Abstract
The concept of the brain as an immune privileged organ is rapidly evolving in light of new findings outlining the sophisticated relationship between the central nervous and the immune systems. The role of T cells in brain development and function, as well as modulation of behavior has been demonstrated by an increasing number of studies. Moreover, recent studies have redefined the existence of a brain lymphatic system and the presence of T cells in specific brain structures, such as the meninges and choroid plexus. Nevertheless, much information is needed to further the understanding of brain T cells and their relationship with the central nervous system under non-inflammatory conditions. In the present study we employed the Rag2−/− mouse model of lymphocyte deficiency and reconstitution by adoptive transfer to study the temporal and anatomical expansion of T cells in the brain under homeostatic conditions. Lymphopenic Rag2−/− mice were reconstituted with 10 million lymphoid cells and studied at one, two and four weeks after transfer. Moreover, lymphoid cells and purified CD4+ and CD8+ T cells from transgenic GFP expressing mice were used to define the neuroanatomical localization of transferred cells. T cell numbers were very low in the brain of reconstituted mice up to one week after transfer and significantly increased by 2 weeks, reaching wild type values at 4 weeks after transfer. CD4+ T cells were the most abundant lymphocyte subtype found in the brain followed by CD8+ T cells and lastly B cells. Furthermore, proliferation studies showed that CD4+ T cells expand more rapidly than CD8+ T cells. Lymphoid cells localize abundantly in meningeal structures, choroid plexus, and circumventricular organs. Lymphocytes were also found in vascular and perivascular spaces and in the brain parenchyma across several regions of the brain, in particular in structures rich in white matter content. These results provide proof of concept that the brain meningeal system, as well as vascular and perivascular spaces, are homing sites of lymphocytes and suggest the possibility of a brain specific T cell subtype.
Keywords: T cells, Brain, Endogenous Proliferation, Homeostatic Proliferation, Neurovascular, Meninges
Introduction
An increasing number of studies indicate that T cells may perform diverse functions in the brain in addition to their classical role of immune surveillance and pathogen clearance. T cells have been implicated in complex brain processes including memory (Brynskikh et al., 2008; Derecki et al., 2010; Kipnis et al., 2004; Kipnis et al., 2012; Ziv et al., 2006), emotional behavior (Brachman et al., 2015; Kim et al., 2012; Lewitus et al., 2008), stress responsiveness (Clark et al., 2014c), as well as neurogenesis (Wolf et al., 2009; Ziv et al., 2006) and brain development and brain sexual differentiation (Rilett et al., 2015). The existence of a brain lymphatic system, recently re-defined by two independent studies (Aspelund et al., 2015; Louveau et al., 2015), provides the anatomical basis to support the concept that cellular interaction of T cells with CNS elements are likely part of natural physiological processes of the brain. This in turn provides additional support for a role of lymphocytes and T cells in neurobehavioral function. All these studies show that CD3+ T cells are present in specific structures of the brain constituting a brain lymphatic system anatomically related to the meninges and the choroid plexus, where they may influence brain homeostasis through the production of cytokines such as interleukin-4 (IL-4) (Derecki et al., 2010; Kipnis et al., 2012; Radjavi et al., 2014).
Much information on T cell function in the brain has been gained by comparing immunocompetent wild type rodents with lymphocyte deficient animals. The effects on neurobehavioral functions of several lymphocyte subsets has been studied by adoptive transfer of functional cells into lymphocyte deficient animals, a process referred to as reconstitution by adoptive transfer (Riddell et al., 1994). The beneficial effects of lymphocytes and CD4+ T cells on hippocampal dependent memory processes (Radjavi et al., 2014), neurogenesis (Wolf et al., 2009; Ziv et al., 2006) and emotional behavior (Brachman et al., 2015; Rattazzi et al., 2013) has been reported by several of these types of studies. Moreover, detrimental effects on emotionality have also been shown for inflammatory Th17 cells (Beurel et al., 2013) while some discrepancies exist about the specific roles of regulatory T cells in behavior (Cohen et al., 2006; Kim et al., 2012). These studies have employed different models of lymphocyte deficiency, some of which are the result of deleting genes that are expressed in the CNS. For example, the Rag1 gene, which is also expressed in the CNS, has been proposed to modulate the expression of behavior (McGowan et al., 2011; Rattazzi et al., 2013).
Lymphocyte deficient Rag2−/− mice are widely employed to study T cell differentiation and function (Dasgupta et al., 2011; Dorsey et al., 2013; Spanopoulou, 1996). Functional T and B cell deficiency is produced by deletion of the recombination activation gene 2 (RAG2) necessary for the V[D]J re-arrangement process of the T and B cell receptor (Shinkai et al., 1992). There is increasing interest in the use of this model to study the role of T cells on brain function and behavior (Brachman et al., 2015; Clark et al., 2014a; Clark et al., 2014c; McGowan et al., 2011; Rattazzi et al., 2013) due to the restricted expression of the Rag2−/− gene in peripheral immune cells (Chun et al., 1991; Clark et al., 2014b). These mice are good acceptors of functional lymphocytes. T cells in particular were shown to proliferate and expand in peripheral tissues and organs (Min et al., 2004). This process, initially called homeostatic expansion in a lymphopenic setting (Goldrath et al., 2000; Murali-Krishna and Ahmed, 2000), has been shown to involve two distinct proliferative responses of T cells. A rapid proliferative response that is independent of interleukin-7 (IL-7) and a slower response dependent on IL-7 (Min et al., 2004; Min and Paul, 2005; Min et al., 2005; Troy and Shen, 2003). The first response has been referred to as endogenous proliferation and the second as homeostatic proliferation (Min and Paul, 2005). To our knowledge, there is no information on the profiles of brain T cell expansion and anatomical localization in the model of adoptive transfer in immune deficient mice. Thus, the objective of the present studies was to provide a temporal and anatomical characterization of lymphocytes, and in particular CD4+ and CD8+ T cells, in the brain during endogenous and homeostatic expansion in lymphopenic Rag2−/− mice. The results of the present studies provide proof of concept that T cells home and expand into the brain under homeostatic conditions and localize mostly in the brain lymphatic system. They also reveal a significant degree of interaction with vascular and perivascular cells across the entire brain during this process.
