Abstract
Glomerulonephritis is one of the most severe manifestations of systemic lupus erythematosus, with considerable morbidity and mortality. There remains a major unmet need for successful management of lupus nephritis. TAM family receptor tyrosine kinases (Mer and Axl) play an important role in the maintenance of immune homeostasis in the kidney. Mer is constitutively expressed in the glomeruli; Axl expression is inducible in glomeruli under inflammatory conditions. To investigate the distinct functions of Axl and Mer in lupus nephritis, we compared the severity of nephrotoxic serum glomerulonephritis in WT, Axl-KO, Mer-KO, and Axl/Mer-KO mice. Mer-KO mice developed severe glomerulonephritis, with significantly decreased survival and increased blood urea nitrogen levels, compared to WT mice given the same treatment. However, nephrotoxic serum-treated Axl-KO mice had significantly increased survival rates and improved renal function as compared to similarly treated WT, Mer-KO, and Axl/Mer-KO mice. Interestingly, mice lacking both Axl and Mer developed kidney inflammation comparable to WT mice. Western blot analysis revealed significantly increased Stat3 phosphorylation and caspase-1 activation in the kidneys of nephritic Mer-KO mice. In contrast, Axl deficient nephrotoxic serum-injected mice showed decreased Akt phosphorylation and Bcl-xl upregulation. Thus, the reciprocal activation of Axl and Mer receptor tyrosine kinases has a major impact on the outcome of renal inflammation.
Keywords: glomerulonephritis, anti-GBM, Axl, Mer
Introduction
Systemic lupus erythematosus (SLE) is a multi-system autoimmune disease. Renal involvement occurs in most patients, and glomerulonephritis is a major cause of morbidity (1). Diffuse proliferative glomerulonephritis (DPGN) carries the worst prognosis, resulting in 11-48% of patients developing end stage renal disease by five years (2). Immune complex deposition in the kidney initiates an inflammatory cascade that causes glomerular disease, but there are many modulating factors, including products of the innate immune system (3). Nephrotoxic serum (NTS)-mediated anti-glomerular basement membrane (anti-GBM) disease is a well-studied mouse model of immune-mediated glomerulonephritis, characterized by rapidly progressive renal dysfunction (4). Glomerular injury causes proteinuria and reduced glomerular filtration. Increasing evidence indicates that multiple influences, including chemokines, cytokines, neutrophils/macrophages, and genetic factors determine the severity of disease (4-7).
The innate and adaptive immune systems play important roles in the pathogenesis of glomerulonephritis. Disease susceptibility, however, is also influenced by factors intrinsic to the kidney itself. For example, intrinsic expression of tumor necrosis factor (TNF) receptor 2 on glomerular endothelial cells promotes monocyte recruitment into the kidney during anti-GBM nephritis (8). It has also been observed that mesangial cell production of pro-inflammatory cytokines following TLR3 engagement contributes to nephritis (9). A further example is the finding that resident renal cells are the dominant source of IFN-I (Type I interferon) in the anti-GBM nephritis model. Elimination of the IFN receptor ameliorates anti-GBM disease (10), underscoring the importance of IFN-I in this nephritis model.
The role of TAM (Tyro-3, Axl, and Mer) receptor tyrosine kinases signaling as negative regulators of inflammation has drawn much attention (11-13). TAM receptors are expressed primarily by myeloid cells of the immune system and achieve their immune regulatory action through phagocytosis of apoptotic debris, upregulation of key signaling suppressors, and secretion of anti-inflammatory cytokines (11, 12, 14, 15). Protein S and Gas 6 (growth arrest specific protein 6) were identified in the mid-90s as ligands for TAM receptors (16). Macrophage expression of Mer and Axl is required for the Gas6-dependent phagocytosis of apoptotic cells and regulation of immune responses (17-19). Mice lacking Mer single or TAM triple receptors developed mild or severe lupus-like autoimmune disorders, respectively (14, 15). Mer is highly expressed in glomeruli and functions in the suppression of inflammatory responses and in the clearance of apoptotic cells (5, 14). Mer-KO mice respond to inflammatory stimuli by producing increased proinflammatory mediators such as IL-1, IL-6, and TNFα (5, 20, 21). Axl, on the other hand, mediates cell survival signaling in many cell types. Binding of Gas6 to Axl leads to receptor dimerization, autophosphorylation, and activation of downstream signaling pathways including PI3 kinase/AKT and MAPKs/ERKs, resulting in pro-survival and proliferative responses, respectively (22). In both mice and humans, Axl and Gas6 are expressed by glomerular mesangial cells and by tubular cells (23-25). Previous studies using Gas6−/− mice showed a pathogenic role for Gas6 in NTS-induced nephritis and streptozotocin-induced diabetic nephropathy (26, 27). Loss of Gas6 protected against mesangial cell proliferation and glomerular hypertrophy, and led to improved proteinuria and survival (26). These data suggest that inhibitors of the Gas6/Axl pathway may be of therapeutic benefit in renal pathologies. However, the molecular regulation of these events and the progression of glomerulonephritis are not fully elucidated. In addition, the TAM-independent function of Gas6 as a coagulant versus its TAM-dependent function, as an anti-inflammatory agent, has not been experimentally dissociated.
Our previous work showed that there is accelerated and more severe NTS-nephritis in the absence of Mer (5). We now show that Axl-KO mice, in contrast, develop reduced renal inflammation with significantly improved survival rate and reduced blood urea nitrogen (BUN), as compared to NTS-treated WT, Mer-KO, and Axl/Mer-double knockout (A/M-KO) mice. Decreased Akt phosphorylation and ablated Bcl-xl expression were seen in the kidneys of Axl deficient NTS-injected mice. Furthermore, Mer deficiency led to activation of Stat3 and down-regulation of SOCS3.
