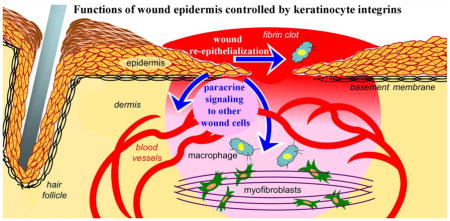
Abstract
During cutaneous wound healing, keratinocyte proliferation and migration are critical for re-epithelialization. In addition, the epidermis secretes growth factors, cytokines, proteases, and matricellular proteins into the wound microenvironment that modify the extracellular matrix and stimulate other wound cells that control the inflammatory response, promote angiogenesis, and facilitate tissue contraction and remodeling. Wound keratinocytes express at least seven different integrins - the major cell adhesion receptors for the extracellular matrix - that collectively control essential cell-autonomous functions to ensure proper re-epithelialization, including migration, proliferation, survival, and basement membrane assembly (Fig. 1, arrow 1). Moreover, it has become evident in recent years that some integrins can regulate paracrine signals from wound epidermis that stimulate other wound cells involved in angiogenesis, contraction and inflammation (Fig. 1, arrows 2 and 3). Importantly, it is likely that abnormal integrin expression or function in the epidermis contributes to wound pathologies such as over-exuberant healing (e.g., hypertrophic scar formation) or diminished healing (e.g., chronic wounds). In this review, we discuss current knowledge of integrin function in the epidermis, which implicates them as attractive therapeutic targets to promote wound healing or treat wound pathologies. We also discuss challenges that arise from the complex roles that multiple integrins play in wound epidermis, which may be regulated through extracellular matrix remodeling that determines ligand availability. Indeed, understanding how different integrin functions are temporally coordinated in wound epidermis, and which integrin functions go awry in pathological wounds, will be important to determine how best to target them clinically to achieve maximum therapeutic benefit.
Keywords: Integrin, Wound healing, Epidermis, Keratinocyte, Extracellular matrix
Graphical Abstract
Introduction
The skin consists of an underlying dermal layer that provides tissue strength and compliance, covered by the stratified epidermis that forms the outer barrier. When the skin is injured, the constituent cells undergo a highly coordinated wound healing response to repair the damaged tissue, close the wound, and restore barrier function (Gurtner et al., 2008). Wound healing is a tightly regulated process that can be divided into distinct, but overlapping stages of hemostasis/clotting, inflammation, re-epithelialization, and tissue remodeling (Gurtner et al., 2008; Martin, 1997). Throughout these stages, the extracellular matrix (ECM) undergoes continuous and temporally regulated changes in composition and structure that provide critical regulatory cues to a variety of wound cells to ensure proper healing. Importantly, abnormalities in mechanical and chemical properties of the ECM contribute to wound pathologies, such as chronic wounds or hypertrophic scars (Kenny and Connelly, 2015; Wong et al., 2013).
In addition to their well characterized roles in wound re-epithelialization, epidermal keratinocytes play a major role in modifying the wound microenvironment through secretion of extracellular factors (e.g., growth factors, extracellular proteases, ECM-associated proteins) that stimulate other cells to facilitate wound healing, including endothelial cells that drive angiogenesis, and fibroblasts/myofibroblasts that deposit a scar and promote contraction (Gurtner et al., 2008; Martin, 1997; Santoro and Gaudino, 2005). In non-wounded skin, basal keratinocytes of the stratified epidermis are adherent to a basement membrane (BM), which is a specialized, sheet-like ECM that consists mainly of laminins (LNs), type IV collagen, and type VII collagen and separates the epidermis from the sub-adjacent connective ECM of the dermis (Burgeson and Christiano, 1997). In addition to maintaining epidermal-dermal adhesion, the BM provides cues to keratinocytes that help control epidermal differentiation, stratification, and hair development. In wounded skin, keratinocytes that are proximal to the injury proliferate and migrate over the changing provisional ECM, which is initially composed of extravasated plasma proteins (e.g., fibrinogen, plasma fibronectin, vitronectin), and is gradually transformed by local expression of cellular fibronectin, type I collagen, and other matrix proteins. Eventual reassembly of an intact BM during wound re-epithelialization is essential to restore barrier function and structural support to the neo-epidermis.
Integrins are the major cell surface receptors for epidermal adhesion to the both the BM in non-wounded skin and the provisional ECM in wounded skin (Hynes, 2002). As discussed in the following sections, integrins control a wide variety of keratinocyte functions during normal wound healing, including migration, proliferation, survival, BM regeneration, and paracrine induction of angiogenesis. Integrin-mediated interactions of keratinocytes with their adjacent ECM are dynamic and bidirectional throughout wound healing, and abnormal integrin expression or function in wound pathologies are likely to contribute to over-exuberant healing (e.g., hypertrophic scars) or diminished healing (e.g., chronic wounds) (reviewed in (Koivisto et al., 2014)). This review will focus on what we currently know about the regulatory roles that integrins play in wound keratinocytes, with regard to both cell-autonomous functions and epidermal functions that modify the wound microenvironment (illustrated in Fig. 1). As space limitations preclude a comprehensive review of the extensive literature in this field, we direct the reader to other reviews for additional coverage of relevant topics (Kenny and Connelly, 2015; Koivisto et al., 2014; Longmate and DiPersio, 2014; Margadant et al., 2010).
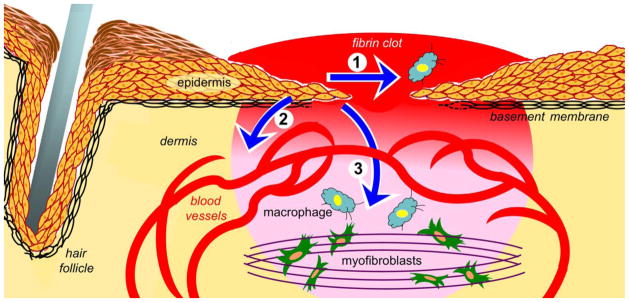
Fig. 1.
Illustration depicts functions of wound epidermis that may be controlled by keratinocyte integrins. Arrow 1 indicates wound re-epithelialization, which is driven by keratinocyte proliferation, local matrix remodeling, and migration. Arrow 2 indicates paracrine signaling from the epidermis to vascular endothelial cells that promotes wound angiogenesis. Arrow 3 indicates paracrine signaling from the epidermis to other wound cells, including inflammatory cells (blue cells) and fibroblasts/myofibroblasts that promote wound contraction (green cells). The wound bed is indicated in red-to-pink gradient.
Overview of integrin function in cell adhesion and signal transduction
All integrins are heterodimeric glycoproteins consisting of an α and a β subunit. Eight different β subunits can each partner in limited combinations with 18 α subunits to form 24 distinct integrins with different, albeit often overlapping ligand-binding specificities (Hynes, 2002). Both α and β subunits consist of a large extracellular domain, a single-pass transmembrane domain, and a relatively short cytoplasmic domain of ~20–70 amino acids (the exception is the β4 subunit cytoplasmic domain of over 1000 amino acids). In addition to binding ECM ligands through their extracellular domains, many integrins are simultaneously linked to the actin cytoskeleton via scaffolding proteins (e.g., talin, vinculin) that bind to their cytoplasmic domains, thereby mediating a physical, transmembrane linkage between the ECM and the cytoskeleton that manifests as focal adhesions (Delon and Brown, 2007; Hynes, 2002; Iwamoto and Calderwood, 2015; Liu et al., 2000). Similarly, the epidermal integrin, α6β4, mediates a linkage between the ECM and the keratin cytoskeleton within hemidesmosomes, which are essential for stable adhesion of the basal keratinocyte layer (Litjens et al., 2006).
Integrins regulate bidirectional signal transduction across the plasma membrane through direct or indirect interactions of their cytoplasmic domains with a wide variety of intracellular signaling and adaptor proteins, such as talin, integrin-linked kinase (ILK), vinculin, kindlins, paxillin, focal adhesion kinase (FAK), and Src. The literature on integrin-mediated signal transduction has been reviewed extensively (Hynes, 2002; Iwamoto and Calderwood, 2015; Legate and Fassler, 2009; Schwartz and Ginsberg, 2002; Winograd-Katz et al., 2014), and it was recently estimated that the integrin “adhesome network” includes more than 180 potential signaling and adaptor proteins (Zaidel-Bar and Geiger, 2010). Here, we will discuss FAK activation as an instructive example of “outside-in” integrin signaling that feeds into a number of downstream pathways (Cary and Guan, 1999; Mitra and Schlaepfer, 2006). Integrin-mediated cell adhesion can stimulate FAK auto-phosphorylation on Y397, which creates a high-affinity binding site for the Src-homology 2 (SH2) domain of Src (or another Src-family kinase). Upon binding to FAK, Src phosphorylates additional FAK tyrosines (e.g., Y861, Y925) to create binding sites for other kinases or adaptors, such as GRB2, phosphatidylinositol 3-kinase (PI3-K), and p130CAS, thereby linking the integrin/FAK/Src complex to downstream effectors such as mitogen-activated protein kinases (e.g., ERK, JNK, and p38), Rho family GTPases (e.g., CDC42, Rho, RAC1), or AKT (Cary and Guan, 1999; Mitra and Schlaepfer, 2006). Several keratinocyte integrins can activate FAK (e.g., α3β1, α5β1, αv integrins), and FAK signaling can regulate a number of keratinocyte functions in vitro, including migration, proliferation, survival, and ECM assembly/remodeling (Choma et al., 2007; Essayem et al., 2005; Kim et al., 2000; Manohar et al., 2004; McLean et al., 2004; Yurko et al., 2001). Surprisingly, wound studies performed in mice with keratinocyte-restricted deletion of FAK revealed no effect on wound re-epithelialization in vivo, although FAK function in the epidermis was linked to keratinocyte survival, stem cell proliferation, and tumorigenesis (Essayem et al., 2005; McLean et al., 2004). Further investigation is required to determine FAK involvement in other epidermal wound functions, such as ECM remodeling or paracrine signaling to other wound cells (see below).
Importantly, some integrins undergo lateral associations with other cell surface proteins, such as members of the tetraspanin family, growth factor receptors, caveolin, or urokinase receptor (uPAR) (Berditchevski, 2001; Chapman et al., 1999; Comoglio et al., 2003; Hemler, 2005; Porter and Hogg, 1998; Salanueva et al., 2007; Wei et al., 2001). Such interactions may regulate integrin activation or function by altering their affinity or avidity for ligands, turnover on the cell surface, subcellular localization to lipid rafts or other compartments, or ability to recruit signaling adaptors. Of particular relevance to wound healing, the epidermal integrins α3β1 and α6β4 can each bind to the CD151 tetraspanin protein (Kazarov et al., 2002; Yang et al., 2008; Yauch et al., 2000), and integrin/CD151 complexes have been linked to both intracellular signaling pathways (Yauch et al., 1998) and epithelial cell motility (Winterwood et al., 2006). In addition, coordinated interaction of CD151 with keratinocyte integrins α3β1 and α6β4 has been linked to hemidesmosome maturation (Sterk et al., 2000). Of note, CD151 is upregulated in wound epidermis and important for wound healing (Cowin et al., 2006). Further study is required to elucidate the mechanisms whereby integrin/CD151 complexes may contribute to wound healing.
