SUMMARY
CD8 T cells must integrate antigenic and inflammatory signals to differentiate into efficient effector and memory T cells able to protect us from infections. The mechanisms by which T cell receptor (TCR) signaling and pro-inflammatory cytokine receptor signaling cooperate in these processes are poorly defined. Herein, we show that IL-12 and other pro-inflammatory cytokines transduce signals through the TCR signalosome in a manner that requires Fyn activity and self-peptide-MHC (self-pMHC) interactions. This mechanism is crucial for CD8 innate T cell functions. Loss of Fyn activity or blockade of self-pMHC interactions severely impaired CD8 T cell IFNγ and NKG2D expression, proliferation and cytotoxicity upon cytokine mediated bystander activation. Most importantly, in the absence of self-pMHC interactions, CD8 memory T cells fail to undergo bystander activation upon an unrelated infection. Thus, CD8 T cell bystander activation while independent of cognate antigen still requires self-pMHC and TCR signaling.
INTRODUCTION
In the context of infection, pathogen specific CD8 T cells integrate TCR, IL-12 and/or Type I IFN pro-inflammatory cytokine signals to differentiate and exert effector functions(1). This ultimately aids in the clearance of the pathogen and ensures the generation of pathogen-specific memory T cells. It is unclear how TCR and pro-inflammatory cytokine signals synergize within the CD8 T cell to dictate its fate and its response along a pathogenic immune response. TCR and pro-inflammatory cytokine receptor signaling pathways are indeed unique and independent of each other. Thus, upon antigen recognition by the TCR, the ITAMs of the TCR/CD3 complex become phosphorylated by Src kinases (Fyn and Lck) in a process that is enhanced by the co-receptor CD8(2). This results in the recruitment and activation of the kinase ZAP-70. Activated ZAP-70, then, mediates the phosphorylation of the LAT/SLP-76 signalosome which nucleates the activation of several signaling pathways including NFκB, MAPKs, and PI3K/Akt/mTOR(3). By contrast, IL-12R or Type I IFNR signaling involves the activation of Tyk2 and Janus kinases, which leads to the recruitment, phosphorylation and dimerization of STAT proteins to regulate gene expression(4). Crosstalk between cytokine receptors and the TCR for activation of downstream p38MAPK, PI3K/Akt/mTOR, signaling pathways has been previously reported for IL-12(5, 6). Similarly, cooperation of both signals at the level of gene regulation and chromatin remodeling has also been shown(7). However, whether TCR and cytokine receptor signals are able to cooperate in the most membrane proximal signaling events or share similar early signaling intermediates is unknown.
Effector and memory CD8 T cells can be activated in the absence of cognate antigen by pro-inflammatory cytokines such as IL-12, type I IFNs and IL-18 in a process referred to as bystander or innate CD8 activation(8, 9). This type of CD8 activation is relevant in the context of heterologous infections, where memory CD8 T cells raised against a specific pathogen can become activated upon infection with an unrelated pathogen, even in the absence of cross-reactivity(8, 10). CD8 T cell bystander activation results in rapid IFNγ production, proliferation and upregulation of NKG2D and CD25(11). In some cases, this is beneficial as it can lead to increased protective immunity against pathogens and tumors(8).
Mechanistically, it remains unclear how cytokine mediated CD8 bystander activation occurs. Expression of IL-12R or Type I IFNR is required on the surface of effector or memory CD8 T cells(8) that are undergoing bystander activation, suggesting that canonical Jak/STAT signaling is involved. In agreement with this, a partial role for STAT4 in innate activation of LCMV-specific CD8 effector cells has recently been shown. Yet, a detailed map of the signaling events required is missing(12). On the other hand, it has also been reported that cytokine signaling can regulate the sensitivity of TCR to self(13). Hence, while it is generally accepted that cognate antigen is not required for bystander CD8 T cell activation, a potential role for TCR signaling may still be argued and has not been fully addressed to date.
In this study, we hypothesized that pro-inflammatory cytokine signals crosstalk with very proximal TCR signaling events to modulate CD8 T cell responses. We have investigated the ability of different cytokines to induce activation of TCR’s membrane proximal signaling events. Our data, unexpectedly, shows that pro-inflammatory cytokine receptor signals utilize components exclusive to the TCR/CD3 signalosome to mediate bystander effector/memory CD8 T cell responses.
MATERIALS AND METHODS
Mice and reagents
C57BL/6 (B6), B6Rag−/−, B6β2m−/−, B6.SJL (CD45.1+), OT-I mice were bred and maintained in accordance with University of Missouri Office of Animal Resources Animal Care and Use Committee. Lymph Nodes and spleens from OT-I IL-12Rβ1−/− were provided by Mathew Mescher (University of Minnesota). OT-I Lckind and OT-I FynKO lymph nodes and spleens were provided by Rose Zamoyska (University of Edinburgh). C57BL/6 MHC class I (Db−/−, Kb−/− CD3δ−/−) deficient mouse hosts were from Adam Schrum and Diana Gil (Mayo Clinic, MN). OVA peptide was from New England Peptides. Mouse IL-6, IL-7, IL-10, IL-12 and IL-15, were from PeproTech. IL-23, NKG2D (CX5), ICAM-1 (YN1), Eomes (Dan11) and anti-Vα2 (B20.1) were from eBioscience. IL-18 was from Medical and Biological Laboratories and IFNα was from PBL Assay Science. IL-2 (clone X63), anti- phosphotyrosine (4G10) and anti-CD3ζ (H146) antibodies were produced from hybridoma cultures. Antibodies anti-phospho-Erk (197G2), p38, Akt, STAT-4 (D2E4), p65, mTOR, Src (D49G4) and anti- Lck, Fyn and cleaved Caspase 3 were from Cell Signaling. Anti-α-tubulin was from Sigma. Antibodies for immunoprecipitation of Fyn (15) and Lck (3A5) as well as T-bet (4B10) were from Santa Cruz Biotechnologies. Antibodies against CD8β (53-5.8), IL-12Rβ1, anti-CD3ε (2C11), IFNγ (XMG1.2), CD8α, CD4, CD25, CD44, CD62L, CD69 as well as Annexin V and 7-AAD were from BD. Antibody against H-2 Kb bound to SIINFEKL (25-D1.16) was from BioLegend whereas blocking antibody anti-H-2Kb (Y3) was a gift from Marc Jenkins (University of Minnesota. CFSE dye was from EMD Millipore. Granzyme B from Invitrogen, Src Family Kinase inhibitor PP2 was from Enzo, Jak2 (B42) and Tyk2 (A1) Tyrphostin inhibitors were from Sigma.
CTL culture, stimulations and Immunoblotting
1 × 107/mL splenocytes from polyclonal B6 mice or OT-I rag+/+ mice were activated with 1 µg/mL anti-CD3ε and anti-CD28 (eBioscience) or 20nM of OVA peptide (OVAp) respectively, in complete media to establish CTL cultures. IL-2 (50 U/mL) was added at 24 hour and cells were kept at a concentration of 1 × 106/mL until the time of IL-12 stimulation. At day 2, cell cultures are washed and resuspended in fresh complete media with IL-2. At day 3, CTLs were purified by ficoll, re-suspended at 2.5 × 107/mL without IL-2 and tested for residual presence of the antigen and/or pre-treated with blocking antibodies or small molecule inhibitors as indicated. For in vitro biochemical studies, day 3 CTLs were stimulated with IL-12 (20ng/mL) unless otherwise indicated, followed by lysis in ice-cold buffer (25 mM Tris pH 7.6, 150 mM NaCl, 5 mM EDTA, 1 mM EGTA, 1 mM β-glycerophosphate, 1 % IGEPAL, 1ug/mL each of Leupeptin, pepstatin and aprotinin, 1 mM NaF, 1mM Na3VO4, 1 mM PMSF) for 1 h. Cell lysates were subjected to PAGE-SDS and immunoblotting as in (14).
Densitometry
Densitometry of protein bands in immunoblots was performed on non-saturated films as defined by Image J software and determined using Image J and Photoshop CC2015. Identification of the phosphorylated protein from total phosphotyrosine blots (4G10/P-Y) was performed by molecular weight and after reprobing and blotting with specific antibodies. Densitometry values shown in the figures is relative to unstimulated cells and was corrected for loading control protein shown below the phosphorylated protein blot in the figures. Loading controls for phosphorylated bands were determined on the same membrane after stripping and reprobing for the unphosphorylated proteins with antibodies specific for the protein in question and/or tubulin. When this was not possible, loading was assessed on parallel gels from the same experiment run with equal volumes and in the same order.
Flow cytometry
Surface and intracellular staining was performed as in (15). Brefeldin A (3µg/mL) was used for the last 2–6 hours of culture prior to IFNγ staining. For proliferation assays, cells were labeled cells with 1µM CFSE as described previously in (14). Data were acquired either on a FACSCalibur or LSR II (BD) and analyzed with FlowJo software (TreeStar).
