Abstract
A classical hallmark of acute inflammation is neutrophil infiltration of tissues, a multi-step process that involves sequential cell-cell interactions of circulating leukocytes with interleukin (IL)-1- or tumor necrosis factor-α (TNF)-activated microvascular endothelial cells (ECs) and pericytes (PCs) that form the wall of the postcapillary venules. The initial infiltrating cells accumulate perivascularly in close proximity to PCs. IL-17, a pro-inflammatory cytokine that acts on target cells via a heterodimeric receptor formed by IL-17RA and IL-17RC subunits, also promotes neutrophilic inflammation but its effects on vascular cells are less clear. We report that both cultured human ECs and PCs strongly express IL-17RC and, while neither cell type expresses much IL-17RA, PCs express significantly more than ECs. IL-17, alone or synergistically with TNF, significantly alters inflammatory gene expression in cultured human PCs but not ECs. RNA-seq analysis identifies many IL-17-induced transcripts in PCs encoding proteins known to stimulate neutrophil-mediated immunity. Conditioned media (CM) from IL-17-activated PCs, but not ECs, induce pertussis toxin-sensitive neutrophil polarization, likely mediated by PC-secreted chemokines, and also stimulate neutrophil production of pro-inflammatory molecules, including TNF, IL-1α, IL-1β, and IL-8. Furthermore, IL-17-activated PCs but not ECs can prolong neutrophil survival by producing G-CSF and GM-CSF, delaying the mitochondria outer membrane permeabilization and caspase 9 activation. Importantly, neutrophils exhibit enhanced phagocytic capacity after activation by CM from IL-17-treated PCs. We conclude that PCs, not ECs, are the major target of IL-17 within the microvessel wall and that IL-17-activated PCs can modulate neutrophil functions within the perivascular tissue space.
Introduction
The sine qua non of inflammation is leukocyte transmigration across the vessel wall to enter involved tissues, a process orchestrated by changes in the local microvasculature (1). Leukocyte extravasation typically occurs in postcapillary venules, the walls of which are composed of a luminal endothelial cell (EC) lining supported by abluminal pericytes (PCs) that are in contact with and share a common basement membrane with the ECs. Interactions between ECs and leukocytes have been the previous focus of inflammation research; only very recently have PCs also been recognized as active regulators of leukocyte trafficking and modulators of leukocyte effector functions in the perivascular space (2-4). After breaching the endothelial barrier, extravasating neutrophils crawl on postcapillary venular PCs, a process mediated by interactions between PC intercellular adhesion molecule-1 (ICAM-1) with neutrophil macrophage-1 antigen (Mac-1) and lymphocyte function-associated antigen 1 (LFA-1), and use gaps created by PCs to escape from the venular wall (5). The influence of PCs on neutrophils may extend beyond the time of direct contact-mediated interactions. In arterioles and capillaries, for example, PCs provide guidance cues for the migration of newly extravasated neutrophils and macrophages within interstitium (3). Moreover, PC-derived paracrine signals likely continue to target perivascular accumulations of leukocytes over the course of an inflammatory reaction.
The focus on ECs was made possible by the development of reproducible culture systems of human ECs and key findings from such in vitro experiments have been extensively confirmed in vivo (1, 6). The changes that occur in ECs at sites of inflammation have been described as “activation” and are largely induced by potent pro-inflammatory cytokines such as tumor necrosis factor-α (TNF) or interleukin (IL)-1 species. IL-17 is a pro-inflammatory cytokine secreted predominately by Th17 cells, but also can be produced by other immune cells such as γδ T cells, natural killer cells, type 3 innate lymphocytes and a subset of neutrophils. IL-17 plays a major role in neutrophil-mediated defensive immunity, particularly in the settings of extracellular bacterial and fungal infections. IL-17 signals through a heterodimeric receptor consisting of IL-17RA and IL-17RC subunits. IL-17RA is widely expressed by many cell types, with particularly high expression by leukocytes (7), whereas IL-17RC is predominately expressed by non-hematopoietic cells (8). Neutrophils lack IL-17RC and do not respond to IL-17 (9). It is therefore widely assumed that IL-17, like IL-1 and TNF, will exert its pro-inflammatory effects through activation of ECs. However, we and others have noted that effects of IL-17 on human ECs appear weak compared to the responses by several other stromal cell types such as fibroblasts, synoviocytes or vascular smooth muscle cells (10-12). The effect of IL-17 on PCs, which are related to but distinct from smooth muscle cells, has not been previously examined. Here we report that IL-17 potently activates PCs to express gene products that affect neutrophils and suggest that PCs, rather than ECs, may be the principal microvascular cell type responsible for the effects of IL-17 in neutrophilic inflammation.
Materials & Methods
Cells
All human cells were obtained through protocols approved by the Yale University Institutional Review Board. Human umbilical vein endothelial cells (HUVECs), human placental pericytes (PCs), human dermal microvascular endothelial cells (HDMECs), and human neutrophils were isolated and cultured using methods previously described (2, 13-15). The neutrophil population derived from this protocol is >99.9% CD15+ and CD68−, indicating the purity of isolated cells. Neutrophils were freshly isolated and used immediately for all experiments.
Generation of EC or PC Conditioned Media
Unstimulated or cytokine-stimulated EC or PC conditioned media (CM) were generated by treating confluent ECs or PCs grown in 6-well plates with IL-17 (100ng/ml, R&D), TNF (20ng/ml, Gibco), or both for 6 hours, and subsequently removing the recombinant cytokines by thorough washing with EC or PC media. New media were then added to ECs or PCs to collect freshly secreted proteins for 24 hours. CM was filtered using Acrodisc Syringe Filters with HT Tuffryn Membrane (Pall Corporation) to remove any debris.
IL-17 Receptor Analysis
For flow cytometry experiments, cells were stained with anti-human IL-17RA (Biolegend), IL-17RB (R&D), IL-17RC (R&D), IL-17RD (R&D), IL-17RE (Novus) or the appropriate isotype control antibodies. Samples stained with unconjugated primary antibodies were then incubated with the appropriate conjugated secondary antibodies (Life technologies) before acquisition on an LSR II flow cytometer (BD). For Western blot analysis, vascular cell monolayers were washed in ice cold PBS twice and lysed in RIPA buffer (Sigma). Samples were then mixed with Laemmli's sample buffer and heated at 95°C for 10 minutes prior to loading. After electrophoresis, samples were transferred onto a PVDF membrane for 90 minutes at 4°C. Membranes were blocked with TBST containing either 5% BSA or 5% non-fat dry milk, and anti-human IL-17RA (Cell signaling), IL-17RC (Novus), or β-actin (Sigma) were added for overnight incubation at 4°C. Bound antibodies were visualized with anti-mouse or anti-rabbit HRP-conjugated antibodies (ThermoScientific) and SuperSignal Femto or Pico West (Pierce). Densitometry was performed using NIH Image J software.