Materials and Methods
1. Animals and tissue processing
Six to eight week old C57Bl/6 wild type mice were obtained from Taconic Farms (Germantown, NY) and used as donors of lymphocytes (n = 22 females) or for control reference group (n = 8, males and 8 females) in flow cytometry experiments. Six week old transgenic C57BL/6-Tg(CAG-EGFP)10sb/J male mice (n = 22) were obtained from Jackson laboratories (Farmington, CT. Stock #003291) and used as donors of lymphocytes for fluorescent microscopy analyses. Six to eight week old Rag2−/− mice (n = 22 females and 22 males) were obtained from Taconic Farms (Germantown, NY) and used as recipients of lymphocytes or purified T cells. After arrival, all mice were housed in microisolator cages under strict sanitary conditions and allowed to acclimate for one week before any procedures. Mice were euthanized by CO2 inhalation followed by cervical dislocation (donors) or were anesthetized with isoflurane followed by perfusion with 20 ml PBS (Fisher Scientific, Waltham MA) and 0.01% heparin to remove blood from the brain. Brains were immediately dissected by splitting the skull along the sagittal suture and across the interparietal bone. As the brain was removed the dura mater, whose major connections to the skull were severed, was also collected. The underside of the skullcap and base of the skull were inspected to insure that the meninges were fully removed. The tissue was directly placed in DMEM (Corning Cellgro, Tewksbury, MA) supplemented with 5% foetal bovine serum (FBS) (Gemini Bioproducts, Sacramento CA) and freshly processed for mononuclear cell (MNC) preparation. Spleens and lymph nodes were collected during the dissection procedure and also processed for lymphocyte preparation. Groups of mice reconstituted with lymphocytes from transgenic mice were additionally perfused with 30 ml ice cold 4% paraformaldehyde in phosphate buffered saline. The brains were extracted with dissections intended to preserve as much of the meninges attached to the brain. For confocal microscopy, the brains were post-fixed 24 h in 4% PFA at 4° C and transferred to a 30% sucrose solution until they sunk and then processed for sectioning. For two-photon microscopy the brains were post fixed one to two days in 4% PFA at 4° C and then transferred to PBS for one day. They were then lightly embedded in 10% porcine gelatin in PBS, chilled and then fixed in 4% PFA for an additional day and finally transferred to PBS. All procedures were carried out under approved IACUC protocols and institutional guidelines at the University of Maryland, School of Medicine.
2. Preparation of peripheral lymphoid and T cells for adoptive transfer
All experiments involving adoptive transfer of lymphoid cells were carried out on a 1:1 donor to recipient basis according to sex. Experiments of adoptive transfer of purified CD4+ and CD8+ T cells were carried out on a 2:1 donor to recipient basis. Lymph nodes (LNs: cervical, brachial, inguinal and mesenteric) were processed from each mouse in 5 ml DMEM and centrifuged at 400 g (1500 rpm) for 5 min. Red blood cells were removed by using ACK lysis buffer (Quality Biological INC, Gaithersburg, Maryland). The pellet was resuspended with 5 ml PBS and passed through a 40 μm cell strainer to make a single cell suspension. Dead cells were stained with Trypan Blue (Corning Cellgro) and live cells counted using a hemocytometer. For specific CD4+ and CD8+ reconstitution, T cells were further isolated by negative selection using the EasySep T cell isolation kit specific for CD4+ or CD8+ T cells (Stem Cell Technologies, Vancouver, BC, Canada) following manufacturer’s guidelines for manual separation. Rag2−/− mice were reconstituted with 10 million cells in a total volume of 200 μl via tail vein injections and used for flow cytometry analysis at one, two and four weeks after reconstitution and at two and four weeks after for fluorescent microscopy studies.
3. Preparation of mononuclear cells from whole brains
Whole brains including the meninges were collected and finely sectioned and passed through a cell strainer (70 μm) (Fisher Scientific) to make a single cell suspension. The cells were precipitated by centrifugation and washed twice in 5% FBS in DMEM (Corning Cellgro). After centrifugation, cells were resuspended in 10 ml 30% Percoll (Sigma Aldrich) in PBS and gently layered on top of 70% Percoll in PBS creating a gradient. The Percoll gradient was centrifuged at 500 g for 30 min and the brain mononuclear cells (MNCs) obtained from the layer formed between the 30–70% Percoll layers. The yield and quality of the MNC preparation was determined by staining the cells with 0.4% Trypan Blue in PBS and counting them under a microscope using a hemocytometer. The yield per single brain using this method was 685,000 ± 29,000 cells (SEM).
4. Flow cytometry
Cells from the LNs, Spleen (Spl) and brain MNCs were washed with 200 μl PBS two times in 96 wells plates. The cells were re-suspended in 50 μl of staining buffer (PBS+0.5% FBS) containing 1:100 diluted fluorescence conjugated anti-mouse antibody combination including: anti-CD8-FITC (BD Biosciences, cat #553030), anti-CD8-PerCP-Cy5.5 (BD Biosciences, cat #561109), anti-CD44–APC (BD Biosciences, cat #559250), anti-CD4-APC (BD Biosciences, cat #553051), anti-CD4-PE (BD Biosciences, cat #553730), anti-CD19-PE-Cy7 (BD Biosciences, cat #552854), anti-CD3-eFluor 450 (eBioscience, 48-0032-82), anti-NK1.1-PerCP-Cy5.5 (BD Biosciences, cat #561111), anti-NK1.1-APC(BD Biosciences, cat #550627), Viabilty-7-AAD (BD Biosciences, cat #555816) and anti-CD16/CD32 (Fc blocker, BD Biosciences, cat #553142). Cells were mixed with different combinations of antibody cocktails and incubated at 4 °C in the dark for 30 min. The cells were then washed three times with staining buffer and re-suspended in a final volume of 200 μl of staining buffer. Single-cell suspensions from individual mice were analysed on BD™ LSR II flow cytometer (BD Biosciences, San Jose, California) with a window of 200,000 events in the live lymphocyte gate and data processed using FlowJo version 10 software (Tree Star, Ashland, Oregon) using biexponential scaling generating data per single mouse, which was used for further statistical comparisons.
5. Proliferation Assay
Proliferation was assessed by carboxyfluorescein succinimidyl ester (CFSE) assay. Whole lymphoid cells were made into a single-cell suspension (106/ml) in PBS and incubated with 5 μM CFSE (BioLegend, San Diego CA) in the dark for 10 minutes at 37 °C. Cells were washed with 44 ml of ice cold RPMI-1640 media (10% FBS) and then with 44 ml of fresh RPMI-1640 media (10% FBS). The pellet was re-suspended in PBS and 10 million CFSE labelled lymphoid cells were adoptive transferred to Rag2−/− mice. Spleens, lymph nodes and brains from reconstituted mice were collected at each experimental time point and CFSE dilution of lymphocyte subset analysed by flow cytometry as described above.