Cytotoxicity assay
Briefly, CFSE and cleaved-caspase 3-based assay was designed following previous published work(13, 16). Day 4 OT-I CTLs pretreated with or without PP2 were stimulated or not with IL-12 (20ng/mL). They were then plated together with CFSE-labeled (250 nM) EL-4 targets at different effector to target ratios (determined by flow cytometry), spun and incubated at 37°C for 5 hours. CFSE labeled targets pulsed with null peptide and 1%Triton treated CFSE labeled targets were used as negative (spontaneous lysis) and positive controls (maximal lysis) respectively. % Specific Lysis was calculated as described in (15). Sample values were performed in triplicate in 96-well U-bottom plates for each independent experiment.
Immunoprecipitation
1.5 × 107 cells were stimulated, lysed in a 1% NP-40 buffer and subjected to immunoprecipitation with anti-Fyn or anti-Lck antibodies as described in (17).
Adoptive transfers and Infections
B6.SJL (CD45.1+) mice containing or not OT-1 donor cells were immunized with Listeria Monocytogenes (LM) expressing OVA (1 × 104 CFU/mouse) or VSV or VSV-OVA (2 × 106 pfu/mouse) and used for in vitro and in vivo experiments at memory. For these experiments OT-1 or polyclonal CD8 memory T cells were enriched by negative selection (Miltenyi) from spleen and lymph nodes and gated on CD44hi, IL-7Rhi, Kb-VSV or -OVA tetramer+ CD8+. For heterologous infections, 5 × 104 polyclonal VSV specific memory cells purified cells were adoptively transferred to congenic B6 or MHC class I deficient hosts. The next day, mice were infected with 1 × 105 CFU LM. Spleen and lymph nodes were harvested 16 hours later for ex vivo analysis.
Statistics
Statistical tests were performed with Prism software (Graphpad) and unless otherwise indicated are two-tailed, unpaired Student’s t-tests assuming unequal variance with α set to P ≤ 0.05.
RESULTS
IL-12 and other pro-inflammatory cytokines induce TCR signal transduction
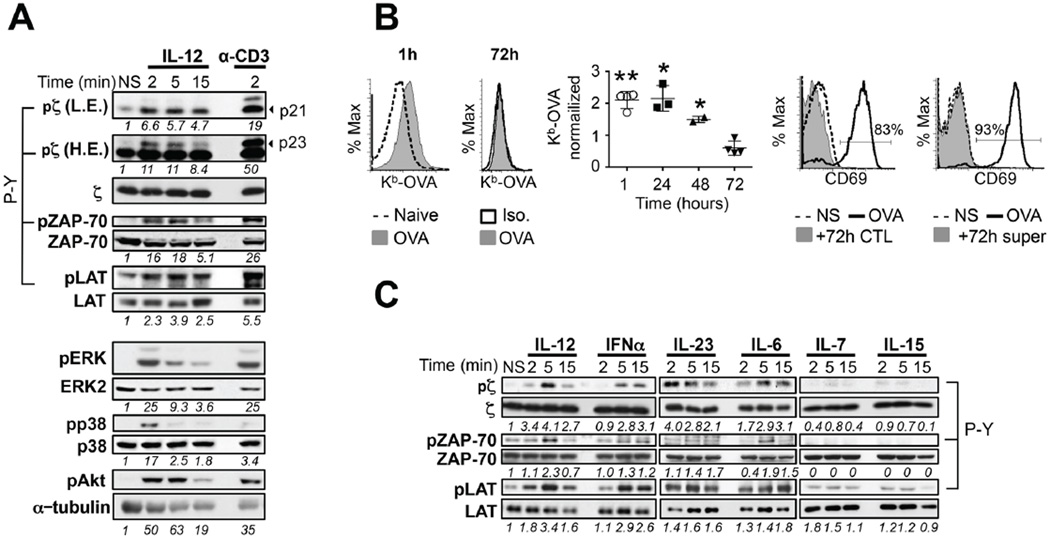
IL-12 and Type I IFN consolidate the programming initiated by antigenic and costimulatory signals by increasing histone acetylation and allowing continued expression of genes key for effector function and memory development(7). We tested whether antigenic and inflammatory cytokine signals would also cooperate at the most membrane proximal events. For this, we stimulated OT-1 CD8 effector T cells (CTLs) with IL-12 or anti-CD3 (positive control). Strikingly, we observed that IL-12-stimulated CTLs were able to induce the phosphorylation of CD3ζ (in both forms p21 and p23), an exclusive component of the TCR/CD3 complex that is required to initiate TCR signaling(18) (Fig. 1A). Transduction of TCR signaling was productive, as we could detect downstream phosphorylation of ZAP-70, its substrate LAT and also induction of the signaling pathways ERK, NFκB, Akt and p38MAPK (Fig. 1, 5A). This was not due, though, to residual presence of the antigen OVA at the time of IL-12 stimulation (72h), as Kb-OVA surface expression in the 72h OT-1 CTL culture was undetectable and co-cultured of these cells or culture supernatants with naïve OVA specific OT-1 T cells did not up-regulate expression of the early activation marker CD69 (Fig. 1B).
Figure 1. Pro-inflammatory cytokines activate TCR signaling in CD8 T cells.
(A) Day 3 OT-I CTLs were stimulated or not (NS) with 10 ng/mL IL-12 or through the TCR with cross-linked anti-CD3 antibodies for the indicated times. Tyrosine phosphorylation of p21 and p23 forms of CD3ζ (pζ), ZAP-70 (pZAP-70) and LAT (pLAT) was determined by immunoblot with anti-phosphoTyr antibody (4G10 or P-Y). Densitometry values are shown separately for the p21 and p23 forms. Phosphorylation (p-) of ERK, p38 and Akt was determined by immunoblot with antibodies specific for the phosphorylated forms of the kinases. Loading controls were assessed on parallel gels for CD3ζ, ZAP-70 and LAT and on the same membrane after stripping for ERK2, p38 and tubulin. Low (L.E.) and high (H.E.) exposures are displayed for CD3ζ. (B) Histograms for Kb-OVA expression at 1h and 72h OT-1 CTL cultures were obtained upon staining with the Kb-OVA specific antibody 25-D1.16 (Left). OVA peptide bound to H-2Kb expression normalized to non-pulsed OVA T cell cultures is shown in the graph as fold induction (Middle). Naive congenic OT-I T cells were co-cultured with day 3 OT-I CTLs or supernatant from day 3 OT-1 CTL cultures for 3 hours. CD69 expression was measured on naïve congenic OT-1 cells by flow cytometry. CD69 expression of OT-1 naïve cells stimulated with 20 nM OVA is shown as a positive control. Data are representative of n≥ 3 independent experiments. (C) Day 3 Polyclonal CTLs were purified by magnetic bead enrichment and stimulated or not with 10 ng/mL of the indicated cytokine or 5000 U/mL IFNα for the indicated time. Immunoblots were performed as in (A). CD3ζ, ZAP-70 and LAT loading controls as in (A). Shown are representatives of n=3–9 experiments per cytokine. Densitometry values of phosphorylated protein normalized to total protein and relative to their respective NS controls are shown below the panel. All graphs show mean±SD. * p < 0.05 or **p= 0.005.
