Abstract
A preclinical prototype of a transcutaneous thermal therapy system has been developed for the targeted treatment of breast cancer cells using focused microwaves as an adjuvant to radiation, chemotherapy, and high intensity focused ultrasound (HIFU). The prototype system employs a 2D array of tapered microstrip patch antennas operating at 915 MHz to focus continuous-wave microwave energy transcutaneously into the pendent breast suspended in a coupling medium. Prior imaging studies are used to ascertain the material properties of the breast tissue, and this data is incorporated into a multiphysics model. Time-reversal techniques are employed to find a solution (relative amplitudes and phase) for focusing at a given location. Modeling tests of this time-reversal focusing method have been performed which demonstrate good targeting accuracy within heterogeneous breast tissue. Experimental results using the laboratory prototype to perform focused heating in tissue-mimicking gelatin phantoms have demonstrated 1.5 cm diameter focal spot sizes and differential heating at the desired focus sufficient to achieve an antitumor effect confined to the target region.
Index Terms: Microwave therapy, hyperthermia, microstrip patch antennas, microwave imaging, time-reversal
I. Introduction
Thermal therapies–particularly the use of thermal ablation (including microwave, RF, cryoablation, and high intensity focused ultrasound [1]), hyperthermia, and heat activated drug delivery in the treatment of cancer–have been the focus of increasing laboratory and clinical research. In the various ablation methods, either significantly elevated temperatures (typically above 50 °C) generated by electromagnetic waves or ultrasound, or freezing temperatures generated by liquid nitrogen are used to cause very rapid and localized tissue destruction. In the case of hyperthermia, moderately elevated temperatures (typically between 40–45°C) have been shown to achieve cytotoxic effects that render cancer cells more vulnerable to radiotherapy [2] and chemotherapy [3], as well as inducing both apoptotic and necrotic cell death given a sufficient thermal dose [4]–[6].
Motivated by this knowledge, researchers have developed microwave systems capable of inducing localized hyperthermia as an adjuvant cancer treatment modality. Laboratory and clinical studies of a system using a 2-element adaptive phased array and an invasive electric-field probe to focus within a compressed breast have been reported by Dooley et al. [7] and Vargas et al. [8]. In addition, Stauffer et al. [9], [10] and Dewhirst et al. [11] have demonstrated MR-guided phased arrays for the treatment of locally advanced breast cancer, chest wall recurrence, and osteosarcomas. Several groups have also begun clinical testing of temperature-triggered drug delivery using Low Temperature-Sensitive Liposomes activated with hyperthermia [12], [13] and RF ablation [14]. A summary of the methods and recent progress in heat activated drug delivery can be found in [15].
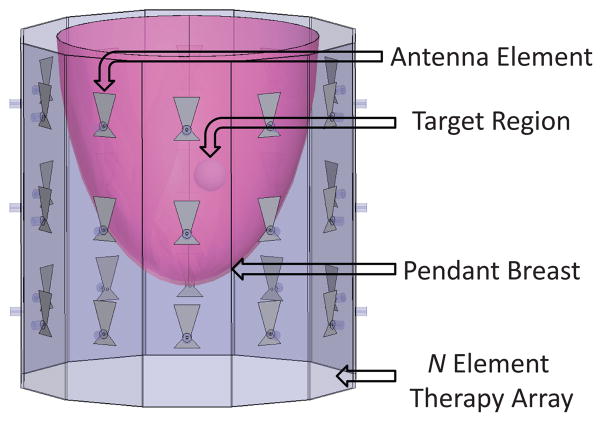
With these potential applications in mind, a preclinical prototype of a noninvasive thermal therapy system has been developed for the targeted treatment of breast cancer cells using focused microwaves. In this system, an array of antennas operating at 915 MHz is used to focus continuous-wave microwave energy transcutaneously into the pendent breast suspended in a coupling medium (shown in Figure 1). Prior imaging studies are used to ascertain the material properties of the breast tissue, and this data is incorporated into a multiphysics model. Time-reversal techniques (described in section II-A) are then employed to find a solution (relative amplitudes and phase) for focusing at a given location, resulting in maximal thermal dose at the tumor location. Using this system, focal spot sizes in the array plane of approximately 1.5 cm in diameter have been achieved (discussed in section III-B), and significant differential heating in the target region has been observed in focused heating tests within tissue-mimicking gelatin phantoms (details in section III-C).
Fig. 1.