Quantitative real-time RT-PCR analysis
To isolate RNA from ECs, PCs, or neutrophils, cells were washed with sterile PBS and then processed using the RNeasy Mini kit (QIAGEN), according to the manufacturer’s protocol. The High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) was used to synthesize cDNA from RNA. All quantitative real-time RT-PCR reactions were assembled with TaqMan Gene Expression Master Mix and predeveloped Taqman gene expression probes (Applied Biosystems). Reactions were analyzed on a CFX96 Real Time system using CFX Manager Software (Bio-Rad Laboratories). Gene expression levels were normalized to GAPDH.
ELISA
For measurement of pro-inflammatory cytokine production by ECs and PCs, cultured cells were treated with 20ng/ml of IL-17, 20ng/ml of TNF, or both for 24 hours. Fresh supernatant was collected and assayed for IL-6, IL-8, G-CSF, and GM-CSF using Duoset ELISA Development System (R&D), according to manufacturer’s instructions.
RNA-seq library preparation and data analysis
Human placental PCs were stimulated with 20ng/ml of IL-17 (R&D), TNF (Gibco), or both for 12 hours. Total RNA was purified using the RNeasy Mini kit (QIAGEN), in which an on-column DNase treatment was included. Purified RNA was submitted to the Yale Center for Genomic Analysis where it was subjected to mRNA isolation and library preparation. Libraries were pooled, 6 samples per lane, and sequenced on an Illumina HiSEQ 2500 (75bp paired end reads), and aligned using STAR to the GRCh37 (hg19) reference genome. A count-based differential expression protocol was adapted for this analysis (16); mappable data were counted using HTSeq, and imported into R for differential expression analysis using DESeq2. To find differentially regulated sets of genes for signature generation, an absolute 1.5 fold-change difference between samples and FDR (Benjamini-Hochberg) ≤ 0.1 was used. Raw sequencing data were deposited to the Sequence Read Archive (SRP078508; http://www.ncbi.nlm.nih.gov/sra/SRP078508).
Gene Set Enrichment Analysis (GSEA)
Gene sets from the MSigDB immunologic signatures collection (C2, C5, C7) were chosen for testing of enrichment of each stimulated condition versus unstimulated control from the RNA-seq data. R-GSEA code was used to analyze the data, using the signal to noise ranking metric and 100,000 permutations of the gene labels for statistical significance testing. Results were visualized using R, with the running enrichment score (ES), member position by the signal to noise ranking metric (barcode), and ranking metric scale shown in output plots.
Neutrophil Polarization & Actin Quantification
Neutrophils were incubated in control, EC, or PC CM for 8 hours, followed by fixation with 4% paraformaldehyde. In indicated experiments, neutrophils were pre-treated with 200ng/ml of pertussis toxin (Sigma-Aldrich) for 90 minutes prior to incubation in CM. For polarization studies, neutrophils were imaged on a Zeiss Axiovision inverted microscope immediately after fixation. Cell borders were manually outlined and fit to an ellipse using ImageJ, and the parameters of the ellipse were measured. Polarization of the cells, as determined by changes in aspect ratio (the ratio of the length of the major axis to the minor axis), was measured. For actin quantification, cells were mounted on slides using Vectashield Hardset Mounting Medium with Phalloidin (Vector Laboratories). Mounted cells were fluorescently imaged and analyzed with ImageJ. As described above, individual cells were outlined and fit to an ellipse, and the phalloidin intensity along the major axis was measured. The side with the most intense phalloidin staining was selected as the starting point. For immunofluorescence analysis of F-actin in these neutrophils, cells were first stained with anti-human F-actin (Novus) followed by an anti-mouse IgM secondary antibody (Molecular Probes) prior to imaging on a fluorescence microscope.
Neutrophil Survival
Neutrophils incubated in control or cytokine-activated EC or PC CM, or media containing human recombinant cytokines (IL-6, IL-8, CXCL1, and GM-CSF from R&D; G-CSF from Gibco), were collected at the indicated time. Where indicated, G-CSF and GM-CSF were depleted from PC CM by immunoadsorption. G-CSF (eBioscience) and GM-CSF (R&D) antibodies were incubated in PC CM (20ug/ml) for 30 minutes at 37°C, followed by removal of the antigen-antibody complexes using Dynabeads Pan Mouse IgG (Life Technologies). For survival studies, cells were washed with PBS and then stained with FITC-conjugated Annexin V and Propidium Iodide (PI) (Life Technologies). After 15 minutes of incubation at 25°C, samples are analyzed on an LSRII flow cytometer (BD). BrdU Staining Kit for Flow Cytometry (eBioscience), JC-1 Mitochondrial Membrane Potential Assay Kit (Cayman Chemical), and Green FLICA Caspase 9 Assay Kit are used for measurement of neutrophil proliferation, mitochondria outer membrane permeabilization, and caspase 9 activation, respectively. These experiments were conducted following manufacturers’ instructions.
Phagocytosis
Neutrophils were pre-treated with control M199 media, unstimulated PC CM, or IL-17-stimulated PC CM for 12 hours. Neutrophils were then co-incubated with pHrodo Green S. aureus BioParticles Conjugate (Molecular Probes) in Live Cell Imaging Solution (Molecular Probes) for 90 minutes at 37 °C. Flow cytometry was performed immediately for quantitative measurements of phagocytosis.
Statistical analysis
Data are expressed as mean ± SEM. Statistical analyses were performed using GraphPad Prism software. Unpaired t-tests were used to make statistical comparisons between two groups. Comparisons between multiple groups were performed using one-way ANOVA with Tukey’s test for post-hoc analysis. In all experiments, p < 0.05 was considered statistically significant.
Results
IL-17 receptor expression differs between ECs and PCs
To determine the potential of vascular cells to respond to IL-17, we first examined the expression level of IL-17RA and IL-17RC on cultured HUVECs, HDMECs, and human placental PCs. Flow cytometric analysis showed that PCs reproducibly express higher level of IL-17RA than HUVECs and HDMECs on the cell surface although the level of expression is low in all three cell types. In contrast, all three cell types express robust surface level of IL-17RC, but the expression level on ECs is higher and more homogeneous than that of PCs (Table I, Supplemental Fig. 1A-B). Supporting our findings by flow cytometry, Western blot analysis demonstrated that PCs express higher level of IL-17RA than ECs. Both PCs and ECs express IL-17RC and the level of expression by HUVECs is higher than that of PCs (Supplemental Fig. 1C). Consistent with previous reports (9), IL-17RA is expressed at high levels on neutrophils, whereas IL-17RC is absent, confirming the validity of the reagents used for flow cytometry (Supplemental Fig. 1B). As some cytokine receptors on vascular cells may be modulated by cytokines (17), we examined this possibility but found that neither TNF nor IFN-γ treatments modulated IL-17 receptor expression on ECs or PCs (Supplemental Fig. 1D). Both ECs and PCs express IL-17RD but neither cell types express IL-17RB nor IL-17RE (Supplemental Fig. 1E), although the role of these receptors in IL-17-induced inflammatory responses is unclear.