6. Confocal microscopy
Brains were serially cryo-sectioned at 60 μm thickness in the coronal plane from the olfactory bulbs to the end of the cerebellum and stored in long-term cryoprotectant (ethylene-glycol & normal sucrose). Every fourth section was directly mounted onto gelatin-coated slides, stained in 1:10000 solution of Draq5 fluorescent nuclear stain (Cell Signaling Technology, Danvers, MA) in PBS, dried and mounted with Fluorosave (EMD Millipore, Darmstadt, Germany). Sections were imaged using a Zeiss LSM 780 (Carl Zeiss Microscopes) with GFP fingerprinting at low resolution and Zeiss LSM 710 at high resolution under the control of Zen imaging software. GFP/Draq5 confocal microscopy was performed using a Zeiss 0.80 NA 20x objective for low resolution images or a 1.1 NA 40x water objective for higher resolution. Larger format images were collected using the tiled z-stack capability of Zen software, while high resolution images were collected as single z-stacks. GFP was excited at 488 nm and observed either beyond 505 nm for when low background was desired or beyond 490 nm when high autofluorescent background was desired to demonstrate the presence of vessels. Draq5 nuclear stain was excited at 633 nm and observed beyond 700 nm. Additional cryo-sections for vascular imaging were stained with DyLight 594-labeled Lycopersicon esculentum (Tomato) Lectin (Vector Laboratories, Burlingame, CA). Sections were washed in 0.2% Triton X-100 (Sigma Aldrich) in PBS for 1 h, PBS for 2 h, stained 2 h in 1:500 lectin in PBS, washed 4 times over 2 h, stained with Hoechst 33342 (Invitrogen, Grand Island NY) 1:10000 in PBS 15 min, and then washed in PBS for 30 min. Sections were mounted in Fluor-Glo media (Valley Scientific, Mayville PA). Hoechst was excited at 950 nm, GFP at 488 nm, and DyLight 594 at 594 nm wavelengths and imaged using a 40x 1.1 NA water objective. Low resolution images showing GFP-labeled cells were processed using Zen software. GFP fingerprinting was used to separate GFP signal from background (pseudo-coloured red). High resolution images were processed to give optimal contrast using Zen, Fiji image processing software (Schindelin et al., 2012), and Adobe Photoshop (Adobe Systems Inc., San Jose CA). Custom colour lookup tables were used for rendering of vascular Dylight 594 to achieve optimal contrast.
7. Two-photon microscopy
Brains were cut into coronal slabs of approximately 1 mm in thickness using a brain matrix (Ted Pella Inc., Redding CA), and the slabs were then soaked in Draq5 fluorescent nuclear stain (1:4000) in PBS overnight, then cleared in a solution of 40% glycerol (Sigma), 20% thiodiethanol (Sigma) and 4x PBS for at least 2 h at room temperature and held in clearing media until mounted. The brain slabs were mounted in chamber slides containing clearing media and then coverslipped. GFP/Draq5 two-photon (2P) microscopy was performed using a Zeiss LSM 710 fitted with a Chameleon pulsed laser controlled by Zen imaging software. GFP was excited in 2P mode at 810 nm and observed from 515–650 nm, while Draq5 was excited in single photon mode at 633 nm and observed at 650–750 nm. Tissue samples viewed in 2P mode were routinely imaged up to 800 μm from the nominal top surface of the tissue. Data was processed using Imaris software (v. 7.1.1, Bitplane, Concord MA). For quantification, hippocampal volume on large tiled z-stacks was manually traced and covered with an isosurface that was then used as a clipping mask to show only the relevant volume. Hippocampal tissue volume was based on the volume of the 3D isosurface. Cells were manually counted, and cell densities calculated by dividing total cells by tissue volume. Individual cell images were manually adjusted to show optimal contrast using Imaris and Adobe Photoshop.
8. Statistical Analysis
A one-way ANOVA with Fisher’s LSD post hoc analysis was used to compare population distributions of T cells within the lymph nodes, spleen and brain. A Student’s t test was used to compare male versus female data in wild type mice. Data are presented as the mean ± standard error of the mean (SEM); a p ≤ 0.05 was considered significant. Statistical analysis was conducted with GraphPad Prism 6 (GraphPad Software, Inc., La Jolla CA).
Results
1. Characterization of lymphocyte subtypes in the brain of C57Bl/6 wild type mice
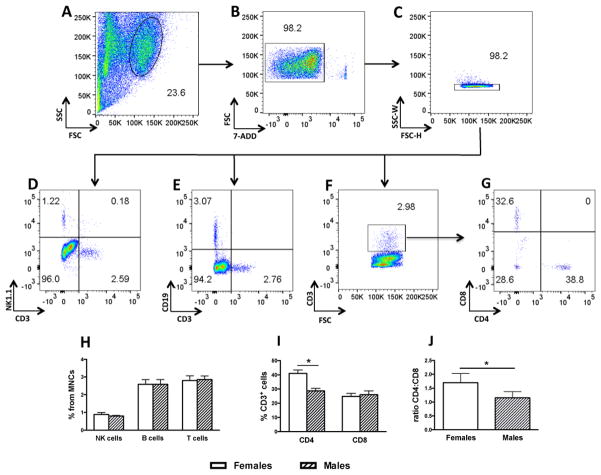
Brain lymphocyte subsets were first characterized in MNC preparations from individual brains of immunocompetent female C57Bl/6 (n = 8) and male (n = 8) WT mice. This established a reference point on the proportion of naturally occurring lymphocyte subsets in the brain and the consistency and sensitivity of the method. Total MNCs were gated on size by scatter (FSC vs SCC) and the lymphocyte gate further evaluated for live cells on the negative 7-AAD population (Fig. 1A, B). Single cells were further defined within the negative 7-AAD population using SSC-W/FSC-H gating (Fig. 1C). Lymphoid cells were plotted for natural killer (NK) cells (NK1.1+CD3−) (Fig. 1D), B cells (CD19+CD3−) (Fig. 1 E), and T cells (CD3+) (Fig. 1F). T cells were further analysed within the CD3+ gate as CD4+ and CD8+ positive cells (Fig. 1G). Percentages for each lymphocyte subtype for both male and female combined were 0.86 ± 0.07 SEM for NK cells, 2.6 ± 0.17 SEM for B cells and 2.82 ± 0.18 SEM for T cells with no sex differences observed (Fig. 1H). The proportion of CD4+ T cells within the CD3+ population was 41.03 ± 2.4 for females and 32.23 ± 2.5 for males [unpaired t-test, (t = 2.34, df = 14; p < 0.05) (Fig. 1I). The proportion of CD8+ T cells within the CD3+ population was 24.86 ± 2 for females and 29.65 ± 3.2 for males (n.s). The ratio of CD4:CD8 T cell for females was 1.7 ± 0.1 and 1.1 ± 0.1 for males (t = 3.2, df = 10; p < 0.01) (Fig. 1J). Rag2−/− mice under basal, non-reconstituted conditions were confirmed devoid of differentiated CD3+/CD4+ and CD3+/CD8+ cell in both the brain and LNs (Suppl. Fig. 1).
Figure 1.
Representative dot plots from a single mouse showing the gating strategy for lymphocyte subtype characterization in MNCs from wild type (WT) mice (n = 8 females and 8 males). A) Lymphocyte gate from brain MNCs. B) Live cells gate (7-ADD-negative). C) Singlet separation by SSC-W/FSC-H. D) NK cells (NK1.1+CD3−). E) B cells (CD19+CD3 −). F) T cells (total CD3+). G) T cell subpopulation in the CD3+ gate for CD4 and CD8. H) Proportion of NK, B and T cells in female and male brain MNCs. I) Proportion of CD3+/CD4+ and CD3+/CD8+ in female and male brain MNCs. J) CD4:CD8 ration in CD3 the fraction of female and male brain MNCs. * p < 0.05.
