Abstract
Persistent neurotoxic side effects of Oxaliplatin (OX) chemotherapy, including sensory ataxia, limit the efficacy of treatment and significantly diminish patient quality of life. The common explanation for neurotoxicity is neuropathy, however the degree of neuropathy varies greatly among patients and appears insufficient in some cases to fully account for disability. We recently identified an additional mechanism that might contribute to sensory ataxia following OX treatment. In the present study, we tested whether that mechanism, selective modification of sensory signaling by muscle proprioceptors might result in behavioral deficits in rats. OX was administered once per week for seven weeks (cumulative dose i.p. 70 mg/kg) to adult female Wistar rats. Throughout and for three weeks following treatment, behavioral analysis was performed daily on OX and sham control rats. Compared to controls, OX rats demonstrated errors in placing their hind feet securely and/or correctly during a horizontal ladder rung task. These behavioral deficits occurred together with modification of proprioceptor signaling that eliminated sensory encoding of static muscle position while having little effect on encoding of dynamic changes in muscle length. Selective inability to sustain repetitive firing in response to static muscle stretch led us to hypothesize that OX treatment impairs specific ionic currents, possibly the persistent inward Na currents (NaPIC) that are known to support repetitive firing during static stimulation in several neuron types, including the class of large diameter dorsal root ganglion cells that includes muscle proprioceptors. We tested this hypothesis by determining whether the chronic effects of OX on the firing behavior of muscle proprioceptors in vivo were mimicked by acute injection of NaPIC antagonists. Both riluzole and phenytoin, each having multiple drug actions but having only antagonist action on NaPIC in common, reproduced selective modification of proprioceptor signaling observed in OX rats. Taken together, these findings lead us to propose that OX chemotherapy contributes to movement disability by modifying sensory encoding, possibly via a chronic neurotoxic effect on NaPIC in the sensory terminals of muscle proprioceptors.
Keywords: chemotherapy, sensorimotor deficits, neurotoxicity, muscle proprioceptors, neuropathy
1. Introduction
Neurotoxicity is the most significant side effect diminishing quality of life and limiting use of the anticancer agents commonly used to treat the most frequently occurring cancers (Argyriou et al., 2014; Cavaletti, 2014). Whereas treatment with, for example, the third generation platinum compound oxaliplatin (OX) can achieve high rates of disease free survival from colorectral cancer (Andre et al., 2009), OX treatment is often accompanied by severe and persistent neurotoxic symptoms, which grow more prevalent with the increasing long-term survival of the cancer patient population (Aaronson et al., 2014; Fehrenbacher, 2015). Accumulating doses of OX can result in numbness, and tingling in toes and feet (Argyriou et al., 2012; Avan et al., 2015), as well as sensory ataxia with imbalance and falls (Bennett et al., 2012; Tofthagen et al., 2012), some or all of which can persist in a substantial number of patients for years after the cessation of chemotherapy (Kiernan, 2007; Park et al., 2011b; Briani et al., 2014). Finding a solution to this urgent and growing problem is hindered by inadequate understanding of the pathogenesis of neurotoxic symptoms.
Two mechanisms are commonly held accountable for long-term chemotherapy-related neurotoxic symptoms. One is peripheral sensory neuropathy that manifests as reduction in sensory nerve evoked compound action potentials (SNAPs) and as substantial epidermal denervation (Krishnan et al., 2005; Argyriou et al., 2008; Burakgazi et al., 2011). These indications of cell death and/or axonal degeneration of primary sensory neurons establish the occurrence of receptor denervation that could readily produce sensorimotor aberrations. However, the degree of neuropathy varies widely as evidenced by disparity between patient-reported symptoms and the amount of neuropathy on examination (Park et al., 2013; Cavaletti, 2014). In fact neuropathy has even been found absent in a subset of patients who nonetheless report significant symptoms (Burakgazi et al., 2011). Thus, neuropathy cannot be solely responsible for sensorimotor dysfunction and additional explanations are required. The other contributor to neurotoxic symptoms is chemotherapy-induced modification of ion channels (Park et al., 2011a; Park et al., 2013). For example, OX modification of voltage-gated Na channels is held responsible for the hyper-excitability of sensory axons associated with chronic pain occurring with OX treatment (Adelsberger et al., 2000; Grolleau et al., 2001; Benoit et al., 2006; Krishnan et al., 2006; Park et al., 2009, 2011a; Sittl et al., 2012). It is unknown whether interference with axonal signaling is sufficient to explain the long-term persistence of wide-ranging sensory symptoms induced by chemotherapy, but correlation exists between the severity of acute changes in excitability and development of chronic sensory impairment (Park et al., 2009).
With interest in discovering the mechanism underlying disability with proprioceptive function, i.e. the sensory ataxia with postural instability and clumsiness (Bennett et al., 2012; Kneis et al., 2015) that can be induced by chemotherapy and continue long after its removal, we developed a rodent model of chronic OX treatment. This model reproduced the absence of neuropathy observed in some OX patients, but it revealed chronic dysfunction in proprioceptive signaling (Bullinger et al., 2011). Sensory neurons supplying muscle spindles showed no evidence of degeneration of either axons or sensory terminals, but their firing failed to signal static muscle length as it does normally. The chronic effect of OX was selective for static muscle position leaving unaffected the encoding of dynamic muscle stretch and the capacity of the axon to conduct action potentials to the central nervous system. These observations led us to the novel hypothesis that OX neurotoxicity chronically impairs ion channels in the sensory endings of muscle spindle receptors that are responsible for selected features of sensory transduction or encoding of muscle position. The present study was designed to take the first steps at localizing the mechanism. In addition, we sought evidence that this model would cause proprioceptive-like disorders in rats as in humans.
Preliminary findings were presented in a conference proceedings report, Vincent et al. 2015.
2. Methods
2.1 Animal use
Adult female Wistar rats (250–300g, Charles Rivers Laboratory Wilmington, VA) were housed in regularly cleaned cages in a temperature and light controlled environment, fed and watered ad libitum, monitored for wellbeing, and used experimentally, all as approved by Wright State University Laboratory Animal Care and Use Committee. Rats were randomly divided into two groups. One group of 15 rats (5 sham controls and 10 OX) was used to study chronic effects of chronic OX treatment. These rats were subjected to repeated measures described below over a period of 13 weeks before being studied a few days later in terminal electrophysiological experiments. A different group of 22 rats was used to test the capacity for acute injection of pharmacological agents having known drug actions to mimic the electrophysiological effects of OX in terminal experiments. Following terminal experiments, rats were overdosed with isoflurane anesthesia and their hearts surgically removed.
2.2 Chronic OX studies
These studies were designed to test effects of chronic OX treatment. Oxaliplatin (BIOTANG, Lexington, MA) dissolved in 5% dextrose was administered via intraperitoneal (IP) injection once per week (except week 4 when OX was unexpectedly unavailable) in single doses of 10mg/kg to reach a cumulative dose of 70 mg/kg over 8 weeks. Ten rats received these injections concurrently with five rats that served as sham controls receiving only vehicle (5% dextrose i.p.) on the same schedule. OX effects were assessed by studying rats over a period of 14 weeks spanning periods before, during and after injections. Throughout this period, each rat was closely and frequently monitored for pain or distress. No individual rat reached set criteria established for early removal from the study, e.g. 20% weight loss, vocalization, failure to drink or groom, severe lethargy, self-mutilation, uncontrollable infection.
Repeated measures of body weight, motor task performance, and tail nerve conduction were obtained from all rats before electrophysiological recordings were made in terminal experiments, all as described below.
2.3 Behavioral testing and other repeated measures
Over the course of the study all 15 animals were handled by two individuals who were responsible for all behavioral testing, weight measurements, SNAP recordings, and OX treatment. The ladder rung walking task was chosen for its validated use to detect and describe sensorimotor deficits (Metz and Whishaw, 2002, 2009; Akay et al., 2014). All 15 rats were introduced and trained on the horizontal ladder run apparatus in a single session lasting 4 hours one week prior to the start of the study. Rats were trained to traverse the apparatus in the same direction in order to reach their home cage. Behavioral testing was performed once per week at the same time of day on different days each week, and lasted approximately 4 hours. During a test session, each rat traversed the apparatus 5 times. On separate days animal weight and SNAPs were recorded (see below). Behavioral sessions were video recorded using a high definition camera (Samsung F90 HD Camcorder) aimed toward the left side of the animal; a mirror was placed on the right side of the apparatus to allow clear visibility of the right hind limb (Fig. 1B). All videos were imported onto a laptop computer and exported as QuickTime videos that were stored for analysis. The person performing all analysis was blinded to which rats received treatment. All results were compiled and were subsequently decoded for statistical analyses.
Figure 1. Movement disability in OX-treated rats.
Rats were scored for errors in hind foot placement while walking on unevenly spaced ladder rungs in sessions spanning 11 weeks. (A, C) For untreated control rats (n=5) data were pooled and plotted as percent change in order to illustrate improvement (training effect) relative to initial task sessions (time 0); mean values (open black circles joined by lines) are bracketed for 95% CI. For each one of 10 OX-treated rats (identified by red number) mean percent change is plotted per task session, and the schedule for OX injection is noted by blue dots. (A) Secure hind foot placement was scored by errors/steps in rung contact multiplied by −1 and computed as percent change from the pre-treatment value for each OX treated rat. (B) Photograph shows left hind limb slip in double image (simultaneous side and underneath views) of OX-treated rat walking on ladder rungs. (C) Correct hind foot placement was scored by errors/steps in correctly placing the trailing hind foot on the same rung as the ipsilateral forelimb multiplied by −1 and computed as percent change from the pre-treatment value for each OX treated rat. (D) Plot of secure vs correct hind foot placement (unlike Figs. 1A and 1C, values were calculated as percent change from untreated control mean value at week 11); box at origin encompasses variation pooled for untreated control rats.
