Abstract
60–70 % of IFN-γ−/− NOD.H-2h4 mice given NaI-supplemented water develop a slow onset autoimmune thyroid disease, characterized by thyrocyte epithelial cell hyperplasia/ proliferation (TEC H/P). TEC H/P develops much earlier in CD28−/− mice and nearly 100 % (both sexes) have severe TEC H/P at 4 months of age. Without NaI supplementation, 50% of 5–6 month old CD28−/−IfFN-γ−/− mice develop severe TEC H/P, and 2–3 weeks of NaI is sufficient for optimal development of severe TEC H/P. Mice with severe TEC H/P are hypothyroid and normalization of serum thyroxine (T4) levels does not reduce TEC H/P. Activated CD4+ T cells are sufficient to transfer TEC H/P to SCID recipients. Thyroids of mice with TEC H/P have infiltrating T cells and expanded numbers of proliferating thyrocytes that highly express CD40. CD40 facilitates, but is not required for development of severe TEC H/P, as CD40−/−IFN-γ−/−CD28−/− mice develop severe TEC H/P. Accelerated development of TEC H/P in IFN-γ−/− CD28−/− mice is a result of reduced Treg numbers as CD28−/− mice have significantly fewer Tregs, and transfer of CD28-positive Tregs inhibits TEC H/P. Essentially all female IFN-γ−/− CD28−/−NOD.H-2h4 mice have substantial lymphocytic infiltration of salivary glands and reduced salivary flow by 6 months of age, thereby providing an excellent new model of autoimmune exocrinopathy of the salivary gland. This is one of very few models where autoimmune thyroid disease and hypothyroidism develop in most mice by 4 months of age. This model will be useful for studying the effects of hypothyroidism on multiple organ systems.
Keywords: Autoimmunity, thyroid, hypothyroidism, fibrosis, salivary gland dysfunction, regulatory T cell, mouse
Introduction
IFN-γ−/− NOD.H-2h4 mice given NaI in their drinking water for >6 months develop an autoimmune thyroid disease, thyrocyte epithelial cell (TEC) hyperplasia and proliferation (TEC H/P). The primary feature that distinguishes TEC H/P from other autoimmune thyroid diseases is that, in addition to infiltration of the thyroid by inflammatory cells and production of autoantibodies, there is extensive hyperplasia and proliferation of TECs or thyrocytes (1) . TEC proliferation is inhibited by a direct effect of IFN-γ on TEC (2), and TEC H/P does not develop if IFN-γ is present (3). The extensive proliferation and destruction of thyroid follicles results in low thyroid function (hypothyroidism), with low levels of serum thyroxine (T4). There is also extensive fibrosis, and the thyroid cellular infiltrate includes CD4+ and CD8+ T cells, macrophages, dendritic cells and eosinophils (1, 4). TEC H/P is a well-characterized animal model of autoimmune thyroid disease that is very useful for addressing mechanisms underlying development of abnormal proliferation and fibrosis due to autoimmunity. It can also be used to investigate mechanisms by which abnormal proliferative lesions develop a senescent phenotype (5) or progress to neoplasia. A major drawback of the TEC H/P model described in our previous studies using CD28+/+ IFN-γ−/− NOD.H-2h4 mice is that thyroid lesions develop in only 60–70% of mice after >6mo exposure to NaI in the water (3). Therefore, a model in which TEC H/P develops earlier and in a higher percentage of mice would greatly facilitate further studies.
CD28, expressed by most T cells, is responsible for the classical “two-signal” model of naïve lymphocyte activation, in which full activation of T cells requires both TCR/MHC engagement and signaling through CD28. Absence of CD28 signaling can lead to anergy or apoptosis of T cells in response to TCR stimulation (6, 7). However in several models of spontaneous autoimmune disease, including iodine-facilitated spontaneous autoimmune thyroiditis (I-SAT) (8), diabetes (9–12) and pancreatitis (13), disease is accelerated when CD28 is absent. Development of earlier and/or more severe autoimmune disease in mice lacking CD28 is due primarily to reduced numbers of functional Tregs (14). CD28 is required for thymic development of natural T regs and is also required to maintain peripheral Tregs (14, 15). A major factor that controls Treg numbers is reduced CD28-dependent IL-2 production that inhibits Treg survival and self-renewal (14). Our unpublished studies indicated that transient depletion of Tregs using anti-CD25 mAb increased the incidence of severe TEC H/P in CD28+IFN-γ−/− mice given NaI water for ≥7 mo. However, these effects were relatively modest and did not lead to earlier development of TEC H/P in most mice (our unpublished results). We hypothesized that a prolonged and permanent reduction of Tregs would occur with genetic deletion of CD28, and would result in an increased incidence and/or earlier development of TEC H/P. To test this hypothesis, IFN-γ-positive CD28−/− NOD.H-2h4 mice, generated in our laboratory for another project (8), were crossed with IFN-γ−/− NOD.H-2h4 mice to generate mice lacking both CD28 and IFN-γ. Genetic deletion of CD28 had profound effects on the development of severe TEC H/P such that severe TEC H/P developed in essentially 100% of IFN-γ−/− CD28−/−NOD.H-2h4 mice (both sexes) 6–8 wk after NaI supplementation of the drinking water. TEC H/P in this strain was severe enough to result in low serum thyroid hormone levels, thus providing an important clinical correlate of Hashimoto’s thyroiditis that is lacking in many other mouse models of autoimmune thyroid disease. Because mice with severe TEC H/P become hypothyroid at a relatively young age, this mutant mouse strain will be useful for studying the effects of hypothyroidism and normalization of thyroid hormone levels on other organs/systems such as the cardiovascular system (16, 17).
The mutant mouse described here provides a robust experimental model to examine the role of CD4+ vs. CD8+ T cells and CD40 expression in the thyroid in TEC H/P development and provide new information regarding the role of NaI in induction and maintenance of this autoimmune thyroid disease. These questions could not be addressed in CD28-positive IFN-γ−/− mice because of the much longer induction period and lower incidence of disease.
In addition to early development of severe TEC H/P, nearly all IFN-γ−/− CD28−/−NOD.H-2h4 females spontaneously develop inflammation of the salivary gland by 4 mo of age. Salivary gland inflammation greatly increases at 6 mo of age and is accompanied by significant salivary hypofunction, a clinical manifestation of the human autoimmune disease Sjogren’s syndrome (SS). Some mice of both sexes also had inflammation of the pancreas that occasionally progressed to diabetes. IFN-γ−/−CD28−/−NOD.H-2h4 mice provide a robust model for studying mechanisms involved in development of autoimmune thyroid disease accompanied by hypothyroidism, and may also be very useful as a new animal model of autoimmune exocrinopathy of the salivary gland.
Materials and Methods
Mice
IFN-γ−/− NOD.H-2h4 SCID and IFN-γ−/− NOD.H-2h4 mice (MMRRC 037140) ((18) were generated in the University of Missouri animal facility as previously described (1). CD28-deficient IFN-γ−/− NOD.H-2h4 mice (MMRRC 037411) were generated by crossing CD28−/− IFN-γ+/+ NOD.H-2h4 mice (8) with IFN-γ−/− NOD.H-2h4 mice. The F1 mice were crossed and the resulting offspring were selected for homozygous expression of the IFN-γ and CD28 mutations by PCR analysis of tail DNA using primers described on the Jackson Laboratories web site. For some experiments, the IFN-γ−/−CD28−/− mice were crossed with IFN-γ−/− CD40−/− mice (MMRRC 037143) (18–20) to generate IFN-γ−/− CD28−/−CD40−/− mice (MMRRC 037143). Mice expressing eGFP in Foxp3+ Tregs were generated in our animal facility by crossing previously generated WT Foxp3-GFP NOD.H-2h4 mice (8) with IFN-γ−/− NOD.H-2h4 mice. WT NOD.H-2h4 mice (Jax 00447) and C57Bl/6 (B6) mice were from the Weisman colony at the University of Missouri. All animal protocols were approved by the University of Missouri Animal Care and Use Committee. Mouse strains with MMRRC numbers are available to other investigators through the Mutant Mouse and Rat Resource Center (MMRRC).
Administration of NaIin the drinking water
Development of severe TEC H/P in 60–70% of IFN-γ−/− CD28-positive NOD.H-2h4 mice requires NaI supplementation of the drinking water for >6 months (1, 3). In this study, most mice were given 0.08 % NaI in their drinking water beginning at 6–7 wk of age. For some experiments, mice were not given NaI water or were given NaI water for 2–4 wk and then maintained on plain water. In other experiments, mice were given NaI water for 6–7 wk, and maintained on plain water for the duration of the experiment. Both male and female mice were used, but all mice in an individual experiment were the same sex.
Administration of thyroxine (T4) in the drinking water
In some experiments, mice were given NaI water for various periods of time. After they were determined to be hypothyroid (serum T4 <3 μg/dL of serum), they were given plain water (no added NaI) or plain water containing T4 (Sigma, St. Louis, MO) at a predetermined optimal concentration of 25 ng/ml of water. They were maintained on water containing T4 or plain water without T4 for the duration of the experiment. Serum T4 concentrations were determined in all mice before addition of T4 to the water and again when the experiment was terminated.
Serum T4 Assay
Blood was collected from the retro-orbital plexus immediately before collection of thyroids and, in some experiments, before addition of T4 to the drinking water. Serum T4 levels were measured by ELISA using a T4 test kit (Leinco, St. Louis, MO) according to the manufacturer’s protocol. Results are expressed as μgT4/dL of serum. Hypothyroid mice are defined as having <3 μg/dL T4 in serum. Values for normal mouse serum ranged from 4–8 μg/dL. As previously reported (20), serum T4 levels highly correlate with TEC H/P severity scores. Only mice with few or no residual normal thyroid follicles (severity scores of 5+ or 4+ scores with <20% residual normal thyroid follicles) have low serum T4.