Table I.
Flow Cytometric Analysis of IL-17 Receptors on Human Cells
| Cell Type | IL-17RA | IL-17RC |
|---|---|---|
| PC | 324 ± 55.99 | 1115 ± 227 |
| HUVEC | 149 ± 10.24 | 12136 ± 2082 |
| HDMEC | 109 ± 10.44 | 26851 ± 2633 |
| neutrophil | 1181± 68.83 | 75.33± 22.67 |
IL-17R subunits on human PCs, ECs and neutrophils were analyzed by flow cytometry. The results shown are the corrected median fluorescence intensity (MFI) expressed in arbitrary units of fluorescence [n=3, SEM].
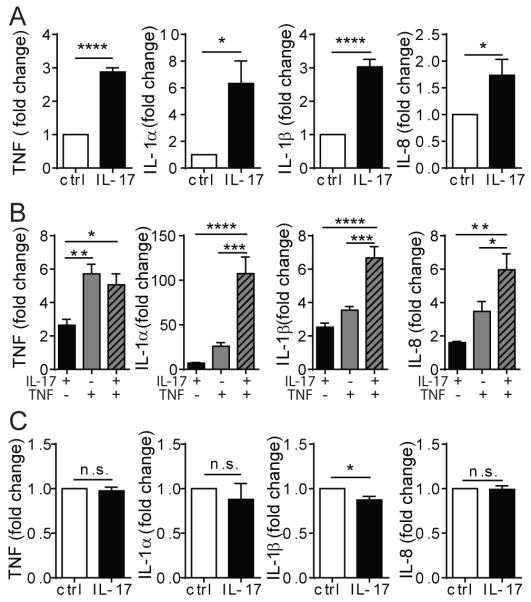
IL-17 induces cultured human PCs, but not ECs, to produce pro-inflammatory molecules
To investigate how vascular cells respond to IL-17, we initially examined the effects of IL-17A homodimers (commonly referred to simply as IL-17) on expression of IL-6 and IL-8, two inflammatory proteins that are strongly induced by TNF or IL-1 in both ECs and PCs. IL-17 does not significantly alter expression of mRNA or protein secretion for these molecules in either HUVECs or HDMECs, but does significantly induce both mRNA and protein expression in PCs (Fig. 1, Supplemental Fig. 2A). Since IL-17 has been reported to act synergistically with TNF in other cell types (18, 19), meaning that the level of induced target proteins by combining these two cytokines is greater than can be elicited with maximal concentrations of either cytokine alone, we next tested whether IL-17 and TNF would act synergistically in microvascular cells. In this analysis, we additionally considered the induction of CXCL5 and CCL20 which have been associated with IL-17-mediated inflammatory responses. As expected, TNF alone triggers potent induction of transcripts encoding IL-6, IL-8, CXCL5, and CCL20 in HUVECs, but the further addition of IL-17 produced only a small increase above that induce by TNF alone that generally did not reach statistical significance (Fig. 2A). A similar lack of synergism was observed in HDMECs (Fig. 2B). In contrast, IL-17 acts synergistically with TNF to induce expression of all four transcripts by PCs above that induced by maximally active concentrations of TNF or IL-17 alone (Fig. 2C). Similar interactions (or lack thereof) were observed in ECs and PCs stimulated with suboptimal dosages of IL-17 and TNF (Supplemental Fig. 2B).
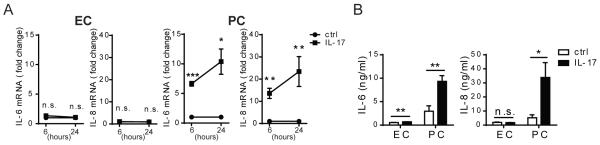
Figure 1. IL-17 activates PCs, but not ECs, to produce pro-inflammatory molecules.
(A) HUVEC and PC production of IL-6 and IL-8 transcripts after 6h or 24 h of IL-17 (20ng/ml) treatment assessed by qRT-PCR and normalized to GAPDH. Fold increase of the IL-17-stimulated conditions was calculated based on unstimulated controls of the respective cell type. Unstimulated control ECs and PCs express comparable levels of IL-6 and IL-8 transcripts. The threshold cycle numbers (Ct) for all conditions were below 30 cycles. (B) HUVEC and PC production of IL-6 and IL-8 proteins assessed by ELISA after 24h of IL-17 (20ng/ml) treatment. [n=3-6, t-test, SEM] *p<0.05, **p<0.01, ***p<0.001.
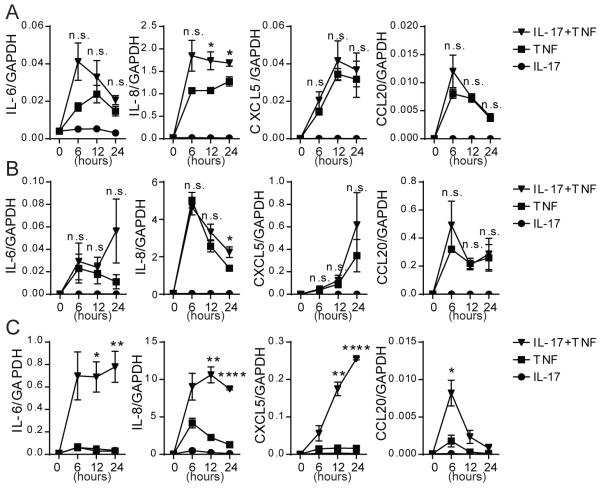
Figure 2. IL-17 and TNF synergistically upregulate pro-inflammatory gene expression in PCs.
Production of IL-6, IL-8, CXCL5, and CCL20 transcripts by HUVECs (A), HDMECs (B), and PCs (C) after stimulation with 20ng/mL of recombinant human IL-17 (circle), TNF (square), or IL-17+TNF (inverted triangle) for the indicated time. To determine the presence of synergistic interactions, transcript levels from IL-17+TNF conditions were compared to the sum of the transcript levels from IL-17 alone and TNF alone [n=3, t-test, SEM]. *p<0.05, **p<0.01, ****p <0.0001.
The actions of IL-17A homodimers may be shared by IL-17F homodimers or by IL-17A/F heterodimers; all three forms signal through the same IL-17RA/RC heterodimeric receptors, but IL-17F has been shown to bind to IL-17RC with higher affinity than to the IL-17RA component of the receptor complex (20). Since ECs uniformly express a higher level of IL-17RC than do PCs, we examined if ECs might preferentially respond to IL-17A/F or IL-17F vs. IL-17A. In fact, ECs are also unresponsive to IL-17A/F or IL-17F stimulation when these forms of IL-17 are added alone (Fig. 3A) or in combination with TNF (Supplemental Fig. 2C). IL-17A/F, but not IL-17F does appear to activate PCs to express pro-inflammatory genes, albeit with lower potency than IL-17A and the level of induction does not reach statistical significance (Fig. 3B). However, both IL-17A/F and IL-17F significantly enhance the response of TNF to turn on gene expression, although these other forms of IL-17 do not show the same level of synergy observed when IL-17A and TNF are combined (Supplemental Fig. 2C).