Materials and Methods
Animals
The WT C57BL/6J (B6) mice were originally purchased from Jackson Laboratories (Bar Harbor, ME). Axl-KO mice were a gift from Dr. Carla V. Rothlin (Yale University, Department of Immunobiology) (28). Axl-KO mice were then bred onto Mer-KO mice (29) to generate the Axl/Mer double knock out mice, designated as A/M-KO mice. All mice were fully backcrossed onto B6 background (n>9) and were housed under specific pathogen-free conditions at the animal facilities of Temple University. All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Nephrotoxic serum (NTS)-induced nephritis
NTS serum was prepared as previously described (5). In brief, mice glomeruli were isolated by differential sieving and sonicated. Sheep were hyperimmunized with mouse glomeruli. The NTS was heat-inactivated and absorbed with murine blood cells. Age- and sex-matched mice (WT, Axl-KO, Mer-KO, and A/M-KO) were injected intravenously with 7.5ml/kg of nephrotoxic serum prepared from sheep (5). Mice were then followed with measurement of proteinuria (Uristix, Bayer Corporation, Elkhart, IN) and serum urea level using Urea Nitrogen Direct kit (Stanbio Laboratory, Boerne, TX). These animals were euthanized to obtain renal specimens at days 3-, 7-, and 14-post injection.
Kidney histology
Kidneys from each animal were embedded in OCT medium and snap-frozen in liquid nitrogen. Cryostat sections (4 μm) were stained with H&E and periodic acid-Schiff (PAS) for routine histology. The expression of proliferating cell nuclear antigen (PCNA, Invitrogen, CA) was evaluated by immunostaining. Pathological evaluation by light microscopy was done in a blinded manner.
Immunofluorescent staining
Kidney sections were incubated with 1% BSA in PBS. Sample sections were then blocked with 3% BSA in PBS. Immune complex deposition was evaluated by incubating sections from day 3 and day 14 with FITC conjugated anti-sheep and anti-mouse antibodies, respectively. Slides were mounted in anti-fading aqueous mounting medium (Biomeda Corp., Foster City, CA), and images were acquired using an Olympus BX60 fluorescence microscope equipped with camera (Center Valley, PA).
Western blot analysis
Protein from kidney tissues was extracted using a RIPA lysis buffer, and Western blot analysis was performed. After blocking non-specific binding with 5% (w/v) dry milk, membranes were incubated with the primary antibodies (anti-Mer and phospho-Mer, Axl, Bcl-2, Bcl-6, Bcl-xl, Akt and phospho-Akt, Stat3 and phospho-Stat3, SOCS3, and Caspase 1), followed by HRP-conjugated secondary antibody. All antibodies were purchased from Cell Signaling Technology (Danvers, MA) unless otherwise noted. Signals were detected using ECL. Quantitative analysis of images was performed using ImageStudio software (LI-COR Biotechnology, Lincoln, NE). For Axl phosphorylation, immunoprecipitation was performed at 4°C for 2 hr using 1 μg of anti-phosphotyrosine antibody (Biolegend, San Diego, CA). Immune complexes were collected with 50 mg of protein A-Sepharose beads (GE-Healthcare Biosciences, Pittsburgh, PA). Proteins were immunoblotted with goat rabbit anti-mouse Axl antibody.
RT-PCR
Total RNA was isolated using RNA Mini Kit (Ambion, Carlsbad, CA) from the renal cortex portion of experimental mice. cDNA was then synthesized from 2 μg of total RNA using High Capacity RNA-to-cDNA Kit (Applied Biosystems Inc. Foster City, CA). Real-time PCR was performed in StepOnePlus Applied Biosystem Real-Time machine. Primers for IL-6, IFN-γ, and GAPDH were also purchased from Applied Biosystems. Fold changes were calculated using the C method as previous reported (5).
Statistical analysis
Experiments were repeated 2-3 times and at least 5 mice were analyzed from each group. Study groups were analyzed for normal distribution using Kolmogorov-Smirnov test. Comparisons between two groups passing normality were assessed using the parametric Student t test. Data are expressed as the arithmetic mean ± SEM. Western blot data were analyzed using ImageStudio software (Li-Cor, Lincoln, NE). Intensity differences between groups were tested using the Mann-Whitney U test. Data are shown as median with interquartile range. The BUN determinations were compared using 2-way NOVA with repeated measures. A p value of less than 0.05 was considered statistically significant. Analyses were completed using Prism 6.0 for Mac (GraphPad Software) or Microsoft Excel.
Results
Expression and activation of Axl and Mer in the kidneys of nephritic mice
Mer is constitutively expressed at high levels in the kidneys of naïve mice (30, 31). In contrast, Axl expression is not detectable in the kidneys of naïve mice, yet it is up-regulated under inflammatory conditions (26, 32). We previously showed upregulation of Mer in the glomeruli of NTS-injected mice (5). In this study, we analyzed Mer and Axl activation/phosphorylation by Western blot under naïve and inflammatory conditions. Baseline expression of Mer in the kidneys of naïve WT and Axl-KO mice was associated with low-level phosphorylation (Fig. 1A), indicating a housekeeping function of Mer in the kidney. Upon injection of NTS, Mer expression/phosphorylation was significantly upregulated in the WT kidneys (Fig. 1A and B). Axl was not expressed in unmanipulated normal kidney (Fig. 1A control). Moderate Axl expression/activation was observed at early stages of kidney inflammation (Fig. 1A). High levels of Axl expression and activation were detected in the WT kidney at later stages of nephritis (Fig. 1B). Interestingly, Mer phosphorylation (day 3) in the kidneys of Axl-KO nephritic mice and Axl phosphorylation (day 14) in the kidney of Mer-KO nephritic mice were significantly lower than that in the kidneys of WT nephritic mice (Fig. 1). These data may reflect a functional interaction between Mer and Axl. Taken together, we showed for the first time Mer and Axl can be phosphorylated (activated) in the kidney of NTS injected mice and that their expression/activation levels may be regulated by each other.
Figure 1. Expression and activation of Mer and Axl in mouse kidney.