TEC H/P severity scoring
Thyroids were removed, one thyroid lobe was fixed in formalin, sectioned, and stained with H&E. Thyroid histopathology was scored for the extent of thyroid follicular cell hyperplasia/proliferation using a scale of 0 to 5+ as previously described (1, 18). All slides were read in a blinded manner by two investigators, one with no knowledge of the experimental details. Briefly, a score of 0 indicates a normal thyroid, and 0+ indicates mild follicular changes and/or a few inflammatory cells infiltrating the thyroid. A 1+ score indicates cellular infiltrates with at least 125 cells with hyperplastic changes sufficient to cause replacement of several follicles. A 2+ score represents 10–20 foci of cellular infiltration, with hyperplastic changes causing replacement or destruction of up to one fourth of the gland, 3+ indicates that one fourth to one half of the gland has hyperplastic changes, and 4+ indicates that greater than one half of the gland has hyperplasia. Thyroids with a score of 5+ have few or no remaining normal follicles. Mice with TEC H/P graded 4–5+ had widespread clusters of proliferating thyrocytes with lymphocyte infiltration, and areas of proliferating thyrocytes surrounded by collagen. All thyroids with mild or severe hyperplasia also had infiltrating lymphocytes.
Evaluation of salivary gland and pancreas infiltration
Submandibular salivary glands, pancreas, liver and kidney were removed from some mice when thyroids were collected. They were fixed in formalin, sectioned and stained with H&E. The extent of lymphocyte infiltration inthe salivary glands was scored by counting the number of foci/lymphocyte aggregates consisting of >50 lymphocytes (9, 21, 22). All scoring was done in a blinded manner using the microscope and MetaMorph software indicated below. Focus scores are defined as the number of lymphocyte foci (>50 cells) per 4mm2 of tissue (22). Images of whole submandibular gland sections were generated by stitching together multiple 40X magnification images using a Zeiss Axiovert 200M microscope and MetaMorph software at the University of Missouri Molecular Cytology Core Facility. Pancreas inflammation (insulitis) was scored as previously described (9). Blood glucose levels were assessed using test strips and an Accu-Chek monitoring system. Mice with blood glucose levels >300mg/dL were considered to be diabetic (9).
Saliva collection
Mice were anesthetized with Avertin and an endotracheal tube (PE50 polyethylene tubing) was inserted through a 2 cm mid-ventral incision to prevent aspiration. Saliva secretion was induced by i.p. injection of 0.25 mg/kg carbachol. Saliva was collected from the oral cavity for 15 min using a pipet tip and placed in a pre-weighed Eppendorf tube. Results are presented both as μl saliva/g body weight and as μl saliva/15 min.
Evaluation of fibrosis
The extent of fibrosis in thyroids of mice with TEC H/P was evaluated in a blinded manner by examination of H&E stained thyroid sections and confirmed in some mice by staining with Masson’s Trichrome as previously described (1, 20).
In vivo depletion of CD4+ and/or CD8+ T cells
CD4+ and/or CD8+ T cells were depleted using anti-CD4 (GK1.5; BioXCell, West Lebanon, NH) or anti-CD8 (116–13.1; Harlan Laboratories, Indianapolis, IN). Antibodies were administered i.p. (250 μg/injection) beginning at 5–6 wk of age. Control mice were given rat IgG (250 μg i.p.) at the same intervals. Mice were given NaI water one week later, and antibody injections were repeated every 10–12 days until termination of the experiment when mice were 15 wk old. When thyroids were removed, splenic CD4+ and CD8+ T cells were analyzed by flow cytometry. Only mice with few or no residual cells of the depleted subset are included in the figures.
Cell culture and adoptive transfer of TEC H/P
In some experiments, TEC H/P was induced by adoptive transfer of 72 hr cultured splenocytes or purified T cells from mice with severe (4–5+) TEC H/P to IFN-γ−/− NOD.H-2h4 SCID mice as previously described in detail (1, 4, 23). Recipients were given NaI water, and thyroid histopathology was determined at various times as indicated in the figures. In some experiments, T cells in recipient spleens were depleted using anti-CD4 and/or anti-CD8 as indicated above. Antibodies or rat IgG were injected beginning 6 or 30 days after cell transfer and injections were repeated every 2 wk as indicated in the legend to Figure 2. Thyroids were removed 28 or 60 days after cell transfer.
T cell purification
In some experiments, cultured splenocytes were separated into CD4+ and CD8+ subsets using CD4 or CD8 T cell isolation kits (Stem Cell, Vancouver, BC) according to the manufacturer’s protocols. Purity of isolated cells (always >95%) was determined by flow cytometry. Purified cells were transferred i.v. to SCID recipients (1.5 x 106 per recipient). Recipients of CD8+ T cells were given a single injection of anti-CD4 and recipients of CD4+ T cells were given a single injection of anti-CD8 the day after cell transfer to deplete any residual cells of the unwanted subset. Recipients were given NaI water and thyroids were removed 28 days later. Recipient spleens were analyzed by flow cytometry to ensure that only the transferred T cell subset was present.
Suppression of TEC H/P by adoptive transfer of Tregs
For Treg transfers, splenocytes from CD28-positive IFN-γ−/− NOD.H-2h4 mice expressing Foxp3 eGFP were sorted into GFP-positive and GFP-negative fractions using a MoFlo XDP cell sorter (Beckman Coulter, Brea, CA). GFP-positive Treg (>98% pure) or control GFP-negative cells (1x106) were transferred i.v. into IFN-γ−/− CD28−/−NOD.H-2h4 recipients that were irradiated (300 Gy) using an X-RAD 320 irradiator (Precision X-ray, New Branford, CT) 3–6 hr before Treg transfer. Mice were given NaI water, and transfer of Tregs or control GFP-negative cells was repeated 3 wk later without irradiation. TEC H/P severity was determined by histology 6–8 wk later. The percentages of donor (CD28-positive) and recipient (CD28-negative) Foxp3+ splenic T cells were determined at this time.
Flow cytometry
For analysis of sorted Foxp3+ Tregs, cells were stained with anti-CD28-FITC, anti-CD4-PerCp Cy5.5, and anti-Foxp3-allophycocyanin (all from eBioscience). Intracellular staining was done after surface staining using a Foxp3 intracellular staining kit (eBioscience). To determine total numbers of Foxp3+ Tregs, the number of spleen cells was multiplied by the total percentage of CD4+Foxp3+ cells detected by flow cytometry. Flow cytometry was done using a Cyan ADP flow cytometer (Beckman Coulter) and data analyzed using FlowJo (TreeStar Inc). For assessment of CD4+ and CD8+ T cells in spleens of antibody treated mice or separated T cell subsets, cells were stained with PE-anti-CD4 (RMA-4; eBioscience or Biolegend) or PE anti-CD8 (53.6; eBioscience or Biolegend). Samples were analyzed on a FACSCalibur (BD Bioscience) and data analyzed using FlowJo.
Immunohistochemistry
Frozen or formaldehyde fixed paraffin sections of thyroids were blocked with 5% BSA in PBS, and endogenous peroxidase was inhibited by incubation with 0.3% H2O2 for 30 min. Anti-TTF-1 (H-190, Santa Cruz Biotechnology), anti-CD40 (1C10; eBioscience for frozen sections or C-20 (sc975; Santa Cruz for paraffin sections), anti-human CD3 (rabbit polyclonal, Dako), anti-CD4 (clone GK1.5, supernatant) or anti-CD8 (clone 53.6, supernatant) were used as primary Ab. For staining of frozen sections (CD4, CD8 and CD40), biotinylated anti-rat IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) was used as secondary Ab (1:500), followed by avidin-HRP binding using a Vectastain Elite PK-6100 kit (Vector Laboratories, Burlingame, CA). Peroxidase activity was visualized using a Vector Nova-Red Substrate Kit (Vector). TTF1, CD3 and CD40 staining of paraffin sections was done by IDEXX RADIL, Columbia, MO. They were developed with biotinylated anti-rabbit or anti-goat IgG at previously determined optimal concentrations followed by avidin-HRP and visualized using diaminobenzidine tetrahydrochloride (DAB) as the chromogen.
Statistical analysis
Nonparametric Mann-Whitney test was used to determine significance of severity scores. Student’s t-test, was used for all other data including saliva flow and serum T4 analyses, using analysis software included with GraphPad Prism 4 (GraphPad Software Inc, La Jolla, CA). Groups were considered statistically significant when p values were < 0.05.
Results
Rapid development of severe TEC H/P in IFN-γ −/ CD28−/− - NOD.H-2h4 mice
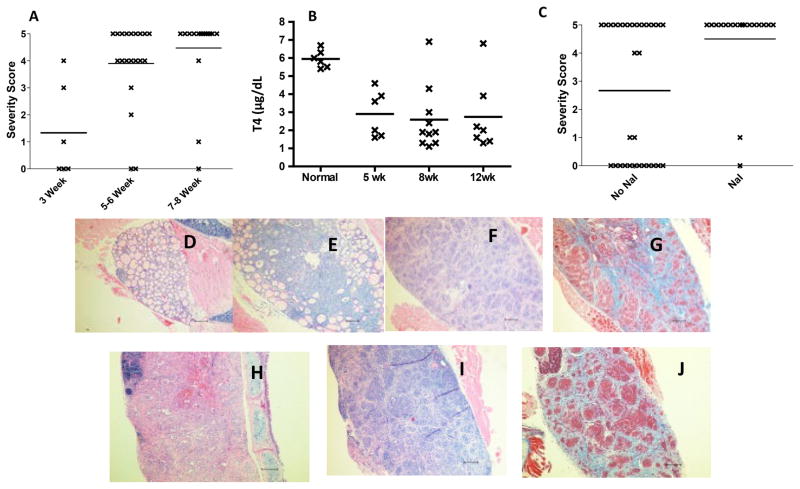
To test the hypothesis that genetic deletion of CD28 would lead to earlier development of severe TEC H/P, IFN-γ−/−CD28−/−NOD.H-2h4 mice, 6–8 wk old, were given 0.08% NaI in their drinking water. Thyroids were removed 3, 5–6 and 7–8 wk later, and TEC H/P severity scores were determined by histology. IFN-γ−/− CD28−/− mice developed severe (4–5+ severity scores) TEC H/P at a greatly accelerated rate compared to CD28-positive mice (1, 3). After 5–6 wk on NaI-supplemented water, ~80% of CD28−/− mice had severe (4–5+) TEC H/P, and after 7–8 wk, nearly 100% of them had severe TEC H/P (Figure 1A). After 7–8 wk, all mice with 5+ severity scores and those with strong 4+ severity scores (10–20% residual normal follicles) had low serum T4 (1–3 μg/dL), i.e. they were hypothyroid. Mice with 4+ severity scores and 25–50% residual normal thyroid follicles and those with lower scores had normal serum T4 (≥3 μg/dL (Fig. 1B). By comparison, only 60–70% of CD28-positive IFN-γ−/−- NOD.H-2h4 mice develop severe TEC H/P after prolonged (6–7 mo) administration of NaI water (1). More importantly, at 3–4 mo of age (age of mice in Fig. 1A after 6–8 wk on NaI water), ≤20% of CD28-positive IFN-γ−/− NOD.H-2h4 mice have severe TEC H/P (1, 3). Therefore, genetic deletion of CD28 greatly accelerates earlier development and increases the incidence of severe TEC H/P in IFN-γ−/− NOD.H-2h4 mice.