Figure 3. IL-17A more potently activates PCs compared to IL-17A/F and IL-17F.
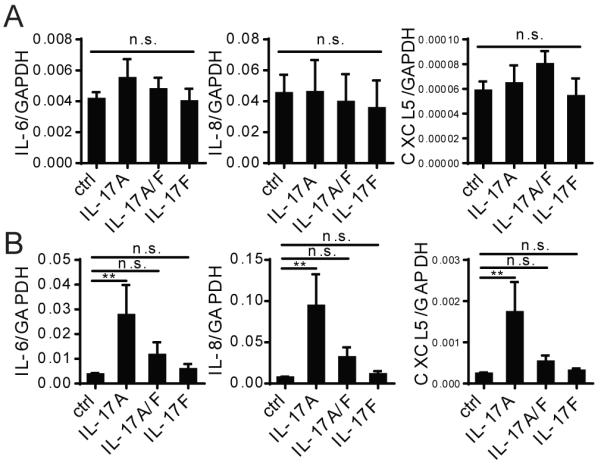
HUVECs (A) and PCs (B) were stimulated with 20ng/ml of recombinant human IL-17A, IL-17A/F, or IL-17F for 12 h, and the level of IL-6, IL-8, and CXCL5 mRNA were quantified by qRT-PCR [n=3, one-way ANOVA and Tukey’s multiple comparisons test, SEM]. **p<0.01.
RNA sequencing reveals the complex pattern of IL-17 actions on PCs
We initially focused on target genes previously associated with IL-17 responses in other cell types. To acquire an unbiased view of the effects that IL-17 has upon the inflammatory properties of PCs, we performed RNA sequencing (RNA-seq) to profile the transcriptome of nonactivated, IL-17-, TNF-, and IL-17+TNF-activated PCs. Differential expression analysis of each of the activated conditions against nonactivated PCs revealed that IL-17 significantly upregulates 42 genes compared to 610 with TNF stimulation, and that there is substantial overlap in the genes upregulated by IL-17 or TNF alone (Fig. 4A). While TNF in general is a more potent cytokine than IL-17 at upregulating genes in PCs, a small number of genes, such as Csf3, Cebpd, and Nfkbiz, are more strongly induced by IL-17. Overall, IL-17-activation of PCs induced an increase in multiple pro-inflammatory factors that are most relevant in the context of neutrophil-mediated immunity, including G-CSF (Csf3), GM-CSF (Csf2), CXCL-1 (Cxcl1), CXCL-6 (Cxcl6), and IL-8 (Cxcl8) (Fig. 4B). All of these pro-inflammatory genes are also synergistically upregulated by combining IL-17 with TNF (Supplemental Fig. 3A). Interestingly, stimulating PCs with both IL-17 and TNF resulted in the upregulation of 562 genes that were not significantly upregulated by either cytokine alone (Fig. 4A). Furthermore, co-stimulation of PCs with IL-17 and TNF also appeared to attenuate upregulation of some genes due to TNF stimulation alone, including MMP9 (Mmp9) and M-CSF (Csf1) (Supplemental Fig. 3B). qRT-PCR verified changes in gene expression of selected candidates identified by RNA-seq (Supplemental Fig. 3C). The enhanced expression of G-CSF and GM-CSF and reduced expression of M-CSF is consistent with a selective role of IL-17 for neutrophilic rather than monocytic inflammation. We also examined the endothelial gene expression of a list of selected inflammation-related genes up-regulated in IL-17-activated PCs. In contrast to PCs, ECs do not produce these inflammation-related transcripts after IL-17 stimulation (Supplemental Fig. 3D).
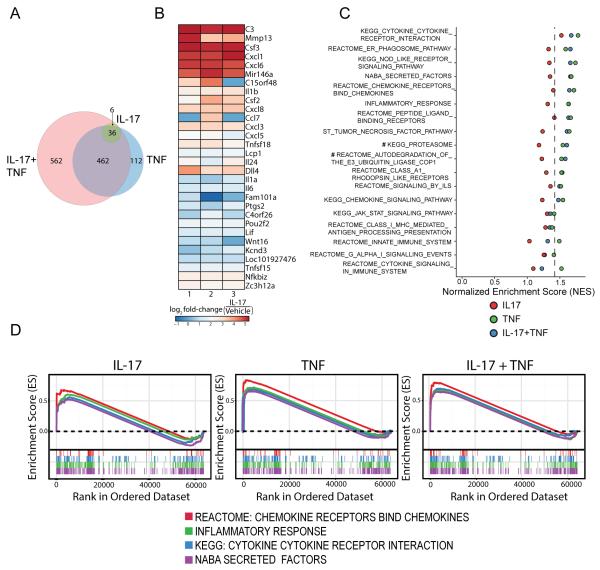
Figure 4. RNA sequencing analysis reveals the complex pattern of IL-17 actions on PCs.
(A) Differential expression analysis of the PC transcriptome after stimulation with IL-17, TNF, or IL-17+TNF for 12 h (20ng/ml for each cytokine). (B) Heat map visualization of the IL-17-activated PC gene signatures. (C) Gene Set Enrichment Analysis (GSEA) of IL-17-, TNF-, or IL-17+TNF- stimulated PCs using gene sets derived from biochemical pathways (C2, C5) and immunological signatures (C7) curated by MSigDB. Plot shows normalized enrichment scores (NES) of selected statistically significant and biologically relevant gene sets. Values right of the dotted line attain FDR < 0.2, with the exception of the TNF group for the two gene sets denoted with #. (D) GSEA leading edge analysis of cytokine-stimulated PCs using four selected gene sets related to neutrophil function and inflammation (Chemokine Receptors Bind Chemokines, Inflammatory Response, Cytokine-Cytokine Receptor Interaction, and Secreted Factors), showing their enrichment scores (where a positive score indicates 'enrichment' (higher expression) in the given cytokine-stimulated samples relative to the unstimulated control). TNF- and IL-17+TNF-activated PCs demonstrate significant enrichment of these gene sets (FDR < 0.02, except for gene set Chemokine Receptors Bind Chemokines in IL-17+TNF-treated PCs, which has a FDR of 0.128).