Ladder Rung: Horizontal Apparatus
The ladder rung apparatus was built as previously described (Metz and Whishaw, 2002), consisting of two Plexiglas walls, 1m × 0.3m, connected at the bottom with 1/8 inch (~3mm) diameter steel rods (rungs). Rungs were set at irregular intervals, with a maximum of 5 cm and a minimum of 1 cm distance between rungs. Holes drilled for rungs were 1 cm apart, across the entirety of the Plexiglas, allowing for different iterations (6 iterations were used in total) of rung patterns, preventing animals from learning any single pattern.
Analysis of Hind Foot Placement
Two measures of hind foot placement: secure placement, and correct placement were analyzed in control and OX treated animals. All steps taken during ladder walking, from first to last were included in the analysis (Antonow-Schlorke et al., 2013). Once analyzed, the data were averaged and are presented as percent of change, week 0 scores were analyzed, averaged and are represented as zero.
Analysis of Secure Placement
Secure hind foot placement was assessed using the seven-category scale scoring system introduced by Metz and Whishaw (Metz and Whishaw, 2002, 2009). The scoring system, which distinguishes misses, slips, and placement errors in rung contact was slightly modified by assigning a score of 0 whenever the foot entirely missed a rung, whether or not it was associated with a deep fall. Based on the scoring system above, error was defined as a score of 0–2. The total number of errors in hind foot placement was calculated with no distinction between right and left, and the mean error/step ratio was calculated for all five trails and represented as percent of error. This number was multiplied by −1 in order to convert the values into a measure of the number of secure placements rather than the number of errors per steps. Thus an increase in the number of errors/steps corresponds to a decrease in secure hind foot placement of the hind foot.
Analysis of Correct Placement
During ladder rung walking, normal rats tended to place their trailing hind foot on the exact same rung as the ipsilateral front foot, defined here as correct placement of the hind foot; sequential placement of the ipsilateral hind foot on a different rung was defined as an error in placement and supposed to reflect impaired proprioceptive ability. Placing the hind foot on an incorrect rung, and then replacing to the correct rung, was still considered an error in placement. Correct hind foot placement was defined as the number of errors/steps multiplied by −1. The total number of errors was counted and expressed as an average of error/step ratio for both hind limbs. Multiplying this number by −1 gives a value for correct placement. Thus as the number of errors/steps increases, the value for correct hind foot placement decreases.
Sensory Nerve Action Potentials (SNAPs)
At the end of each behavioral test session, rats were temporarily anesthetized with isoflurane inhalation via nose cone (2–4% in 100% O2) in order to record SNAPs as described in our earlier reports (Novak et al., 2009; Bullinger et al., 2011). Two pairs of needle electrodes were inserted sub-dermally in the tail: one pair near the base of the tail for recording SNAPs (compound action potentials) evoked by electrical stimulation through the other pair located 2 cm distally. A ground electrode was inserted near the recording electrodes. An electrical stimulus of 10 mA was applied to elicit and record the SNAP using a Nicolette Viking Quest nerve conduction machine (Natus, Pleasanton, CA). Before removal of electrodes, a permanent marker was used to record the position of the electrodes, enabling exact placement of the electrodes for the duration of the study.
2.4 Terminal Experiments
Electromechanical responses of individual proprioceptors were examined electrophysiologically in terminal experiments performed on all rats, including those used to study the behavioral effects of OX treatment and those dedicated solely for studying the acute effects of riluzole and phenytoin on sensory signaling.
Anesthesia and Vitals
Rats were deeply anesthetized by isoflurane inhalation throughout the entire terminal experiment, beginning with induction in a closed chamber (5% in 100% O2) and continuing with delivery via a tracheal cannula (1.5–2.5% in 100% O2). Subcutaneous injections of lactated ringer solution were given to maintain adequate fluid levels and blood pressure. Respiratory rate, heart rate, oxygen saturation, and pCO2 were monitored to ensure anesthesia and overall animal health. Body temperature was recorded via a rectal probe, and maintained between 36–38°C with heated water pads and a heat lamp.
Surgical Dissection
The left hind limb and lumbosacral spinal cord were surgically exposed as needed to record the firing of individual sensory neurons in response to controlled muscle stretch (Haftel et al., 2004; Bullinger et al., 2011). Briefly, each rat was placed in a rigid stereotaxic frame, and the legs were secured with ankle and knee joints fixed at angles of approximately 90° and 120°, respectively. The left triceps surae muscles (medial and lateral gastrocnemius and soleus muscles) were partially freed of from surrounding connective tissue, marked for their resting length (Lr) measured with the leg in its fixed joint positions, and then detached from the calcaneus. The distal end of the severed Achilles tendon was tied directly to the lever of a force and length-sensing servomotor (Model 305B–LR, Aurora Scientific Inc.), which provided for application of controlled muscle stretch while recording muscle length and force. Triceps surae nerves were freed from the surrounding tissue and placed on a unipolar stimulating electrode, and other nerves in the left hind limb were crushed, including common peroneal, sural, and posterior tibial nerves. A laminectomy was performed from T10-S1, and the dura mater was removed to expose the spinal cord and dorsal roots. Skin flaps were tied up in the back and hind limb to create pools for mineral oil, in order to prevent the tissue desiccation. During certain experiments (see Results) ventral roots were sectioned to eliminate gamma motor influence over muscle spindles.
Data Collection from Proprioceptors
Action potentials from the axons of individual muscle proprioceptors were recorded with glass microelectrodes (filled with 2M K+ Acetate) driven into dorsal roots supported on bipolar hook electrodes. Sensory axons were randomly sampled and selected for recording when they displayed orthodromic action potentials with a conduction delay of <3 ms following peripheral electrical stimulation of triceps surae nerves. Individual experiments yielded intra-axonal records from between 10–40 triceps surae muscle proprioceptors. In rats selected for pharmacologic investigation, data were collected from multiple proprioceptors sampled both before and beginning 45 mins following i.p. injection of either riluzole or phenytoin
Sensory axons were designated as either muscle spindle or tendon organ proprioceptors, respectively, depending upon whether they paused or accelerated firing during electrically evoked twitch contractions of the triceps surae muscles. Further study of proprioceptor firing was based on responses to muscle stretch including ramp-hold-release (3 mm, 20 mm/s ramp, 1 sec hold), three successive triangular ramp stretches (4 mm/s, 3 mm), vibration (50–250 Hz, 80 µm), and ramp-hold-release (3 mm, 20 mm/s ramp, 1 sec hold) with superimposed 100 Hz vibration (80 µm) during the 1 sec hold phase. The ramp portions of ramp-hold-release and triangular stretch represented fast and slow ramps respectively. Muscle spindle proprioceptors that did exhibit an initial high frequency burst at the beginning of the ramp or triangular stretch and 1:1 firing entrainment during ≥ 100Hz vibration were designated group Ia, and as while those that did not exhibit meet these criteria were identified as group II. All stretches were performed at resting length (Lr) of 10 g ± 3 g, Lr+ 10 g, and Lr+ 20 g. Intra-axonal records of action potentials and of muscle length and force were digitized (20 kHz), and stored on a computer for later analysis using Spike2 software.
Dynamic and static responsiveness of proprioceptors were measured as different parameters of firing occurring, respectively during changing and constant muscle length. Dynamic response parameters included rate of initial burst firing at the onset of fast ramp stretches, the rate of firing rates at the peak of ramp stretch (peak firing rate). Dynamic thresholds were recorded as the length change necessary to generate sustained repetitive firing during fast and slow ramps. Measures of static responsiveness were measured during the hold phase of ramp-hold-release stretches as the duration and average rate of repetitive firing.
2.5 Acute Pharmacological Studies
Twenty two rats having received no prior treatment or study were used to measure proprioceptor firing responses in terminal electrophysiological study exactly as described above. Individual muscle proprioceptors were randomly sampled both before and after i.p. injection of riluzole (16 rats), phenytoin (4 rats), or vehicle alone (DMSO in saline). Riluzole was given in doses of 4 mg/kg (4 rats), 6 mg/kg (8 rats), and 10 mg/kg (4 rats), and phenytoin was given in a single dose of 20 mg/kg dose. Vehicle consisted of 1 mL DMSO combined with 4 mL 0.9% saline.
2.6 Statistical Analysis
Statistical comparisons of control versus treatment groups were made for various parameters of proprioceptor encoding with nested ANOVA and Tukey’s honestly significant difference post hoc tests. (Statistica software, Statsoft Inc, Tulsa, OK). The level of significance for all statistical tests was set at P 0.05. All values are reported as either means ± SE, or means ± SD.
3. Results
3.1 Proprioceptive movement deficits in OX-treated rats
In previous study of chronic OX-treatment in rats, we demonstrated defects in sensory signaling by muscle proprioceptors (Bullinger et al., 2011). Because these signals are critical for detecting and guiding limb position and movement, we predicted deficits in movements that rely on sensory feedback about limb position (see Introduction). In order to assess sensorimotor function in rats, we tested their performance in walking over horizontally arranged ladder rungs (Metz and Whishaw, 2002, 2009). As rats move with vision directed forward, success in walking relies on proprioceptive function that assists with foot placement on rungs that pass out of sight (Fig. 1B).
Performance measures in ladder rung walking are illustrated in Fig. 1. All values are expressed as percent change per rat from scores obtained in early task sessions (time 0). Plots for secure (Fig. 1A) and correct hind foot placement (Fig. 1C) illustrate sensorimotor function for each OX-treated rat over time in relation to the mean and 95% confidence intervals computed from values pooled from all five sham control rats. A general pattern emerged over time with values for secure and correct hind foot placement falling below control confidence intervals, despite some tendency for both OX and control rats to improve performance with time. Divergence from control values appeared as early as one week after the first OX dose and continued through the last measurements made 3 weeks after OX-treatment was discontinued.
