Abstract
A variety of growth factors are being explored as therapeutic agents relevant to the axonal and oligodendroglial deficits that occur as a result of demyelinating lesions. This review focuses on five such proteins that are present in the lesion site and impact oligodendrocyte regeneration. It then presents approaches that are being exploited to manipulate the lesion environment affiliated with multiple neurodegenerative diseases and suggests that the utility of these approaches can extend to demyelination. Challenges are to further understand the roles of specific growth factors on a cellular and tissue level. Emerging technologies can then be employed to optimize the use of growth factors to ameliorate the deficits associated with demyelinating degenerative diseases.
Keywords: FGF, IGF-I, PDGF, CNTF, BDNF, Demyelinating disease, Cuprizone, EAE, Lysolecithin, Therapeutic approaches to degenerative disease, MS
1. Introduction
The demyelinating disease, Multiple Sclerosis or MS, presents as a highly variable disorder. Nevertheless, most patients initially exhibit relapsing-remitting disease that becomes progressive in a subset of these individuals. In some patients disease progression is constant from the time of onset (Lublin & Reingold 1996). Traditionally MS is thought to be caused by peripheral T-cells that become activated, enter the brain and become re-activated by antigen presenting cells, leading to a succession of inflammatory events (Wekerle & Lassman 2006). Therefore, most therapies that have been directed to MS are anti-inflammatory in nature. Unfortunately, these therapies have limited effectiveness. This may be due to the axonal- and oligodendroglial degenerative changes that also occur in demyelinating lesions and result in failure of oligodendrocytes to remyelinate the axons. For remyelination to occur oligodendrocyte progenitors must migrate to the active lesion site, differentiate and remyelinate the denuded axons (Richardson et al 2011). In MS it has been estimated that approximately 70% of lesions that are demyelinated contain progenitor cells or premyelinating oligodendrocytes, suggesting an inability to differentiate, while approximately 30% of lesions contain few progenitors, suggesting an inability to migrate to the lesion site (Boyd et al 2013, Chang et al 2002, Lucchinetti et al 1999). This review focuses on growth factors that impact the regenerative processes that go awry and have been implicated in the pathology of MS (Mirowska-Guzel 2009). It is suggested that these may be possible therapeutic agents (Woodruff & Franklin 1997). The review then goes on to present approaches that may prove useful in the delivery of these factors, first in models of disease and then in clinical cases (see figure 1). The challenges are to understand roles of these factors on a cellular and tissue level and to manipulate the relevant factors at the demyelinated sites in a manner that enhances regeneration.
Figure 1.
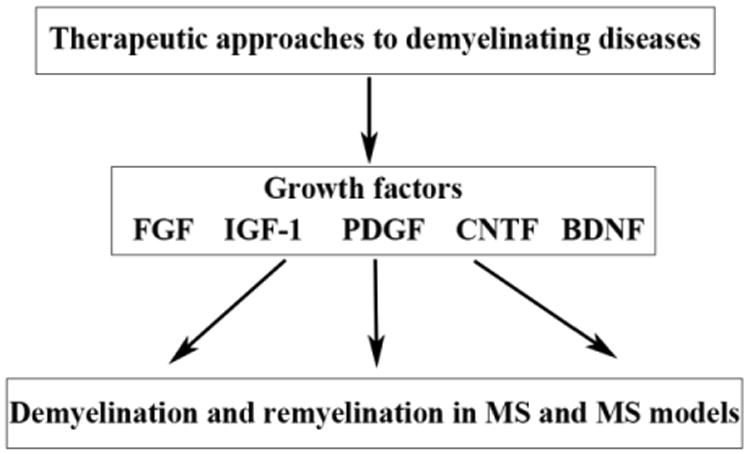
This figure presents the structure of the review. First the review indicates roles of the growth factors FGF2, IGF-I, PDGF, CNTF and BDNF on demyelination and remyelination in MS and MS models. It then details therapeutic approaches that are being used to manipulate levels of these factors within the brain and possibly impact demyelinating disease.
Before beginning, please note that because of space constraints, the growth factors chosen for discussion are limited to those long known to be present in demyelinating lesions and to impact the regenerative processes. For a more comprehensive list of factors that impact remyelination, please see the excellent reviews that cover multiple factors in detail (Chew & DeBoy 2015, Clemente et al 2013, Gallo & Deneen 2014, Hamby & Sofroniew 2010, Moore et al 2011).
2. Growth factors known to impact oligodendrocytes in demyelinating pathologies
2.1. Fibroblast growth factor (FGF)
One of the first growth factor families to be examined for effects on oligodendrocytes is FGF. FGF is a large family of factors that includes 22 members with multiple receptors to which these factors bind, making the study of FGF complex (Turner et al 2015). The most well examined FGF with respect to MS is FGF2 (also called basic FGF) that binds to four membrane-bound receptors (FGFR1-FGFR4). Oligodendrocyte lineage cells express FGFR1-3 (Bansal et al 1996). While FGF2's actions are diverse, effects mediated by specific receptors are less so and are found to regulate distinct aspects of oligodendrocyte development and myelination (Fortin et al 2005, Furusho et al 2011, Oh et al 2003).
In MS patients the disease impacts FGF2 and its receptors, suggesting a role for this factor. In particular, FGF2 is elevated in the cerebrospinal fluid (CSF) and serum of MS patients who exhibit relapsing remitting MS (RR-MS) or secondary progressive disease (SP-MS) (Harirchian et al 2012, Sarchielli et al 2008, Su et al 2006), however, note Mori et al, 2014 who did not find significant changes in FGF2 (Mori et al 2014). Within the brain FGF2 is expressed in active lesions and in the periphery of chronic lesions. More specifically, FGF2 is found in perivascular astrocytes in normal appearing gray matter where the blood brain barrier has been disrupted and in microglia/macrophages associated with the lesion sites. The receptor, FGFR1, is found in oligodendrocyte precursor cells recruited toward the chronic-active lesions, in perivascular astrocytes and in neurons (Clemente et al 2011).
The findings using MS brains are consistent with results seen in demyelination models which also exhibit increased levels of FGF2 and FGFRs in lesion sites (Armstrong et al 2002, Gudi et al 2011, Hinks & Franklin 1999, Liu et al 1998, Messersmith et al 2000). The significance of these increases has been evaluated by elevating levels of FGF2 after demyelination. Injections of FGF2 reverse deficits in myelin basic protein (MBP) gene expression and axonal function after lysolecithin treatment of the optic chiasm of adult mice (Dehghan et al 2012). It also increases MBP gene expression after lysolecithin treatment of the hippocampus of adult rats (Azin et al 2015). Similarly, intrathecal injection of a viral vector coding for FGF2 ameliorates clinical scores, reverses deficits in numbers of oligodendrocyte precursors and oligodendrocytes, and decreases the immune response in an experimental autoimmune encephalomyelitis (EAE) model (Ruffini et al 2001). Consistent with this is the observation that FGF2 -/- mice exhibit more severe EAE than do FGF2+/+ mice in a manner associated with increased activity of the immune system and reduced remyelination (Rottlaender et al 2011). These experiments that examine higher vs lower levels of FGF suggest that FGF enhances repair.
On the other hand are studies that suggest that endogenous FGF2 may inhibit remyelination in a demyelinating lesion. Thus, using the cuprizone model or the murine hepatitis virus strain A59 model (MHV-A59), increased numbers of oligodendrocytes are apparent in the lesioned white matter in FGF2-/- mice. This finding is consistent with culture results that suggest that cells in a depleted FGF environment increase differentiation (Armstrong et al 2002). Moreover, during recovery following 6 weeks of cuprizone treatment (acute treatment) numbers of oligodendrocytes are increased in the FGF2 -/- mice (Murtie et al 2005). When mice are subjected to chronic cuprizone treatment for 12 weeks and are then allowed to recover, similar results are noted. FGF-/- mice exhibit a recovery of oligodendrocytes and of myelin while FGF +/+ mice are inhibited in their ability to do so (Armstrong et al 2006, Tobin et al 2011).
Complementary to this are studies that assess the consequences of deleting FGF receptors associated with oligodendrocyte lineage cells. FGFR1 was deleted in PLP +cells during 12 weeks of cuprizone treatment and then the mice were allowed to recover. Remyelination and axonal integrity are enhanced during the recovery period (Zhou et al 2012). In contrast, when both FGFR1 and FGFR2 were knocked out in CNP+ or Olig1+ oligodendrocyte lineage cells in the chronic cuprizone model, recovery from the demyelination is impaired (Furusho et al 2015). In particular, fewer mature oligodendrocytes are present and myelin oligodendrocyte glycoprotein (MOG) levels are reduced.
In sum, experiments evaluating the action of FGF have yielded conflicting results. It has been suggested that this is due to the fact that FGF2 plays multiple roles. For example, it is known that FGF2 can impact not only oligodendrocytes, but also astrocytes, microglia, neurons and hematopoietic cells in a lesion site. The roles of these cells can be altered by the type of lesion and these distinct cells can themselves influence the response to FGF2 (Furusho et al 2015). Moreover, specific FGFRs are known to play different roles (Fortin et al 2005). How the receptors are affected by the lesion under immune conditions or more vs less severe demyelinating conditions may influence their response to FGF.
2.2. Insulin-like growth factor (IGF)
The story of the role of IGF is, as in the case of FGFs, also complicated. This family includes IGF-I and IGF-II. The ligands mediate their actions through two receptors, IGF type 1 receptor (IGF-1R) and IGF type 2 receptor (IGF-2R). In addition, the availability of the ligands is dependent, in part, on six IGF-binding proteins (IGFBPs) and proteases that cleave the IGFBPs (Chesik et al 2007). IGF-I is known to support oligodendrocyte progenitor cell cycle progression, myelin formation and oligodendrocyte survival (Beck et al 1995, Carson et al 1993, McMorris et al 1990, Min et al 2012, Roth et al 1995). It binds and mediates these effects through the IGF-1R (Zeger et al 2007).
In MS patients, IGF-I, IGF-1R, and IGFBPs are found in oligodendrocytes within normal appearing white matter and in chronic demyelinated plaques and are enhanced in areas surrounding the demyelinating plaques (Wilczak et al 2008, Wilczak & De Keyser 1997). This state in MS suggests that the IGF system is an important player in MS disease. On the other hand, circulating IGF-I levels in the serum and CSF are not different in MS patients when they are compared to control (Pirttila et al 2004, Poljakovic et al 2006, Wilczak et al 2005), although the IGFBP-3 levels are increased in MS patient serum, which may account for reduced bioavailability of IGF-I (Lanzillo et al 2011).
IGF signaling molecules increase in animal models of MS. In the cuprizone-induced demyelinating model, IGF-I mRNA and protein are strongly upregulated in astrocytes and microglia of the demyelinated area (Gudi et al 2011, Komoly et al 1992). Similarly in the EAE model, IGF-I is increased in reactive astrocytes in demyelinating lesions and IGF-1R is expressed in oligodendrocytes and neurons (Liu et al 1994). When subjected to cuprizone, overexpression of IGF-I protects mature oligodendrocytes from apoptosis (Mason et al 2000), while ablation of the IGF-1R results in poor recovery from demyelination (Mason et al 2003), suggesting a role of IGF-I in treating demyelinating disease.