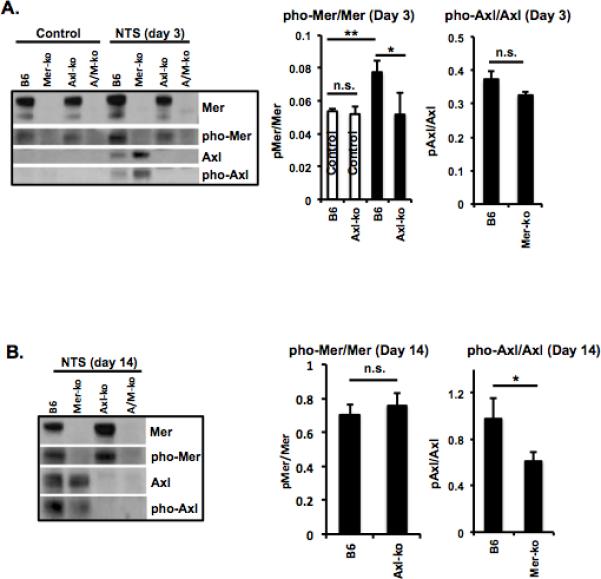
Kidneys from control (control serum-treated) and experimental (NTS-treated) mice were cut into small pieces (≤1mm) and homogenized in RIPA buffer. Mer and Axl expression and phosphorylation were analyzed by Western blot at day 3 (A) and day 14 (B) after injection. Densitometry analyses to quantify the ratio of phosphorylated Axl(Mer) to total Axl(Mer), respectively, is shown at the bottom. Representative Western images are shown. Experiments were independently repeated at least 2 times. Values are expressed as median with interquartile range. Statistical test is the Mann-Whitney test. *p<0.05.
Axl deficiency leads to nephritis resistance and enhanced survival, but Mer deficiency leads to more severe nephritis and decreased survival
We have previously shown that Mer-KO mice developed significantly more severe NTS-induced nephritis compared to WT mice (5). In this report, we expand on that observation by including Axl-KO and A/M-KO mice. Four groups of mice (WT, Axl-KO. Mer-KO, and A/M-KO) were subjected to NTS injection. As expected, Mer-KO mice developed severe glomerulonephritis, with significantly decreased survival and increased BUN levels as compared to WT mice given the same treatment ((5) and Fig. 2). Necrotic cell death was also evident in the glomeruli of Mer-KO mice with nephritis (Fig. 3A). However, Axl-KO mice were protected from nephritis and had a significantly reduced BUN (Fig. 2A) and increased survival rate (Fig. 2B) compared to the WT, Mer-KO and A/M-KO mice injected with the same serum. Interestingly, Axl and Mer double deficient mice showed an intermediate phenotype between Axl- and Mer-single knock out mice. Though BUN levels of A/M-KO mice were similar to NTS-injected WT mice (Fig. 2A), A/M-KO mice exhibited a trend for reduced survival (Fig. 2B). Pathological changes were similar between NTS-injected A/M-KO and WT mice (Fig. 3A). The differences in renal function and survival were not due to variations in the amount of NTS administered nor to anti-donor antibodies generated by host mice (Fig. 3B).
Figure 2. Opposing role of Axl and Mer in mice injected with NTS.
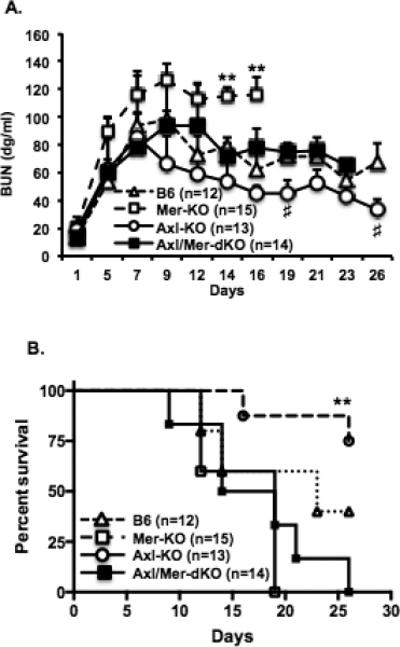
WT, Axl-KO, Mer-KO, and A/M-KO mice were injected with NTS (7.5 ml/kg of mouse body weight). A. Blood was collected by tail vein sampling during the study. Sera were prepared and stored at −20°C. BUN levels were determined using the BUN enzymatic end point test kit (Stanbio Labs). BUN was analyzed by two-way Anova with repeated measures. B. Survival rate was followed after NTS injection and analyzed with the Kaplan-Meier estimate. Data are representative of two independent experiments. **p<0.01, Mer-KO vs. B6; #P<0.05, Axl-KO vs. B6.
Figure 3. Pathological changes in the kidney of nephritic mice lacking Axl or Mer.
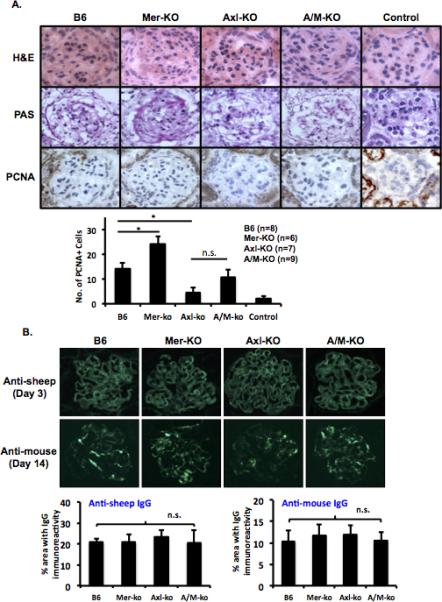
Kidneys were embedded in OCT medium and snap frozen in liquid nitrogen. Kidney sections (4μm) were prepared at different time points as indicated. A. H&E (day 14, top row), PAS (day 14, middle row), and PCNA (day 7, bottom row) stainings were performed. B. Kidney sections (4μm) were stained with FITC conjugated anti-sheep (top) and anti-mouse IgG (bottom) antibodies. Images were taken with an Olympus camera and are representative of at least three different sections from individual mice in each experiment and were evaluated in two independent experiments. Control group was treated with the same volume of sheep serum. All other groups were treated with NTS serum. Magnification: x400.
Axl-mediated glomerular cell proliferation in NTS-nephritic mice
Axl regulates proliferation and survival in many types of cancer cells (22). Gas6 induces massive proteinuria and glomerular cell proliferation and Gas6−/− mice develop less mortality and proteinuria when nephritis is induced (26). In vitro studies showed that interfering with the Gas6/Axl pathway inhibits mesangial cell proliferation (32). We wondered if preservation of kidney function observed in Axl-KO mice injected with NTS was due to the inhibition of glomerular cell proliferation. We examined cell proliferation in Axl-KO mice, WT mice, Mer-KO mice, and A/M-KO mice during the early stage of NTS-nephritis. Immunostaining for PCNA on day 7 showed intense nuclear staining in the glomeruli of WT mice (Fig. 3A). NTS-injected Mer-KO mice showed significantly increased glomerular proliferation compared to the WT mice (Fig. 3A). In contrast, and consistent with previous results from Gas6−/− mice (26), numbers of PCNA-positive cell per glomerular cross section were significantly lower in Axl-KO mice compared to NTS-injected WT and Mer-KO mice (Fig. 3A). Though there was a trend for increased proliferation in the glomeruli of A/M-KO mice compared to the glomeruli of Axl-KO mice, statistical analysis revealed no significant difference between Axl-KO and A/M-KO mice given the same treatment (Fig. 3A).