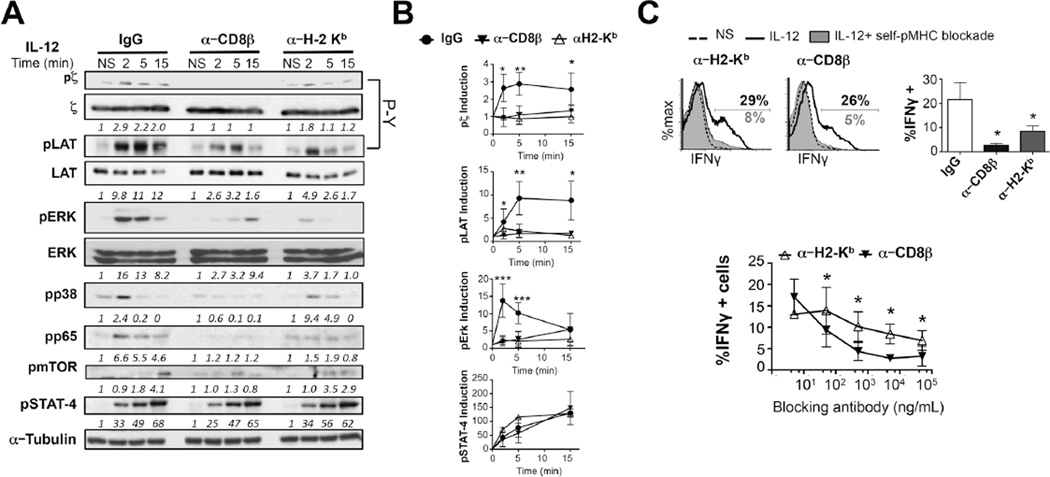
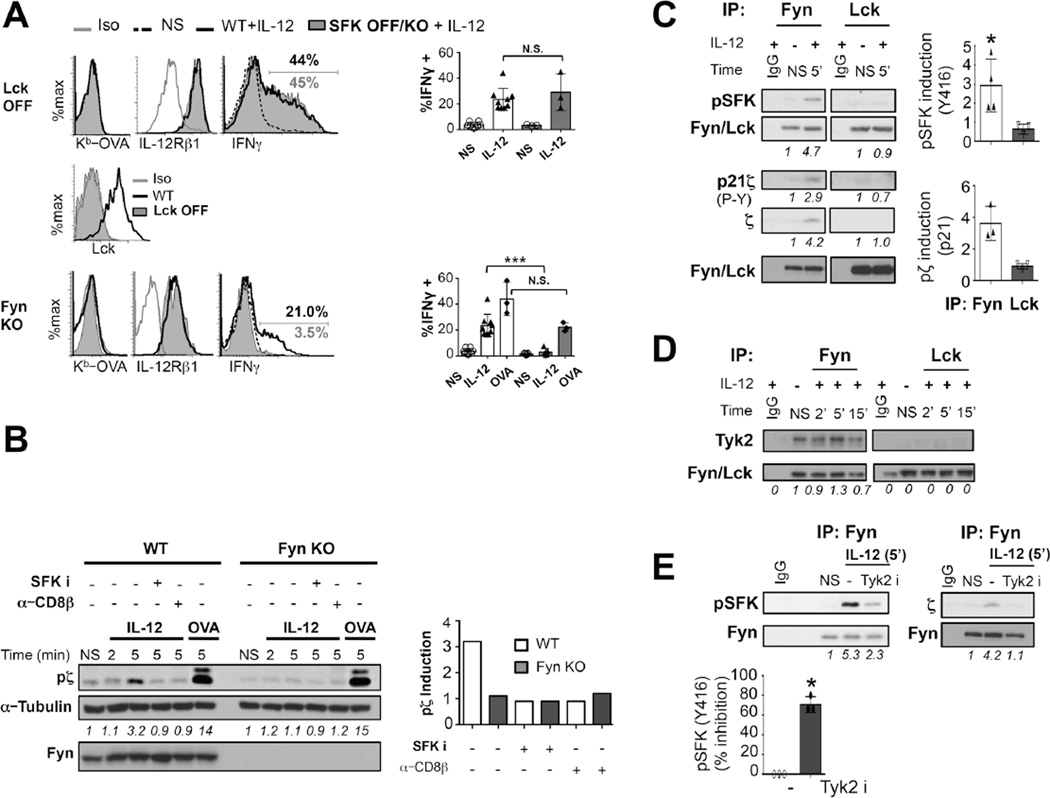
Figure 5. Self-pMHC, and CD8 are required for IL-12-dependent TCR signaling and bystander IFNγ expression.
(A) OT-I CTLs were pre-treated with blocking antibodies against CD8β (5 µg/mL) or H-2Kb (5µg/mL) or isotype controls (IgG) previous to stimulation with 10 ng/mL IL-12. Induction in the levels of pζ (4G10), pZAP-70, pLAT, pERK, p-p38MAPK, p-p65, p-mTOR (S2481) and pSTAT4 were assessed by immunoblot. p21CD3 ζ, LAT and ERK loading was determined following stripping of the same membranes. (B) Densitometry values of each of these phosphoproteins relative to the NS control and normalized to total level of the protein or α-tubulin are shown below the panels or in the graphs. (C) OT-1CTLs from (A) were treated with 5–50,000 ng/mL of the blocking antibodies followed by 6 h of IL-12 stimulation. IFNγ expression was assessed by flow cytometry. All graphs show mean±SD. Data is representative of 3–6 independent experiments. *p < 0.05 **p < 0.005 or ***p < 0.0005. N.S. (non significant).
Next, we tested whether other cytokines could induce TCR signaling. We stimulated polyclonal CTLs with cytokines for which they express receptors (IL-12, IFNα, IL-23, IL-6, IL-7 or IL-15) (Supplemental Fig. 1). Not only IL-12 but also the pro-inflammatory cytokines, IFNα, IL-23 and IL-6 induced the phosphorylation of CD3ζ, ZAP-70 and LAT between 1.5–4 fold over non-stimulated control cells. IL-12 and IL-23 were the most potent stimulators of TCR signaling. On the contrary, homeostatic cytokines IL-7 and IL-15 did not induce any detectable phosphorylation of CD3ζ, ZAP-70 or LAT (Fig. 1C, Supplemental Fig. 1). These data show, for the first time, that pro-inflammatory cytokines can induce TCR signaling in effector CD8 T cells in the absence of cognate antigen.
IL-12 dependent TCR signaling requires IL-12R expression and Src kinases activity
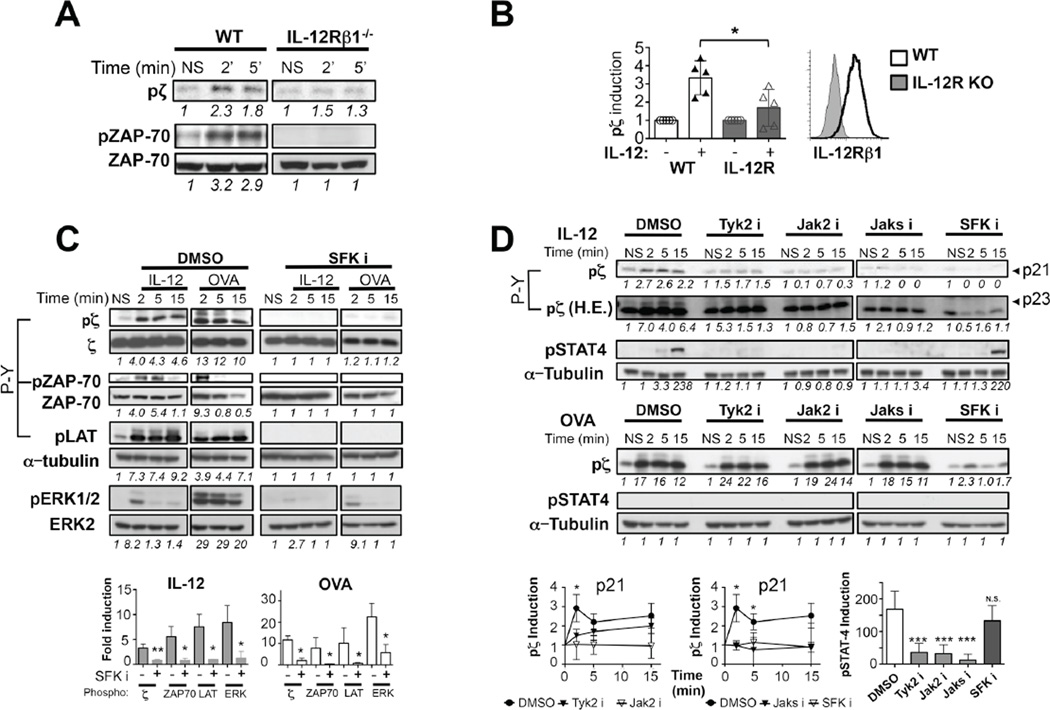
Since IL-12 is a key cytokine in effector and memory CD8 T cell differentiation(19), we focused on IL-12 to gain a deeper understanding of how pro-inflammatory cytokines crosstalk with TCR signaling. We investigated the role of IL-12R in enabling IL-12 to induce TCR signaling. IL-12R deficient CTLs exhibited a severe reduction in the phosphorylation of CD3ζ or ZAP-70 (Fig. 2A,B). This demonstrates that IL-12R expression is required for the induction of TCR signaling upon IL-12 stimulation.
Figure 2. IL-12-dependent TCR signaling requires IL-12R and both, Janus and Src Family Kinase activities.
(A–B) Day 3 OT-I or OT-IxIL12Rβ1−/− CTLs were stimulated or not (NS) with 10 ng/mL IL-12 for indicated times. Tyr phosphorylation of p21 and p23 CD3ζ and ZAP-80 was determined by immunoblot with P-Y, 4G10 antibody. Loading controls were determined in a parallel gel. IL-12Rβ1 expression was assessed by flow cytometry. (C,D) OT-I CTLs were pre-treated with DMSO vehicle control, or inhibitors to SFK (SFKi, 10 µM PP2), Jak2 (100 µM Jak2 i), Tyk2 (100 µM Tyk2 i), or both kinases (50µM each, Jaks i). Then T cells were stimulated or not (NS) with 10 (C) or 1 ng/mL IL-12 (D) or Kb-OVA tetramer for indicated times and levels of pζ, pZAP-70, pLAT, pERK1/2 and pSTAT4 were determined by immunoblot with 4G10 antibody. Tubulin loading controls (C, D) were performed on the same membrane. CD3ζ and ZAP-70 blots in (C) were determined in a parallel gel. Densitometry values for the phosphorylated p21 and p23 forms of CD3ζ are shown in (D). Graphs depict densitometry data for phosphorylated protein relative to their respective NS controls, normalized to loading control. Data is shown for specific time points: 2 minutes (ζ, & ERK), 5 minutes (ZAP-70) or 15 minutes (LAT and STAT4) upon IL-12 stimulation. All graphs show mean±SD. Data is representative of 3–6 independent experiments. * p < 0.05, ** p=0.005 and ***p=0.0005.