Closer inspection of Fig 1 shows the following: First, OX-rats exhibited considerable variability both among individuals and within individuals across time. Compare, for example, secure hind foot placement (Fig. 1A) that was poor throughout and after treatment for OX-rat #3, not distinguishable from normal for OX-rat #5 until treatment was complete, and never abnormal for OX-rat #2. In this respect, our rat model reproduces the high degree of variability also observed for the OX patient population, in which subjective assessment of motor ability varies between and within individuals (Bennett et al., 2012). Second, Fig. 1A shows that values for secure hind foot placement in OX-rats rarely exceeded control means and were never higher than the control 95% confidence intervals. This contrasts with values for correct hind foot placement (Fig. 1C), which for some OX rats (#4 and #8) were better than normal. Thus, rats capable of correct placement were unable to make secure contact, e.g. slips occurred as shown in Fig. 1B. Although these differences between performance in secure vs correct hind foot placement may prove to be important if verified in a larger study, there was nonetheless qualitative agreement between these measures in 7/10 OX rats at the time of the last performance measurement (Fig. 1D).
OX-rats were routinely observed for a number of additional factors that might have contributed to poor sensorimotor performance. Although OX-rats did not increase body weight as seen in the sham control rats over the study period, both sham control and OX-rats gained weight. Mean body weight increased 20% for sham controls compared to 10.5% for OX-rats. Following each treatment, OX rats were also less active and had lower appetites. Otherwise, OX-rats exhibited no deterioration in general condition, e.g. severe lethargy, piloerection or failure to groom. During behavioral test sessions, OX animals exhibited similar climbing and gripping capabilities as control rats. From the latter observations, we rule out weakness as the basis for poorer than normal motor performance.
Severe neuropathy, involving dying-back degeneration extending into the peripheral nerve was assessed by measuring SNAPs from the rats’ most distal extremity, the tail, at the end of each behavioral test session. We found no significant difference (p>0.05) between OX and control rats over time consistent with our earlier study (Bullinger et al., 2011). Even by the end of behavioral testing, OX-rats were not different than control, respectively, in either SNAP amplitude (mean ± SD; 0.30 ± 0.04 mV, 0.33 ± 0.08 mV) or latency (mean ± SD; 0.80 ± 0.08 ms, 0.82 ± 0.08 ms). These findings rule out the occurrence of neuropathy severe enough to cause axon degeneration in parent sensory nerves, as it does in some though not all OX patients (Burakgazi et al., 2011). However, this measure does not assess degeneration of the distal most endings of sensory neurons (see Discussion).
3.2 Proprioceptor impairment occurred together with proprioceptive movement deficits
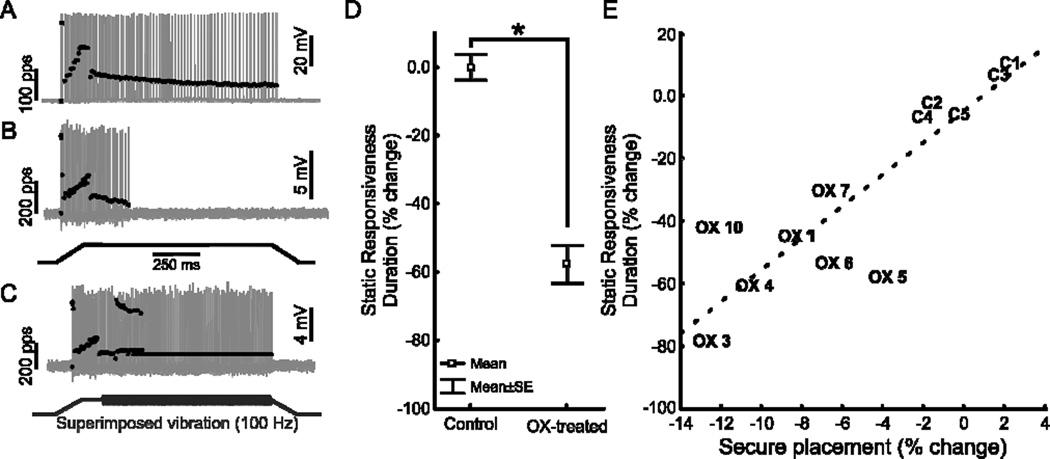
As a test of our hypothesis that proprioceptive encoding deficits contribute to poor motor performance, we performed in vivo electrophysiological studies on all OX treated rats at the conclusion of behavioral evaluation in weeks 12 and 13 (cf Fig. 1A,C time scale). Analyses focused on group Ia muscle spindle proprioceptors, for which per-animal sample sizes were largest. Fig. 2 compares firing behavior of Ia proprioceptors sampled from sham control and OX-rats. A conspicuous group difference was found in static responsiveness, i.e. the firing that occurs during the static (hold) phase of muscle stretch. Records taken from a control rat (Fig. 2A) show that repetitive firing was sustained throughout static stretch, which is typical for normal Ia proprioceptors and which classifies them as slowly adapting sensory neurons (Lewin and McMahon, 1991). In sharp contrast, records from an OX-rat (Fig. 2B) show that firing was absent during most of static stretch. The reduction in static responsiveness for OX rats as a group averaged >50% (Fig. 2D, Table 1). These findings corroborate the abnormally low static responsiveness that we reported earlier for OX treated rats (Bullinger et al., 2011).
Figure 2. OX treatment shortens firing duration during static muscle stretch.
Representative records of Ia proprioceptors firing (grey traces show intra-axonal records of action potentials, black dots indicate instantaneous firing rate) in response to ramp-hold-release muscle stretch (B bottom trace; 3mm, 20mm/s, 1s hold phase). (A) In an untreated control rat, Ia fired during fast-dynamic (ramp) phase and throughout static (hold) phase of stretch. (B) In a rat 4 weeks after 8-week treatment course of OX, Ia fired similar to normal during fast-dynamic phase but stopped firing during the static phase of muscle stretch. (C) For the same Ia proprioceptor as in B, vibration superimposed on muscle stretch (bottom trace; 100Hz, 80µm vibration during stretch hold phase) restored firing. (D) Mean ± SE for duration of static-phase firing expressed as percent change for pooled samples of Ia proprioceptors in OX-treated rats (71 Ia’s in 10 rats) relative to control rats (52 Ia’s in 5 rats). (E) Comparison of mean static-phase firing (n>4 Ia proprioceptors per animal) and secure hind foot placement for individual control (C) and OX (OX) animals expressed as a percent change from mean control values. Secure hind foot placement during horizontal ladder rung task is positively correlated with the duration of static responsiveness (r2=0.76, p<0.01, dashed line).
Table 1. Muscle length encoding by Ia muscle spindle proprioceptors.
Rats in oxaliplatin (OX) study were all subject to the same procedures, i.e. chronic measurement of motor performance and terminal measurement of proprioceptor encoding; 10 received repeated injections of vehicle + OX and 5 received injections of vehicle alone. All rats in riluzole (Ril) study, were examined only in terminal experiments to obtain measurement of proprioceptor encoding before and after Ril injection.
| OX Control | OX (70mg/kg) | Riluzole Control | Riluzole (6mg/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Means ± SD | n | Means ± SD | n | Means ± SD | n | Means ± SD | n | ||
| Dynamic Responsiveness | |||||||||
| Fast ramp | |||||||||
| Dynamic firing threshold, mm | 0.15 ±0.23 | 52 | 0.13 ±0.27 | 71 | 0.16 ±0.14 | 66 | 0.19 ±0.34 | 34 | |
| Initial Burst, pulses/sec | 215.26 ± 190.80 | 52 | 315.02 ±204.91 | 71 | 358.05 ± 133.11 | 32 | 425.97 ± 90.23 | 19 | |
| Peak firing rate, pulses/sec | 243.63 ± 60.35 | 52 | 190.85 ±77.38* | 71 | 158.99 ±72.58 | 66 | 130.89 ±48.49 | 34 | |
| Dynamic index | 171.09 ±46.24 | 48 | 201.90 ± 100.88 | 26 | 128.75 ±66.50 | 47 | 103.43 ±52.44 | 17 | |
| Slow ramp | |||||||||
| Dynamic firing threshold, mm | 0.71 ±0.29 | 51 | 1.15 ±0.56* | 58 | 1.19 ±0.37 | 62 | 1.55 ±0.62 | 33 | |
| Reduced dyanmic response | 28.45 ± 9.47 | 51 | 20.10 ±8.92* | 58 | 17.77 ±5.95 | 66 | 13.85 ±6.17* | 34 | |
| Static Responsiveness | |||||||||
| Hold | |||||||||
| Average firing rate, Pulses/sec | 73.59 ± 38.86 | 52 | 16.69± 27.17* | 71 | 28.22 ± 26.61 | 66 | 14.26 ± 20.69* | 34 | |
| Duration sustained firing, ms | 896.84 ± 246.41 | 52 | 378.61 ±418.50* | 71 | 746.38 ±315.39 | 66 | 422.56 ± 422.56* | 34 | |
n, number of afferents pooled within groups. Nested ANOVA and Tukey’s honestly significant difference post hoc tests were used to test for the significance of group differences (*p<0.05)
The average magnitudes of impairment in proprioceptor signaling and behavioral performance were compared among individual OX rats. Fig. 2E shows a significant positive correlation between how securely the hind foot was placed on the rungs and the duration of Ia afferent static responsiveness (r2=0.76, p<0.01). Static responsiveness was not significantly related to correct hind foot placement.