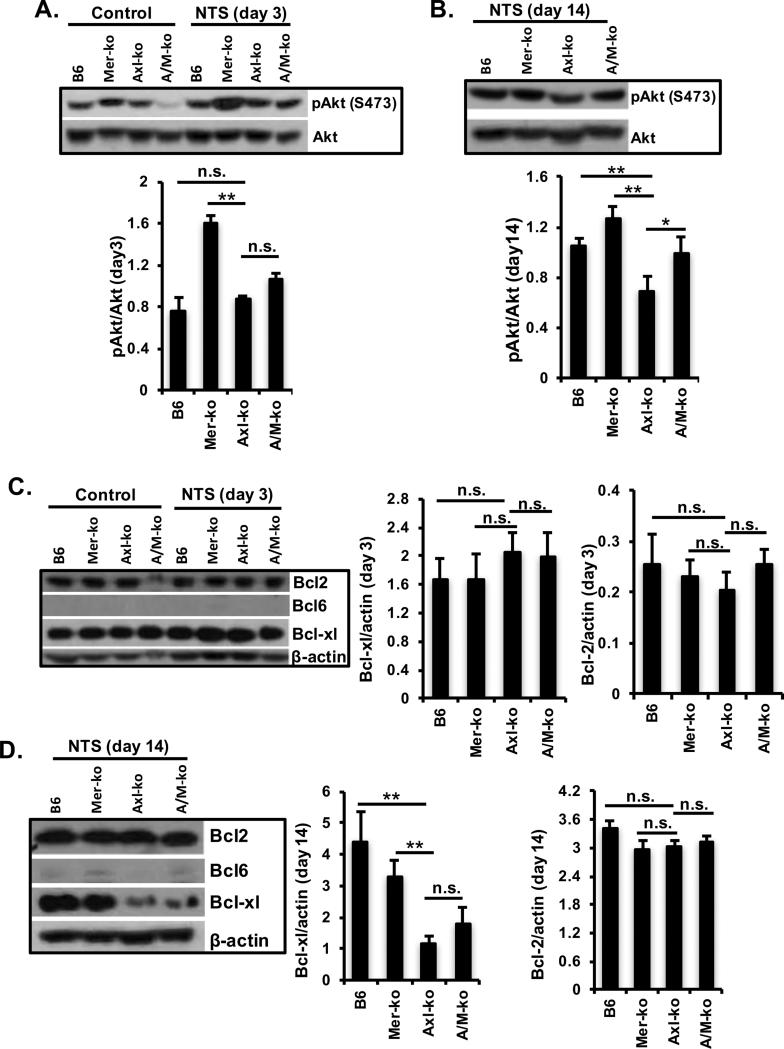
Ameliorated kidney function in Axl-KO mice was associated with Akt phosphorylation and Bcl-xl upregulation
Axl activation results in upregulation of the MAPK and PI3K/Akt signaling pathway, thereby promoting cell survival (22, 33). We observed no differences in MAPK expression and activation in the kidney when all the groups of experimental mice with nephritis were examined (data not shown). Western blot analysis of protein extracts from kidneys showed that Axl activation is associated with Akt phosphorylation and Bcl-xl upregulation after NTS injection (Fig. 4). Kidney Akt phosphorylation was no different between WT and Axl-KO mice 3 days after NTS injection (Fig. 4A). However, there was a significant decrease of Akt phosphorylation in the kidneys of Axl-KO mice when compared to the Mer-KO mice (Fig. 4A). By day 14, Akt phosphorylation was enhanced in the kidneys of WT mice, while Akt phosphorylation remained unchanged in the kidney Axl-KO mice, which resulted in a significant lower level of kidney Akt phosphorylation in the Axl-KO mice compared to all other three groups of mice under the same treatment (Fig. 4B). Bcl-2, Bcl-6, and Bcl-xl were all analyzed by Western blot. Bcl-2 remained at a constitutively high level in the kidneys of all control and experimental mice (Fig. 4C and D). Renal Bcl-6 was not detectable in any of the mice. However, Bcl-xl expression was high in the kidneys of all groups of mice, including the control group, at day 3 (Fig. 4C). Surprisingly, a significant decrease of Bcl-xl expression was observed in the kidneys of Axl-KO mice at day14 after NTS injection as compared to the WT and Mer-KO mice (Fig. 4D). Taken together, data support a housekeeping function of Bcl-2 in the kidney and an Axl-dependent regulatory role of Bcl-xl during renal inflammation.
Figure 4. Axl-mediated Akt and Bcl-xl activation in the kidney of nephritic mice.
Glomerulonephritis was induced as described. Kidney samples were homogenized and prepared in RIPA buffer. Immunoblotting analysis compared expression and activity of Axl signaling pathway molecules (Akt) between WT, Axl-KO, Mer-KO, and A/M-KO mice at day 3 (A) and day 14 (B). Bcl2, Bcl-6, and Bcl-xl expression were also analyzed at day 3 (C) and day 14 (D) after injection. Densitometry analysis to quantify the ratio of phosphorylated Akt to total Akt and Bcl proteins to β-actin, respectively, is shown. Representative Western images are shown. Experiments were independently repeated at least 2 times. Values are expressed as median with interquartile range. Statistical test is the Mann-Whitney test. **p<0.01, *p<0.05.