Gene Set Enrichment Analysis (GSEA) using gene sets derived from biochemical pathways (C2, C5) and immunological signatures (C7) curated by MSigDB provided further insights into the cytokine responses of PCs (21). In general, pathways pertaining to immune responses and immunological signaling pathways associated with inflammation, such as Innate Immune System, NOD-like Receptor Signaling, JAK-STAT Signaling, and Tumor Necrosis Factor pathways were affected (Fig. 4C). Consistent with the differential expression analysis, IL-17 is overall a weaker pro-inflammatory inducer than TNF in PCs. In this analysis, IL-17 stimulation alone did not induce any significant gene set enrichment (with the exception of Cytokine-Cytokine Receptor Interaction, FDR < 0.1), whereas both TNF and IL-17+TNF showed a significant upregulation of the majority of these gene sets. Four selected gene sets related to inflammation and neutrophil function (Chemokine Receptors Bind Chemokines, Inflammatory Response, Cytokine-Cytokine Receptor Interaction, and Secreted Factors) were selected for leading edge analysis of the enrichment scores. These four gene sets do not reach statistical significance in their enrichment in IL-17-treated PCs, although they do trend towards enrichment, whereas the TNF- and IL-17+TNF-activated PCs show robust enrichment of these gene sets associated with inflammation and neutrophil functions (FDR < 0.15) (Fig. 4D). Overall, these data further verify that IL-17 actions are more selective than those of TNF and the genes in PCs influenced by IL-17 appear to be more specific for actions on neutrophils.
IL-17-activated PCs induce neutrophil polarization
We next investigated if there are in fact functional changes in neutrophil behaviors mediated by IL-17-induced PC paracrine signals. First we tested whether IL-17-activated PCs induce neutrophil polarization, a response required for neutrophil crawling and migration early in neutrophil extravasation and a key indicator of neutrophil activation. Freshly isolated human neutrophils were incubated in conditioned media (CM) from control or IL-17-activated PCs. IL-17-activated but not control PC CM induced neutrophil front-tail polarization, as confirmed by the increase in neutrophil aspect ratio (Fig. 5A). Neutrophil polarization is dependent on F-actin reorganization and the formation of an F-actin-rich lamellipodia and a posterior uropod. Neutrophils exposed to IL-17-treated PC CM exhibit dynamic shift of actin distribution from diffuse to focal shown by staining with either phalloidin or with antibody to F-actin (Fig. 5B, 5C). In contrast to the effects of IL-17-treated PC CM, IL-17-treated EC CM have no effect on neutrophil morphology or actin distribution (Fig. 5A-C). To ensure the observed phenotype is not the result of potential residual recombinant IL-17 cytokine in the CM, we treated neutrophils directly with recombinant human IL-17 and found that it does not induce neutrophil polarization, consistent with a previous report showing that neutrophils, which lack expression of the IL-17RC component of the receptor, are unresponsive to IL-17 activation (9). Neutrophil polarization and actin redistribution are typically mediated by chemokines. As suggested by our RNA-seq data, PC CM likely contains too many different neutrophil-activating chemokines to determine which ones might induce polarization. However, all of these chemokines act through pertussis toxin (PT)-sensitive G protein coupled receptors. Therefore, to confirm that this response is chemokine-induced, we tested whether neutrophil polarization observed in IL-17-activated PC CM could be inhibited by PT. As predicted, PT-treated neutrophils do not undergo polarization in IL-17-activated PC CM, supporting the conclusions that the chemokines present in PC CM are biologically active and that neutrophil polarization is mediated by chemokines secreted by IL-17-activated PCs (Fig. 5D). Neither untreated nor PT-treated neutrophils were polarized by control EC or IL-17-stimulated EC CM.
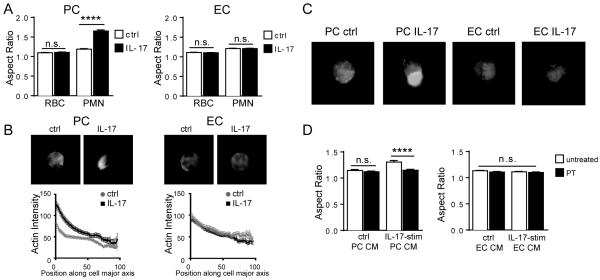
Figure 5. CM from IL-17-activated PCs induces neutrophil polarization.
Aspect ratios (A) and phalloidin staining with quantification of F-actin localization (B) of neutrophils incubated in control or IL-17-stimulated PC or EC CM [n=6, t-test, SEM]. Residual red blood cells (RBC) served as internal controls for analysis of aspect ratios to ensure morphological changes were not the result of cell mounting or staining. (C) Representative images demonstrating immunofluorescence staining of F-actin in neutrophils cultured in the indicated CM [n=3]. (D) Aspect ratios of untreated or PT-treated neutrophils incubated in CM of control or IL-17-stimulated PCs or ECs [n=2-4, one-way ANOVA, SEM]. **** p<0.0001.
Neutrophils produce pro-inflammatory molecules in response to IL-17-activated PCs
Neutrophil secretion of cytokines, chemokines, proteases, and other signaling moieties furnishes them with the potential to drive local inflammation and tissue remodeling. IL-17-activated PC CM induces neutrophils to synthesize IL-1α, IL-1β, TNF, and IL-8 (Fig. 6A) but not IL-6, IL-17, IL-18, IFN-γ, or IL-4. IL-17+TNF-treated PC CM more potently induce neutrophil production of IL-1α, IL-1β, TNF, and IL-8 (Fig. 6B). Once again, IL-7-activated EC CM fail to activate neutrophils to produce any of the above effector molecules (Fig. 6C).
Figure 6. CM from IL-17-activated and/or TNF-activated PCs induces transcripts of pro-inflammatory molecules in neutrophils.
