Abstract
Profilin 1, cofilin 1, and vasodialator stimulated phosphoprotein (VASP) are actin binding proteins (ABP) which regulate actin remodelling and facilitate cancer cell metastases. MiR~17–92 is highly expressed in metastatic tumors and profilin1 and cofilin1 are predicted targets. Docosahexaenoic acid (DHA) inhibits cancer cell proliferation and adhesion. These studies tested the hypothesis that the metastatic phenotype is driven by changes in ABPs including alternative phosphorylation and/or changes in subcellular localization. Additionally, we tested the efficacy of DHA supplementation to attenuate or inhibit these changes. Human lung cancer tissue sections were analyzed for F-actin content and expression and cellular localization of profilin1, cofilin1 and VASP (S157 or S239 phosphorylation). The metastatic phenotype was investigated in A549 and MLE12 cells lines using 8 Br-cAMP as a metastasis inducer and DHA as a therapeutic agent. Migration was assessed by wound assay and expression measured by western blot and confocal analysis. MiR~17–92 expression was measured by qRT-PCR. Results indicated increased expression and altered cellular distribution of profilin1/VASPpS157 but no changes in cofilin1/VASPpS239 in the human malignant tissues compared to normal tissues. In A549 and MLE12 cells, the expression patterns of profilin1/VASPpS157 or cofilin1/VASPpS239 suggested an interaction in regulation of actin dynamics. Furthermore, DHA inhibited cancer cell migration and viability, ABP expression and cellular localization, and modulated expression of miR~17–92 in A549 cells with minimal effects in MLE12 cells. Further investigations are warranted to understand ABP interactions, changes in cellular localization, regulation by miR~17–92, and DHA as a novel therapeutic.
Keywords: docosahexaenoic acid, lung cancer, migration, miR~17–92, actin binding proteins, metastasis, actin dynamics
Introduction
Metastasis to distant organs and persistent survival of cancer cells reflect high disease cancer mortality rates (1–3). Overall, statistics project a total of 1,665,540 new cases associated with cancer in the United States in 2014 (4). Although death rates declined 20% from 1991 to 2009, cancer still imposes an intolerable socio-economical toll (1600 deaths/day) (4–6). Unfortunately, curative chemotherapeutic approaches have not emerged and current adjuvant chemotherapies are largely inefficient against cancer cells with the complete malignant phenotype, increasing median survival by only a few years and with a high frequency of residual micro metastasis and relapse (7–10). Overall, approximately 50% of surgically treated patients suffer recurrent disease due to resistance to cell death (apoptosis) and permanent cell cycle kinetics changes (1, 8, 11–13). Taken together, these considerations underscore the urgent need for unraveling fundamental signaling mechanisms involved in cancer cell survival and metastasis that could be translated into novel therapeutics.
The process of metastasis is facilitated by defects in actin dynamics which disrupt normal cell homeostasis promoting changes in cell polarity, adhesion, and proliferation (14–17). Actin dynamics are also known to affect cancer cell survival where increased F-actin turnover promotes cell viability and decreased F-actin turnover inhibits it through apoptosis (18–20). The actin binding proteins (ABPs) vasodilator-stimulated phosphoprotein (VASP), profilin 1, and cofilin 1 are key facilitators of actin dynamics (21–23). Under homeostatic conditions VASP, profilin 1, and cofilin 1 are regulated through a feedback mechanism involving actin polymerization/stabilization or depolymerization/degradation. Profilin 1 recruits ATP-actin monomers to the proline-rich region (PPR) of VASP, a scaffolding ABP, and aids in actin polymerization through the formation of long actin filaments. Alternatively, cofilin 1 binds to actin depolymerizing factor (ADF) and disassembles actin filaments without capping or by creating positive ends on the filament fragments. These events involve an ATP-to-ADP exchange, which precipitates the recycling of actin filaments to reassemble and polymerize. In response to changes in the cellular microenvironment, profilin 1 converts the ADP-actin monomers to ATP-actin monomers to be reused for actin polymerization. In this way, actin binding protein mediated-actin dynamics regulate cellular homeostasis and maintain cytoskeletal structure (17, 21, 22, 24–31). During metastasis, actin cytoskeletal remodeling at the leading edges of cancer cells facilitate invasive organelle (lamelipodia, filopodia and invadopodia) formation and matrix degradation (15, 32–34).
The complexity of ABPs regulation and the control of malignant cell behaviors are demonstrated in Figure 6. In cancer cells, especially as they transform toward metastasis, dramatic changes occur disrupting internal signaling cascades which in turn accelerate cellular machinery, including actin dynamics, to meet the demand for increased proliferation and migration. VASP can be phosphorylated at S157 via PKA (protein kinase A) or S239 via PKG (protein kinase G). Under resting conditions phosphorylation of VASP at S157 promotes VASP activity while phosphorylation at S239 facilitates homeostasis in the presence of a stimulus due to changes in the cellular environment. Thus actin polymerization via VASPpS157 is pro-metastatic/anti-apoptotic while VASPpS239 is anti-metastatic/pro-apoptotic in cancer cells. A number of actin binding proteins including profilin 1 and cofilin 1 are associated with VASP and are likely involved in the metastatic process but their interactions with each other and VASP are not fully understood. The current investigation explores these associations and speculates that they constitute a “switch on or off” mechanism for actin dynamics and thus unregulated proliferation and migration. In summary, we and others have shown VASPpS157 as pro-carcinogenic, promoting protrusion, remodeling, and cell survival (20, 23, 35). In contrast, VASPpS239 has been shown to be anti-carcinogenic, promoting F- actin de-polymerization, cytoskeletal stabilization, and apoptosis (23, 34).
Fig 6. Schematic presentation of hypothesis.
Cancer cell migration and viability is induced and suppressed by the proposed biochemical circuit comprising profilin1/pVASP-S157 and cofilin1/pVASP-S239. DHA suppressed metastatic phenotypes through negatively or positively effecting signaling. DHA also suppressed miR-17–92 cluster levels in cancer cells. But how DHA affects profilin1/cofilin1 interaction with VASP phosphorylation or miRs with ABPs complexes in cancer metastasis and cell survival is not known.
MicroRNAs (miRs) are attractive molecular therapeutic targets against cancer growth and metastasis (36). MiR-17~92, a cluster of six microRNAs encoded by a single transcript, is highly expressed in malignant tumors and may function as an oncogene by negatively regulating tumor suppressor genes and/or genes that control cell proliferation, differentiation, or apoptosis (36–38). Of relevance, ABPs like profilin 1 and cofilin 1 are among the predicted targets of miR~17–92 (predicted by Target Scan). Therefore, we investigated miR-17~92 cluster expression in relation to changes in VASP phosphorylation and cofilin 1 and profilin 1 expression.
