Abstract
From the moment we are born, every injury to the skin has the potential to form a scar, many of which can impair form and/or function. As such, scar management constitutes a billion-dollar industry. Yet effectively promoting scarless wound healing remains an elusive goal. The complex interactions of wound healing contribute to our inability to recapitulate scarless wound repair as it occurs in nature, such as in fetal skin and oral mucosa. However, many new advances have occurred over recent years, some of which have translated scientific findings from bench to bedside. In vivo lineage tracing has helped establish a variety of novel cellular culprits that may act as key drivers of the fibrotic response. These newly characterized cell populations present further targets for therapeutic intervention, some of which have already demonstrated promising results in animal models. Here, we discuss several recent studies that identify exciting approaches to diminishing scar formation (Fig. 1). Particular attention will also be paid to the canonical Wnt/β-catenin signaling pathway, which plays an important role in both embryogenesis and tissue repair. New insights into the differential effects of Wnt signaling on heterogeneous fibroblast and keratinocyte populations within the skin further demonstrate methods by which wound healing can be redirected to a more fetal, scarless phenotype.
Keywords: Wound healing, Fibroblast, Scarless, Wnt, β-catenin
INTRODUCTION
Human skin has evolved the remarkable ability to repair itself after injury. An unavoidable consequence of the skin’s rapid wound healing process is the formation of fibrotic scar tissue. While the presence of a scar for some is merely a cosmetic concern, scars of the face and large burn scars can be severely debilitating. Children are particularly prone to the effects of scarring and suffer from long-term physical (Sheridan, et al., 2000) and psychological (Robert, et al., 1999) harm after developing large scars from surgery or burns. In the United States, $7.5 billion is spent annually on burn care (Finkelstein, et al., 2006) and much of the effort spent treating burn patients is related to the burn scar. Considering non-burn scars, 80 million surgical incisions (CDC, 2010, Cullen, et al., 2009) and 12 million traumatic lacerations (Singer, et al., 1997) occur in the United States annually, resulting in a potential $12 billion market for the treatment of the resulting scars (Sen, et al., 2009).
The ability to cause cutaneous injuries to heal without fibrosis would revolutionize the care of lacerations, incisions, and burns, and would alleviate an enormous amount of suffering. A major advance in the science of scarring took place when it was recognized that the skin of the fetus in mammals heals without scar early in gestation. The oral mucosa demonstrates a similar privileged scarless phenotype following wounding. Unfortunately, wound therapies that result in tissue regeneration in place of physiological reparative healing with scar formation have yet to be realized clinically.
The more recent appreciation for the functional and topographical heterogeneity of cells collectively known as fibroblasts has advanced our ability to more specifically target cells implicated in wound healing, with fewer systemic consequences. While relevant therapeutic interventions are fairly novel, the generalizability of characterizing and targeting specific subsets of cell populations is likely to open many new doors in treating fibrotic disease. In preparation for a proliferation of such studies, we present an overview of our current ability to modulate the wound healing process in favor of a regenerative, scarless phenotype. Special attention will be paid to canonical Wnt/β-catenin signaling, long understood to influence both embryological development and wound healing, as many recent studies have focused on its influence over specific subsets of cells within the dermis and epidermis.
HETEROGENEITY OF SKIN CELL POPULATIONS
Recent advances in stem cell biology have added insight into our understanding of wound healing. The discovery of long-lived epithelial stem cells (ESCs) in the hair follicle bulge region led to the hypothesis that development and repair were dependent on these cells. However, fate-mapping experiments revealed ESCs in the bulge region do not normally contribute to the epidermis, but respond to wounding by generating short-lived transient amplifying cells (TACs) responsible for acute wound repair. (Ito, et al., 2005) Recently, the cellular heterogeneity of the skin interfollicular epidermis (IFE) has been examined using clonal fate analysis and is shown to consist of slow-cycling stem cells and committed progenitor cells. After wounding, only stem cells contribute substantially to the repair and long-term regeneration of the tissue. (Mascre, et al., 2012)
In the dermis, a population of mesenchymal cells, known as dermal papilla (DP) cells are thought to regulate hair follicle growth and development and serve as a reservoir of multipotent stem cells (Driskell, et al., 2011). Another population of cells, known as skin-derived precursors (SKPs), derive from Sox2+ follicle-associated dermal precursors and have been shown to contribute to dermal maintenance, wound healing, and hair follicle morphogenesis. SKPs self-renew and maintain their pluripotency, exhibiting functional properties of an adult dermal stem cell. (Biernaskie, et al., 2009) Recently, Driskell et al. have shown that dermal fibroblasts arise from two distinct lineages that form the upper and lower dermis. While the upper papillary dermis contributes to re-epithelization and hair follicle formation, it is the lower dermis that comprises the reticular fibroblasts that synthesize the bulk of the fibrillar extracellular matrix (ECM) and mediates dermal repair after wounding. These fibroblasts are the first to infiltrate the wound bed during healing, and they demonstrate a myofibroblastic phenotype with α-smooth muscle actin (α-SMA) expression. (Driskell, et al., 2013)
The appreciation for the functional heterogeneity of dermal fibroblasts has led to advances in our understanding of their responses to signaling pathways during the wound healing process. The distinct lineages of dermal fibroblasts demonstrate differential responses to epidermal Wnt/β-catenin paracrine signaling. Specifically, epidermal Hedgehog (Hh) promotes papillary fibroblast proliferation whereas transforming growth factor-β (TGF-β) meditates ECM remodeling the reticular dermis. Pharmacological inhibition of either TGF-β or Hh was shown to specifically target these fibroblast lineages. (Lichtenberger, et al., 2016) While the authors did not directly study wound healing and scar formation, their findings did corroborate the findings of Rinkevich et al. as they relate to a fibroblast lineage originating from Engrailed-1 (En1)-expressing progenitors. Both the reticular fibroblasts of Lichtenberger, which co-express Sca1 and CD26, and the CD26+ fibroblasts derived from En1+ embryonic cells, were found to comprise 70–80% of all dermal fibroblasts. It is these cells that are responsible for the bulk of connective tissue deposition in the dorsal dermis leading to scar formation. (Lichtenberger, et al., 2016, Rinkevich, et al., 2015) Overall, these studies have improved our understanding of stem cell biology and wound healing, giving hope for improving aberrant wound repair.