Functions of epidermal integrins in wound healing
Integrin expression patterns within developing and adult epidermis have been documented in both humans and rodent models, and many aspects appear to have been conserved in evolution (Margadant et al., 2010; Watt, 2002). Within the stratified epidermis, integrin expression is restricted to the basal keratinocyte layer that is attached to the BM, and it is down-regulated as differentiating keratinocytes move upwards into the suprabasal layers (Watt, 2002). Studies using global or keratinocyte-specific knockout mice have revealed important roles for integrins in the epidermis (Margadant et al., 2010). Indeed, the general importance of β1 subfamily integrins was revealed through keratinocyte-restricted knockout of the Itgb1 gene (encoding the β1 subunit), which leads to an array of skin defects that includes reduced proliferation, loss of sebaceous glands and hair follicles, disorganized BM, and impaired wound re-epithelialization (Brakebusch et al., 2000; Grose et al., 2002; Raghavan et al., 2000). Of note, wound re-epithelialization defects were due mainly to severely impaired keratinocyte migration, whereas keratinocyte proliferation within the wound epidermis was not compromised and was even increased in later wounds (Grose et al., 2002). Importantly, however, subsequent studies showed that regeneration of wound epidermis in these mice most likely occurs through outgrowth of keratinocytes that have escaped Cre-mediated recombination, reflecting an essential role for β1 integrins in re-epithelialization (Piwko-Czuchra et al., 2009). Interestingly, knockout of any individual α subunit gene (i.e., deletion a specific αβ heterodimer) leads to only a subset of the defects seen in β1-null mice, indicating that different integrins have unique, albeit sometimes overlapping roles. Somewhat surprisingly, knockout mice with epidermis-specific deletion of either the β1 subunit (i.e., all β1 integrins) or individual integrins (α3β1, α6β4, α2β1, α9β1, or αvβ5) displayed mild or no defects in epidermal stratification or differentiation (Brakebusch et al., 2000; DiPersio et al., 1997; DiPersio et al., 2000b; Grenache et al., 2007; Huang et al., 2000; Raghavan et al., 2000; Singh et al., 2009; Zweers et al., 2007), indicating that epidermal development is not dependent on any particular integrin(s).
During wound healing, some integrins display persistent or enhanced expression (e.g., α3β1, α6β4, α2β1, α9β1, and αvβ5), while others are expressed de novo (e.g., α5β1 and αvβ6) (Thomas et al., 2006; Watt, 2002). As a group, these integrins can bind to a wide variety of ECM ligands that appear in the wound bed, including fibronectin (α5β1, α9β1, αvβ6), collagen (α2β1), vitronectin (αvβ5), tenascin (α9β1, αvβ6), and LN-332 that is deposited by migrating keratinocytes (α3β1, α6β4) (Margadant et al., 2010; Nguyen et al., 2001; Thomas et al., 2006; Watt, 2002). Many of these integrins have been shown to control keratinocyte motility in culture (Carter et al., 1990a; Carter et al., 1990b; Choma et al., 2004; Frank and Carter, 2004; Grose et al., 2002; Pilcher et al., 1997; Sehgal et al., 2006), and their potential to influence migration through traction generation and signaling is obvious (Ridley et al., 2003). Consistently, epidermal deletion of all β1 integrins together reduced wound re-epithelialization (Grose et al., 2002). Yet, the importance of individual integrins for wound re-epithelialization in vivo remains ambiguous, as wounds of adult mice with global or keratinocyte-specific deletion of individual integrins (e.g., αvβ6, αvβ5, α3β1, α2β1, or α9β1) showed surprisingly mild or no effects on epidermal migration (Grenache et al., 2007; Huang et al., 2000; Margadant et al., 2009; Singh et al., 2009; Zweers et al., 2007). In some cases, discordant results from in vitro and in vivo studies probably reflect the greater complexity of the wound ECM in vivo, where multiple integrin-ligand interactions might compensate for loss of a single interaction.
In the following sections, we will briefly discuss what is currently known about functions of individual integrins within the epidermis (summarized in Table 1). However, given the repertoire of integrins expressed in wound epidermis, it is important to keep in mind that different integrins may undergo complex interactions with potential for cumulative effects (either synergistic or opposing) on keratinocyte functions. Therefore, it is likely that coordinated regulation of different integrins during wound healing, perhaps achieved through the temporal and/or spatial regulation of their ECM ligands, is important to achieve proper wound outcome.
Table 1.
Summary of epidermal integrins, their ECM ligands, and their known functions in the skin
| Integrin | ECM ligands in skin | Known functions in unwounded epidermis | Known functions in wound epidermis |
|---|---|---|---|
| α3β1 | LN (mainly LN-332) | Expressed constitutively. Essential for basement membrane organization during skin development, and epidermal-dermal adhesion in newborn mice. | Required for maturation of the basement membrane, epidermal-dermal adhesion, and pararcine stimulaton of angiogenesis. May modulate epidermal migration. |
| α6β4 | LN (mainly LN-332) | Expressed constitutively. Essential for hemidesmosome formation and epidermal-dermal adhesion. | Presumably required for hemidesmosome assembly and epidermal adhesion following re-epithelialization. Roles in epidermal migration are unclear. |
| α9β1 | FN (via EDA segment), TN, OPN, TSP, ADAMs, EMILIN1, VEGF-C & -D | Expressed constitutively at low levels. Not essential for epidermal development or adhesion. | Upregulated. Essential for normal keratinocyte proliferation during wound re-epithelialization. |
| α2β1 | collagens | Expressed constitutively. Not essential for epidermal development or adhesion. | Upregulated. No essential roles reported, but may contribute to epidermal migration over collagen. |
| α5β1 | FN (via RGD) | Expressed at very low levels. Not essential for epidermal development or adhesion. | Upregulated. No essential roles reported, but likely contributes to epidermal migration on fibronectin. |
| αvβ6 | FN, TN, LAP of TGFβ-l and -3 (via RGD) | Restricted to follicular cells. Not essential for epidermal development or adhesion, although required in juvenile mice for normal hair growth. | Upregulated. Required for efficient wound healing in aged mice. May regulate ECM proteolysis, keratinocyte survival, and keratinocyte-mediated activation of latent TGFβ. Over-expression associated with chronic wounds. |
| αvβ5 | VN (via RGD) | Expressed at low levels. Not essential for epidermal development or adhesion. | Upregulated. May contribute to keratinocyte-mediated activation of latent TGFβ. |
Table adapted from Longmate and DiPersio (2014). See text for supporting literature. ECM, extracellular matrix; LN, laminin; FN, fibronectin; TGFβ, transforming growth factor β; TN, tenascin-C, OPN, osteopontin; VEGF, vascular endothelial cell growth factor; TSP, thrombospondin-1; LAP, latency-associated proteins; VN, vitronectin.
The laminin-binding integrins, α3β1 and α6β4
LN-332 is the main adhesive ligand in resting epidermis, and its effects on keratinocyte behavior are mediated through binding to integrins α3β1 and α6β4 (Carter et al., 1991; Longmate and DiPersio, 2014; Margadant et al., 2010; Nguyen et al., 2000). α6β4 is an essential component of the intermediate filament-associated hemidesmosomes that anchor the epidermis to the dermis (Litjens et al., 2006; Margadant et al., 2010). In contrast, α3β1 is associated with actin-based adhesions (i.e., focal adhesions) from within which it can initiate FAK/Src or other signaling pathways that promote keratinocyte motility and survival (Carter et al., 1990a; Choma et al., 2007; Choma et al., 2004; Litjens et al., 2006; Manohar et al., 2004). While α3β1 and α6β4 can both bind LN-332, these two integrins have distinct functions. For example, LN-332 proteolysis has differential effects on α3β1 or α6β4 binding (Goldfinger et al., 1998), and antagonistic roles of these two integrins in cell migration have been described (Russell et al., 2003). Further evidence of distinct roles for α3β1 and α6β4 comes from comparing variants of the human blistering skin disease, junctional epidermolysis bullosa (JEB), which have been linked to mutations in the α3, α6 or β4 integrin subunits, or in the individual chains of LN-332 itself (Has et al., 2012; Kiritsi et al., 2013; Ruzzi et al., 1997; Vidal et al., 1995). Indeed, differences between these JEB variants, and the corresponding knockout mice, reveal different mechanisms of blistering at the epidermal-dermal junction. For example, newborn mice with homozygous null mutations in the genes that encode either the α6 or β4 subunit (Dowling et al., 1996; Georges-Labouesse et al., 1996; van der Neut et al., 1996), or newborn human patients with loss-of-function mutations in α6β4 (Ruzzi et al., 1997; Vidal et al., 1995), show extensive epidermal blistering due to loss of hemidesmosomes and detachment from the BM. In contrast, absence of α3β1 due to null or loss-of-function mutation of the gene encoding the α3 subunit causes relatively minor skin blisters in newborn mice (DiPersio et al., 1997) or young patients (Has et al., 2012), which form due to BM rupture without loss of α6β4-mediated attachment to LN-332 (DiPersio et al., 1997; DiPersio et al., 2000b), revealing a distinct role for α3β1 in maintaining BM integrity.
Hemidesmosomes are dissassembled during wound re-epithelialization, then reassembled after wound closure to restore stable adhesion of the neo-epidermis (Litjens et al., 2006). Hemidesmosome disassembly may involve Src-mediated phosphorylation of the β4 cytoplasmic domain, possibly triggered by epidermal growth factor (EGF) or other signals from the wound microenvironment (Mariotti et al., 2001). While the role for α6β4 in stable epidermal adhesion has been long established, the extent to which this integrin may regulate intracellular signaling pathways in keratinocytes is less clear (Margadant et al., 2010). Moreover, while α6β4 can regulate motility of cultured keratinocytes (Russell et al., 2003; Sehgal et al., 2006), pro-migratory roles during wound re-epithelialization have not been described.
Integrin α3β1 can regulate a number of keratinocyte functions, including ECM organization (deHart et al., 2003; Hamelers et al., 2005), survival (Manohar et al., 2004), proliferation (Gonzales et al., 1999), and expression/secretion/activity of ECM proteases or pro-angiogenic factors (DiPersio et al., 2000a; Ghosh et al., 2000; Mitchell et al., 2009; Sugiura and Berditchevski, 1999). However, roles for α3β1 in keratinocyte migration have been unclear, as some in vitro studies reported pro-migratory roles (Choma et al., 2007; Choma et al., 2004; Frank and Carter, 2004), while others reported that α3β1 dampens directional motility (deHart et al., 2003; Margadant et al., 2009). These discordant findings could be due to species-specific differences between rodent and human keratinocytes, and/or variation among models with regard to deposition of endogenous ECM ligands for other integrins (Hamill et al.; Nguyen et al., 2000). In any case, knockout studies revealed that α3β1 is not essential for epidermal closure in wounds of adult mice (Margadant et al., 2009; Mitchell et al., 2009); in fact, deletion of α3β1 from epidermis slightly enhanced re-epithelialization (Margadant et al., 2009). Interestingly, the rate of epidermal closure was reduced within transplanted skin grafts from neonatal mice with global deletion of α3 (Reynolds et al., 2008), possibly reflecting a role for α3β1 in wound re-epithelialization of sub-adult mice. Alternatively, α3β1 may have important roles in other cellular compartments of full-thickness skin grafts (Reynolds et al., 2008), illustrating the importance of investigating integrin functions from within specific cellular compartments of the wound microenvironment.
Although α3β1 is dispensable for wound re-epithelialization in adult mice, it is essential for the regeneration of a stable BM during wound healing. Indeed, mice lacking epidermal α3β1 display blisters in re-epithelialized wounds due to BM rupture, similar to skin blisters that of α3-null neonatal mice, indicating that the developmental role for α3β1 in maintaining BM integrity is recapitulated in adult wound healing (Longmate et al., 2014). Interestingly, blistering in both neonatal skin and adult wounds was linked to reduced expression of the matricellular protein, fibulin-2 (Longmate et al., 2014), which can bind to the LN-γ2 chain near the N-terminus (Utani et al., 1997) and has been implicated in stable incorporation of LN-332 into the developing BM (Gagnoux-Palacios et al., 2001). Of note, α3-null keratinocytes showed reduced fibulin-2 gene expression, providing a potential mechanism for loss of BM integrity (Longmate et al., 2014; Missan et al., 2014). Proteolytic processing of the LN-γ2 chain was also impaired in wounds of mice with α3-null epidermis, and in α3-null keratinocytes cultured in high calcium (Longmate et al., 2014), and proteolytic cleavage of γ2 within LN-332 may regulate interactions with fibulin-2 (Aumailley et al., 2003; Gagnoux-Palacios et al., 2001; Sasaki et al., 2001; Utani et al., 1997). Thus, α3β1-dependent processing of LN-332 may be important for regulating BM maturation through modulation of key ECM linkages. A role for α3β1 in ECM assembly/organization is supported further by studies showing that it regulates matrix metalloproteinases (MMPs) and other extracellular proteases (Ghosh et al., 2000; Iyer et al., 2005; Sugiura and Berditchevski, 1999), as well as LN-332 deposition/organization by keratinocytes (deHart et al., 2003). Finally, as we discuss later, recent studies have identified an important role for epidermal α3β1 in the paracrine induction of wound angiogenesis through secretion of soluble factors that stimulate endothelial cell function (Mitchell et al., 2009)(W. M. Longmate and C. M. DiPersio, unpublished).