3.3 Mechanisms of impaired proprioceptor signaling in OX-treated rats
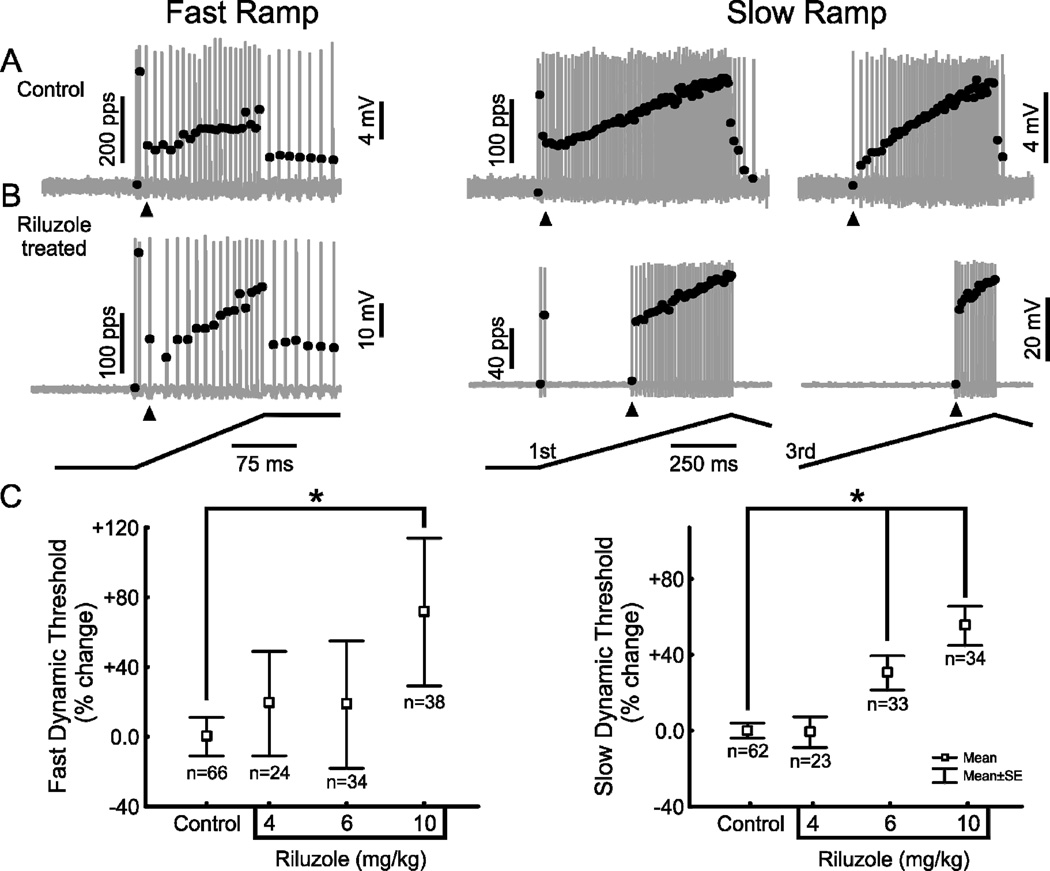
At the time during static muscle stretch when Ia proprioceptors failed to fire, superimposition of mechanical vibration, a fast-dynamic muscle stretch was perfectly effective in eliciting firing (Fig. 2C). Thus, the loss of static responsiveness was not attributable to inability either to generate or conduct spikes centrally, since the Ia afferent remained responsive to fast dynamic mechanical stimulation produced by muscle vibration. Instead we speculated that reduced static responsiveness originated from impairment of underlying persistent inward current mechanisms. If this were the case, then additional signs of firing impairment would be expected, as they are with suppression of persistent inward currents in other neuronal systems (Harvey et al., 2006). Specifically, we expected that the threshold for repetitive firing in OX rats would remain unchanged during fast ramp stretch (20 mm/s), but would increase during for slow ramp stretch (4 mm/s). Results illustrated in Fig. 3 confirmed our expectation. Threshold for repetitive-firing increased by about 60% with slow- but not fast-dynamic stretch in OX-rats (Fig. 3C,F, Table 1). Comparison of Fig 3A and 3B provides insight into the change in threshold. In control rats, repetitive firing is immediately preceded by an initial burst of rapid firing, which occurs at the onset of fast-dynamic stretch and in the first of three sequential slow-dynamic triangular muscle stretches (Fig. 3A; Haftel et al. 2004). That initial burst was not lost in OX-rats (Fig. 3B), and it occurred at the same threshold for control and OX-rats (see Table 1). These findings indicate that in OX rats, increased threshold was specific to repetitive firing and was not an expression of general insensitivity to mechanical stimulation.
Figure 3. OX-treatment increases repetitive firing threshold during slow- but not fast-dynamic muscle stretch.
Threshold for repetitive firing was measured from Ia proprioceptors during fast ramps (20mm/s; left column) and slow ramps (4mm/s in 3 successive triangular stretches; right column). Action potential firing and firing rate in response to muscle stretch (traces described in Fig. 2) were taken from one Ia proprioceptor in a control rat (A) and one in an OX-treated rat (B). Repetitive firing threshold (arrow heads) after OX-treatment was similar to control for fast ramps, but higher than control for slow ramps. Note that threshold of the transient initial burst was unchanged. (C) Mean ± SE for repetitive firing threshold for fast (left) and slow (right) ramps expressed as percent change for pooled samples of Ia proprioceptors in OX-treated rats (58 Ia’s in 10 rats) relative to control rats (51 Ia’s in 5 rats).
3.4 Pharmacological block of NaPIC mimics proprioceptor impairment with OX
Repetitive firing in response to a static stimulus relies in many neurons on persistent inward current mediated by Na channels (Crill, 1996; Lee and Heckman, 1998, 2001; Harvey et al., 2006). If OX treatment altered proprioceptor signaling through this mechanism, then we expected to mimic its effect by reducing sodium persistent inward current (NaPIC) in normal rats. Riluzole was selected for its known effects in blocking NaPIC and repetitive firing in the large diameter class of DRG neurons that include muscle proprioceptors (Xie et al., 2011).
Fig. 4 illustrates the effect of acute riluzole injection on the static responsiveness of Ia proprioceptors in normal rats. For Ia proprioceptors sampled from a single rat, comparison of Fig. 4A with 4B demonstrates a reduction in static responsiveness similar to that shown for OX treatment (Fig. 2). Fig. 4D demonstrates that the reduction in static responsiveness was dose dependent. By comparison, OX reduced static responsiveness by about 60% (Fig. 2.; Table 1), an amount found for riluzole concentrations between 6–10mg/Kg. If we speculate that OX acts through the same mechanism as riluzole, possibly block of NaPIC, then we would suggest that NaPIC is only partially impaired by OX treatment.
Figure 4. Riluzole mimics OX-treatment in shortening firing duration during static muscle stretch.
Representative records (as described in Fig. 2) of Ia proprioceptor responses to muscle stretch in one rat studied (A) before and (B,C) after Riluzole injection (6mg/kg i.p.). Similar to the effect of OX-treatment (Fig. 2), acute Riluzole injection shortened static responsiveness, but had no apparent effect on dynamic responsiveness exhibited during fast-ramp stretch or vibration. (D) Reduction in duration of static responsiveness (expressed as percent change from control) for Ia proprioceptors was dose dependent.
Additional effects of acute riluzole were studied as they were for OX treatment. Fig. 4C shows that Ia proprioceptors remained sensitive to fast-dynamic mechanical stimulation produced by muscle vibration, just as in OX-rats (cf. Fig 2C). Fig. 5 shows that riluzole increased the threshold for repetitive firing in response to slow stretches, and affected fast stretches only at the highest dose (cf. Fig 3 for OX rats). Table 1 compares riluzole (at the intermediate dose of 6mg/kg) and OX treatment for their effects on additional Ia firing parameters. The match is nearly identical between those parameters that either were or were not significantly different relative to control. The one exception was peak firing rate which was nominally reduced by riluzole, but not significantly so as it was with OX treatment. Collectively, these findings are consistent with the possibility that OX treatment impairs proprioceptor signaling by reducing NaPIC.
Figure 5. Riluzole mimics the OX-treatment increase in repetitive-firing threshold during slow-dynamic muscle stretch.
Representative records of Ia proprioceptor firing in response to muscle stretch (cf. Fig. 3) in a rat studied (A) before and (B) after riluzole injection (6mg/kg i.p.). Repetitive firing threshold (arrow heads) was similar before and after acute Ril injection for fast ramps, but was substantially higher for slow ramps. Note that threshold of the transient initial burst was unchanged. (C) Mean ± SE repetitive firing threshold for fast (left) and slow (right) ramps expressed as percent change for pooled samples of Ia proprioceptors after relative to before Ril-injection.
Table 1 revealed differences between the control groups for riluzole vs. OX that bear consideration. Riluzole control values were taken from Ia proprioceptors sampled from single rats before riluzole injection, and those values are similar to control values recorded from untreated rats in an earlier study (Bullinger et al., 2011). However, the OX controls were different from riluzole controls for nearly every parameter. We can only speculate that the OX controls were influenced by the behavioral study protocol or by vehicle injection, nonetheless, these data provide the appropriate controls for OX treated rats, which underwent the same protocol.
Riluzole might have reduced Ia static responsiveness through its known action of blocking NaPIC in motoneurons (Harvey et al., 2006; Theiss et al., 2007). Through this action, riluzole might decrease activity of gamma motoneurons, which play a critical role in setting Ia sensitivity to muscle stretch (Crowe and Matthews, 1964; Matthews, 1972). To determine whether a loss of gamma modulation of muscle spindle gain might underlie OX-induced encoding defects, we sectioned ventral roots (where gamma motoneuron axons exit the spinal cord) and recorded spindle encoding in both controls and riluzole injected rats. Ventral root section had no significant effect (p>0.05) on Ia static responsiveness. In control rats, there was no significant difference in the duration of static responsiveness with ventral roots intact (746 ± 315 ms) or ventral roots cut (790 ± 269 ms). Neither did the effect of riluzole depend on gamma motoneuron input, since riluzole reduced the duration of static responsiveness to levels that were not significantly different (p>0.05) with ventral roots intact (423 ± 438 ms) or cut (518 ± 404 ms).
Mechanistic interpretation of the riluzole effect is confounded by its multiple drug actions. In addition to blocking NaPIC, riluzole activates calcium dependent potassium channels (BK/SK) (Grunnet et al., 2001; Wang et al., 2008), inhibits voltage gated Ca 2+ channels (Huang et al., 1997; Stefani et al., 1997), and modulates glutamate signaling and release (Doble, 1996; Lamanauskas and Nistri, 2008; Bellingham, 2011, 2013), all of which are present in muscle spindles (Bewick and Banks, 2015). In attempt to narrow the set of possible explanations for the riluzole effect, we tested acute injection of phenytoin, which also blocks NaPIC (Lampl et al., 1998; Zeng et al., 2005), but does not share the other actions with riluzole. The effects of phenytoin on Ia proprioceptor firing were similar to those of riluzole and OX treatment. Fig. 6 shows the shortening of firing duration during static muscle stretch described above for OX treatment and matched to the effect of riluzole at its intermediate dose (6mg/Kg). The reproduction of impaired Ia firing in OX-rats by two drugs having predominantly only NaPIC blockade in common leads us to the parsimonious though provisional conclusion that OX reduces static and slow-dynamic responsiveness of Ia proprioceptors by reducing NaPIC in the proprioceptor’s peripheral nerve terminals.