Focused Microwave Therapy of the Breast: General Approach
Based on these results, this system has the potential to offer improved targeting and delivery of focused heating over current microwave thermal therapy systems, without the need for invasive probes. Upon completion of an optimized clinical version, this system will have the potential to be used in neoadjuvant thermal treatment for presurgical tumor reduction, minimally invasive tumor ablation, locally enhanced drug delivery, and in postoperative adjuvant therapy to reduce local recurrence.
II. Methods
Development of the preclinical microwave thermal therapy prototype system was completed in a series of stages. First, an initial 2D antenna array was designed, and a full wave forward model of the microwave therapy array was created. Following that, modeling tests of the time-reversal focusing method were performed to ensure good targeting accuracy within heterogeneous breast tissue. Next, the focusing ability of the system was characterized by measuring the microwave power distribution within the therapy chamber using a coaxial probe. Finally, tissue-mimicking gelatin phantoms were developed and experiments were performed to test differential heating capability at the desired focus, along with the system’s ability to achieve a tumoricidal thermal dose confined to the target region.
A. Image Based Time-Reversal Focusing
An early study by Surowiec et al. [16] proposed a three element focused hyperthermia system that identified the need to account for the various effects of source and array geometry on focusing. Since that study, researchers have developed various methods of computing the relative magnitude and phase parameters necessary to focus microwave energy at the target. In the clinical system reported by Fenn, Dooley, Vargas, et al., an invasive electric-field probe placed within the compressed breast is used to focus a 2-element adaptive phased array [17], [18]. Recent theoretical studies have also reported less invasive methods including the use of a deformable mirror method [19] and transmit beamforming methods [20]. Finally, there are time-reversal techniques which are rooted in theory previously developed for ultrasound focusing [21], such as those discussed in Guo et al. [22] and Kosmas et al. [23] which.
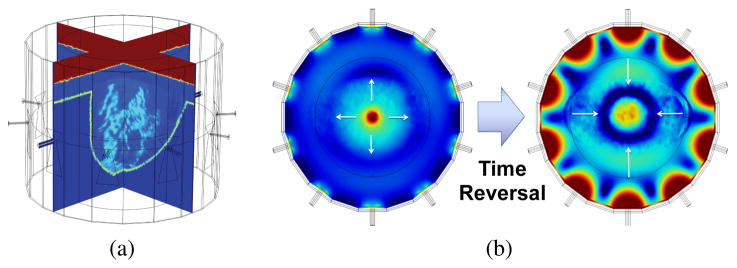
In the patient-specific variant of the time-reversal method employed here, the predicted microwave tissue properties from a prior imaging study (e.g. ultrasound, MRI, CT, or possibly microwave imaging) are incorporated into a full wave forward model of the microwave therapy array (Figure 2a). A synthetic dipole point source polarized along the axis of the cylindrical cavity) is then excited at the target location within the breast (as shown in the field distribution on the left in Figure 2b). The relative phase of the electric field at each antenna feed location after traveling from the point source excitation through the intervening breast tissue and coupling fluid is computed and stored. The complex phase information is then conjugated (time-reversed) to produce the phase delays needed to electronically steer the focus of the microwave antenna array back to the inclusion location (as shown in the field distribution on the right in Figure 2b). A table of phase delays needed to focus within the therapy chamber can then be generated by repeating this procedure for the set of desired focal locations. In the experiments that follow, this was done on a 1.5 cm uniform grid of 49 possible focal states.
Fig. 2.
(a) Sample Imported MR Image of Fatty Breast (b) Patient Specific Time-Reversal Focusing Using Imported MR Image. Left: Outgoing field distribution due to synthetic dipole placed at the center of the array. Right: Incoming field distribution focused using time-reversed phase data.
B. System Design
As shown in Figure 3, the prototype microwave therapy system consists of a signal generator, a 12-channel power divider and electronically controlled phase shifter network, 12 microwave amplifiers providing 10 watts of power per channel, and a 15 cm diameter cylindrical cavity containing a 12 element antenna array. The signal generator provides a low-power 915 MHz continuous microwave source, which is first equally divided to each channel using the Minicircuits power divider (ZN12PD-252-S+). Each channel is then simultaneously phase-delayed using a series of two electronically controlled phase shifters (Minicircuits JSPH-1000) by an amount determined with the time-reversal focusing method discussed previously. Each of the appropriately delayed signals are then amplified with a 10 Watt Minicircuits broadband amplifier (ZHL-10W-2G+) and transmitted into the therapy chamber using a microstrip patch antenna designed to operate with maximum efficiency at 915 MHz. The loss through the power divider/phase shifter network was measured to be 14.5 dB and is compensated for using the signal generator. The power at the output of each amplifier was measured to be 40.7 dBm (11.75 W) using a spectrum analyzer.