Tyrosine Phosphorylation of the ITAMs in the CD3 chains of the TCR/CD3 complex is mediated by Src kinases Lck and Fyn(20, 21). We stimulated CTLs with IL-12 or antigen in the presence or absence of the specific Src kinase family inhibitor (SFKi) PP2, which is widely used to inhibit TCR signaling(22). Similar to OVA stimulation, the Src kinase inhibitor also blocked the ability of IL-12 to induce the phosphorylation of CD3ζ, ZAP-70, LAT and ERK (Fig. 2C). Together these data indicate that IL-12 signals require IL-12R and Src kinase activity to induce TCR signaling.
IL-12 induction of TCR signaling is dependent on both Src and Jak2/Tyk2 kinase activities
All the cytokines able to induce TCR signaling such as Type I IFN, IL-23, IL-12 and IL-6 signal through Jak2/ Tyk2(23). We tested whether the activity of these kinases was necessary to induce TCR signaling. Since Jak2 deficient animals are not viable(24), we stimulated CTLs with IL-12 in the presence or absence of a specific Jak2 and/or Tyk2 inhibitors. Importantly, IL-12-mediated phosphorylation of CD3ζ (p21 and p23 forms) was reduced to basal levels when Jak2, Tyk2 or both activities were inhibited. However and in contrast to Src inhibition, blocking of Jak2/Tyk2 did not affect CD3ζ phosphorylation (p21 and p23) upon strong antigenic stimulation (Fig. 2D).
Canonical Jak2/Tyk2 signaling leads to STAT4 phosphorylation(5). Phospho-STAT4 levels were severely reduced in IL-12 stimulated CTLs in the absence of Jak2 or Tyk2 kinase activity. By contrast, inhibition of Src kinase activity did not change the ability of IL-12 to induce the phosphorylation of STAT4 (Fig. 2D). Collectively, these data show that IL-12 utilizes JAK2/Tyk2 signaling to trigger two independent pathways: a Src kinase-independent pathway that supports STAT4 activation and a Src kinase-dependent pathway that enables TCR signaling and is STAT4 independent.
IL-12 mediated CD8 effector functions require TCR signaling
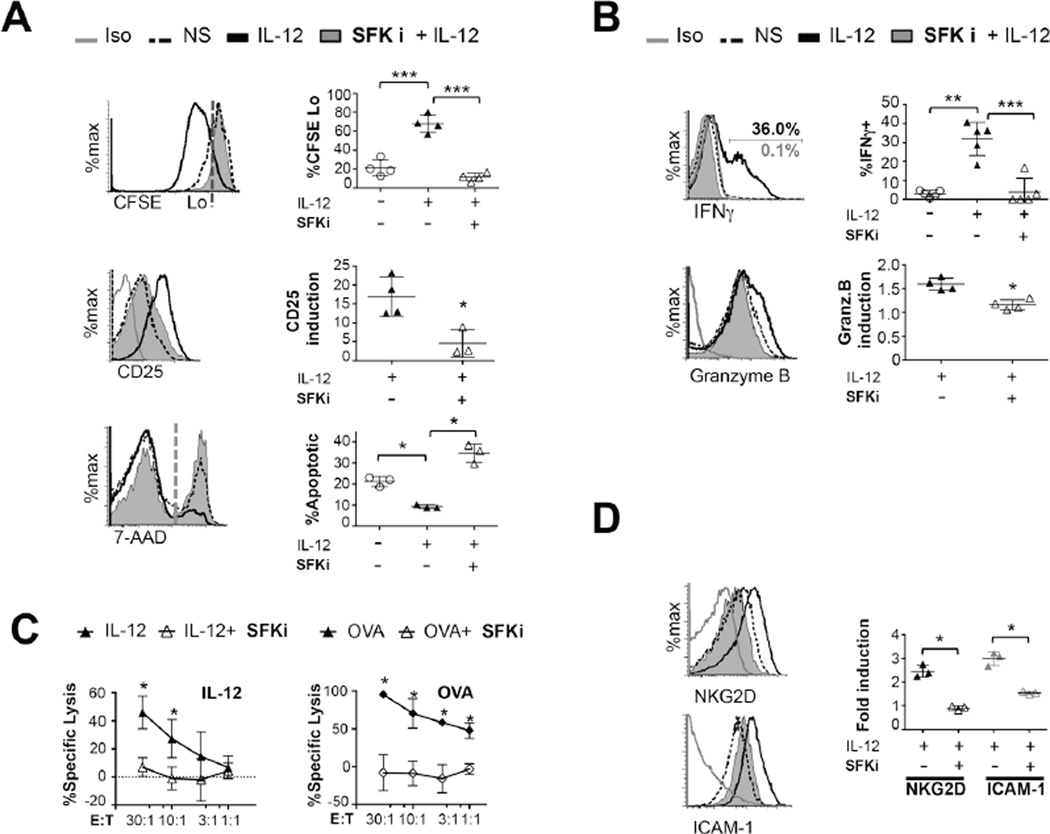
To test the role of TCR signaling in bystander CD8 activation, we evaluated several bystander T cell responses(25) in conditions where TCR signaling had been inhibited. Given that TCR and Lck deficient T cells cannot mature(26, 27), we used PP2 to inhibit TCR signaling. CTLs exposed to IL-12 were prompted to divide. However, cell division and IL-2Rα chain (CD25) upregulation were blocked when TCR signaling was inhibited (Fig. 3A). This was consistent with the idea that IL-12 supports cell division through maintained expression of IL-2R(28). Src kinase activity was also required for IL-12 to support T cell survival, as shown by the increased frequency of apoptotic cells in the presence of PP2 (Fig. 3A). Remarkably, CD8 T cells were unable to induce any expression of IFNγ or Granzyme B upon IL-12 stimulation when TCR signaling was blocked (Fig. 3B).
Figure 3. IL-12 mediated bystander CD8 T cell responses require TCR signaling.
(A) OT-I CTLs were labeled with CFSE before treating with vehicle or PP2 followed by stimulation or not (NS) for 48 hours with IL-12 and assessed for CFSE dilution or CD25 expression or apoptosis (frequency of 7-AAD+ cells) by flow cytometry. Induction of CD25 expression is normalized to the NS control. (B) OT-1 CTLs were stimulated for 6 hours with IL-12 before assessing intracellular IFNγ or Granzyme B expression. (C) Day 4 OT-1CTLs were incubated with 20ng/mL IL-12 or not (dotted line) for 16 hours before treatment with SFK inhibitor. CD8 effectors were then mixed at indicated ratios with CFSE-labeled EL-4 target cells either pulsed or not with OVAp and cytotoxicity was measured. Data is representative of 3–5 independent experiments. (D) OT-I CTLs were pre-treated with vehicle or PP2 followed by IL-12 stimulation for 48h and assessed for surface NKG2D and ICAM-1. (E) by flow cytometry. Graphs show induction of the expression of the proteins indicated relative to NS controls or frequency of cells expressing CFSElow, 7ADD, IFNγ, GranzymeB. All graphs show mean±SD. Data is representative of 3–4 independent experiments. *p < 0.05, **p=0.005 or *** p=0.0005.
Next, we tested the role of Src kinases in IL-12 induced bystander cytotoxicity. Markiewicz et al. have previously shown that exposure to IL-12 increases the sensitivity of CTLs to kill cognate antigen or self-antigen pulsed targets(13). IL-12-treated CTLs were able to induce approximately 45% killing in non-antigen pulsed syngeneic targets at a 30:1 effector to target ratio versus 100% killing observed for OVA-pulsed targets. In both conditions, IL-12 treatment in the presence of Src family kinases inhibitors led to no detectable cytotoxicity (Fig. 3C).
The ability of IL-12 to enable CD8 T cells to kill targets correlates with an increase in LFA-1 activation and ICAM-1 upregulation(13). We observed that while ICAM-1 upregulation was blocked with PP2, LFA-1 expression remained unchanged. In agreement with Markiewicz et al. we did not detect differences in conjugate formation in the presence of IL-12 and in the absence of cognate antigen (Fig. 3D and Supplemental Fig. 2). NKG2D is induced in bystander activation, can enhance synapse formation and increase the stimulatory potency of weak peptides(25, 29–31). IL-12 induction of NKG2D was also reduced in the absence of Src activity (Fig. 3D). Overall, these data suggest that IL-12 requires Src kinase activity to increase synapse formation via LFA-1-ICAM-1 and NKG2D interactions. In summary, inhibition of Src kinase activity, which abrogates the IL-12 mediated induction of TCR signaling, severely abolished bystander CD8 T cell functions.