SCARLESS WOUND HEALING AS IT OCCURS IN NATURE
Fetal scarless wound healing
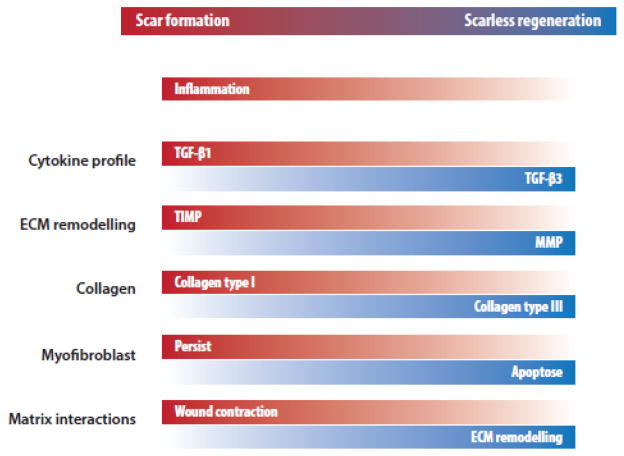
Interestingly, wounds in the early, but not late, mammalian gestational period heal without the formation of scar in a process resembling regeneration (Gurtner, et al., 2008). Although, the exact mechanism is yet unknown, there are many characteristics of the fetal skin that differentiate it from adult skin, both at the cellular and tissue levels (Fig. 2). One notable feature is the decreased tensile strength in early fetal skin. Based on this observation, a study by Wong et al. has elucidated focal adhesion kinase (FAK) as the pathway that ties mechanotransduction with fibrosis. FAK, through extracellular-related kinase (ERK), triggers the secretion of monocyte chemoattractant protein-1 (MCP-1), which has been associated with a number of human fibrotic disorders (Wynn, 2008). By knocking out or inhibiting various components of the inflammatory FAK-ERK-MCP-1 pathway, scar formation was attenuated. (Wong, et al., 2012) This work has translated to the development of a stress-shielding device that has been shown in two randomized controlled clinical trials to reduce scar formation (Lim, et al., 2014, Longaker, et al., 2014). Early gestational cells may also modify their embryonic niche in a way that promotes regenerative healing, while the cells of late gestation may be restricted to reparative healing. As an example, human keratinocytes from mid-gestation have the ability to modulate fibroblast gene expression, causing increased proliferation and migration relative to co-culture with late-gestation fibroblasts (Wang, et al., 2015). Moreover, proteome analysis of cultured fibroblasts revealed different patterns of protein expression when comparing adult and fetal fibroblasts, further supporting scar formation during wound healing as a cell-intrinsic property (Ho, et al., 2014).
Figure 2. Features of reparative scar formation and scarless regeneration in wound healing.
ECM, extracellular matrix; MMP, matrix metalloproteinase; TGF-β, transforming growth factor beta; TIMP, tissue inhibitor of metalloproteinase.
Oral mucosa
In adult mammals, healing of the oral mucosa provides the closest example of regenerative healing as it demonstrates very minimal scarring. Inflammation at the wound bed is known to contribute to the formation of a scar. Both oral mucosa and fetal skin may be considered as “protected environments” for wound healing. Human fetal skin was observed to heal without scar when transplanted subcutaneously into immunodeficient nude mice, while cutaneous transplantation of age-matched fetal skin resulted in scar formation (Lorenz, et al., 1992). Similarly, in the oral cavity salivary mucins form hydrophilic viscoelastic gels, which in turn act as barriers protecting the underlying mucosa from mechanical damage and direct interaction with pathogens and other harmful substances (Amerongen and Veerman, 2002). However, the role of this “protected environment” was determined to be necessary but insufficient to achieve scarless wound healing, given that adult skin transplanted to the fetus heals with a scar following incisional wounding (Longaker, et al., 1994).
Analysis of healthy human oral mucosa revealed significantly lower numbers of neutrophils and macrophages, compared to healthy skin (Glim, et al., 2015). However, the authors were unable to study these differences in acute wounds. The attenuated inflammatory environment of the oral mucosa likens it to the fetal wound healing environment and may partially explain the similarities in reduced scar formation. ECM composition prior to wounding has also been hypothesized as a contributor to privileged scarless healing. Relative to skin, the oral mucosa exhibits increased fibronectin, its splice variant ED-A, and chondroitin sulfate in the former. Pro-fibrotic myofibroblast α-SMA expression relies on cell surface ED-A fibronectin interaction in addition to TGF-β1 (Tomasek, et al., 2002). Conversely, the presence of elastin was more pronounced in the skin. Additionally, the rate of proliferation was higher in oral keratinocytes, with keratinocytes located in the dermis demonstrating increased differentiation. (Glim, et al., 2014) The unique characteristics of the oral mucosa relative to the cutaneous skin contribute to its ability to heal with minimal scarring. However, significant advances are required to establish this privileged healing in cutaneous wounds.
RECENT ADVANCES IN SCARLESS WOUND HEALING RESEARCH
Administration of therapeutics with known anti-inflammatory activity
Fetal scarless wound healing is associated with significantly reduced inflammation, whereas pathological scarring often involves an unregulated inflammatory response (Larson, et al., 2010). Therefore, significant effort has been devoted to modulating the inflammatory processes that may negatively impact wound healing. Utilization of physiological anti-inflammatory molecules offers the potential to direct wound healing down regenerative pathways. For example, systemic administration of the neuropeptide α-melanocyte-stimulating hormone (α-MSH) led to a more regenerative healing process characterized by less macroscopic scar formation as well as organization of collagen fibers more closely mimicking unwounded skin (de Souza, et al., 2015). While this effect required administration of α-MSH prior to wounding, prophylactic administration of anti-fibrotic agents is not without clinical utility, particularly in cases such as scar revision surgery.
Fibroblast growth factor 9 (Fgf9) also provides a means for modulating the inflammatory environment, for example by promoting monocyte to M2 macrophage differentiation in cardiac ischemic injury (Singla, et al., 2015). Importantly in cutaneous injury, Fgf9 from dermal γδ T cells was shown by Gay et al. to facilitate follicle neogenesis. In humans, wound healing with scar formation occurs without regeneration of hair follicles. Given that Fgf9 overexpression potentiates increased follicle neogenesis in mice, these findings may translate to therapies promoting regenerative healing. (Gay, et al., 2013)
In a study by Morris et al. the authors aimed to better understand how wound size and inflammation impact fetal ability to undergo regenerative healing rather than scar formation. Larger (8 mm) wounds created in fetal lambs were found to heal with scar formation while 2 mm wounds healed without scar. These findings also correlated with different gene expression profiles and increased inflammation in large fetal wounds. Administration of lentiviral IL-10 resulted in restoration of normal skin architecture and attenuation of pro-inflammatory gene expression. (Morris, et al., 2014) However, use of viral vectors is greatly limited in clinical use due to the risks of oncogenesis. Low concentrations of recombinant human interkleukin-10 injected intradermally in humans prior to incision showed improved macroscopic scar appearance at 12 months (Kieran, et al., 2013).