Figure 1.
Rapid development of severe TEC H/P in IFN-γ−/− CD28−/− NOD.H-2h4 mice. A) IFN-γ−/−CD28−/− mice (both sexes) were given NaI water at 7–8 wk of age. Thyroids were removed 3, 5–6 or 7–8 wk later as indicated. TEC H/P severity scores of individual mice are shown. N =6 (3 wk); 20 (5–6 wk) and 19 (7–8 wk). B) Serum T4 levels for some mice in Fig. 1A. N=6 (normal mouse); N= 6 (5 wk); N= 9 (8 wk) and N= 6 (12 wk). Serum T4 is significantly reduced in all groups compared to normal mice (p<0.05) C) IFN-γ−/− CD28−/− mice (both sexes) were maintained on plain water (no NaI) or given NaI water at 7–8 wk of age. Thyroids were removed when mice were 5–6 mo old. Mice given NaI water had a significantly higher incidence of severe (4–5+) TEC H/P compared to those not given NaI water (p< 0.01). N=30 (no NaI) and N =18 (NaI). D -J) H&E (D-F, H, I) or Trichrome (G, J) stained sections of thyroids from IFN-γ−/− CD28−/− NOD.H-2h4 mice given NaI water for 3 (D, 0+ severity), 5 (E, 4+ severity), or 8 wk (F,G 5+ severity) or from mice maintained 6 mo on plain water (H, I, J, 5+ severity). Note the extensive proliferation of thyrocytes with virtually complete obliteration of normal follicles, and fibrosis (blue). Pictures are representative of at least 6 mice examined in D, G and J and 20 or more for other panels. Magnification: X100.
NaI supplementation promotes but is not required for development of severe TEC H/P
NaI supplementation of the drinking water is absolutely required for development of severe TEC H/P in CD28-positive IFN-γ−/− NOD.H-2h4 mice, and CD28-positive IFN-γ−/− NOD.H-2h4 mice maintained on plain water for ≥12 mo do not develop severe TEC H/P (Braley-Mullen, H., unpublished results). Because genetic deletion of CD28 promotes earlier development and increases the incidence of severe TEC H/P (Fig. 1A), we hypothesized that IFN-γ−/−CD28−/− mice might develop severe TEC H/P without NaI supplementation of the water. To address this, thyroids from IFN-γ−/− CD28−/− mice, 5–6 mo old, were evaluated for TEC H/P (Fig. 1C). Approximately 50% of mice (both sexes) not given NaI water had severe TEC H/P, and all mice with 5+ severity scores had low serum T4 (not shown). The incidence of severe TEC H/P was similar in a smaller cohort of 7–8 mo old mice (not shown). Mice older than 8 mo were not studied. Nearly all 5–6 mo old CD28−/− mice given NaI water beginning at 7–8 wk of age had severe TEC H/P (Fig. 1C), and as shown in Fig. 1A, most younger (13–15 wk) mice given NaI for 5–8 wk also had severe TEC H/P. Therefore, NaI supplementation of the drinking water is not absolutely required for development of severe TEC H/P in IFN-γ−/− CD28−/− NOD.H-2h4 mice, but it clearly increases the incidence and promotes earlier development of severe TEC H/P. As noted in our previous studies with CD28-positive IFN-γ−/− NOD.H-2h4 mice (1, 20), most mice have severe (4–5+) or no or mild (0–1+) TEC H/P, and this is also true for IFN-γ−/− CD28−/− NOD.H-2h4 mice. Only a few mice have intermediate (2–3+) TEC H/P severity scores even when thyroids are examined early (Fig. 1A). Thyroid histology of IFN-γ−/− CD28−/− NOD.H-2h4 mice with TEC H/P is indistinguishable from that of CD28-positive IFN-γ−/− mice (Fig. 1C–F), and histology is identical in mice given NaI or plain water (Fig. 1E, F vs 1G, H, I). The primary pathologic feature is massive proliferation of thyrocytes and infiltration of inflammatory cells including CD4+ and CD8+ T cells, macrophages and dendritic cells (not shown). All thyroids have significant numbers of infiltrating eosinophils because IFN-γ is absent. Collagen deposition (fibrosis) is extensive in most thyroids with 5+ severity scores including mice not given NaI water (Fig. 1F, I).
Iodine promotes initiation of TEC H/P but lesions progress and are maintained in the absence of added iodine
Early development of severe TEC H/P in a high percentage of IFN-γ−/− CD28−/− mice requires NaI supplementation of the drinking water (Fig. 1B). Mice with very severe TEC H/P have low serum T4 (Fig. 1B). Thyroid lesions and hypothyroidism persist for life and thyroid fibrosis becomes more extensive over time (our unpublished results). We used multiple approaches in attempts to promote resolution of severe TEC H/P and reversal of hypothyroidism. No approaches were successful unless the intervention began before thyroid lesions became very severe (e.g. Fig. 2). It was suggested that removing excess iodine from the water and/or normalizing serum T4 levels might facilitate resolution of TEC H/P. Several approaches were used to address this question. To determine if removing NaI from the water before severe TEC H/P developed limits progression of TEC H/P, mice were given NaI water for 2 wk, then given plain water for 6 wk. Most mice (12 of 14) developed severe TEC H/P when given NaI water for only 2 wk if they were maintained on plain water for an additional 6 wk (Table I). Similarly, 16 of 18 mice given NaI water for 3–4 wk developed severe TEC H/P if maintained an additional 3–4 wk on plain water (Table I, line 2). Disease severity scores remained constant after 15 wk on plain water (Table I, line 3). Therefore, after effector T cell activation is initiated (a process promoted by NaI supplementation), iodine has little or no influence on further progression of TEC H/P. Importantly, 4 wk of NaI water did not provide sufficient time for development of severe TEC H/P (Table I, line 4). After 4 wk, at least 3–4 wk on plain water was required for maximal disease development (Table I, line 3). Together, these results indicate that after T cell activation is initiated and facilitated by exposure to NaI, iodine supplementation is not required for further progression of thyroid lesions to maximal severity.
Table I.
NaI supplementation of the water for 2–4 wk is sufficient for maximal development of severe TEC H/P
| TEC H/P Severity Score b | |||||||
|---|---|---|---|---|---|---|---|
| NaI (wk)a | Plain (wk)a | 0 | 1+ | 2+ | 3+ | 4+ | 5+ |
| 2 | 6 | 6 | 0 | 2 | 0 | 6 | 6 |
| 3–4 | 4 | 1 | 0 | 0 | 1 | 5 | 11 |
| 3–4 | 15 | 1 | 0 | 0 | 0 | 2 | 8 |
| 4 c | 0 | 2 | 2 | 0 | 1 | 0 | 0 |
| 8 | 0 | 0 | 1 | 0 | 0 | 2 | 8 |
Groups of IFN-γ−/−CD28−/− NOD.H-2h4 mice, 6 wk of age, were given NaI in their water for the indicated time. Mice in lines 1–3 were then maintained on plain water (no NaI) as indicated before thyroids were removed.
Numbers of mice with the indicated TEC H/P severity scores.
Thyroids were removed after 4 wk on NaI water, indicating that disease is not fully developed when mice in lines 1–3 were removed from NaI supplementation.
As shown above (Fig. 1B), mice with severe TEC H/P have low serum T4 levels. To determine if normalization of serum T4 levels and/or removal of excess iodine from the water would result in reduced TEC H/P severity, mice were given NaI water for 4–14 wk. Blood was collected to determine serum T4 levels, and groups of mice were maintained on plain water (no added NaI) or plain water to which 25 ng/ml thyroxine (T4) was added. Thyroids were removed 4–10 wk later, and blood was collected to measure serum T4 levels. Because mice with low serum T4 (<3 μg/dL) always have severe TEC H/P (18, 20); (Fig. 1B), this provided a way to ensure that mice had very severe TEC H/P when T4 administration began. This is important because serum T4 levels provide a way to determine disease severity without sacrificing the mouse, thus increasing the usefulness of this model for further studies. The results (Table II) indicate that TEC H/P severity was essentially unchanged after serum T4 levels were normalized for several weeks. Note that while serum T4 levels in most mice given exogenous T4 was in the range of 4–8 μg/dL reported for normal mice in Fig. 1B and in earlier studies (20), a few mice had higher T4 levels (11–16 μg/dL). They lost minimal weight, and appeared identical to both hypothyroid and euthyroid mice. Their thyroid histology was indistinguishable from that of all other mice with severe TEC H/P (not shown), which is not surprising because T4 was provided exogenously and was not produced by the thyroid. These results indicate that reversing the hypothyroid status did not influence how long TEC H/P lesions were maintained.
Table II.
Normalization of serum T4 by administration of thyroxine does not influence the maintenance of severe TEC H/P
| Serum T4c | |||||
|---|---|---|---|---|---|
| NaI (wk)a | Plain(wk)a | T4 (wk)a | 4–5+ TEC H/Pb | Before | After |
| 4 | 10 | 0 | 5/5 | ND | 1.3±0 |
| 4 | 0 | 10 | 8/10 | 1.6±0.1 | 8.2±2.3 |
| 14 | 0 | 0 | 8/8 | ND | 1.6±0.8 |
| 8 | 6 | 0 | 5/6 | 3.9±2.8 * | 2.1±1.9 * |
| 8 | 0 | 6 | 12/12 | 1.1±1.0 | 9.8±3.3 |
Groups of IFNγ−/− CD28−/− mice were given NaI water for the indicated number of weeks. They were subsequently maintained as indicated on plain water (no NaI) or plain water containing thyroxine (25ng/ml) before thyroids were removed.