Integrin α9β1
Integrin α9β1 is expressed constitutively in the epidermis and is upregulated during wound healing, although it remains relatively understudied compared with other epidermal integrins. A major ligand of α9β1 within the wound bed is the EDA/EIIIA segment of cellular fibronectin, while other potential ligands found in the wound include tenascin-C, thrombospondin-1, EMILIN1, osteopontin, ADAMs proteins, and vascular endothelial growth factor (VEGF) -C and -D (Hoye et al., 2012; Margadant et al., 2010; Shinde et al., 2008; Singh et al., 2004). Studies in epidermis-specific α9 knockout mice revealed that α9β1 is important for proliferation of wound keratinocytes. Indeed, α9β1-deficient epidermis showed significantly impaired keratinocyte proliferation, while the rate of epidermal migration over the wound was not diminished, causing reduced thickness of the neo-epidermis and delayed differentiation (Singh et al., 2009). Interestingly, recent studies from our group indicate a complex interplay between integrins α9β1 and α3β1 during wound healing, wherein α9β1 exerts a transdominant-inhibitory effect over the pro-angiogenic functions of α3β1 (see below), possibly controlled through temporal regulation of α9β1 ligands within the wound bed (W. M. Longmate and C. M. DiPersio, unpublished).
Integrin α2β1
The collagen-binding integrin, α2β1, is expressed constitutively in epidermis and upregulated in wounds. Mice that are homozygous for a global α2-null mutation display normal skin development without overt defects in wound re-epithelialization or contraction, indicating that this integrin is not essential for wound closure per se (Chen et al., 2002; Zweers et al., 2007). However, α 2-null mice show enhanced wound angiogenesis, reduced mast cell infiltration into wounds, and reduced tensile strength of healed wounds, indicating complex roles for α2β1 in wound healing (Grenache et al., 2007; Zweers et al., 2007). It remains to be determined whether wound healing defects in α2-null mice are due to absence of α2β1 from the epidermis or from other wound cells, as this integrin has not been deleted specifically from the epidermis.
Integrin α5β1
Integrin α5β1 is expressed at very low levels in unwounded epidermis, but it is upregulated in healing wounds where it may promote keratinocyte migration over cellular fibronectin (via binding to the RGD site) that is deposited into the provisional wound ECM by wound macrophages and fibroblasts (Brown et al., 1993; Ffrench-Constant et al., 1989). However, it remains unknown whether epidermal α5β1 is required for in vivo wound healing, since the α5-null mutation is embryonic lethal (Yang et al., 1993), and wound studies in epidermis-specific α5 knockout mice have not been reported. Thus, it remains possible that defective epidermal migration reported in wounds of β1-deficient mice (Grose et al., 2002) is due largely to loss of α5β1, since to date no other α subunit knockout has produced the β1-null phenotype.
The αv integrins
Members of the αv integrin subfamily display dynamic expression patterns in the transition from unwounded to wounded skin (Koivisto et al., 2014). In unwounded epidermis, αvβ5 and αvβ8 are expressed at low levels, while αvβ6 is restricted to hair follicle stem cells (Breuss et al., 1995; Hamidi et al., 2000; Jones et al., 1997; Margadant et al., 2010). However, αvβ6 and αvβ5 are both upregulated in wound epidermis (Breuss et al., 1995; Hamidi et al., 2000; Jones et al., 1997; Margadant et al., 2010), where each may bind to RGD motifs within several ECM proteins (e.g., fibronectin, tenascin, vitronectin) (Hynes, 2002; Thomas et al., 2006). Interestingly, mice lacking αvβ6 (i.e., β6-null) displayed an age-dependent delay in wound healing (AlDahlawi et al., 2006). In addition to controlling cell migration over the provisional ECM, the de novo expression of αvβ6 may protect keratinocytes from undergoing anoikis (i.e., apoptosis triggered by altered adhesion during ECM remodeling), as this integrin can activate AKT survival pathways in epithelial cells (Janes and Watt, 2004). αvβ6 can also regulate extracellular proteases such as MMP-9 (Thomas et al., 2001a), MMP-3 (Ramos et al., 2002), and uPA (Ahmed et al., 2002), suggesting roles in ECM remodeling. Moreover, αvβ6 can bind and activate the ECM-bound reservoir of latent TGFβ, which is secreted as a complex of latency-associated protein (LAP) and latent TGFβ-binding protein (LTBP) that is covalently linked to fibronectin (Sheppard, 2005; Taipale et al., 1994). Indeed, αvβ6 (and perhaps to a lesser extent αvβ5) can bind the RGD motif within LAP to trigger a conformational change that activates latent TGFβ1 or TGFβ3 complex (Annes et al., 2004; Munger et al., 1999; Sheppard, 2005), suggesting that induction of αvβ6 during wound healing may provide a temporally controlled mechanism for local TGFβ activation.
Epidermal integrins regulate crosstalk to other cellular compartments of the wound
An extensive network of communication exists between the different cell types within the wound microenvironment, and mounting evidence supports the concept that integrins from within neutrophils, monocytes, fibroblasts, or endothelial cells can control crosstalk to keratinocytes that influences wound re-epithelialization (Koivisto et al., 2014). Importantly, the epidermis is also well known to send paracrine signals to other cellular compartments of the skin (Fig. 1), although mechanisms that regulate signaling in this direction remain unclear (Ghahary and Ghaffari, 2007; Nowinski et al., 2004; Werner et al., 2007; Zigrino et al., 2012). The following sections will focus on how keratinocyte integrins may regulate paracrine signals that emanate from the epidermis and influence functions of other wound cells. As we discuss below, such integrin-dependent intercellular crosstalk might be propagated through secretion of diffusable growth factors, altered physical properties of the ECM (e.g., matrix proteolysis or mechanical signaling), or proteolytic release of ECM-bound growth factors or bioactive fragments.
Paracrine signaling to the wound vasculature
The granulation phase of wound healing involves the growth of new vasculature through angiogenesis, which is important for delivering oxygen, nutrients and immune cells to the wound bed that are critical for the repair process. Later, during the tissue remodeling phase, the vasculature regresses to restore normal vessel density, thereby preventing tissue hyperoxia (Johnson and DiPietro, 2013). Control of blood vessel density involves the proliferation, migration, and apoptosis of endothelial cells, which can be regulated through accessibility of angiogenic growth factors such as basic fibroblast growth factor (FGF-2), EGF, platelet-derived growth factor (PDGF), and VEGF. Importantly, many pro-angiogenic growth factors are provided to endothelial cells by other wound cells, including neutrophils, platelets, and keratinocytes, representing pathways of intercellular communication that control wound angiogenesis. Moreover, defects in these pathways may contribute to vascular abnormalities associated with chronic wounds (Eming et al., 2007; Eming et al., 2014; Johnson and Wilgus, 2014; Koh and DiPietro, 2011; Martinez et al., 2015; Nissen et al., 1998).
While it has long been known that the epidermis secretes factors that can influence wound angiogenesis (Santoro and Gaudino, 2005; Singer and Clark, 1999), recent studies from our group have linked keratinocyte integrins to the paracrine stimulation of endothelial cells. For example, keratinocyte α3β1 promotes the expression and secretion of mitogen-regulated protein 3 (MRP3), a pro-angiogenic factor that stimulates endothelial cell migration and wound angiogenesis (Mitchell et al., 2009). Moreover, keratinocyte α3β1 promotes expression of MMP-9 (Iyer et al., 2005; Lamar et al., 2008), which has pro-angiogenic functions in wound healing and other tissue remodeling processes (Bergers et al., 2000; Hattori et al., 2009; McCawley and Matrisian, 2001; Yu and Stamenkovic, 2000). In contrast, wound studies performed in α2-null mice indicate an anti-angiogenic role for α2β1, although the cellular compartment from within which this regulation occurs remains unclear due to the global nature of this knockout model (Zweers et al., 2007). Interestingly, integrin α6β4 has been shown to regulate the expression of both α3β1 and α2β1 in keratinocytes (Kligys et al., 2012), raising the possibility that complex interplay between different epidermal integrins may control wound angiogenesis. Along similar lines, our group recently determined that keratinocyte α9β1 suppresses the pro-angiogenic, paracrine functions of α3β1, suggesting that functional coordination of different keratinocyte integrins can regulate intercellular communication that controls vascular density (W. M. Longmate and C. M. DiPersio, unpublished). Since the appearance of ECM ligands for different epidermal integrins is dynamic throughout the stages of wound healing, we speculate that temporally coordinated integrin activation may control both angiogenic growth at early stages and vascular normalization at later stages.
Paracrine signaling to wound fibroblasts/myofibroblasts
There is considerable evidence that paracrine signaling from the epidermis to fibroblasts/myofibroblasts can influence wound contraction, and that loss of such intercellular crosstalk is associated with fibrosis and hypertrophic scar formation (Ghahary and Ghaffari, 2007; Werner et al., 2007). Indeed, studies in co-culture models have shown that keratinocyte-secreted factors (e.g., growth factors, cytokines, ECM components, MMPs) can have both positive and negative effects on fibroblast genes and functions (Nowinski et al., 2004; Shephard et al., 2004; Werner et al., 2007). For example, keratinocyte-derived TGFβ promoted α-smooth muscle actin (α-SMA) expression and myofibroblast differentiation (Hata et al., 2014), while secretion of interleukin-1 (IL-1) by keratinocytes at early time points in co-culture suppressed α-SMA expression (Shephard et al., 2004). In another example, the keratinocyte-derived anti-fibrogenic factor, stratifin, up-regulated MMP-1 expression in fibroblasts via a p38/MAPK pathway (Lam et al., 2005), possibly leading to less collagen deposition and reduced dermal fibrosis (Rahmani-Neishaboor et al., 2012). Keratinocytes also produce PDGF (Ansel et al., 1993), which has been shown to stimulate chemotaxis, proliferation, and gene expression in fibroblast in vitro, and enhance the influx of fibroblasts and extracellular matrix deposition during wound healing in vivo (Pierce et al., 1991). Moreover, it was shown recently that treatment of mouse corneal stromal fibroblasts with PDGF, in combination with TGFβ, can stimulate differentiation to myofibroblasts in vitro (Singh et al., 2014). Other fibroblast responses to keratinocyte-released factors include down-regulation of type I collagen expression/synthesis in response to collagen-inhibitory factors (CIFs) (Ghaffari et al., 2009), and up- regulation of cyclooxygenase-2 (Cox-2) expression and prostaglandin E2 (PGE2) production in response to IL-1α (Sato et al., 1997). Interestingly, the latter response may trigger a feedback loop, as fibroblast- derived PGE2 has recently been shown to inhibit TGFβ-mediated myofibroblast differentiation in an autocrine manner (Penke et al., 2014).
Given the roles that keratinocyte integrins play in the paracrine induction of wound angiogenesis (see above), we speculate that they may also regulate paracrine pathways that control fibroblast/myofibroblast function in wounds. Consistently, some keratinocyte integrins have been shown to modulate gene expression and/or bioavailability of ECM proteases that are already known to stimulate wound fibroblasts. For example, keratinocyte integrins αvβ6 and α3β1 can induce expression and secretion of MMP-9, MMP-3, uPA, and/or other extracellular proteases (Ghosh et al., 2000; Iyer et al., 2005; Ramos et al., 2002; Thomas et al., 2001b) into the wound stroma. Indeed, recruitment of MMP-9 to the fibroblast surface triggers TGFβ activation, which coupled with mechanical tension and other ECM signals can induce myofibroblast differentiation (Dayer and Stamenkovic, 2015; Kobayashi et al., 2014; Van De Water et al., 2013). Other studies support integrin-mediated induction of IL-1α from the epidermis (Hobbs and Watt, 2003). Additional evidence that integrins may control epithelial-to-myofibroblast crosstalk comes from studies in a mouse lung fibrosis model, which showed that deletion of integrin α3β1 from lung epithelial cells led to diminished numbers of myofibroblasts (Kim et al., 2009).