In contrast to results with cuprizone, conflicting effects have been reported when IGF-I is injected to EAE rodents. For example, in agreement with the cuprizone results, in acute rat EAE IGF-I, injected peripherally during the onset of disease, reduces maximum clinical scores as well as lesion severity, and promotes a faster recovery (Liu et al 1997, Liu et al 1995). It also reduces the size and number of demyelinating lesions and upregulates myelin related protein mRNAs (Yao et al 1995, Yao et al 1996). In a chronic relapsing mouse EAE model, IGF-I treatment also reduces clinical deficits (Li et al 1998). However, other studies using the mouse EAE model reveal only a transient reduction of clinical scores and minor improvement in remyelination when IGF-I is infused during the acute phase, and no effect when IGF-I is infused during the chronic phase (Cannella et al 2000). Moreover, the injection of IGF-1 adeno-associated virus (AAV) into the quadriceps muscle has no effect on clinical scores of EAE when it is administered before disease onset and worsens the symptoms when it is administered post-disease onset (Genoud et al 2005). Similarly, oligodendrocyte remyelination is not improved when AAV-IGF-I is injected into the lysolecithin-induced demyelination lesion in aged rats (O'Leary et al 2002). In a clinical study of rhIGF-I in MS patients, 6 months of subcutaneous injection of rhIGF-I does not provide significant improvement to disease progress compared to baseline (Frank et al 2002).
Thus, as was the case with FGF, the results of experiments on IGF have been complicated. The differential results obtained may be due to the complexity of the different demyelinating models. Moreover, differences noted with cuprizone may be due to the lack of the immune component affiliated with EAE, MS or aged animals. Clearly, increased understanding of how the lesion environment is impacted in different models of disease would be helpful in delineating the cellular responses to IGF-I.
2.3. Platelet Derived Growth Factor (PDGF)
Experiments defining effects of PDGF, although not completely consistent, have yielded results that suggest it may be useful, particularly early in the progression of disease. The PDGF family includes four members with PDGF-AA being the protein that has been implicated in impacting oligodendrocyte progenitor cell proliferation and differentiation (Calver et al 1998, Reigstad et al 2005). PDGF-AA binds to platelet derived growth factor receptor α (PDGFR α) (Antoniades 1991). PDGFRα+ cells have been known for some time to be expressed by progenitor and preoligodendrocyte lineage cells (Ellison & de Vellis 1994)
In MS PDGFRα+ cells are increased within white matter lesions. The PDGFRα+ cells express Ki-67, a nuclear cell proliferation marker, suggesting that they proliferate in response to PDGF (Maeda et al 2001). In agreement with this possibility, high levels of PDGF in CSF are associated with full recovery from RR-MS while poor recovery is evident in patients with low levels of PDGF (Mori et al 2014). Interesting, PDGF is reported to decrease with prolonged disease duration (Harirchian et al 2012) and in the CSF of patients with primary progressive MS (Mori et al 2013). Studies of MS models suggest that sources of PDGF may be peripheral immune cells (Koehler et al 2008). The authors suggest that enhancing levels of PDGF may be a valuable approach that can reduce clinical consequences of neuronal damage in progressive phases of MS (Mori et al 2013).
This possibility was investigated using lysolecithin or cuprizone models of demyelination. A PDGF injection to a lysolecithin lesion elicits an initial increase in oligodendrocytes, remyelinated axons, and a decrease in the size of the lesion. Proliferation of oligodendrocyte progenitors is also increased (Allamargot et al 2001). However, this increase is short lived and after 3 months the control group that did not receive PDGF catches up to the PDGF group (Allamargot et al 2001). In complementary studies by others where mice were treated with cuprizone for 4 or 6 weeks, or received a lysolecithin injection, overexpression of PDGF-A increases the density of total oligodendrocytes. However, unlike the situation in the studies above, there is no effect on remyelination in the lysolecithin lesion where it was evaluated at 14 days or 5 weeks (Woodruff et al 2004). This may be due to the different methods of increasing PDGF.
When effects of overexpression of PDGF were assessed with acute treatment of cuprizone, elevated PDGF results in increases in the progenitors. However, PDGF overexpression does not elicit increases in oligodendrocyte progenitors after 12 week treatment with cuprizone or when mice are removed from the cuprizone for 6 weeks following 12 week treatment, indicating that effects of PDGF on progenitors may have disappeared. Nevertheless, enhanced numbers of PLP mRNA+ cells are evident during the recovery phase in mice with elevated PDGF (Vana et al 2007). Interestingly, this appears to be due to a reduction in apoptosis in oligodendrocytes (Vana et al 2007).
The results, then, largely agree that PDGF increases progenitors, at least during early phases of a demyelinating lesion. Later effects of the factor may be due to a reversal of cell death. The data are consistent with studies that elicited reduced PDGF signaling. For example, PDGFRα+/- mice exhibit decreases in oligodendrocyte progenitors after 5 weeks of cuprizone treatment (Murtie et al 2005). Moreover, intraperitoneal (ip) injections of trapidil, a potential PDGF antagonist, to rats elicits a decrease in remyelinating axons and thinner myelin sheaths in response to lysolecithin (McKay et al 1997).
2.4. Ciliary neurotrophic factor (CNTF)
Studies with CNTF suggest its possible utility as a therapeutic agent. CNTF is a neurotrophic cytokine that belongs to the IL-6 family. CNTF binds to its receptor CNTFRα, which is membrane bound by glycosyl-phosphatidylinositol linkage. Because CNTFRα lacks an intracellular domain, two other receptor proteins LIFRβ and gp130 are recruited to the receptor complex and are required for CNTF signal transduction (Davis et al 1993b, Davis et al 1991, Ip et al 1993). In adult rats, CNTFRα is found mainly in the CNS and skeletal muscles (Davis et al 1991). CNTFRα can also be cleaved off the membrane and exist as a soluble factor mediating the CNTF response (Davis et al 1993a). Components of the CNTF receptor complex are found on neurons and oligodendrocytes in the brain (Dutta et al 2007, Watanabe et al 1996).
Originally identified as a trophic factor to support neurons, CNTF has multiple functions on oligodendrocyte lineage cells that suggest an important role in myelin repair. For example, CNTF can promote oligodendrocyte progenitor cell proliferation, survival, and maturation (Barres et al 1996, Barres et al 1993, Louis et al 1993, Stankoff et al 2002). Further evidence implies the importance of CNTF in MS by showing that CNTF and the CNTF receptor complex are strongly upregulated in the cerebral cortex of MS patients (Dutta et al 2007). Consistent with this finding, CNTF mRNA is highly upregulated during demyelination and remyelination in the cuprizone-demyelinated mouse corpus callosum where it is found in microglia (Tanaka et al 2013). It is also found in astrocytes during remyelination of MHV-A59 treated spinal cord (Albrecht et al 2003)
Although not a risk factor for MS, the homozygous null mutation of CNTF in humans (CNTF-/-) is associated with earlier disease onset compared to normal CNTF in MS patients (Giess et al 2002). Similarly, knockout animal studies show that CNTF-deficient mice experience a more severe disease course with poorer recovery in EAE (Linker et al 2002). This is associated with reduced oligodendrocyte progenitor cell proliferation, increased oligodendrocyte apoptosis, axonal damage and myelin dystrophy (Linker et al 2002). In addition, CNTF neutralizing antibodies reduce migration of oligodendrocyte progenitors toward a lysolecithin lesion in the corpus callosum of an adult animal (Vernerey et al 2013). In contrast to these human and rodent studies, another report indicates that although similar percentages of the CNTF gene mutation are noted between MS patients and controls, no correlation is found between null CNTF and the age of disease onset (Hoffmann & Hardt 2002, Hoffmann et al 2002).
CNTF has been applied in animal models of MS and shows some promising results. Daily ip administration of CNTF (days 8-24 post immunization) significantly ameliorates EAE clinical scores, as well as increases numbers of oligodendrocytes, NG2 cells, axons and neurons (Kuhlmann et al 2006), although effects return to control level after the injection is stopped. Continuous pump infusion of CNTF decreases demyelination, protects neurons and improves peak clinical scores (Fang et al 2013). Intravenous (iv) delivery of human mesenchymal stem cells (MSCs) overexpressing CNTF to EAE mice significantly delays the onset of EAE, improves clinical symptoms and reduces demyelination (Lu et al 2009). CNTF application also protects retinal ganglion cells from apoptosis in acute rat EAE neuritis (Maier et al 2004). On the other hand, in one study nanospheres containing CNTF are ineffective in enhancing numbers of oligodendrocyte progenitors or postmitotic oligodendrocytes following an ethidium bromide demyelinating lesion (Talbott et al 2007).
In sum, the studies using CNTF are generally in agreement that, because of its benefits in demyelinating disease, CNTF is a therapeutic target that should be pursued. However, this is mentioned with the caution that although not associated with MS, a clinical trial of recombinant human CNTF indicated no benefits for ALS patients, with dose-associated adverse effects, and even death, reported (Miller et al 1996). A further caution is noted with respect to a rat model of spinal cord injury where transplanted astrocytes derived from CNTF-treated glial-restricted precursors resulted in hypersensitivity to pain (Davies et al 2008).
2.5. Brain derived neurotrophic factor (BDNF)
Studies of BDNF also suggest its possible utility as a therapeutic target. BDNF is a member of the neurotrophin gene family that includes NGF, BDNF, NT3 and NT4/5. BDNF binds specifically to trkB receptors that have been found on oligodendrocytes and oligodendrocyte progenitor cells (Vondran et al 2010, Wong et al 2013). Although it is well known for its actions on neurons (Huang & Reichardt 2001, Thoenen 2000), only relatively recently has its role on oligodendrocytes been appreciated, particularly after demyelination. This is due in part to the observation that it has relatively selective actions regionally, particularly during development. For example, in culture BDNF enhances numbers of oligodendrocytes of the basal forebrain, but not the cortex (Du et al 2003). In vivo, BDNF deficits in BDNF+/- mice are associated with decreases in myelin proteins in oligodendrocytes of the spinal cord and optic nerve early in development. These myelin deficits recover as the animal ages (Xiao et al 2010). In the basal forebrain, in contrast, the deficits are apparent throughout life (Vondran et al 2010). With respect to the corpus callosum, BDNF+/- mice do not show deficits in oligodendrocytes in the adult. However, effects of a reduction in BDNF on oligodendrocyte proliferation and differentiation are clearly observed after demyelination (VonDran et al 2011).
In the case of MS, levels of BDNF generally are reported to be elevated in peripheral blood mononuclear cells and serum, particularly during the relapsing phase of RR-MS (Caggiula et al 2005, Sarchielli et al 2002), but note (Azoulay et al 2008, Azoulay et al 2005) that report that less BDNF is in the serum of RR-MS patients with no difference when remission and relapse phases are compared and a reduced level of secreted BDNF from RR-MS patients in remission compared to control patients. In MS BDNF is expressed not only in peripheral blood mononuclear cells (Caggiula et al 2005, Gielen et al 2003, Kerschensteiner et al 1999, Petereit et al 2003, Sarchielli et al 2002) but also in astrocytes, infiltrating lymphocytes and microglia/macrophages in the brain within perivascular areas and in lesions (Kerschensteiner et al 1999, Stadelmann et al 2002). It is reported that immune cells also express trkB receptors, suggesting that they can respond to BDNF (De Santi et al 2009) (although note the work of (Stadelmann et al 2002) that suggests the trkB receptors are not on the immune cells). During SP-MS less BDNF is in CSF than in the CSF from RR-MS patients assessed during the stable phase of the disease (Sarchielli et al 2002), indicating that in SP-MS a deficit in BDNF may be affiliated with disease progression.