Fyn is required for CD8 bystander activation
We then tested IL-12- mediated bystander activation in CD8 T cells that lack expression of either Lck or Fyn. Lck inducible CD8 T cells (Lckind) have an inducible transgene and express Lck only in the presence of doxycycline(32),. Uninduced LckOFF OT-1 CTLs were tested for expression of Lck, IL-12R and their ability to induce IFNγ upon IL-12 stimulation in comparison with doxycycline treated Lck-expressing controls. Interestingly, IL-12 stimulated LckOFF OT-1 CTLs expressed levels of IFNγ similar to their Lck sufficient counterparts (Fig. 4A). Conversely, we found that OT-1 FynKO CTLs were unable to express IFNγ upon IL-12 stimulation (Fig. 4A). This was not due to a complete impairment of OT-1 FynKO cells to signal through the TCR as OVA stimulated OT-1FynKO cells were able to induce IFNγ expression to levels similar to their WT counterparts (Fig. 4A). Furthermore, IL-12 stimulated-FynKO CD8 T cells also fail to induce CD3ζ phosphorylation (Fig. 4B), indicating that Fyn is involved in the induction of TCR signaling upon IL-12 stimulation.
Figure 4. Fyn is required for IL-12-dependent TCR signaling and bystander CD8 induction of IFNγ expression.
(A) OT-I or OT-IxLckind or OT-IxFynKO CTLs were generated upon OVA stimulation. On day 2, doxycycline was withdrawn from a cohort of the Lckind cultures (Lck-OFF). At day 3 CTLs were assessed for Kb-OVA, Lck and IL-12R expression before treating or not (NS) with 20 ng/mL IL-12 or congenic OVAp-pulsed APCs for 6 hours. Frequency of OT-1 cells expressing IFNγ expression is shown. (B) OT-1WT or FynKO CTLs were pre-treated with DMSO vehicle control, PP2 or anti-CD8β blocking antibody (5 µg/mL) and stimulated or not (NS) with 10 ng/mL IL-12 or Kb-OVA tetramer to determine levels of p21 CD3ζ by immunoblot (4G10 blot). Tubulin loading control was determined on the same membrane and Fyn control was obtained after stripping of the P-Y blot. Numbers and graph show densitometry values relative to NS control and corrected for loading control (α-tubulin). (C) Fyn (left) and Lck (right) immunoprecipitations were performed on lysates from OT-I CTLs stimulated with IL-12 after preclearing with beads and IgG controls. Immunoblots showing levels of pY416 SFK, p21ζ (4G10) or (D) Tyk2 pulled down with Fyn and Lck are shown. Extracts from an IgG precleared stimulated sample are shown as controls. Upon IL-12 stimulation (5ng/mL) in the absence (−) or presence of Tyk2 inhibitor (Tyk2 i), Fyn was immunoprecipitated and pY416 SFK and p21 CD3ζ were assessed by immunoblot. The percent inhibition of Fyn phosphorylation was measured relative to vehicle treated controls normalized to total Fyn. Fyn loading was determined after stripping on the same membrane. Data is representative n≥ 3 independent experiments with n=3 mice per group. All graphs show mean±SD. *** p=0.0005. N.S. (non significant).
Fyn kinase has been shown to interact with CD3ζ and contribute to its phosphorylation(20). Thus, we performed Fyn and Lck immunoprecipitations from CTL cell lysates that had or not been stimulated with IL-12. We found that IL-12 stimulation led to an induction in the association of CD3ζ and phospho-CD3ζ to Fyn but not to Lck (Fig. 4C). Tyk2 was constitutively associated to Fyn and was not found in Lck immunoprecipitates (Fig. 4D). However, Tyk2 activity was required for Fyn phosphorylation on Y146 and for Fyn to associate to CD3ζ (Fig. 4E). This suggests that in effector CD8 T cells, IL-12 stimulation connects the activity of Tyk2 with Fyn’s ability to induce TCR signaling through CD3ζ. These data confirm the role of Fyn in CD8 bystander activation and provides part of the mechanism by which IL-12 is able to transduce TCR signals.
Blocking self-pMHC-TCR interactions inhibits IL-12-dependent TCR signaling and bystander CD8 activation
Src kinases could be involved in IL-12R signaling in a TCR independent manner. To further clarify this and gain insight into a potential role for self-pMHC interactions in bystander CD8 T cell activation, we inhibited TCR signaling using two approaches different from Src inhibition. We pre-treated CTLs with anti-Kb (Y3) or anti-CD8β (53-5.8) to block TCR-pMHC interactions(33) and evaluated IL-12 mediated bystander CD8 activation. Blocking TCR-self-pMHC interactions or CD8-MHC interactions caused a severe reduction in the levels of phopho-CD3ζ, ZAP-70 and LAT induced upon IL-12 stimulation. This also led to lower levels of phospho-ERK1/2, -p38MAPK and -p65 NFκB, however, phosphorylation of STAT4 was not affected (Fig. 5A,B). Blockade of TCR-self-pMHC interactions also decreased the expression of IFNγ upon IL-12 stimulation (Fig. 5C). Collectively, these data demonstrate that self-pMHC interactions are required for IL-12 to modulate TCR signaling and to support innate CD8 effector functions, such as IFNγ.
Role of IL-12-dependent TCR signaling in bystander memory CD8 T cell activation
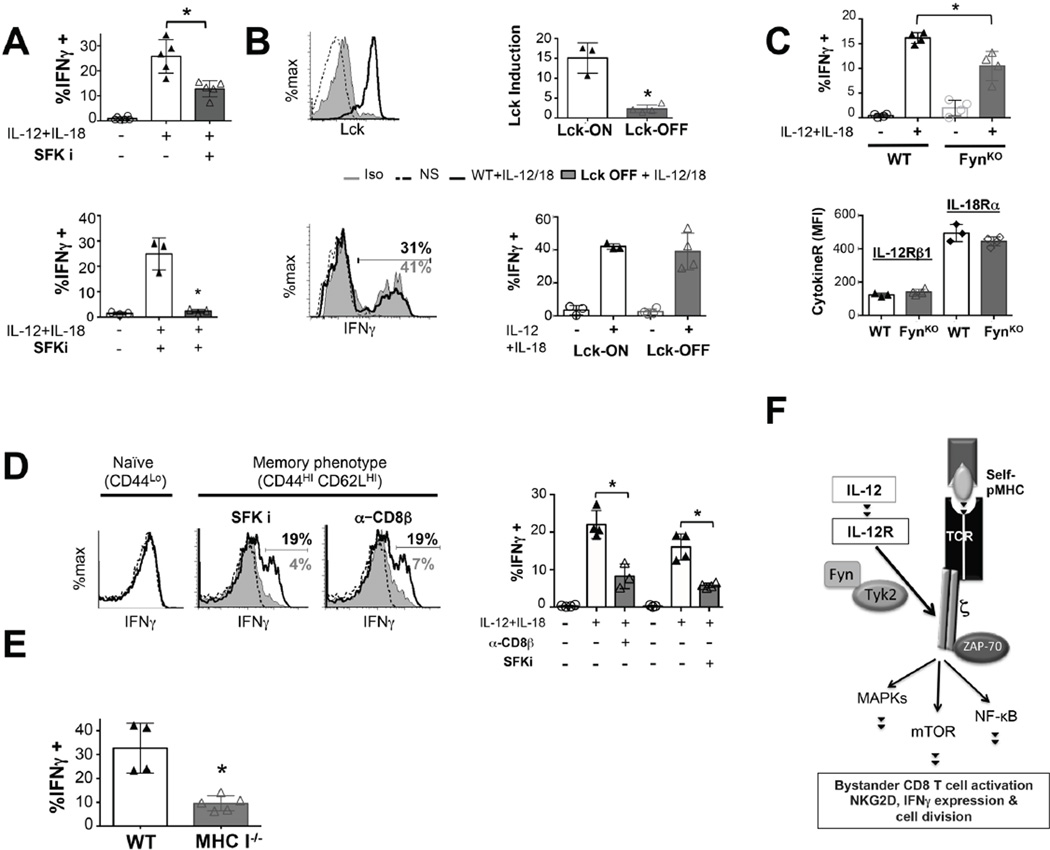
CD8 memory T cells can be induced to produce IFNγ by IL-12 and IL-18(34). We evaluated whether bystander activation of memory T cells requires TCR signaling. PP2 treatment caused a significant reduction in the ability of OT-1 memory cells to produce IFNγ upon IL-12/18 stimulation (Fig. 6A). However, Lck kinase activity was not required, since OT-1 Lckind memory cells expressed similar levels of IFNγ regardless of the expression of Lck (Fig. 6B). By contrast, we observed a significant reduction in the frequency of FynKO memory T cells expressing IFNγ upon bystander activation. This was not due to defects in IL-12R or IL-18R levels at memory (Fig. 6C). Src inhibition also abrogated the ability of polyclonal CD44hi CD62Lhi memory phenotype T cells and polyclonal VSV specific memory T cells to express IFNγ upon cytokine stimulation, confirming this phenomenon is not restricted to monoclonal OT-1 transgenic memory T cells but, most importantly it also occurs in more physiological conditions where polyclonal memory T cells are generated(Fig. 6A,D). Thus, similar to effector T cells, memory CD8 T cells also require Src activity for IL-12 dependent bystander IFNγ expression.