Fig. 3.
2D Microwave Thermal Therapy System Prototype (a) Signal Generator (b) 12 Channel Electronic Phase Control (c) 10 Watt per Channel Amplifiers (120 W Total) (d) 12 Element Therapy Array
1) Microstrip Antenna Array
In microwave thermal therapy, as in near-field microwave imaging, antennas must be designed to radiate either directly into tissue, or into a matching fluid medium designed to mimic the dielectric properties of the tissue. Also, mutual impedance effects can negatively impact antenna performance in a variety of ways (shift resonant frequency, alter radiation pattern, reduce matching performance). Furthermore, these effects can influence each antenna in an array differently, depending on the geometry of the antenna and the array. Since it is not feasible to account for all of these effects analytically, the general strategy was to use an iterative combination of analytical methods and computer simulation in order to design the proof of concept array. First, a standard model of a rectangular patch antenna was used to make an initial design intended to resonate at 915 MHz (ISM Band). Then, the geometry was modified with a linear taper to account for the effect of the dielectric loading of the biological tissue on the input impedance. This taper was subsequently optimized through an iterative process of simulations guided by the analytical model. Finally, the array geometry and antenna spacing were optimized using a similar iterative process of computer simulation. Further details can be found in [24], [25].
2) Emulsion Matching Medium and Gelatin Phantoms
A surfactant-stabilized two-phase oil-in-water emulsion is used as a matching medium designed for optimal coupling of microwave power between the array and the breast. Oil-in-water emulsions were chosen due to their straightforward fabrication from readily available, inexpensive materials, their relatively low attenuation, low viscosity for ease of circulation, and the ability to adjust their dielectric properties to match the tissue of interest simply by varying the ratio of oil and water. The emulsion coupling medium used in the current therapy system is composed of 60% vegetable oil, 36% DI water, and 4% HLB10 surfactant (46% SPAN 80 and 54% TWEEN 80) and was emulsified using an ultrasonic sonicator. The dielectric propeties of the emulsion coupling medium were measured using the Agilent 85070E open-ended coaxial dielectric probe kit to be εr = 22.9 and σ = 0.07 at 915 MHz. In order to dissipate the heat generated by the absorption of the significant electromagnetic energy present in the immediate near field of the antenna elements, the emulsion matching medium was circulated and cooled using an ice water bath. Both heterogeneous (Figure 4a) and homogeneous (Figure 4b) solid phantoms were created with a range of dielectric properties by adding unflavored gelatin to emulsions with varying ratios of oil/water that were heated to slightly below boiling. Further details of the emulsion coupling fluid and tissue mimicking phantoms can be found in [26].
Fig. 4.
(a) Initial Temperature Measurements With Digital Probe Thermometers in 50/50 Oil-in-Water Gelatin Phantom with 30/70 Oil-in-Water Cylindrical Inclusion. (b) Thermistor Array Above Homogeneous 50/50 Oil-in-Water Gelatin Phantom in 60/40 Matching Fluid.
C. System Characterization
1) Characterization of Microwave Power Delivery
In order to evaluate the focusing ability of the system, the microwave power distribution within the therapy chamber filled with homogeneous coupling fluid was measured using a coaxial probe (shown in Figure 5). The probe was manually positioned on a 2D grid with 5 mm spacing, and the field for 49 electronically scanned focal states was measured at each position. From this data microwave power distribution maps were created for each focal state, and the results are discussed in section III-B.
Fig. 5.
Coaxial Probe for Mapping Microwave Power Distribution in the Plane of the Therapy Array (shown in photo without coupling fluid for clarity).
2) Characterization of Thermal Distribution
A series of focused heating experiments were conducted on a number of tissue-mimicking gelatin phantoms. To initially confirm that focused heating was occurring at the desired location, temperature measurements were taken after a series of heating times using simple digital probe thermometers at several sample locations within a gelatin phantom and surrounding emulsion matching medium (shown in Figure 4a). Following these simple temperature probe experiments, a linear array of thermistors (as shown in Figure 4b) was used to map the temperature distribution throughout the therapy array for a number of gelatin phantoms, focal locations, and heating times. This was accomplished by first orienting the 7 element thermistor array along one of the principal axes of the cavity. The array was continually lowered by hand to 20 points in depth at 0.5 cm increments, where the temperature measured by each thermistor was recorded. This was done for 7 different positions across the cavity, spaced at 1.5 cm increments, in order to create a 7 × 7 × 20 array of temperature samples which span the volume of the gelatin phantoms as well as a portion of the surrounding coupling medium.