As was the case with the study of multiple factors, studies using MS samples prompted examination of the roles of BDNF in models of demyelination. When the cuprizone model was evaluated, BDNF +/- mice exhibit greater deficits in myelin proteins when compared to BDNF +/+ mice during both the demyelinating and recovery phases. The deficit in BDNF is also associated with deficits in levels of progenitor cell proliferation that occur in response to this demyelinating model (Tsiperson et al 2015, VonDran et al 2011). Conversely, when BDNF levels are increased in the cuprizone model by enhancing astrocyte-derived BDNF, levels of myelin proteins are increased (Fulmer et al 2014).
In EAE, BDNF delivered into the brain through transformed MSCs, reduces the clinical score, decreases inflammation and apoptosis, as well as demyelination (Makar et al 2009, Makar et al 2008). In complementary study, injection of BDNF overexpressing T cells reduces clinical scores and axonal damage (Linker et al 2010). Conversely, induced deficits in BDNF, including those deficits directed to GFAP+ astrocytes or lck/lysM+ immune cells results in increased severity of the EAE and axonal damage (Lee et al 2012, Linker et al 2010).
When considered together, these studies consistently indicate that BDNF may enhance the ability of the CNS to withstand a demyelinating lesion. Interestingly, similar effects of BDNF have been reported with respect to spinal cord injury where elevated BDNF is associated with increases in oligodendrocyte lineage cells and myelin protein (McTigue et al 1998). However, the work of Javeri et al (Javeri et al 2010) should be noted which found that BDNF+/- mice have reduced EAE, suggesting that under some circumstances BDNF may have negative consequences.
This review has outlined advances made in our understanding of the role of a cadre of growth factors in reversing deficits in oligodendrocytes that may lead to the repair of a demyelinating lesion. As noted, within the study of each growth factor differences in effect are reported, due undoubtedly to the different models being evaluated at different times in the de-and re-myelination processes and complexities inherent therein. Clearly, further study in which effects on specific cells can be better defined and in which examination of the detailed cellular responses of the lesion site itself is carefully explored will be necessary to more precisely define the roles of individual growth factors. Nevertheless, because of results suggesting that these factors do impact demyelinating disease models, parallel studies are warranted to evaluate how levels of growth factors can be enhanced appropriately to ameliorate disease. As noted below, these approaches have primarily been utilized in many models of neurodegenerative disease. We suggest, and in some cases it has been shown, that such manipulations may be also appropriate for MS.
3. Approaches to the therapeutic application of growth factors
When addressing the problem of treating brain disease with growth factors, issues include how to first, enhance levels of growth factors within specific regions of the central nervous system and second, how to optimize entry of these factors from the periphery. While some reports indicate that growth factors do not cross the blood brain barrier in vivo (Pardridge 2002), others report that the ability to cross the barrier may exist (Pan et al 1998), but differs depending on the growth factor in question (Poduslo & Curran 1996). As indicated in the following section, many of the therapies that are being tested focus on these issues.
3.1. Introduction of growth factors into the central nervous system by injection of viral vectors or encapsulated engineered cells
The use of viral vectors to introduce specific growth factors into the central nervous system is being explored in multiple neurodegenerative disease models (Bartus et al 2007, Gasmi et al 2007, Gelfand & Kaplitt 2013, Giralt et al 2011, Nagahara & Tuszynski 2011) and is even being tested in humans (Tuszynski et al 2015). Relevant to the present review, this approach is being explored in the EAE model (Nygardas et al 2013, Ruffini et al 2001). Vectors used are modified viruses that can carry growth factor genes to the brain and spinal cord and include herpes simplex virus type-1 (HSV-1), lentivirus and AAV. This approach is used to deliver FGF-2 (Ruffini et al 2001), IGF-1 (Tsai et al 2012), BDNF and NGF (Ruitenberg et al 2004, Tuszynski et al 2015, Ziemlinska et al 2014) or a combination of factors (Paradiso et al 2009) to lesioned areas, suggesting that it may also be useful in the case of MS.
Concerns with this approach involve the need to inject the vectors into the CNS, complicating its use when multiple injections are necessary. A related strategy that is being pursued is to deliver growth factors in microspheres directly into the brain. The microspheres have the ability to release the factors over a prolonged time course. Data is accumulating that indicates that NGF and GDNF delivered this way may ameliorate signs of disease in models of Alzheimer's disease or Parkinson's Disease (Garbayo et al 2009, Gu et al 2009).
In a related approach cells genetically manipulated to produce growth factors are being encapsulated with immunoisolatory, semipermeable membranes to limit humoral rejection. The encapsulated cells are implanted directly into the brain where they produce factors over prolonged times. Encapsulated cells secreting CNTF reverse signs of disease in models of Huntington's disease, Alzheimer's disease and retinitis pigmentosa (Emerich et al 1997a, Emerich et al 1996, Emerich & Thanos 2006, Emerich et al 1997b, Garcia et al 2010, Tao et al 2002), while cells secreting GDNF rescue neurons in models of amyotrophic lateral sclerosis (Sagot et al 1996) Interestingly, CNTF producing capsules were implanted into Huntington disease and retinitis pigmentosa patients with promising results (Bachoud-Levi et al 2000, Bloch et al 2004, Kauper et al 2012, Sieving et al 2006).
3.2. Approaches to enable peptide growth factors to cross the blood brain barrier
As noted above, growth factors are large proteins that do not cross the blood brain barrier efficiently; hence, they are not ideal for application in the periphery without any modification. A method that is now being developed to approach this problem is to incorporate growth factors such as FGF-2 into nanoparticles able to cross the blood brain barrier. In a recent report, nanoparticles were conjugated with antibodies directed against transferrin receptor-1, a peptide endogenously expressed on brain endothelial cells. When the nanoparticles were injected to an animal exposed to a stroke model, receptor mediated transcytosis occurs across the blood barrier and FGF-2 is able to enter the brain, resulting in reduction in the size of the injury (Yemisci et al 2015). Additional approaches, such as using glucose transporters (Bonina et al 2003, Patching 2016) or gene therapy with AAV9 (Dayton et al 2012), are also seeking to develop systemic gene delivery strategies to bypass the barrier.
Using a similar tactic, cell-penetrating peptides are being developed as vectors for delivery of peptides across the blood brain barrier and into cells (Kristensen et al 2016). In 1988 it was reported that the Human Immunodeficiency Virus Trans-activator of transcription (Tat) protein can cross cell membranes (Frankel & Pabo 1988). Further study identified a second protein, the Drosophila antennapedia Homeodomain protein as having similar properties (Joliot et al 1991). Subsequently, the peptide sequence that is necessary to ensure membrane penetration in the Drosophila antennapedia Homeodomain protein was identified and is known as penetratin (Derossi et al 1994). Other peptides are now been recognized as having similar capabilities. This strategy is being used to introduce BDNF as well as glial cell line-derived neurotrophic factor (GDNF) into the brain undergoing degeneration. When the core functional domain of BDNF was fused with a TAT transduction domain and injected intraperitoneally, it is able to cross the blood brain barrier in two Alzheimer's disease models, improve memory deficits, stimulate trkB, as well as downstream signaling cascades, reverse synaptic deficits and reduce A beta and tau pathology (Wu et al 2015). When GDNF was fused to TAT and intravenously injected into mice subjected to ischemia, neuronal apoptosis is reduced (Kilic et al 2005). The utility of this approach, although promising, is limited by the instability of the cell-penetrating peptides and studies are ongoing to address this issue.
3.3. Mimetics as alternatives to growth factors
A different strategy is the development of small molecules that can cross the blood brain barrier and mimic the effects of the growth factor of interest. An example of this approach is the use of an NCAM-derived FGFR1 agonist, fibroblast growth loop (FGL) that is derived from the NCAM FGFR1 binding site (Kiselyov et al 2003). Neuroprotective effects of FGL are reported in a model of Alzheimer's disease, elicited upon intranasal and subcutaneous administration (Klementiev et al 2007). The drug is also effective in reversing inflammatory events and glial cell activation noted in aged rats (Downer et al 2009, Ojo et al 2011), but has adverse effects on hippocampal neurons in young animals (Ojo et al 2013). Nevertheless, it is reported to be in clinical trials in Alzheimer's patients (Woodbury & Ikezu 2014).
BDNF mimetics have been generated. In these cases small molecules were developed that mimic a region of BDNF that binds to its receptor, trkB (Massa et al 2010, Wong et al 2014). While one of these mimetics promotes myelination of oligodendrocytes in culture (Wong et al 2014), another has been applied intranasally and restores motor learning deficits following traumatic brain injury (Massa et al 2010). Other approaches to enhancing BDNF's actions involve the development of chimeric peptides formed by the conjugation of BDNF to a monoclonal antibody to the blood brain barrier transferrin receptor. As was the situation using nanoparticles above, this antibody enhances the travel of BDNF into the brain, resulting in protection from ischemia (Zhang & Pardridge 2001).
3.4. The use of intranasal delivery or mesenchymal stem cells (MSCs) to deliver growth factors and enhance repair
A different approach to enhancing growth factor transport across the blood brain barrier is the use of intranasal administration. In this approach, growth factors are delivered to the brain through the nasal epithelium. Proteins applied to the epithelium are then transported through the olfactory and trigeminal nerve pathways to reach the brain and spinal cord. Successful delivery of BDNF, CNTF (Alcala-Barraza et al 2010, Vaka et al 2012), IGF-I (Thorne et al 2004), and FGF-2 (Feng et al 2012) is reported in rodents. Intranasal administration of proteins is also used in humans (Reger et al 2006) and in EAE models of MS (Li et al 2014).
MSCs are also used in a number of disease models to enhance repair, including models of MS and MS itself. In these cases the MSCs are injected into the CNS or peripherally (Gharibi et al 2015, Karussis et al 2010). Their ability to reverse injury sites is due to the fact that MSCs can migrate preferentially to regions where lesions are evident (Cohen 2013) and to modulate immune responses through the release of growth factors (Gerdoni et al 2007, Zappia et al 2005). MSCs do not elicit their effects through their differentiation into other cell types (Gerdoni et al 2007). Of interest to this review, recent work using the cuprizone and EAE models indicate that MSCs have effects on neurons and oligodendrocytes that are coincident with increases in BDNF (El-Akabawy & Rashed 2015, Kassis et al 2008, Zhang et al 2005), consistent with the possibility that one of the growth factors they produce is BDNF. Other studies have enhanced this potential by engineering the ability of MSCs to produce increased levels of growth factors. In particular, stem cells engineered to express increased levels of BDNF (BDNF-MSC) that are delivered to EAE animals elicit decreased EAE clinical scores, reduce immune response and cell death and reduce demyelination (Makar et al 2014, Makar et al 2008). Similarly, when BDNF-MSCs are injected intraspinally to the lesioned spinal cord, increased recovery occurs (Gransee et al 2015). In a final example, MSCs engineered to overexpress CNTF also ameliorate EAE and effects of demyelination. The data suggest that trophic effects of MSCs can be augmented by their manipulation to produce specific growth factors.
3.5. Drugs that are being used for MS may also make use of growth factors
Interestingly, multiple MS drugs developed because of their immunomodulatory characteristics appear to elicit their effects, in part, through the action of growth factors that impact remyelination in MS models. This has been most clearly observed in the case of BDNF. An excellent example is the role of glatiramer acetate (GA), also known as copaxone (Cop-1), identified because of its actions to inhibit the progression of EAE, and now known to be an effective therapy for MS.