Number of mice in each group with severe (strong 4+ or 5+) TEC H/P.
Mean serum T4 levels of individual mice determined when they began receiving plain or T4 supplemented water (before) or when thyroids were removed (after). Before values were not determined (ND) for mice in lines 1 and 3 but always fall within the range of 4–8 μg/dL determined for mice without TEC H/P (as in Fig. 1B) and as reported previously (1) Serum T4 is expressed as mean μg T4 per dL of serum ± SEM.
One mouse in this group had no disease and normal serum T4 (7.4 before and 6.5 after).
T cell requirement for development and transfer of severe TEC H/P in CD28−/− mice
T cells are required for development of severe TEC H/P in CD28-positive IFN-γ−/− NOD.H-2h4 mice (4), and severe TEC H/P can be transferred to SCID recipients with purified splenic T cells from donors with severe TEC H/P (4). In our earlier experiments, it was not feasible to determine which T cell subset was required for initial development of severe TEC H/P in donor mice, because of the long induction period and variable disease incidence. Because severe TEC H/P develops in most CD28−/− mice given NaI water for 6–8 wk (Fig. 1A), antibody-mediated depletion of CD4+ and/or CD8+ T cells was done to determine which subset was most important for initial development of TEC H/P. Groups of CD28−/− mice, 6–7 wk old, were given rat IgG, anti-CD4, anti-CD8 or both antibodies (250 μg) every 10–12 days. One wk after the first injection, mice were given NaI water and thyroids were removed 7 wk later. TEC H/P severity was minimally affected by depletion of either subset alone, but development of severe TEC H/P was inhibited in most mice after depletion of both CD4+ and CD8+ T cell subsets (Fig. 2A). Depletion of the appropriate T cell subset with the single antibody or both antibodies was essentially complete as determined by flow cytometry of splenocytes when thyroids were removed (data not shown). The two mice with 4+ TEC H/P in the group given both antibodies did not have more residual splenic T cells, so it is unknown why their disease was minimally suppressed. These results indicate that severe TEC H/P in IFN-γ−/−CD28−/−mice is dependent on both CD4+ and CD8+ T cells for optimal development.
Severe TEC H/P can be transferred to SCID recipients by T cells from donors with severe TEC H/P but not by T cells from donors with no or mild TEC H/P (1) This is also true using donor T cells from IFN-γ−/− CD28−/− mice (our unpublished results). To determine if T cell depletion inhibits TEC H/P induced by activated T cells, splenocytes from IFN-γ−/−CD28−/− donors with severe TEC H/P were transferred to SCID recipients, and CD4+ and/or CD8+ T cells were depleted by administration of anti-CD4 and/or anti-CD8 6 or 30 days later. Depletion of both T cell subsets starting 6 days after cell transfer inhibited development of severe TEC H/P in most mice up to 60 days after cell transfer (Fig. 2B). However, T cell depletion had no effect if it was delayed until 30 days after cell transfer, when recipients already had severe TEC H/P (Fig. 2B), suggesting T cells are not required for maintenance of thyroid lesions. Unexpectedly, depletion of CD4+ T cells was as effective as depletion of both T cell subsets (Fig. 2C), suggesting that CD8+ T cells are not required when TEC H/P is induced by activated T cells. To address this directly, splenocytes from CD28−/− donors with severe TEC H/P were cultured as before, and separated into purified CD4+ or CD8+ subsets (see Methods). Recipients of purified CD4+ T cells, unseparated splenocytes or a mixture of CD4+ and CD8+ T cells all developed severe TEC H/P 4 wk later, while recipients of purified CD8+ T cells did not develop TEC H/P (Fig. 2C). The results were unexpected because CD8+ T cells are the major T cell subset in spleens and thyroids of SCID recipients of splenocytes from IFN-γ−/− CD28−/− donors (not shown), and our previous results with CD28-positive IFN-γ−/− mice indicated that purified CD8+ T cells transferred TEC H/P more effectively than CD4+ T cells (4, 18). We have no explanation as to why activated CD4+ and not CD8+ T cells transfer severe TEC H/P when CD28 is absent. However, these results were highly reproducible and are consistent with the antibody depletion results in Fig. 2B.
Figure 2.
T cell requirement differs for initial development of TEC H/P (A) vs. induction of TEC H/P by splenocytes or transferred T cells (B, C). A, Naïve IFN-γ−/−CD28−/− NOD.H-2h4 males, 5–6 wk old, were given NaI water. One wk later, groups of mice were given rat IgG (Iso), anti-CD4 (GK1.5), anti-CD8 (116–13.1) or both antibodies every 10–12 days (see Methods). Thyroids were removed after 8 wk on NaI water (15 wk of age). Anti-CD4 or anti-CD8 alone had little effect on TEC H/P severity in most mice (p >0.1). Depletion of both CD4+ and CD8+ T cells significantly inhibited TEC H/P development (p < 0.0001). N=8 (Iso), 7 (anti-CD8), 9 (anti-CD4) and 9 (anti-CD4 + 8). B, splenocytes from IFN-γ−/−CD28−/−I females with severe TEC H/P were cultured and transferred to female SCID recipients (see Methods). Recipients were given NaI water and thyroids were removed 28 or 60 days later as indicated. Groups of recipients were given rat IgG (Con), anti-CD4 or anti-CD4 and anti-CD8 beginning 6 or 30 days after cell transfer as indicated. Antibody injections were repeated at 12–14 day intervals. Thyroids were removed from all treated mice at day 60. Mice given anti-CD4 or anti-CD4 and anti-CD8 at day 6 (before recipients had severe disease) developed minimal TEC H/P at day 60 (p < 0.001), whereas delaying T cell depletion until day 30 when recipients had severe TEC H/P had no effect (p > 0.1). N= 9 (Con, Day 30); 10 (Con, Day 60); 5 (anti-CD4 day 6); 9 (anti-CD4 and 8, day 6,) C, 5 (anti-CD4 and 8 day 30). D, Splenocytes from IFN-γ−/− CD28−/− females were cultured 72 hr. Cells were separated into CD4+ or CD8+ subsets as described in Methods, and transferred to SCID females. Recipients were given NaI water and thyroids were removed 28 days later. Splenocytes and CD4+T cells, but not CD8+, T cells induced severe TEC H/P in recipient mice. N= 8 (spl); 12 (CD4+); 11 (CD8+) and 8 (CD4 and CD8). Results are representative of two (C) or three (A, B) separate experiments. Thyroids and spleens of recipients of CD4+ T cells had no detectable CD8+ T cells (not shown).
T cells and other cells in thyroids of IFN-γ−/− CD28−/− mice with severe TEC H/P
The characteristic thyroid pathology of TEC H/P in CD28-positive mice includes proliferation of thyrocytes (TEC), extensive fibrosis, infiltration of lymphocytes (1), and increased CD40 expression by thyrocytes (18). To determine if thyroid pathology in CD28−/− mice is similar to TEC H/P in CD28-positive IFN-γ−/− NOD.H-2h4 mice, thyroids from IFN-γ−/− CD28−/− mice given NaI water were examined by immunohistochemistry. Thyroid transcription factor (TTF-1) is a transcription factor expressed in the thyroid (24). If proliferating TEC are derived from epithelial cells, they will be TTF-1--positive. However, since they express p63 (5) and resemble thyroid solid cell nests histologically, they could be derived from ultimobrachial bodies, and would be TTF-1 negative (24). To distinguish between these possibilities, thyroids were stained with anti-TTF-1 (Supplemental Fig. 1 A–D). TECs from normal mice (not shown) and normal nonproliferating TECs express TTF-1 (not shown). Similarly, proliferating TEC from mice with severe TEC H/P (Supplemental Fig. 1 A–D) and nonproliferating TEC in their thyroids (Suppl. Fig. 1 A, C, D; arrows in A, C, D) highly express TTF1, indicating that proliferating TEC in mice with TEC H/P are derived from thyroid epithelial cells and not from ultimobrachial bodies.
Thyroids from IFN-γ−/− CD28−/−mice with severe TEC H/P have infiltrating lymphocytes. Since TEC H/P is a T cell-dependent autoimmune disease, some thyroid infiltrating cells should be T cells. Consistent with this expectation, thyroids from IFN-γ−/− CD28−/− mice had many CD3+ cells (Suppl. Fig. 1 E–H) including both CD4+ and CD8+ T cells (Suppl. Fig. 1 I–L). Macrophages, eosinophils and dendritic cells are also present in thyroids of mice with TEC H/P (4, 20). Thyroids of mice with TEC H/P have very few B220+ B cells (18), distinguishing TEC H/P from I-SAT in WT NOD.H-2h4 mice (18, 25). Thyrocytes of CD28-positive IFN-γ−/− mice with TEC H/P highly express CD40 and our previous studies indicated that CD40 expression by thyrocytes was important for development of severe TEC H/P (19). Proliferating thyrocytes in IFN-γ−/− CD28−/− mice with severe TEC H/P also highly express CD40 (Suppl. Fig. 1 N–P), whereas thyroids with minimal inflammation and non-proliferating thyrocytes expressed minimal CD40 (Suppl. Fig. 1 M). Therefore, thyroid infiltrates in IFN-γ−/− CD28−/− mice with severe TEC H/P are indistinguishable from those in CD28-positive mice.
