photobleaching and saturation of the CCD camera. This is particularly important when cargo molecules partition between lysosomes and recycling endosomes in the same experiment.
The equilibration time required for clamping the intracellular pH to 6.5 may vary according to the vesicles and cell analyzed. Therefore it is important to ascertain that the fluorescence ratio reached a steady-state value by repeated image acquisition during a ~5 min time period.
Use the image obtained at 495 nm excitation wavelengths for the selection since the signal to noise ratio is better at 495 nm than at 440 nm. Identify as many vesicles as possible. Usually we avoid analyzing vesicles residing in close proximity to the plasma membrane.
Stripping of cell surface Ab could be accomplished by incubating the cells in 1 ml ice-cold medium at pH 2.5 for 2 min (0.2 M acetic acid, 0.2 M NaCl, adjust to pH 2.5 with NaOH), wash with 1.5 ml ice-cold PBS++ twice and then incubate the cells for 3 min in 1 ml cold Recovery buffer (150 mM NaCl, 20 mM HEPES, 1 mM CaCl2, 5 mM KCl, 0.1 mM MgCl2, adjust to pH 7.4 with NaOH). These steps should be repeated three-four times at least.
The combination of dyes increases the pH-sensitivity at acid pH, since the pKa of Oregon-Green is ~ 4.7 (32). The Oregon-Green/FITC-dextran loading protocol provided similar lysosomal pHv values to that obtained with FITC-dextran alone, suggesting that the extrapolating procedure for pHv < 5.4 in the presence of FITC alone is fairly reliable (21).
It is critical to use identical or very similar acquisition parameters, including exposure time, light intensities and camera gain, during the calibration and the FRIA experiment. If the emitted fluorescence light intensities are either below or above the detection limit, adjust the illumination light intensity, camera gain and/or exposure time appropriately.
To monitor the non-specific effect of Hrs down-regulation, it is recommended to determine the lysosomal delivery kinetics of various lysosomal cargoes (e.g. Lamp1, Lamp2 and CD63 and the fluid-phase marker dextran). This would rule out non-specific effects as described previously (22).
Due to the rapid recycling and extracellular dissociation of the FITC-Tf from the Tf-receptor, incubation of cells at 37°C should be avoided in the absence of labelled Tf. To accomplish this goal, the chase of labelled CFTR is performed in the presence of fluorophore-conjugated Tf.
References
- 1.Mukherjee S, Ghosh R, Maxfield F. Endocytosis. Physiol. Rev. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- 2.Sharma M, Pampinella F, Nemes C, Benharouga M, So J, Du K, Bache KG, Papsin B, Zerangue N, Stenmark H, Lukacs GL. Misfolding diverts CFTR from recycling to degradation: quality control at early endosomes. J Cell Biol. 2004;164:923–33. doi: 10.1083/jcb.200312018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun-Wada GH, Wada Y, Futai M. Diverse and essential roles of mammalian vacuolar-type proton pump ATPase: toward the physiological understanding of inside acidic compartments. Biochim Biophys Acta. 2004;1658:106–14. doi: 10.1016/j.bbabio.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- 5.Katzmann DJ, Odorizzi G, Emr SD. Receptor downregulation and multivesicular-body sorting. Nat Rev Mol Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 6.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 7.von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–45. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janvier K, Bonifacino JS. Role of the endocytic machinery in the sorting of lysosome-associated membrane proteins. Mol Biol Cell. 2005;16:4231–42. doi: 10.1091/mbc.E05-03-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marks MS, Woodruff L, Ohno H, Bonifacino JS. Protein targeting by tyrosine- and di-leucine-based signals: evidence for distinct saturable components. J Cell Biol. 1996;135:341–54. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh P, Dahms NM, Kornfeld S. Mannose 6-phosphate receptors: new twists in the tale. Nat Rev Mol Cell Biol. 2003;4:202–12. doi: 10.1038/nrm1050. [DOI] [PubMed] [Google Scholar]
- 11.Humphrey JS, Peters PJ, Yuan LC, Bonifacino JS. Localization of TGN38 to the trans-Golgi network: involvement of a cytoplasmic tyrosine-containing sequence. J Cell Biol. 1993;120:1123–35. doi: 10.1083/jcb.120.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonifacino JS, Rojas R. Retrograde transport from endosomes to the trans-Golgi network. Nat Rev Mol Cell Biol. 2006;7:568–79. doi: 10.1038/nrm1985. [DOI] [PubMed] [Google Scholar]
- 13.Lukacs GL, Segal G, Kartner N, Grinstein S, Zhang F. Constitutive internalization of cystic fibrosis transmembrane conductance regulator occurs via clathrin- dependent endocytosis and is regulated by protein phosphorylation. Biochem J. 1997;328(Pt 2):353–61. doi: 10.1042/bj3280353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weixel KM, Bradbury NA. Mu 2 binding directs the cystic fibrosis transmembrane conductance regulator to the clathrin-mediated endocytic pathway. J Biol Chem. 2001;276:46251–9. doi: 10.1074/jbc.M104545200. [DOI] [PubMed] [Google Scholar]