Mer-deficiency is associated with Stat3 phosphorylation and SOCS3 down-regulation
Signaling pathways downstream of Mer activation have been studied by multiple groups (30, 31). However, most of the results came from the receptor chimeras (34). The inhibitory role of TAM in inflammatory responses is linked to Stat1 phosphorylation and SOCS1/3 upregulation (12). Mer-mediated inhibition of LPS-induced inflammatory response is also Stat1 and SOCS1/3 dependent. Surprisingly, Stat1 phosphorylation was not observed in the kidneys of nephritic mice, regardless of high levels of Stat1 protein (data not shown). On the other hand, Stat3 was significantly activated in the kidneys of Mer-KO mice compared to WT, Axl-KO, and A/M-KO mice after NTS injection (Fig. 5A and B). Stat3 activation was associated with SOCS3 down regulation in the kidneys of the same mice. Consistent with previous findings, data shown here suggest a negative regulatory role for SOCS3 on Stat3 activation (35, 36). In addition, our previous data showed that Stat3 activation in the kidney was associated with severe nephritis in chronic graft-versus-host disease (cGVHD) (35). A small increase in pStat3 was also noted in the Axl-KO nephritic mice (Fig. 5A and B). A possible cross-regulation between Mer and Axl was indicated in the study by Seitz et al (19). Results in figure 1 may also suggest the same phenomenon. It is possible that in Axl-KO mice, Mer may not function as in the WT kidney. Impaired Mer function may lead to an increased level of Axl-KO mice kidney after NTS injection.
Figure 5. Stat pathway and caspase1 activation in the kidney of Mer-KO mice with nephritis.
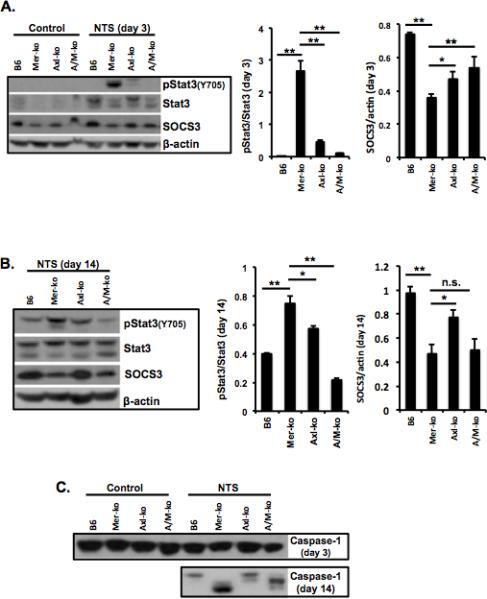
Kidney samples were prepared in RIPA buffer. Analysis of phosphorylation status of Stat3 and expression levels of SOCS3 at day 3 (A) and day 14 (B), and caspase-1 activation (C) during the development of NTS-nephritis was done by Western immunoblotting. Densitometry analysis to quantify ratio of phosphorylated Stat3 to total Stat3 and SOCS3 proteins to β-actin, respectively, is shown at the bottom. Representative Western images are shown. Experiments were independently repeated at least 2 times. Values are expressed as median with interquartile range. Statistical test is the Mann-Whitney test. **p<0.01, *p<0.05.
TAM receptors are known to mediate the homeostatic phagocytosis of apoptotic cells and moderate immune responses. We previously reported a significant increase of apoptotic cells in the kidneys of nephritic Mer-KO mice. Caspase 3 functions primarily in cell death execution (37), while caspase 1 is best established as the protease responsible for the processing of IL-1 (38). Activated caspases can be detected as cleaved fragments. We wondered if TAM deficiency alters the expression and activation of caspases involved in the process of apoptotic cell death and inflammatory cytokine production. Expression and activation of caspase 3 were no different in the kidneys of NTS injected mice among all groups (data not shown). Figure 5C showed high level cleavages of caspase 1 in the kidneys of Mer-KO and A/M-KO mice after NTS injection. Axl-KO mice displayed minimum caspase 1 cleavage in the kidney 14 days after nephritis induction (Fig. 5C). Taken together, data indicate a Mer-dependent caspase 1 inhibition in NTS-nephritis.
Differentially regulated inflammatory cytokines in nephritic Mer-KO and Axl-KO mice
Cytokines are known to play an important role during renal inflammation. Both Mer and Axl were shown to regulate inflammatory cytokine profile changes. We previously showed increased proinflammatory cytokines in the kidneys of Mer-KO mice after NTS injection. Consistent with the previous finding, we detected significantly increased IL-6 mRNA in the kidneys of Mer-KO mice compared to the WT mice 7 days after NTS administration (Fig. 6A). We also detected a significantly higher level of IFN-γ in Mer-KO nephritic mice compared to all other three groups (Fig. 6B). Levels of both IL-6 and IFN-γ were significantly lower in the kidneys of nephritic Axl-KO mice as compared to Mer-KO mice given the same treatment (Fig. 6). However, mRNA levels of IFN-γ were similar between WT, Axl-KO, and A/M-KO mice (Fig. 6B). Lower expression of inflammatory cytokines was observed in the kidneys of Axl-KO mice, when nephritis was induced, consistent with the higher survival rate and lower levels of BUN.
Figure 6. Measurement of IL-6 and IFN-γ gene expression from experimental mice with nephritis.
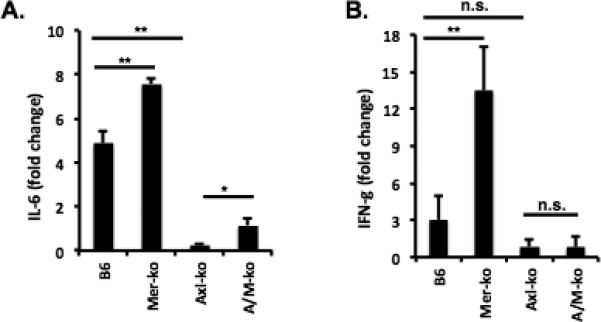
Three kidney samples from each group were homogenized for total mRNA preparation at day 8 after NTS injection. mRNA levels of IL-6 (A) and IFN-γ (B) were analyzed by real-time PCR. The fold changes are with respect to mRNA levels on control mice and were calculated after normalization against GAPDH message. One of two independent experiments is shown. Results represent the means ± SEM. *P<0.05, **P < 0.01.