Development of severe TEC H/P in CD28−/− mice lacking CD40
Our previous studies indicated that CD40 expression by proliferating thyrocytes is important and may be essential for development of severe TEC H/P in CD28-positive mice, since CD40−/− mice and bone marrow chimeras lacking CD40 expression in thyroids are resistant to TEC H/P (19). Therefore, we hypothesized that IFN-γ−/−CD40−/−CD28−/− NOD.H-2h4 would not develop TEC H/P. To address this, CD40−/−CD28−/− mice were generated as described in Methods. Mice were given NaI water at 7–8 wk of age, and thyroids were removed 6 and 8 wk later (Suppl. Fig. 2 A, B). CD40−/− CD28 −/−mice given NaI water for 6 wk did not have TEC H/P, but many that were given NaI water for 8 wk developed severe TEC H/P. The incidence of severe TEC H/P in CD40−/−CD28−/− −/− mice given NaI water for 8 wk was not significantly different from that in a cohort of age and sex-matched CD40-positive CD28−/− mice (p = 0.08), although more CD40−/− mice had milder lesions (Suppl. Fig. 2B). Histologically, thyroids of CD40−/−CD28−/− mice with severe TEC H/P were indistinguishable from those in CD28−/−CD40-positive mice (Fig. 3D, E), except they did not express CD40 (Suppl. Fig. 2 F, G). They had comparable infiltration of CD3+ T cells (Suppl. Fig. 2 H). Splenocytes and CD4+ T cells, but not CD8+ T cells, from CD40−/−CD28−/− donors with severe TEC H/P transferred severe TEC H/P to SCID recipients (Suppl. Fig. 2C), indicating that TEC H/P in CD40−/− mice is induced by purified T cells and is likely autoimmune. CD40-positive CD28−/− mice produce low levels of antithyroglobulin autoantibodies, levels of which correlate with TEC H/P severity scores (Braley-Mullen, unpublished results) whereas antithyroglobulin antibodies were not detectable in CD40−/−CD28−/− mice (not shown). Therefore when CD28 is absent, expression of CD40 by thyrocytes is not absolutely required for severe TEC H/P to develop, although CD40 clearly facilitates earlier development of and a higher incidence of severe TEC H/P, and is required for development of detectable antithyroglobulin antibody.
Figure 3.
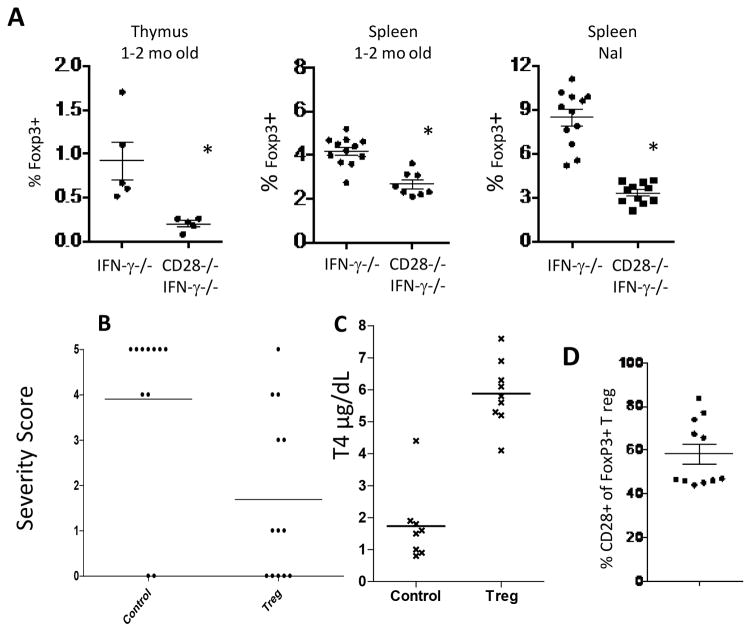
IFN-γ−/− CD28−/− NOD.H-2h4 mice have fewer Tregs than CD28-positive IFN-γ−/− mice, and CD28-positive Tregs suppress TEC H/P in CD28−/− mice. A, Foxp3+ CD4+ T cells in thymus and spleens of CD28−/− and CD28-positive IFN-γ−/− mice were determined by flow cytometry. Treg numbers are significantly lower (p <0.05) both for naïve CD28−/− mice and for CD28−/− mice with TEC H/P (NaI group) compared to CD28-positive mice. B, IFN-γ−/− CD28−/− females, 6 wk of age, were irradiated (300Gy) and injected i.v. with control GFP-negative cells or sorted Foxp3+Tregs from Foxp3-GFP CD28-positive mice. Mice were given NaI water. 3 wk later, they received a second injection of sorted GFP+CD28+ Tregs or control GFP-negative cells. Thyroids were removed 4–5 wk after the second injection of Tregs. Results are representative of three independent experiments. Recipients of CD28-positive Tregs developed less severe TEC H/P (p< 0.0001) compared to recipients of Foxp3-negative T cells. N= 11 (control) and 12 (Treg recipients). C, CD28−/− recipients of CD28-positive Tregs have normal serum T4 levels while recipients of non-Treg cells have low serum T4 levels. T4 results are from some of the mice in Fig. 3B. T4 values for recipients of Foxp3+ Tregs are significantly higher than for recipients of non-Tregs (p< 0.0001). N= 8 (control) and 9 (Treg). D, Persistence and expansion of transferred CD28+ Tregs in CD28−/− mice. Sorted Foxp3+ cells from Foxp3GFP CD28-positive mice were transferred to CD28−/− mice as in B. Mice were given NaI water and GFP+ (Foxp3+) CD28+CD4+ T cells in the spleen were enumerated by flow cytometry 6–8 wk after transfer. Many T regs in recipient spleens were of donor origin. No GFP+ cells were detected in CD28−/− mice not given Tregs (not shown). Results are representative of 2 separate experiments. N = 11.
IFN-γ−/− CD28−/− mice have fewer Tregs than CD28-positive IFN-γ−/− mice and Tregs from CD28-positive mice suppress TEC H/P development in CD28−/− mice
CD28 regulates Treg homeostasis, influencing both thymic development (14) and peripheral homeostasis (26, 27) Reduced Treg numbers in CD28−/− NOD mice account for their increased susceptibility to diabetes (26, 27), and reduced numbers of functional Tregs account for the increased severity of I-SAT in CD28−/− IFN-γ-positive NOD.H-2h4 mice (8). To test the hypothesis that reduced functional Tregs account for the increased susceptibility of IFN-γ−/− CD28−/− mice to TEC H/P, splenic Foxp3+ T regs were enumerated by flow cytometry in naïve mice 1–2 mo old and in mice with TEC H/P. Naive IFN-γ−/− CD28−/− mice had significantly fewer thymic and splenic Foxp3+ Tregs than CD28-positive IFN-γ−/− mice (Fig. 3A). After being given NaI water to allow for development of TEC H/P, IFN-γ−/− CD28−/− mice had significantly fewer Foxp3+ T regs compared to CD28-positive mice (Fig. 3A). Reduced numbers and/or function of Tregs in CD28−/− mice is largely responsible for their earlier development and increased incidence of severe TEC H/P, because transfer of sorted T reg from Foxp3-GFP CD28-positive IFN-γ−/− mice significantly reduced the incidence and severity of TEC H/P induced by transferred CD28−/− effector T cells (Fig. 3B). Transfer of CD28-positive Tregs resulted in normalization of thyroid function, as Treg recipients had normal serum T4, whereas recipients of Foxp3-negative T cells from CD28-positive mice were hypothyroid (Fig. 3C). The transferred CD28-positive Tregs expanded and persisted in the recipient spleens, comprising 50–80% of the total splenic Tregs 5 wk after transfer (Fig. 3D). These results indicate that the rapid development of severe TEC H/P and hypothyroidism in IFN-γ−/−CD28−/− mice is due, at least in part, to reduced numbers and/or function of T regs.
IFN-γ−/− CD28−/− NOD.H-2h4 mice have robust lymphocytic infiltration of salivary glands
NOD mice that spontaneously develop diabetes commonly have inflammation in other organs including the thyroid (28) and salivary gland (29, 30). WT NOD.H-2h4 mice, closely related to NOD mice, develop I-SAT when given NaI in their water (18), and many WT NOD.H-2h4 female mice have salivary gland infiltration at 10–12 mo of age (29) with ectopic follicles that increase in size over time (31). We previously reported that IFN-γ-positive CD28−/−NOD.H-2h4 mice have a higher incidence of salivary gland infiltration than WT (CD28-positive) NOD.H-2h4 mice (8). CD28-positive IFN-γ−/− NOD.H-2h4 mice have a low incidence of lymphocyte infiltration in organs other than the thyroid (our unpublished results). Because IFN-γ−/−CD28−/− NOD.H-2h4 mice have a high incidence of early and severe TEC H/P, it was important to determine if other organs had lymphocyte infiltration. To address this, pancreas, submandibular salivary glands, liver and kidney were examined in some 6–7 mo old IFN-γ−/− CD28−/− mice. There were no infiltrates in liver tor kidney (not shown). Pancreas infiltration was present in 50–60% of 5–7 mo old IFN-γ−/−CD28−/− mice of both sexes (not shown), and a comparable percentage of retired breeders, 6–7 mo of age. Unexpectedly, about 10% of retired breeders, both sexes, had diabetes (blood glucose ≥300 mg/dL) despite absence of the MHC class II molecules associated with susceptibility to diabetes. Because the incidence was low, this was not addressed further.