Paracrine signaling to inflammatory cells
It is well known that chemokines produce by keratinocytes can recruit inflammatory cells to the wound, and that misregulation of this process contributes to prolonged inflammation, impaired reepithelialization, and altered myofibroblast differentiation (reviewed in (Van Linthout et al., 2014)). For example, interferon inducible protein-10 (IP-10/CXCL-10) is upregulated in keratinocytes during wound healing, and its overexpression results in an intensified immune response that is associated with delayed reepithelialization and a prolonged granulation phase, as well as reduced angiogenesis (Barrientos et al., 2008). In another example, macrophage chemoattractant protein (MCP-1/CCL2) that is produced by wound keratinocytes serves to recruit monocytes/macrophages, T-cells, and mast cells (DiPietro, 1995). Moreover, persistent expression of MCP-1 leads to a prolonged inflammatory response in chronic wounds (Wetzler et al., 2000), and MCP-1 knockout mice display delayed wound healing (Low et al., 2001). While roles for epidermal integrins in paracrine signaling to inflammatory cells have not been explored extensively, treatment of epithelial cells with an antibody that targets integrin α3β1 was shown to suppress the induction of MCP-1, IL-6 and IL-8 that occurred in response to treatment with IL-1 (Lubin et al., 2003). Moreover, expression of a β1 integrin transgene in the suprabasal layers of the epidermis enhanced IL-1α secretion, providing a potential signal for crosstalk to inflammatory cells (Hobbs and Watt, 2003).
Potential mechanisms of integrin-mediated, long-distance signaling from the epidermis to other wound cells
Signaling through growth factors and cytokines
Regulating the bioavailability of growth factors, cytokines or chemokines is a critical point of control over the inflammatory phase of wound healing, and failure to control or resolve inflammation can contribute to both chronic wounds and fibrotic pathologies. The cytokine network is also critical for controlling protease-mediated degradation of fibrillar collagen and other matrix proteins that is required for the remodeling phase of wound healing (Singer and Clark, 1999). Although the epidermis is spatially separated from the stromal compartment of the skin, keratinocyte-derived growth factors and cytokines can diffuse to other cellular compartments within the wound microenvironment. Importantly, some epidermal integrins can modulate growth factor/cytokine production that can then stimulate distal wound cells such as fibroblasts, endothelial cells, and inflammatory cells, thereby providing a means of integrin-dependent paracrine signaling. For example, as already mentioned, we reported that keratinocyte α3β1 promotes MRP3 secretion, which stimulates pro-angiogenic functions of endothelial cells (Mitchell et al., 2009). As also mentioned, expression of β1 integrin in suprabasal keratinocytes leads to IL-1 expression (Hobbs and Watt, 2003), which stimulates fibroblast growth and collagen synthesis (Sauder et al., 1990), and may regulate TGFβ-mediated myofibroblast differentiation (Shephard et al., 2004). Epidermal-derived cytokines and growth factors may also feedback to regulate epidermal integrin expression. For example, α6β4-mediated activation of latent TGFβ in keratinocytes is associated with up-regulation of some epidermal integrins (e.g., α5β1, α2β1, αvβ5, αvβ6) and down-regulation of others (e.g., α3β1) (Zambruno et al., 1995), raising the possibility that α6β4/TGFβ signaling indirectly regulates integrin-dependent paracrine pathways that stimulate other cells. These complex regulatory interactions between growth factors/cytokines and epidermal integrins indicate the likely importance of tight spatial-temporal control over these interactions, perhaps through bioavailability of key factors.
Signaling through extracellular proteases
Proteolytic remodeling of the ECM is an essential feature of normal wound healing (Steffensen et al., 2001), and defects in ECM organization are associated with chronic wounds (Agren and Werthen, 2007; Lobmann et al., 2002). Indeed, MMPs are involved in all phases of wound healing, and abnormal MMP expression/function is well known to contribute to the pathogenesis of chronic wounds (Lobmann et al., 2002). It is well established that some keratinocyte integrins (e.g., α3β1, αvβ6) can regulate the expression or function of matrix-degrading extracellular proteases (e.g., MMP-9, uPA or uPAR) (Ghosh et al., 2000; Iyer et al., 2005; Thomas et al., 2001b), which may in turn control ECM remodeling and/or proteolytic release of ECM-bound growth factors or bioactive peptides that are crucial for proper wound healing (Gill and Parks, 2008; McCawley and Matrisian, 2001; Page-McCaw et al., 2007; Schenk and Quaranta, 2003). It follows that defects in integrin-dependent pathways of protease regulation in the epidermis may contribute to the pathogenesis of abnormal wound healing through effects on other cells in the wound microenvironment (Agren and Werthen, 2007; Lobmann et al., 2002). For example, as described above, an α3β1-to-MMP-9 signaling axis (Iyer et al., 2005) may contribute to pro-angiogenic communication from the epidermis to the wound vasculature (Mitchell et al., 2009). Similar communication may occur from the epidermis to dermal fibroblasts/myofibroblasts, given the important roles that some MMPs play in myofibroblast differentiation and wound contraction (Hattori et al., 2009; Kobayashi et al., 2014; Mirastschijski et al., 2004).
Other potential mechanisms
There are other potential mechanisms of integrin-mediated, long-distance signaling from the epidermis that are relatively unexplored but warrant some discussion. For example, mechanical signaling, or mechanotransduction, is accomplished through ability of the constituent cells of a tissue to both exert force on the ECM and sense the ability of the ECM to resist that force (i.e., its stiffness) (Hinz, 2010; Van De Water et al., 2013). In their capacity as physical linkers of the ECM to the cytoskeleton, integrins function as transducers of mechanical signals across the plasma membrane (Hynes, 2002). This function can be influenced by changes in chemical or physical properties of the ECM that alter its stiffness, such as changes in organization of collagen fibers, fibronectin, or other matrix components that can be brought about by post-translational modifications, altered crosslinking, or proteolysis (Humphrey et al., 2014; Wells, 2008). Importantly, the biomechanical properties/stiffness of the ECM changes dramatically during the course of wound healing, as it progresses from the initial fibrin clot, to the provisional ECM of the granulation tissue, and finally to the remodeled stromal ECM and regenerated BM. Wound cells respond to these changes (Hinz, 2010), and increasing evidence links wound pathogenesis to alterations in ECM compliance and mechanical tension within the wound bed (Wong et al., 2011). Such alterations may be caused by changes in cell-generated forces, defective ECM deposition, or mis-regulated proteolysis of the ECM (Hermes et al., 2011; Lancerotto et al., 2012; Shirakawa and Isseroff, 2005; Trengove et al., 1999; Wong et al., 2013).
Since keratinocytes respond to matrix stiffness through alterations in migration and proliferation (Evans et al., 2013; Wang et al., 2012; Zarkoob et al., 2015)(reviewed in (Kenny and Connelly, 2015)), one could speculate that keratinocyte integrins can also transduce mechanical signals from the epidermis that influence behaviors of other wound cells (Kenny and Connelly, 2015). Indeed, epidermal keratinocytes generate relatively high intercellular tension, which is enhanced by cell-cell adhesion within the collectively migrating sheet of cells (Vedula et al., 2014), raising the possibility that forces transduced to the adjacent wound ECM may modulate its stiffness. In addition, keratinocytes might indirectly modulate stiffness of the wound ECM through release of matrix-degrading proteases. However, the extent to which mechanical signaling and ECM stiffness are an important mode of communication from the epidermis to other wound cells requires further investigation. Such studies will certainly require the development of cell culture or organotypic models wherein mechanical stimuli can be isolated from, and controlled independently of, diffusible factors (e.g. growth factors, cytokines) that also control wound healing (Kenny and Connelly, 2015).
A growing literature supports the cellular shedding of extracellular vesicles, including microvesicles and exosomes, as another potential mechanism of intercellular communication (for a review see (Camussi et al., 2010)). Such cell-to-cell crosstalk may occur through the ability of microvesicles/exosomes to directly stimulate receptors on target cells, or through their capacity to transport cargos that alter target cell function (e.g., membrane receptors, signaling proteins, mRNAs, or microRNAs). Interestingly, tumor cell-derived exosomes have been shown to trigger fibroblast-to-myofibroblast differentiation through transport of TGFβ (Webber et al., 2010). Similarly, keratinocytes have been shown to release exosomes/microvesicles that stimulate wound healing-associated genes (e.g., MMPs, interleukins, TGFβ signaling genes) and functions in dermal fibroblasts, suggesting a potential mechanism of paracrine signaling during wound healing (Chavez-Munoz et al., 2009). Interestingly, recent studies in cancer models have implicated important roles for integrins, or extracellular matrix proteins, in exosome-mediated signaling (Fedele et al., 2015; Sung et al., 2015), raising the intriguing possibility that epidermal integrins play a similar role in paracrine crosstalk from keratinocytes to other wound cells.
Implications for pathological wound healing
As discussed above, normal acute wounds heal by a series of overlapping phases (i.e., inflammation, proliferative phase and tissue remodeling) that optimize a prompt return to tissue homeostasis. Each of these phases entails dynamic and balanced interactions between soluble mediators (e.g., growth factors) extracellular matrix components and both resident and infiltrating cells. When these wound dynamics are disrupted, pathological wound healing ensues. The wound pathologies are manifested along a spectrum that includes, at one end, wounds that do not heal well (e.g., chronic wounds) and at the other end wounds that heal over-exuberantly (e.g., hypertrophic scars and keloids). While ongoing work in many labs has identified potential therapies, there has been a relatively low rate of success, renewing calls for robust cellular and molecular biology to support the development of novel therapies (Eming et al., 2014; Nunan et al., 2014). In addition to the cell-autonomous functions of integrins in wound keratinocytes, we believe that roles for integrins in paracrine signaling from wound epidermis are understudied. In the previous sections, we reviewed how keratinocyte integrins may regulate both autocrine and paracrine functions during wound healing (Fig. 1). In the current section, we will illuminate important areas in which disrupted keratinocyte integrin interactions with ECM may contribute to wound pathologies.
Chronic wounds
The term “chronic wounds” encompasses wound pathologies in which a defect in barrier function - normally assembled by keratinocytes occurs. An alarming increase in the number of chronic wounds has become apparent in recent years and is the focus of much concern, particularly in view of the rising tide of diabetic patients (Sen et al., 2009) (Eming et al., 2014). These wound pathologies occur in patients in which vascular function is compromised, either as a consequence of chronic venous insufficiency (e.g., venous leg ulcers), reduced arterial blood supply secondary to diabetes (e.g, foot ulcers), or pressure ulcers occurring in areas of skin that are compressed against underlying bone. Chronic wounds are characterized by a hyper-proliferative but stationary epidermis, persistent bacterial infection and inflammation and a compromised wound vasculature. Moreover, recent gene expression analyses have revealed a different synthetic program in keratinocytes at the non-healing edges of chronic wounds compared to normal keratinocytes (Pastar et al., 2008). Of note, chronic wounds often display dramatic alterations in epidermal integrin expression, such as increased αvβ6 and decreased α5β1 expression, and these changes are likely to play causal roles in wound pathology ((Hakkinen et al., 2004; Koivisto et al., 2014; Morgan et al., 2004; Widgerow, 2013)). Below, we speculate on several areas in which keratinocyte integrin-ECM interactions may be critical in chronic wound pathologies.
With regard to wound infections, it is now well understood that bacteria exploit host ECM components and other proteins in order to colonize tissues (Foster et al., 2014). Among these are ECM proteins that are prominent in healing wounds, such as plasma and cellular forms of fibronectin. Cellular fibronectin includes an alternatively spliced segment, termed EDA (or EIIIA) that is undetectable in unwounded skin (Singh et al., 2004). Our recent work has demonstrated an important role for the EDA segment of fibronectin in promoting biofilm formation (Oliver-Kozup et al., 2013). The EDA segment binds to integrin α9β1, which promotes keratinocyte proliferation (Liao et al., 2002; Shinde et al., 2008; Singh et al., 2009), and we have observed that α9β1 exerts transdominant inhibition of keratinocyte integrin α3β1 function (W. M. Longmate and C. M. DiPersio, unpublished). Therefore, it will be important to determine if and how bacterial biofilm formation in chronic wounds disrupts normal interactions between keratinocyte integrins, and how such disruption impacts paracrine signaling via fibronectin- and integrin-dependent pathway(s). Because keratinocytes secrete paracrine mediators (e.g., IL-1, TNFα) that are known components of innate immunity pathways (see for example, see (Feldmeyer et al., 2010)), analyses of integrin-dependent mechanisms that regulate keratinocyte cytokine production in chronic inflammatory settings will likely prove important. The presence of persistent inflammation during wound infection also entails alterations in the spectrum of proteases elaborated by both immune cells and keratinocytes. Although there are clearly many proteases (e.g., serine proteases, MMPs) that are regulated by integrins and have critical roles, recent work from our group supports a model in which dysregulation of α3β1-ECM interactions could influence MMP-9-dependent homeostasis (Iyer et al., 2005; Lamar et al., 2008; Missan et al., 2015).