Docosahexaenoic acid (DHA), a polyunsaturated fatty acid and nutritional supplement, plays a role in normal development (39) and has been shown to inhibit cancer cell proliferation, invasion and survival (40, 41). DHA has been and is currently being investigated as a potential therapeutic for cancer. Kim et al. and Jing et al. demonstrated that DHA induced apoptosis and autophagy in cancer cells through AMPK activation and PI3K/Akt inhibition, which led to suppression of mTOR signaling pathways (42, 43). Others have identified DHA-mediated changes in caspase 3 or disruptions in tumor suppressor expression (44). Our studies provide evidence that DHA alters the assembly of actin binding proteins which has direct implications on cell migration.
The current studies were designed to test the hypothesis that the metastatic phenotype is driven by changes in actin binding proteins which include alternative phosphorylation and/or alternative subcellular localization. Additionally, we will test the efficacy of DHA supplementation to attenuate or inhibit these changes. Overall, we propose that regulation of profilin1/VASPpS157, cofilin1/VASPpS239, and/or miR-17~92 cluster are innovative and novel molecular target(s) in cancer metastases and that DHA is a concept based therapeutic for inhibiting metastasis and prolonged cell viability through modulation of actin dynamics.
Materials and Methods
Actin binding protein expression and cellular localization in human lung biopsy samples (Immunofluorescence (IF) staining)
Human lung cancer tissue slides were obtained from the Lung Cancer Biospecimen Research Network (LCBRN), University of Virginia, Charlottesville, VA. Normal (n = 4) and malignant (n = 4) lung tissue slides were processed for immunofluorescence labeling using standard protocols (45). Tissue sections were stained with profilin 1, cofilin 1, VASP, and VASPpS239 (dilution, 1: 500) (Cell Signaling Technology, Inc., Danvers, MA) and anti-human rabbit monoclonal antibodies targeting VASPpS157 (dilution, 1: 500) (Santa Cruz, Dallas, TX) followed by secondary antibodies Alexa 488 labeled IgG (1:1000) and nuclei were stained with DAPI (Invitrogen, Carlsbad, CA). Images (4 from each slide) were taken using Carl Zeiss’s Axio Scope A1 Polarized Light Fluorescent Microscope (Carl Zeiss, Jena, Germany) with 400X magnification and identical settings. Intensity of image color was quantified using NIH Image J software using identical background settings. The results expressed as intensity/cell.
Cell culture and treatment
Invasive lung cancer cells (A549, ATCC® CCL-185™) were obtained from American Type Culture Collection (ATCC, Manassas, VA) in fall of 2013. At the time of purchase, the cells were cultured for two passages and frozen in liquid nitrogen for future use. For the present experiments, a new vial was thawed and the cells were authenticated at that time using the vendor’s recommendations which included morphology, growth curve analyses, and mycoplasma detection. Cells used for the current studies were between passages 4–8 after purchase from the vendor. Non-invasive lung epithelial cells (MLE12, ATCC® CRL-2110™) were obtained from American Type Culture Collection (ATCC, Manassas, VA) in 2011. At the time of purchase, the cells were cultured for two passages and then frozen in liquid nitrogen. Periodic refreezing of early passages was performed to maintain the integrity of the cell line. For the present experiments, a new vial at passage 8 was thawed and the cells were authenticated at that time using the vendor’s recommendations which included morphology, growth curve analyses, and mycoplasma detection. Cells used for these studies were between passages 9–15 after purchase from the vendor. Cells were cultured (at 37 °C) in a humidified atmosphere containing 5% CO2. A549 cells were grown in DMEM 1X with 10% FBS supplemented with 4.5 g/L-glucose while MLE12 cells were maintained in HITES media as previously described (46). At ~80% confluence, cells were treated for 24 h with either PBS (the vehicle control), cell permeant analogs of cAMP (8Br-cAMP, 300 μM) (Sigma Chemical Co., St. Louis, MO), DHA alone (60 μM), or cAMP and DHA.
F-actin content
F-actin content in tissue and cells was estimated by using Alexa-633 tagged phalloidin (Invitrogen, Eugene, Oregon) and DAPI (Invitrogen, Carlsbad, CA) for the nucleus. Images were acquired in a blinded fashion using a confocal microscope (LSM510, Carl Zeiss, Jena, Germany) at 400× magnification. Identical confocal settings were applied to acquire images across all conditions. F-actin content/cell was quantified using NIH Image J analysis.
Cell migration analysis (wound assay)
Metastatic potential of cancer cells was assessed by cell migration using wound assay. Cells were plated in Falcon’s six well plate and treated with 8 Br-cAMP and/or DHA before (pretreatment) and after (post-treatment) wound induction. A wound was induced by scratching the cell monolayer using sterile 20 μl pipete tip. Briefly, 5 wounds/well were made at 5 random places and the exterior of the plate marked with permanent marker. The photographs of five wounds from each treatment at 0 h and 24 h were taken at the same place (identified by marks on the plate) with Olympus BX51 compound microscope at 10X magnification. The analysis of the wound was carried out with NIH Image J software by measuring the distance between the edges of wound as a measure of cell migration into the wound.
Cell Apoptosis
Cellular apoptosis was measured by staining 8 Br-cAMP and/or DHA treated cells with cl-caspase-3 anitbodies (1:200) followed by flow cytometry. Cell lysates were analyzed by western blot for expression of the apoptosis proteins caspase-3, caspase-9, and BAX using the respective antibodies (1:500).
Actin binding protein localization in cells (confocal)
Actin binding protein localization within cells was measured at the leading front of wounds by confocal microscopy. Cells (MLE12 and A549) were plated in 6-well plates containing cover slips (Corning Incorporated Corning, New York, NY) at 1 × 105 cells/well and treated as previously described. After completion of treatments a wound was induced; after an additional 24 h of wound closure the cover slips were harvested and cells were stained with anti-profilin 1 (1:500) and anti-cofilin 1 (1:500) followed by secondary antibody, Alexa 488 labeled IgG (1:1000), and nuclei were stained with DAPI (Invitrogen, Carlsbad, CA). Confocal microscopy (LSM510, Carl Zeiss, Jena, Germany) was used to acquire Images (200X) in a blinded fashion using identical confocal settings for all images. Percent (%) change in protein intensity was quantified using NIH Image J analysis.