Findings of upregulated stromal cell-derived factor 1 (SDF-1)/C-X-C chemokine receptor type 4 (CXCR-4) after burn injury and subsequent hypertrophic scar formation have also led to novel therapeutic targets as CXCR-4 antagonism inhibits inflammatory cell recruitment and fibroblast activation. In this case, Ding et al. transplanted split-thickness human skin grafts into full-thickness wounds in nude mice. Serial injection of the CXCR-4 antagonist CTCE-9908 resulted in grafts that remained flat, with only limited contraction, contrary to findings in controls demonstrating more hypertrophic scars. Results of associated gene and protein expression studies, coupled with analysis of tissue architecture and cell recruitment, collectively suggest that CXCR-4 antagonism exhibits a potent anti-fibrotic and pro-regenerative phenotype. (Ding, et al., 2014) Limiting the fibrotic response following skin grafting has the potential to reduce functional deficits due to contractures, particularly when grafts are placed over mobile joints. Furthermore, such therapies may preclude the need to harvest full-thickness skin grafts, which demonstrate less contraction relative to split-thickness skin grafts.
Cellular targets for scarless wound healing
Advances in developmental biology, by lineage tracing and transplantation assays, have allowed researchers to identify populations of cells, contributing to scar formation. Dulauroy et al. utilized transient expression of a disintegrin and metalloprotease 12 (ADAM12) to distinguish a pro-inflammatory subset of perivascular cells that are activated upon acute injury in muscle and dermis. Ablating these cells, or knocking down ADAM12, was sufficient to decrease fibrosis and scarring. (Dulauroy, et al., 2012) Recent studies by Rinkevich et al. have further elucidated the heterogenic nature of dermal fibroblast populations. Of particular interest, fibroblasts originating from En1-lineages have been identified as the main culprits in cutaneous scarring that occurs beyond early gestation. These cells act as the major contributor of connective tissue in scar formation. Concomitant expression of CD26 (also known as dipeptidyl peptidase-4, DPP4) surface marker allowed selective abrogation of the En1-fibroblast lineage with diprotin A. Treatment with this small molecule reduced cutaneous scarring secondary to full-thickness excisional wounding without compromising the integrity of the healed tissue. (Rinkevich, et al., 2015) In vivo experiments have demonstrated the ability of DPP4 inhibitors to curb the fibrogenic phenotypes of keloid-derived fibroblasts as well as normal fibroblasts. The underlying mechanisms associated with the observed decrease in collagen production and TGF-β1 expression involve the pro-fibrotic pp38 and pERK1/2 pathways. (Thielitz, et al., 2008)
Myofibroblasts are key players in the normal wound healing response, contributing to wound contraction and ECM production (Gurtner, et al., 2008). Cells demonstrating persistent myofibroblast-like phenotypes are key contributors to fibrosis, associated with excess ECM deposition and disinhibited contraction make them ideal targets to reduce scar formation (Powell, et al., 1999). However, normally as the scar enters the maturation phase, these cells largely undergo apoptosis (Desmouliere, et al., 1995). Reversal of the myofibroblast phenotype may also contribute to decreased numbers of these cells (Desai, et al., 2014, Maltseva, et al., 2001). Additionally, myofibroblasts (or myofibroblast-like cells) have also been determined to be a functionally heterogeneous cell population, with numerous potential precursors, including fibroblasts, mesenchymal stem cells (MSCs), smooth muscle cells, endothelial cells, and fibrocytes (Hinz, et al., 2007). Adipocytes have also been found to appear simultaneously during wound healing. While it is unclear if fibroblasts and adipocytes share a common progenitor, Schmidt et al. have demonstrated that dermal adipocytes, via intercellular communication, are necessary for fibroblast recruitment in wound healing (Schmidt and Horsley, 2013). Thus, experimental interventions have been targeted at a variety of cell populations, both directly and indirectly responsible for scar formation.
Studies conducted by Desai et al. have demonstrated both the ability to direct differentiation of myofibroblast precursors (adipose-derived mesenchymal stem cells, ASCs) away from a myofibroblastic phenotype, as well as reverse differentiate cells towards a more fibroblast-like phenotype. Specifically, they showed that myofibroblastic cells treated with basic fibroblast growth factor (bFGF) led to diminished expression of α-SMA, collagen I, and fibronectin, as well as a loss of focal adhesions and stress fibers. These cells were also more migratory, associated with tenascin-C and vimentin upregulation, in-line with a more fibroblast-like phenotype. Their findings suggest that the myofibroblast differentiation process is not terminal, elucidating bFGF as a phenotypic reversing agent. (Desai, et al., 2014)
Extracorporeal shockwaves (ESW), more commonly used in urological or orthopedic interventions, may also have a role in reducing scar formation. ESW treatment caused myofibroblast precursors to differentiate into more fibroblast-like cells with lower contractility and higher migration potential, simultaneously reducing α-SMA and type I collagen expression. (Rinella, et al., 2016)
Stem cells and scarless wound healing
Stem cell-based wound therapies are generally directed at treating recalcitrant wounds, while their potential for scarless wound healing has been less well established in vivo. Stem cells have certainly demonstrated an ability to modulate the wound environment to improve healing, in part by reducing inflammation (Corcione, et al., 2006, Di Nicola, et al., 2002, Loots, et al., 1998). However, whether this translates to significant decreases in scarring is unclear as a result of sometimes conflicting findings (Doi, et al., 2016, Sabapathy, et al., 2014). Furthermore, practical barriers associated with stem cell-based therapies may restrict their utility in pursuit of scarless wound healing (Nuschke, 2014). Despite the potential limitations of direct stem cell-based scarless wound therapies, one recent study by Li et al. demonstrated the potential benefits of conditioned media from umbilical cord (UC)-MSC cultures. Dermal fibroblasts under the paracrine influence of these cells exhibited characteristics reminiscent of fetal fibroblasts, such as low capacity to form myofibroblasts, a decreased TGF-β1/TGF-β3 ratio, and increased expression of enzymes involved in ECM remodeling (matrix metalloproteinases, MMPs). Wounds treated with the UC-MSC conditioned media also healed faster with decreased collagen accumulation. (Li, et al., 2015) Additionally, in vitro studies have shown that human amniotic fluid-derived MSC conditioned media has the potential to inhibit the pro-fibrotic actions of TGF-β1 and even reverse the myofibroblast phenotype to a fibroblast-like state. Conditioned media from ASCs produced similar results, but to a lesser extent. (Mia and Bank, 2015) Taken together, these findings suggest that stem cells may be able to promote a scarless phenotype without the requirement of direct inoculation to the wound bed, drastically reducing the risk of resultant oncogenesis (Fig. 3).