In recent data, cultured GA-specific T cells are shown to release BDNF in rats and in humans in response to GA (Kipnis et al 2000, Ziemssen et al 2002). Moreover, in an EAE mouse transferred GA-specific T cells express BDNF when they migrate to the brain, putting them in position to impact local cells (Aharoni et al 2003). That they may do so in response to GA is indicated by studies in which BDNF is deleted from immune cells in EAE mice. While GA reduces clinical signs in these animals, deletion of BDNF from immune cells results in a more severe disease course and loss of axons, suggesting that GA elicits its effects through BDNF (Linker et al 2010). This observation may have relevance to MS as it is noted that reduced levels of BDNF in serum and CSF of relapsing remitting MS patients are elevated by GA treatment (Azoulay et al 2005)
Complementary studies using GA suggest that GA may elevate other growth factors as well. For example, GA-stimulated T cells release PDGF and IGF-I into culture medium (Skihar et al 2009). This conditioned medium elevates numbers of cultured oligodendrocyte progenitors. Moreover, after a lysolecithin lesion GA treatment elevates oligodendrocyte progenitors in lesions of the spinal cord coincident with increases in IGF-I, as well as BDNF (Skihar et al 2009) and GA treatment during development enhances numbers of oligodendrocyte progenitors as well as mature oligodendrocytes and myelin in a manner coincident with increases in IGF-I and BDNF (From et al 2014).
Other MS drugs involve BDNF in their actions. Laquinimod elevates BDNF levels in the serum of MS patients (Thone et al 2012). In a manner analogous to that of GA, deletion of BDNF from immune cells inhibits protective effects of laquinimod in an EAE mouse (Thone et al 2012). Fingolimod, also used to treat MS patients, elevates BDNF in the brain of mice and in mouse models of disease (Deogracias et al 2012). In the case of the MS drug, alemtuzumab, MBP stimulated peripheral blood mononuclear cells from patients who are treated with the drug, release BDNF, CNTF and PDGF into medium. When neuronal cultures are grown in this conditioned medium, there is an increase in numbers of neurons and axonal length that is reversed by anti-BDNF or anti-CNTF, suggesting that the factors are at least partially responsible for the effects of the conditioned medium (Jones et al 2010).
PDGF function may also play a role in the action of another MS drug. The human monoclonal IgM (rHIgM22), a drug used in clinical trials, enhances remyelination in the demyelinating Theiler's murine encephalomyelitis virus model and the lysolecithin model (Bieber et al 2002, Warrington et al 2007). When mixed cultures that include OPCs are exposed to rHIgM22, OPC proliferation is enhanced in a process mediated by PDGFRα, suggesting the role of this factor in the drug's actions (Watzlawik et al 2013).
4. Summary
A number of growth factors are now known to directly impact oligodendrocytic responses to demyelination through the activation of their receptors on oligodendrocyte progenitor cells or postmitotic cells. There is hope that these factors may be used therapeutically to influence repair in addition to the anti-immune therapies currently in use. Because of a growing literature that suggests the importance of these proteins in demyelination as well as multiple other degenerative diseases, a number of promising therapeutic approaches are being developed to elevate the growth factors within the damaged CNS. It is suggested that these approaches will also be useful in attacking demyelinating diseases such as MS.
Highlights.
Growth factors that affect the proliferating and maturing oligodendrocyte lineage cells within a demyelinating lesion may impact repair.
These factors include FGF-2, IGF-I, PDGF, CNTF and BDNF.
Therapeutic approaches that are being developed to manipulate and deliver growth factors within the lesion site of multiple neurodegenerative diseases may also influence demyelinating lesions and be useful to diseases such as MS.
Acknowledgments
The work by the Dreyfus laboratory cited in this review was supported by NMSS grant RG 4257B4/1, NIH R01 NS036647 and NIH P01 HD023315.
Abbreviations
- AAV
adeno-associated virus
- BDNF
brain-derived neurotrophic factor
- CNP
2′,3′-cyclic neucleotide 3′-phosphodiesterase
- CNS
central nervous system
- CNTF
ciliary neurotrophic factor
- CNTFRα
ciliary neurotrophic factor receptor alpha
- Cop-1
copaxone
- CSF
cerebrospinal fluid
- EAE
experimental autoimmune encephalomyelitis
- FGF
fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- FGL
fibroblast growth loop
- GA
glatiramer acetate
- GDNF
glial cell line-derived neurotrophic factor
- GFAP
glial fibrillary acidic protein
- HSV-1
herpes simplex virus type-1
- IGF-I
insulin-like growth factor 1
- IGFBP
insulin-like growth factor binding protein
- IGF1R
insulin-like growth factor type 1 receptor
- IL-6
interleukin-6
- IP
intraperitoneal
- IV
intravenous
- LIFRβ
leukemia inhibitory factor receptor beta
- MBP
myelin basic protein
- MHV-A59
murine hepatitis virus strain A59
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- MSCs
mesenchymal stem cells
- NCAM
neural cell adhesion molecule
- NGF
nerve growth factor
- NT3
neurotrophin-3
- NT4/5
neurotrophin-4/5
- Olig1
oligodendrocyte transcription factor 1
- PDGF
platelet derived growth factor
- PDGFRα
platelet derived growth factor receptor alpha
- PLP
proteolipid protein
- rHIgM22
recombinant human monoclonal IgM 22
- RR-MS
relapsing remitting multiple sclerosis
- SP-MS
secondary progressive multiple sclerosis
- trkB
tyrosine receptor kinase B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aharoni R, Kayhan B, Eilam R, Sela M, Arnon R. Glatiramer acetate-specific T cells in the brain express T helper 2/3 cytokines and brain-derived neurotrophic factor in situ. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14157–62. doi: 10.1073/pnas.2336171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht PJ, Murtie JC, Ness JK, Redwine JM, Enterline JR, et al. Astrocytes produce CNTF during the remyelination phase of viral-induced spinal cord demyelination to stimulate FGF-2 production. Neurobiology of disease. 2003;13:89–101. doi: 10.1016/s0969-9961(03)00019-6. [DOI] [PubMed] [Google Scholar]
- Alcala-Barraza SR, Lee MS, Hanson LR, McDonald AA, Frey WH, 2nd, McLoon LK. Intranasal delivery of neurotrophic factors BDNF, CNTF, EPO, and NT-4 to the CNS. Journal of drug targeting. 2010;18:179–90. doi: 10.3109/10611860903318134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allamargot C, Pouplard-Barthelaix A, Fressinaud C. A single intracerebral microinjection of platelet-derived growth factor (PDGF) accelerates the rate of remyelination in vivo. Brain research. 2001;918:28–39. doi: 10.1016/s0006-8993(01)02761-5. [DOI] [PubMed] [Google Scholar]
- Antoniades HN. PDGF: a multifunctional growth factor. Bailliere's clinical endocrinology and metabolism. 1991;5:595–613. doi: 10.1016/s0950-351x(10)80005-9. [DOI] [PubMed] [Google Scholar]
- Armstrong RC, Le TQ, Flint NC, Vana AC, Zhou YX. Endogenous cell repair of chronic demyelination. Journal of neuropathology and experimental neurology. 2006;65:245–56. doi: 10.1097/01.jnen.0000205142.08716.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RC, Le TQ, Frost EE, Borke RC, Vana AC. Absence of fibroblast growth factor 2 promotes oligodendroglial repopulation of demyelinated white matter. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:8574–85. doi: 10.1523/JNEUROSCI.22-19-08574.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azin M, Mirnajafi-Zadeh J, Javan M. Fibroblast Growth Factor-2 Enhanced The Recruitment of Progenitor Cells and Myelin Repair in Experimental Demyelination of Rat Hippocampal Formations. Cell journal. 2015;17:540–456. doi: 10.22074/cellj.2015.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azoulay D, Urshansky N, Karni A. Low and dysregulated BDNF secretion from immune cells of MS patients is related to reduced neuroprotection. Journal of neuroimmunology. 2008;195:186–93. doi: 10.1016/j.jneuroim.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Azoulay D, Vachapova V, Shihman B, Miler A, Karni A. Lower brain-derived neurotrophic factor in serum of relapsing remitting MS: reversal by glatiramer acetate. Journal of neuroimmunology. 2005;167:215–8. doi: 10.1016/j.jneuroim.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Bachoud-Levi AC, Deglon N, Nguyen JP, Bloch J, Bourdet C, et al. Neuroprotective gene therapy for Huntington's disease using a polymer encapsulated BHK cell line engineered to secrete human CNTF. Human gene therapy. 2000;11:1723–9. doi: 10.1089/10430340050111377. [DOI] [PubMed] [Google Scholar]
- Bansal R, Kumar M, Murray K, Morrison RS, Pfeiffer SE. Regulation of FGF receptors in the oligodendrocyte lineage. Molecular and cellular neurosciences. 1996;7:263–75. doi: 10.1006/mcne.1996.0020. [DOI] [PubMed] [Google Scholar]
- Barres BA, Burne JF, Holtmann B, Thoenen H, Sendtner M, Raff MC. Ciliary neurotrophic factor enhances the rate of oligodendrocyte generation. Molecular and cellular neurosciences. 1996;8:146–56. doi: 10.1006/mcne.1996.0053. [DOI] [PubMed] [Google Scholar]
- Barres BA, Schmid R, Sendnter M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–95. doi: 10.1242/dev.118.1.283. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Herzog CD, Bishop K, Ostrove JM, Tuszynski M, et al. Issues regarding gene therapy products for Parkinson's disease: the development of CERE-120 (AAV-NTN) as one reference point. Parkinsonism & related disorders. 2007;13(Suppl 3):S469–77. doi: 10.1016/S1353-8020(08)70052-X. [DOI] [PubMed] [Google Scholar]
- Beck KD, Powell-Braxton L, Widmer HR, Valverde J, Hefti F. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron. 1995;14:717–30. doi: 10.1016/0896-6273(95)90216-3. [DOI] [PubMed] [Google Scholar]
- Bieber AJ, Warrington A, Asakura K, Ciric B, Kaveri SV, et al. Human antibodies accelerate the rate of remyelination following lysolecithin-induced demyelination in mice. Glia. 2002;37:241–9. doi: 10.1002/glia.10033. [DOI] [PubMed] [Google Scholar]
- Bloch J, Bachoud-Levi AC, Deglon N, Lefaucheur JP, Winkel L, et al. Neuroprotective gene therapy for Huntington's disease, using polymer-encapsulated cells engineered to secrete human ciliary neurotrophic factor: results of a phase I study. Human gene therapy. 2004;15:968–75. doi: 10.1089/hum.2004.15.968. [DOI] [PubMed] [Google Scholar]
- Bonina F, Puglia C, Rimoli MG, Melisi D, Boatto G, et al. Glycosyl derivatives of dopamine and L-dopa as anti-Parkinson prodrugs: synthesis, pharmacological activity and in vitro stability studies. Journal of drug targeting. 2003;11:25–36. doi: 10.1080/1061186031000086090. [DOI] [PubMed] [Google Scholar]
- Boyd A, Zhang H, Williams A. Insufficient OPC migration into demyelinated lesions is a cause of poor remyelination in MS and mouse models. Acta neuropathologica. 2013;125:841–59. doi: 10.1007/s00401-013-1112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula M, Batocchi AP, Frisullo G, Angelucci F, Patanella AK, et al. Neurotrophic factors and clinical recovery in relapsing-remitting multiple sclerosis. Scandinavian journal of immunology. 2005;62:176–82. doi: 10.1111/j.1365-3083.2005.01649.x. [DOI] [PubMed] [Google Scholar]