Actin binding protein expression
Actin binding protein (profilin 1, cofilin 1, VASP, VASPpS157 and VASPpS239) expression was measured in total cell lysates by western blot. SDS sample buffer was used to prepare total cell lysates and proteins were separated by SDS-PAGE, transferred to nitrocellulose membranes, and probed with anti-human rabbit monoclonal antibodies targeting profilin 1, cofilin 1, VASP, VASPpS157 or VASPpS239 (dilution, 1:1000) and mouse monoclonal antibodies targeting β-actin (1:10,000) (Abcam, Cambridge, MA). Finally, membranes were probed with horseradish peroxidase-conjugated secondary antibody (dilution, 1:1000) (BD Pharminogen®, Franklin lakes, NJ) for 1 h at room temperature and specific bands were visualized employing AmershamTMECLTM Prime Western Blotting Detection Reagent (GE Healthcare, Buckinghamshire, UK) and the band intensity was measured by densitometry.
MiR~17–92 analysis
Treated A549 and MLE12 cells were harvested in TRIzol® Reagent (Life Technologies; Carlsbad, CA) followed by microRNA isolation using Qiagen Rneasy RNA/miRNA prep protocol (Valencia, CA) in accordance with the manufacturer’s instructions. Quantitation of miR-17~92 cluster was done by qRT-PCR using TaqMan® MicroRNA Assays (Life Sciences, Grand Island, NY).
Statistical Analysis
Experiments were repeated a total of three times. Data presented represent means ± SEM. Data were analyzed by student t-test, one-way ANOVA or two-way ANOVA (with Tukey’s post hoc) depending upon the format of the data. A value of p<0.05 was considered significant.
Results
Expression of F-actin, cofilin 1, profilin 1, and VASP in lung cancer tissues
F-actin, cofilin 1, profilin 1, VASP, VASPpS157 and VASPpS239 immunofluorescence was measured in the normal portion, at the tumor leading edges (invasive tumor), and in the mid portion (mid tumor) of the tissue section. The area of the tissue sections having cancerous growth was located and the center, where cells were densely populated, was identified as the mid-tumor. The edges of these areas where the cells are less dense, loosely held, and migrating to the interstitial spaces, were identified as the tumor leading edge (26). Cellular distribution patterns showed profilin 1 primarily in the cytoplasm of normal cells (yellow arrows) but at the cellular edges and in the nucleus of tumor leading edge cells (pink arrows), and primarily in the nucleus of mid tumor cells (yellow arrows). Cofilin 1 was observed in the perinuclear area and at cell boundaries (white arrows) in normal and mid-tumor cells but was also present in the nuclear region (yellow arrow) at the tumor leading edge (Figure 1a). Accordingly, VASPpS157 and VASPpS239 showed cellular distributions similar to profilin 1 and cofilin 1. Higher expression of profilin 1, VASP, and VASPpS157 (Figure 1b), and F-actin content (Figure 1c) were observed at the tumor leading edge than in the mid tumor or normal tissue. Lower profilin1 expression was observed in the mid tumor compared to control (Figure 1 b). No changes in cofilin 1 or VASPpS239 levels were detected.
Fig 1. Human lung cancer tissue and A549 cells express of F-actin, cofilin 1, profilin 1, VASP, VASPpS157, and VASPpS239.
F-actin content and profilin 1, cofilin 1, and phosphorylated VASP expression were assessed in human autopsy samples using florescent microscopy and Image J software (a and b). Images are 400X magnification. Green, profilin 1 or cofilin 1, stained with specific antibodies against each protein; red, F-actin stained with phalloidin, and blue, nuclei stained with DAPI (c). Expression of F-actin by fluorescent intensity (d) and of profilin 1, cofilin 1, or VASP protein levels were measured by Western blot in untreated A549 and MLE12 cells (e). Data for human tissues ((a) or (b)) were analyzed by one-way ANOVA for each protein individually and further analyzed by Tukey’s post hoc. For expression in A549 or MLE12 cells ((d) or (e)) data were analyzed by t-test. Statistical significance was noted as follows: For ANOVA; (*) indicates different than control; (#) indicates different than tumor leading edge; For t-test; (*) p<0.05, and (***) p<0.001, n= 4 tissue sections from different donors and n= 3 experiments for cells.
Expression of F-actin, cofilin 1, profilin1, VASP, and VASPpS157 in A549 and MLE12 cells
F-actin content in MLE12 and A549 cells was measured by immunofluorescence using confocal microscopy and digital analysis. At 24 h post-treatment greater F-actin content was observed in the A549 cells than in the MLE12 cells (Figure 1d). Likewise, higher expression of profilin 1 and VASPpS157 and lower expression of cofilin 1 and VASPpS239 was observed in A549 cells compared to MLE12 cells (Figure 1e).
A549 cells exhibited nuclear localization of ABPs, higher miR~17–92 expression, greater migration, and less apoptosis than MLE12 cells
Cellular distribution and nuclear localization of profilin 1 and cofilin 1 in A549 and MLE12 cells were examined at the leading edge of cell growth after a wound. In MLE12 cells, confocal microscopy indicated cofilin 1 expression was localized at the cell boundary between cell-cell connections (white arrows). In A549 cells, greater levels of cofilin 1 fluorescence were observed in the cytoplasm at the perinuclear area (pink arrows), at the forward edge of wound healing progression, and at the cell boundary at cell-cell interactions. Cofilin 1 fluorescence was also observed in the nucleus, especially in the regions where cells were in dense colonies in both cell lines (Figure 2a). Profilin 1 immunofluorescence was observed around the cell boundary at the cell-cell interactions in MLE12 cells (white arrows) (Figure 2a). A549 cells had more profilin1 immunofluorescence near the leading edges of the cell at the forward front of the wound (pink arrows), while cells further from the wound front demonstrated more nuclear expression (yellow arrows).
Fig 2. Invasive cancer cells, A549 showed nuclear localization of ABPs, higher miR~17–92 expression, greater migration, and less apoptosis than non-invasive, MLE12 cells.
Subcellular distribution was analyzed by confocal microscopy (cofilin1 and profilin1 green; DAPI is in pseudo-red). Cofilin 1 is present around the nucleus (pink arrows) in A549 cells, on the cell boundary (white arrows) in MLE12 cells, and in the nucleus (yellow arrow) at colony formation region of both cells. Profilin1 is present in the nucleus in A549 cells preceding the wound front and in the cell edges at wound front (pink arrows); and on the cell boundary in MLE12 cells (white arrows). Images are 200X magnification (a). Western blot analyses of cofilin 1, profilin 1 or VASP in membranous (ME), nuclear soluble (NE(s)) and nuclear chromatin (NE (ch)) fractions (b). MiR~17–92 cluster were measured by qRT-PCR (c). A549 or MLE12 cells were also processed for cell migration by the wound-healing assay (d). Apoptosis was assessed by flow cytometry for caspase-3 expression or by western analysis for cl-Casp-3, Casp-9, or BAX proteins (e). Data were analyzed by t-test for each individual protein and significance is indicated * p<0.05, ** p<0.005, and *** p<0.001, n=3 experiments.