While temperature probes are used here, in the clinical setting an integrated, noninvasive method of real time thermal monitoring will be needed. One possible method would involve using a ring array of transducer elements integrated into the current microwave array to perform ultrasonic transmission tomography. Using this method, the opposite sign of the thermal dependence of speed of sound in fat and water bearing tissues can be accounted for given the substantially different speeds of sound of these two tissue classes [27], [28]. Imaging temperature with smaller aperture ultrasound is also possible, e.g. [29]–[31], as is microwave radiometry [32]. While real time absolute temperature measurement is desirable, ultrasonic or other imaging of a final endpoint, such as elasticity changes from thermal necrosis may be sufficient as well.
III. Results and Discussion
A. Antenna Performance
The tapered microstrip antennas used in the therapy array were designed using Ansoft HFSS to have optimal efficiency when radiating at 915 MHz into a coupling medium with dielectric properties of εr = 23 and σ = 0.06. A comparison of the HFSS predicted and measured reflection coefficients for each of the 12 therapy antennas is shown in Figure 6, demonstrating that all of the antennas are well matched (reflection coefficient less than −15 dB) at 915 MHz radiating into the coupling medium.
Fig. 6.
HFSS Predicted vs. Measured Antenna Reflection Coefficients
B. Array Focusing
1) Synthetic Focusing Tests in Anatomically Realistic Numerical Breast Phantoms
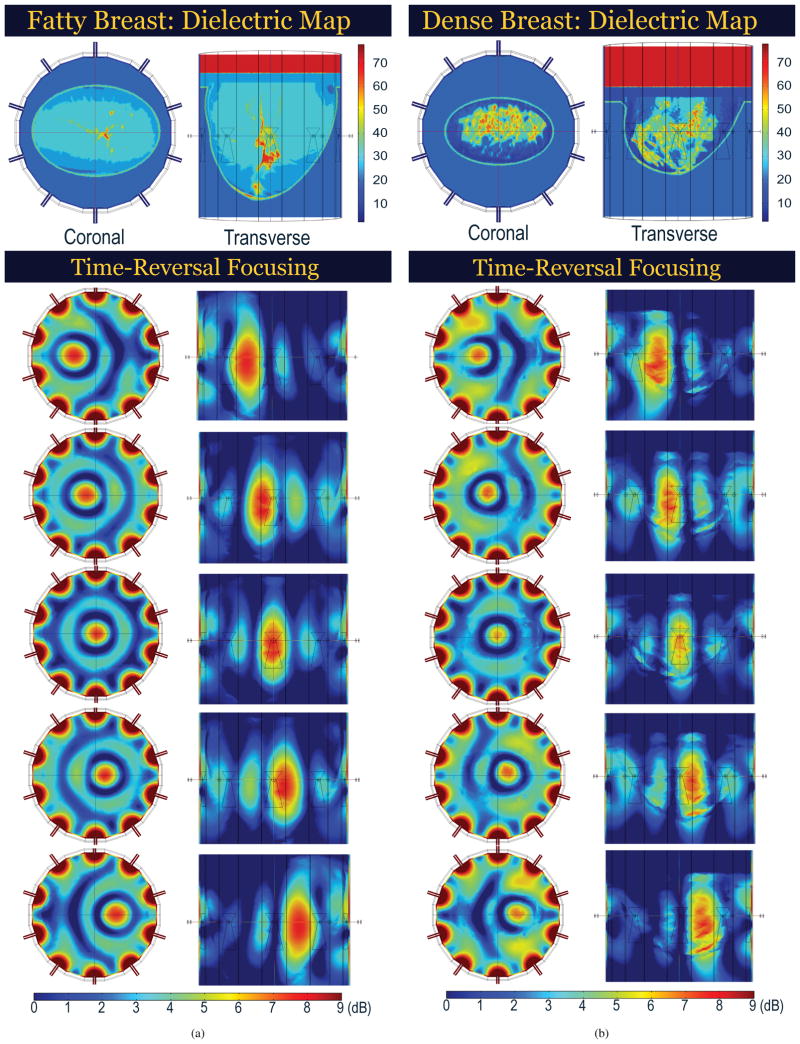
The focusing capabilities of the time-reversal method were first tested synthetically using a 10 element array to focus within a variety of anatomically realistic numerical breast phantoms. These modeling tests were performed by importing MRI-based numerical breast phantoms [33] that spanned the range of BIRADS breast types [34] into COMSOL, which was then used to simulate the interaction of the time-reversal-focused electromagnetic waves with the reported [35], [36] electrical properties (Table I) of the various breast tissue types. These results (shown in Figure 7) demonstrate the ability of the system to achieve focusing within a 1.5 cm target region–for breasts of widely differing density and heterogeneity–without prohibitive microwave power delivery to other regions of the breast.