Figure 6. Phenytoin shortens firing duration during static muscle stretch.
(A) Representative records of a Ia proprioceptor firing (top grey trace action potentials with instantaneous firing rate indicated by superimposed black dots) in response to ramp-hold-release muscle stretch (bottom trace; 3mm, 20mm/s, 1s hold phase) following phenytoin injection (20mg/kg i.p.). Similar to the acute effect of riluzole (cf Fig. 4), acute phenytoin injection shortened static responsiveness, but had little effect on dynamic responsiveness exhibited during fast-ramp stretch or vibration (data not shown). (B) Duration of static responsiveness (expressed as percent change from control) significantly reduced for 25 Ia proprioceptors measured in rats injected with phenytoin compared with 21 Ia afferents in control rats.
3.5 Encoding deficits are present in other muscle proprioceptors
The large diameter group II muscle spindle afferents and group Ib tendon organ afferents are also muscle proprioceptors that provide essential feedback regarding muscle length and force (Matthews, 1972; Jami, 1992). Fig. 7 illustrates the effects of OX treatment and riluzole on static responsiveness of these two proprioceptors. For group II proprioceptors, static responsiveness shifted, but only slightly and insignificantly toward lower than control values in OX rats, although riluzole in the highest dose studied here produced a substantial and significant decrease. For group Ib proprioceptors, there was a significant reduction in responsiveness with OX treatment and with acute injection of riluzole. This finding had unique value in establishing that impaired sensory firing did not require the functional and structural specializations that are present in muscle spindles but absent in tendon organs (Hunt, 1974). We suggest, therefore, that these findings localize impairment of static responsiveness to the nerve terminals of proprioceptors.
Figure 7. Effects of OX treatment and riluzole on group II spindle and Ib tendon-organ proprioceptors.
(A) OX treatment shortened firing duration during static muscle stretch nominally for II’s (p=0.077) and significantly for Ib’s. (B) Acute riluzole injection also tended to shorten firing at the highest doses.
4. DISCUSSION
Recently our laboratory discovered that chronic treatment with oxaliplatin in rodents produces a selective deficit in sensory encoding by muscle proprioceptors (Bullinger et al., 2011). In particular, sensory neurons supplying muscle spindle receptors lose their ability to signal stationary muscle position. Given the primacy of proprioceptor signals for informing the central nervous system about limb position (Prochazka and Ellaway, 2012; Proske and Gandevia, 2012), we predicted, and in the present study we demonstrated that movements relying on proprioceptive feedback were impaired in OX-treated rats. This finding motivated us to further explore the cellular mechanisms underlying impaired sensory signaling. Our findings suggested that OX treatment chronically impairs proprioceptors through ion-channel mechanisms, which present possible therapeutic targets for relieving some of the disabling effects of OX chemotherapy.
4.1 Movement Disability with Proprioceptor Impairment
Sensory information provided by muscle proprioceptors is indispensable to the production of normal movements and postures of body and limbs (Prochazka and Ellaway, 2012; Proske and Gandevia, 2012). These receptors are distributed in nearly all mammalian skeletal muscles where they encode dynamic and static parameters of muscle length and force. Muscle proprioceptors are the major source of sensory feedback used to control movements that are unassisted by visual input or vestibular sense, e.g. reaching for an object outside the visual field. Sensory feedback from muscle proprioceptors is critical not only for making rapid adjustments to unexpected movement conditions (Nichols et al., 1999; Shemmell et al., 2010), but also for maintaining body schema, i.e. a sensorimotor representation of the spatial relations between body parts that is used in guiding voluntary movement (Ivanenko et al., 2011). It follows then that any conditions which interrupt sensory detection, encoding or signal transmission to the central nervous system have the potential to result in postural instability, clumsiness, and ataxia as has been found under a variety of conditions in rodents, cats, and humans (Krinke et al., 1985; Abelew et al., 2000; Sghirlanzoni et al., 2005).
In rats receiving chronic OX treatment, we observed abnormal motor behavior characterized as difficulty with achieving proper limb placement. The following observations suggest a link to altered proprioceptor signaling. First, the behavioral task that OX-treated rats struggled with was one that accentuated reliance on proprioceptor signaling. Specifically, rats walked on ladder rungs, a task that required them to position hind feet on unevenly spaced rungs that passed outside their visual field as they stepped forward; rung positions were randomly changed between sessions to prevent advanced knowledge of their position (Metz and Whishaw, 2002, 2009). This task exposed substantial errors in placing the foot securely and correctly beginning within the first two weeks of OX treatment and extending until the last measurements taken at three weeks after OX treatment. Disability was not attributable to muscle weakness, for which we found no evidence in the rats’ general behavior, e.g. climbing onto objects, or recovering from missteps or falls on the ladder rung apparatus. Second, the same OX rats that performed poorly in the proprioceptive movement task also exhibited impaired sensory signaling in terminal electrophysiological study of muscle proprioceptors. In fact, we found a significant correlation in the magnitude of abnormalities measured in animal and proprioceptor behavior. Third, difficulties with limb placement tested in the ladder rung test are also found in mice in which muscle spindles are selectively eliminated by experimental genetic mutation (Akay et al., 2014). These observations support a possible causal relationship between movement disorders and modified proprioceptor firing observed here for OX treated rats.
4.2 Assessing Patient Relevance
With OX chemotherapy, patients report difficulties often characterized as proprioceptive disability, including instability when walking and clumsiness with manipulating objects (Bennett et al., 2012). There are good reasons to propose that these movement deficits originate from impaired signaling by muscle proprioceptors. As muscle proprioceptors are the sole source for detecting and signaling of muscle position and force in rats and all other mammalian species studied thus far (Matthews, 1972; Jami, 1992), so too are they the origin of this information in humans. Muscle spindles and tendon organs in humans resemble those in rats and other animals in their structure and neural innervation (Swash and Fox, 1972; Nitatori, 1988; Eriksson et al., 1994; Liu et al., 2003) and in their encoding of dynamic and static parameters of muscle conditions, including their sustained (slowly adapting) firing during fixed muscle length or force (Edin and Vallbo, 1990b, a; Macefield, 2005). Experimental manipulations of proprioceptor signaling in humans, e.g. temporarily silencing muscle proprioceptors by nerve compression (Walsh et al., 2010; Inui et al., 2011) demonstrate their necessity for normal movement. Additionally, destruction of muscle proprioceptors in human victims of large fiber neuropathy leads to clumsiness, ataxia and inability to properly position limbs outside visual field (Rothwell et al., 1982). These observations establish strong parallels between humans and another animal species with respect to proprioceptor signaling and its requirement in producing normal movement, and they support our assertion that the effects of OX on proprioceptive signaling and behavior that we observe in rats are likely to have relevance for patients.
4.3 Role of Neuropathy
Signaling by primary sensory afferents might be obstructed through any one of several processes and locations, ranging from sensory detection or encoding at afferent endings to conduction and transmission by afferent axons and central synapses. The best documented cause of chronic sensory impediment by OX chemotherapy is neuropathy, occurring as a length-dependent dying back from the distal ends of the longest sensory axons supplying distal limbs, e.g. feet and hands (Avan et al., 2015). The possibility that neuropathy contributed to present results cannot be definitively discounted. Although we found no evidence of neuropathy in SNAPs measured from the long sensory axons in the rat tail, we did not directly test for histological signs of degeneration in sensory nerve terminals. While acknowledging uncertainty, the following several observations suggested to us that neuropathy was probably not a major contributor to our results. First, none of the proprioceptors sampled from OX rats generated action potentials in response to electrical stimulation of nerve (1 cm proximal to muscle entry) without also firing in response to muscle stretch, thereby giving no evidence that sensory terminals had degenerated in advance of parent axons. Second, direct histological examination in our earlier study showed that the sensory innervation of muscle spindles was not retracted and was grossly normal in appearance (Bullinger et al. 2011). It is important to note, however, that the cumulative dose of OX was raised from 40mg in that previous study to 70mg in the present study, in order to better match the dosage in humans. A cumulative dose of 70mg/kg in rats is equivalent to approximately 415mg/m2 in humans (Freireich et al., 1966; Reagan-Shaw et al., 2008), a dose that is at just threshold for the earliest detection of neuropathy measured from changes in SNAP amplitude (Grothey, 2003) and well below levels, >700mg/m2, that produce robust neuropathy (Argyriou et al., 2008; Avan et al., 2015). Thus we may not expect the higher dose used in the present study to produce substantially more neuropathy in our OX treated rats, and this supposition is supported by the similarity in OX effects on the mechano-responsiveness of proprioceptors sampled in this and our previous study (cf Table 1 from each paper). Third, the chronic effects of OX were stereotypical in affecting static firing exclusively and did not express widely varying changes in firing behavior that one would expect from individual proprioceptors in different stages of sensory terminal degeneration. None of these observations are easily reconciled as evidence for neuropathy.