2. Time course of lymphocyte expansion in the brain of Rag2−/− mice
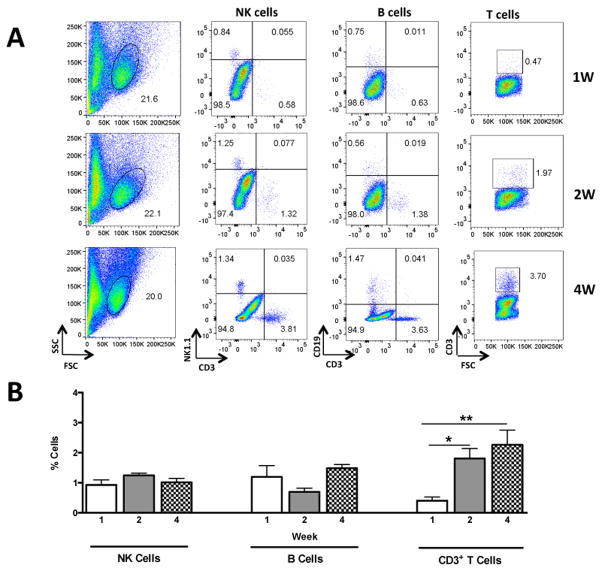
The relative proportion of lymphocyte subtypes and NK cells were compared in MNCs from the brain, LNs and Spl of reconstituted Rag2−/− mice (n = 15 females, 5 per time point). Following the labelling and gating strategy outlined above, NK, B and T cells were analysed at one, two and four weeks after reconstitution. CD3+ T cells were very low in the MNC fraction of the brain at one week after transfer (Fig. 1) while they were abundant in the LN (Suppl. Fig. 2) and Spl (Supp. Fig. 3). A significant increase in CD3+ T cells was found in the brain at two weeks after transfer [F (2, 12) = 9.52; p < 0.005] with maximal numbers at four weeks while they remained constant in the LNs and Spl at these time points (Fig. 2A, B and Suppl. Fig. 2A, B; 3A, B). The proportion of B cells was found to be very low in the brain at one and two weeks after transfer with an increase at four weeks, nevertheless this increase was not significant (Fig. 2A, B). In contrast, the proportion of B cells increased in the LNs [F (2, 12) = 4.81; p < 0.02] and remained unchanged in the Spl (Suppl. Fig 2A, B; and Suppl. Fig. 3A, B). The proportion of NK cells remained unchanged in the brain during the course of lymphocyte expansion while they decreased in peripheral organs with minimal values at four weeks after transfer (Suppl. Fig. 2A, B; and Suppl. Fig. 3A, B). These results indicate that T cells are the most abundant type of lymphocytes in the brain during endogenous and homeostatic expansion in Rag2−/− mice.
Figure 2.
Representative dot plots from a single mouse showing the time course of lymphocyte expansion in the brain of Rag2−/− mice reconstituted with 10 million lymphoid cells (n = 15 females). A) Representative dot plots outlining lymphoid subsets including NK cells (NK1.1+CD3−), B cells (CD19+CD3−) and T cells (total CD3+) at one, two and four weeks after transfer used for quantifications shown in panel B. * p < 0.05; ** p < 0.01.
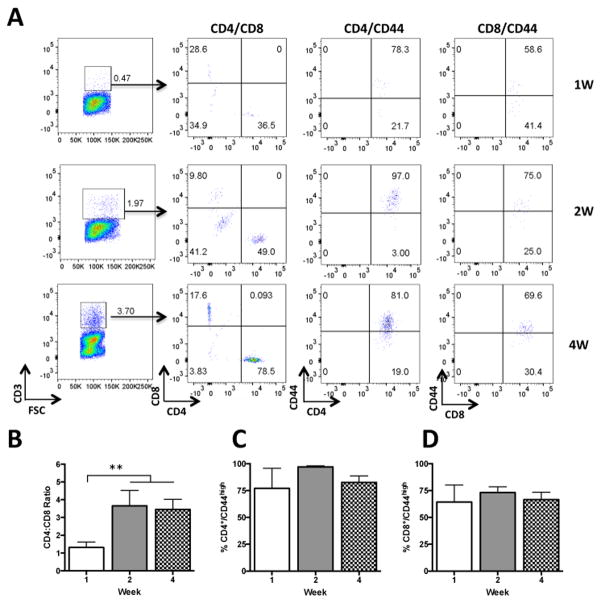
The proportion of CD4+ and CD8+ T cells were further compared within the CD3+ population. The ratio of CD4+ and CD8+ T cells in the brain was about 1:1 at one week and significantly changed at two and four weeks after transfer [F (2, 12) = 4.12; p < 0.05] with CD4+ being on average about 3.5:1 more abundant than CD8+ T cells (Figure 3A, B). The ratio in peripheral organs was about 2:1 from week one and remained constant through the testing period (Suppl. Fig. 4A, B; and Suppl. Fig. 5A, B). T cells were also analysed for the memory marker CD44 known to increase during homeostatic expansion (Murali-Krishna and Ahmed, 2000). The proportion of CD4+/CD44high and CD8+/CD44high remained constant through time points in the brain and Spl while they increased in the LNs (Fig. 3A, C, D and Suppl. Fig. 4A, C, D and Suppl. Fig. 5A, C, D). The majority of cells in the brain were found to be CD44high ranging from 77 to 97 % for CD4+ and 64 to 73 % for CD8+ across different time points (Fig 3 C, D). These results indicate that CD4+/CD44high cells are the most abundant T cell type in the brain during endogenous and homeostatic expansion and that both CD4+ and CD8+ have a memory-like phenotype.
Figure 3.
Representative dot plots from a single mouse showing time course of T cell expansion in the brain of Rag2−/− mice reconstituted with 10 million lymphoid cells (n = 15 females). A) Representative dot plots in the CD3+ gate for the proportion of CD4+ and CD8+ T cells used for quantification shown in panel B and proportions of CD4/CD44high shown in panel C and CD8/CD44high shown in panel D. ** p < 0.01.