Discussion
The TAM receptors have emerged as critical players in the immune response. Axl and Mer have been shown to segregate into distinct niches of expression and function in tolerance induction and inhibition of inflammation (39). We found that Axl and Mer target different pathways in the course of renal inflammation: Mer acts mainly in apoptotic cell clearance and inhibition of inflammatory cytokines, whereas Axl activation promotes glomerular cell proliferation. Mer expression was consistently high in the kidney and rose still higher after NTS injection. Axl expression was undetectable in the kidneys of naïve mice, but rose promptly upon inflammatory insult. It is noteworthy that Axl activation was significantly lower in the kidneys of Mer-KO nephritic mice, compared to Axl activation in the kidneys of nephritic WT mice, and vice versa, Mer activation was significantly lower in the kidneys of Axl-KO mice. Similarly, Seitz et al reported a diminished Mer phosphorylation from Axl/Tyro3−/− mice. Together, data suggest a functional interaction between these receptors.
Mer-deficiency associated Stat3 activation in the kidney represents a novel finding. Mer-mediated inhibition of inflammatory responses involves Stat1 activation and SOCS1/3 expression (12). Activation of the Stat pathway (Stat5 and Stat6) was also noted in Jurkat cells stimulated with Gas6 (40). We expected to see a decrease of Stat1 phosphorylation in the kidneys of Mer-KO mice after NTS injection. Instead, we observed a significant increase in Stat3 activation. Enhanced Stat3 phosphorylation was associated with SOCS3 down regulation, which reinforces the inhibitory role of SOCS3 in Stat3 activation. Similarly, enhanced Stat3 phosphorylation was observed in the kidneys of Stat1-KO mice when lupus-like cGVHD was induced (35). Stat3 activation in the kidneys of nephritic Mer-KO mice was probably due to a negative feedback from Mer-mediated Stat1 activation, as Stat1 and Stat3 reciprocally regulate each other, and play opposite roles in several aspects of inflammation (41).
Caspases are a family of endoproteases that have critical roles in maintaining homeostasis through regulating inflammation and cell death (42), representing two essential axes in glomerulonephritis. Activation of caspase1 requires cleavage, leading to the rapid secretion of IL-1β. An additional consequence of caspase1 activation in macrophages is rapid and caspase-1-dependent cell death (43). Increased IL-1 secretion and enhanced apoptotic cell death were reported previously in the kidneys of Mer-KO mice injected with NTS (35). Strikingly, Caspase1 activation (cleavage) was only observed in the kidneys of the nephritic mice lacking the Mer receptor, indicating a Mer-dependent caspase1 inhibition.
Two major ligands have been identified mediating TAM receptor signaling. Both ligands are secreted by multiple cell types and are present in mouse and human blood (44). Our data showed that Mer-mediated macrophage phagocytosis of apoptotic cells is Gas6-dependent (17). Protein S, on the other hand, was also shown to drive Mer-dependent phagocytosis in mouse bone marrow-derived macrophages (45). However, only Gas6 was able to drive Axl-dependent phagocytosis (39). A novel function of Gas6 in the development of nephritis has been studied (46). Gas6 was also shown to promote mesangial cell proliferation in in vitro study (47). Data published to date suggest that Axl-mediated glomerular proliferation is Gas6 dependent.
Increased serum levels of soluble Mer and Axl have been observed in lupus patients by several groups (48-50). Lee et al (51) found that elevated levels of soluble TAM receptors were more evident in patients with progressive renal failure. Significantly increased levels of TAM may reflect decreased membrane TAM receptors, which represent important factors in phagocytic clearance of apoptotic cells generated during inflammation, including nephritis. The importance of Gas6/Axl pathway has also been implicated in many types of kidney diseases (25). Inhibition of Gas6 has been proved to be beneficial in nephritis (26, 32). However, Gas6, a coagulation factor in the blood, plays an important role in platelet aggregation (52). Long-term inhibition of Gas6 may cause severe side effects, including prolonged prothrombin times, anemia, and bleeding tendency. In the present work, we showed a significantly improved survival rate and preservation of renal function in Axl-KO mice compared to all other groups of mice given the same disease induction. These findings suggest that agents blocking Axl function might be useful in treating nephritis. Several therapeutics targeting Axl are under development in the field of cancer research (53). Recently, three small kinase inhibitors for Axl have entered clinical trials. However, not all inhibitors have activity limited to Axl. Systemic administration of Axl inhibitors requires close monitoring, as Mer plays an opposite effect in renal inflammation, due to its anti-inflammatory activities. Results from the clinical trials in cancer research have a potential therapeutic application in lupus nephritis patients. Further investigations on the role of the Gas6/Axl pathway in human kidney diseases and the development of specific antagonists targeting the pathway are warranted.
Acknowledgement
We are grateful to Dr. Philip L. Cohen for critical reading of the manuscript and to Dr. Daohai Yu for the assistance with statistical analysis.
This work was supported by NIH grant DK095067 to W.H.S.