TABLE I.
Dielectric Values Used in Numerical Breast Phantoms
| Material | εr | σ (S/m) |
|---|---|---|
|
| ||
| Glandular Tissue (1.1, 1.2, 1.3) | 55, 45, 35 | 0.6, 0.5, 0.4 |
| Transitional Tissue (2) | 25 | 0.3 |
| Adipose Tissue (3.1, 3.2, 3.3) | 15, 10, 5 | 0.2, 0.15, 0.1 |
| Skin | 40 | 1.0 |
| Muscle (Chest Wall) | 65 | 2.0 |
| Matching Fluid | 24 | 0.5 |
Fig. 7.
COMSOL Predicted Microwave Power Distributions for Time-Reversal Focusing in (a) Fatty and (b) Dense Breast (10 Element Array). Elongation in Transverse Plane due to 2D Array Focusing.
2) Experimental Focusing Tests in Coupling Media
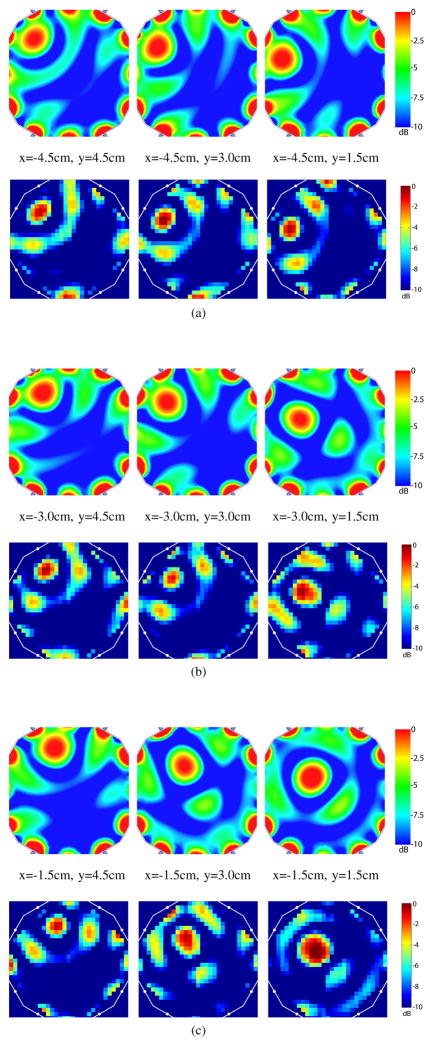
The microwave power distribution for 49 focal states was measured using a coaxial probe that was manually positioned on a 2D grid with 5 mm spacing (see Figure 5). Microwave power distribution maps for 9 sample focal states are shown in Figure 8. These results suggest that using the current time-reversal method with a 12 element, 15 cm diameter cylindrical array (each element 7.5 cm from the center) can achieve suitable focusing within a circular region approximately 10 cm in diameter. This is evident by good targeting accuracy, focal spot size and shape, and power delivered to the target location in this region. However, near the perimeter of the chamber, we observe reduced targeting accuracy, deformed and reduced focal spots, and significant side lobes, which are expected. These limitations can be overcome with additional elements or modifications to the geometry of the array as needed for a given focusing requirement.
Fig. 8.
Microwave Power Distributions on a Subgrid with 1.5 cm Spacing: Simulated Results on Top vs. Measured Results on Bottom. (a) x=−4.5 cm (b) x=−3.0 cm (c) x=−1.5 cm.
C. Thermal Distribution in Tissue Mimicking Phantoms
In the first series of phantom heating experiments, temperature measurements were taken using digital probe thermometers at sample locations within a gelatin phantom and surrounding emulsion matching medium (see Figure 4a). Following these simple temperature probe experiments, a linear array of thermistors (see Figure 4b) was used to map the temperature distribution throughout the phantom and surrounding fluid for a number of gelatin phantoms, focal locations, and heating times. The results of both the thermistor array mapping and the digital probe measurements for a focal spot offset 3 cm from the center of the therapy chamber are presented in Figure 9. In these tests, as in others performed on phantoms of different sizes and composition (some containing cancer mimicking inclusions), significant differential heating was observed at the target location.