Dysregulation of angiogenesis and compromised wound vasculature is a cardinal feature of chronic wounds. Wound keratinocytes have long been known to secrete angiogenic factors (Johnson and Wilgus, 2014), yet the mechanisms that regulate this process remain poorly understood. As already discussed, keratinocyte integrin α3β1 governs the expression of pro-angiogenic factors, including MRP-3 (Mitchell et al., 2009) and MMP-9 (Iyer et al., 2005), suggesting a potential role for disrupted keratinocyte integrin function in modulating angiogenesis in chronic wounds.
Scarring pathologies
The healing that follows deep traumatic or serious burn injury is often complicated by the elaboration of keloids or hypertrophic scars (Ehrlich et al., 1994). Keloids are abnormal scars that contain abundant collagen deposits, extend beyond the boundary of the original wound, rarely regress and occur in individuals who are genetically predisposed. Surgical resection reinitiates this process, and keloids have a high recurrence rate despite adjunct treatments including intralesional steroid treatment (Andrews et al., 2016). In contrast, hypertrophic scars are elevated above the skin surface and are characterized by redness and itching, but they remain within the boundaries of the initial injury. Although they often regress spontaneously over time, these scars can undergo disfiguring contractures that can limit motion, particularly over joints. Surgical scar revision remains a mainstay therapy, although many other therapeutic approaches have been attempted (Reish and Eriksson, 2008). Interestingly, occlusive dressings have been effective in both controlled animals studies and prospective, randomized studies (Mustoe and Gurjala, 2011). Importantly, these dressings are thought to promote epidermal function by prompt restoration of barrier function, thereby reducing water loss and potentially reducing epidermal paracrine signals to inflammatory cells and other wound cells (Mustoe and Gurjala, 2011; Reish and Eriksson, 2008). Given the emerging roles for keratinocyte integrins in controlling epidermal paracrine signals, their potential roles in hypertrophic scarring should not be overlooked and warrants investigation.
Concluding remarks
In summary, it has become clear in recent years that integrins control both autocrine and paracrine functions of wound keratinocytes that extend well beyond established roles in epidermal adhesion and migration (Fig. 1, arrow 1). Indeed, keratinocyte integrins also play important roles in enabling the epidermis to modify the wound microenvironment, either through alterations in the wound ECM or through communication with other wound cells. It is clear that certain epidermal integrins can influence angiogenesis by promoting paracrine pathways that stimulate endothelial cells (Fig. 1, arrow 2), and it seems likely that such paracrine stimulation extends to other wound cells, such as fibroblasts/myofibroblasts or inflammatory cells (Fig. 1, arrow 3). Since many epidermal functions are altered in wound pathologies such as chronic wounds and hypertrophic scars, there are promising opportunities for basic research on integrin-mediated keratinocyte-ECM interactions that may provide a foundation for novel wound healing therapies. Indeed, results of genetic studies in mice support the concept that wound re-epithelialization might be facilitated through therapeutic manipulation of epidermal integrins. However, this strategy will likely require combinatorial targeting of several integrins with both distinct and overlapping functions. In addition, targeting gene regulatory functions of epidermal α3β1 (and possibly other integrins) may allow the therapeutic modulation of gene groups with predicted roles in altering the wound microenvironment, including secreted growth factors, cytokines, proteases, or ECM/matricellular proteins. Nevertheless, while good progress has been made towards identifying wound-related functions of individual integrins, we are only beginning to understand how the functions of multiple integrins are coordinated within the epidermis to ensure normal wound healing. Formidable challenges lie ahead as we attempt to translate this knowledge into the development of therapeutic approaches to treat wound healing deficiencies in the clinic. Adding to this challenge, it is likely that integrin-mediated control over diverse epidermal wound functions is precisely timed, in order to maintain properly coordinated regulation of epidermal function throughout the stages of wound healing.
Acknowledgments
Acknowledgements and Funding Information:
The authors are grateful to members of the DiPersio and Van De Water laboratories, as well as to other colleagues at Albany Medical College, for valuable discussions and insights. Research was supported by NIH grants from NIAMS to L. Van De Water and C. M. DiPersio (R01AR063778), and from NCI to C. M. DiPersio (R01CA129637). We offer our apologies to the many researchers whose valuable contributions to the field could not be cited due to space constraints.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Agren MS, Werthen M. The extracellular matrix in wound healing: a closer look at therapeutics for chronic wounds. The international journal of lower extremity wounds. 2007;6:82–97. doi: 10.1177/1534734607301394. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Pansino F, Clyde R, Murthi P, Quinn MA, Rice GE, Agrez MV, Mok S, Baker MS. Overexpression of alpha(v)beta6 integrin in serous epithelial ovarian cancer regulates extracellular matrix degradation via the plasminogen activation cascade. Carcinogenesis. 2002;23:237–244. doi: 10.1093/carcin/23.2.237. [DOI] [PubMed] [Google Scholar]
- AlDahlawi S, Eslami A, Hakkinen L, Larjava HS. The alphavbeta6 integrin plays a role in compromised epidermal wound healing. Wound Repair Regen. 2006;14:289–297. doi: 10.1111/j.1743-6109.2006.00123.x. [DOI] [PubMed] [Google Scholar]
- Andrews JP, Marttala J, Macarak E, Rosenbloom J, Uitto J. Keloids: The paradigm of skin fibrosis - Pathomechanisms and treatment. Matrix Biol. 2016 doi: 10.1016/j.matbio.2016.01.013. [Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansel JC, Tiesman JP, Olerud JE, Krueger JG, Krane JF, Tara DC, Shipley GD, Gilbertson D, Usui ML, Hart CE. Human keratinocytes are a major source of cutaneous platelet-derived growth factor. J Clin Invest. 1993;92:671–678. doi: 10.1172/JCI116636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumailley M, El Khal A, Knoss N, Tunggal L. Laminin 5 processing and its integration into the ECM. Matrix Biol. 2003;22:49–54. doi: 10.1016/s0945-053x(03)00013-1. [DOI] [PubMed] [Google Scholar]
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Berditchevski F. Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci. 2001;114:4143–4151. doi: 10.1242/jcs.114.23.4143. [DOI] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C, Grose R, Quondamatteo F, Ramirez A, Jorcano JL, Pirro A, Svensson M, Herken R, Sasaki T, Timpl R, Werner S, Fassler R. Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J. 2000;19:3990–4003. doi: 10.1093/emboj/19.15.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108:2241–2251. doi: 10.1242/jcs.108.6.2241. [DOI] [PubMed] [Google Scholar]
- Brown LF, Dubin D, Lavigne L, Logan B, Dvorak HF, Van de Water L. Macrophages and fibroblasts express embryonic fibronectins during cutaneous wound healing. Am J Pathol. 1993;142:793–801. [PMC free article] [PubMed] [Google Scholar]
- Burgeson RE, Christiano AM. The dermal-epidermal junction. Curr Opin Cell Biol. 1997;9:651–658. doi: 10.1016/s0955-0674(97)80118-4. [DOI] [PubMed] [Google Scholar]
- Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–848. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- Carter WG, Kaur P, Gil SG, Gahr PJ, Wayner EA. Distinct functions for integrins α3β1 in focal adhesions and a6b4/bullous antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990a;111:3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter WG, Ryan MC, Gahr PJ. Epiligrin, a new cell adhesion ligand for integrin α3β1 in epithelial basement membranes. Cell. 1991;65:599–610. doi: 10.1016/0092-8674(91)90092-d. [DOI] [PubMed] [Google Scholar]
- Carter WG, Wayner EA, Bouchard TS, Kaur P. The role of integrins α2β1 and α3β1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990b;110:1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cary LA, Guan JL. Focal adhesion kinase in integrin-mediated signaling. Front Biosci. 1999;4:D102–113. doi: 10.2741/cary. [DOI] [PubMed] [Google Scholar]
- Chapman HA, Wei Y, Simon DI, Waltz DA. Role of urokinase receptor and caveolin in regulation of integrin signaling. Thromb Haemost. 1999;82:291–297. [PubMed] [Google Scholar]
- Chavez-Munoz C, Kilani RT, Ghahary A. Profile of exosomes related proteins released by differentiated and undifferentiated human keratinocytes. J Cell Physiol. 2009;221:221–231. doi: 10.1002/jcp.21847. [DOI] [PubMed] [Google Scholar]
- Chen J, Diacovo TG, Grenache DG, Santoro SA, Zutter MM. The alpha(2) integrin subunit-deficient mouse: a multifaceted phenotype including defects of branching morphogenesis and hemostasis. Am J Pathol. 2002;161:337–344. doi: 10.1016/s0002-9440(10)64185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choma DP, Milano V, Pumiglia KM, DiPersio CM. Integrin alpha3beta1-dependent activation of FAK/Src regulates Rac1-mediated keratinocyte polarization on laminin-5. J Invest Dermatol. 2007;127:31–40. doi: 10.1038/sj.jid.5700505. [DOI] [PubMed] [Google Scholar]
- Choma DP, Pumiglia K, DiPersio CM. Integrin α3β1 directs the stabilization of a polarized lamellipodium in epithelial cells through activation of Rac1. J Cell Sci. 2004;117:3947–3959. doi: 10.1242/jcs.01251. [DOI] [PubMed] [Google Scholar]
- Comoglio PM, Boccaccio C, Trusolino L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr Opin Cell Biol. 2003;15:565–571. doi: 10.1016/s0955-0674(03)00096-6. [DOI] [PubMed] [Google Scholar]
- Cowin AJ, Adams D, Geary SM, Wright MD, Jones JC, Ashman LK. Wound healing is defective in mice lacking tetraspanin CD151. J Invest Dermatol. 2006;126:680–689. doi: 10.1038/sj.jid.5700142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer C, Stamenkovic I. Recruitment of Matrix Metalloproteinase-9 (MMP-9) to the Fibroblast Cell Surface by Lysyl Hydroxylase 3 (LH3) Triggers Transforming Growth Factor-beta (TGF-beta) Activation and Fibroblast Differentiation. J Biol Chem. 2015;290:13763–13778. doi: 10.1074/jbc.M114.622274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deHart GW, Healy KE, Jones JC. The role of alpha3beta1 integrin in determining the supramolecular organization of laminin-5 in the extracellular matrix of keratinocytes. Exp Cell Res. 2003;283:67–79. doi: 10.1016/s0014-4827(02)00028-9. [DOI] [PubMed] [Google Scholar]
- Delon I, Brown NH. Integrins and the actin cytoskeleton. Curr Opin Cell Biol. 2007;19:43–50. doi: 10.1016/j.ceb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- DiPersio CM, Hodivala-Dilke KM, Jaenisch R, Kreidberg JA, Hynes RO. α3β1 integrin is required for normal development of the epidermal basement membrane. J Cell Biol. 1997;137:729–742. doi: 10.1083/jcb.137.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio CM, Shao M, Di Costanzo L, Kreidberg JA, Hynes RO. Mouse keratinocytes immortalized with large T antigen acquire α3β1 integrin-dependent secretion of MMP-9/gelatinase B. J Cell Sci. 2000a;113:2909–2921. doi: 10.1242/jcs.113.16.2909. [DOI] [PubMed] [Google Scholar]
- DiPersio CM, van der Neut R, Georges-Labouesse E, Kreidberg JA, Sonnenberg A, Hynes RO. alpha3beta1 and alpha6beta4 integrin receptors for laminin-5 are not essential for epidermal morphogenesis and homeostasis during skin development. J Cell Sci. 2000b;113:3051–3062. doi: 10.1242/jcs.113.17.3051. [DOI] [PubMed] [Google Scholar]
- DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock. 1995;4:233–240. [PubMed] [Google Scholar]
- Dowling J, Yu Q-C, Fuchs E. β4 integrin is required for hemidesmosomal formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich HP, Desmouliere A, Diegelmann RF, Cohen IK, Compton CC, Garner WL, Kapanci Y, Gabbiani G. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994;145:105–113. [PMC free article] [PubMed] [Google Scholar]
- Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol. 2007;127:514–525. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- Eming SA, Martin P, Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Science Translational Medicine. 2014;6:265–266. doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essayem S, Kovacic-Milivojevic B, Baumbusch C, McDonagh S, Dolganov G, Howerton K, Larocque N, Mauro T, Ramirez A, Ramos DM, Fisher SJ, Jorcano JL, Beggs HE, Reichardt LF, Ilic D. Hair cycle and wound healing in mice with a keratinocyte-restricted deletion of FAK. Oncogene. 2005;25:1081–108. doi: 10.1038/sj.onc.1209130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans ND, Oreffo RO, Healy E, Thurner PJ, Man YH. Epithelial mechanobiology, skin wound healing, and the stem cell niche. Journal of the Mechanical Behavior of Biomedical Materials. 2013;28:397–409. doi: 10.1016/j.jmbbm.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Fedele C, Singh A, Zerlanko BJ, Iozzo RV, Languino LR. The alphavbeta6 integrin is transferred intercellularly via exosomes. J Biol Chem. 2015;290:4545–4551. doi: 10.1074/jbc.C114.617662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmeyer L, Werner S, French LE, Beer HD. Interleukin-1, inflammasomes and the skin. Eur J Cell Biol. 2010;89:638–644. doi: 10.1016/j.ejcb.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C, Van de Water L, Dvorak HF, Hynes RO. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989;109:903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TJ, Geoghegan JA, Ganesh VK, Hook M. Adhesion, invasion and evasion: the many functions of the surface proteins of Staphylococcus aureus. Nature Reviews Microbiology. 2014;12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DE, Carter WG. Laminin 5 deposition regulates keratinocyte polarization and persistent migration. J Cell Sci. 2004;117:1351–1363. doi: 10.1242/jcs.01003. [DOI] [PubMed] [Google Scholar]
- Gagnoux-Palacios L, Allegra M, Spirito F, Pommeret O, Romero C, Ortonne JP, Meneguzzi G. The short arm of the laminin gamma2 chain plays a pivotal role in the incorporation of laminin 5 into the extracellular matrix and in cell adhesion. J Cell Biol. 2001;153:835–850. doi: 10.1083/jcb.153.4.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin α6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- Ghaffari A, Kilani RT, Ghahary A. Keratinocyte-conditioned media regulate collagen expression in dermal fibroblasts. J Invest Dermatol. 2009;129:340–347. doi: 10.1038/jid.2008.253. [DOI] [PubMed] [Google Scholar]
- Ghahary A, Ghaffari A. Role of keratinocyte-fibroblast cross-talk in development of hypertrophic scar. Wound Repair Regen. 2007;15(Suppl 1):S46–53. doi: 10.1111/j.1524-475X.2007.00225.x. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Brown R, Jones JC, Ellerbroek SM, Stack MS. Urinary-type plasminogen activator (uPA) expression and uPA receptor localization are regulated by alpha 3beta 1 integrin in oral keratinocytes. J Biol Chem. 2000;275:23869–23876. doi: 10.1074/jbc.M000935200. [DOI] [PubMed] [Google Scholar]
- Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40:1334–1347. doi: 10.1016/j.biocel.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfinger LE, Stack MS, Jones JCR. Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol. 1998;141:255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales M, Haan K, Baker SE, Fitchmun M, Todorov I, Weitzman S, Jones JCR. A cell signal pathway involving laminin-5, α3β1 integrin, and mitogen-activated protein kinase can regulate epithelial cell proliferation. Mol Biol Cell. 1999;10:259–270. doi: 10.1091/mbc.10.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenache DG, Zhang Z, Wells LE, Santoro SA, Davidson JM, Zutter MM. Wound healing in the alpha2beta1 integrin-deficient mouse: altered keratinocyte biology and dysregulated matrix metalloproteinase expression. J Invest Dermatol. 2007;127:455–466. doi: 10.1038/sj.jid.5700611. [DOI] [PubMed] [Google Scholar]
- Grose R, Hutter C, Bloch W, Thorey I, Watt FM, Fassler R, Brakebusch C, Werner S. A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development. 2002;129:2303–2315. doi: 10.1242/dev.129.9.2303. [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Hakkinen L, Koivisto L, Gardner H, Saarialho-Kere U, Carroll JM, Lakso M, Rauvala H, Laato M, Heino J, Larjava H. Increased expression of beta6-integrin in skin leads to spontaneous development of chronic wounds. Am J Pathol. 2004;164:229–242. doi: 10.1016/s0002-9440(10)63113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelers IH, Olivo C, Mertens AE, Pegtel DM, van der Kammen RA, Sonnenberg A, Collard JG. The Rac activator Tiam1 is required for α3β1-mediated laminin-5 deposition, cell spreading, and cell migration. J Cell Biol. 2005;171:871–881. doi: 10.1083/jcb.200509172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi S, Salo T, Kainulainen T, Epstein J, Lerner K, Larjava H. Expression of alpha(v)beta6 integrin in oral leukoplakia. Br J Cancer. 2000;82:1433–1440. doi: 10.1054/bjoc.1999.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill KJ, Hopkinson SB, Hoover P, Todorovic V, Green KJ, Jones JC. Fibronectin expression determines skin cell motile behavior. J Invest Dermatol. 2012;132:448–457. doi: 10.1038/jid.2011.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Has C, Sparta G, Kiritsi D, Weibel L, Moeller A, Vega-Warner V, Waters A, He Y, Anikster Y, Esser P, Straub BK, Hausser I, Bockenhauer D, Dekel B, Hildebrandt F, Bruckner-Tuderman L, Laube GF. Integrin alpha3 mutations with kidney, lung, and skin disease. N Engl J Med. 2012;366:1508–1514. doi: 10.1056/NEJMoa1110813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata S, Okamura K, Hatta M, Ishikawa H, Yamazaki J. Proteolytic and non-proteolytic activation of keratinocyte-derived latent TGF-beta1 induces fibroblast differentiation in a wound-healing model using rat skin. Journal of pharmacological sciences. 2014;124:230–243. doi: 10.1254/jphs.13209fp. [DOI] [PubMed] [Google Scholar]
- Hattori N, Mochizuki S, Kishi K, Nakajima T, Takaishi H, D’Armiento J, Okada Y. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am J Pathol. 2009;175:533–546. doi: 10.2353/ajpath.2009.081080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–811. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- Hermes O, Schlage P, auf dem Keller U. Wound degradomics - current status and future perspectives. Biol Chem. 2011;392:949–954. doi: 10.1515/BC.2011.092. [DOI] [PubMed] [Google Scholar]
- Hinz B. The myofibroblast: paradigm for a mechanically active cell. J Biomech. 2010;43:146–155. doi: 10.1016/j.jbiomech.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Hobbs RM, Watt FM. Regulation of interleukin-1alpha expression by integrins and epidermal growth factor receptor in keratinocytes from a mouse model of inflammatory skin disease. J Biol Chem. 2003;278:19798–19807. doi: 10.1074/jbc.M300513200. [DOI] [PubMed] [Google Scholar]
- Hoye AM, Couchman JR, Wewer UM, Fukami K, Yoneda A. The newcomer in the integrin family: integrin alpha9 in biology and cancer. Advances in biological regulation. 2012;52:326–339. doi: 10.1016/j.jbior.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Huang X, Griffiths M, Wu J, Farese RV, Jr, Sheppard D. Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol Cell Biol. 2000;20:755–759. doi: 10.1128/mcb.20.3.755-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Iwamoto DV, Calderwood DA. Regulation of integrin-mediated adhesions. Curr Opin Cell Biol. 2015;36:41–47. doi: 10.1016/j.ceb.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer V, Pumiglia K, DiPersio CM. α3β1 integrin regulates MMP-9 mRNA stability in immortalized keratinocytes: a novel mechanism of integrin-mediated MMP gene expression. J Cell Sci. 2005;118:1185–1195. doi: 10.1242/jcs.01708. [DOI] [PubMed] [Google Scholar]
- Janes SM, Watt FM. Switch from alphavbeta5 to alphavbeta6 integrin expression protects squamous cell carcinomas from anoikis. J Cell Biol. 2004;166:419–431. doi: 10.1083/jcb.200312074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, DiPietro LA. Apoptosis and angiogenesis: an evolving mechanism for fibrosis. FASEB J. 2013;27:3893–3901. doi: 10.1096/fj.12-214189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KE, Wilgus TA. Vascular endothelial growth factor and angiogenesis in the regulation of cutaneous wound repair. Adv Wound Care. 2014;3:647–661. doi: 10.1089/wound.2013.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J, Watt FM, Speight PM. Changes in the expression of alpha v integrins in oral squamous cell carcinomas. J Oral Pathol Med. 1997;26:63–68. doi: 10.1111/j.1600-0714.1997.tb00023.x. [DOI] [PubMed] [Google Scholar]
- Kazarov AR, Yang X, Stipp CS, Sehgal B, Hemler ME. An extracellular site on tetraspanin CD151 determines alpha 3 and alpha 6 integrin-dependent cellular morphology. J Cell Biol. 2002;158:1299–1309. doi: 10.1083/jcb.200204056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny FN, Connelly JT. Integrin-mediated adhesion and mechano-sensing in cutaneous wound healing. Cell Tissue Res. 2015;360:571–582. doi: 10.1007/s00441-014-2064-9. [DOI] [PubMed] [Google Scholar]
- Kim LT, Wu J, Bier-Laning C, Dollar BT, Turnage RH. Focal adhesion kinase up-regulation and signaling in activated keratinocytes. J Surg Res. 2000;91:65–69. doi: 10.1006/jsre.2000.5914. [DOI] [PubMed] [Google Scholar]
- Kim Y, Kugler MC, Wei Y, Kim KK, Li X, Brumwell AN, Chapman HA. Integrin alpha3beta1-dependent beta-catenin phosphorylation links epithelial Smad signaling to cell contacts. J Cell Biol. 2009;184:309–322. doi: 10.1083/jcb.200806067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiritsi D, Has C, Bruckner-Tuderman L. Laminin 332 in junctional epidermolysis bullosa. Cell Adh Migr. 2013;7:135–141. doi: 10.4161/cam.22418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligys KR, Wu Y, Hopkinson SB, Kaur S, Platanias LC, Jones JC. alpha6beta4 integrin, a master regulator of expression of integrins in human keratinocytes. J Biol Chem. 2012;287:17975–17984. doi: 10.1074/jbc.M111.310458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Kim H, Liu X, Sugiura H, Kohyama T, Fang Q, Wen FQ, Abe S, Wang X, Atkinson JJ, Shipley JM, Senior RM, Rennard SI. Matrix metalloproteinase-9 activates TGF-beta and stimulates fibroblast contraction of collagen gels. Am J Physiol Lung Cell Mol Physiol. 2014;306:L1006–1015. doi: 10.1152/ajplung.00015.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TJ, DiPietro LA. Inflammation and wound healing: the role of the macrophage. Expert Rev Mol Med. 2011;13:e23. doi: 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivisto L, Heino J, Hakkinen L, Larjava H. Integrins in Wound Healing. Adv Wound Care. 2014;3:762–783. doi: 10.1089/wound.2013.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam E, Kilani RT, Li Y, Tredget EE, Ghahary A. Stratifin-induced matrix metalloproteinase-1 in fibroblast is mediated by c-fos and p38 mitogen-activated protein kinase activation. J Invest Dermatol. 2005;125:230–238. doi: 10.1111/j.0022-202X.2005.23765.x. [DOI] [PubMed] [Google Scholar]