While evidence for neuropathy in patients is in many cases incontrovertible and undoubtedly a significant contributor to disability (Argyriou et al., 2014; Avan et al., 2015), additional chronic effects and their importance cannot be dismissed. For example, pain and allodynia observed following OX (Kiernan, 2007; Fehrenbacher, 2015) are suspected to result, not from neuropathy, but instead from sensory axon hyper-excitability (Park et al., 2009). Neither does neuropathy explain disability reported by patients for whom there is no evidence for a decrease either in SNAPs or in the density of epidermal nerve terminals (Burakgazi et al. 2011). When significant neuropathy does occur, it may not act alone in causing disability. Considering the possibility that OX affects proprioceptor signaling in patients, as we show here for rodents, we expect involvement of proprioceptors distributed throughout the body. Proprioceptors reside in trunk and proximal limbs muscles (Banks, 2006), and the sensory feedback they supply supports sundry movements and postures (Proske and Gandevia, 2009; Roden-Reynolds et al., 2015). Normal walking, for example, relies heavily on sensory feedback from proprioceptors in hip muscles (Roden-Reynolds et al., 2015). Impaired signaling from those sites would be readily capable of contributing to imbalance, difficulty walking, and clumsiness variously described by OX patients. These proximal origins of movement disability originating proximally would be masked, however, and mistakenly assigned to proprioceptors in distal extremities denervated by length-dependent neuropathy, because we commonly interact with the environment through hands and feet. Sensation from the feet is critical for weight-bearing activities (Meyer et al., 2004) and from hands for skilled grasping (Witney et al., 2004). All considered, it seems reasonable, and not disproven that factors in addition to distal neuropathy contribute significantly to chronic disability. This notion could be critically assessed by discriminating tests for proprioceptive disorders in the trunk, shoulders and hips.
4.4 A Mechanism for OX Modification of Proprioceptor Encoding
Several mechanisms apart from neuropathy might have resulted in selective modification of static firing by proprioceptors following OX treatment. Reduced static firing might have derived from one or another of the numerous specializations of muscle spindle receptors. For example, OX might have impaired static firing by reducing gamma motor drive to the spindle’s intrafusal muscle fibers (Matthews, 1962; Boyd and Ward, 1975; Banks et al., 1997). This possibility seemed unlikely, however, given our demonstration that complete experimental elimination of gamma motor input was not capable of significantly reducing proprioceptor responsiveness. More generally, we were able to rule out the necessity of specializations unique to muscle spindles by demonstrating that static responsiveness was equally impaired in Ib proprioceptors that provide the sole neural innervation to relatively non-specialized tendon organ receptors (Hunt, 1974; Jami, 1992; Zelena, 1994). The proprioceptors’ parent axons, spanning the distance from muscle to dorsal roots, were fully competent to generate and conduct action potentials from nerve terminals to the spinal cord, even at sustained, high frequency in response to rapid oscillations in muscle length, i.e. vibrations. Finally, the generation of action potentials with high fidelity in response to each length oscillation throughout bouts of muscle vibration demonstrated that the underlying ionic currents generated either in mechano-transduction or in action potential encoding were not chronically disabled by OX (Bewick and Banks, 2015). Elimination of these mechanisms promoted the idea that abbreviated static responsiveness and increased threshold for repetitive firing involved sustained ionic currents and channels normally generated during static and dynamic muscle stretch. The suspect channels are ones that contribute to currents underlying the receptor potential produced through mechano-transduction (Hunt et al., 1978) and/or currents required to sustain repetitive firing of action potentials for encoding steady depolarization.
The ion channels that support sustained repetitive firing during steady depolarization in muscle proprioceptors have not been identified. In numerous other neurons, repetitive firing to sustained and slow depolarization rests on properties of Na channels that inactivate slowly and thus generate persistent inward currents (NaPIC) (Crill, 1996; Lee and Heckman, 1998, 2001; Harvey et al., 2006). One of these channels, NaV1.6 is expressed by large diameter DRG neurons (Black et al., 1996; Rush et al., 2007; Chung et al., 2015), which include muscle proprioceptors as well as primary sensory neurons supplying skin. Expression of NaV1.6 by the cell body is matched by expression in the nerve terminals of Merkel cells (Lesniak et al., 2014), which like proprioceptors are slowly adapting (Iggo and Muir, 1969; Zimmerman et al., 2014). Furthermore, both NaV1.6 and NaPIC respond acutely to OX (Sittl et al., 2012). Collectively, these findings led us to predict that OX might abbreviate static responsiveness and increase the dynamic threshold of for repetitive firing by compromising NaPIC in the nerve terminals of proprioceptors.
We assessed the importance of NaPIC by testing whether a known pharmacologic antagonist would mimic the OX effect on static responsiveness and the dynamic threshold for repetitive firing. We selected riluzole for its action as a blocker of NaPIC (Benoit and Escande, 1991; Urbani and Belluzzi, 2000; Lamanauskas and Nistri, 2008; Bellingham, 2011) in DRG neurons (Xie et al., 2011). Acute i.p. injection of riluzole in normal rats reproduced essentially all of the effects that chronic OX had or did not have on proprioceptor encoding. While having no effect on the normal generation or conduction of action potentials evoked by muscle vibration, riluzole exhibited a dose dependent reduction in static responsiveness. Riluzole also increased repetitive firing threshold during slow ramps as it did in OX treated rats, and at higher doses increased repetitive firing threshold during fast ramps. These findings supported our hypothesis, however, riluzole’s additional drug actions, e.g. activation of K+ current via BK channels (Grunnet et al., 2001; Wang et al., 2008) confound interpretation. Although there is as yet no more specific NaV1.6 antagonist, there is anti-epileptic drug, phenytoin, which shares only riluzole’s block of NaPIC, but none of its other drug actions (Lampl et al., 1998; Zeng et al., 2005). Previous study has established that in cats, phenytoin reduced the static responsiveness of muscle spindles (Anderson and Raines, 1974). Our finding that acute administration of phenytoin to normal rats also abbreviated static responsiveness bolsters the plausibility that NaPIC supports static firing in proprioceptors and that it may be the target of chronic OX.
The route by which chronic OX treatment might compromise NaPIC remains to be determined. Some studies (Sittl et al., 2012) though not all (Wu et al., 2009) demonstrate that OX acutely increases and prolongs NaPIC in DRG neurons. In addition, NaV1.6 provides a route through which mechanical allodynia develops, presumably from increased excitability of large diameter sensory neurons, within 24 hours of intra-plantar injection of OX in mice (Deuis et al., 2014). These acute effects of OX are opposite to the decreased excitability we observe in muscle proprioceptors. We did find, however, that acute administration of high doses of OX produced spontaneous firing in normal rats, and we are currently testing whether this acute increase in excitability might trigger a compensatory and chronic decrease in excitability. If this is the case then blocking acute hyper-excitability is a potential treatment that may prevent chronic effects on proprioception.
5. Conclusion
Our findings demonstrate that oxaliplatin treatment has the potential to cause chronic movement disorders by a novel mechanism. In our rodent model of OX neurotoxicity, movement deficits in tasks relying on proprioception co-existed with impaired sensory encoding that selectively diminished the signal for static or slowly changing muscle position. We suggest that proprioceptor signaling is diminished by a chronic effect of OX that impairs NaPIC. Identification of this independent target of OX neurotoxicity offers a novel target for therapeutic intervention.
In the absence of neuropathy Oxaliplatin causes lasting behavioral deficits in rats
Behavioral deficits are associated with partial deletion of proprioceptor encoding
Blocking NaPIC reproduces Oxaliplatin’s partial deletion of proprioceptor encoding
Chronic defects in patients following Oxaliplatin may result from NaPIC dysfunction
Acknowledgments
This work was supported by National Institute of Neurological Disorders and Stroke (NINDS) Grant P01NS057228. We are grateful to Lori Goss for her technical assistance and to Dario Carrasco for his critical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaronson NK, Mattioli V, Minton O, Weis J, Johansen C, Dalton SO, Verdonck-de Leeuw IM, Stein KD, Alfano CM, Mehnert A, de Boer A, van de Poll-Franse LV. Beyond treatment - Psychosocial and behavioural issues in cancer survivorship research and practice. EJC supplements : EJC : official journal of EORTC, European Organization for Research and Treatment of Cancer [et al] 2014;12:54–64. doi: 10.1016/j.ejcsup.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abelew TA, Miller MD, Cope TC, Nichols TR. Local loss of proprioception results in disruption of interjoint coordination during locomotion in the cat. Journal of neurophysiology. 2000;84:2709–2714. doi: 10.1152/jn.2000.84.5.2709. [DOI] [PubMed] [Google Scholar]
- Adelsberger H, Quasthoff S, Grosskreutz J, Lepier A, Eckel F, Lersch C. The chemotherapeutic oxaliplatin alters voltage-gated Na(+) channel kinetics on rat sensory neurons. European journal of pharmacology. 2000;406:25–32. doi: 10.1016/s0014-2999(00)00667-1. [DOI] [PubMed] [Google Scholar]
- Akay T, Tourtellotte WG, Arber S, Jessell TM. Degradation of mouse locomotor pattern in the absence of proprioceptive sensory feedback. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16877–16882. doi: 10.1073/pnas.1419045111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RJ, Raines A. Suppression by diphenylhydantoin of afferent discharges arising in muscle spindles of the triceps surae of the cat. The Journal of pharmacology and experimental therapeutics. 1974;191:290–299. [PubMed] [Google Scholar]