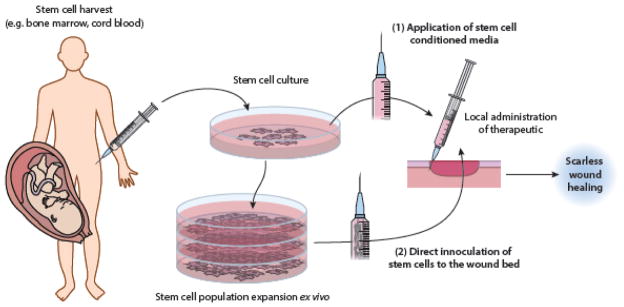
Figure 3. Stem cell-based therapies to promote scarless wound healing.
Schematic showing general principles of two cell-based therapeutic methodologies, (1) application of stem cell conditioned media and (2) direct application of stem cells to the wound bed. The poor survivability of mesenchymal stem cells (MSCs) transplanted to the wound bed has prompted development of other novel therapies that take advantage of the paracrine mechanisms of action of these cells. Application of conditioned media from umbilical cord blood-derived MSC (UCB-MSC) culture is one such example (Doi, et al., 2016).
Wnt and regeneration
Analysis of gene expression in fetal mouse keratinocytes and fibroblasts at embryonic day (E)16 and E18 time points, straddling the transition from scarless to scar forming repair, has revealed numerous differentially regulated pathways, such as canonical Wnt/β-catenin signaling; many others have yet to be examined under the lens of wound healing (Hu, et al., 2014). These pathways create numerous novel targets to modulate the wound healing process towards a more scarless phenotype (Fig. 4).
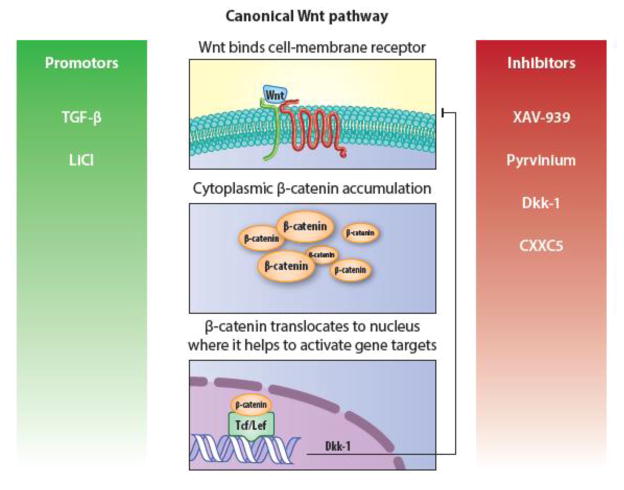
Figure 4. Brief overview of canonical Wnt signaling and its associated targets.
Wnt binding results in cytoplasmic accumulation of β-catenin, which is degraded in the absence of ligand binding. Subsequently, β-catenin translocates to the nucleus where it interacts with T cell-specific transcription factor/lymphoid enhancer-binding factor (Tcf/Lef) in order to modulate gene expression. CXXC5 (CXXC Finger Protein 5); Dkk-1, Dickkopf-1; LiCl, lithium chloride; TGF-β, transforming growth factor beta.
Wnt signaling is a key component of embryological development but is also involved in all phases of the wound healing response. Of the various subsets of Wnt signaling pathways, the canonical Wnt pathway is most relevant to tissue regeneration and repair, and will be the focus of this section. In this pathway, Wnt ligand binding at the cell surface leads to cytoplasmic β-catenin accumulation, which would otherwise be degraded by the APC/Axin/GSK3 complex. β-catenin subsequently translocates to the nucleus to exert its effects as a transcriptional co-activator. (Houschyar, et al., 2015) This section will focus on the influence of Wnt signaling in wound healing as it pertains to scar formation. For more in-depth review of the role of Wnt signaling in tissue renewal and regeneration, the reader is directed to the following review article (Clevers, et al., 2014).
Multiple studies have elucidated a link between TGF-β and the canonical Wnt/β-catenin pathway in fibrosis. For example, analysis of pathological scars (hypertrophic scars and keloids) uncovered upregulated Wnt signaling secondary to TGF-β (Sato, 2006). Even in normal wound healing with scar formation, β-catenin levels have been shown to double during the proliferative phase (Cheon, et al., 2002). Another study found that human keloids display Wnt-3a overexpression, inducing fibroblasts of endothelial origin to transition to mesenchymal cells, which in turn lead to collagen accumulation (Lee, et al., 2015b). While Wnt-3a downregulates CD31 expression, it is unclear if these cells are directly related to the aforementioned ADAM12+ lineage, which are CD31− (Dulauroy, et al., 2012). Future studies may translate our understanding of these endothelial or perivascular progenitors to eliminate fibrosis in wound healing.
While TGF-β signaling is central to the pro-fibrotic fibroblast phenotype, several studies have shown that the Wnt pathway is an important mediator. For example, Cheon et al. demonstrated that TGF-β1 is unable to promote hyperplastic wounds in transgenic mice lacking β-catenin (Cheon, et al., 2006). Akmetshina et al. found that TGF-β downregulates the Dickkopf-1 (Dkk-1) Wnt-antagonist, thereby upregulating the canonical Wnt signaling pathway. This claim was further substantiated by enhanced Wnt and decreased Dkk-1 expression in fibrotic human tissue samples. It thus follows that overexpression of endogenous Dkk-1 in a transgenic mouse line prevented bleomycin-induced fibrosis. (Akhmetshina, et al., 2012) However, topical application of Dkk-1 in a full-thickness excisional model inhibited wound healing (Shi, et al., 2015). Therefore, harnessing Dkk-1 for therapeutic intervention will require further insight into its mechanism of action, particularly as it relates to fibrotic etiology and means of administration.
Modulation of the Wnt/β-catenin pathway is not restricted to the use of endogenous mediators. Bastakoty et al. demonstrated the potential therapeutic nature of small molecules promote a regenerative wound healing phenotype. Topical application of Wnt pathway inhibitors (XAV-939, a tankyrase inhibitor, and pyrvinium, a casein kinase activator) led to formation of rete pegs and dermal papillae following wound closure. These structures are found in intact skin rather than a mature scar. Scar formation was also inhibited, as demonstrated by fewer myofibroblasts expressing α-SMA and reduced type I collagen synthesis. Wnt activation with lithium chloride (LiCl) in turn reduced regenerative repair. (Bastakoty, et al., 2015) However, in a full thickness excisional wound model in rats, topical LiCl application demonstrated reduction in gross scar appearance without impacting time to wound closure (Shi, et al., 2015). As in the case with therapeutic Dkk-1 administration, more studies are needed to produce the desired regenerative healing phenotype in vivo.