- Lamar JM, Iyer V, DiPersio CM. Integrin alpha3beta1 potentiates TGFbeta-mediated induction of MMP-9 in immortalized keratinocytes. J Invest Dermatol. 2008;128:575–586. doi: 10.1038/sj.jid.5701042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancerotto L, Bayer LR, Orgill DP. Mechanisms of action of microdeformational wound therapy. Seminars in Cell and Developmental Biology. 2012;23:987–992. doi: 10.1016/j.semcdb.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Legate KR, Fassler R. Mechanisms that regulate adaptor binding to beta-integrin cytoplasmic tails. J Cell Sci. 2009;122:187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. The EIIIA segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem. 2002;277:14467–14474. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- Litjens SH, de Pereda JM, Sonnenberg A. Current insights into the formation and breakdown of hemidesmosomes. Trends Cell Biol. 2006;16:376–383. doi: 10.1016/j.tcb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Liu S, Calderwood DA, Ginsberg MH. Integrin cytoplasmic domain-binding proteins. J Cell Sci. 2000;113:3563–3571. doi: 10.1242/jcs.113.20.3563. [DOI] [PubMed] [Google Scholar]
- Lobmann R, Ambrosch A, Schultz G, Waldmann K, Schiweck S, Lehnert H. Expression of matrix-metalloproteinases and their inhibitors in the wounds of diabetic and non-diabetic patients. Diabetologia. 2002;45:1011–1016. doi: 10.1007/s00125-002-0868-8. [DOI] [PubMed] [Google Scholar]
- Longmate LM, DiPersio CM. Integrin regulation of epidermal functions in wounds. Advances in Wound Care. 2014;3:229–246. doi: 10.1089/wound.2013.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmate WM, Monichan R, Chu ML, Tsuda T, Mahoney MG, DiPersio CM. Reduced fibulin-2 contributes to loss of basement membrane integrity and skin blistering in mice lacking integrin alpha3beta1 in the epidermis. J Invest Dermatol. 2014;134:1609–1617. doi: 10.1038/jid.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low QE, Drugea IA, Duffner LA, Quinn DG, Cook DN, Rollins BJ, Kovacs EJ, DiPietro LA. Wound healing in MIP-1alpha(−/−) and MCP-1(−/−) mice. Am J Pathol. 2001;159:457–463. doi: 10.1016/s0002-9440(10)61717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin FD, Segal M, McGee DW. Regulation of epithelial cell cytokine responses by the alpha3beta1 integrin. Immunology. 2003;108:204–210. doi: 10.1046/j.1365-2567.2003.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar A, Shome SG, Lamar J, Stirling L, Iyer V, Pumiglia K, DiPersio CM. Alpha 3 beta 1 integrin promotes keratinocyte cell survival through activation of a MEK/ERK signaling pathway. J Cell Sci. 2004;117:4043–4054. doi: 10.1242/jcs.01277. [DOI] [PubMed] [Google Scholar]
- Margadant C, Charafeddine RA, Sonnenberg A. Unique and redundant functions of integrins in the epidermis. FASEB J. 2010;24:4133–4152. doi: 10.1096/fj.09-151449. [DOI] [PubMed] [Google Scholar]
- Margadant C, Raymond K, Kreft M, Sachs N, Janssen H, Sonnenberg A. Integrin alpha3beta1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci. 2009;122:278–288. doi: 10.1242/jcs.029108. [DOI] [PubMed] [Google Scholar]
- Mariotti A, Kedeshian PA, Dans M, Curatola AM, Gagnoux-Palacios L, Giancotti FG. EGF-R signaling through Fyn kinase disrupts the function of integrin alpha6beta4 at hemidesmosomes: role in epithelial cell migration and carcinoma invasion. J Cell Biol. 2001;155:447–458. doi: 10.1083/jcb.200105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. Wound healing-aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- Martinez CE, Smith PC, Palma Alvarado VA. The influence of platelet-derived products on angiogenesis and tissue repair: a concise update. Frontiers in Physiology. 2015;6:290. doi: 10.3389/fphys.2015.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–540. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- McLean GW, Komiyama NH, Serrels B, Asano H, Reynolds L, Conti F, Hodivala-Dilke K, Metzger D, Chambon P, Grant SG, Frame MC. Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 2004;18:2998–3003. doi: 10.1101/gad.316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirastschijski U, Haaksma CJ, Tomasek JJ, Agren MS. Matrix metalloproteinase inhibitor GM 6001 attenuates keratinocyte migration, contraction and myofibroblast formation in skin wounds. Exp Cell Res. 2004;299:465–475. doi: 10.1016/j.yexcr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Missan D, Mitchell K, Subbaram S, DiPersio CM. Integrin α3β1 signaling through MEK/ERK determines alternative polyadenylation of the MMP-9 mRNA transcript in immortalized mouse keratinocytes. PLoS One. 2015;10:e0119539. doi: 10.1371/journal.pone.0119539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missan DD, Chittur SV, DiPersio CM. Regulation of fibulin-2 gene expression by integrin α3β1 contributes to the invasive phenotype of transformed keratinocytes. J Invest Dermatol. 2014;134:2418–27. doi: 10.1038/jid.2014.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K, Szekeres C, Milano V, Svenson KB, Nilsen-Hamilton M, Kreidberg JA, DiPersio CM. Alpha3beta1 integrin in epidermis promotes wound angiogenesis and keratinocyte-to-endothelial-cell crosstalk through the induction of MRP3. J Cell Sci. 2009;122:1778–1787. doi: 10.1242/jcs.040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18:516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Morgan MR, Thomas GJ, Russell A, Hart IR, Marshall JF. The integrin cytoplasmic-tail motif EKQKVDLSTDC is sufficient to promote tumor cell invasion mediated by matrix metalloproteinase (MMP)-2 or MMP-9. J Biol Chem. 2004;279:26533–26539. doi: 10.1074/jbc.M401736200. [DOI] [PubMed] [Google Scholar]
- Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, Pittet JF, Kaminski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- Mustoe TA, Gurjala A. The role of the epidermis and the mechanism of action of occlusive dressings in scarring. Wound Repair Regen. 2011;19(Suppl 1):s16–21. doi: 10.1111/j.1524-475X.2011.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen NN, Polverini PJ, Koch AE, Volin MV, Gamelli RL, DiPietro LA. Vascular endothelial growth factor mediates angiogenic activity during the proliferative phase of wound healing. Am J Pathol. 1998;152:1445–1452. [PMC free article] [PubMed] [Google Scholar]
- Nguyen BP, Ren XD, Schwartz MA, Carter WG. Ligation of integrin alpha 3beta 1 by laminin 5 at the wound edge activates Rho-dependent adhesion of leading keratinocytes on collagen. J Biol Chem. 2001;276:43860–43870. doi: 10.1074/jbc.M103404200. [DOI] [PubMed] [Google Scholar]
- Nguyen BP, Ryan MC, Gil SG, Carter WG. Deposition of laminin 5 in epidermal wounds regulates integrin signaling and adhesion. Curr Opin Cell Biol. 2000;12:554–562. doi: 10.1016/s0955-0674(00)00131-9. [DOI] [PubMed] [Google Scholar]
- Nowinski D, Lysheden AS, Gardner H, Rubin K, Gerdin B, Ivarsson M. Analysis of gene expression in fibroblasts in response to keratinocyte-derived factors in vitro: potential implications for the wound healing process. J Invest Dermatol. 2004;122:216–221. doi: 10.1046/j.0022-202X.2003.22112.x. [DOI] [PubMed] [Google Scholar]
- Nunan R, Harding KG, Martin P. Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Disease Models and Mechanisms. 2014;7:1205–1213. doi: 10.1242/dmm.016782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver-Kozup H, Martin KH, Schwegler-Berry D, Green BJ, Betts C, Shinde AV, Van De Water L, Lukomski S. The group A streptococcal collagen-like protein-1, Scl1, mediates biofilm formation by targeting the extra domain A-containing variant of cellular fibronectin expressed in wounded tissue. Mol Microbiol. 2013;87:672–689. doi: 10.1111/mmi.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastar I, Stojadinovic O, Tomic-Canic M. Role of keratinocytes in healing of chronic wounds. Surgical Technology International. 2008;17:105–112. [PubMed] [Google Scholar]
- Penke LR, Huang SK, White ES, Peters-Golden M. Prostaglandin E2 inhibits alpha-smooth muscle actin transcription during myofibroblast differentiation via distinct mechanisms of modulation of serum response factor and myocardin-related transcription factor-A. J Biol Chem. 2014;289:17151–17162. doi: 10.1074/jbc.M114.558130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce GF, Mustoe TA, Altrock BW, Deuel TF, Thomason A. Role of platelet-derived growth factor in wound healing. J Cell Biochem. 1991;45:319–326. doi: 10.1002/jcb.240450403. [DOI] [PubMed] [Google Scholar]
- Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, Parks WC. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. J Cell Biol. 1997;137:1445–1457. doi: 10.1083/jcb.137.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwko-Czuchra A, Koegel H, Meyer H, Bauer M, Werner S, Brakebusch C, Fassler R. β1 integrin-mediated adhesion signalling is essential for epidermal progenitor cell expansion. PLoS One. 2009;4:e5488. doi: 10.1371/journal.pone.0005488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JC, Hogg N. Integrins take partners: cross-talk between integrins and other membrane receptors. Trends Cell Biol. 1998;8:390–396. doi: 10.1016/s0962-8924(98)01344-0. [DOI] [PubMed] [Google Scholar]
- Raghavan S, Bauer C, Mundschau G, Li Q, Fuchs E. Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J Cell Biol. 2000;150:1149–1160. doi: 10.1083/jcb.150.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani-Neishaboor E, Hartwell R, Jalili R, Jackson J, Brown E, Ghahary A. Localized controlled release of stratifin reduces implantation-induced dermal fibrosis. Acta Biomaterialia. 2012;8:3660–3668. doi: 10.1016/j.actbio.2012.06.025. [DOI] [PubMed] [Google Scholar]
- Ramos DM, But M, Regezi J, Schmidt BL, Atakilit A, Dang D, Ellis D, Jordan R, Li X. Expression of integrin beta 6 enhances invasive behavior in oral squamous cell carcinoma. Matrix Biol. 2002;21:297–307. doi: 10.1016/s0945-053x(02)00002-1. [DOI] [PubMed] [Google Scholar]
- Reish RG, Eriksson E. Scars: a review of emerging and currently available therapies. Plast Reconstr Surg. 2008;122:1068–1078. doi: 10.1097/PRS.0b013e318185d38f. [DOI] [PubMed] [Google Scholar]
- Reynolds LE, Conti FJ, Silva R, Robinson SD, Iyer V, Rudling R, Cross B, Nye E, Hart IR, DiPersio CM, Hodivala-Dilke KM. alpha3beta1 integrin-controlled Smad7 regulates reepithelialization during wound healing in mice. J Clin Invest. 2008;118:965–974. doi: 10.1172/JCI33538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Russell AJ, Fincher EF, Millman L, Smith R, Vela V, Waterman EA, Dey CN, Guide S, Weaver VM, Marinkovich MP. Alpha 6 beta 4 integrin regulates keratinocyte chemotaxis through differential GTPase activation and antagonism of alpha 3 beta 1 integrin. J Cell Sci. 2003;116:3543–3556. doi: 10.1242/jcs.00663. [DOI] [PubMed] [Google Scholar]
- Ruzzi L, Gagnoux-Palacios L, Pinola M, Belli S, Meneguzzi G, D’Alessio M, Zambruno G. A homozygous mutation in the integrin a6 gene in junctional epidermolysis bullosa with pyloric atresia. J Clin Invest. 1997;99:2826–2831. doi: 10.1172/JCI119474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salanueva IJ, Cerezo A, Guadamillas MC, del Pozo MA. Integrin regulation of caveolin function. J Cell Mol Med. 2007;11:969–980. doi: 10.1111/j.1582-4934.2007.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005;304:274–286. doi: 10.1016/j.yexcr.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Gohring W, Mann K, Brakebusch C, Yamada Y, Fassler R, Timpl R. Short arm region of laminin-5 gamma2 chain: structure, mechanism of processing and binding to heparin and proteins. J Mol Biol. 2001;314:751–763. doi: 10.1006/jmbi.2001.5176. [DOI] [PubMed] [Google Scholar]