3. T cell proliferation
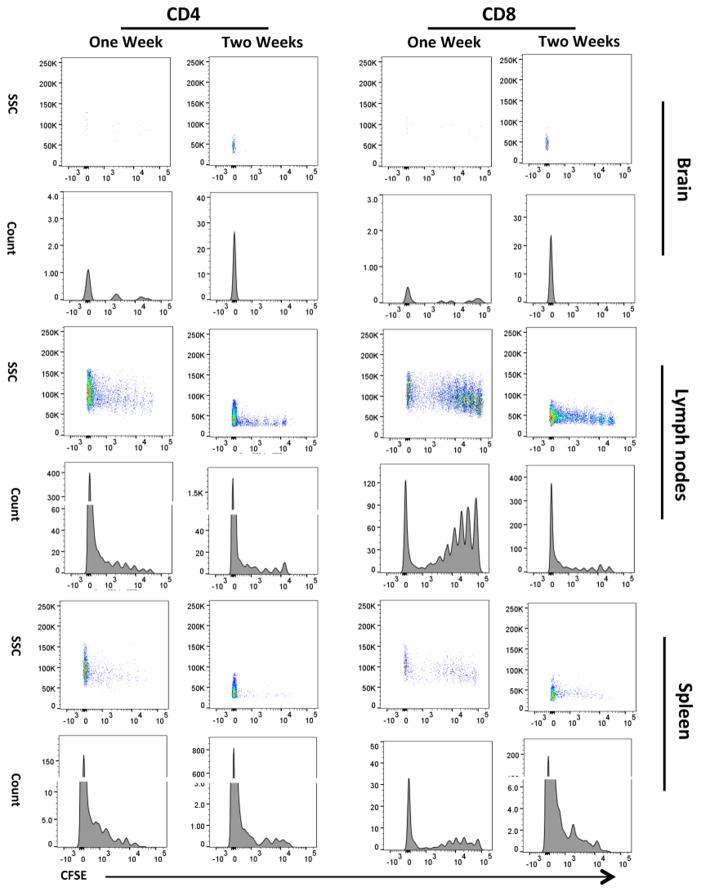
T cell proliferation in reconstituted Rag2−/− mice was assessed at one and two weeks (n = 8 females, 4 per time-point) after transfer by CFSE dilution of CD3+/CD4+ and CD3+/CD8+ T cells following the gating strategies outlined previously. CFSE signal in theses cell isolated from the brain was low at one week and undetectable at two weeks after transfer (Fig. 4), while the numbers of CD4+ and CD8+ cells significantly increased (Suppl. Fig. 6) indicating that the majority of T cells in the brain were daughter cells. CFSE fluorescence was still detectable in CD4+ and CD8+ cells in the LNs and Spl at one and two weeks after transfer indicating the occurrence of several cell divisions of the original population (Fig 4). These results indicate that the majority of CD3+/CD4+ and CD3+/CD8+ T cells in the brain are newly expanded cells.
Figure 4.
Carboxyfluorescein succinimidyl ester (CFSE) proliferation assay in Rag2−/− mice reconstituted with 10 million lymphoid cells at one (n = 4 females) and two (n = 4 females) weeks after transfer. Proliferation was analysed in CD4+ and CD8+ T cells in the brain, lymph nodes (LN) and spleen (Spl). Shown are representative density plots in the upper panels and histograms in the lower panels on each tissue analysed.
4. Neuroanatomical localization of lymphoid cells during homeostatic expansion
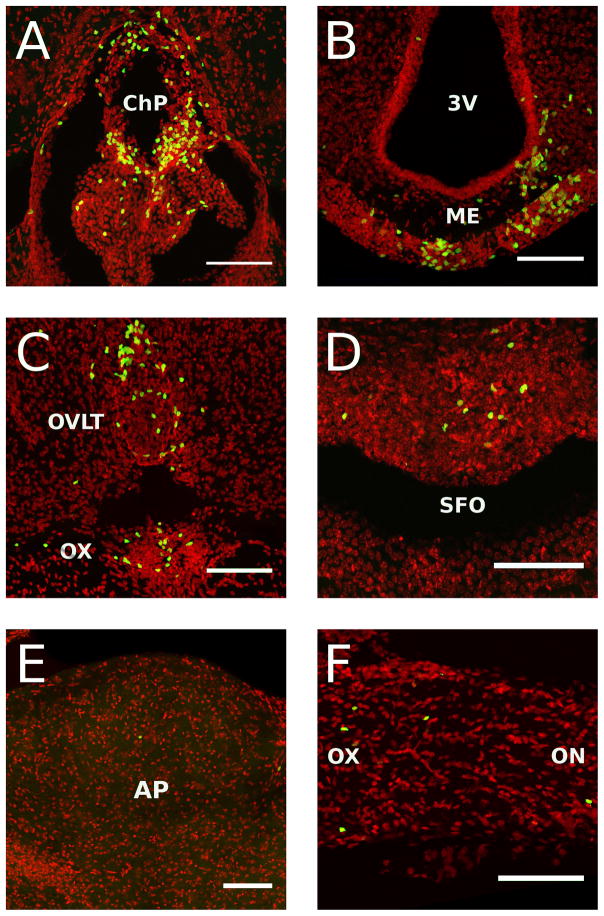
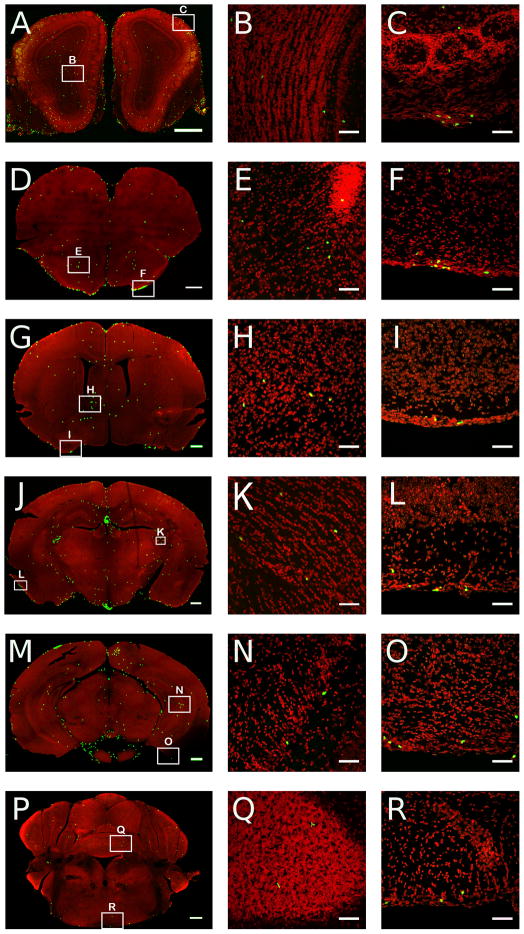
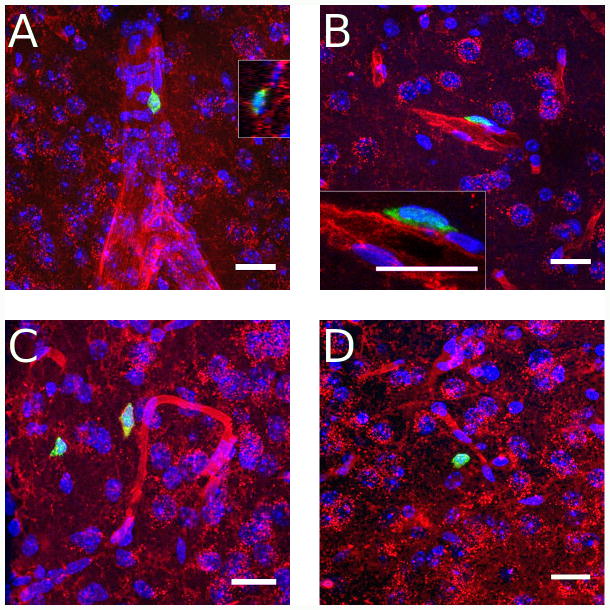
These studies involved the identification in the brain of Rag2−/− mice (n = 4 males) of GFP positive lymphoid cells from transgenic immunocompetent donors at four weeks after transfer using confocal microscopy. Coronal serial sections of 60 μm thickness were obtained through the entire brain and every fourth sections directly mounted on microscope slides and used for microscopic analyses. Randomly selected mice (n = 3 males) were used for flow cytometry analysis to confirm that the proportion of different types of cells was comparable to those obtained in previous experiments (Suppl. Fig. 7). GFP positive lymphoid cells were clearly visible throughout the brain with the highest density of cells found in the choroid plexus and circumventricular organs (CVOs), which lack the blood brain barrier (BBB) (Fig. 5). They were densely found in the choroid plexus (ChP, Fig 5A), median eminence (ME, Fig. 5B), organum vasculosum of the lamina terminalis (OVLT, Fig. 5C), and subfornical organ (SFO, Fig. 5D). They were also consistently found, but with less density, in the area postrema (AP, Fig 5E) and across the optic tract (OT) and optic chiasm (OX) (Fig. 5F). GFP positive lymphoid cells were also densely observed in the meninges across all levels of the brain (Fig 6). Scattered lymphoid GFP+ cells were also found across the brain tissue with no particular specific anatomical distribution. Nevertheless, they were consistently found in the olfactory bulbs (Fig 6A, B), anterior olfactory nucleus (AON) (Fig 6D, E), bed nucleus of the stria terminalis (BNST) (Fig. 6G, H), fimbria of the hippocampus (Fig. 6J, K), and across different layers of the cortex (Fig. 6M, N). Analyses at high magnification revealed a diverse morphology of GFP positive cells with apparent cellular interactions with other cell types (Suppl. Fig. 8). The anatomical relationship of GFP+ lymphoid cells with respect to vascular elements was analysed in tomato lectin stained sections. Cells were found inside the lumen of vessels (Fig. 7A), in perivascular spaces (Fig. 7B), and outside blood vessels in the parenchyma of the brain (Fig. 7 C, D). This pattern of lymphoid cell distribution was invariant for the meninges, ChP, CVOs and OT and OX in all cases analyzed. GFP+ cells were also consistently found in the white matter, for example in structures such as the fimbria, but with variant distributions across individual cases. Similarly, cells were always found in the parenchyma across the brain structures shown, but with a significant degree of variability in the density and grouping of cells.