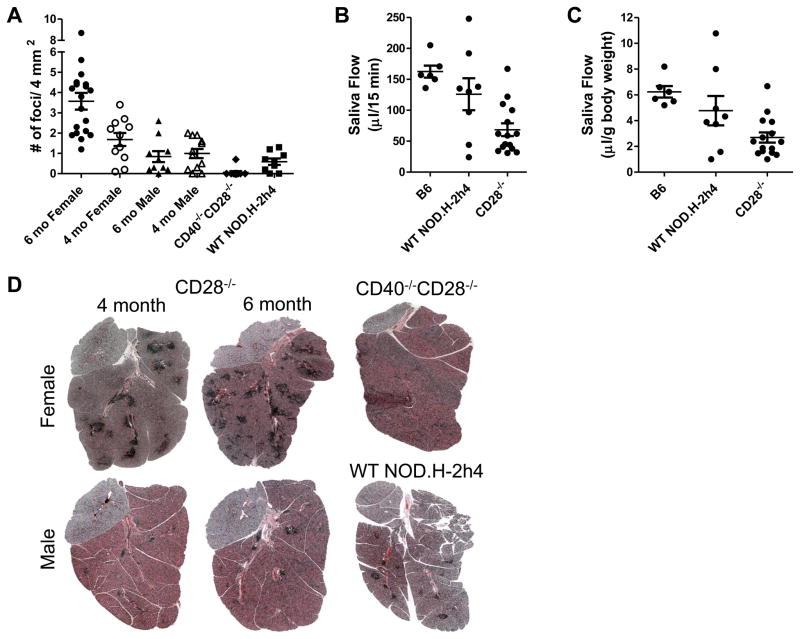
Of particular interest, nearly 100% of female IFN-γ−/− CD28−/− NOD.H-2h4 mice had moderate to extensive lymphocyte infiltration in their submandibular salivary glands at 4 mo of age, and the number and size of lymphocytic foci increased substantially at 6 mo of age (Fig. 4A). Although TEC H/P develops equally in both males and females, salivary gland infiltration was more predominant in females (Fig.4) as previously reported by others for WT NOD.H-2h4 mice (28, 30). Most males also had salivary gland lymphocyte infiltration, but the areas of infiltrate were smaller and less numerous than in females, and the number of foci did not change between 4 and 6 mo of age (Fig.4A and D). CD40−/−CD28−/− females had almost no salivary gland infiltrates (Fig. 4A, D), indicating that development of SS-like lesions in CD28−/− mice is CD40-dependent. Interestingly, the size and numbers of lymphocytic foci in salivary glands of female IFN-γ−/− CD28−/− −/− NOD.H-2h4 mice were greater than in a small cohort of age-matched WT NOD.H-2h4 mice, suggesting that IFN-γ−/− CD28−/− mice may provide a more robust model of SS-like disease than WT NOD.H-2h4 mice. Salivary gland function as determined by salivary flow was significantly reduced in female IFN-γ−/− CD28−/− NOD.H-2h4 mice compared to age-matched female WT NOD.H-2h4 mice or nonautoimmune B6 mice (Fig. 4B, C). This was true whether results were presented as total saliva collected in 15 min (Fig. 4B) or amount of saliva per 15 min relative to body weight (Fig. 4C). To further characterize this strain as an animal model of SS, sera from 4 and 6 mo old female IFN-γ−/− CD28−/− mice (N= 10 of each age) were evaluated for anti-Ro and anti-La autoantibodies using ELISA kits from Alpha Diagnostics. Autoantibody levels were low and often not detectable (data not shown). This is not surprising, as CD28/B7 interactions are important for T/B interactions (10), and IFN-γ-positive and IFN-γ−/− CD28−/− NOD.H-2h4 mice have much lower antithyroglobulin autoantibody responses than their CD28-positive counterparts, even though they have severe autoimmune thyroid disease (8, 18).
Figure 4.
Female IFN-γ−/− CD28−/− mice develop SS-like infiltration of the submandibular salivary glands. A, Mean submandibular gland focus scores of 4 and 6 mo old IFN-γ−/− CD28−/− , 4–5 mo IFN-γ−/− CD40−/− and 5 mo WT NOD.H-2h4 mice. N= 17, 6 mo female, N= 11 (4 mo female), N=10 (6 mo male), N= 12 (4 mo male), N= 10 (CD40−/− female) and N= 9 (5 mo WT NOD.H-2h4 female). p< 0.001 (6 mo female vs. 4 mo female; p< 0.001 6 mo female vs. 6 mo male; p= 0.06, 6 mo male vs. 4 mo female; p = 0.08, 4mo female vs. 4 mo male; p< 0.001, 6 mo female vs. CD40−/−CD28−/−; p <0.03, 6 mo female vs. WT NOD.H-2h4; p = 0.05, 4 mo female vs. WT NOD.H-2h4. B,C, Mean salivary flow from the indicated strains (all females 5 mo old) expressed as saliva collected in 15 min (B) or μl saliva collected in 15 min per gram of body weight. N= 6, B6, N = 8, NOD.H-2h4 WT, N= 14, CD28−/− mice. Saliva production in CD28−/− mice is significantly reduced compared to both B6 (p<0.001) and WT NOD.H-2h4 mice (p< 0.02 (B), p<0.047 (C). D, representative H&E stained sections from salivary glands of 4 and 6 mo female and male CD28−/− and CD40−/− and WT NOD.H-2h4 females. Note the fewer and smaller infiltrates in CD28−/−males, CD28−/−CD40−/− females and WT NOD.H-2h4 females compared to CD28−/− females. Magnification, X40. Photos are representative of the mice represented in A.
Discussion
This study describes and characterizes a new mouse model of autoimmune thyroid disease and SS. The model is unique because autoimmune thyroid disease is accompanied by hypothyroidism that develops spontaneously (no requirement for immunization) by 4 mo of age in most mice of both sexes. To our knowledge, this is one of very few murine models where hypothyroidism resulting from thyroid autoimmunity develops in most mice at a relatively young age. Therefore, this model will be useful for addressing basic mechanisms involved in thyroid autoimmunity accompanied by low thyroid hormone levels as occur in humans with Hashimoto’s thyroiditis. Perhaps more importantly, it will be useful for studying effects of low thyroid hormone levels on other organs and systems. Low thyroid hormone levels influence many other systems in the body, including the cardiovascular (17, 32), renal (33) reproductive (34), and immune systems (35). Autoimmunity is a major cause of hypothyroidism in humans. An animal model of autoimmune thyroid disease with low thyroid hormone levels at 4 mo of age provides a unique model for determining how thyroid hormone levels influence, e.g., cardiovascular functions such as vascular stiffening and blood pressure abnormalities in models of diet-induced obesity (36–38). Autoimmunity and hypothyroidism are chronic, persisting for life, providing a large window for testing effects of low thyroid hormone in other systems. In addition, hypothyroidism can be normalized by adding T4 to the drinking water, comparable to treating humans with Synthroid. Therefore, this model provides translational relevance, by determining if normalizing thyroid hormone levels can reverse conditions that are affected by hypothyroidism, such as increased vascular stiffness as a measure of cardiovascular disease.
The autoimmune thyroid disease TEC H/P develops very slowly in IFN-γ−/− NOD.H-2h4 mice that express CD28. NaI supplementation of the drinking water for >6 months is required, and severe TEC H/P with hypothyroidism develops in only 60% of mice (1, 2). In contrast, when CD28 is absent, essentially all IFN-γ−/− NOD.H-2h4 mice of both sexes develop severe TEC H/P and hypothyroidism (low serum T4) by 4 mo of age (Fig. 1). The mechanism by which CD28 deficiency promotes development of spontaneous autoimmune diseases is primarily due to reduced Treg numbers and function in CD28−/− mice (8, 14, 39). Our results are consistent with those studies as transfer of CD28-positive Tregs reduced TEC H/P severity scores and normalized thyroid hormone levels (Fig. 3).
CD28-negative or low T cells are present in humans but not in mice. CD28-negative T cells arise during T cell activation, and are considered to be antigen-experienced highly differentiated cells that play significant roles in several human diseases (40–42). Intriguingly, patients with primary SS have increased percentages of CD8+ CD28-negative T cells that correlate with disease severity (43), and increases in circulating soluble CD28 have been reported in SS and other autoimmune diseases ( (44). Although the model used here has a mutation that does not naturally occur in mice, studying CD28-negative effector cells in a murine autoimmune disease could provide important information relevant for human medicine.
This study provides new information regarding the requirement for NaI supplementation of the drinking water for development of autoimmune thyroid disease in NOD.H-2h4 mice. A common concern with studies of autoimmune thyroid diseases in NOD.H-2h4 mice is the requirement for supraphysiologic concentrations of iodine for early development of autoimmune disease (45–48). Although NaI supplementation facilitates early development of severe TEC H/P in CD28−/− mice, many mice develop severe TEC H/P without iodine supplementation (Fig. 1C), and 2–3 wk of NaI supplementation is sufficient for most mice to develop severe TEC H/P (Table I). Severe TEC H/P with low serum T4 levels then develop optimally with or without NaI supplementation. This is important because iodine administration can be shortened to 2–3 wk, thus helping alleviate concerns that long-term exposure to excess iodine has adverse effects on the thyroid, or could result in the Wolff-Chaikoff effect, a mechanism that prevents thyroids from secreting normal amounts of thyroid hormones when iodine is in excess (46, 49). Our results show that facilitation of autoimmune thyroid disease by administration of iodine is an early event, suggesting that iodine facilitates autoreactive T cell activation, perhaps due, in part, to its ability to be incorporated into a target epitope recognized by autoreactive T cells (45, 50) and/or to facilitate upregulation of MHC or costimulatory molecules such as ICAM-1 on thyrocytes (45, 51). Although several studies have addressed possible underlying mechanisms by which iodine promotes thyroid autoimmunity in NOD.H-2h4 mice, the precise cellular events are poorly understood (reviewed in (45), and beyond the scope of this study.
TECH/P differs from another autoimmune thyroid disease, I-SAT that develops in IFN-γ-positive NOD.H-2h4 mice following NaI supplementation of the drinking water (18, 45), because TEC H/P develops only when IFN-γ is absent (1, 2), raising concerns that this model lacks an appropriate human counterpart since humans are not IFN-γ deficient. However, a murine model of thyroid autoimmunity accompanied by hypothyroidism as occurs in humans can be an excellent model for determining how hypothyroidism developing as a consequence of autoimmunity affects various organs and organ systems. In most other mouse models of autoimmune thyroiditis not requiring immunization, serum T4 levels are normal (8, 18, 45, 47), so those models lack the major biomarker of Hashimoto’s thyroiditis in humans, low serum thyroid hormone levels. This biomarker also allows for assessment of disease severity without sacrificing the mouse, thus increasing the experimental usefulness of this model. WT NOD.H- 2h4 mice with I-SAT do not have low serum T4 levels (18, 47), and cannot be used to study effects of low thyroid hormone levels on other organs or systems.
Several other murine models that spontaneously develop hypothyroidism have been described. Most comparable to ours are CCR7-deficient NOD mice (52) where both sexes spontaneously develop severe thyroid lesions that appear similar to those in CD28−/− NOD.H-2h4 mice. Mice also have thyroid fibrosis, low serum T4 levels, thyroid-infiltrating T cells, and antithyroglobulin antibodies. The disease is autoimmune and transferable to SCID recipients with splenic T cells (52). Unlike IFN-γ−/− CD28−/− NOD.H-2h4 mice, CCR7-deficient NOD mice express IFN-γ, the thyroid infiltrate has a significant B cell component, and they have infiltrates in most organs. The infiltrate in our model is confined to the thyroid, salivary glands and pancreas and B cells are not detected in the thyroid. Thyroid disease in CCR7-deficient NOD mice develops somewhat later and has a lower incidence (ca 70%) (52) compared to that in IFN-γ−/− CD28−/− NOD.H-2h4 mice. Other murine models that spontaneously develop goiter and hypothyroidism include mice expressing transgenes such as IFN-γ or IL-12 in the thyroid (53, 54) and iodine-induced hypothyroidism in SJL mice (55). Those models do not have an autoimmune basis.