After harvesting, cells were fractionated into membrane (ME), nuclear soluble (NE(s), and nuclear chromatin NE(ch) fractions. Western blot analysis of the membrane fraction indicated decreased expression of cofilin 1 and increased expression of both profilin 1 and VASPpS157 in A549 cells compared to MLE12 cells. In the nuclear soluble and chromatin fractions, higher expression of cofilin 1 and profilin1 was observed in the A549 than in the MLE12 cells. Additionally, expression of cofilin 1 and profilin 1 were higher and VASPpS157 was lower in A549 compared to MLE12 cells in the NE(ch) fraction (Figure 2b).
MiR-17~92 cluster expression was quantified using qRT-PCR in MLE12 and A549 cells. Higher levels of miR-17, miR-19b, miR-20a and miR-92 were observed in A549 cells compared to MLE12 (Figure 2c).
Cell migration was measured using a wound healing assay (at 24 h) and apoptosis was measured by both flow cytometry and western blot analysis. A549 cells filled the wound faster than MLE12 cells (Figure 2d) indicating faster migration or metastatic potential. Flow cytometry indicated lower levels of caspase-3 expression in A549 cells than in MLE12 cells. Similarly, western blot indicated that levels of caspase-3, caspase-9, and BAX were lower in A549 cells than MLE12 cells (Figure 2 e and Supplemental Figure 1).
DHA supplementation suppressed migration and induced apoptosis in A549 cells
MLE12 and A549 cells were treated with 8Br-cAMP (cAMP), DHA, or cAMP/DHA for 24 h before induction (pre-treatment) or after induction (post-treatment) of wound. DHA pretreatment and post-treatment had no effect on migration in MLE12 cells but DHA treatment suppressed migration alone and in combination with cAMP in A549 cells (Figure 3a–b). DHA treatment induced caspase-3 immunofluorescence by flow cytometry and also protein expression by western blot. DHA treatment also induced caspase 9 and BAX protein expression in A549 cells (Figure 3c).
Fig 3. DHA supplementation inhibits migration and increases apoptosis in A549 cells.
Cell migration was quantified by the wound-healing assay (a–b). Apoptosis was analyzed by flow cytometry using anti-cl-caspase-3 antibody (1:200) or by western blot for cl-caspase-3, caspase-9, or BAX (c). Data were analyzed by two-way ANOVA with Tukey’s post hoc; * indicates different from control same cell type; # indicates different from different cell type same treatment. Using independent student’s t-tests for differences between individual groups, $indicates differences between groups; @indicates different than cAMP treatment, p<0.05, n=3.
DHA treatment altered F-actin, profilin1, and cofilin1 immunofluorescence
F-actin, profilin 1, and cofilin 1 immunofluorescence were assessed by confocal microscopy in cells pretreated and post-treated with DHA. F-actin fluorescence intensity increased in the MLE12 cells treated with DHA or cAMP/DHA. Trends toward lower levels of F-actin fluorescence were observed with DHA supplementation in A549 cells but no post hoc analysis differences were indicated though values were significant using independent t-test for individual treatments (Figure 4 and Table 1).
Fig 4. DHA treatment reduced F-actin content and alters subcellular distribution of profilin1 and cofilin1.

Confocal microscopy was used to assess F-actin contents and ABPs. F-actin content/cell (400X magnification). Red, F-actin stained with phalloidin (a, top image panel) or cofilin1 and profilin1 green; DAPI is in pseudo-red) (a, middle and lower images panels). Cofilin1 is present around nucleus (pink arrows) and cell edges in A549 cells; and on the cell boundary (blue arrows) and in the nucleus (yellow arrow) at colony formation region in MLE12 cells. Profilin1 is present in the nucleus in A549 cells (yellow arrows); and on the cell boundary in MLE12 cells (white arrows). Images are 40X magnification. Fluorescent intensity was assessed using NIH Image J software (b, table). Data were analyzed by two-way ANOVA with Tukey’s post hoc; * indicates different from control same cell type; # indicates different from different cell type same treatment. Using independent student’s t-tests for differences between individual groups, $indicates differences between groups; @indicates different than cAMP treatment, p<0.05, n=3.
Table 1.
Confocal image fluorescent intensity (Figure 4).
| F-actin | ||||
|---|---|---|---|---|
| Control | DHA | cAMP | cAMP/DHA | |
| Pretreatment | ||||
| MLE12 | 4.00±0.71 | 7.77±0.82$ | 9.71±2.00*$ | 9.27±0.95*$ |
| A549 | 7.56±1.22 | 3.94±0.09$ | 11.45±0.95$ | 7.44±0.95@ |
| Two-way ANOVA: effect of treatment, interaction between cell type and treatment | ||||
| Post-treatment | ||||
| MLE12 | 1.80±0.15 | 8.84±0.69$ | 8.35±0.98$ | 12.95±0.913#$@ |
| A549 | 6.37±1.22 | 2.84±0.56$ | 13.83±1.61$ | 4.27±1.02@ |
| Two-way ANOVA: effect of treatment, interaction between cell type and treatment | ||||
| Cofilin 1 | ||||
| Pretreatment | ||||
| MLE12 | 169.4±34.8 | 399.9±107.4* | 173.5±51.3 | 153.4±15.2 |
| A549 | 174.7±32.7 | 329.7±38.2$ | 125.5±12.5 | 225.8±29.9@ |
| Two-way ANOVA: effect of treatment | ||||
| Post-treatment | ||||
| MLE12 | 422.9±15.0 | 1601.5±446.4$ | 524.8±106.9 | 1683.6±639.0 |
| A549 | 218.2±14.0 | 607.1±104.1$ | 136.2±23.8$ | 145.4±20.5#$ |
| Two-way ANOVA: effect of treatment, effect of cell type, an interaction between cell type and treatment | ||||
| Profilin 1 | ||||
| Pretreatment | ||||
| MLE12 | 21.14±1.00 | 19.61±3.46 | 152.0±21.8$ | 58.92±7.33$@ |
| A549 | 349.2±34.4# | 34.53±8.81#$ | 389.1±66.7# | 46.31±12.41*$@ |
| Two-way ANOVA: effect of treatment, effect of cell type, an interaction between cell type and treatment | ||||
| Post-treatment | ||||
| MLE12 | 42.07±8.23 | 31.92±1.97 | 75.04±8.38$ | 34.74±4.28@ |
| A549 | 51.34±6.46 | 37.33±6.62 | 120.8±18.7*# | 53.03±4.04 |
| Two-way ANOVA: effect of treatment and effect of cell type | ||||
DHA increased cofilin 1 fluorescence near the cell edges, in the perinuclear region and slightly in the nucleus in A549 cells (pink arrow) (Figure and Table 1). Assessment of cofilin 1 immunofluorescence indicated a substantial increase in expression due to DHA treatment in both pretreated and post-treated cells (Figure and Table 1). No individual differences were indicated in the cAMP treatment groups but there was a difference between MLE12 and A549 cells in the cAMP/DHA treated groups.