- Calver AR, Hall AC, Yu WP, Walsh FS, Heath JK, et al. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20:869–82. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- Cannella B, Pitt D, Capello E, Raine CS. Insulin-like growth factor-1 fails to enhance central nervous system myelin repair during autoimmune demyelination. The American journal of pathology. 2000;157:933–43. doi: 10.1016/S0002-9440(10)64606-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ, Behringer RR, Brinster RL, McMorris FA. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993;10:729–40. doi: 10.1016/0896-6273(93)90173-o. [DOI] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. The New England journal of medicine. 2002;346:165–73. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Chesik D, Wilczak N, De Keyser J. The insulin-like growth factor system in multiple sclerosis. International review of neurobiology. 2007;79:203–26. doi: 10.1016/S0074-7742(07)79009-8. [DOI] [PubMed] [Google Scholar]
- Chew LJ, DeBoy CA. Pharmacological approaches to intervention in hypomyelinating and demyelinating white matter pathology. Neuropharmacology. 2015 doi: 10.1016/j.neuropharm.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente D, Ortega MC, Arenzana FJ, de Castro F. FGF-2 and Anosmin-1 are selectively expressed in different types of multiple sclerosis lesions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14899–909. doi: 10.1523/JNEUROSCI.1158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemente D, Ortega MC, Melero-Jerez C, de Castro F. The effect of glia-glia interactions on oligodendrocyte precursor cell biology during development and in demyelinating diseases. Frontiers in cellular neuroscience. 2013;7:268. doi: 10.3389/fncel.2013.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA. Mesenchymal stem cell transplantation in multiple sclerosis. Journal of the neurological sciences. 2013;333:43–9. doi: 10.1016/j.jns.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JE, Proschel C, Zhang N, Noble M, Mayer-Proschel M, Davies SJ. Transplanted astrocytes derived from BMP- or CNTF-treated glial-restricted precursors have opposite effects on recovery and allodynia after spinal cord injury. Journal of biology. 2008;7:24. doi: 10.1186/jbiol85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Ip NY, Stahl N, Scherer S, et al. Released form of CNTF receptor alpha component as a soluble mediator of CNTF responses. Science. 1993a;259:1736–9. doi: 10.1126/science.7681218. [DOI] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Stahl N, Pan L, Taga T, et al. LIFR beta and gp130 as heterodimerizing signal transducers of the tripartite CNTF receptor. Science. 1993b;260:1805–8. doi: 10.1126/science.8390097. [DOI] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Valenzuela DM, Wong VV, Furth ME, et al. The receptor for ciliary neurotrophic factor. Science. 1991;253:59–63. doi: 10.1126/science.1648265. [DOI] [PubMed] [Google Scholar]
- Dayton RD, Wang DB, Klein RL. The advent of AAV9 expands applications for brain and spinal cord gene delivery. Expert opinion on biological therapy. 2012;12:757–66. doi: 10.1517/14712598.2012.681463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Santi L, Annunziata P, Sessa E, Bramanti P. Brain-derived neurotrophic factor and TrkB receptor in experimental autoimmune encephalomyelitis and multiple sclerosis. Journal of the neurological sciences. 2009;287:17–26. doi: 10.1016/j.jns.2009.08.057. [DOI] [PubMed] [Google Scholar]
- Dehghan S, Javan M, Pourabdolhossein F, Mirnajafi-Zadeh J, Baharvand H. Basic fibroblast growth factor potentiates myelin repair following induction of experimental demyelination in adult mouse optic chiasm and nerves. Journal of molecular neuroscience : MN. 2012;48:77–85. doi: 10.1007/s12031-012-9777-6. [DOI] [PubMed] [Google Scholar]
- Deogracias R, Yazdani M, Dekkers MP, Guy J, Ionescu MC, et al. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14230–5. doi: 10.1073/pnas.1206093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. The Journal of biological chemistry. 1994;269:10444–50. [PubMed] [Google Scholar]
- Downer EJ, Cowley TR, Cox F, Maher FO, Berezin V, et al. A synthetic NCAM-derived mimetic peptide, FGL, exerts anti-inflammatory properties via IGF-1 and interferon-gamma modulation. Journal of neurochemistry. 2009;109:1516–25. doi: 10.1111/j.1471-4159.2009.06076.x. [DOI] [PubMed] [Google Scholar]
- Du Y, Fischer TZ, Lee LN, Lercher LD, Dreyfus CF. Regionally specific effects of BDNF on oligodendrocytes. Developmental neuroscience. 2003;25:116–26. doi: 10.1159/000072261. [DOI] [PubMed] [Google Scholar]
- Dutta R, McDonough J, Chang A, Swamy L, Siu A, et al. Activation of the ciliary neurotrophic factor (CNTF) signalling pathway in cortical neurons of multiple sclerosis patients. Brain : a journal of neurology. 2007;130:2566–76. doi: 10.1093/brain/awm206. [DOI] [PubMed] [Google Scholar]
- El-Akabawy G, Rashed LA. Beneficial effects of bone marrow-derived mesenchymal stem cell transplantation in a non-immune model of demyelination. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 2015;198:11–20. doi: 10.1016/j.aanat.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Ellison JA, de Vellis J. Platelet-derived growth factor receptor is expressed by cells in the early oligodendrocyte lineage. Journal of neuroscience research. 1994;37:116–28. doi: 10.1002/jnr.490370116. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Cain CK, Greco C, Saydoff JA, Hu ZY, et al. Cellular delivery of human CNTF prevents motor and cognitive dysfunction in a rodent model of Huntington's disease. Cell transplantation. 1997a;6:249–66. doi: 10.1177/096368979700600308. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Lindner MD, Winn SR, Chen EY, Frydel BR, Kordower JH. Implants of encapsulated human CNTF-producing fibroblasts prevent behavioral deficits and striatal degeneration in a rodent model of Huntington's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:5168–81. doi: 10.1523/JNEUROSCI.16-16-05168.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich DF, Thanos CG. Intracompartmental delivery of CNTF as therapy for Huntington's disease and retinitis pigmentosa. Current gene therapy. 2006;6:147–59. doi: 10.2174/156652306775515547. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Winn SR, Hantraye PM, Peschanski M, Chen EY, et al. Protective effect of encapsulated cells producing neurotrophic factor CNTF in a monkey model of Huntington's disease. Nature. 1997b;386:395–9. doi: 10.1038/386395a0. [DOI] [PubMed] [Google Scholar]
- Fang M, He D, Zhang F, Hu Z, Yang J, et al. Antineuroinflammatory and neurotrophic effects of CNTF and C16 peptide in an acute experimental autoimmune encephalomyelitis rat model. Frontiers in neuroanatomy. 2013;7:4. doi: 10.3389/fnana.2013.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C, Zhang C, Shao X, Liu Q, Qian Y, et al. Enhancement of nose-to-brain delivery of basic fibroblast growth factor for improving rat memory impairments induced by co-injection of beta-amyloid and ibotenic acid into the bilateral hippocampus. International journal of pharmaceutics. 2012;423:226–34. doi: 10.1016/j.ijpharm.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Fortin D, Rom E, Sun H, Yayon A, Bansal R. Distinct fibroblast growth factor (FGF)/FGF receptor signaling pairs initiate diverse cellular responses in the oligodendrocyte lineage. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:7470–9. doi: 10.1523/JNEUROSCI.2120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank JA, Richert N, Lewis B, Bash C, Howard T, et al. A pilot study of recombinant insulin-like growth factor-1 in seven multiple sderosis patients. Mult Scler. 2002;8:24–9. doi: 10.1191/1352458502ms768oa. [DOI] [PubMed] [Google Scholar]
- Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–93. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- From R, Eilam R, Bar-Lev DD, Levin-Zaidman S, Tsoory M, et al. Oligodendrogenesis and myelinogenesis during postnatal development effect of glatiramer acetate. Glia. 2014;62:649–65. doi: 10.1002/glia.22632. [DOI] [PubMed] [Google Scholar]
- Fulmer CG, VonDran MW, Stillman AA, Huang Y, Hempstead BL, Dreyfus CF. Astrocyte-derived BDNF supports myelin protein synthesis after cuprizone-induced demyelination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:8186–96. doi: 10.1523/JNEUROSCI.4267-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusho M, Kaga Y, Ishii A, Hebert JM, Bansal R. Fibroblast growth factor signaling is required for the generation of oligodendrocyte progenitors from the embryonic forebrain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:5055–66. doi: 10.1523/JNEUROSCI.4800-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusho M, Roulois AJ, Franklin RJ, Bansal R. Fibroblast growth factor signaling in oligodendrocyte-lineage cells facilitates recovery of chronically demyelinated lesions but is redundant in acute lesions. Glia. 2015;63:1714–28. doi: 10.1002/glia.22838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Deneen B. Glial development: the crossroads of regeneration and repair in the CNS. Neuron. 2014;83:283–308. doi: 10.1016/j.neuron.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbayo E, Montero-Menei CN, Ansorena E, Lanciego JL, Aymerich MS, Blanco-Prieto MJ. Effective GDNF brain delivery using microspheres--a promising strategy for Parkinson's disease. Journal of controlled release : official journal of the Controlled Release Society. 2009;135:119–26. doi: 10.1016/j.jconrel.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Garcia P, Youssef I, Utvik JK, Florent-Bechard S, Barthelemy V, et al. Ciliary neurotrophic factor cell-based delivery prevents synaptic impairment and improves memory in mouse models of Alzheimer's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7516–27. doi: 10.1523/JNEUROSCI.4182-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasmi M, Herzog CD, Brandon EP, Cunningham JJ, Ramirez GA, et al. Striatal delivery of neurturin by CERE-120, an AAV2 vector for the treatment of dopaminergic neuron degeneration in Parkinson's disease. Molecular therapy : the journal of the American Society of Gene Therapy. 2007;15:62–8. doi: 10.1038/sj.mt.6300010. [DOI] [PubMed] [Google Scholar]
- Gelfand Y, Kaplitt MG. Gene therapy for psychiatric disorders. World neurosurgery. 2013;80:S32 e11–8. doi: 10.1016/j.wneu.2012.12.028. [DOI] [PubMed] [Google Scholar]
- Genoud S, Maricic I, Kumar V, Gage FH. Targeted expression of IGF-1 in the central nervous system fails to protect mice from experimental autoimmune encephalomyelitis. Journal of neuroimmunology. 2005;168:40–5. doi: 10.1016/j.jneuroim.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Gerdoni E, Gallo B, Casazza S, Musio S, Bonanni I, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Annals of neurology. 2007;61:219–27. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- Gharibi T, Ahmadi M, Seyfizadeh N, Jadidi-Niaragh F, Yousefi M. Immunomodulatory characteristics of mesenchymal stem cells and their role in the treatment of multiple sclerosis. Cellular immunology. 2015;293:113–21. doi: 10.1016/j.cellimm.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Gielen A, Khademi M, Muhallab S, Olsson T, Piehl F. Increased brain-derived neurotrophic factor expression in white blood cells of relapsing-remitting multiple sclerosis patients. Scandinavian journal of immunology. 2003;57:493–7. doi: 10.1046/j.1365-3083.2003.01260.x. [DOI] [PubMed] [Google Scholar]