The mutant mouse model described here provides an excellent model for studying two organ-specific autoimmune diseases in the same animal, namely autoimmune thyroid disease and SS-like disease of the salivary gland. The coexistence of both diseases in the same mice is consistent with the relatively frequent coexistence of thyroiditis and SS in humans (56). Interestingly, autoimmune thyroid disease in IFN-γ−/− CD28−/−NOD.H-2h4 mice is equivalent in males and females, but females have a much greater incidence and severity of salivary gland infiltration than do males (Fig. 4). The female preponderance of SS-like lesions was also noted in other mouse models including WT NOD.H-2h4 and NOD mice ((29–31, 57) and is also true in humans (57). Essentially all female IFN-γ−/− CD28−/− NOD.H-2h4 mice have salivary gland infiltrates at 4 mo of age, and the number and size of the infiltrates greatly increases at 6 mo of age (Fig. 4D). NaI supplementation of the water, used to facilitate early development of thyroid lesions, has no influence on development of SS-like lesions, since the incidence and focus scores were comparable in experimental mice and retired breeders not given NaI (not shown). Salivary gland infiltration is greater and develops earlier in female IFN-γ−/−CD28−/−NOD.H-2h4 mice than in WT NOD.H-2h4 mice, also used to study SS (8, 29, 31) (Fig.4A–D). CD28 −/− mice have significant loss of salivary function, a clinical manifestation of SS in humans (57, 58) that exceeds that in WT NOD.H-2h4 mice (Fig. 4B, C). WT NOD.H-2h4 mice have ectopic follicles in their salivary gland infiltrates, shown to be derived from splenic germinal centers (31). Because CD28−/− mice lack germinal centers (59), composition of the lymphocytic infiltrates is likely to differ in salivary glands of WT and CD28−/− mice. Our studies did not address this issue. The IFN-γ−/−CD28−/− model is potentially a novel model of SS since it has an early robust onset of lymphocytic infiltration, correlating with loss of salivary gland function. CD28 deficiency also promotes salivary gland infiltration in WT NOD.H-2h4 mice (8) but infiltration is more extensive in IFN-γ−/−CD28−/− mice (our unpublished results). CD28 deficiency has not been studied in other mouse models of SS. IFN-γ was shown to be crucial for development of the SS phenotype in other mouse models (60, 61), but IFN-γ is clearly not required when CD28 is absent. Studies on the role of Tregs in SS have been inconsistent and often contradictory (62). Therefore the IFN-γ−/−CD28−/− mouse model provides a unique tool for investigating mechanisms involved in SS development. The extent to which this model compares to other mouse models of SS (30, 57, 58, 63) remains to be determined.
Finally, development of several spontaneous autoimmune diseases including diabetes, pancreatitis, thyroiditis and SS-like syndrome is greatly promoted in mice lacking CD28, due primarily to reduced numbers of functional Tregs (7, 12 13, 24). While CD28 costimulation is required for activation of T cells responding to foreign antigens (6, 7, 10), self-reactive effector T cells are effectively activated in the Treg-deficient environment in CD28−/− mice. CD28 costimulation may be less critical when effector T cells are chronically stimulated by self-antigen, and/or other costimulatory molecules might be used for T cell activation in spontaneous autoimmune diseases (6, 14). Importantly, IFN-γ−/−CD28−/− NOD.H-2h4 mice represent a powerful new model for the study of autoimmune thyroid disease with hypothyroidism and for the study of SS-like lesions. Also, because SS and autoimmune thyroid disease both develop spontaneously in this model as in humans, this model will be useful for studying the mechanisms by which hypothyroidism might impact salivary gland function.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant AI 074857 (HBM, P.I.), by the Lottie Caroline Hardy Trust., and by NIH Grants RO1DE007389 and RO1DE02332 from the National Institute of Dental & Craniofacial Research (NIDCR) (GAW, P.I.).
Abbreviations used
- TEC H/P
thyrocyte epithelial cell hyperplasia/proliferation
- TEC
thyroid epithelial cell or thyrocyte
- I-SAT
iodine-facilitated spontaneous autoimmune thyroiditis
- T4
thyroxine
- NaI
sodium iodide, SS, Sjogren’s syndrome, B6, C57Bl/6
- MMRRC
Mutant Mouse and Rat Resource Colony
References
- 1.Yu S, Sharp GC, Braley-Mullen H. Thyroid epithelial cell hyperplasia in IFN-gamma deficient NOD.H-2h4 mice. Clin Immunol. 2006;118:92–100. doi: 10.1016/j.clim.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 2.Yu S, Sharp GC, Braley-Mullen H. Thyrocytes responding to IFN-gamma are essential for development of lymphocytic spontaneous autoimmune thyroiditis and inhibition of thyrocyte hyperplasia. J Immunol. 2006;176:1259–1265. doi: 10.4049/jimmunol.176.2.1259. [DOI] [PubMed] [Google Scholar]
- 3.Yu S, Sharp GC, Braley-Mullen H. Dual roles for IFN-gamma, but not for IL-4, in spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Immunol. 2002;169:3999–4007. doi: 10.4049/jimmunol.169.7.3999. [DOI] [PubMed] [Google Scholar]
- 4.Yu S, Fang Y, Sharav T, Sharp GC, Braley-Mullen H. CD8+ T cells induce thyroid epithelial cell hyperplasia and fibrosis. J Immunol. 2011;186:2655–2662. doi: 10.4049/jimmunol.1002884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciornei, Hong RTS, Fang Y, Zhu Z, Braley-Mullen H. Mechanisms and kinetics of proliferation and fibrosis development in a mouse model of thyrocyte hyperplasia. Cell Immunol. 2016;304–305:16–26. doi: 10.1016/j.cellimm.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol Rev. 2011;241:180–205. doi: 10.1111/j.1600-065X.2011.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2:116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 8.Ellis JS, Hong SH, Zaghouani H, Braley-Mullen H. Reduced effectiveness of CD4+Foxp3+ regulatory T cells in CD28-deficient NOD.H-2h4 mice leads to increased severity of spontaneous autoimmune thyroiditis. J Immunol. 2013;191:4940–4949. doi: 10.4049/jimmunol.1301253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis JS, Wan X, Braley-Mullen H. Transient depletion of CD4+ CD25+ regulatory T cells results in multiple autoimmune diseases in wild-type and B-cell-deficient NOD mice. Immunology. 2013;139:179–186. doi: 10.1111/imm.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 11.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 12.Tang Q, Adams JY, Tooley AJ, Bi M, Fife BT, Serra P, Santamaria P, Locksley RM, Krummel MF, Bluestone JA. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meagher C, Tang Q, Fife BT, Bour-Jordan H, Wu J, Pardoux C, Bi M, Melli K, Bluestone JA. Spontaneous development of a pancreatic exocrine disease in CD28-deficient NOD mice. J Immunol. 2008;180:7793–7803. doi: 10.4049/jimmunol.180.12.7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bour-Jordan H, Bluestone JA. Regulating the regulators: costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009;229:41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- 16.Fazio S, Palmieri EA, Lombardi G, Biondi B. Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm Res. 2004;59:31–50. doi: 10.1210/rp.59.1.31. [DOI] [PubMed] [Google Scholar]
- 17.Grais IM, Sowers JR. Thyroid and the heart. Am J Med. 2014;127:691–698. doi: 10.1016/j.amjmed.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Braley-Mullen H, Yu S. NOD.H-2h4 Mice: An Important and Underutilized Animal Model of Autoimmune Thyroiditis and Sjogren's Syndrome. Adv Immunol. 2015;126:1–43. doi: 10.1016/bs.ai.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Kayes T, Fang Y, Yu S, Downey E, Wang S, Braley-Mullen H. Agonistic anti-CD40 induces thyrocyte proliferation and promotes thyroid autoimmunity by increasing CD40 expression on thyroid epithelial cells. J Immunol. 2013;190:3928–3938. doi: 10.4049/jimmunol.1202929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu S, Downey EF, Braley-Mullen H. Agonistic anti-CD40 promotes early development and increases the incidence of severe thyroid epithelial cell hyperplasia (TEC H/P) in CD4−/− mice. Immun Inflamm Dis. 2013;1:14–25. doi: 10.1002/iid3.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen CQ, Yin H, Lee BH, Chiorini JA, Peck AB. IL17: potential therapeutic target in Sjogren's syndrome using adenovirus-mediated gene transfer. Lab Invest. 2011;91:54–62. doi: 10.1038/labinvest.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iizuka M, Tsuboi H, Matsuo N, Asashima H, Hirota T, Kondo Y, Iwakura Y, Takahashi S, Matsumoto I, Sumida T. A crucial role of RORgammat in the development of spontaneous Sialadenitis-like Sjogren's syndrome. J Immunol. 2015;194:56–67. doi: 10.4049/jimmunol.1401118. [DOI] [PubMed] [Google Scholar]
- 23.Kayes TD, Braley-Mullen H. Culture promotes transfer of thyroid epithelial cell hyperplasia and proliferation by reducing regulatory T cell numbers. Cell Immunol. 2013;285:84–91. doi: 10.1016/j.cellimm.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez LP, Lopez-Marquez A, Santisteban P. Thyroid transcription factors in development, differentiation and disease. Nat Rev Endocrinol. 2015;11:29–42. doi: 10.1038/nrendo.2014.186. [DOI] [PubMed] [Google Scholar]