Figure 5.
High resolution digital reconstructed images of Rag2−/− mice reconstituted with 10 million GFP expressing lymphoid cells at 4 weeks after transfer (n = 4 males). A) Choroid plexus (ChP), B) Median eminence (ME), C) Organum vasculosum of the lamina terminalis (OVLT), D) Subfornical organ (SFO), E) Area postrema (AP), F) Optic nerve (on) and chiasm (ox). 3V: Third ventricle. All scale bars = 50 microns
Figure 6.
Digital reconstructed images across coronal brain sections of Rag2−/− mice reconstituted with 10 million GFP expressing lymphoid cells at 4 weeks after transfer (n = 4 males). Left panels are low and middle and right panels are medium resolution images. Cells shown in low resolution images were expanded by applying a Gaussian blur followed by palette compression to give a saturated green signal for visibility purposes. Thus larger clusters of cells are no longer visible as individual cells. Examples of individual and small clusters of cells are shown at higher resolution across different structures of the brain (middle panels) or the meninges (right panels). Coronal levels A–B: olfactory bulbs; D–E: anterior olfactory nucleus; G–H: bed nucleus of the stria terminalis; J–K: fimbria dorsal hippocampus; M–N: ventral hippocampus; P–Q: cerebellum; C, F, I, L, O: meninges. Scale bars left panels = 500 microns, middle and right panels = 50 microns.
Figure 7.
High resolution digital reconstructed images of Rag2−/− mice reconstituted with 10 million GFP expressing lymphoid cells at 4 weeks after transfer showing the relationship of GFP+ lymphoid cells with vascular structures stained with tomato lectin. A) GFP+ lymphoid cell in a large vessel in the medial amygdala. Inset (right side) shows the cell is in the lumen of the vessel with significant cellular interaction with endothelial cells. B) Perivascular cell in primary motor cortex. Inset shows limbs of GFP+ cell apparently extending around endothelial cells, confirming its perivascular location. C) Lymphoid cells in the parenchyma of the medial posterodorsal amygdaloid nucleus. Note the red lectin staining around the cell plasma membrane of the cell closer to the vessel suggesting that it was at some time in contact with the vascular lumen. D) Extravascular cell in anterior caudate putamen. Blue: Hoechst nuclear stain; Green: GFP; Red: DyLight 594 labeled Lycopersicon esculentum (Tomato) Lectin. All scale bars = 20 microns.
5. Volume quantification of CD4+ and CD8+ T cells by two-photon microscopy
The objective of these experiments was to obtain tissue density values for CD4+ and CD8+ T cells in the brain parenchyma excluding meningeal structures, the choroid plexus and CVOs. The dorsal hippocampus was selected as the region of interest since the boundaries of this structure are easily identifiable in the thick sections used for quantification. Groups of male Rag2−/− mice were reconstituted with 8 to 10 million purified CD4+ (n = 4 males) or CD8+ (n = 4 males) T cells from transgenic GFP-expressing mice and the volume distribution of cells studied by two-photon microscopy. Preliminary examinations confirmed the presence of abundant T cells in the meningeal system with particular consistency in the region of the superior sagittal sinus (Suppl. Fig. 9 & Suppl. Movie 1). Volume quantification in the dorsal hippocampus yielded a total of 518.5 cells/mm3 ± 46.6 for CD4+ and 138.7 cells/mm3 ± 8.4 for CD8+ cells. These numbers correspond to a 3.7:1 ratio, which is comparable to the ratio obtained with flow cytometry experiments in reconstituted female Rag2−/− mice. High magnifications of cellular elements identified during the quantification are shown in Suppl. Fig. 10 and the volume distribution in Suppl. Movie 2 & 3. These studies confirm that CD4+ T cells expand at a higher rate than CD8+ T cells and provide a tissue density value for these cells in brain tissue.