- Giess R, Maurer M, Linker R, Gold R, Warmuth-Metz M, et al. Association of a null mutation in the CNTF gene with early onset of multiple sclerosis. Archives of neurology. 2002;59:407–9. doi: 10.1001/archneur.59.3.407. [DOI] [PubMed] [Google Scholar]
- Giralt A, Carreton O, Lao-Peregrin C, Martin ED, Alberch J. Conditional BDNF release under pathological conditions improves Huntington's disease pathology by delaying neuronal dysfunction. Molecular neurodegeneration. 2011;6:71. doi: 10.1186/1750-1326-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransee HM, Zhan WZ, Sieck GC, Mantilla CB. Localized delivery of brain-derived neurotrophic factor-expressing mesenchymal stem cells enhances functional recovery following cervical spinal cord injury. Journal of neurotrauma. 2015;32:185–93. doi: 10.1089/neu.2014.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Long D, Song C, Li X. Recombinant human NGF-loaded microspheres promote survival of basal forebrain cholinergic neurons and improve memory impairments of spatial learning in the rat model of Alzheimer's disease with fimbria-fornix lesion. Neuroscience letters. 2009;453:204–9. doi: 10.1016/j.neulet.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Gudi V, Skuljec J, Yildiz O, Frichert K, Skripuletz T, et al. Spatial and temporal profiles of growth factor expression during CNS demyelination reveal the dynamics of repair priming. PloS one. 2011;6:e22623. doi: 10.1371/journal.pone.0022623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamby ME, Sofroniew MV. Reactive astrocytes as therapeutic targets for CNS disorders. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2010;7:494–506. doi: 10.1016/j.nurt.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harirchian MH, Tekieh AH, Modabbernia A, Aghamollaii V, Tafakhori A, et al. Serum and CSF PDGF-AA and FGF-2 in relapsing-remitting multiple sclerosis: a case-control study. European journal of neurology. 2012;19:241–7. doi: 10.1111/j.1468-1331.2011.03476.x. [DOI] [PubMed] [Google Scholar]
- Hinks GL, Franklin RJ. Distinctive patterns of PDGF-A, FGF-2, IGF-I, and TGF-beta1 gene expression during remyelination of experimentally-induced spinal cord demyelination. Molecular and cellular neurosciences. 1999;14:153–68. doi: 10.1006/mcne.1999.0771. [DOI] [PubMed] [Google Scholar]
- Hoffmann V, Hardt C. A null mutation in the CNTF gene is not associated with early onset of multiple sclerosis. Archives of neurology. 2002;59:1974. doi: 10.1001/archneur.59.12.1974. author reply 74-5. [DOI] [PubMed] [Google Scholar]
- Hoffmann V, Pohlau D, Przuntek H, Epplen JT, Hardt C. A null mutation within the ciliary neurotrophic factor (CNTF)-gene: implications for susceptibility and disease severity in patients with multiple sclerosis. Genes and immunity. 2002;3:53–5. doi: 10.1038/sj.gene.6363818. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Neurotrophins: roles in neuronal development and function. Annual review of neuroscience. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip NY, McClain J, Barrezueta NX, Aldrich TH, Pan L, et al. The alpha component of the CNTF receptor is required for signaling and defines potential CNTF targets in the adult and during development. Neuron. 1993;10:89–102. doi: 10.1016/0896-6273(93)90245-m. [DOI] [PubMed] [Google Scholar]
- Javeri S, Rodi M, Tary-Lehmann M, Lehmann PV, Addicks K, Kuerten S. Involvement of brain-derived neurotrophic factor (BDNF) in MP4-induced autoimmune encephalomyelitis. Clin Immunol. 2010;137:181–9. doi: 10.1016/j.clim.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Joliot A, Pernelle C, Deagostini-Bazin H, Prochiantz A. Antennapedia homeobox peptide regulates neural morphogenesis. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:1864–8. doi: 10.1073/pnas.88.5.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Anderson JM, Phuah CL, Fox EJ, Selmaj K, et al. Improvement in disability after alemtuzumab treatment of multiple sclerosis is associated with neuroprotective autoimmunity. Brain : a journal of neurology. 2010;133:2232–47. doi: 10.1093/brain/awq176. [DOI] [PubMed] [Google Scholar]
- Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Archives of neurology. 2010;67:1187–94. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis I, Grigoriadis N, Gowda-Kurkalli B, Mizrachi-Kol R, Ben-Hur T, et al. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. Archives of neurology. 2008;65:753–61. doi: 10.1001/archneur.65.6.753. [DOI] [PubMed] [Google Scholar]
- Kauper K, McGovern C, Sherman S, Heatherton P, Rapoza R, et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases. Investigative ophthalmology & visual science. 2012;53:7484–91. doi: 10.1167/iovs.12-9970. [DOI] [PubMed] [Google Scholar]
- Kerschensteiner M, Gallmeier E, Behrens L, Leal VV, Misgeld T, et al. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? The Journal of experimental medicine. 1999;189:865–70. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic E, Kilic U, Hermann DM. TAT-GDNF in neurodegeneration and ischemic stroke. CNS drug reviews. 2005;11:369–78. doi: 10.1111/j.1527-3458.2005.tb00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Yoles E, Porat Z, Cohen A, Mor F, et al. T cell immunity to copolymer 1 confers neuroprotection on the damaged optic nerve: possible therapy for optic neuropathies. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:7446–51. doi: 10.1073/pnas.97.13.7446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov VV, Skladchikova G, Hinsby AM, Jensen PH, Kulahin N, et al. Structural basis for a direct interaction between FGFR1 and NCAM and evidence for a regulatory role of ATP. Structure. 2003;11:691–701. doi: 10.1016/s0969-2126(03)00096-0. [DOI] [PubMed] [Google Scholar]
- Klementiev B, Novikova T, Novitskaya V, Walmod PS, Dmytriyeva O, et al. A neural cell adhesion molecule-derived peptide reduces neuropathological signs and cognitive impairment induced by Abeta25-35. Neuroscience. 2007;145:209–24. doi: 10.1016/j.neuroscience.2006.11.060. [DOI] [PubMed] [Google Scholar]
- Koehler NK, Roebbert M, Dehghani K, Ballmaier M, Claus P, et al. Up-regulation of platelet-derived growth factor by peripheral-blood leukocytes during experimental allergic encephalomyelitis. Journal of neuroscience research. 2008;86:392–402. doi: 10.1002/jnr.21497. [DOI] [PubMed] [Google Scholar]
- Komoly S, Hudson LD, Webster HD, Bondy CA. Insulin-like growth factor I gene expression is induced in astrocytes during experimental demyelination. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:1894–8. doi: 10.1073/pnas.89.5.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen M, Birch D, Morck Nielsen H. Applications and Challenges for Use of Cell-Penetrating Peptides as Delivery Vectors for Peptide and Protein Cargos. International journal of molecular sciences. 2016;17 doi: 10.3390/ijms17020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Remington L, Cognet I, Bourbonniere L, Zehntner S, et al. Continued administration of ciliary neurotrophic factor protects mice from inflammatory pathology in experimental autoimmune encephalomyelitis. The American journal of pathology. 2006;169:584–98. doi: 10.2353/ajpath.2006.051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzillo R, Di Somma C, Quarantelli M, Ventrella G, Gasperi M, et al. Insulin-like growth factor (IGF)-I and IGF-binding protein-3 serum levels in relapsing-remitting and secondary progressive multiple sclerosis patients. European journal of neurology. 2011;18:1402–6. doi: 10.1111/j.1468-1331.2011.03433.x. [DOI] [PubMed] [Google Scholar]
- Lee DH, Geyer E, Flach AC, Jung K, Gold R, et al. Central nervous system rather than immune cell-derived BDNF mediates axonal protective effects early in autoimmune demyelination. Acta neuropathologica. 2012;123:247–58. doi: 10.1007/s00401-011-0890-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Quigley L, Yao DL, Hudson LD, Brenner M, et al. Chronic relapsing experimental autoimmune encephalomyelitis: effects of insulin-like growth factor-I treatment on clinical deficits, lesion severity, glial responses, and blood brain barrier defects. Journal of neuropathology and experimental neurology. 1998;57:426–38. doi: 10.1097/00005072-199805000-00006. [DOI] [PubMed] [Google Scholar]
- Li YH, Yu JZ, Liu CY, Zhang H, Zhang HF, et al. Intranasal delivery of FSD-C10, a novel Rho kinase inhibitor, exhibits therapeutic potential in experimental autoimmune encephalomyelitis. Immunology. 2014;143:219–29. doi: 10.1111/imm.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker RA, Lee DH, Demir S, Wiese S, Kruse N, et al. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: therapeutic implications in a model of multiple sclerosis. Brain : a journal of neurology. 2010;133:2248–63. doi: 10.1093/brain/awq179. [DOI] [PubMed] [Google Scholar]
- Linker RA, Maurer M, Gaupp S, Martini R, Holtmann B, et al. CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nature medicine. 2002;8:620–4. doi: 10.1038/nm0602-620. [DOI] [PubMed] [Google Scholar]
- Liu X, Linnington C, Webster HD, Lassmann S, Yao DL, et al. Insulin-like growth factor-I treatment reduces immune cell responses in acute non-demyelinative experimental autoimmune encephalomyelitis. Journal of neuroscience research. 1997;47:531–8. doi: 10.1002/(sici)1097-4547(19970301)47:5<531::aid-jnr8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Liu X, Mashour GA, Webster HF, Kurtz A. Basic FGF and FGF receptor 1 are expressed in microglia during experimental autoimmune encephalomyelitis: temporally distinct expression of midkine and pleiotrophin. Glia. 1998;24:390–7. doi: 10.1002/(sici)1098-1136(199812)24:4<390::aid-glia4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Liu X, Yao DL, Bondy CA, Brenner M, Hudson LD, et al. Astrocytes express insulin-like growth factor-I (IGF-I) and its binding protein, IGFBP-2, during demyelination induced by experimental autoimmune encephalomyelitis. Molecular and cellular neurosciences. 1994;5:418–30. doi: 10.1006/mcne.1994.1052. [DOI] [PubMed] [Google Scholar]
- Liu X, Yao DL, Webster H. Insulin-like growth factor I treatment reduces clinical deficits and lesion severity in acute demyelinating experimental autoimmune encephalomyelitis. Mult Scler. 1995;1:2–9. doi: 10.1177/135245859500100102. [DOI] [PubMed] [Google Scholar]
- Louis JC, Magal E, Takayama S, Varon S. CNTF protection of oligodendrocytes against natural and tumor necrosis factor-induced death. Science. 1993;259:689–92. doi: 10.1126/science.8430320. [DOI] [PubMed] [Google Scholar]
- Lu Z, Hu X, Zhu C, Wang D, Zheng X, Liu Q. Overexpression of CNTF in Mesenchymal Stem Cells reduces demyelination and induces clinical recovery in experimental autoimmune encephalomyelitis mice. Journal of neuroimmunology. 2009;206:58–69. doi: 10.1016/j.jneuroim.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–11. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain : a journal of neurology. 1999;122(Pt 12):2279–95. doi: 10.1093/brain/122.12.2279. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Solanky M, Menonna J, Chapin J, Li W, Dowling P. Platelet-derived growth factor-alpha receptor-positive oligodendroglia are frequent in multiple sclerosis lesions. Annals of neurology. 2001;49:776–85. doi: 10.1002/ana.1015. [DOI] [PubMed] [Google Scholar]