- Andre T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- Antonow-Schlorke I, Ehrhardt J, Knieling M. Modification of the ladder rung walking task-new options for analysis of skilled movements. Stroke research and treatment. 2013;2013:418627. doi: 10.1155/2013/418627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyriou AA, Bruna J, Marmiroli P, Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): an update. Critical reviews in oncology/hematology. 2012;82:51–77. doi: 10.1016/j.critrevonc.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Argyriou AA, Kyritsis AP, Makatsoris T, Kalofonos HP. Chemotherapy-induced peripheral neuropathy in adults: a comprehensive update of the literature. Cancer management and research. 2014;6:135–147. doi: 10.2147/CMAR.S44261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyriou AA, Polychronopoulos P, Iconomou G, Chroni E, Kalofonos HP. A review on oxaliplatin-induced peripheral nerve damage. Cancer treatment reviews. 2008;34:368–377. doi: 10.1016/j.ctrv.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Avan A, Postma TJ, Ceresa C, Avan A, Cavaletti G, Giovannetti E, Peters GJ. Platinum-induced neurotoxicity and preventive strategies: past, present, and future. The oncologist. 2015;20:411–432. doi: 10.1634/theoncologist.2014-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks RW. An allometric analysis of the number of muscle spindles in mammalian skeletal muscles. Journal of anatomy. 2006;208:753–768. doi: 10.1111/j.1469-7580.2006.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks RW, Hulliger M, Scheepstra KA, Otten E. Pacemaker activity in a sensory ending with multiple encoding sites: the cat muscle spindle primary ending. The Journal of physiology. 1997;498(Pt 1):177–199. doi: 10.1113/jphysiol.1997.sp021850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS neuroscience & therapeutics. 2011;17:4–31. doi: 10.1111/j.1755-5949.2009.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC. Pre- and postsynaptic mechanisms underlying inhibition of hypoglossal motor neuron excitability by riluzole. Journal of neurophysiology. 2013;110:1047–1061. doi: 10.1152/jn.00587.2012. [DOI] [PubMed] [Google Scholar]
- Bennett BK, Park SB, Lin CS, Friedlander ML, Kiernan MC, Goldstein D. Impact of oxaliplatin-induced neuropathy: a patient perspective. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2012;20:2959–2967. doi: 10.1007/s00520-012-1428-5. [DOI] [PubMed] [Google Scholar]
- Benoit E, Escande D. Riluzole specifically blocks inactivated Na channels in myelinated nerve fibre. Pflugers Archiv : European journal of physiology. 1991;419:603–609. doi: 10.1007/BF00370302. [DOI] [PubMed] [Google Scholar]
- Benoit E, Brienza S, Dubois JM. Oxaliplatin, an anticancer agent that affects both Na+ and K+ channels in frog peripheral myelinated axons. General physiology and biophysics. 2006;25:263–276. [PubMed] [Google Scholar]
- Bewick GS, Banks RW. Mechanotransduction in the muscle spindle. Pflugers Archiv : European journal of physiology. 2015;467:175–190. doi: 10.1007/s00424-014-1536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Dib-Hajj S, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel alpha-subunit mRNAs. Brain research Molecular brain research. 1996;43:117–131. doi: 10.1016/s0169-328x(96)00163-5. [DOI] [PubMed] [Google Scholar]
- Boyd IA, Ward J. Motor control of nuclear bag and nuclear chain intrafusal fibres in isolated living muscle spindles from the cat. The Journal of physiology. 1975;244:83–112. doi: 10.1113/jphysiol.1975.sp010785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briani C, Argyriou AA, Izquierdo C, Velasco R, Campagnolo M, Alberti P, Frigeni B, Cacciavillani M, Bergamo F, Cortinovis D, Cazzaniga M, Bruna J, Cavaletti G, Kalofonos HP. Long-term course of oxaliplatin-induced polyneuropathy: a prospective 2-year follow-up study. Journal of the peripheral nervous system : JPNS. 2014;19:299–306. doi: 10.1111/jns.12097. [DOI] [PubMed] [Google Scholar]
- Bullinger KL, Nardelli P, Wang Q, Rich MM, Cope TC. Oxaliplatin neurotoxicity of sensory transduction in rat proprioceptors. Journal of neurophysiology. 2011;106:704–709. doi: 10.1152/jn.00083.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burakgazi AZ, Messersmith W, Vaidya D, Hauer P, Hoke A, Polydefkis M. Longitudinal assessment of oxaliplatin-induced neuropathy. Neurology. 2011;77:980–986. doi: 10.1212/WNL.0b013e31822cfc59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaletti G. Chemotherapy-induced peripheral neurotoxicity (CIPN): what we need and what we know. Journal of the Peripheral Nervous System. 2014;19:66–76. doi: 10.1111/jns5.12073. [DOI] [PubMed] [Google Scholar]
- Chung G, Saito M, Kawasaki Y, Kawano T, Yin D, Lee S, Kogo M, Takada M, Bae YC, Kim JS, Oh SB, Kang Y. Generation of resonance-dependent oscillation by mGluR-I activation switches single spiking to bursting in mesencephalic trigeminal sensory neurons. The European journal of neuroscience. 2015;41:998–1012. doi: 10.1111/ejn.12858. [DOI] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annual review of physiology. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- Crowe A, Matthews PB. The Effects of Stimulation of Static and Dynamic Fusimotor Fibres on the Response to Stretching of the Primary Endings of Muscle Spindles. The Journal of physiology. 1964;174:109–131. doi: 10.1113/jphysiol.1964.sp007476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuis JR, Lim YL, Rodrigues de Sousa S, Lewis RJ, Alewood PF, Cabot PJ, Vetter I. Analgesic effects of clinically used compounds in novel mouse models of polyneuropathy induced by oxaliplatin and cisplatin. Neuro-oncology. 2014;16:1324–1332. doi: 10.1093/neuonc/nou048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–S241. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- Edin BB, Vallbo AB. Dynamic response of human muscle spindle afferents to stretch. Journal of neurophysiology. 1990a;63:1297–1306. doi: 10.1152/jn.1990.63.6.1297. [DOI] [PubMed] [Google Scholar]
- Edin BB, Vallbo AB. Muscle afferent responses to isometric contractions and relaxations in humans. Journal of neurophysiology. 1990b;63:1307–1313. doi: 10.1152/jn.1990.63.6.1307. [DOI] [PubMed] [Google Scholar]
- Eriksson PO, Butler-Browne GS, Thornell LE. Immunohistochemical characterization of human masseter muscle spindles. Muscle & nerve. 1994;17:31–41. doi: 10.1002/mus.880170105. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher JC. Chemotherapy-induced peripheral neuropathy. Progress in molecular biology and translational science. 2015;131:471–508. doi: 10.1016/bs.pmbts.2014.12.002. [DOI] [PubMed] [Google Scholar]
- Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer chemotherapy reports Part 1. 1966;50:219–244. [PubMed] [Google Scholar]
- Grolleau F, Gamelin L, Boisdron-Celle M, Lapied B, Pelhate M, Gamelin E. A possible explanation for a neurotoxic effect of the anticancer agent oxaliplatin on neuronal voltage-gated sodium channels. Journal of neurophysiology. 2001;85:2293–2297. doi: 10.1152/jn.2001.85.5.2293. [DOI] [PubMed] [Google Scholar]
- Grothey A. Oxaliplatin-safety profile: neurotoxicity. Seminars in oncology. 2003;30:5–13. doi: 10.1016/s0093-7754(03)00399-3. [DOI] [PubMed] [Google Scholar]
- Grunnet M, Jespersen T, Angelo K, Frokjaer-Jensen C, Klaerke DA, Olesen SP, Jensen BS. Pharmacological modulation of SK3 channels. Neuropharmacology. 2001;40:879–887. doi: 10.1016/s0028-3908(01)00028-4. [DOI] [PubMed] [Google Scholar]
- Haftel VK, Bichler EK, Nichols TR, Pinter MJ, Cope TC. Movement reduces the dynamic response of muscle spindle afferents and motoneuron synaptic potentials in rat. Journal of neurophysiology. 2004;91:2164–2171. doi: 10.1152/jn.01147.2003. [DOI] [PubMed] [Google Scholar]
- Harvey PJ, Li Y, Li X, Bennett DJ. Persistent sodium currents and repetitive firing in motoneurons of the sacrocaudal spinal cord of adult rats. Journal of neurophysiology. 2006;96:1141–1157. doi: 10.1152/jn.00335.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CS, Song JH, Nagata K, Yeh JZ, Narahashi T. Effects of the neuroprotective agent riluzole on the high voltage-activated calcium channels of rat dorsal root ganglion neurons. The Journal of pharmacology and experimental therapeutics. 1997;282:1280–1290. [PubMed] [Google Scholar]
- Hunt CC, Wilkinson RS, Fukami Y. Ionic basis of the receptor potential in primary endings of mammalian muscle spindles. The Journal of general physiology. 1978;71:683–698. doi: 10.1085/jgp.71.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt CC, McIntyre . Muscle Recpetors. New York: Springer-Verlag; 1974. [Google Scholar]
- Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. The Journal of physiology. 1969;200:763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inui N, Walsh LD, Taylor JL, Gandevia SC. Dynamic changes in the perceived posture of the hand during ischaemic anaesthesia of the arm. The Journal of physiology. 2011;589:5775–5784. doi: 10.1113/jphysiol.2011.219949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanenko YP, Dominici N, Daprati E, Nico D, Cappellini G, Lacquaniti F. Locomotor body scheme. Human movement science. 2011;30:341–351. doi: 10.1016/j.humov.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Jami L. Golgi tendon organs in mammalian skeletal muscle: functional properties and central actions. Physiological reviews. 1992;72:623–666. doi: 10.1152/physrev.1992.72.3.623. [DOI] [PubMed] [Google Scholar]
- Kiernan MC. The pain with platinum: oxaliplatin and neuropathy. European journal of cancer. 2007;43:2631–2633. doi: 10.1016/j.ejca.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Kneis S, Wehrle A, Freyler K, Lehmann K, Rudolphi B, Hildenbrand B, Bartsch HH, Bertz H, Gollhofer A, Ritzmann R. Balance impairments and neuromuscular changes in breast cancer patients with chemotherapy-induced peripheral neuropathy. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2015 doi: 10.1016/j.clinph.2015.07.022. [DOI] [PubMed] [Google Scholar]
- Krinke G, Naylor DC, Skorpil V. Pyridoxine megavitaminosis: an analysis of the early changes induced with massive doses of vitamin B6 in rat primary sensory neurons. Journal of neuropathology and experimental neurology. 1985;44:117–129. [PubMed] [Google Scholar]