(A&B) TNF, IL-1α, IL-1β and IL-8 transcript expression by neutrophils cultured in the indicated PC CM. (C) Effector molecule transcript expression by neutrophils cultured in control or IL-17-stimulated HUVEC CM. [n=4-5; t-test (A&C); one-way ANOVA (B), SEM]. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
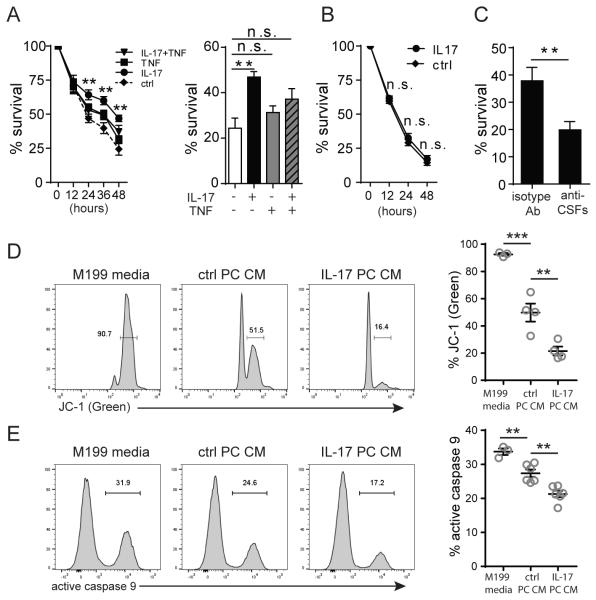
Neutrophil survival is prolonged by IL-17-stimulated PCs
Neutrophils are generally short-lived, but may persist within inflamed tissues for a greater period of time than they normally survive within the circulation. As many extravasated neutrophils can persist in a perivascular location, we next explored whether IL-17-activated PCs influence neutrophil survival. Freshly isolated human neutrophils show progressive loss of viability over 48 hours in standard culture medium as assessed by annexin V and PI staining. We find that basal PCs secrete factors that enhance neutrophil survival, and that IL-17-activated PCs significantly further extend neutrophil viability in comparison to basal PCs (Fig. 7A, Supplemental Fig. 4 A&B). At 48 hours, approximately 45% of neutrophils incubated in the IL-17-activated PC CM were alive compared to fewer than 10% in the control media. In fact, IL-17-activated PCs are superior to TNF-activated or IL-17+TNF-activated PCs in their ability to enhance neutrophil survival (Fig. 7A). BrdU labelling was performed and confirmed that the increased numbers of neutrophils remaining in IL-17-activated PC CM was not due to proliferation. (Supplemental Fig. 4C). In contrast to IL-17-treated PC CM, IL-17-treated EC CM did not enhance neutrophil longevity (Fig. 7B). To identify which paracrine moieties are responsible for the prolonged neutrophil survival in IL-17-activated PC CM, we returned to the RNA-seq analysis of PC gene expression. As survival is more likely to be mediated by a cytokine rather than a chemokine, our RNA-seq data pointed to IL-6, G-CSF, and GM-CSF as candidate effectors in this system. We tested the ability of each of these molecules to modulate neutrophil survival by treating neutrophils with these recombinant cytokines and found that both G-CSF and GM-CSF can significantly prolong neutrophil survival in vitro (Supplemental Fig. 4D) whereas IL-6 does not. As expected, neither IL-8 nor CXCL-1, which are prototypic neutrophil-active chemokines, can prolong neutrophil survival. By ELISA, we confirmed that IL-17 activates PCs to produce and secrete both G-CSF and GM-CSF proteins, and that G-CSF is secreted at a higher level than GM-CSF. On the other hand, ECs secrete neither G-CSF nor GM-CSF after IL-17 stimulation (Supplemental Fig 4E). Immunoadsorption of both G-CSF and GM-CSF from the IL-17-stimulated PC CM abolished prolongation of neutrophil lifespan (Fig. 7C).
Figure 7. Neutrophil survival ex vivo is prolonged by IL-17-activated PC paracrine signals.
(A) Neutrophil survival in control, IL-17-, TNF-, or IL-17+TNF-stimulated PC CM at the indicated time (left) and at 48 h (right) [n=8, * indicates significant difference between the IL-17-stimulated condition versus control; one-way ANOVA, SEM]. (B) Neutrophil survival in control or IL-17-stimulated HUVEC CM [n=6, t-test, SEM]. (C) Neutrophil survival at 48 h in IL-17-stimulated PC CM with or without immunoadsorption of G-CSF and GM-CSF [n=8, t-test, SEM]. (D) Mitochondrial transmembrane potential of neutrophils cultured in control media, control PC CM, or IL-17-stimulated PC CM assessed by flow cytometry after JC-1 staining [n=3-4, one-way ANOVA, SEM]. (E) Flow cytometric measurement of the caspase 9 activation in neutrophils cultured in control media, control PC CM, or IL-17-stimulated PC CM by FAM-LEHD-FMK FLICA staining [n=6-8, one-way ANOVA, SEM]. Data from one neutrophil donor was excluded from the analysis due to extensive caspase 9 activation in all groups at early time points. **p<0.01, ***p<0.001.
To gain mechanistic insights regarding how PC-secreted molecules enhance neutrophil survival, we investigated how the apoptotic pathways of neutrophils may be affected by PC CM. Since previous reports have shown that G-CSF prolong neutrophil survival by inhibiting mitochondria-initiated pathways of apoptosis (22, 23), we examined whether mitochondrial outer membrane permeabilization (MOMP) and cytochrome c-dependent activation of caspase 9 play a role in inhibition of neutrophil apoptosis induced by IL-17-activated PCs. Neutrophils cultured in IL-17-activated PC CM indeed demonstrated delayed mitochondrial outer membrane permeabilization (MOMP) and reduced caspase 9 activity in comparison to neutrophils cultured in control media or basal PC CM (Fig. 7D, 7E).
Neutrophils activated by IL-17-stimulated PCs exhibit enhanced phagocytic capacity
Neutrophils constitute the first line of defense against bacterial and fungal infection, and phagocytosis is a key effector function in this process. We therefore evaluated whether neutrophil phagocytosis can also be modulated by IL-17-activated PCs. Neutrophils pretreated with control media, basal PC CM, or IL-17-activated PC CM were co-incubated with fluorescently labelled Staphylococcus aureus, and the level of phagocytic activity was measured. Neutrophils treated with IL-17-activated PC CM exhibit enhanced phagocytic capacities in comparison to those cultured in control media or basal PC CM (Fig. 8).
Figure 8. Neutrophils activated by IL-17-stimulated PCs exhibit enhanced phagocytic capacity.
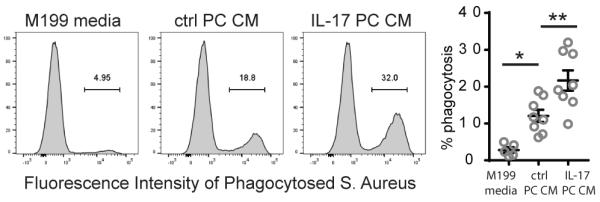
Phagocytosis of fluorescently labelled Staphylococcus Aureus by neutrophils pre-treated with the indicated media or CM. Representative flow cytometry plots are shown (left) and quantification from pooled independent experiments is displayed (right) [n=4-8, one-way ANOVA and Tukey’s multiple comparisons test, SEM]. *p<0.05, **p<0.01.
Discussion
The pro-inflammatory cytokine IL-17 plays a critical role in immune defense against extracellular bacterial and fungal infections principally through the induction and enhancement of neutrophil infiltration into an infected tissue site. The current paradigm of inflammation is focused on cytokine-induced changes in microvascular cells, particularly on ECs of the postcapillary venule, as the orchestrator of leukocyte recruitment. In this study, we examined the effect of IL-17 on the activation of the two principal cell types that form the microvascular wall, namely ECs and PCs. Although IL-17 receptors are ubiquitously expressed, we corroborate previous studies and demonstrate that the strength of IL-17 signaling varies widely between different cell types (10, 11). Specifically, we found that cultured human PCs are much more responsive to IL-17 stimulation in producing pro-inflammatory molecules than are cultured human ECs. Additionally, IL-17 synergizes with TNF to induce the expression of multiple inflammatory cytokines and chemokines in PCs but not ECs. Through RNA-seq analysis of cytokine-stimulated PCs, we identified IL-17-stimulated PCs as source of selected inflammatory gene products, and while IL-17 is less potent than the actions of TNF, its actions were more selective for inducing proteins that influence neutrophilic responses.