Figure 6. Role of Lck, Fyn and self-pMHC in bystander activation of memory CD8 T cells.
1 × 104 naive OT-I WT, OT-1FynKO or OT-I Lckind naïve cells were adoptively transferred to congenic recipient mice and challenged with LM-OVA (1 × 104 CFU). ≥ 30 days post infection, spleens and lymph nodes were harvested and 1 × 105 OT-1 memory T cells (CD44hi, CD8α+, CD45.2+, Kb-OVA tetramer+) were stimulated for 6h with IL-12+IL-18 (10 ng/mL) and assessed for their IFNγ expression by flow cytometry. (A) OT-1 memory T cells generated in response to LM-OVA (top graph) and (bottom graph) polyclonal CD8 Kb-VSVp (peptide) tetramer positive memory T cells (generated upon challenge of C57BL/6 mice with VSV) were pre-treated with PP2. (B) OT-1Lckind memory cells were treated with doxycycline (Lck-ON) or not (Lck-OFF) overnight in the presence of 5 ng/mL IL-7. Prior to inflammatory cytokine treatment, Lck levels were measured in memory cultures by flow cytometry (top panel). (C) OT-I WT of OT-I FynKO memory cells were assessed for expression of IL-12 and IL-18 receptors and % of IFNγ expressing cells was determined by flow cytometry. (D) CD44hi CD62Lhi memory phenotype CD8 T cells from (32 weeks old) B6 mice were pre-treated with blocking anti-CD8-pMHC (5 µg/mL) or PP2 (10 µM) and stimulated with IL-12 & IL-18 (10ng/mL). 6h later IFNγ was assessed by flow cytometry. (E) 5 × 104 congenically marked polyclonal memory T-cells (CD8-enriched, CD45.1+, CD44hi, Kb-VSVp tetramer+) from ≥ 120 days VSV-infected C57BL/6 mice were adoptively transferred to MHC class I sufficient (WT) or deficient (MHC I −/−) hosts and subsequently re-challenged with (1 × 105 CFU) Listeria monocytogenes. 16 hours later cells were harvested from lymph nodes and spleens and de novo intracellular IFNγ synthesis was measured over 3h. by flow cytometry. All graphs show mean±SD and show MFI values for cytokine receptors or frequency of IFNγ positive bystander activated memory T cells. Data representative of at least 3 independent experiments with n= 3–4 mice per group. * p < 0.05. (F) Model of the mechanism by which IL-12 transduces TCR signals to regulate innate CD8 effector functions. In the presence of self-pMHC-TCR interactions, IL-12 binding to IL-12R allows Tyk2-to activate Fyn, which in turn, phosphorylates CD3ζ to induce TCR-dependent signaling pathways such as ERK, NFκB and mTOR. This enables bystander CD8 functions such as NKG2D or IFNγ expression and cell division.
Next, we tested the role of self-pMHC interactions in CD8 memory bystander activation. IFNγ induction was greatly inhibited upon blocking CD8-pMHC-TCR interactions in polyclonal memory phenotype CD8 T cells (CD44hi CD62Lhi) (Fig. 6D) stimulated ex vivo with IL-12+IL-18. Similar results were obtained in vivo for polyclonal VSV specific memory T cells in an heterologous infection with LM(34). When we transferred equal numbers of polyclonal VSV-specific memory CD8 T cells into MHC class I sufficient or deficient congenic hosts and infected them with the unrelated pathogen Listeria monocytogenes, we found that IFNγ was severely reduced in VSV-specific memory T cells harvested from MHC class I deficient hosts (Fig. 6E). Curiously, monoclonal OT-1 memory T cells generated upon infection with VSV-OVA were refractory to the lack of self-pMHC interactions upon infection with Listeria (Supplemental Fig. 3). Overall, these data indicate that innate activation of memory CD8 T cells requires self-pMHC interactions for the expression of IFNγ. We propose the biochemical model shown in Fig. 6F to describe the mechanism by which IL-12 regulates bystander CD8 T cell activation through TCR signaling.
DISCUSSION
While it is well established that cytokines can activate CD8 effector and memory T cells in vitro and in vivo, the mechanism that enables them to cause CD8 T cell activation is far less clear. In this study, we show that contrary to what was assumed, cytokine mediated bystander activation of CD8 T cells is TCR signaling dependent. More specifically, our data indicates that IL-12, a prototype bystander cytokine, even in the absence of cognate antigen, requires TCR signaling to activate CD8 T cells in a process that involves self-pMHC-CD8 interactions and the activation of Tyk2 and Fyn kinase but not STAT-4 phosphorylation. Blocking of any of the former components lead to a complete inhibition of CD8 bystander effector functions.
The data reported here adds a novel aspect to the cooperation of TCR and pro-inflammatory signals beyond the one described at a transcriptional and epigenetic level(35). Our results are consistent with previous reports showing how another pro-inflammatory cytokine, IFNα (also important for full activation of CD8 T cells(35)), is able to induce the activation of components of the TCR signalosome. These studies described how this process was important to enable ERK activation in human cell lines and primary cells. However, they did not provide physiological relevance(36). Our data now show that this inflammatory/antigenic signal crosstalk is crucial for the innate activation of CD8 T cells and provides a more complete mechanism. We conclude that pro-inflammatory cytokine receptors that signal through Tyk2 enable TCR signaling in a process that is dependent on Src activity. We show that in the case of effector CD8 T cells, at least a pool of Fyn is constitutively associated with Tyk2. Once IL-12 mediates activation of Tyk2, Tyk2 facilitates Fyn phosphorylation of Tyr416 (which is required for its kinase activity), Fyn associates and phosphorylates CD3ζ. Hence, this process describes how IL-12R signals can initiate TCR signaling (Fig. 6). While we describe such a mechanism only for IL-12, our results are in agreement with previous reports by Uddin et al. describing a direct interaction of Tyk2 and Fyn in human hematopoietic cell lines upon IFNα stimulation(37). Our data also indicate that self-pMHC interactions are required for IL-12 to induce the phosphorylation of CD3ζ although it is currently unclear whether this implies a physical interaction between the TCR and the IL-12R or this occurs through other indirect mechanisms. We have performed experiments to test a potential direct interaction of the TCR and IL-12R following different approaches. However, the lack of sensitive and specific reagents able to detect mouse IL-12R has precluded the ability to reach a clear conclusion. Interestingly, we have found that IL-12 enhances TCR clustering to levels similar to TCR agonistic ligand stimulation (Supplemental Fig. 2C). This indicates that in addition to the biochemical mechanism described here, IL-12 may be able to enhance self-pMHC-TCR oligomerization at the level of the membrane to facilitate TCR signaling. Alternatively, IL-12 may regulate the ability of CD3ζ to interact with Fyn by recruiting Fyn to specialized locations in the membrane where its acylation, and thereby its interaction with CD3ζ is favored(38). Both mechanisms could contribute to lower the T cell activation threshold for cytokine-mediated IFNγ expression in effector and memory CD8 T cells (2, 39). Future studies beyond the scope of this report will be needed to thoroughly assess these possibilities.
The fact that self-pMHC interactions are required for CD8 bystander activation points to the idea that inflammation could, in some instances, be utilized to improve weak T cell responses. In line with this, Markiewicz and colleagues reported that IL-12 could indeed enhance the response of CTLs to peptides at the range of self (13). Furthermore, inflammatory cytokines such as IL-15 or IL-12 have been shown to improve the bystander response of NK and CD8 effector and memory T cells at the site of tumors(40).
The beneficial role of bystander activation of effector or memory CD8 T cell in viral clearance is well established. Previous studies have also determined that cognate antigen is not required for this process(8). Yet, no conclusive study to date had clearly discarded a role for self-pMHC interactions in bystander activation of effector and memory CD8 T cells. On the contrary, several studies have indeed pointed to a potential contribution. A recent study by Chu et al. suggested that bystander-CTLs did receive TCR signals of limited strength (but below the level of agonist ligands)(25), although the authors did not assess the nature of these signals. Other studies have considered the potential role of self in inflammatory mediated activation of CD8 T cells. However, the diversity in the maturation stages of the CD8 T cells analyzed (naïve versus effector or memory), the cytokines tested and the nature of the bystander responses elicited (proliferation versus cytotoxicity or IFNγ) has made it difficult to reach a clear conclusion. Thus, IL-2 has been suggested to induce bystander activation of naïve CD8 T cells contingent on self-pMHC-TCR interactions(41). Similarly, IL-7/IL-18 can also act on naïve T cells and presumably cooperate with self-peptide MHC interactions to improve TCR signaling(42) and promote proliferation of CD8 T cells. By contrast, Tough and Sprent reported that Interferons drive proliferation of CD44hi CD8 T cells in a self-pMHC independent manner(43). We have found that cytokines IL-2, IL-15 and IL-7 are not capable of transducing TCR signals in effector CD8 T cells that express high levels of these cytokine receptors. Our data, however, suggest that pro-inflammatory cytokines that signal through Tyk2 like IL-12, IFNα, IL-6, -12 and -23 are the main players in TCR signal transduction in CD8 bystander activation.