Discussion
Proliferation of peripheral T cells in a lymphopenic setting, such as Rag2−/− mice, is a well characterized process involving rapid “burst like” proliferative cycles called endogenous proliferation in which cells divide at a ratio of one division per day usually completing 7 division cycles within a week (Min and Paul, 2005). A slower proliferative response in which cells divide one or two times a week occurs in parallel and is defined as homeostatic proliferation (Min et al., 2004; Troy and Shen, 2003). Distinct and common rules govern these processes in CD4+ and CD8+ cells including the requirement or not of IL-7 and the dependence of MHC peptides complexes (Min et al., 2005). These cells acquire markers of memory with subsequent cell division characterized by an increase in the expression of CD44, which is independent of foreign antigen stimulation (Goldrath et al., 2000; Min et al., 2004; Murali-Krishna and Ahmed, 2000). Studies showed that the clonal diversity determined by self antigens is the main mechanism that regulates expansion in which newly formed memory cells limit the proliferation of reactive cells to the same antigen (Min et al., 2005; Troy and Shen, 2003). This process is believed to naturally occur during development of T cells in neonates and to be recapitulated in a lymphopenic setting (Min et al., 2003; Min et al., 2002). Most of the proliferative responses have been shown to occur in the LNs and to a lesser extent in the Spleen (Min and Paul, 2005; Murali-Krishna and Ahmed, 2000). Newly formed memory cells have been shown to home to other peripheral organs and have been found mostly localized in the liver (Min et al., 2004). The profile of T cell proliferation in the LNs and Spleen found in the present study is in line with those referenced above. In the brain, the number of T cells was still very low one week after transfer (less than 0.5% of the MNCs as compared to 3% in WT animals) and significantly increased at two weeks, when the CSFE signal was completely diluted. Taking into account that proliferation in the LNs has completed the cycles of expansion as described in previous studies, the present results indicate that almost all, if not all, T cells found in the brain are newly formed cells, or daughter cells, that have undergone a significant number of cell divisions either in the LNs, the brain or a combination of both. The proportion of memory CD4+/CD44high and CD8+/CD44high T cells in the brain was high from week one indicating that the majority of cells from the beginning of the colonization have acquired a memory-like state. Whether these cells are reactive to brain specific self-antigens or if they migrated to the brain after becoming memory cells was not evaluated in this study. In this regard, it is likely that a diverse population of T cells specific to CNS and non-CNS antigens may be part of the T cell repertoire found in the brain in homeostatic conditions. Our data also indicate that T cells need to undergo a number of cell divisions in the LNs before developing the capacity to reach the brain lymphatic system. This can be related to a number of factors including a required number of peripheral T cells to create a gradient or exposure to CNS specific antigens to allow entrance to the brain lymphatic system, among many other possibilities.
The present study also report an increased ratio of CD4:CD8 cells in the brain of females with respect to male wild type animals. The increased CD4:CD8 ratio found in reconstituted Rag2−/− mice in flow cytometry experiments may have been related to this factor since these experiments were carried out in female mice. Nevertheless, the CD4:CD8 ratio of female reconstituted animals was higher (3.5:1) than those of wild type (1.7:1) female mice. Moreover, when male mice were reconstituted with purified CD4+ or CD8+ T cells the ratio determined by tissue volume quantification was 3.7:1, which is comparable to that of reconstituted female mice. Together, these data strongly indicates that CD4+ T cells are the most abundant cell type in the brain during endogenous and homeostatic expansion in Rag2−/− mice independent of sex. Whether Rag2−/− mice may acquire with time the sex differences in CD4:CD8 ratios seen in WT mice or whether these sex differences in WT are strain specific remain to be determined.
The proportion of B cells in the brain of WT mice is comparable to that of T cells. Nevertheless, B cells in the brain of reconstituted mice never reached WT values during the course of the month period, while they significantly increased in the LNs. This data shows the limited proliferative and/or migratory capacity of brain B cells when they are not stimulated with foreign antigens. NK cells were detected in control non-reconstituted Rag2−/− mice and numbers remained unchanged in the brain while they were significantly reduced in peripheral organs, which is a logical consequence of the expansion of lymphocytes in the periphery. Nevertheless, it is plausible that a different regulatory mechanism of NK cell numbers during expansion of lymphocytes in reconstituted Rag2−/− mice occurs in the brain with respect to peripheral compartments.
The exact localization of T cells in the brain under control non-inflammatory conditions has been difficult to characterize due to a number of technical challenges. The most notorious has been the poor immunoreactivity in brain tissue of most of the cell surface markers of lymphocytes. The best studied marker has been CD3 which has produced reliable data on the presence of CD3+ cells in the choroid plexus and meninges (Derecki et al., 2010; Lewitus et al., 2008). Recently, the study of Antoine Louveau (Louveau et al., 2015), who developed whole mount meningeal lymphatic system preparations provided a good characterization of T cells in the brain and also detailed the flow of these cells between the brain and the cervical lymph nodes. However, the characterization of T cells in perivascular spaces and the brain parenchyma as described in the present study has been more elusive. The approach of using lymphoid cells and T cells from GFP-transgenic mice to trace the anatomical localization revealed a more extensive relationship between these cells and cells of the CNS. The abundant localization in the ME and CVOs is not unexpected, since these structures are devoid of the BBB and in contact with the circulation (Morita et al., 2015). However, the question arises if the localization in perivascular spaces and the brain parenchyma is an epiphenomenon of the adoptive transfer approach in Rag2−/− mice or part of a naturally occurring process. In this regard, experiments of adoptive transfer in neonate mice indicate that the process of T cell proliferation is similar during development of the adaptive immune system suggesting that proliferation in adult lymphopenic mice recapitulate many aspects of T cell development (Min et al., 2004; Min et al., 2003; Min et al., 2002). It is possible then that migration of T cells into the brain perivascular spaces and the brain parenchyma is part of a natural process during development of the adaptive immune system of occupying specific places in the brain. This process may also extend to clinical cases involving recovering from lymphopenia, such as during effective HIV therapy. The traffic of T cells from the periphery to the brain in adult normal brain has been proposed during learning, in which the number of T cells in the choroid plexus has been found to be increased (Derecki et al., 2010), but no information is available on if perivascular and parenchymal T cells also increase or change their position. Whether T cells traffic in and out of perivascular and parenchymal spaces of the brain under non-inflammatory conditions and the specific circumstances of this process is a matter for future studies.
In conclusion, the present study outlines a number of parameters of lymphocyte and T cell proliferation in the brain of Rag2−/− mice of the C57Bl/6 background when reconstituted with 10 million cells. They provide proof of concept that T cells home to and expand in the brain and indicate that under these conditions, T cells require more than two weeks to reach levels comparable to those of wild type mice. They also indicate that CD4+ T cells expand more than any other type of lymphoid cell and reveal that a portion of T cells distribute in perivascular spaces in addition to the brain lymphatic system. This information provides basis for future studies aimed at defining the temporal and anatomical expansion of brain T cells under different experimental conditions and in other models of lymphocyte deficiency. In particular, Rag2−/− mice on a BALB/c background, known to differ on their immune responses with respect to the C57Bl/6 strain. This information may reveal novel mechanisms of interaction of T cells with CNS elements that may help explain the effects of these cells on brain homeostasis and function.
Supplementary Material
Highlights for Review.
Temporal and phenotypic profiles of lymphocyte expansion in lymphopenic mouse brain.
CD4+ T cells are the most abundant lymphocyte subtype in the brain during expansion.
Lymphocytes are mainly localized to the meninges and the choroid plexus.
Lymphocytes are found in several brain structures devoid of blood brain barrier.
Lymphocytes interact with the neurovasculture during expansion in the brain.