The fluorescence patterns of profilin 1 indicated that DHA prevented profilin1 localization to the cell edges while it increased localization in the nucleus in A549 cells. Furthermore, cAMP increased profilin-specific fluorescence and DHA lessened profilin-specific fluorescence with or without co-treatment with cAMP (Figure and Table 1).
DHA altered ABP protein expression and attenuated increases in miR-17 and 19b expression in A549 cells
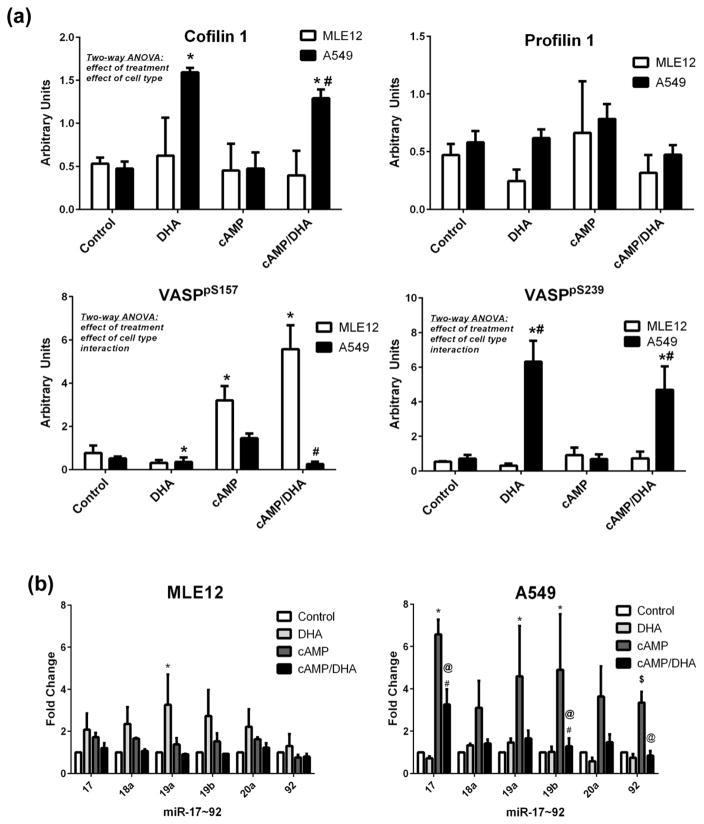
Western blot analysis of whole cell lysate indicated that DHA increased cofilin 1 and VASPpS239 expression in A549 cells (Figure 5a). Alternatively, cAMP alone caused a substantial increase in VASPpS157 expression in MLE12 cells and DHA attenuated the cAMP effect on VASPpS157 expressions (Figure 5a).
Fig 5. DHA modulates expression of cofilin1, VASPpS239, and VASPpS157 and miR~17–92 expressions in A549 cells.
A549 and MLE12 cells were treated as indicated and harvested at 24h for western blot analyses of cofilin 1, profilin 1, or VASP (a). Data were analyzed by two way-ANOVA with Tukey’s post hoc. Additional cells were harvested and mRNA isolated for assessment of the miR 17~92 cluster by TaqMan PCR (b). PCR data were analyzed by one-way ANOVA for each miR in each cell line. Significance is indicated at p<0.05, *different than control; #different between cell types, same treatment. Using independent student’s t-tests for differences between individual groups, $indicates differences between two groups, @indicates different than cAMP treatment by an independent student’s t-test, p<0.05.
The miR-17~92 cluster was analyzed by qRT-PCR in these same cells and the results indicated that MLE12 cells exhibited an increase in mir-19a in cAMP-treated cells but the other miRs were not different. A549 cells exhibited an increase in expression in response to cAMP treatment in miR-17, 19a, 19b and 92. DHA supplementation was able to suppress the increases observed in miR-17, 19b and 92 due to cAMP (Figure 5b).
Subcellular localization of ABPs in response to DHA and/or cAMP
No differences in cofilin 1 expression were observed between cell types or treatments in the ME fraction. A549 cells expressed substantially more cofilin 1 than MLE12 cells in the nuclear fractions, specifically in response to cAMP in the NE(s) fraction and DHA in the NE(ch) fraction (Supplemental Figure 2). Profilin 1 was also more highly expressed in A549 cells than in MLE12 cells in the ME fraction, with a decrease in DHA and increase in the cAMP treated cells, and with a specific increase in cAMP treated cells in the NE(ch) fractions.
VASPpS157 expression was elevated in all fractions and both cells types supplemented with DHA. Of note, VASPpS157 expression was lower in A549 cells than MLE12 cells in the NE(ch) fraction (Supplemental Figure 2). No differences were observed in VASPpS157 expression in the ME or NE(s) fractions; however, there was an effect of cell type with greater expression in MLE12 cells treated with DHA and/or cAMP.
Discussion
Actin remodeling at the leading edges of cancer cells contributes to the formation of cell protrusive structures which facilitate metastasis and increased cell survival (5, 6, 15, 18, 33). Asymmetric phosphorylation of VASP protein at S157 or S239 has been shown to regulate actin turnover and differential phosphorylation of VASP can act in a pro- or anti-metastatic manner to regulate cancer cell viability (20, 34, 35). Actin dynamics are complex processes requiring the recruitment of actin monomers and the formation of filamentous actin (22, 27) indicating that other proteins are likely interacting with VASP in this process.
In human lung normal and malignant biopsy samples, Figurehigher levels of F-actin content, total VASP, VASPp157, and profilin 1 expression were observed at the tumor leading edge than in the normal tissues, confirming the association between increased expression of these APBs and metastatic phenotype (Figure 1 a–c). Interestingly, changes in subcellular localization of profilin 1 and cofilin 1 were observed at the tumor leading edge. In this region, cofilin 1 was found primarily around the nucleus while profilin 1 was primarily found at the invasive cellular edges. Accordingly, VASPpS157 and VASPpS239 indicated cellular distributions similar to profilin1 and cofilin1, respectively. Overall, these observations further support previous findings that profilin1 and VASPpS157 may have a role in increasing F-actin content and enhancing invasive potential in tumor cells while cofilin1 and VASPpS239 may be involved in maintaining homeostasis.