Abbreviations
- SLE
systemic lupus erythematosus
- TAM
Tyro-3, Axl, and Mer
- DPGN
Diffuse proliferative glomerulonephritis
- NTS
Nephrotoxic serum
- GBM
glomerular basement membrane
- BUN
blood urea nitrogen
- PCNA
proliferating cell nuclear antigen
- PAS
periodic acid-Schiff
References
- 1.Davidson A. What is damaging the kidney in lupus nephritis? Nat Rev Rheumatol. 2015 doi: 10.1038/nrrheum.2015.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mok CC, Wong RW, Lai KN. Treatment of severe proliferative lupus nephritis: the current state. Ann Rheum Dis. 2003;62:799–804. doi: 10.1136/ard.62.9.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davidson A, Aranow C. Pathogenesis and treatment of systemic lupus erythematosus nephritis. Curr Opin Rheumatol. 2006;18:468–475. doi: 10.1097/01.bor.0000240356.45550.13. [DOI] [PubMed] [Google Scholar]
- 4.Du Y, Fu Y, Mohan C. Experimental anti-GBM nephritis as an analytical tool for studying spontaneous lupus nephritis. Arch Immunol Ther Exp (Warsz) 2008;56:31–40. doi: 10.1007/s00005-008-0007-4. [DOI] [PubMed] [Google Scholar]
- 5.Shao WH, Zhen Y, Rosenbaum J, Eisenberg RA, McGaha TL, Birkenbach M, Cohen PL. A protective role of Mer receptor tyrosine kinase in nephrotoxic serum-induced nephritis. Clin Immunol. 2010;136:236–244. doi: 10.1016/j.clim.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenderfer SE, Dubinsky WP, Hernandez-Sanabria M, Braun MC. Urine proteome analysis in murine nephrotoxic serum nephritis. Am J Nephrol. 2009;30:450–458. doi: 10.1159/000242430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanafusa N, Sogabe H, Yamada K, Wada T, Fujita T, Nangaku M. Contribution of genetically engineered animals to the analyses of complement in the pathogenesis of nephritis. Nephrol Dial Transplant 17 Suppl. 2002;9:34–36. doi: 10.1093/ndt/17.suppl_9.34. [DOI] [PubMed] [Google Scholar]
- 8.Venkatesh D, Ernandez T, Rosetti F, Batal I, Cullere X, Luscinskas FW, Zhang Y, Stavrakis G, Garcia-Cardena G, Horwitz BH, Mayadas TN. Endothelial TNF receptor 2 induces IRF1 transcription factordependent interferon-beta autocrine signaling to promote monocyte recruitment. Immunity. 2013;38:1025–1037. doi: 10.1016/j.immuni.2013.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka H, Imaizumi T. Inflammatory chemokine expression via Toll-like receptor 3 signaling in normal human mesangial cells. Clin Dev Immunol. 2013;2013:984708. doi: 10.1155/2013/984708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fairhurst AM, Xie C, Fu Y, Wang A, Boudreaux C, Zhou XJ, Cibotti R, Coyle A, Connolly JE, Wakeland EK, Mohan C. Type I interferons produced by resident renal cells may promote end-organ disease in autoantibody-mediated glomerulonephritis. J Immunol. 2009;183:6831–6838. doi: 10.4049/jimmunol.0900742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Rothlin CV, Lemke G. TAM receptor signaling and autoimmune disease. Curr Opin Immunol. 2010;22:740–746. doi: 10.1016/j.coi.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen PL, Caricchio R, Abraham V, Camenisch TD, Jennette JC, Roubey RA, Earp HS, Matsushima G, Reap EA. Delayed apoptotic cell clearance and lupus-like autoimmunity in mice lacking the c-mer membrane tyrosine kinase. J Exp Med. 2002;196:135–140. doi: 10.1084/jem.20012094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu Q, Lemke G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro 3 family. Science. 2001;293:306–311. doi: 10.1126/science.1061663. [DOI] [PubMed] [Google Scholar]
- 16.Rothlin CV, Carrera-Silva EA, Bosurgi L, Ghosh S. TAM receptor signaling in immune homeostasis. Annu Rev Immunol. 2015;33:355–391. doi: 10.1146/annurev-immunol-032414-112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao WH, Zhen Y, Eisenberg RA, Cohen PL. The Mer receptor tyrosine kinase is expressed on discrete macrophage subpopulations and mainly uses Gas6 as its ligand for uptake of apoptotic cells. Clin Immunol. 2009;133:138–144. doi: 10.1016/j.clim.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishi C, Toda S, Segawa K, Nagata S. Tim4- and MerTKmediated engulfment of apoptotic cells by mouse resident peritoneal macrophages. Mol Cell Biol. 2014;34:1512–1520. doi: 10.1128/MCB.01394-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seitz HM, Camenisch TD, Lemke G, Earp HS, Matsushima GK. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J Immunol. 2007;178:5635–5642. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- 20.Guttridge KL, Luft JC, Dawson TL, Kozlowska E, Mahajan NP, Varnum B, Earp HS. Mer receptor tyrosine kinase signaling: prevention of apoptosis and alteration of cytoskeletal architecture without stimulation or proliferation. J Biol Chem. 2002;277:24057–24066. doi: 10.1074/jbc.M112086200. [DOI] [PubMed] [Google Scholar]
- 21.Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162:3498–3503. [PubMed] [Google Scholar]
- 22.Axelrod H, Pienta KJ. Axl as a mediator of cellular growth and survival. Oncotarget. 2014;5:8818–8852. doi: 10.18632/oncotarget.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiebeler A, Park JK, Muller DN, Lindschau C, Mengel M, Merkel S, Banas B, Luft FC, Haller H. Growth arrest specific protein 6/Axl signaling in human inflammatory renal diseases. Am J Kidney Dis. 2004;43:286–295. doi: 10.1053/j.ajkd.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Lee IJ, Hilliard B, Swami A, Madara JC, Rao S, Patel T, Gaughan JP, Lee J, Gadegbeku CA, Choi ET, Cohen PL. Growth arrest-specific gene 6 (Gas6) levels are elevated in patients with chronic renal failure. Nephrol Dial Transplant. 2012;27:4166–4172. doi: 10.1093/ndt/gfs337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yanagita M. Gas6, warfarin, and kidney diseases. Clin Exp Nephrol. 2004;8:304–309. doi: 10.1007/s10157-004-0305-z. [DOI] [PubMed] [Google Scholar]
- 26.Yanagita M, Ishimoto Y, Arai H, Nagai K, Ito T, Nakano T, Salant DJ, Fukatsu A, Doi T, Kita T. Essential role of Gas6 for glomerular injury in nephrotoxic nephritis. J Clin Invest. 2002;110:239–246. doi: 10.1172/JCI14861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagai K, Matsubara T, Mima A, Sumi E, Kanamori H, Iehara N, Fukatsu A, Yanagita M, Nakano T, Ishimoto Y, Kita T, Doi T, Arai H. Gas6 induces Akt/mTOR-mediated mesangial hypertrophy in diabetic nephropathy. Kidney Int. 2005;68:552–561. doi: 10.1111/j.1523-1755.2005.00433.x. [DOI] [PubMed] [Google Scholar]