Considering our studies in the context of heterologous infections, IL-15, IFNα/β, IL-12 and IL-18 could either act on memory CD8 T cells to mediate IFNγ and cytotoxic functions(8, 34) or sensitize these cells to antigen(44). The mechanism behind how these cytokines can enhance responses to antigen is unclear. However, a study by Marshall and colleagues suggested that self-pMHC interactions are important for this process(45). Markiewicz and colleagues also described that IL-12 sensitization to antigen is linked to an enhancement of CD3ζ recruitment to the cSMAC, which may correlate with stronger TCR signaling(13). It might be that pro-inflammatory cytokines use the TCR signaling machinery as a central hub to regulate direct innate activation of CD8 T cells and also to enhance sensitivity of memory T cells to antigen(44). Although we have not formally tested the latter, if true, this would argue for a central role of the TCR in controlling T cell functions at all times.
Aside from IFNγ expression, we have described that IL-12R employs TCR signaling and Src activity to regulate other innate CD8 functions, such as cell division or NKG2D expression. Despite the requirement of CD8 and MHC that we found in our studies, Lck which is associated to CD8 was not involved in cytokine mediated TCR signaling. Instead, we found that Fyn was the src kinase enabling IL-12 to regulate CD8 innate functions. This would be consistent with the idea that Fyn may play a more prevalent role than Lck in low-grade TCR signals(46). It is also remarkable that the role of Fyn in the regulation of bystander CD8 activation is more marked in CTLs than in memory T cells. This may be related to a different biochemical wiring of effector and memory T cells, as suggested by the fact that Lck does not have a role in memory responses (47). Alternatively, it may be a consequence of differences in the cytokine milieu that effector and memory T cells are dependent on to exert their innate functions (48) or the TCR signaling thresholds that are set in the priming conditions of memory T cells (49). Related to the latter, it is surprising that OT-1 memory cells generated against VSV-OVA are bystander activated in a self-pMHC independent fashion while polyclonal VSV CD8 specific memory cells were not. It might be that differences in their original affinities for the antigen(50) or in T cell tuning (51) are responsible for this difference.
It is important to note that the role of TCR signaling described here does not discard an involvement of classic Jak/STAT signaling in the regulation of certain aspects of cytokine mediated innate CD8 activation. Indeed, CTLs primed in the absence of STAT-4 are less responsive to IL-12/IL-18 mediated bystander expression of IFNγ, suggesting STAT-4 is required to program the ability of the CTL to respond to cytokines(12).
What is the impact of innate CD8 activation and what are the potential repercussions of such non-cognate antigen specific mediated T cell responses? A great body of work has demonstrated that the production of IFNγ by inflammation mediated bystander activation of CTLs or memory CD8 T cells offers immediate and significant protection against intracellular pathogens(52). This has also been associated with immunosuppression of allergic responses in the lung(53). Yet, other aspects of innate CD8 activation, such as expression of NKG2D, have been linked to increased immunopathology in Leishmania infection(29) and in celiac disease(54). Expression of CD69 and CD25, on the other side, may warrant CD8 T cells to remain in inflamed tissue and allow for limited IL-2 mediated expansion, enabling antigen non specific tissue resident CD8 T cells to provide with a first barrier of innate protection before the cognate antigen immune response is ready to control infection. Why these innate activated CD8 T cells do not cause overt autoimmunity is currently unknown and deserves further investigation. Our results showing that inflammation mediated bystander activation of CD8 T cells requires TCR signaling and self-peptide MHC interactions suggest that innate activation of CD8 T cells is restricted to self.
Supplementary Material
Acknowledgments
Supported by the University of Missouri Mission Enhancement Fund, the University of Missouri Life Sciences Fellowship (KMK) and by the US National Institute of Health (RO1 AI110420 to E. T.).
We thank Dr. Matt Mescher for providing us with OT-1IL-12R deficient T cells.
REFERENCES
- 1.Gerner MY, Heltemes-Harris LM, Fife BT, Mescher MF. Cutting Edge: IL-12 and Type I IFN Differentially Program CD8 T Cells for Programmed Death 1 Re-expression Levels and Tumor Control. Journal of immunology (Baltimore, Md : 1950) 2013;191:1011–1015. doi: 10.4049/jimmunol.1300652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gascoigne NRJ, Casas J, Brzostek J, Rybakin V. Initiation of TCR Phosphorylation and Signal Transduction. Frontiers in Immunology. 2011;2 doi: 10.3389/fimmu.2011.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith-Garvin J, Koretzky G, Jordan M. T Cell Activation. Annu Rev Immunol. 2009 doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watford WT, Hissong BD, Bream JH, Kanno Y, Muul L, O'Shea JJ. Signaling by IL-12 and IL-23 and the immunoregulatory roles of STAT4. Immunol Rev. 2004;202:139–156. doi: 10.1111/j.0105-2896.2004.00211.x. [DOI] [PubMed] [Google Scholar]
- 6.Rao RR, Li Q, Odunsi K, Shrikant PA. The mTOR Kinase Determines Effector versus Memory CD8+ T Cell Fate by Regulating the Expression of Transcription Factors T-bet and Eomesodermin. Immunity. 2010 doi: 10.1016/j.immuni.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal P, Raghavan A, Nandiwada S, Curtsinger J, Bohjanen P, Mueller D, Mescher M. Gene Regulation and Chromatin Remodeling by IL-12 and Type I IFN in Programming for CD8 T Cell Effector Function and Memory. The Journal of Immunology. 2009;183:1695. doi: 10.4049/jimmunol.0900592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg RE, Forman J. The role of CD8 T cells in innate immunity and in antigen non-specific protection. Curr Opin Immunol. 2006;18:338–343. doi: 10.1016/j.coi.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Freeman BE, Hammarlund E, Raue HP, Slifka MK. Regulation of innate CD8+ T-cell activation mediated by cytokines. Proc Natl Acad Sci U S A. 2012;109:9971–9976. doi: 10.1073/pnas.1203543109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SK, Brehm MA, Welsh RM, Selin LK. Dynamics of memory T cell proliferation under conditions of heterologous immunity and bystander stimulation. J Immunol. 2002;169:90–98. doi: 10.4049/jimmunol.169.1.90. [DOI] [PubMed] [Google Scholar]
- 11.Cox MA, Kahan SM, Zajac AJ. Anti-viral CD8 T cells and the cytokines that they love. Virology. 2013;435:157–169. doi: 10.1016/j.virol.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suarez-Ramirez JE, Tarrio ML, Kim K, Demers DA, Biron CA. CD8 T cells in innate immune responses: using STAT4-dependent but antigen-independent pathways to gamma interferon during viral infection. mBio. 2014;5:e01978–e01914. doi: 10.1128/mBio.01978-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Markiewicz MA, Wise EL, Buchwald ZS, Cheney EE, Hansen TH, Suri A, Cemerski S, Allen PM, Shaw AS. IL-12 enhances CTL synapse formation and induces self-reactivity. Journal of immunology (Baltimore, Md : 1950) 2009;182:1351–1361. doi: 10.4049/jimmunol.182.3.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teixeiro E, Daniels MA, Hausmann B, Schrum AG, Naeher D, Luescher I, Thome M, Bragado R, Palmer E. T cell division and death are segregated by mutation of TCRbeta chain constant domains. Immunity. 2004;21:515–526. doi: 10.1016/j.immuni.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 15.Teixeiro E, Daniels M, Hamilton S, Schrum A, Bragado R, Jameson S, Palmer E. Different T Cell Receptor Signals Determine CD8+ Memory Versus Effector Development. Science (New York, NY) 2009;323:502. doi: 10.1126/science.1163612. [DOI] [PubMed] [Google Scholar]
- 16.Lecoeur H, Fevrier M, Garcia S, Riviere Y, Gougeon ML. A novel flow cytometric assay for quantitation and multiparametric characterization of cell-mediated cytotoxicity. J Immunol Methods. 2001;253:177–187. doi: 10.1016/s0022-1759(01)00359-3. [DOI] [PubMed] [Google Scholar]
- 17.Teixeiro E, Fuentes P, Galocha B, Alarcon B, Bragado R. T cell receptor-mediated signal transduction controlled by the beta chain transmembrane domain: apoptosis-deficient cells display unbalanced mitogen-activated protein kinases activities upon T cell receptor engagement. J Biol Chem. 2002;277:3993–4002. doi: 10.1074/jbc.M107797200. [DOI] [PubMed] [Google Scholar]
- 18.Shores EW, Love PE. Insights into T cell development and signal transduction provided by TCR-zeta chain deficient mice. International reviews of immunology. 1996;13:301–315. doi: 10.3109/08830189609061754. [DOI] [PubMed] [Google Scholar]