Fig. 9.

Focus at x = 3 cm, y = 0 cm: (a) Simulated Microwave Power Distribution (b) Measured Microwave Power Distribution (c) Temperature after 45 minutes of Heating, Measured with Thermistor Array (d) Temperature as a Function of Time, Measured with Digital Probe Thermometers.
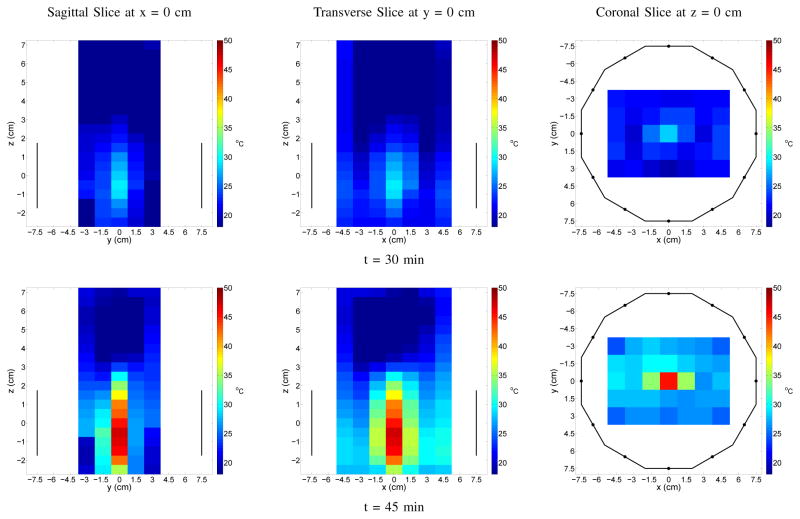
Finally, temperature maps of the focal planes are shown for both a 3 cm offset case (Figure 10) and a center focused case (Figure 11). These results show that the temperature distribution is in good agreement with the earlier microwave power maps. The asymmetry around the focal spots along the x-direction seen in the offset focus case is most likely due to imprecision in the manual placement of the thermistor array. Despite their somewhat low resolution, they confirm the ability of the system to achieve an antitumor effect within a 1.5 cm target region in the array plane without unwanted heating in any other region.
Fig. 10.
Temperature Maps of Focused Heating at x = 3 cm, y = 0 cm in Homogeneous Phantom for 30 and 45 Minute Heating Times. Sagittal and Transverse Slices Show Vertical Extent of the Active Antennas; Coronal Slice Outlines the Chamber.
Fig. 11.
Temperature Maps of Focused Heating at x = 0 cm, y = 0 cm in Homogeneous Phantom for 30 and 45 Minute Heating Times. Sagittal and Transverse Slices Show Vertical Extent of the Active Antennas; Coronal Slice Outlines the Chamber.
IV. Conclusion
A preclinical prototype of a microwave thermal therapy system that employs an image-based time-reversal method has been developed and tested. Simulated and experimental results have demonstrated that such a system can achieve suitable focusing and heating for the potential application of thermal therapy of the breast. Based on the results of these studies, the design and fabrication of a clinically viable focused microwave array is currently underway that will demonstrate full 3D focusing ability using nonlinear array optimization techniques with substantially improved anterior to posterior concentration of heating and more efficient cooling of the coupling fluid. Upon completion, the focused microwave thermal therapy system will have the potential to be used in neoadjuvant tumor treatment prior to surgery, to completely ablate the tumor, to selectively enhance drug delivery and absorption through focused thermal release, or as an adjuvant to postoperative radiation therapy and chemotherapy.
Acknowledgments
The authors would like to thank Brian Fowlkes for his guidance on the system design, Oliver Kripfgans for his assistance with the modeling of numerical breast phantoms, and Clare Ward, Stephen Clarkson, Mario Fabiilli, and Line van Nieuwstadt for their assistance with the development and measurement of the emulsion coupling fluid. Portions of this work were supported by DOD/BCRP (W81XWH-10-1-0514; BC095397) and NIH T32 EB005172.