- 15.Gentzsch M, Chang XB, Cui L, Wu Y, Ozols VV, Choudhury A, Pagano RE, Riordan JR. Endocytic trafficking routes of wild type and DeltaF508 cystic fibrosis transmembrane conductance regulator. Mol Biol Cell. 2004;15:2684–96. doi: 10.1091/mbc.E04-03-0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prince LS, Peter K, Hatton SR, Zaliauskiene L, Cotlin LF, Clancy JP, Marchase RB, Collawn JF. Efficient endocytosis of the cystic fibrosis transmembrane conductance regulator requires a tyrosine-based signal. J Biol Chem. 1999;274:3602–9. doi: 10.1074/jbc.274.6.3602. [DOI] [PubMed] [Google Scholar]
- 17.Swiatecka-Urban A, Duhaime M, Coutermarsh B, Karlson KH, Collawn J, Milewski M, Cutting GR, Guggino WB, Langford G, Stanton BA. PDZ domain interaction controls the endocytic recycling of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277:40099–105. doi: 10.1074/jbc.M206964200. [DOI] [PubMed] [Google Scholar]
- 18.Swiatecka-Urban A, Boyd C, Coutermarsh B, Karlson KH, Barnaby R, Aschenbrenner L, Langford GM, Hasson T, Stanton BA. Myosin VI regulates endocytosis of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2004;279:38025–31. doi: 10.1074/jbc.M403141200. [DOI] [PubMed] [Google Scholar]
- 19.Picciano JA, Ameen N, Grant BD, Bradbury NA. Rme-1 regulates the recycling of the cystic fibrosis transmembrane conductance regulator. Am J Physiol Cell Physiol. 2003;285:C1009–18. doi: 10.1152/ajpcell.00140.2003. [DOI] [PubMed] [Google Scholar]
- 20.Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978;75:3327–31. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barriere H, Nemes C, Du K, Lukacs GL. Plasticity of Poly-Ubiquitin Recognition as Lysosomal Targeting Signals by the Endosomal Sorting Machinery. Mol Biol Cell. 2007 doi: 10.1091/mbc.E07-07-0678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glozman R, Okiyoneda T, Mulvihill CM, Rini JM, Barriere H, Lukacs GL. N-glycans are direct determinants of CFTR folding and stability in secretory and endocytic membrane traffic. J Cell Biol. 2009;184:847–62. doi: 10.1083/jcb.200808124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barriere H, Bagdany M, Bossard F, Okiyoneda T, Wojewodka G, Gruenert D, Radzioch D, Lukacs GL. Revisiting the role of cystic fibrosis transmembrane conductance regulator and counterion permeability in the pH regulation of endocytic organelles. Mol Biol Cell. 2009;20:3125–41. doi: 10.1091/mbc.E09-01-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geisow MJ. Fluorescein conjugates as indicators of subcellular pH. A critical evaluation. Exp Cell Res. 1984;150:29–35. doi: 10.1016/0014-4827(84)90698-0. [DOI] [PubMed] [Google Scholar]
- 25.Harold FM, Baarda JR. Effects of nigericin and monactin on cation permeability of Streptococcus faecalis and metabolic capacities of potassium-depleted cells. J Bacteriol. 1968;95:816–23. doi: 10.1128/jb.95.3.816-823.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tartakoff A, Vassalli P, Detraz M. Comparative studies of intracellular transport of secretory proteins. J Cell Biol. 1978;79:694–707. doi: 10.1083/jcb.79.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erdahl WL, Chapman CJ, Taylor RW, Pfeiffer DR. Effects of pH conditions on Ca2+ transport catalyzed by ionophores A23187, 4-BrA23187, and ionomycin suggest problems with common applications of these compounds in biological systems. Biophys J. 1995;69:2350–63. doi: 10.1016/S0006-3495(95)80104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar KG, Barriere H, Carbone CJ, Liu J, Swaminathan G, Xu P, Li Y, Baker DP, Peng J, Lukacs GL, Fuchs SY. Site-specific ubiquitination exposes a linear motif to promote interferon-alpha receptor endocytosis. J Cell Biol. 2007;179:935–50. doi: 10.1083/jcb.200706034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barriere H, Lukacs GL. Analysis of endocytic trafficking by single-cell fluorescence ratio imaging. Curr Protoc Cell Biol. 2008 doi: 10.1002/0471143030.cb1513s40. Chapter 15, Unit 15 13. [DOI] [PubMed] [Google Scholar]
- 30.Lencer WI, Weyer P, Verkman AS, Ausiello DA, Brown D. FITC-dextran as a probe for endosome function and localization in kidney. Am J Physiol. 1990;258:C309–17. doi: 10.1152/ajpcell.1990.258.2.C309. [DOI] [PubMed] [Google Scholar]
- 31.Falcon-Perez JM, Nazarian R, Sabatti C, Dell'Angelica EC. Distribution and dynamics of Lamp1-containing endocytic organelles in fibroblasts deficient in BLOC-3. J Cell Sci. 2005;118:5243–55. doi: 10.1242/jcs.02633. [DOI] [PubMed] [Google Scholar]
- 32.Delmotte C, Delmas A. Synthesis and fluorescence properties of Oregon Green 514 labeled peptides. Bioorg Med Chem Lett. 1999;9:2989–94. doi: 10.1016/s0960-894x(99)00512-0. [DOI] [PubMed] [Google Scholar]