To further investigate these findings, we performed in-vitro studies using invasive lung cancer cells, A549, and non-invasive mouse lung epithelial cells, MLE12. One of the limitations to our study is the use of alveolar type II cells from two different species. While we acknowledge that the species differences may play a role in the responses of these particular cells, both of these cell lines are derived from alveolar type II cells, are highly characterized, are the topics of numerous publications, and have been used previously to contrast cancer verses non-cancer lung epithelial cells (47, 48). A549 cells are derived from a lung carcinoma and possess the invasive characteristics of cancer cells while MLE12 cells are immortalized with the integration of the SV40 large T antigen and are not “normal cells” but are non-invasive and non-cancerous in nature. Because of their extensive characterization and the cell type similarities, but distinct differences in the invasive nature, we chose to use these cells types in our investigations.
A comparative analysis of both cell lines showed that A549 cells also had higher F-actin content, greater VASPpS157 and profilin1 expression, and less VASPpS239 and cofilin1 expression than MLE12 cells (Figure 1d–e). Furthermore, A549 cells had increased migration and decreased apoptosis compared to MLE12 cells (Figure 2 d–e). Previous studies have reported the same increased level of VASPpS157 and decreased level of VASPpS239 in cancer cells or tissues compared to normal cells or tissues (20, 34). In addition, profilin 1 and cofilin 1 have also been shown to regulate cancer cell migration and viability in a similar manner (28, 49). Confocal analysis of cells at the wound edge confirmed the western blot findings of higher profilin 1 and lower cofilin 1 levels in A549 cells than MLE12 cells (Figure 2 a). Interestingly, A549 cells had higher profilin 1 cytoplasmic expression at the leading edges of the invasive cells at the front of wound (similar to the findings in human cancer tissues) while cofilin1 expression was localized to the nuclear region. Western blot analyses of membranous, nuclear soluble, and nuclear chromatin fractions in each cell line revealed higher profilin1/VASPpS157 and lower cofilin1 expression associated with the cell membrane as observed by microscopy (Figure 2 b). These findings further suggest a possible interaction of profilin1 with VASPpS157 and cofilin1 with VASPpS239 in regulation of actin dynamics at the cellular leading edges during migration.
DHA supplementation has been previously shown to inhibit cancer cell adhesion, proliferation and invasiveness (38, 40). We propose the basic concept that in cancer cells, disease progression involves changes in actin binding protein-mediated actin dynamic which facilitates metastasis and increases cell survival. In addition, cancer cells develop mechanisms to suppress apoptotic pathways to further expedite the pro-proliferative phenotype. Our data indicate that the therapeutic potential of DHA supplementation affects both of these basic events. Thus DHA could be considered a concept based therapy to treat the metastatic phenotype. The results of wound studies indicated that DHA treatment significantly inhibited migration and enhanced the expression of apoptosis markers in A549 cells (Figure 3 a–c). In confocal analysis, DHA reduced profilin 1 and F-actin content, induced cofilin1 expression, and attenuated the effect of cAMP in the A549 cells. Furthermore, DHA induced cofilin1 and VASPpS239 while it suppressed VASPpS157 expression in A549 cells (Figure 5 a). The finding that DHA reversed the effect of cAMP in A549 cells suggests that DHA is affecting upstream secondary messengers which mediate PKA/PKG activities and alter responses to stimuli. Remarkably, DHA changed the subcellular localization of ABPs in parallel to its effect on metastatic phenotype and cell viability. DHA prevented profilin 1 localization to the cell edges while it increased its localization into the nucleus in A549 cells and increased cofilin 1 expression near the cell edges and in the nuclear region (Figure 4a). Together, these data support our hypothesis that DHA modulates ABPs and suppresses cell migration and viability in A549 cells. The effects of DHA treatment in subcellular fractions indicated enhanced expression of all ABPs in A549 cells than in MLE12 cells with the exception of VASPpS239 (Supplemental figure 2 a–b). In all ABPs tested, DHA induced an increase in expression in A549 cells that was greater than cAMP induction except for cofilin1 in NE(s).
Analysis of microRNAs revealed higher levels miR-17, miR-19b, miR-20a, and miR-92 in A549 cells than MLE12 (Figure 2c) which may be linked to induction of malignant behavior (37, 50–53). Moreover, analysis of miRs in cAMP-treated A549 cells indicated a further increase in miR expression (Figure 5b). DHA reversed the impact of cAMP on these miRs in comparison to cAMP treatment alone. Target Scan predicted and previous studies reported, profilin 1 and cofilin 1 as potential targets of miR-92 and -19a/b respectively, and miR-17 as a regulator of ECM facilitating metastatic potential of cancer cells (36, 37). We postulate that this may be one mechanism by which the increases in nuclear expression of profilin 1, VASPpS157 and cofilin 1 are regulated, specifically in response to the invasive phenotype.
Together these findings confirm that localization of profilin1/VASPpS157 from cytoplasm to nucleus and of cofilin1/VASPpS239 to the cell leading edge is related to actin content and thus cell migration and apoptotic cell death of cancer cells. Furthermore, higher expression of miRs in the 17~92 cluster in A549 cells and a DHA-mediated attenuation of the effect of cAMP on the expression of these miRs in A549 cells (Figure 5b) further strengthens our prediction of possible association/cross talk between ABPs expression and miR~17–92 expression.
Conclusion
Overall, our study postulated that profilin 1 and cofilin 1 may be interacting with phosphorylated VASP and that these interactomes may migrate to different subcellular regions to regulate actin dynamics (Figure 6). Interestingly, greater expression of miRs-17, -19a, -19b and -92 in A549s cells correspond with high expression of cofilin 1 and profilin 1. Finally, our data provide evidence that DHA influences the regulation of actin dynamics, cell migration, and cell viability specifically in cancer cells via ABPs expression, cellular localization, and miR~17–92 cluster expression. Further, these data provide evidence that DHA could be developed as an innovative, anti-cancer therapy with minimal effects on normal cell biology and that ABPs interactome and miR cross talk could be established as novel anti-metastatic cancer therapeutic approaches.
Supplementary Material
Acknowledgments
The authors acknowledge the financial support of NIH funding R01AT006880 to Dr. Lynette Rogers and Molly Augustine for manuscript editing.