- 19.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009;182:2786–2794. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol Rev. 2009;228:9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 21.Nika K, Soldani C, Salek M, Paster W, Gray A, Etzensperger R, Fugger L, Polzella P, Cerundolo V, Dushek O, Höfer T, Viola A, Acuto O. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity. 2010;32:766–777. doi: 10.1016/j.immuni.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Casas J, Brzostek J, Zarnitsyna VI, Hong JS, Wei Q, Hoerter JA, Fu G, Ampudia J, Zamoyska R, Zhu C, Gascoigne NR. Ligand-engaged TCR is triggered by Lck not associated with CD8 coreceptor. Nature communications. 2014;5:5624. doi: 10.1038/ncomms6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker SJ, Rane SG, Reddy EP. Hematopoietic cytokine receptor signaling. Oncogene. 2007;26:6724–6737. doi: 10.1038/sj.onc.1210757. [DOI] [PubMed] [Google Scholar]
- 24.Neubauer H, Cumano A, Muller M, Wu H, Huffstadt U, Pfeffer K. Jak2 deficiency defines an essential developmental checkpoint in definitive hematopoiesis. Cell. 1998;93:397–409. doi: 10.1016/s0092-8674(00)81168-x. [DOI] [PubMed] [Google Scholar]
- 25.Chu T, Tyznik AJ, Roepke S, Berkley AM, Woodward-Davis A, Pattacini L, Bevan MJ, Zehn D, Prlic M. Bystander-activated memory CD8 T cells control early pathogen load in an innate-like, NKG2D-dependent manner. Cell Rep. 2013;3:701–708. doi: 10.1016/j.celrep.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Molina TJ, Kishihara K, Siderovski DP, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann KU, Veillette A, et al. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 27.Love PE, Shores EW, Johnson MD, Tremblay ML, Lee EJ, Grinberg A, Huang SP, Singer A, Westphal H. T cell development in mice that lack the zeta chain of the T cell antigen receptor complex. Science. 1993;261:918–921. doi: 10.1126/science.7688481. [DOI] [PubMed] [Google Scholar]
- 28.Starbeck-Miller GR, Xue HH, Harty JT. IL-12 and type I interferon prolong the division of activated CD8 T cells by maintaining high-affinity IL-2 signaling in vivo. J Exp Med. 2014;211:105–120. doi: 10.1084/jem.20130901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crosby EJ, Goldschmidt MH, Wherry EJ, Scott P. Engagement of NKG2D on bystander memory CD8 T cells promotes increased immunopathology following Leishmania major infection. PLoS Pathog. 2014;10:e1003970. doi: 10.1371/journal.ppat.1003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markiewicz MA, Carayannopoulos LN, Naidenko OV, Matsui K, Burack WR, Wise EL, Fremont DH, Allen PM, Yokoyama WM, Colonna M, Shaw AS. Costimulation through NKG2D enhances murine CD8+ CTL function: similarities and differences between NKG2D and CD28 costimulation. J Immunol. 2005;175:2825–2833. doi: 10.4049/jimmunol.175.5.2825. [DOI] [PubMed] [Google Scholar]
- 31.Cemerski S, Das J, Giurisato E, Markiewicz MA, Allen PM, Chakraborty AK, Shaw AS. The balance between T cell receptor signaling and degradation at the center of the immunological synapse is determined by antigen quality. Immunity. 2008;29:414–422. doi: 10.1016/j.immuni.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legname G, Seddon B, Lovatt M, Tomlinson P, Sarner N, Tolaini M, Williams K, Norton T, Kioussis D, Zamoyska R. Inducible expression of a p56Lck transgene reveals a central role for Lck in the differentiation of CD4 SP thymocytes. Immunity. 2000;12:537–546. doi: 10.1016/s1074-7613(00)80205-8. [DOI] [PubMed] [Google Scholar]
- 33.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J Exp Med. 2000;191:335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Curtsinger JM, Mescher MF. Inflammatory cytokines as a third signal for T cell activation. Curr Opin Immunol. 2010;22:333–340. doi: 10.1016/j.coi.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevens CN, Simeone A-M, John S, Ahmed Z, Lucherini OM, Baldari CT, Ladbury JE. T-cell receptor early signalling complex activation in response to interferon-alpha receptor stimulation. Biochem J. 2010;428:429–437. doi: 10.1042/BJ20091660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uddin S, Sher DA, Alsayed Y, Pons S, Colamonici OR, Fish EN, White MF, Platanias LC. Interaction of p59fyn with interferon-activated Jak kinases. Biochem Biophys Res Commun. 1997;235:83–88. doi: 10.1006/bbrc.1997.6741. [DOI] [PubMed] [Google Scholar]
- 38.van't Hof W, Resh MD. Dual fatty acylation of p59(Fyn) is required for association with the T cell receptor zeta chain through phosphotyrosine-Src homology domain-2 interactions. J Cell Biol. 1999;145:377–389. doi: 10.1083/jcb.145.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Minguet S, Swamy M, Alarcon B, Luescher IF, Schamel WW. Full activation of the T cell receptor requires both clustering and conformational changes at CD3. Immunity. 2007;26:43–54. doi: 10.1016/j.immuni.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 40.Tietze JK, Wilkins DE, Sckisel GD, Bouchlaka MN, Alderson KL, Weiss JM, Ames E, Bruhn KW, Craft N, Wiltrout RH, Longo DL, Lanier LL, Blazar BR, Redelman D, Murphy WJ. Delineation of antigen-specific and antigen-nonspecific CD8(+) memory T-cell responses after cytokine-based cancer immunotherapy. Blood. 2012;119:3073–3083. doi: 10.1182/blood-2011-07-369736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho J-H, Boyman O, Kim H-O, Hahm B, Rubinstein MP, Ramsey C, Kim DM, Surh CD, Sprent J. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J Exp Med. 2007;204:1787–1801. doi: 10.1084/jem.20070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walsh MC, Pearce EL, Cejas PJ, Lee J, Wang LS, Choi Y. IL-18 synergizes with IL-7 To drive slow proliferation of naive CD8 T cells by costimulating self-peptide-mediated TCR signals. J Immunol. 2014;193:3992–4001. doi: 10.4049/jimmunol.1400396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272:1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 44.Richer MJ, Nolz JC, Harty JT. Pathogen-specific inflammatory milieux tune the antigen sensitivity of CD8(+) T cells by enhancing T cell receptor signaling. Immunity. 2013;38:140–152. doi: 10.1016/j.immuni.2012.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marshall HD, Prince AL, Berg LJ, Welsh RM. IFN-alpha beta and self-MHC divert CD8 T cells into a distinct differentiation pathway characterized by rapid acquisition of effector functions. J Immunol. 2010;185:1419–1428. doi: 10.4049/jimmunol.1001140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang Q, Subudhi SK, Henriksen KJ, Long CG, Vives F, Bluestone JA. The Src family kinase Fyn mediates signals induced by TCR antagonists. J Immunol. 2002;168:4480–4487. doi: 10.4049/jimmunol.168.9.4480. [DOI] [PubMed] [Google Scholar]
- 47.Tewari K, Walent J, Svaren J, Zamoyska R, Suresh M. Differential requirement for Lck during primary and memory CD8+ T cell responses. Proc Natl Acad Sci USA. 2006;103:16388–16393. doi: 10.1073/pnas.0602565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richer MJ, Pewe LL, Hancox LS, Hartwig SM, Varga SM, Harty JT. Inflammatory IL-15 is required for optimal memory T cell responses. J Clin Invest. 2015;125:3477–3490. doi: 10.1172/JCI81261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hogquist KA, Jameson SC. The self-obsession of T cells: how TCR signaling thresholds affect fate 'decisions' and effector function. Nat Immunol. 2014;15:815–823. doi: 10.1038/ni.2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knudson KM, Goplen NP, Cunningham CA, Daniels MA, Teixeiro E. Low-Affinity T Cells Are Programmed to Maintain Normal Primary Responses but Are Impaired in Their Recall to Low-Affinity Ligands. Cell Reports. 2013;4:554–565. doi: 10.1016/j.celrep.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 51.Fulton RB, Hamilton SE, Xing Y, Best JA, Goldrath AW, Hogquist KA, Jameson SC. The TCR's sensitivity to self peptide-MHC dictates the ability of naive CD8(+) T cells to respond to foreign antigens. Nat Immunol. 2015;16:107–117. doi: 10.1038/ni.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lertmemongkolchai G, Cai G, Hunter CA, Bancroft GJ. Bystander activation of CD8+ T cells contributes to the rapid production of IFN-gamma in response to bacterial pathogens. J Immunol. 2001;166:1097–1105. doi: 10.4049/jimmunol.166.2.1097. [DOI] [PubMed] [Google Scholar]
- 53.Marsland BJ, Scanga CB, Kopf M, Le Gros G. Allergic airway inflammation is exacerbated during acute influenza infection and correlates with increased allergen presentation and recruitment of allergen-specific T-helper type 2 cells. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2004;34:1299–1306. doi: 10.1111/j.1365-2222.2004.02021.x. [DOI] [PubMed] [Google Scholar]
- 54.Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, Raulet DH, Lanier LL, Groh V, Spies T, Ebert EC, Green PH, Jabri B. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21:357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.