A recent study by Lee et al. demonstrates the balance that must be struck in regulating pathways of wound healing, such as Wnt, in the pursuit of improved wound healing. Here the authors demonstrated that CXXC5, a zinc finger family protein, inhibits β-catenin and blocks wound healing in part by decreasing contraction, migration, and collagen production in dermal fibroblasts/myofibroblast-like features of dermal fibroblasts. The authors subsequently developed synthetic peptides to block interactions between CXXC5 and Dishevelled (Dvl), a mediator of Wnt signaling, which together lead to β-catenin degradation. While topical treatment of cutaneous wounds in mice resulted in faster healing, which could be further accelerated with positive Wnt regulators, this therapy may result in more scar formation. However, the authors did not quantify final scar size across treatment and control groups. (Lee, et al., 2015a)
Not only do cells exhibit different genetic expression profiles as they transition into the adult scar-forming phenotype, developmental stage and tissue of origin dramatically impact how fibroblasts respond to Wnt signaling (Lam and Gottardi, 2011). For example, Carre et al. elucidated differences between mouse embryonic (E16.5) fibroblasts and postnatal (P1) fibroblasts following stimulation with Wnt-3a. Wnt-3a increased postnatal, but not fetal, fibroblast proliferation, with concomitant collagen type I synthesis. Furthermore, recombinant Wnt-3a produced similar findings to administration of pro-fibrotic TGf-β1 and also shifted postnatal fibroblast expression of TGF-β isoforms towards that of scarring wounds, with increased TGF-β1 and decreased TGF-β3. Finally, based on gene expression analyses, endogenous Wnt signaling was found to be elevated, but to a substantially lesser degree in fetal cutaneous wound repair compared with adult skin. (Carre, et al., 2010)
Despite these findings, by inducing sustained epidermal β-catenin signaling, Collins et al. observed increased fibroblast proliferation and enhanced remodeling of the dermal ECM at levels akin to neonatal dermis. The connective tissue composition also more closely resembled developing skin. (Collins, et al., 2011) Furthermore, the activation of epidermal Wnt/β-catenin signaling can cause expansion of the upper dermal fibroblast lineage, which in turn allows hair follicle growth not traditionally observed in physiological wound healing (Driskell, et al., 2013, Gurtner, et al., 2008). This observation has led some to conclude that the upper dermal fibroblasts could be utilized as a means for resolving scar formation and promoting scarless wound healing, given their more abated fibrillary collagen production relative to the lower dermis lineage (Driskell and Watt, 2015).
Many Wnt studies in skin have focused on its role in hair follicle neogenesis and its involvement in cutaneous homeostasis and regeneration. While researchers such as Bastakoty et al. specifically targeted the dermis with topical application of Wnt modulators/inhibitors, many other studies into the skin regenerative phenotype have focused on follicular neogenesis, relying on epithelial or follicle-specific paracrine Wnt signaling (Bastakoty, et al., 2015, Ito, et al., 2007). Following full-thickness wound epithelialization, Ito et al. noted that formation of de novo hair follicles both requires Wnt signaling and is promoted by Wnt overexpression. Given that mammalian hair follicles are believed to form only during embryonic development, the authors postulated that wounding could induce an embryonic phenotype in the skin, which in turn can be regulated by Wnt proteins. (Ito, et al., 2007) Specific models used to study the impact of Wnt in scar formation may differ in part due to the transient mode of action and the different means by which this pathway is manipulated experimentally. De la Roche et al. noted that long-term Wnt activation limits the efficacy of small molecule Wnt inhibitors (de la Roche, et al., 2014). The differential outcomes of Wnt stimulation based on target tissues, partly related to the mode of Wnt pathway modulation, is likely to account for some of the aforementioned discrepancies relating to upregulation of the canonical pathway either promoting or inhibiting scarless wound healing.
MicroRNA (miRNA)
MicroRNA (miRNA) gene therapies are another more recent avenue for potential therapeutic intervention. These molecules exert an inhibitory role on mRNA transcription within eukaryotic cells, effectively silencing genes at a post-transcriptional level (Sen and Ghatak, 2015). Comparison of genome-wide miRNA expression between mid- (E16) and late-gestational (E19) mouse skin discovered global repression of these molecules at the earlier time point, which is known to heal without scar formation (Cheng, et al., 2010). In human fetal keratinocytes, Zhao et al. established the miR-34 family as potential candidates for scarless wound healing. Expression of these miRNAs was significantly lower in late- as compared to mid-gestational keratinocytes. This family may also heavily suppress genes involved throughout the TGF-β pathway. (Zhao, et al., 2015) Numerous tools have been developed to modulate cell and tissue miRNA levels, several of which have been directed towards regenerative wound healing. For example, miR-145, has been found at three times normal levels in hypertrophic scars and pro-fibrotic TGF-β1-induced myofibroblasts. Using a commercial inhibitor of miR-145, Gras et al. were able to show a significant decrease in type I collagen expression, TGF-β1 secretion, and contractility in skin myofibroblasts. (Gras, et al., 2015) Additionally, miRNAs can be combined with biomimetic scaffolds to enhance wound healing, furthering their clinical potential (Monaghan, et al., 2014). Future in vivo studies will hopefully enumerate the clinical potential for other miRNA-based therapies.
CONCLUSION
Aberrant wound healing, scarring, and fibrosis results in a tremendous burden on public health. Thankfully in this regard, recent advances in stem cell and developmental biology have elucidated novel pathways and cell types involved in scarring and fibrosis. However, to accelerate generalizability and translation, future studies aimed at assessing therapeutic potential for scarless wound healing would benefit from a more standardized model. It is not uncommon for decreased scarring to be interpreted from histological data showing less collagen staining. While collagen accumulation is a fundamental component of scar formation, clinically speaking, it is the macroscopic cutaneous scarring that most negatively impacts form and function following wound healing. Therefore, studies would benefit from a macroscopic assessment of scar size in wound healing models. Additionally, translation of research findings may be aided through utilization of a humanized wound model, relying either on porcine tissue or splinted murine skin, as loose-skinned animals heal primarily by contraction whereas humans heal by granulation tissue formation (Galiano, et al., 2004). Increased standardization and rigor in defining what truly promotes scarless wound healing in vivo will also improve generalizability and translatability of findings.