References
- 1.Kennedy JE. High-intensity focused ultrasound in the treatment of solid tumours. Nature Reviews Cancer. 2005;5:321–327. doi: 10.1038/nrc1591. [DOI] [PubMed] [Google Scholar]
- 2.Vernon CC, et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: results from five randomized controlled trials. Int J Radiat Oncol Biol Phys. 1996;35:731–744. doi: 10.1016/0360-3016(96)00154-x. [DOI] [PubMed] [Google Scholar]
- 3.Vujaskovic Z, et al. A Phase I/II Study of Neoadjuvant Liposomal Doxorubicin, Paclitaxel, and Hyperthermia in Locally Advanced Breast Cancer. Int J Hyperthermia. 2010;26(5):514–521. doi: 10.3109/02656731003639364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Critical Reviews in Oncology/Hematology. 2002 Jul;43(1):33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 5.Dewhirst MW, Viglianti BL, Lora-Michiels M, Hansen M, Hoopes PJ. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia. 2003;19:267–294. doi: 10.1080/0265673031000119006. [DOI] [PubMed] [Google Scholar]
- 6.Jones E, Thrall D, Dewhirst MW, Vujaskovic Z. Prospective thermal dosimetry: the key to hyperthermias future. Int J Hyperthermia. 2006;22:247–253. doi: 10.1080/02656730600765072. [DOI] [PubMed] [Google Scholar]
- 7.Dooley WC, Vargas HI, Fenn AJ, Tomaselli MB, Harness JK. Focused microwave thermotherapy for preoperative treatment of invasive breast cancer: a review of clinical studies. Ann Surg Oncol. 2010;17:1076–1093. doi: 10.1245/s10434-009-0872-z. [DOI] [PubMed] [Google Scholar]
- 8.Vargas HI, Dooley WC, Gardner RA, Gonzalez KD, Venegas R, Heywang-Kobrunner SH, Fenn AJ. Focused microwave phased array thermotherapy for ablation of early-stage breast cancer: results of thermal dose escalation. Ann Surg Oncol. 2004;11:139–146. doi: 10.1245/aso.2004.03.059. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Vogel M, Maccarini PF, Stakhursky V, Soher BJ, Das S, Arabe OA, Joines WT, Stauffer PR. Improved Hyperthermia Treatment Control using SAR/Temperature Simulation and PRFS Magnetic Resonance Thermal Imaging. Int J Hyperthermia. 2011;27(1):86–99. doi: 10.3109/02656736.2010.501509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stauffer PR, et al. Conformal Microwave Array (CMA) Applicators for Hyperthermia of Diffuse Chestwall Recurrence. Int J Hyperthermia. 2010;26(7):686–698. doi: 10.3109/02656736.2010.501511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng KS, Dewhirst MW, Stauffer PR, Das S. Effective learning strategies for real-time image-guided adaptive control of multiple source hyperthermia applicators. Medical Physics. 2010;37(3):1285–1297. doi: 10.1118/1.3302829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vujaskovic Z, Kim DW, Jones E, et al. A phase I/II study of neoad-juvant liposomal doxorubicin, paclitaxel, and hyperthermia in locally advanced breast cancer. Int J Hyperthermia. 2010;26:514–521. doi: 10.3109/02656731003639364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phase 1/2 study of ThermoDox with approved hyperthermia in treatment of breast cancer recurrence at the chest wall (DIGNITY). Bethesda, MD. NCT00826085. ClinicalTrials.gov
- 14.Phase 3 study of ThermoDox with radiofrequency ablation (RFA) in treatment of hepatocellular carcinoma (HCC). Bethesda, MD. NCT00617981. ClinicalTrials.gov
- 15.Landon CD, Park J, Needham D, Dewhirst MW. Nanoscale Drug Delivery and Hyperthermia: The Materials Design and Preclinical and Clinical Testing of Low Temperature-Sensitive Liposomes Used in Combination with Mild Hyperthermia in the Treatment of Local Cancer. The Open Nanomedicine Journal. 2011;3:38–64. doi: 10.2174/1875933501103010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surowiec A, Bicher HI. Heating characteristics of the TRIPAS hyperthermia system for deep seated malignancy. Journal of Microwave Power and Electromagnetic Energy. 1995;30(3):135–40. doi: 10.1080/08327823.1995.11688269. [DOI] [PubMed] [Google Scholar]
- 17.Fenn AJ, Wolf GL, Fogle RM. An adaptive microwave phased array for targeted heating of deep tumours in intact breast: animal study results. Int J Hyperthermia. 1999;15:45–61. doi: 10.1080/026567399285846. [DOI] [PubMed] [Google Scholar]