Unlike other prototype pro-inflammatory cytokines, such as TNF and IL-1β, which primarily act on ECs to promote inflammatory programs, our previous work indicated that IL-17 seem to preferentially act on mural cells of large vessels (i.e. vascular smooth muscle cells) rather than on ECs (11). PCs are the mural cells of the microvasculature, and they are located at the interface of circulating blood and the interstitium, and such strategic positioning enables them to influence trafficking and effector functions of the newly extravasated leukocytes. However, due to the lack of well-defined PC markers and technical difficulties in isolation of PCs, the role of PCs in inflammation has been largely ignored in comparison to the wealth of information regarding EC responses. While recent in situ observations have implicated PCs as essential modulators in the inflammatory process (3, 5), direct analysis of the PC response to cytokines is more easily studied in cell culture. We recently developed a simple protocol to isolate PCs from human placental microvessels and have used these cells to characterize effects of IFN-γ, IL-1, and TNF on the inflammatory functions of this cell type (2, 4, 14, 24). Here we extend these studies to examine the effects of IL-17 on PCs. A potential limitation of this study is that PCs are likely to be heterogeneous. It will be important to determine if PCs from different tissues or even from different parts of the microvasculature from the same tissue behave the same way. That being said, ECs are also heterogeneous but tend to assume a similar phenotype in culture, and studies with HUVECs have successfully predicted most inflammatory responses attributable to ECs of various tissues. The same may be true of human placental PCs.
The strength of IL-17 signaling actually varies widely among different cell types and among different species. Compared to other cell types (such as skin fibroblasts or synoviocytes), human ECs only weakly respond to IL-17 if at all. Stromal cells that are similar to PCs in ontogeny, such as fibroblasts, may produce 20-fold more of IL-6 than human brain ECs after same dosage of IL-17 stimulation (10). Species differences may also be relevant as mouse aortic ECs are at least 10-fold more responsive to IL-17 than human aortic ECs (25). On the other hand, human lung microvascular ECs do appear to express IL-17RA and respond to IL-17 activation, although the magnitude of the responses observed while comparable to TNF in this cell type, are quite weak by comparisons to TNF responses in other studies (26, 27). It has also been reported that HUVECs expressed CCL20 and adhesion molecules (ICAM-1 and VCAM-1) after IL-17 stimulation, but the magnitude of expression observed in this study was again very limited (28). These data cumulatively suggest that while some EC types may respond to IL-17, the magnitude of the human EC response is very small and unlikely to account for the powerful role that IL-17 exhibits in neutrophilic inflammation. As neutrophils lack IL-17RC and do not respond directly to IL-17, we reasoned that some other vascular cell type might play this role. In this study, we identified microvascular PCs as the probable cellular target of IL-17-driven neutrophil responses.
While recent studies on neutrophil-PC interaction have focused on neutrophil recruitment, the anatomic position of PCs points to an additional role of IL-17-activated PCs in influencing behaviors of leukocytes in the interstitium. It has been shown that PCs provide guidance cues that enable effective interstitial migration of extravasated macrophages and neutrophils to sterile inflammatory foci, a process dependent on both direct PC-leukocyte interaction and PC-secreted chemoattractants, such as macrophage migration-inhibitory factor. Compared to macrophages and neutrophils that do not encounter capillary and arteriolar PCs during interstitial migration, those that do interact with PCs undertake a more target-directed and expedited route to reach the site of tissue necrosis (3). In this study, we identified important interstitial neutrophil functions that can be modulated by IL-17-activated PCs. Specifically, IL-17 activates PCs to create a perivascular milieu that induces neutrophil polarization, a process that involves F-actin reorganization and is required for effective interstitial migration. During an infection, neutrophils are one of the earliest immune cells to arrive in the involved tissues, and play important role in recruitment of other immune cell types. Neutrophils normally contribute to this process by secretion of cytokines and chemokines, including IL-8, which recruits additional neutrophils, or TNF and IL-1β, which act on other cell types to drive pro-inflammatory programs. We found that IL-17-stimulated PCs induce neutrophil synthesis of TNF, IL-1α, IL-1β, and IL-8. Finally, we also demonstrate that IL-17-activated PC can prolong neutrophil functions by extending their otherwise limited lifespan. It has been previously shown that resting PC produce factors that prolong neutrophil survival (3), but this function is greatly enhanced by IL-17-mediated activation, and unlike induction of chemokines, IL-17-stimulated PCs may be more potent than either TNF-stimulated PCs or IL-17+TNF-stimulated PCs. We further show that IL-17-activated PCs prolong neutrophil survival by secreting G-CSF and GM-CSF, and that the mitochondria death pathways were inhibited in neutrophils exposed to CM from IL-17-activated PCs. Finally, we demonstrate that soluble factors produced by IL-17-activated PCs may also enhance phagocytic activities of neutrophils, suggesting an important role of PCs in optimizing pathogen clearance in the context of IL-17-mediated immunity.
While neutrophils are key early responders to infection, their role in IL-17-mediated chronic autoimmunity, largely mediated by T cells, is less clear. Recent evidence demonstrates essential roles of neutrophils in recruitment of adaptive immune cells by generating a migratory path enriched with neutrophil-derived chemokines that attract T cells into affected tissues (29). However, the full extent to which IL-17-mediated neutrophil actions are linked to T cell immune responses, particularly in the context of IL-17-driven autoimmune diseases, remains poorly understood. The roles that PCs might play in these chronic inflammatory reactions will be an important area of future research.
In summary, we have provided the first report on the role of IL-17-stimulated PCs in an inflammatory response, namely enhancing neutrophil function by influencing their activation, cytokine production, survival, and phagocytic capacity. We also showed that PCs are much more potent responders to IL-17 stimulation than are ECs, suggesting that IL-17 primarily activates PCs to drive pro-inflammatory processes at microvascular sites. Findings from this study offer important mechanistic insights on how PCs may contribute to an inflammatory response and identify IL-17-activated PCs as new targets for development of anti-inflammatory therapeutics.
Supplementary Material
Acknowledgements
We thank Louise Camera-Benson and Gwendoline Arrington-Davis for assistance with EC cell culture.
Source of support: This work is supported by National Institutes of Health (NIH) grant R01-HL051014 to J.S. Pober. R. Liu was supported by an NIH Medical Scientist Training Program grant (T32-GM007205) and is currently supported by an NIH National Research Service Award predoctoral fellowship (F31-HL129563).