- Krishnan AV, Goldstein D, Friedlander M, Kiernan MC. Oxaliplatin-induced neurotoxicity and the development of neuropathy. Muscle & nerve. 2005;32:51–60. doi: 10.1002/mus.20340. [DOI] [PubMed] [Google Scholar]
- Krishnan AV, Goldstein D, Friedlander M, Kiernan MC. Oxaliplatin and axonal Na+ channel function in vivo. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:4481–4484. doi: 10.1158/1078-0432.CCR-06-0694. [DOI] [PubMed] [Google Scholar]
- Lamanauskas N, Nistri A. Riluzole blocks persistent Na+ and Ca2+ currents and modulates release of glutamate via presynaptic NMDA receptors on neonatal rat hypoglossal motoneurons in vitro. The European journal of neuroscience. 2008;27:2501–2514. doi: 10.1111/j.1460-9568.2008.06211.x. [DOI] [PubMed] [Google Scholar]
- Lampl I, Schwindt P, Crill W. Reduction of cortical pyramidal neuron excitability by the action of phenytoin on persistent Na+ current. The Journal of pharmacology and experimental therapeutics. 1998;284:228–237. [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Bistability in spinal motoneurons in vivo: systematic variations in persistent inward currents. Journal of neurophysiology. 1998;80:583–593. doi: 10.1152/jn.1998.80.2.583. [DOI] [PubMed] [Google Scholar]
- Lee RH, Heckman CJ. Essential role of a fast persistent inward current in action potential initiation and control of rhythmic firing. Journal of neurophysiology. 2001;85:472–475. doi: 10.1152/jn.2001.85.1.472. [DOI] [PubMed] [Google Scholar]
- Lesniak DR, Marshall KL, Wellnitz SA, Jenkins BA, Baba Y, Rasband MN, Gerling GJ, Lumpkin EA. Computation identifies structural features that govern neuronal firing properties in slowly adapting touch receptors. eLife. 2014;3:e01488. doi: 10.7554/eLife.01488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin GR, McMahon SB. Physiological properties of primary sensory neurons appropriately and inappropriately innervating skin in the adult rat. Journal of neurophysiology. 1991;66:1205–1217. doi: 10.1152/jn.1991.66.4.1205. [DOI] [PubMed] [Google Scholar]
- Liu JX, Thornell LE, Pedrosa-Domellof F. Muscle spindles in the deep muscles of the human neck: a morphological and immunocytochemical study. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2003;51:175–186. doi: 10.1177/002215540305100206. [DOI] [PubMed] [Google Scholar]
- Macefield VG. Physiological characteristics of low-threshold mechanoreceptors in joints, muscle and skin in human subjects. Clinical and experimental pharmacology & physiology. 2005;32:135–144. doi: 10.1111/j.1440-1681.2005.04143.x. [DOI] [PubMed] [Google Scholar]
- Matthews PB. The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Quarterly journal of experimental physiology and cognate medical sciences. 1962;47:324–333. doi: 10.1113/expphysiol.1962.sp001616. [DOI] [PubMed] [Google Scholar]
- Matthews PBC. Mammalian Muscle Receptors and Their Central Actions. Baltimore: The Williams and Wilkins Company; 1972. pp. 1–59. [Google Scholar]
- Metz GA, Whishaw IQ. Cortical and subcortical lesions impair skilled walking in the ladder rung walking test: a new task to evaluate fore- and hindlimb stepping, placing, and co-ordination. Journal of neuroscience methods. 2002;115:169–179. doi: 10.1016/s0165-0270(02)00012-2. [DOI] [PubMed] [Google Scholar]
- Metz GA, Whishaw IQ. The ladder rung walking task: a scoring system and its practical application. Journal of visualized experiments : JoVE. 2009 doi: 10.3791/1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer PF, Oddsson LI, De Luca CJ. The role of plantar cutaneous sensation in unperturbed stance. Experimental brain research. 2004;156:505–512. doi: 10.1007/s00221-003-1804-y. [DOI] [PubMed] [Google Scholar]
- Nichols TR, Cope TC, Abelew TA. Rapid spinal mechanisms of motor coordination. Exercise and sport sciences reviews. 1999;27:255–284. [PubMed] [Google Scholar]
- Nitatori T. The fine structure of human Golgi tendon organs as studied by three-dimensional reconstruction. Journal of neurocytology. 1988;17:27–41. doi: 10.1007/BF01735375. [DOI] [PubMed] [Google Scholar]
- Novak KR, Nardelli P, Cope TC, Filatov G, Glass JD, Khan J, Rich MM. Inactivation of sodium channels underlies reversible neuropathy during critical illness in rats. The Journal of clinical investigation. 2009;119:1150–1158. doi: 10.1172/JCI36570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC. Oxaliplatin-induced neurotoxicity: changes in axonal excitability precede development of neuropathy. Brain : a journal of neurology. 2009;132:2712–2723. doi: 10.1093/brain/awp219. [DOI] [PubMed] [Google Scholar]
- Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC. Dose effects of oxaliplatin on persistent and transient Na+ conductances and the development of neurotoxicity. PloS one. 2011a;6:e18469. doi: 10.1371/journal.pone.0018469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SB, Lin CS, Krishnan AV, Goldstein D, Friedlander ML, Kiernan MC. Long-term neuropathy after oxaliplatin treatment: challenging the dictum of reversibility. The oncologist. 2011b;16:708–716. doi: 10.1634/theoncologist.2010-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SB, Goldstein D, Krishnan AV, Lin CS, Friedlander ML, Cassidy J, Koltzenburg M, Kiernan MC. Chemotherapy-induced peripheral neurotoxicity: a critical analysis. CA: a cancer journal for clinicians. 2013;63:419–437. doi: 10.3322/caac.21204. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Ellaway P. Sensory systems in the control of movement. Comprehensive Physiology. 2012;2:2615–2627. doi: 10.1002/cphy.c100086. [DOI] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The kinaesthetic senses. The Journal of physiology. 2009;587:4139–4146. doi: 10.1113/jphysiol.2009.175372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: their roles in signaling body shape, bodyposition and movement, and muscle force. Physiological reviews. 2012;92:1651–1697. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Roden-Reynolds DC, Walker MH, Wasserman CR, Dean JC. Hip proprioceptive feedback influences the control of mediolateral stability during human walking. Journal of neurophysiology. 2015;114:2220–2229. doi: 10.1152/jn.00551.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Day BL, Obeso JA, Thomas PK, Marsden CD. Manual motor performance in a deafferented man. Brain : a journal of neurology. 1982;105(Pt 3):515–542. doi: 10.1093/brain/105.3.515. [DOI] [PubMed] [Google Scholar]
- Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their roles in electrogenesis within dorsal root ganglion neurons. The Journal of physiology. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sghirlanzoni A, Pareyson D, Lauria G. Sensory neuron diseases. The Lancet Neurology. 2005;4:349–361. doi: 10.1016/S1474-4422(05)70096-X. [DOI] [PubMed] [Google Scholar]
- Shemmell J, Krutky MA, Perreault EJ. Stretch sensitive reflexes as an adaptive mechanism for maintaining limb stability. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2010;121:1680–1689. doi: 10.1016/j.clinph.2010.02.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sittl R, Lampert A, Huth T, Schuy ET, Link AS, Fleckenstein J, Alzheimer C, Grafe P, Carr RW. Anticancer drug oxaliplatin induces acute cooling-aggravated neuropathy via sodium channel subtype Na(V)1.6-resurgent and persistent current. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6704–6709. doi: 10.1073/pnas.1118058109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani A, Spadoni F, Bernardi G. Differential inhibition by riluzole, lamotrigine, and phenytoin of sodium and calcium currents in cortical neurons: implications for neuroprotective strategies. Experimental neurology. 1997;147:115–122. doi: 10.1006/exnr.1997.6554. [DOI] [PubMed] [Google Scholar]
- Swash M, Fox KP. Muscle spindle innervation in man. Journal of anatomy. 1972;112:61–80. [PMC free article] [PubMed] [Google Scholar]
- Theiss RD, Kuo JJ, Heckman CJ. Persistent inward currents in rat ventral horn neurones. The Journal of physiology. 2007;580:507–522. doi: 10.1113/jphysiol.2006.124123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofthagen C, Overcash J, Kip K. Falls in persons with chemotherapy-induced peripheral neuropathy. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2012;20:583–589. doi: 10.1007/s00520-011-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbani A, Belluzzi O. Riluzole inhibits the persistent sodium current in mammalian CNS neurons. The European journal of neuroscience. 2000;12:3567–3574. doi: 10.1046/j.1460-9568.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- Walsh LD, Gandevia SC, Taylor JL. Illusory movements of a phantom hand grade with the duration and magnitude of motor commands. The Journal of physiology. 2010;588:1269–1280. doi: 10.1113/jphysiol.2009.183038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Lin MW, Lin AA, Wu SN. Riluzole-induced block of voltage-gated Na+ current and activation of BKCa channels in cultured differentiated human skeletal muscle cells. Life sciences. 2008;82:11–20. doi: 10.1016/j.lfs.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Witney AG, Wing A, Thonnard JL, Smith AM. The cutaneous contribution to adaptive precision grip. Trends in neurosciences. 2004;27:637–643. doi: 10.1016/j.tins.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Wu SN, Chen BS, Wu YH, Peng H, Chen LT. The mechanism of the actions of oxaliplatin on ion currents and action potentials in differentiated NG108-15 neuronal cells. Neurotoxicology. 2009;30:677–685. doi: 10.1016/j.neuro.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Xie RG, Zheng DW, Xing JL, Zhang XJ, Song Y, Xie YB, Kuang F, Dong H, You SW, Xu H, Hu SJ. Blockade of persistent sodium currents contributes to the riluzole-induced inhibition of spontaneous activity and oscillations in injured DRG neurons. PloS one. 2011;6:e18681. doi: 10.1371/journal.pone.0018681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelena J. Nerves and Mechanoreceptors. London: Chapman and Hall; 1994. [Google Scholar]
- Zeng J, Powers RK, Newkirk G, Yonkers M, Binder MD. Contribution of persistent sodium currents to spike-frequency adaptation in rat hypoglossal motoneurons. Journal of neurophysiology. 2005;93:1035–1041. doi: 10.1152/jn.00831.2004. [DOI] [PubMed] [Google Scholar]
- Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science. 2014;346:950–954. doi: 10.1126/science.1254229. [DOI] [PMC free article] [PubMed] [Google Scholar]