Acknowledgments
Supported by the National Institute of Mental Health Research Grant R01MH097676 (OppNet) and VA merit award grant BX000935 to LHT. Confocal microscopy studies were supported in part by instrumentation grants NIH S10 RR024550 and NIH S10 OD016374 to Johns Hopkins University. We would like to thank Dr. Ferenc Livak, Director, University of Maryland Marlene and Stewart Greenebaum Cancer Center Flow Cytometry Shared Service for his help with flow cytometry acquisition and analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aspelund A, Antila S, Proulx ST, Karlsen TV, Karaman S, Detmar M, Wiig H, Alitalo K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. The Journal of experimental medicine. 2015;212:991–999. doi: 10.1084/jem.20142290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, Jope RS. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biological psychiatry. 2013;73:622–630. doi: 10.1016/j.biopsych.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman RA, Lehmann ML, Maric D, Herkenham M. Lymphocytes from chronically stressed mice confer antidepressant-like effects to naive mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:1530–1538. doi: 10.1523/JNEUROSCI.2278-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brynskikh A, Warren T, Zhu J, Kipnis J. Adaptive immunity affects learning behavior in mice. Brain, behavior, and immunity. 2008;22:861–869. doi: 10.1016/j.bbi.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Chun JJ, Schatz DG, Oettinger MA, Jaenisch R, Baltimore D. The recombination activating gene-1 (RAG-1) transcript is present in the murine central nervous system. Cell. 1991;64:189–200. doi: 10.1016/0092-8674(91)90220-s. [DOI] [PubMed] [Google Scholar]
- Clark SM, Michael KC, Klaus J, Mert A, Romano-Verthelyi A, Sand J, Tonelli LH. Dissociation between sickness behavior and emotionality during lipopolysaccharide challenge in lymphocyte deficient Rag2 mice. Behavioural brain research. 2014a;278C:74–82. doi: 10.1016/j.bbr.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SM, Sand J, Francis TC, Nagaraju A, Michael KC, Keegan AD, Kusnecov A, Gould TD, Tonelli LH. Immune status influences fear and anxiety responses in mice after acute stress exposure. Brain, behavior, and immunity. 2014b;38:192–201. doi: 10.1016/j.bbi.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Ziv Y, Cardon M, Kaplan Z, Matar MA, Gidron Y, Schwartz M, Kipnis J. Maladaptation to mental stress mitigated by the adaptive immune system via depletion of naturally occurring regulatory CD4+CD25+ cells. Journal of neurobiology. 2006;66:552–563. doi: 10.1002/neu.20249. [DOI] [PubMed] [Google Scholar]
- Dasgupta P, Chapoval SP, Smith EP, Keegan AD. Transfer of in vivo primed transgenic T cells supports allergic lung inflammation and FIZZ1 and Ym1 production in an IL-4Ralpha and STAT6 dependent manner. BMC immunology. 2011;12:60. doi: 10.1186/1471-2172-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derecki NC, Cardani AN, Yang CH, Quinnies KM, Crihfield A, Lynch KR, Kipnis J. Regulation of learning and memory by meningeal immunity: a key role for IL-4. The Journal of experimental medicine. 2010;207:1067–1080. doi: 10.1084/jem.20091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey NJ, Chapoval SP, Smith EP, Skupsky J, Scott DW, Keegan AD. STAT6 controls the number of regulatory T cells in vivo, thereby regulating allergic lung inflammation. Journal of immunology. 2013;191:1517–1528. doi: 10.4049/jimmunol.1300486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. The Journal of experimental medicine. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Lee H, Lee G, Oh SJ, Shin MK, Shim I, Bae H. CD4+CD25+ regulatory T cell depletion modulates anxiety and depression-like behaviors in mice. PloS one. 2012;7:e42054. doi: 10.1371/journal.pone.0042054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Gadani S, Derecki NC. Pro-cognitive properties of T cells. Nature reviews. Immunology. 2012;12:663–669. doi: 10.1038/nri3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus GM, Cohen H, Schwartz M. Reducing post-traumatic anxiety by immunization. Brain, behavior, and immunity. 2008;22:1108–1114. doi: 10.1016/j.bbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, Derecki NC, Castle D, Mandell JW, Lee KS, Harris TH, Kipnis J. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–341. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Hope TA, Meck WH, Kelsoe G, Williams CL. Impaired social recognition memory in recombination activating gene 1-deficient mice. Brain research. 2011;1383:187–195. doi: 10.1016/j.brainres.2011.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, Foucras G, Meier-Schellersheim M, Paul WE. Spontaneous proliferation, a response of naive CD4 T cells determined by the diversity of the memory cell repertoire. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3874–3879. doi: 10.1073/pnas.0400606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min B, McHugh R, Sempowski GD, Mackall C, Foucras G, Paul WE. Neonates support lymphopenia-induced proliferation. Immunity. 2003;18:131–140. doi: 10.1016/s1074-7613(02)00508-3. [DOI] [PubMed] [Google Scholar]
- Min B, Paul WE. Endogenous proliferation: burst-like CD4 T cell proliferation in lymphopenic settings. Seminars in immunology. 2005;17:201–207. doi: 10.1016/j.smim.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Min B, Sempowski GD, Paul WE. Neonates support “homeostatic” proliferation. Advances in experimental medicine and biology. 2002;512:91–95. [PubMed] [Google Scholar]
- Min B, Yamane H, Hu-Li J, Paul WE. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. Journal of immunology. 2005;174:6039–6044. doi: 10.4049/jimmunol.174.10.6039. [DOI] [PubMed] [Google Scholar]
- Morita S, Furube E, Mannari T, Okuda H, Tatsumi K, Wanaka A, Miyata S. Heterogeneous vascular permeability and alternative diffusion barrier in sensory circumventricular organs of adult mouse brain. Cell and tissue research. 2015 doi: 10.1007/s00441-015-2207-7. [DOI] [PubMed] [Google Scholar]
- Murali-Krishna K, Ahmed R. Cutting edge: naive T cells masquerading as memory cells. Journal of immunology. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- Radjavi A, Smirnov I, Kipnis J. Brain antigen-reactive CD4+ T cells are sufficient to support learning behavior in mice with limited T cell repertoire. Brain, behavior, and immunity. 2014;35:58–63. doi: 10.1016/j.bbi.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattazzi L, Piras G, Ono M, Deacon R, Pariante CM, D’Acquisto F. CD4(+) but not CD8(+) T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice. Translational psychiatry. 2013;3:e280. doi: 10.1038/tp.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell SR, Walter BA, Gilbert MJ, Greenberg PD. Selective reconstitution of CD8+ cytotoxic T lymphocyte responses in immunodeficient bone marrow transplant recipients by the adoptive transfer of T cell clones. Bone marrow transplantation. 1994;14(Suppl 4):S78–84. [PubMed] [Google Scholar]
- Rilett KC, Friedel M, Ellegood J, MacKenzie RN, Lerch JP, Foster JA. Loss of T cells influences sex differences in behavior and brain structure. Brain, behavior, and immunity. 2015;46:249–260. doi: 10.1016/j.bbi.2015.02.016. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: an open-source platform for biological-image analysis. Nature methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Spanopoulou E. Cellular and molecular analysis of lymphoid development using Rag-deficient mice. International reviews of immunology. 1996;13:257–288. doi: 10.3109/08830189609061752. [DOI] [PubMed] [Google Scholar]
- Troy AE, Shen H. Cutting edge: homeostatic proliferation of peripheral T lymphocytes is regulated by clonal competition. Journal of immunology. 2003;170:672–676. doi: 10.4049/jimmunol.170.2.672. [DOI] [PubMed] [Google Scholar]
- Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. Journal of immunology. 2009;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nature neuroscience. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.