- Maier K, Rau CR, Storch MK, Sattler MB, Demmer I, et al. Ciliary neurotrophic factor protects retinal ganglion cells from secondary cell death during acute autoimmune optic neuritis in rats. Brain Pathol. 2004;14:378–87. doi: 10.1111/j.1750-3639.2004.tb00081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makar TK, Bever CT, Singh IS, Royal W, Sahu SN, et al. Brain-derived neurotrophic factor gene delivery in an animal model of multiple sclerosis using bone marrow stem cells as a vehicle. Journal of neuroimmunology. 2009;210:40–51. doi: 10.1016/j.jneuroim.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Makar TK, Nimmagadda VK, Trisler D, Bever CT., Jr Cell-based delivery of brain-derived neurotrophic factor in experimental allergic encephalomyelitis. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2014;34:641–7. doi: 10.1089/jir.2013.0160. [DOI] [PubMed] [Google Scholar]
- Makar TK, Trisler D, Sura KT, Sultana S, Patel N, Bever CT. Brain derived neurotrophic factor treatment reduces inflammation and apoptosis in experimental allergic encephalomyelitis. Journal of the neurological sciences. 2008;270:70–6. doi: 10.1016/j.jns.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Mason JL, Xuan S, Dragatsis I, Efstratiadis A, Goldman JE. Insulin-like growth factor (IGF) signaling through type 1 IGF receptor plays an important role in remyelination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:7710–8. doi: 10.1523/JNEUROSCI.23-20-07710.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JL, Ye P, Suzuki K, D'Ercole AJ, Matsushima GK. Insulin-like growth factor-1 inhibits mature oligodendrocyte apoptosis during primary demyelination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:5703–8. doi: 10.1523/JNEUROSCI.20-15-05703.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massa SM, Yang T, Xie Y, Shi J, Bilgen M, et al. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. The Journal of clinical investigation. 2010;120:1774–85. doi: 10.1172/JCI41356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JS, Blakemore WF, Franklin RJ. The effects of the growth factor-antagonist, trapidil, on remyelination in the CNS. Neuropathology and applied neurobiology. 1997;23:50–8. [PubMed] [Google Scholar]
- McMorris FA, Furlanetto RW, Mozell RL, Carson MJ, Raible DW. Regulation of oligodendrocyte development by insulin-like growth factors and cyclic nucleotides. Annals of the New York Academy of Sciences. 1990;605:101–9. doi: 10.1111/j.1749-6632.1990.tb42385.x. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:5354–65. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messersmith DJ, Murtie JC, Le TQ, Frost EE, Armstrong RC. Fibroblast growth factor 2 (FGF2) and FGF receptor expression in an experimental demyelinating disease with extensive remyelination. Journal of neuroscience research. 2000;62:241–56. doi: 10.1002/1097-4547(20001015)62:2<241::AID-JNR9>3.0.CO;2-D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RG, Petajan JH, Bryan WW, Armon C, Barohn RJ, et al. A placebo-controlled trial of recombinant human ciliary neurotrophic (rhCNTF) factor in amyotrophic lateral sclerosis. rhCNTF ALS Study Group. Annals of neurology. 1996;39:256–60. doi: 10.1002/ana.410390215. [DOI] [PubMed] [Google Scholar]
- Min J, Singh S, Fitzgerald-Bocarsly P, Wood TL. Insulin-like growth factor I regulates G2/M progression through mammalian target of rapamycin signaling in oligodendrocyte progenitors. Glia. 2012;60:1684–95. doi: 10.1002/glia.22387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirowska-Guzel D. The role of neurotrophic factors in the pathology and treatment of multiple sclerosis. Immunopharmacology and immunotoxicology. 2009;31:32–8. doi: 10.1080/08923970802379819. [DOI] [PubMed] [Google Scholar]
- Moore CS, Abdullah SL, Brown A, Arulpragasam A, Crocker SJ. How factors secreted from astrocytes impact myelin repair. Journal of neuroscience research. 2011;89:13–21. doi: 10.1002/jnr.22482. [DOI] [PubMed] [Google Scholar]
- Mori F, Nicoletti CG, Rossi S, Motta C, Kusayanagi H, et al. Growth factors and synaptic plasticity in relapsing-remitting multiple sclerosis. Neuromolecular medicine. 2014;16:490–8. doi: 10.1007/s12017-014-8297-7. [DOI] [PubMed] [Google Scholar]
- Mori F, Rossi S, Piccinin S, Motta C, Mango D, et al. Synaptic plasticity and PDGF signaling defects underlie clinical progression in multiple sclerosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:19112–9. doi: 10.1523/JNEUROSCI.2536-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtie JC, Zhou YX, Le TQ, Vana AC, Armstrong RC. PDGF and FGF2 pathways regulate distinct oligodendrocyte lineage responses in experimental demyelination with spontaneous remyelination. Neurobiology of disease. 2005;19:171–82. doi: 10.1016/j.nbd.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nature reviews Drug discovery. 2011;10:209–19. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- Nygardas M, Paavilainen H, Muther N, Nagel CH, Roytta M, et al. A herpes simplex virus-derived replicative vector expressing LIF limits experimental demyelinating disease and modulates autoimmunity. PloS one. 2013;8:e6420. doi: 10.1371/journal.pone.0064200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary MT, Hinks GL, Charlton HM, Franklin RJ. Increasing local levels of IGF-I mRNA expression using adenoviral vectors does not alter oligodendrocyte remyelination in the CNS of aged rats. Molecular and cellular neurosciences. 2002;19:32–42. doi: 10.1006/mcne.2001.1062. [DOI] [PubMed] [Google Scholar]
- Oh LY, Denninger A, Colvin JS, Vyas A, Tole S, et al. Fibroblast growth factor receptor 3 signaling regulates the onset of oligodendrocyte terminal differentiation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:883–94. doi: 10.1523/JNEUROSCI.23-03-00883.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo B, Gabbott PL, Rezaie P, Corbett N, Medvedev NI, et al. An NCAM mimetic, FGL, alters hippocampal cellular morphometry in young adult (4 month-old) rats. Neurochemical research. 2013;38:1208–18. doi: 10.1007/s11064-012-0908-9. [DOI] [PubMed] [Google Scholar]
- Ojo B, Rezaie P, Gabbott PL, Cowely TR, Medvedev NI, et al. A neural cell adhesion molecule-derived peptide, FGL, attenuates glial cell activation in the aged hippocampus. Experimental neurology. 2011;232:318–28. doi: 10.1016/j.expneurol.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–61. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- Paradiso B, Marconi P, Zucchini S, Berto E, Binaschi A, et al. Localized delivery of fibroblast growth factor-2 and brain-derived neurotrophic factor reduces spontaneous seizures in an epilepsy model. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7191–6. doi: 10.1073/pnas.0810710106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge WM. Blood-brain barrier drug targeting enables neuroprotection in brain ischemia following delayed intravenous administration of neurotrophins. Advances in experimental medicine and biology. 2002;513:397–430. doi: 10.1007/978-1-4615-0123-7_15. [DOI] [PubMed] [Google Scholar]
- Patching SG. Glucose Transporters at the Blood-Brain Barrier: Function, Regulation and Gateways for Drug Delivery. Molecular neurobiology. 2016 doi: 10.1007/s12035-015-9672-6. [DOI] [PubMed] [Google Scholar]
- Petereit HF, Lindemann H, Schoppe S. Effect of immunomodulatory drugs on in vitro production of brain-derived neurotrophic factor. Mult Scler. 2003;9:16–20. doi: 10.1191/1352458503ms869oa. [DOI] [PubMed] [Google Scholar]
- Pirttila T, Vanhatalo S, Turpeinen U, Riikonen R. Cerebrospinal fluid insulin-like growth factor-1, insulin growth factor binding protein-2 or nitric oxide are not increased in MS or ALS. Acta neurologica Scandinavica. 2004;109:337–41. doi: 10.1111/j.1600-0404.2004.00223.x. [DOI] [PubMed] [Google Scholar]
- Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain research Molecular brain research. 1996;36:280–6. doi: 10.1016/0169-328x(95)00250-v. [DOI] [PubMed] [Google Scholar]
- Poljakovic Z, Zurak N, Brinar V, Korsic M, Basic S, Hajnsek S. Growth hormone and insulin growth factor-I levels in plasma and cerebrospinal fluid of patients with multiple sclerosis. Clinical neurology and neurosurgery. 2006;108:255–8. doi: 10.1016/j.clineuro.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Frey WH, 2nd, Baker LD, Cholerton B, et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiology of aging. 2006;27:451–8. doi: 10.1016/j.neurobiolaging.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Reigstad LJ, Varhaug JE, Lillehaug JR. Structural and functional specificities of PDGF-C and PDGF-D, the novel members of the platelet-derived growth factors family. The FEBS journal. 2005;272:5723–41. doi: 10.1111/j.1742-4658.2005.04989.x. [DOI] [PubMed] [Google Scholar]
- Richardson WD, Young KM, Tripathi RB, McKenzie I. NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron. 2011;70:661–73. doi: 10.1016/j.neuron.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GA, Spada V, Hamill K, Bornstein MB. Insulin-like growth factor I increases myelination and inhibits demyelination in cultured organotypic nerve tissue. Brain research Developmental brain research. 1995;88:102–8. doi: 10.1016/0165-3806(95)00088-u. [DOI] [PubMed] [Google Scholar]
- Rottlaender A, Villwock H, Addicks K, Kuerten S. Neuroprotective role of fibroblast growth factor-2 in experimental autoimmune encephalomyelitis. Immunology. 2011;133:370–8. doi: 10.1111/j.1365-2567.2011.03450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffini F, Furlan R, Poliani PL, Brambilla E, Marconi PC, et al. Fibroblast growth factor-II gene therapy reverts the clinical course and the pathological signs of chronic experimental autoimmune encephalomyelitis in C57BL/6 mice. Gene therapy. 2001;8:1207–13. doi: 10.1038/sj.gt.3301523. [DOI] [PubMed] [Google Scholar]
- Ruitenberg MJ, Blits B, Dijkhuizen PA, te Beek ET, Bakker A, et al. Adeno-associated viral vector-mediated gene transfer of brain-derived neurotrophic factor reverses atrophy of rubrospinal neurons following both acute and chronic spinal cord injury. Neurobiology of disease. 2004;15:394–406. doi: 10.1016/j.nbd.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Sagot Y, Tan SA, Hammang JP, Aebischer P, Kato AC. GDNF slows loss of motoneurons but not axonal degeneration or premature death of pmn/pmn mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:2335–41. doi: 10.1523/JNEUROSCI.16-07-02335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarchielli P, Di Filippo M, Ercolani MV, Chiasserini D, Mattioni A, et al. Fibroblast growth factor-2 levels are elevated in the cerebrospinal fluid of multiple sclerosis patients. Neuroscience letters. 2008;435:223–8. doi: 10.1016/j.neulet.2008.02.040. [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Greco L, Stipa A, Floridi A, Gallai V. Brain-derived neurotrophic factor in patients with multiple sclerosis. Journal of neuroimmunology. 2002;132:180–8. doi: 10.1016/s0165-5728(02)00319-3. [DOI] [PubMed] [Google Scholar]
- Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:3896–901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skihar V, Silva C, Chojnacki A, Doring A, Stallcup WB, et al. Promoting oligodendrogenesis and myelin repair using the multiple sclerosis medication glatiramer acetate. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17992–7. doi: 10.1073/pnas.0909607106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadelmann C, Kerschensteiner M, Misgeld T, Bruck W, Hohlfeld R, Lassmann H. BDNF and gp145trkB in multiple sclerosis brain lesions: neuroprotective interactions between immune and neuronal cells? Brain : a journal of neurology. 2002;125:75–85. doi: 10.1093/brain/awf015. [DOI] [PubMed] [Google Scholar]