Footnotes
“The authors disclose no potential conflicts of interest”
Financial Information:
L.K. Rogers, NIH NCCIH/ODS R01AT006880
References
- 1.Pantel K, Cote RJ, Fodstad O. Detection and clinical importance of micrometastatic disease. J Natl Cancer Inst. 1999;91:1113–24. doi: 10.1093/jnci/91.13.1113. [DOI] [PubMed] [Google Scholar]
- 2.Pein M, Oskarsson T. Microenvironment in metastasis: roadblocks and supportive niches. A Review in the Theme: Cell and Molecular Processes in Cancer Metastasis. Am J Physiol Cell Physiol. 2015 doi: 10.1152/ajpcell.00145.2015. ajpcell 00145 2015. [DOI] [PubMed] [Google Scholar]
- 3.Hunter K. The role of individual inheritance in tumor progression and metastasis. Journal of molecular medicine. 2015;93:719–25. doi: 10.1007/s00109-015-1299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 5.Cresanta JL. Epidemiology of cancer in the United States. Prim Care. 1992;19:419–41. [PubMed] [Google Scholar]
- 6.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 7.Barreto SG, Pawar S, Shah S, Talole S, Goel M, Shrikhande SV. Patterns of failure and determinants of outcomes following radical re-resection for incidental gallbladder cancer. World journal of surgery. 2014;38:484–9. doi: 10.1007/s00268-013-2266-4. [DOI] [PubMed] [Google Scholar]
- 8.Estes NC, Giri S, Fabian C. Patterns of recurrence for advanced colon cancer modified by whole abdominal radiation and chemotherapy. The American surgeon. 1996;62:546–49. discussion 9–50. [PubMed] [Google Scholar]
- 9.Petrecca K, Guiot MC, Panet-Raymond V, Souhami L. Failure pattern following complete resection plus radiotherapy and temozolomide is at the resection margin in patients with glioblastoma. Journal of neuro-oncology. 2013;111:19–23. doi: 10.1007/s11060-012-0983-4. [DOI] [PubMed] [Google Scholar]
- 10.Petruzzelli GJ, Howell JB, Pederson A, Origitano TC, Byrne RW, Munoz L, et al. Multidisciplinary treatment of olfactory neuroblastoma: Patterns of failure and management of recurrence. American journal of otolaryngology. 2015;36:547–53. doi: 10.1016/j.amjoto.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Nini A, Gandaglia G, Fossati N, Suardi N, Cucchiara V, Dell’Oglio P, et al. Patterns of Clinical Recurrence of Node-positive Prostate Cancer and Impact on Long-term Survival. European urology. 2015 doi: 10.1016/j.eururo.2015.04.035. [DOI] [PubMed] [Google Scholar]
- 12.Pancione M, Giordano G, Remo A, Febbraro A, Sabatino L, Manfrin E, et al. Immune escape mechanisms in colorectal cancer pathogenesis and liver metastasis. J Immunol Res. 2014;2014:686879. doi: 10.1155/2014/686879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. The EMBO journal. 2011;30:3667–83. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albiges-Rizo C, Destaing O, Fourcade B, Planus E, Block MR. Actin machinery and mechanosensitivity in invadopodia, podosomes and focal adhesions. J Cell Sci. 2009;122:3037–49. doi: 10.1242/jcs.052704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mejillano MR, Kojima S, Applewhite DA, Gertler FB, Svitkina TM, Borisy GG. Lamellipodial versus filopodial mode of the actin nanomachinery: pivotal role of the filament barbed end. Cell. 2004;118:363–73. doi: 10.1016/j.cell.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Morra L, Moch H. Periostin expression and epithelial-mesenchymal transition in cancer: a review and an update. Virchows Arch. 2011;459:465–75. doi: 10.1007/s00428-011-1151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thompson O, Moghraby JS, Ayscough KR, Winder SJ. Depletion of the actin bundling protein SM22/transgelin increases actin dynamics and enhances the tumourigenic phenotypes of cells. BMC Cell Biol. 2012;13:1. doi: 10.1186/1471-2121-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gourlay CW, Ayscough KR. The actin cytoskeleton: a key regulator of apoptosis and ageing? Nature reviews Molecular cell biology. 2005;6:583–9. doi: 10.1038/nrm1682. [DOI] [PubMed] [Google Scholar]
- 19.Posey SC, Bierer BE. Actin stabilization by jasplakinolide enhances apoptosis induced by cytokine deprivation. The Journal of biological chemistry. 1999;274:4259–65. doi: 10.1074/jbc.274.7.4259. [DOI] [PubMed] [Google Scholar]
- 20.Ali M, Rogers LK, Pitari GM. Serine phosphorylation of vasodilator-stimulated phosphoprotein (VASP) regulates colon cancer cell survival and apoptosis. Life Sci. 2015;123:1–8. doi: 10.1016/j.lfs.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Pfaendtner J, De La Cruz EM, Voth GA. Actin filament remodeling by actin depolymerization factor/cofilin. Proc Natl Acad Sci U S A. 2010;107:7299–304. doi: 10.1073/pnas.0911675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quinlan MP. Vinculin, VASP, and profilin are coordinately regulated during actin remodeling in epithelial cells, which requires de novo protein synthesis and protein kinase signal transduction pathways. J Cell Physiol. 2004;200:277–90. doi: 10.1002/jcp.20009. [DOI] [PubMed] [Google Scholar]
- 23.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annual review of cell and developmental biology. 2003;19:541–64. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 24.Benz PM, Blume C, Seifert S, Wilhelm S, Waschke J, Schuh K, et al. Differential VASP phosphorylation controls remodeling of the actin cytoskeleton. J Cell Sci. 2009;122:3954–65. doi: 10.1242/jcs.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang CY, Leu JD, Lee YJ. The actin depolymerizing factor (ADF)/cofilin signaling pathway and DNA damage responses in cancer. International journal of molecular sciences. 2015;16:4095–120. doi: 10.3390/ijms16024095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiotaki R, Polioudaki H, Theodoropoulos PA. Differential nuclear shape dynamics of invasive andnon-invasive breast cancer cells are associated with actin cytoskeleton organization and stability. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2014;92:287–95. doi: 10.1139/bcb-2013-0120. [DOI] [PubMed] [Google Scholar]
- 27.Teng B, Lukasz A, Schiffer M. The ADF/Cofilin-Pathway and Actin Dynamics in Podocyte Injury. Int J Cell Biol. 2012;2012:320531. doi: 10.1155/2012/320531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bae YH, Ding Z, Das T, Wells A, Gertler F, Roy P. Profilin1 regulates PI(3,4)P2 and lamellipodin accumulation at the leading edge thus influencing motility of MDA-MB-231 cells. Proc Natl Acad Sci U S A. 2010;107:21547–52. doi: 10.1073/pnas.1002309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray NW, Kruchten AE, Chen J, McNiven MA. A dynamin-3 spliced variant modulates the actin/cortactin-dependent morphogenesis of dendritic spines. J Cell Sci. 2005;118:1279–90. doi: 10.1242/jcs.01711. [DOI] [PubMed] [Google Scholar]