- 28.Bosurgi L, Bernink JH, Delgado Cuevas V, Gagliani N, Joannas L, Schmid ET, Booth CJ, Ghosh S, Rothlin CV. Paradoxical role of the proto-oncogene Axl and Mer receptor tyrosine kinases in colon cancer. Proc Natl Acad Sci U S A. 2013;110:13091–13096. doi: 10.1073/pnas.1302507110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shao WH, Eisenberg RA, Cohen PL. The Mer receptor tyrosine kinase is required for the loss of B cell tolerance in the chronic graft-versus-host disease model of systemic lupus erythematosus. J Immunol. 2008;180:7728–7735. doi: 10.4049/jimmunol.180.11.7728. [DOI] [PubMed] [Google Scholar]
- 30.Graham DK, Bowman GW, Dawson TL, Stanford WL, Earp HS, Snodgrass HR. Cloning and developmental expression analysis of the murine c-mer tyrosine kinase. Oncogene. 1995;10:2349–2359. [PubMed] [Google Scholar]
- 31.Graham DK, Dawson TL, Mullaney DL, Snodgrass HR, Earp HS. Cloning and mRNA expression analysis of a novel human protooncogene, c-mer. Cell Growth Differ. 1994;5:647–657. [PubMed] [Google Scholar]
- 32.Yanagita M, Ishii K, Ozaki H, Arai H, Nakano T, Ohashi K, Mizuno K, Kita T, Doi T. Mechanism of inhibitory effect of warfarin on mesangial cell proliferation. J Am Soc Nephrol. 1999;10:2503–2509. doi: 10.1681/ASN.V10122503. [DOI] [PubMed] [Google Scholar]
- 33.Lemke G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol. 2013;5:a009076. doi: 10.1101/cshperspect.a009076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cummings CT, Deryckere D, Earp HS, Graham DK. Molecular pathways: MERTK signaling in cancer. Clin Cancer Res. 2013;19:5275–5280. doi: 10.1158/1078-0432.CCR-12-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao WH, Gamero AM, Zhen Y, Lobue MJ, Priest SO, Albandar HJ, Cohen PL. Stat1 Regulates Lupus-like Chronic Graft-versus-Host Disease Severity via Interactions with Stat3. J Immunol. 2015;195:4136–4143. doi: 10.4049/jimmunol.1501353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong F, Jaruga B, Kim WH, Radaeva S, El-Assal ON, Tian Z, Nguyen VA, Gao B. Opposing roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation by SOCS. J Clin Invest. 2002;110:1503–1513. doi: 10.1172/JCI15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- 38.Denes A, Lopez-Castejon G, Brough D. Caspase-1: is IL-1 just the tip of the ICEberg? Cell Death Dis. 2012;3:e338. doi: 10.1038/cddis.2012.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zagorska A, Traves PG, Lew ED, Dransfield I, Lemke G. Diversification of TAM receptor tyrosine kinase function. Nat Immunol. 2014;15:920–928. doi: 10.1038/ni.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandao LN, Winges A, Christoph S, Sather S, Migdall-Wilson J, Schlegel J, McGranahan A, Gao D, Liang X, Deryckere D, Graham DK. Inhibition of MerTK increases chemosensitivity and decreases oncogenic potential in T-cell acute lymphoblastic leukemia. Blood Cancer J. 2013;3:e101. doi: 10.1038/bcj.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wan CK, Andraski AB, Spolski R, Li P, Kazemian M, Oh J, Samsel L, Swanson PA, 2nd, McGavern DB, Sampaio EP, Freeman AF, Milner JD, Holland SM, Leonard WJ. Opposing roles of STAT1 and STAT3 in IL- 21 function in CD4+ T cells. Proc Natl Acad Sci U S A. 2015;112:9394–9399. doi: 10.1073/pnas.1511711112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Man SM, Kanneganti TD. Converging roles of caspases in inflammasome activation, cell death and innate immunity. Nat Rev Immunol. 2016;16:7–21. doi: 10.1038/nri.2015.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winkler S, Rosen-Wolff A. Caspase-1: an integral regulator of innate immunity. Semin Immunopathol. 2015;37:419–427. doi: 10.1007/s00281-015-0494-4. [DOI] [PubMed] [Google Scholar]
- 44.Linger RM, Keating AK, Earp HS, Graham DK. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu H, Shacter E. Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells. Nat Immunol. 2003;4:87–91. doi: 10.1038/ni871. [DOI] [PubMed] [Google Scholar]
- 46.Yanagita M. The role of the vitamin K-dependent growth factor Gas6 in glomerular pathophysiology. Curr Opin Nephrol Hypertens. 2004;13:465–470. doi: 10.1097/01.mnh.0000133981.63053.e9. [DOI] [PubMed] [Google Scholar]
- 47.Yanagita M, Arai H, Ishii K, Nakano T, Ohashi K, Mizuno K, Varnum B, Fukatsu A, Doi T, Kita T. Gas6 regulates mesangial cell proliferation through Axl in experimental glomerulonephritis. Am J Pathol. 2001;158:1423–1432. doi: 10.1016/S0002-9440(10)64093-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zizzo G, Guerrieri J, Dittman LM, Merrill JT, Cohen PL. Circulating levels of soluble MER in lupus reflect M2c activation of monocytes/macrophages, autoantibody specificities and disease activity. Arthritis Res Ther. 2013;15:R212. doi: 10.1186/ar4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ballantine L, Midgley A, Harris D, Richards E, Burgess S, Beresford MW. Increased soluble phagocytic receptors sMer, sTyro3 and sAxl and reduced phagocytosis in juvenile-onset systemic lupus erythematosus. Pediatr Rheumatol Online J. 2015;13:10. doi: 10.1186/s12969-015-0007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu H, Sun X, Zhu L, Hu F, Shi L, Fan C, Li Z, Su Y. Different expression patterns and clinical significance of mAxl and sAxl in systemic lupus erythematosus. Lupus. 2014;23:624–634. doi: 10.1177/0961203314520839. [DOI] [PubMed] [Google Scholar]
- 51.Lee IJ, Hilliard BA, Ulas M, Yu D, Vangala C, Rao S, Lee J, Gadegbeku CA, Cohen PL. Monocyte and plasma expression of TAM ligand and receptor in renal failure: Links to unregulated immunity and chronic inflammation. Clin Immunol. 2015;158:231–241. doi: 10.1016/j.clim.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Angelillo-Scherrer A, de Frutos P, Aparicio C, Melis E, Savi P, Lupu F, Arnout J, Dewerchin M, Hoylaerts M, Herbert J, Collen D, Dahlback B, Carmeliet P. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat Med. 2001;7:215–221. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 53.Myers SH, Brunton VG, Unciti-Broceta A. AXL Inhibitors in Cancer: A Medicinal Chemistry Perspective. J Med Chem. 2015 doi: 10.1021/acs.jmedchem.5b01273. [DOI] [PubMed] [Google Scholar]