- Sato T, Kirimura Y, Mori Y. The co-culture of dermal fibroblasts with human epidermal keratinocytes induces increased prostaglandin E2 production and cyclooxygenase 2 activity in fibroblasts. J Invest Dermatol. 1997;109:334–339. doi: 10.1111/1523-1747.ep12335935. [DOI] [PubMed] [Google Scholar]
- Sauder DN, Kilian PL, McLane JA, Quick TW, Jakubovic H, Davis SC, Eaglstein WH, Mertz PM. Interleukin-1 enhances epidermal wound healing. Lymphokine Research. 1990;9:465–473. [PubMed] [Google Scholar]
- Schenk S, Quaranta V. Tales from the crypt[ic] sites of the extracellular matrix. Trends Cell Biol. 2003;13:366–375. doi: 10.1016/s0962-8924(03)00129-6. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4:E65–68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- Sehgal BU, DeBiase PJ, Matzno S, Chew TL, Claiborne JN, Hopkinson SB, Russell A, Marinkovich MP, Jones JC. Integrin beta4 regulates migratory behavior of keratinocytes by determining laminin-332 organization. J Biol Chem. 2006;281:35487–35498. doi: 10.1074/jbc.M606317200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard P, Martin G, Smola-Hess S, Brunner G, Krieg T, Smola H. Myofibroblast differentiation is induced in keratinocyte-fibroblast co-cultures and is antagonistically regulated by endogenous transforming growth factor-beta and interleukin-1. Am J Pathol. 2004;164:2055–2066. doi: 10.1016/s0002-9440(10)63764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D. Integrin-mediated activation of latent transforming growth factor beta. Cancer Metastasis Rev. 2005;24:395–402. doi: 10.1007/s10555-005-5131-6. [DOI] [PubMed] [Google Scholar]
- Shinde AV, Bystroff C, Wang C, Vogelezang MG, Vincent PA, Hynes RO, Van De Water L. Identification of the peptide sequences within the EIIIA (EDA) segment of fibronectin that mediate integrin alpha9beta1-dependent cellular activities. J Biol Chem. 2008;283:2858–2870. doi: 10.1074/jbc.M708306200. [DOI] [PubMed] [Google Scholar]
- Shirakawa M, Isseroff RR. Topical negative pressure devices: use for enhancement of healing chronic wounds. Arch Dermatol. 2005;141:1449–1453. doi: 10.1001/archderm.141.11.1449. [DOI] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Singh V, Barbosa FL, Torricelli AA, Santhiago MR, Wilson SE. Transforming growth factor β and platelet-derived growth factor modulation of myofibroblast development from corneal fibroblasts in vitro. Exp Eye Res. 2014;120:152–160. doi: 10.1016/j.exer.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Chen C, Pal-Ghosh S, Stepp MA, Sheppard D, Van De Water L. Loss of integrin alpha9beta1 results in defects in proliferation, causing poor re-epithelialization during cutaneous wound healing. J Invest Dermatol. 2009;129:217–228. doi: 10.1038/jid.2008.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P, Reimer CL, Peters JH, Stepp MA, Hynes RO, Van De Water L. The spatial and temporal expression patterns of integrin alpha9beta1 and one of its ligands, the EIIIA segment of fibronectin, in cutaneous wound healing. J Invest Dermatol. 2004;123:1176–1181. doi: 10.1111/j.0022-202X.2004.23485.x. [DOI] [PubMed] [Google Scholar]
- Steffensen B, Hakkinen L, Larjava H. Proteolytic events of wound-healing--coordinated interactions among matrix metalloproteinases (MMPs), integrins, and extracellular matrix molecules. Crit Rev Oral Biol Med. 2001;12:373–398. doi: 10.1177/10454411010120050201. [DOI] [PubMed] [Google Scholar]
- Sterk LM, Geuijen CA, Oomen LC, Calafat J, Janssen H, Sonnenberg A. The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin alpha6beta4 and may regulate the spatial organization of hemidesmosomes. J Cell Biol. 2000;149:969–982. doi: 10.1083/jcb.149.4.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Berditchevski F. Function of α3β1-tetraspan protein complexes in tumor cell invasion. Evidence for the role of the complexes in production of matrix metalloproteinase 2 (MMP-2) J Cell Biol. 1999;146:1375–1389. doi: 10.1083/jcb.146.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Directional cell movement through tissues is controlled by exosome secretion. Nature Communications. 2015;6:7164. doi: 10.1038/ncomms8164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taipale J, Miyazono K, Heldin CH, Keski-Oja J. Latent transforming growth factor-beta 1 associates to fibroblast extracellular matrix via latent TGF-beta binding protein. J Cell Biol. 1994;124:171–181. doi: 10.1083/jcb.124.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GJ, Lewis MP, Hart IR, Marshall JF, Speight PM. AlphaVbeta6 integrin promotes invasion of squamous carcinoma cells through up-regulation of matrix metalloproteinase-9. Int J Cancer. 2001a;92:641–650. doi: 10.1002/1097-0215(20010601)92:5<641::aid-ijc1243>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Thomas GJ, Nystrom ML, Marshall JF. Alphavbeta6 integrin in wound healing and cancer of the oral cavity. J Oral Pathol Med. 2006;35:1–10. doi: 10.1111/j.1600-0714.2005.00374.x. [DOI] [PubMed] [Google Scholar]
- Thomas GJ, Poomsawat S, Lewis MP, Hart IR, Speight PM, Marshall JF. alpha v beta 6 Integrin upregulates matrix metalloproteinase 9 and promotes migration of normal oral keratinocytes. J Invest Dermatol. 2001b;116:898–904. doi: 10.1046/j.1523-1747.2001.01352.x. [DOI] [PubMed] [Google Scholar]
- Trengove NJ, Stacey MC, MacAuley S, Bennett N, Gibson J, Burslem F, Murphy G, Schultz G. Analysis of the acute and chronic wound environments: the role of proteases and their inhibitors. Wound Repair Regen. 1999;7:442–452. doi: 10.1046/j.1524-475x.1999.00442.x. [DOI] [PubMed] [Google Scholar]
- Utani A, Nomizu M, Yamada Y. Fibulin-2 binds to the short arms of laminin-5 and laminin-1 via conserved amino acid sequences. J Biol Chem. 1997;272:2814–2820. doi: 10.1074/jbc.272.5.2814. [DOI] [PubMed] [Google Scholar]
- Van De Water L, Varney S, Tomasek JJ. Mechanoregulation of the Myofibroblast in Wound Contraction, Scarring, and Fibrosis: Opportunities for New Therapeutic Intervention. Adv Wound Care. 2013;2:122–141. doi: 10.1089/wound.2012.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Neut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]
- Van Linthout S, Miteva K, Tschope C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. 2014;102:258–269. doi: 10.1093/cvr/cvu062. [DOI] [PubMed] [Google Scholar]
- Vedula SR, Hirata H, Nai MH, Brugues A, Toyama Y, Trepat X, Lim CT, Ladoux B. Epithelial bridges maintain tissue integrity during collective cell migration. Nature Materials. 2014;13:87–96. doi: 10.1038/nmat3814. [DOI] [PubMed] [Google Scholar]
- Vidal F, Aberdam D, Miquel C, Christiano AM, Pulkkinen L, Uitto J, Ortonne JP, Meneguzzi G. Integrin beta 4 mutations associated with junctional epidermolysis bullosa with pyloric atresia. Nat Genet. 1995;10:229–234. doi: 10.1038/ng0695-229. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang G, Luo X, Qiu J, Tang C. Substrate stiffness regulates the proliferation, migration, and differentiation of epidermal cells. Burns. 2012;38:414–420. doi: 10.1016/j.burns.2011.09.002. [DOI] [PubMed] [Google Scholar]
- Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21:3919–3926. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber J, Steadman R, Mason MD, Tabi Z, Clayton A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 2010;70:9621–9630. doi: 10.1158/0008-5472.CAN-10-1722. [DOI] [PubMed] [Google Scholar]
- Wei Y, Eble JA, Wang Z, Kreidberg JA, Chapman HA. Urokinase receptors promote beta1 integrin function through interactions with integrin alpha3beta1. Mol Biol Cell. 2001;12:2975–2986. doi: 10.1091/mbc.12.10.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells RG. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394–1400. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- Werner S, Krieg T, Smola H. Keratinocyte-fibroblast interactions in wound healing. J Invest Dermatol. 2007;127:998–1008. doi: 10.1038/sj.jid.5700786. [DOI] [PubMed] [Google Scholar]
- Wetzler C, Kampfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol. 2000;115:245–253. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- Widgerow AD. Chronic wounds - is cellular ‘reception’ at fault? Examining integrins and intracellular signalling. International wound journal. 2013;10:185–192. doi: 10.1111/j.1742-481X.2012.00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd-Katz SE, Fassler R, Geiger B, Legate KR. The integrin adhesome: from genes and proteins to human disease. Nat Rev Mol Cell Biol. 2014;15:273–288. doi: 10.1038/nrm3769. [DOI] [PubMed] [Google Scholar]
- Winterwood NE, Varzavand A, Meland MN, Ashman LK, Stipp CS. A critical role for tetraspanin CD151 in alpha3beta1 and alpha6beta4 integrin-dependent tumor cell functions on laminin-5. Mol Biol Cell. 2006;17:2707–2721. doi: 10.1091/mbc.E05-11-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong VW, Akaishi S, Longaker MT, Gurtner GC. Pushing back: wound mechanotransduction in repair and regeneration. J Invest Dermatol. 2011;131:2186–2196. doi: 10.1038/jid.2011.212. [DOI] [PubMed] [Google Scholar]
- Wong VW, Gurtner GC, Longaker MT. Wound healing: a paradigm for regeneration. Mayo Clin Proc. 2013;88:1022–1031. doi: 10.1016/j.mayocp.2013.04.012. [DOI] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Yang XH, Richardson AL, Torres-Arzayus MI, Zhou P, Sharma C, Kazarov AR, Andzelm MM, Strominger JL, Brown M, Hemler ME. CD151 accelerates breast cancer by regulating alpha 6 integrin function, signaling, and molecular organization. Cancer Res. 2008;68:3204–3213. doi: 10.1158/0008-5472.CAN-07-2949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, Berditchevsky F, Harler MB, Reichner J, Hemler ME. Highly stoichiometric, stable, and specific association of integrin α3β1 with CD151 provides a major link to phosphatidylinositol 4-kinase, and may regulate cell migration. Mol Biol Cell. 1998;9:2751–2765. doi: 10.1091/mbc.9.10.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yauch RL, Kazarov AR, Desai B, Lee RT, Hemler ME. Direct extracellular contact between integrin α3β1 and TM4SF protein CD151. J BiolChem. 2000;275:9230–9238. doi: 10.1074/jbc.275.13.9230. [DOI] [PubMed] [Google Scholar]
- Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- Yurko MA, O’Toole EA, Woodley DT. Phosphorylation of focal adhesion kinase (pp125(FAK)) is increased in human keratinocytes induced to migrate by extracellular matrices. J Cell Physiol. 2001;188:24–32. doi: 10.1002/jcp.1093. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Geiger B. The switchable integrin adhesome. J Cell Sci. 2010;123:1385–1388. doi: 10.1242/jcs.066183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambruno G, Marchisio PC, Marconi A, Vaschieri C, Melchiori A, Giannetti A, De Luca M. Transforming growth factor-beta 1 modulates beta 1 and beta 5 integrin receptors and induces the de novo expression of the alpha v beta 6 heterodimer in normal human keratinocytes: implications for wound healing. J Cell Biol. 1995;129:853–865. doi: 10.1083/jcb.129.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarkoob H, Bodduluri S, Ponnaluri SV, Selby JC, Sander EA. Substrate stiffness affects human keratinocyte colony formation. Cellular and Molecular Bioengineering. 2015;8:32–50. doi: 10.1007/s12195-015-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigrino P, Ayachi O, Schild A, Kaltenberg J, Zamek J, Nischt R, Koch M, Mauch C. Loss of epidermal MMP-14 expression interferes with angiogenesis but not with re-epithelialization. Eur J Cell Biol. 2012;91:748–756. doi: 10.1016/j.ejcb.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Zweers MC, Davidson JM, Pozzi A, Hallinger R, Janz K, Quondamatteo F, Leutgeb B, Krieg T, Eckes B. Integrin alpha2beta1 is required for regulation of murine wound angiogenesis but is dispensable for reepithelialization. J Invest Dermatol. 2007;127:467–478. doi: 10.1038/sj.jid.5700546. [DOI] [PubMed] [Google Scholar]