- Stankoff B, Aigrot MS, Noel F, Wattilliaux A, Zalc B, Lubetzki C. Ciliary neurotrophic factor (CNTF) enhances myelin formation: a novel role for CNTF and CNTF-related molecules. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:9221–7. doi: 10.1523/JNEUROSCI.22-21-09221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su JJ, Osoegawa M, Matsuoka T, Minohara M, Tanaka M, et al. Upregulation of vascular growth factors in multiple sclerosis: correlation with MRI findings. Journal of the neurological sciences. 2006;243:21–30. doi: 10.1016/j.jns.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Talbott JF, Cao Q, Bertram J, Nkansah M, Benton RL, et al. CNTF promotes the survival and differentiation of adult spinal cord-derived oligodendrocyte precursor cells in vitro but fails to promote remyelination in vivo. Experimental neurology. 2007;204:485–9. doi: 10.1016/j.expneurol.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Murakami K, Bando Y, Yoshida S. Minocycline reduces remyelination by suppressing ciliary neurotrophic factor expression after cuprizone-induced demyelination. Journal of neurochemistry. 2013;127:259–70. doi: 10.1111/jnc.12289. [DOI] [PubMed] [Google Scholar]
- Tao W, Wen R, Goddard MB, Sherman SD, O'Rourke PJ, et al. Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Investigative ophthalmology & visual science. 2002;43:3292–8. [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and activity-dependent plasticity. Progress in brain research. 2000;128:183–91. doi: 10.1016/S0079-6123(00)28016-3. [DOI] [PubMed] [Google Scholar]
- Thone J, Ellrichmann G, Seubert S, Peruga I, Lee DH, et al. Modulation of autoimmune demyelination by laquinimod via induction of brain-derived neurotrophic factor. The American journal of pathology. 2012;180:267–74. doi: 10.1016/j.ajpath.2011.09.037. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–96. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Tobin JE, Xie M, Le TQ, Song SK, Armstrong RC. Reduced axonopathy and enhanced remyelination after chronic demyelination in fibroblast growth factor 2 (Fgf2)-null mice: differential detection with diffusion tensor imaging. Journal of neuropathology and experimental neurology. 2011;70:157–65. doi: 10.1097/NEN.0b013e31820937e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai LK, Chen YC, Cheng WC, Ting CH, Dodge JC, et al. IGF-1 delivery to CNS attenuates motor neuron cell death but does not improve motor function in type III SMA mice. Neurobiology of disease. 2012;45:272–9. doi: 10.1016/j.nbd.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Tsiperson V, Huang Y, Bagayogo I, Song Y, VonDran MW, et al. Brain-derived neurotrophic factor deficiency restricts proliferation of oligodendrocyte progenitors following cuprizone-induced demyelination. ASN neuro. 2015;7 doi: 10.1177/1759091414566878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CA, Eren-Kocak E, Inui EG, Watson SJ, Akil H. Dysregulated fibroblast growth factor (FGF) signaling in neurological and psychiatric disorders. Seminars in cell & developmental biology. 2015 doi: 10.1016/j.semcdb.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuszynski MH, Yang JH, Barba D, U HS, Bakay RA, et al. Nerve Growth Factor Gene Therapy: Activation of Neuronal Responses in Alzheimer Disease. JAMA neurology. 2015;72:1139–47. doi: 10.1001/jamaneurol.2015.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaka SR, Murthy SN, Balaji A, Repka MA. Delivery of brain-derived neurotrophic factor via nose-to-brain pathway. Pharmaceutical research. 2012;29:441–7. doi: 10.1007/s11095-011-0572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vana AC, Flint NC, Harwood NE, Le TQ, Fruttiger M, Armstrong RC. Platelet-derived growth factor promotes repair of chronically demyelinated white matter. Journal of neuropathology and experimental neurology. 2007;66:975–88. doi: 10.1097/NEN.0b013e3181587d46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernerey J, Macchi M, Magalon K, Cayre M, Durbec P. Ciliary neurotrophic factor controls progenitor migration during remyelination in the adult rodent brain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:3240–50. doi: 10.1523/JNEUROSCI.2579-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vondran MW, Clinton-Luke P, Honeywell JZ, Dreyfus CF. BDNF+/- mice exhibit deficits in oligodendrocyte lineage cells of the basal forebrain. Glia. 2010;58:848–56. doi: 10.1002/glia.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VonDran MW, Singh H, Honeywell JZ, Dreyfus CF. Levels of BDNF impact oligodendrocyte lineage cells following a cuprizone lesion. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:14182–90. doi: 10.1523/JNEUROSCI.6595-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington AE, Bieber AJ, Ciric B, Pease LR, Van Keulen V, Rodriguez M. A recombinant human IgM promotes myelin repair after a single, very low dose. Journal of neuroscience research. 2007;85:967–76. doi: 10.1002/jnr.21217. [DOI] [PubMed] [Google Scholar]
- Watanabe D, Yoshimura R, Khalil M, Yoshida K, Kishimoto T, et al. Characteristic localization of gp130 (the signal-transducing receptor component used in common for IL-6/IL-11/CNTF/LIF/OSM) in the rat brain. The European journal of neuroscience. 1996;8:1630–40. doi: 10.1111/j.1460-9568.1996.tb01307.x. [DOI] [PubMed] [Google Scholar]
- Watzlawik JO, Warrington AE, Rodriguez M. PDGF is required for remyelination-promoting IgM stimulation of oligodendrocyte progenitor cell proliferation. PloS one. 2013;8:e5514. doi: 10.1371/journal.pone.0055149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wekerle H, Lassman H. The immunology of inflammatory demyelinating disease. In: Compston A, Confavreux C, Lassmann H, McDonald I, Miller D, Noseworthy J, Smith K, Werkle H, editors. McAlpine's Multiple Sclerosis 4th ed. Churchill Livingston Elsevier; Linn, MO USA: 2006. pp. 491–555. [Google Scholar]
- Wilczak N, Chesik D, Hoekstra D, De Keyser J. IGF binding protein alterations on periplaque oligodendrocytes in multiple sclerosis: implications for remyelination. Neurochemistry international. 2008;52:1431–5. doi: 10.1016/j.neuint.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Wilczak N, De Keyser J. Insulin-like growth factor-I receptors in normal appearing white matter and chronic plaques in multiple sclerosis. Brain research. 1997;772:243–6. doi: 10.1016/s0006-8993(97)00940-2. [DOI] [PubMed] [Google Scholar]
- Wilczak N, Ramsaransing GS, Mostert J, Chesik D, De Keyser J. Serum levels of insulin-like growth factor-1 and insulin-like growth factor binding protein-3 in relapsing and primary progressive multiple sclerosis. Mult Scler. 2005;11:13–5. doi: 10.1191/1352458505ms1123oa. [DOI] [PubMed] [Google Scholar]
- Wong AW, Giuffrida L, Wood R, Peckham H, Gonsalvez D, et al. TDP6, a brain-derived neurotrophic factor-based trkB peptide mimetic, promotes oligodendrocyte myelination. Molecular and cellular neurosciences. 2014;63:132–40. doi: 10.1016/j.mcn.2014.10.002. [DOI] [PubMed] [Google Scholar]
- Wong AW, Xiao J, Kemper D, Kilpatrick TJ, Murray SS. Oligodendroglial expression of TrkB independently regulates myelination and progenitor cell proliferation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:4947–57. doi: 10.1523/JNEUROSCI.3990-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodbury ME, Ikezu T. Fibroblast growth factor-2 signaling in neurogenesis and neurodegeneration. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2014;9:92–101. doi: 10.1007/s11481-013-9501-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff RH, Franklin RJ. Growth factors and remyelination in the CNS. Histology and histopathology. 1997;12:459–66. [PubMed] [Google Scholar]
- Woodruff RH, Fruttiger M, Richardson WD, Franklin RJ. Platelet-derived growth factor regulates oligodendrocyte progenitor numbers in adult CNS and their response following CNS demyelination. Molecular and cellular neurosciences. 2004;25:252–62. doi: 10.1016/j.mcn.2003.10.014. [DOI] [PubMed] [Google Scholar]
- Wu Y, Luo X, Liu X, Liu D, Wang X, et al. Intraperitoneal Administration of a Novel TAT-BDNF Peptide Ameliorates Cognitive Impairments via Modulating Multiple Pathways in Two Alzheimer's Rodent Models. Scientific reports. 2015;5:1503. doi: 10.1038/srep15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J, Wong AW, Willingham MM, van den Buuse M, Kilpatrick TJ, Murray SS. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neuro-Signals. 2010;18:186–202. doi: 10.1159/000323170. [DOI] [PubMed] [Google Scholar]
- Yao DL, Liu X, Hudson LD, Webster HD. Insulin-like growth factor I treatment reduces demyelination and up-regulates gene expression of myelin-related proteins in experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:6190–4. doi: 10.1073/pnas.92.13.6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao DL, Liu X, Hudson LD, Webster HD. Insulin-like growth factor-I given subcutaneously reduces clinical deficits, decreases lesion severity and upregulates synthesis of myelin proteins in experimental autoimmune encephalomyelitis. Life sciences. 1996;58:1301–6. doi: 10.1016/0024-3205(96)00095-1. [DOI] [PubMed] [Google Scholar]
- Yemisci M, Caban S, Gursoy-Ozdemir Y, Lule S, Novoa-Carballal R, et al. Systemically administered brain-targeted nanoparticles transport peptides across the blood-brain barrier and provide neuroprotection. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2015;35:469–75. doi: 10.1038/jcbfm.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–61. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- Zeger M, Popken G, Zhang J, Xuan S, Lu QR, et al. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007;55:400–11. doi: 10.1002/glia.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Li Y, Chen J, Cui Y, Lu M, et al. Human bone marrow stromal cell treatment improves neurological functional recovery in EAE mice. Experimental neurology. 2005;195:16–26. doi: 10.1016/j.expneurol.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Pardridge WM. Conjugation of brain-derived neurotrophic factor to a blood-brain barrier drug targeting system enables neuroprotection in regional brain ischemia following intravenous injection of the neurotrophin. Brain research. 2001;889:49–56. doi: 10.1016/s0006-8993(00)03108-5. [DOI] [PubMed] [Google Scholar]
- Zhou YX, Pannu R, Le TQ, Armstrong RC. Fibroblast growth factor 1 (FGFR1) modulation regulates repair capacity of oligodendrocyte progenitor cells following chronic demyelination. Neurobiology of disease. 2012;45:196–205. doi: 10.1016/j.nbd.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemlinska E, Kugler S, Schachner M, Wewior I, Czarkowska-Bauch J, Skup M. Overexpression of BDNF increases excitability of the lumbar spinal network and leads to robust early locomotor recovery in completely spinalized rats. PloS one. 2014;9:e88833. doi: 10.1371/journal.pone.0088833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemssen T, Kumpfel T, Klinkert WE, Neuhaus O, Hohlfeld R. Glatiramer acetate-specific T-helper 1- and 2-type cell lines produce BDNF: implications for multiple sclerosis therapy. Brain-derived neurotrophic factor. Brain : a journal of neurology. 2002;125:2381–91. doi: 10.1093/brain/awf252. [DOI] [PubMed] [Google Scholar]