Abbreviations
- ECs
endothelial cells
- PCs
pericytes
- HDMECs
human dermal microvascular endothelial cells
- CM
conditioned media
- PT
pertussis toxin
References
- 1.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nature reviews. Immunology. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 2.Lauridsen HM, Pober JS, Gonzalez AL. A composite model of the human postcapillary venule for investigation of microvascular leukocyte recruitment. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2014;28:1166–1180. doi: 10.1096/fj.13-240986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, Hepper I, Lauber K, Walzog B, Massberg S. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and 'instruct' them with pattern-recognition and motility programs. Nat Immunol. 2013;14:41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 4.Ayres-Sander CE, Lauridsen H, Maier CL, Sava P, Pober JS, Gonzalez AL. Transendothelial migration enables subsequent transmigration of neutrophils through underlying pericytes. PloS one. 2013;8:e60025. doi: 10.1371/journal.pone.0060025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Proebstl D, Voisin MB, Woodfin A, Whiteford J, D'Acquisto F, Jones GE, Rowe D, Nourshargh S. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. The Journal of experimental medicine. 2012;209:1219–1234. doi: 10.1084/jem.20111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cines DB, Pollak ES, Buck CA, Loscalzo J, Zimmerman GA, McEver RP, Pober JS, Wick TM, Konkle BA, Schwartz BS, Barnathan ES, McCrae KR, Hug BA, Schmidt AM, Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 7.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nature reviews. Immunology. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho AW, Shen F, Conti HR, Patel N, Childs EE, Peterson AC, Hernandez-Santos N, Kolls JK, Kane LP, Ouyang W, Gaffen SL. IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. Journal of immunology. 2010;185:1063–1070. doi: 10.4049/jimmunol.0903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, Cassatella MA. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115:335–343. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 10.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. The Journal of experimental medicine. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao DA, Eid RE, Qin L, Yi T, Kirkiles-Smith NC, Tellides G, Pober JS. Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection. The Journal of experimental medicine. 2008;205:3145–3158. doi: 10.1084/jem.20081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosteen MH, Tritsaris K, Hansen AJ, Dissing S. IL-17A potentiates TNFalpha-induced secretion from human endothelial cells and alters barrier functions controlling neutrophils rights of passage. Pflugers Archiv : European journal of physiology. 2014;466:961–972. doi: 10.1007/s00424-013-1354-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gimbrone MA, Jr., Cotran RS, Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. The Journal of cell biology. 1974;60:673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maier CL, Shepherd BR, Yi T, Pober JS. Explant outgrowth, propagation and characterization of human pericytes. Microcirculation. 2010;17:367–380. doi: 10.1111/j.1549-8719.2010.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark PR, Kim RK, Pober JS, Kluger MS. Tumor necrosis factor disrupts claudin-5 endothelial tight junction barriers in two distinct NF-kappaB-dependent phases. PloS one. 2015;10:e0120075. doi: 10.1371/journal.pone.0120075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anders S, McCarthy DJ, Chen Y, Okoniewski M, Smyth GK, Huber W, Robinson MD. Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nature protocols. 2013;8:1765–1786. doi: 10.1038/nprot.2013.099. [DOI] [PubMed] [Google Scholar]
- 17.Karmann K, Hughes CC, Schechner J, Fanslow WC, Pober JS. CD40 on human endothelial cells: inducibility by cytokines and functional regulation of adhesion molecule expression. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:4342–4346. doi: 10.1073/pnas.92.10.4342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Mei J, Gonzales L, Yang G, Dai N, Wang P, Zhang P, Favara M, Malcolm KC, Guttentag S, Worthen GS. IL-17A and TNF-alpha exert synergistic effects on expression of CXCL5 by alveolar type II cells in vivo and in vitro. Journal of immunology. 2011;186:3197–3205. doi: 10.4049/jimmunol.1002016. [DOI] [PubMed] [Google Scholar]
- 19.Katz Y, Nadiv O, Beer Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: a possible role as a "fine-tuning cytokine" in inflammation processes. Arthritis and rheumatism. 2001;44:2176–2184. doi: 10.1002/1529-0131(200109)44:9<2176::aid-art371>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Kuestner RE, Taft DW, Haran A, Brandt CS, Brender T, Lum K, Harder B, Okada S, Ostrander CD, Kreindler JL, Aujla SJ, Reardon B, Moore M, Shea P, Schreckhise R, Bukowski TR, Presnell S, Guerra-Lewis P, Parrish-Novak J, Ellsworth JL, Jaspers S, Lewis KE, Appleby M, Kolls JK, Rixon M, West JW, Gao Z, Levin SD. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. Journal of immunology. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Raam BJ, Drewniak A, Groenewold V, van den Berg TK, Kuijpers TW. Granulocyte colony-stimulating factor delays neutrophil apoptosis by inhibition of calpains upstream of caspase-3. Blood. 2008;112:2046–2054. doi: 10.1182/blood-2008-04-149575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tehranchi R, Fadeel B, Forsblom AM, Christensson B, Samuelsson J, Zhivotovsky B, Hellstrom-Lindberg E. Granulocyte colony-stimulating factor inhibits spontaneous cytochrome c release and mitochondria-dependent apoptosis of myelodysplastic syndrome hematopoietic progenitors. Blood. 2003;101:1080–1086. doi: 10.1182/blood-2002-06-1774. [DOI] [PubMed] [Google Scholar]
- 24.Maier CL, Pober JS. Human placental pericytes poorly stimulate and actively regulate allogeneic CD4 T cell responses. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:183–189. doi: 10.1161/ATVBAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mai J, Nanayakkara G, Lopez-Pastrana J, Li X, Li YF, Wang X, Song A, Virtue A, Shao Y, Shan H, Liu F, Autieri MV, Kunapuli SP, Iwakura Y, Jiang X, Wang H, Yang XF. Interleukin-17A Promotes Aortic Endothelial Cell Activation via Transcriptionally and Post-translationally Activating p38 Mitogen-activated Protein Kinase (MAPK) Pathway. The Journal of biological chemistry. 2016;291:4939–4954. doi: 10.1074/jbc.M115.690081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roussel L, Houle F, Chan C, Yao Y, Berube J, Olivenstein R, Martin JG, Huot J, Hamid Q, Ferri L, Rousseau S. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. Journal of immunology. 2010;184:4531–4537. doi: 10.4049/jimmunol.0903162. [DOI] [PubMed] [Google Scholar]
- 27.Yeh M, Leitinger N, de Martin R, Onai N, Matsushima K, Vora DK, Berliner JA, Reddy ST. Increased transcription of IL-8 in endothelial cells is differentially regulated by TNF-alpha and oxidized phospholipids. Arteriosclerosis, thrombosis, and vascular biology. 2001;21:1585–1591. doi: 10.1161/hq1001.097027. [DOI] [PubMed] [Google Scholar]
- 28.Xing X, Yang J, Yang X, Wei Y, Zhu L, Gao D, Li M. IL-17A induces endothelial inflammation in systemic sclerosis via the ERK signaling pathway. PloS one. 2013;8:e85032. doi: 10.1371/journal.pone.0085032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim K, Hyun YM, Lambert-Emo K, Capece T, Bae S, Miller R, Topham DJ, Kim M. Neutrophil trails guide influenza-specific CD8(+) T cells in the airways. Science. 2015;349:aaa4352. doi: 10.1126/science.aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.