- 18.Gardner RA, Vargas HI, Block JB, Vogel CL, Fenn AJ, Kuehl GV, Doval Focused microwave phased array thermotherapy for primary breast cancer. Ann Surg Oncol. 2002;9:326–332. doi: 10.1007/BF02573866. [DOI] [PubMed] [Google Scholar]
- 19.Arunachalam K, Udpa SS, Udpa L. Computational feasibility of deformable mirror microwave hyperthermia technique for localized breast tumors. Int J Hyperthermia. 2007;23:577–589. doi: 10.1080/02656730701727484. [DOI] [PubMed] [Google Scholar]
- 20.Converse M, Bond EJ, Van Veen BD, Hagness SC. A computational study of ultrawideband versus narrowband microwave hyperthermia for breast cancer treatment. Microw Theory Tech. 2006;54:2169–2180. [Google Scholar]
- 21.Fink M, et al. Time reversed acoustics. Rep Prog Phys. 2000;63:1933–1995. [Google Scholar]
- 22.Guo B, Xu L, Li J. Time reversal based hyperthermia treatment of breast cancer. Microw Opt Tech Lett. 2005 Nov;47(4):335–338. [Google Scholar]
- 23.Kosmas P, Zastrow E, Hagness SC, Van Veen BD. A Computational Study of Time Reversal Techniques for Ultra-Wideband Microwave Hyperthermia Treatment of Breast Cancer. IEEE/SP 14th Workshop on Statistical Signal Processing; 26–29 Aug. 2007.pp. 312–316. [Google Scholar]
- 24.Stang JP, Joines WT. Tapered microstrip patch antenna array for microwave breast imaging. Proc IEEE MTT-IMS Digest. 2008 [Google Scholar]
- 25.Stang JP, Joines WT, Liu QH, Ybarra GA, Yuan M, Leonhardt I. Tapered microstrip patch antenna array for use in breast cancer screening via 3D active microwave imaging. Proc IEEE APS/URSI. 2009 [Google Scholar]
- 26.Stang JP, Ward C, van Nieuwstadt L, Fabiilli M, Haynes M, Carson P, Moghaddam M. Customizable emulsion matching media and tissue mimicking phantoms for microwave breast imaging and therapy. Phys Med Biol. in preparation (draft available) [Google Scholar]
- 27.Johnson SA, Christensen DA, Baxter B. Nonintrusive acoustic temperature tomography for measurement of microwave and ultrasound-induced hyperthermia. Journal of Bioengineering. 1977;1(5–6):555–570. [Google Scholar]
- 28.Jovanovifa I, Hormati A, Littrup P, Duric N, Rama O, Vetterli M. Temperature monitoring during tissue freezing using ultrasound speed measurements. Soc Photo-Optical Instr Engrs. 2009;7265 [Google Scholar]
- 29.VanBaren P, Ebbini ES. Multipoint temperature control during hyperthermia treatments: Theory and simulation. IEEE Trans Biomed Eng. 1995;42(8):818–827. doi: 10.1109/10.398643. [DOI] [PubMed] [Google Scholar]
- 30.Seip R, Ebbini ES. Noninvasive estimation of tissue temperature response to heating fields using diagnostic ultrasound. IEEE Trans Biomed Eng. 1995;42(8):828–839. doi: 10.1109/10.398644. [DOI] [PubMed] [Google Scholar]
- 31.Liu D, Ebbini ES. Real-time 2-D temperature imaging using ultrasound. IEEE Trans Biomed Eng. 2010;57(1):12–16. doi: 10.1109/TBME.2009.2035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arunachalam K, et al. Characterization of a digital microwave radiometry system for noninvasive thermometry using a temperature-controlled homogeneous test load. Phys Med Biol. 2008 Jul 21;53(14):3883–901. doi: 10.1088/0031-9155/53/14/011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zastrow E, Davis SK, Lazebnik M, Kelcz F, Van Veen BD, Hagness SC. Database of 3D Grid-Based Numerical Breast Phantoms for use in Computational Electromagnetics Simulations. http://uwcem.ece.wisc.edu/MRIdatabase/
- 34.D’Orsi CJ, Bassett LW, Berg WA. Atlas. 4. Reston, VA: ACR; 2003. Breast Imaging Reporting and Data System (BI-RADS) [Google Scholar]
- 35.Lazebnik M, et al. A large-scale study of the ultrawideband microwave dielectric properties of normal breast tissue obtained from reduction surgeries. Phys Med Biol. 2007;52:2637–2656. doi: 10.1088/0031-9155/52/10/001. [DOI] [PubMed] [Google Scholar]
- 36.Lazebnik M, et al. A large-scale study of the ultrawideband microwave dielectric properties of normal, benign and malignant breast tissues obtained from cancer surgeries. Phys Med Biol. 2007;52:6093–6115. doi: 10.1088/0031-9155/52/20/002. [DOI] [PubMed] [Google Scholar]