While stem cell-based therapies may provide a more complex mélange of factors to promote scarless wound healing, there exist many barriers to their utility. By definition stem cells are multipotent or pluripotent and thus carry a potential risk of tumor formation. These cells also exhibit difficulty with survival and engraftment following administration. However, the latter may be less of an issue given their largely paracrine mechanisms of action and therapeutic benefits of stem cell conditioned media. (Cerqueira, et al., 2015) Conversely, molecular therapies offer increased specificity in their targets, allowing for more predictable effects. However, individual agents may be inadequate to successfully augment the complex wound healing process to the more privileged regenerative phenotype. Combinations of endogenous and exogenous wound healing modulators may likely prove to be the most practical means of successfully and reproducibly achieving scarless wound repair in patients. As our understanding of regenerative over reparative healing increases, translation of an improved understanding of scarring from fundamental science can result in novel clinical therapeutics.
Figure 1. Recent approaches to reducing scar formation.
Schematic showing novel scientific approaches for decreasing scar formation, including targeting pro-fibrotic cell populations based on surface molecule expression. Modulation of cellular mechanotransduction pathways are another means to reduce scar formation, both at the molecular level, or macroscopically with dressings designed to offload tension at cutaneous wound sites. ADAM12, a disintegrin and metalloprotease 12; DPP4, dipeptidyl peptidase-4; FAK, focal adhesion kinase.
Acknowledgments
Funding: This work was supported in part by the California Institute for Regenerative Medicine (CIRM) Clinical Fellow training grant TG2-01159 (to M.S.H.), the Stanford University School of Medicine Transplant and Tissue Engineering Fellowship Award (to M.S.H.), the American Society of Maxillofacial Surgeons (ASMS)/Maxillofacial Surgeons Foundation (MSF) Research Grant Award (to M.S.H., H.P.L., and M.T.L.), the American College of Surgeons Resident Research Scholarship (to C.D.M.), the Howard Hughes Medical Institute (to L.A.B.), NIH grant R01 GM087609 (to H.P.L.), a gift from Ingrid Lai and Bill Shu in honor of Anthony Shu (to H.P.L.), the Hagey Laboratory for Pediatric Regenerative Medicine and The Oak Foundation (to H.P.L. and M.T.L.), NIH grant U01 HL099776 (to M.T.L.), and the Gunn/Olivier fund (to M.T.L.).
Footnotes
Financial Disclosure: The authors do not have any conflicting financial interests to disclose.
References
- Akhmetshina A, Palumbo K, Dees C, Bergmann C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, Schneider H, Sadowski A, Riener M-O, MacDougald OA, Distler O, Schett G, Distler JHW. Activation of canonical Wnt signalling is required for TGF-β-mediated fibrosis. Nat Commun. 2012;3:735. doi: 10.1038/ncomms1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amerongen AV, Veerman EC. Saliva--the defender of the oral cavity. Oral Dis. 2002;8:12–22. doi: 10.1034/j.1601-0825.2002.1o816.x. [DOI] [PubMed] [Google Scholar]
- Bastakoty D, Saraswati S, Cates J, Lee E, Nanney LB, Young PP. Inhibition of Wnt/beta-catenin pathway promotes regenerative repair of cutaneous and cartilage injury. FASEB J. 2015;29:4881–4892. doi: 10.1096/fj.15-275941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J, Paris M, Morozova O, Fagan BM, Marra M, Pevny L, Miller FD. SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell stem cell. 2009;5:610–623. doi: 10.1016/j.stem.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carre AL, James AW, MacLeod L, Kong W, Kawai K, Longaker MT, Lorenz HP. Interaction of wingless protein (Wnt), transforming growth factor-beta1, and hyaluronan production in fetal and postnatal fibroblasts. Plast Reconstr Surg. 2010;125:74–88. doi: 10.1097/PRS.0b013e3181c495d1. [DOI] [PubMed] [Google Scholar]
- CDC Centers for Disease Control and Prevention. Procedures by selected patient characteristics—Number by procedure category and age. 2010. National hospital discharge survey: 2010 table. [Google Scholar]
- Cerqueira MT, Pirraco RP, Marques AP. Stem Cells in Skin Wound Healing: Are We There Yet? Advances in Wound Care. 2015 doi: 10.1089/wound.2014.0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Yu H, Deng S, Shen G. MicroRNA profiling in mid- and late-gestational fetal skin: implication for scarless wound healing. Tohoku J Exp Med. 2010;221:203–209. doi: 10.1620/tjem.221.203. [DOI] [PubMed] [Google Scholar]
- Cheon SS, Cheah AY, Turley S, Nadesan P, Poon R, Clevers H, Alman BA. beta-Catenin stabilization dysregulates mesenchymal cell proliferation, motility, and invasiveness and causes aggressive fibromatosis and hyperplastic cutaneous wounds. Proc Natl Acad Sci U S A. 2002;99:6973–6978. doi: 10.1073/pnas.102657399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheon SS, Wei Q, Gurung A, Youn A, Bright T, Poon R, Whetstone H, Guha A, Alman BA. Beta-catenin regulates wound size and mediates the effect of TGF-beta in cutaneous healing. Faseb j. 2006;20:692–701. doi: 10.1096/fj.05-4759com. [DOI] [PubMed] [Google Scholar]
- Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- Collins CA, Kretzschmar K, Watt FM. Reprogramming adult dermis to a neonatal state through epidermal activation of β-catenin. Development. 2011;138:5189–5199. doi: 10.1242/dev.064592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- Cullen KA, Hall MJ, Golosinskiy A. Ambulatory surgery in the United States, 2006. National health statistics reports. 2009:1–25. [PubMed] [Google Scholar]
- de la Roche M, Ibrahim AE, Mieszczanek J, Bienz M. LEF1 and B9L shield beta-catenin from inactivation by Axin, desensitizing colorectal cancer cells to tankyrase inhibitors. Cancer Res. 2014;74:1495–1505. doi: 10.1158/0008-5472.CAN-13-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza KS, Cantaruti TA, Azevedo GM, Jr, Galdino DA, Rodrigues CM, Costa RA, Vaz NM, Carvalho CR. Improved cutaneous wound healing after intraperitoneal injection of alpha-melanocyte-stimulating hormone. Exp Dermatol. 2015;24:198–203. doi: 10.1111/exd.12609. [DOI] [PubMed] [Google Scholar]
- Desai VD, Hsia HC, Schwarzbauer JE. Reversible modulation of myofibroblast differentiation in adipose-derived mesenchymal stem cells. PLoS One. 2014;9:e86865. doi: 10.1371/journal.pone.0086865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmouliere A, Redard M, Darby I, Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995;146:56–66. [PMC free article] [PubMed] [Google Scholar]
- Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, Grisanti S, Gianni AM. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99:3838–3843. doi: 10.1182/blood.v99.10.3838. [DOI] [PubMed] [Google Scholar]
- Ding J, Ma Z, Liu H, Kwan P, Iwashina T, Shankowsky HA, Wong D, Tredget EE. The therapeutic potential of a C-X-C chemokine receptor type 4 (CXCR-4) antagonist on hypertrophic scarring in vivo. Wound Repair Regen. 2014;22:622–630. doi: 10.1111/wrr.12208. [DOI] [PubMed] [Google Scholar]
- Doi H, Kitajima Y, Luo L, Yan C, Tateishi S, Ono Y, Urata Y, Goto S, Mori R, Masuzaki H, Shimokawa I, Hirano A, Li TS. Potency of umbilical cord blood- and Wharton’s jelly-derived mesenchymal stem cells for scarless wound healing. Sci Rep. 2016;6:18844. doi: 10.1038/srep18844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. Journal of cell science. 2011;124:1179–1182. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Lichtenberger BM, Hoste E, Kretzschmar K, Simons BD, Charalambous M, Ferron SR, Herault Y, Pavlovic G, Ferguson-Smith AC, Watt FM. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature. 2013;504:277–281. doi: 10.1038/nature12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driskell RR, Watt FM. Understanding fibroblast heterogeneity in the skin. Trends Cell Biol. 2015;25:92–99. doi: 10.1016/j.tcb.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Dulauroy S, Di Carlo SE, Langa F, Eberl G, Peduto L. Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med. 2012;18:1262–1270. doi: 10.1038/nm.2848. [DOI] [PubMed] [Google Scholar]
- Finkelstein E, Corso PS, Miller TR. The incidence and economic burden of injuries in the United States. Oxford University Press; Oxford ; New York: 2006. [Google Scholar]
- Galiano RD, Michaels Jt, Dobryansky M, Levine JP, Gurtner GC. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004;12:485–492. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, Holler PD, Ito M, Yang Z, Treffeisen E, Kim CD, Nace A, Zhang X, Baratono S, Wang F, Ornitz DM, Millar SE, Cotsarelis G. Fgf9 from dermal [gamma][delta] T cells induces hair follicle neogenesis after wounding. Nat Med. 2013;19:916–923. doi: 10.1038/nm.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glim JE, Beelen RH, Niessen FB, Everts V, Ulrich MM. The number of immune cells is lower in healthy oral mucosa compared to skin and does not increase after scarring. Archives of oral biology. 2015;60:272–281. doi: 10.1016/j.archoralbio.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Glim JE, Everts V, Niessen FB, Ulrich MM, Beelen RH. Extracellular matrix components of oral mucosa differ from skin and resemble that of foetal skin. Archives of oral biology. 2014;59:1048–1055. doi: 10.1016/j.archoralbio.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Gras C, Ratuszny D, Hadamitzky C, Zhang H, Blasczyk R, Figueiredo C. miR-145 Contributes to Hypertrophic Scarring of the Skin by Inducing Myofibroblast Activity. Mol Med. 2015;21:296–304. doi: 10.2119/molmed.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S, Marcal H, Foster LJ. Towards scarless wound healing: a comparison of protein expression between human, adult and foetal fibroblasts. Biomed Res Int. 2014;2014:676493. doi: 10.1155/2014/676493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houschyar KS, Momeni A, Pyles MN, Maan ZN, Whittam AJ, Siemers F. Wnt signaling induces epithelial differentiation during cutaneous wound healing. Organogenesis. 2015;11:95–104. doi: 10.1080/15476278.2015.1086052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MS, Januszyk M, Hong WX, Walmsley GG, Zielins ER, Atashroo DA, Maan ZN, McArdle A, Takanishi DM, Jr, Gurtner GC, Longaker MT, Lorenz HP. Gene expression in fetal murine keratinocytes and fibroblasts. J Surg Res. 2014;190:344–357. doi: 10.1016/j.jss.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11:1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- Ito M, Yang Z, Andl T, Cui C, Kim N, Millar SE, Cotsarelis G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature. 2007;447:316–320. doi: 10.1038/nature05766. [DOI] [PubMed] [Google Scholar]
- Kieran I, Knock A, Bush J, So K, Metcalfe A, Hobson R, Mason T, O’Kane S, Ferguson M. Interleukin-10 reduces scar formation in both animal and human cutaneous wounds: results of two preclinical and phase II randomized control studies. Wound repair and regeneration. 2013;21:428–436. doi: 10.1111/wrr.12043. [DOI] [PubMed] [Google Scholar]
- Lam AP, Gottardi CJ. beta-catenin signaling: a novel mediator of fibrosis and potential therapeutic target. Curr Opin Rheumatol. 2011;23:562–567. doi: 10.1097/BOR.0b013e32834b3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg. 2010;126:1172–1180. doi: 10.1097/PRS.0b013e3181eae781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kim MY, Kim HY, Lee YM, Kim H, Nam KA, Roh MR, Min do S, Chung KY, Choi KY. The Dishevelled-binding protein CXXC5 negatively regulates cutaneous wound healing. J Exp Med. 2015a;212:1061–1080. doi: 10.1084/jem.20141601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WJ, Park JH, Shin JU, Noh H, Lew DH, Yang WI, Yun CO, Lee KH, Lee JH. Endothelial-to-mesenchymal transition induced by Wnt 3a in keloid pathogenesis. Wound Repair Regen. 2015b;23:435–442. doi: 10.1111/wrr.12300. [DOI] [PubMed] [Google Scholar]
- Li M, Luan F, Zhao Y, Hao H, Liu J, Dong L, Fu X, Han W. Mesenchymal stem cell-conditioned medium accelerates wound healing with fewer scars. Int Wound J. 2015 doi: 10.1111/iwj.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenberger BM, Mastrogiannaki M, Watt FM. Epidermal [beta]-catenin activation remodels the dermis via paracrine signalling to distinct fibroblast lineages. Nat Commun. 2016;7 doi: 10.1038/ncomms10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim AF, Weintraub J, Kaplan EN, Januszyk M, Cowley C, McLaughlin P, Beasley B, Gurtner GC, Longaker MT. The embrace device significantly decreases scarring following scar revision surgery in a randomized controlled trial. Plast Reconstr Surg. 2014;133:398–405. doi: 10.1097/01.prs.0000436526.64046.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longaker MT, Rohrich RJ, Greenberg L, Furnas H, Wald R, Bansal V, Seify H, Tran A, Weston J, Korman JM, Chan R, Kaufman D, Dev VR, Mele JA, Januszyk M, Cowley C, McLaughlin P, Beasley B, Gurtner GC. A randomized controlled trial of the embrace advanced scar therapy device to reduce incisional scar formation. Plast Reconstr Surg. 2014;134:536–546. doi: 10.1097/PRS.0000000000000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longaker MT, Whitby DJ, Ferguson MW, Lorenz HP, Harrison MR, Adzick NS. Adult skin wounds in the fetal environment heal with scar formation. Ann Surg. 1994;219:65–72. doi: 10.1097/00000658-199401000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol. 1998;111:850–857. doi: 10.1046/j.1523-1747.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- Lorenz HP, Longaker MT, Perkocha LA, Jennings RW, Harrison MR, Adzick NS. Scarless wound repair: a human fetal skin model. Development. 1992;114:253–259. doi: 10.1242/dev.114.1.253. [DOI] [PubMed] [Google Scholar]