- 25.Hong SH, Braley-Mullen H. Follicular B cells in thyroids of mice with spontaneous autoimmune thyroiditis contribute to disease pathogenesis and are targets of anti-CD20 antibody therapy. J Immunol. 2014;192:897–905. doi: 10.4049/jimmunol.1301628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bour-Jordan H, Salomon BL, Thompson HL, Szot GL, Bernhard MR, Bluestone JA. Costimulation controls diabetes by altering the balance of pathogenic and regulatory T cells. J Clin Invest. 2004;114:979–987. doi: 10.1172/JCI20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bour-Jordan H, Blueston JA. CD28 function: a balance of costimulatory and regulatory signals. J Clin Immunol. 2002;22:1–7. doi: 10.1023/a:1014256417651. [DOI] [PubMed] [Google Scholar]
- 28.Bernard NF, Ertug F, Margolese H. High incidence of thyroiditis and anti-thyroid autoantibodies in NOD mice. Diabetes. 1992;41:40–46. doi: 10.2337/diab.41.1.40. [DOI] [PubMed] [Google Scholar]
- 29.Cihakova D, Talor MV, Barin JG, Baldeviano GC, Fairweather D, Rose NR, Burek CL. Sex differences in a murine model of Sjogren's syndrome. Annals of the New York Academy of Sciences. 2009;1173:378–383. doi: 10.1111/j.1749-6632.2009.04760.x. [DOI] [PubMed] [Google Scholar]
- 30.Delaleu N, Nguyen CQ, Peck AB, Jonsson R. Sjogren's syndrome: studying the disease in mice. Arthritis research & therapy. 2011;13:217. doi: 10.1186/ar3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karnell JL, Mahmoud TI, Herbst R, Ettinger R. Discerning the kinetics of autoimmune manifestations in a model of Sjogren's syndrome. Molecular immunology. 2014;62:277–282. doi: 10.1016/j.molimm.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 32.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725–1735. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]
- 33.Hataya Y, Igarashi S, Yamashita T, Komatsu Y. Thyroid hormone replacement therapy for primary hypothyroidism leads to significant improvement of renal function in chronic kidney disease patients. Clin Exp Nephrol. 2013;17:525–531. doi: 10.1007/s10157-012-0727-y. [DOI] [PubMed] [Google Scholar]
- 34.Krassas GE, Poppe K, Glinoer D. Thyroid function and human reproductive health. Endocr Rev. 2010;31:702–755. doi: 10.1210/er.2009-0041. [DOI] [PubMed] [Google Scholar]
- 35.De Vito P, Balducci V, Leone S, Percario Z, Mangino G, Davis PJ, Davis FB, Affabris E, Luly P, Pedersen JZ, Incerpi S. Nongenomic effects of thyroid hormones on the immune system cells: New targets, old players. Steroids. 2012;77:988–995. doi: 10.1016/j.steroids.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 36.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low-Dose Mineralocorticoid Receptor Blockade Prevents Western Diet-Induced Arterial Stiffening in Female Mice. Hypertension. 2015;66:99–107. doi: 10.1161/HYPERTENSIONAHA.115.05674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jia G, Habibi J, Bostick BP, Ma L, DeMarco VG, Aroor AR, Hayden MR, Whaley-Connell AT, Sowers JR. Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension. 2015;65:531–539. doi: 10.1161/HYPERTENSIONAHA.114.04737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang J, Zheng X, Sun M, Wang Z, Fu Q, Shi Y, Cao M, Zhu Z, Meng C, Mao J, Yang F, Huang X, Xu J, Zhou H, Duan Y, He W, Zhang M, Yang T. Low serum free thyroxine concentrations associate with increased arterial stiffness in euthyroid subjects: a population-based cross-sectional study. Endocrine. 2015;50:465–473. doi: 10.1007/s12020-015-0602-1. [DOI] [PubMed] [Google Scholar]
- 39.Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest. 2013;123:580–593. doi: 10.1172/JCI65013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Strioga M, Pasukoniene V, Characiejus D. CD8+ CD28 and CD8+ CD57+ T cells and their role in health and disease. Immunology. 2011;134:17–32. doi: 10.1111/j.1365-2567.2011.03470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mou D, Espinosa J, Lo DJ, Kirk AD. CD28 Negative T Cells: Is Their Loss Our Gain? American Journal of Transplantation. 2014;14:2460–2466. doi: 10.1111/ajt.12937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maly K, Schirmer M. The story of CD4+ CD28- T cells revisited: solved or still ongoing? Journal of immunology research. 2015;2015:348746. doi: 10.1155/2015/348746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smolenska Z, Pawlowska J, Zdrojewski Z, Daca A, Bryl E. Increased percentage of CD8+CD28- T cells correlates with clinical activity in primary Sjogren's syndrome. Cell Immunol. 2012;278:143–151. doi: 10.1016/j.cellimm.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 44.Hebbar M, Jeannin P, Magistrelli G, Hatron PY, Hachulla E, Devulder B, Bonnefoy JY, Delneste Y. Detection of circulating soluble CD28 in patients with systemic lupus erythematosus, primary Sjogren's syndrome and systemic sclerosis. Clin Exp Immunol. 2004;136:388–392. doi: 10.1111/j.1365-2249.2004.02427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kolypetri P, King J, Larijani M, Carayanniotis G. Genes and Environment as Predisposing Factors in Autoimmunity: Acceleration of Spontaneous Thyroiditis by Dietary Iodide in NOD.H2(h4) Mice. Int Rev Immunol. 2015;34:542–556. doi: 10.3109/08830185.2015.1065828. [DOI] [PubMed] [Google Scholar]
- 46.Leung AM, Braverman LE. Consequences of excess iodine. Nat Rev Endocrinol. 2014;10:136–142. doi: 10.1038/nrendo.2013.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teng X, Shan Z, Teng W, Fan C, Wang H, Guo R. Experimental study on the effects of chronic iodine excess on thyroid function, structure, and autoimmunity in autoimmune-prone NOD.H-2h4 mice. Clin Exp Med. 2009;9:51–59. doi: 10.1007/s10238-008-0014-0. [DOI] [PubMed] [Google Scholar]
- 48.Rapoport B, Aliesky HA, Banuelos B, Chen CR, McLachlan SM. A Unique Mouse Strain That Develops Spontaneous, Iodine-Accelerated, Pathogenic Antibodies to the Human Thyrotrophin Receptor. J Immunol. 2015 doi: 10.4049/jimmunol.1500126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Markou K, Georgopoulos N, Kyriazopoulou V, Vagenakis AG. Iodine-Induced hypothyroidism. Thyroid : official journal of the American Thyroid Association. 2001;11:501–510. doi: 10.1089/105072501300176462. [DOI] [PubMed] [Google Scholar]
- 50.Kolypetri P, Carayanniotis K, Rahman S, Georghiou PE, Magafa V, Cordopatis P, Carayanniotis G. The Thyroxine-Containing Thyroglobulin Peptide (aa 2549–2560) Is a Target Epitope in Iodide-Accelerated Spontaneous Autoimmune Thyroiditis. J Immunol. 2014 doi: 10.4049/jimmunol.1400561. [DOI] [PubMed] [Google Scholar]
- 51.Sharma RB, Alegria JD, Talor MV, Rose NR, Caturegli P, Burek CL. Iodine and IFN-gamma synergistically enhance intercellular adhesion molecule 1 expression on NOD.H2h4 mouse thyrocytes. J Immunol. 2005;174:7740–7745. doi: 10.4049/jimmunol.174.12.7740. [DOI] [PubMed] [Google Scholar]
- 52.Martin AP, Marinkovic T, Canasto-Chibuque C, Latif R, Unkeless JC, Davies TF, Takahama Y, Furtado GC, Lira SA. CCR7 deficiency in NOD mice leads to thyroiditis and primary hypothyroidism. J Immunol. 2009;183:3073–3080. doi: 10.4049/jimmunol.0900275. [DOI] [PubMed] [Google Scholar]
- 53.Kimura H, Kimura M, Westra WH, Rose NR, Caturegli P. Increased thyroidal fat and goitrous hypothyroidism induced by interferon-gamma. Int J Exp Pathol. 2005;86:97–106. doi: 10.1111/j.0959-9673.2005.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kimura H, Tzou SC, Rocchi R, Kimura M, Suzuki K, Parlow AF, Rose NR, Caturegli P. Interleukin (IL)-12-driven primary hypothyroidism: the contrasting roles of two Th1 cytokines (IL-12 and interferon-gamma) Endocrinology. 2005;146:3642–3651. doi: 10.1210/en.2005-0275. [DOI] [PubMed] [Google Scholar]
- 55.Li HS, Carayanniotis G. Induction of goitrous hypothyroidism by dietary iodide in SJL mice. Endocrinology. 2007;148:2747–2752. doi: 10.1210/en.2007-0082. [DOI] [PubMed] [Google Scholar]
- 56.Jara LJ, Navarro C, Brito-Zerón dMP, García-Carrasco M, Escárcega RO, Ramos-Casals M. Thyroid disease in Sjögren’s syndrome. Clinical Rheumatology. 2007;26:1601–1606. doi: 10.1007/s10067-007-0638-6. [DOI] [PubMed] [Google Scholar]
- 57.Chiorini JA, Cihakova D, Ouellette CE, Caturegli P. Sjogren syndrome: advances in the pathogenesis from animal models. J Autoimmun. 2009;33:190–196. doi: 10.1016/j.jaut.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen L, Suresh L, Li H, Zhang C, Kumar V, Pankewycz O, Ambrus JL., Jr IL-14 alpha, the nexus for primary Sjogren's disease in mice and humans. Clin Immunol. 2009;130:304–312. doi: 10.1016/j.clim.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 59.Ferguson SE, Han S, Kelsoe G, Thompson CB. CD28 is required for germinal center formation. J Immunol. 1996;156:4576–4581. [PubMed] [Google Scholar]
- 60.Maria NI, Vogelsang P, Versnel MA. The clinical relevance of animal models in Sjogren's syndrome: the interferon signature from mouse to man. Arthritis research & therapy. 2015;17:172. doi: 10.1186/s13075-015-0678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cha S, Brayer J, Gao J, Brown V, Killedar S, Yasunari U. A dual role for interferon-gamma in the pathogenesis of Sjogren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand J Immunol. 2004:60. doi: 10.1111/j.0300-9475.2004.01508.x. [DOI] [PubMed] [Google Scholar]
- 62.Alunno A, Carubbi F, Bistoni O, Caterbi S, Bartoloni E, Mirabelli G, Cannarile F, Cipriani P, Giacomelli R, Gerli R. T Regulatory and T Helper 17 Cells in Primary Sjogren's Syndrome: Facts and Perspectives. Mediators of inflammation. 2015;2015:243723. doi: 10.1155/2015/243723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Woods LT, Camden JM, Batek JM, Petris MJ, Erb L, Weisman GA. P2X7 receptor activation induces inflammatory responses in salivary gland epithelium. American journal of physiology Cell physiology. 2012;303:C790–801. doi: 10.1152/ajpcell.00072.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.