- 30.Mathew S, George SP, Wang Y, Siddiqui MR, Srinivasan K, Tan L, et al. Potential molecular mechanism for c-Src kinase-mediated regulation of intestinal cell migration. The Journal of biological chemistry. 2008;283:22709–22. doi: 10.1074/jbc.M801319200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siton O, Bernheim-Groswasser A. Reconstitution of actin-based motility by vasodilator-stimulated phosphoprotein (VASP) depends on the recruitment of F-actin seeds from the solution produced by cofilin. The Journal of biological chemistry. 2014;289:31274–86. doi: 10.1074/jbc.M114.586958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Armando Gagliardi P, Puliafito A, di Blasio L, Chianale F, Somale D, Seano G, et al. Real-time monitoring of cell protrusion dynamics by impedance responses. Scientific reports. 2015;5:10206. doi: 10.1038/srep10206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochimica et biophysica acta. 2007;1773:642–52. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuzga DS, Pelta-Heller J, Li P, Bombonati A, Waldman SA, Pitari GM. Phosphorylation of vasodilator-stimulated phosphoprotein Ser239 suppresses filopodia and invadopodia in colon cancer. Int J Cancer. 2012;130:2539–48. doi: 10.1002/ijc.26257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anton KA, Sinclair J, Ohoka A, Kajita M, Ishikawa S, Benz PM, et al. PKA-regulated VASP phosphorylation promotes extrusion of transformed cells from the epithelium. J Cell Sci. 2014;127:3425–33. doi: 10.1242/jcs.149674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Developmental biology. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 37.Jevnaker AM, Khuu C, Kjole E, Bryne M, Osmundsen H. Expression of members of the miRNA17–92 cluster during development and in carcinogenesis. J Cell Physiol. 2011;226:2257–66. doi: 10.1002/jcp.22562. [DOI] [PubMed] [Google Scholar]
- 38.Li X, Yang H, Tian Q, Liu Y, Weng Y. Upregulation of microRNA-17–92 cluster associates with tumor progression and prognosis in osteosarcoma. Neoplasma. 2014;61:453–60. doi: 10.4149/neo_2014_056. [DOI] [PubMed] [Google Scholar]
- 39.Horrocks LA, Yeo YK. Health benefits of docosahexaenoic acid (DHA) Pharmacological research : the official journal of the Italian Pharmacological Society. 1999;40:211–25. doi: 10.1006/phrs.1999.0495. [DOI] [PubMed] [Google Scholar]
- 40.Blanckaert V, Ulmann L, Mimouni V, Antol J, Brancquart L, Chenais B. Docosahexaenoic acid intake decreases proliferation, increases apoptosis and decreases the invasive potential of the human breast carcinoma cell line MDA-MB-231. International journal of oncology. 2010;36:737–42. doi: 10.3892/ijo_00000549. [DOI] [PubMed] [Google Scholar]
- 41.Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, et al. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:6530–5. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jing K, Song KS, Shin S, Kim N, Jeong S, Oh HR, et al. Docosahexaenoic acid induces autophagy through p53/AMPK/mTOR signaling and promotes apoptosis in human cancer cells harboring wild-type p53. Autophagy. 2011;7:1348–58. doi: 10.4161/auto.7.11.16658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim N, Jeong S, Jing K, Shin S, Kim S, Heo JY, et al. Docosahexaenoic Acid Induces Cell Death in Human Non-Small Cell Lung Cancer Cells by Repressing mTOR via AMPK Activation and PI3K/Akt Inhibition. BioMed research international. 2015;2015:239764. doi: 10.1155/2015/239764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun SN, Jia WD, Chen H, Ma JL, Ge YS, Yu JH, et al. Docosahexaenoic acid (DHA) induces apoptosis in human hepatocellular carcinoma cells. International journal of clinical and experimental pathology. 2013;6:281–9. [PMC free article] [PubMed] [Google Scholar]
- 45.Snegovskikh V, Mutlu L, Massasa E, Taylor HS. Identification of putative fallopian tube stem cells. Reproductive sciences. 2014;21:1460–4. doi: 10.1177/1933719114553448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Farrell MR, Rogers LK, Liu Y, Welty SE, Tipple TE. Thioredoxin-interacting protein inhibits hypoxia-inducible factor transcriptional activity. Free radical biology & medicine. 2010;49:1361–7. doi: 10.1016/j.freeradbiomed.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kato A, Okura T, Hamada C, Miyoshi S, Katayama H, Higaki J, et al. Cell stress induces upregulation of osteopontin via the ERK pathway in type II alveolar epithelial cells. PloS one. 2014;9:e100106. doi: 10.1371/journal.pone.0100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herrera I, Cisneros J, Maldonado M, Ramirez R, Ortiz-Quintero B, Anso E, et al. Matrix metalloproteinase (MMP)-1 induces lung alveolar epithelial cell migration and proliferation, protects from apoptosis, and represses mitochondrial oxygen consumption. The Journal of biological chemistry. 2013;288:25964–75. doi: 10.1074/jbc.M113.459784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sidani M, Wessels D, Mouneimne G, Ghosh M, Goswami S, Sarmiento C, et al. Cofilin determines the migration behavior and turning frequency of metastatic cancer cells. J Cell Biol. 2007;179:777–91. doi: 10.1083/jcb.200707009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen R, Greenberg E, Nemlich Y, Schachter J, Markel G. miR-17 regulates melanoma cell motility by inhibiting the translation of ETV1. Oncotarget. 2015;6:19006–16. doi: 10.18632/oncotarget.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Danielson LS, Reavie L, Coussens M, Davalos V, Castillo-Martin M, Guijarro MV, et al. Limited miR-17–92 overexpression drives hematologic malignancies. Leukemia research. 2015;39:335–41. doi: 10.1016/j.leukres.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li H, Bian C, Liao L, Li J, Zhao RC. miR-17-5p promotes human breast cancer cell migration and invasion through suppression of HBP1. Breast cancer research and treatment. 2011;126:565–75. doi: 10.1007/s10549-010-0954-4. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Yang S, Yan W, Yang J, Qin YJ, Lin XL, et al. MicroRNA-19 triggers epithelial-mesenchymal transition of lung cancer cells accompanied by growth inhibition. Laboratory investigation; a journal of technical methods and pathology. 2015;95:1056–70. doi: 10.1038/labinvest.2015.76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.