- Maltseva O, Folger P, Zekaria D, Petridou S, Masur SK. Fibroblast Growth Factor Reversal of the Corneal Myofibroblast Phenotype. Investigative Ophthalmology & Visual Science. 2001;42:2490–2495. [PubMed] [Google Scholar]
- Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohee S, Sotiropoulou PA, Simons BD, Blanpain C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489:257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- Mia MM, Bank RA. Paracrine Factors of Human Amniotic Fluid-Derived Mesenchymal Stem Cells Show Strong Anti-Fibrotic Properties by Inhibiting Myofibroblast Differentiation and Collagen Synthesis. Journal of Stem Cell Research & Therapy. 2015;05 [Google Scholar]
- Monaghan M, Browne S, Schenke-Layland K, Pandit A. A collagen-based scaffold delivering exogenous microrna-29B to modulate extracellular matrix remodeling. Mol Ther. 2014;22:786–796. doi: 10.1038/mt.2013.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MW, Jr, Allukian M, 3rd, Herdrich BJ, Caskey RC, Zgheib C, Xu J, Dorsett-Martin W, Mitchell ME, Liechty KW. Modulation of the inflammatory response by increasing fetal wound size or interleukin-10 overexpression determines wound phenotype and scar formation. Wound Repair Regen. 2014;22:406–414. doi: 10.1111/wrr.12180. [DOI] [PubMed] [Google Scholar]
- Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis. 2014;10:29–37. doi: 10.4161/org.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. American Journal of Physiology - Cell Physiology. 1999;277:C1–C19. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- Rinella L, Marano F, Berta L, Bosco O, Fraccalvieri M, Fortunati N, Frairia R, Catalano MG. Extracorporeal shockwaves modulate myofibroblast differentiation of adipose-derived stem cells. Wound Repair Regen. 2016 doi: 10.1111/wrr.12410. [DOI] [PubMed] [Google Scholar]
- Rinkevich Y, Walmsley GG, Hu MS, Maan ZN, Newman AM, Drukker M, Januszyk M, Krampitz GW, Gurtner GC, Lorenz HP, Weissman IL, Longaker MT. Skin fibrosis. Identification and isolation of a dermal lineage with intrinsic fibrogenic potential. Science. 2015;348:aaa2151. doi: 10.1126/science.aaa2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert R, Meyer W, Bishop S, Rosenberg L, Murphy L, Blakeney P. Disfiguring burn scars and adolescent self-esteem. Burns. 1999;25:581–585. doi: 10.1016/s0305-4179(99)00065-0. [DOI] [PubMed] [Google Scholar]
- Sabapathy V, Sundaram B, VMS, Mankuzhy P, Kumar S. Human Wharton’s Jelly Mesenchymal Stem Cells plasticity augments scar-free skin wound healing with hair growth. PLoS One. 2014;9:e93726. doi: 10.1371/journal.pone.0093726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M. Upregulation of the Wnt/beta-catenin pathway induced by transforming growth factor-beta in hypertrophic scars and keloids. Acta Derm Venereol. 2006;86:300–307. doi: 10.2340/00015555-0101. [DOI] [PubMed] [Google Scholar]
- Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development (Cambridge, England) 2013;140:1517–1527. doi: 10.1242/dev.087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Ghatak S. miRNA control of tissue repair and regeneration. Am J Pathol. 2015;185:2629–2640. doi: 10.1016/j.ajpath.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan RL, Hinson MI, Liang MH, Nackel AF, Schoenfeld DA, Ryan CM, Mulligan JL, Tompkins RG. Long-term outcome of children surviving massive burns. Jama. 2000;283:69–73. doi: 10.1001/jama.283.1.69. [DOI] [PubMed] [Google Scholar]
- Shi Y, Shu B, Yang R, Xu Y, Xing B, Liu J, Chen L, Qi S, Liu X, Wang P, Tang J, Xie J. Wnt and Notch signaling pathway involved in wound healing by targeting c-Myc and Hes1 separately. Stem Cell Res Ther. 2015;6:120. doi: 10.1186/s13287-015-0103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ, Hollander JE, Quinn JV. Evaluation and management of traumatic lacerations. N Engl J Med. 1997;337:1142–1148. doi: 10.1056/NEJM199710163371607. [DOI] [PubMed] [Google Scholar]
- Singla DK, Singla RD, Abdelli LS, Glass C. Fibroblast growth factor-9 enhances M2 macrophage differentiation and attenuates adverse cardiac remodeling in the infarcted diabetic heart. PLoS One. 2015;10:e0120739. doi: 10.1371/journal.pone.0120739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielitz A, Vetter RW, Schultze B, Wrenger S, Simeoni L, Ansorge S, Neubert K, Faust J, Lindenlaub P, Gollnick HP, Reinhold D. Inhibitors of dipeptidyl peptidase IV-like activity mediate antifibrotic effects in normal and keloid-derived skin fibroblasts. J Invest Dermatol. 2008;128:855–866. doi: 10.1038/sj.jid.5701104. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu X, Zhang D, Wang X, Zhao F, Zhang T, Wang R, Lin X, Shi P, Pang X. Phenotypic and functional modulation of 20–30 year old dermal fibroblasts by mid- and late-gestational keratinocytes in vitro. Burns. 2015;41:1064–1075. doi: 10.1016/j.burns.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Wong VW, Rustad KC, Akaishi S, Sorkin M, Glotzbach JP, Januszyk M, Nelson ER, Levi K, Paterno J, Vial IN, Kuang AA, Longaker MT, Gurtner GC. Focal adhesion kinase links mechanical force to skin fibrosis via inflammatory signaling. Nat Med. 2012;18:148–152. doi: 10.1038/nm.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Wang Z, Lang H, Liu X, Zhang D, Wang X, Zhang T, Wang R, Shi P, Pang X. Dynamic Expression of Novel MiRNA Candidates and MiRNA-34 Family Members in Early- to Mid-Gestational Fetal Keratinocytes Contributes to Scarless Wound Healing by Targeting the TGF-beta Pathway. PLoS One. 2015;10:e0126087. doi: 10.1371/journal.pone.0126087. [DOI] [PMC free article] [PubMed] [Google Scholar]